Abstract
AIM:
To establish pancreatic alpha-cell mass in lean non-diabetic humans over the adult lifespan, performed as a follow up study to beta cell mass across the adult human lifespan.
METHODS:
We examined human pancreatic autopsy tissue from 66 lean non-diabetic individuals aged from 30–102 years, grouped into deciles: 3rd(30-39 years), 4th(40-49 years), 5th(50-59 years), 6th(60-69 years), 7th(70-79 years), 8th(80-89 years) and 9th deciles(90+ years). Sections of pancreas were immunostained for glucagon and analyzed for fractional alpha-cell area. Population-based pancreatic volume data was used to calculate alpha-cell mass.
RESULTS:
With advanced age, the exocrine pancreas undergoes atrophy demonstrated by increased fat area (as % exocrine area) (0.05±0.01 vs 1.6±0.7% fat area of total exocrine pancreas, 3rd vs 9th decile, p<0.05). Consequently, islet density increases with age (2.7±0.4 vs 10.5±3.3 islets/mm2, 3rd vs 9th decile, p<0.05). Alpha-cell fractional area increases with advanced age (0.34±0.05% vs 0.73±0.26%, 3rd vs 9th decile, p<0.05). However, alpha-cell mass remains constant at ~190mg throughout the adult lifespan in lean non-diabetic humans. Within islets, alpha-cell distribution between mantle and core is unchanged across deciles (1862±220 vs 1945±200 vs 1948±139 alpha-cells in islet mantle/mm2, 3rd vs 6th vs 9th decile, p=0.93 and 1912±442 vs 1449±123 vs 1514±168 alpha-cells in islet core/mm2, 3rd vs 6th vs 9th decile, p=0.47), suggesting that human islets retain their structural organization in the setting of age-related exocrine atrophy.
CONCLUSIONS:
Consistent with our previous findings for beta-cell mass, alpha-cell mass remains constant in humans, even with advanced age. Pancreatic endocrine cells are much more robustly preserved than exocrine cells in aged humans and islets maintain their structural integrity throughout life.
Keywords: Aging, Alpha cell mass, Human, Pancreas
INTRODUCTION
Alpha-cells are the second most abundant cell type in the pancreatic islets, predominantly expressing and secreting glucagon. The coordinated secretion of glucagon and insulin by alpha- and beta-cells, respectively, is the principal controller of glycemia under physiological conditions. Both cell types respond oppositely to changes in blood glucose concentration: while hypoglycemic conditions induce alpha-cell secretion, beta-cells release insulin when glucose levels increase 1, 2. Dysfunction or loss of these cells disrupts the circulating levels of insulin and glucagon that may ultimately lead to the development of diabetes 3. Experimental and clinical evidence supports the ‘bi-hormonal hypothesis’ 4, 5 of diabetes where, along with insulin deficiency or resistance, an absolute or relative excess of glucagon plays a role in the development of this metabolic disorder. For example, in type 2 diabetes (T2D), the impairment of insulin release as well as the development of insulin resistance is often accompanied by increased levels of glucagon in the fasting and postprandial states 6, 7
Because of the limited availability of human pancreas, knowledge about alpha-cell mass during the adult lifespan is lacking. Studies performed in different ethnic populations have shown marked variability in determining alpha-cell mass. For example, alpha-cell mass remained unchanged in non-diabetic and T2D European subjects, though, due to a deficiency of beta-cells, the ratio of alpha: beta cells was altered in the setting of T2D 8. In contrast, a significant decrease in alpha-cell mass was observed in a Japanese population with T2D compared to their non-diabetic counterparts 9 whilst studies performed in a Korean population revealed a significant increase in alpha-cell fractional area in patients with T2D compared to non-diabetic controls 10. Recently, alpha-cell mass was determined in patients with type 1 diabetes (T1D) using automated morphometric analysis on pancreatic tissue from the nPOD repository and demonstrated a reduced alpha-cell mass, though similar alpha-cell area, in T1D versus non-diabetic donors 11. The reported variability in determining alpha-cell mass may stem from the particular subject or population characteristics and/or methodological differences 12. The use of differing anti-glucagon antibodies in the above-mentioned studies further complicates between-study comparisons. Moreover, previous studies involved relatively small numbers of lean non-diabetic subjects of mixed ages (for example, 26 non-diabetic subjects were included in both the European 8 and the Japanese studies) 9. In addition, two of the previous studies included control subjects who were obese 8, 11 , further confounding the results as it has been reported alpha-cell mass is increased in obesity 13. Therefore, studies with larger cohorts of non-diabetic control subjects are needed to better define age-related changes in alpha-cell mass in humans.
In humans, after the age of sixty years, the exocrine pancreas undergoes a progressive decline in volume with an accompanying progressive increase in fibrosis 14, 15. Despite the decline in exocrine pancreas with advanced age, beta-cell mass is remarkably preserved 15, 16. To date, it is not known if this is also true of alpha-cells. In order to establish the mass and pattern of distribution of alpha cells within islets as well as in the ductal compartment in human pancreas across the adult lifespan, we studied sections of human pancreas immunostained for glucagon from the Mayo Clinic autopsy archives from 66 individuals aged from 30 to 102 years as a follow up study to our publication on beta cell mass across the adult human lifespan 16.
MATERIALS AND METHODS
Study design.
Sections of pancreas were obtained from the Mayo Clinic autopsy archives, from autopsies occurring from 1990 to 2006, from 66 lean, non-diabetic adult Caucasian human subjects aged from 30–102 years, and grouped into deciles: 3rd (30-39 years), 4th (40-49 years), 5th (50-59 years), 6th (60-69 years), 7th (70-79 years), 8th (80-89 years) and 9th deciles (90+ years). Since, at autopsy, the pancreas is not routinely dissected free from retroperitoneal tissues to permit measurement of pancreas weight, we made use of a prior report that established population data for pancreas volume by age and body mass index from abdominal computed tomography (CT) scans in 1,887 adults 15 to compute a pancreas volume for each individual. In the previous study by Saisho et al, beta-cell mass was calculated as a product of fractional beta-cell area and pancreas weight (assuming 1cm3 pancreas = 1g) 16. Using the same formulae, we determined alpha-cell mass. Beta-cell area and beta-cell mass were previously reported in these subjects 16. All pancreatic tissue samples were collected from the tail of pancreas.
Human subjects (Table 1).
Table 1.
Demographic data and assessment of pancreatic dysplasia in the human subjects.
| Decile 30-39 |
Age (years) |
BMI | Gender | Pancreatic dysplasia |
|---|---|---|---|---|
| 1 | 30 | 20.3 | M | x |
| 2 | 30 | 22.6 | M | x |
| 3 | 32 | 20.4 | F | x |
| 4 | 33 | 22.3 | M | x |
| 5 | 35 | 23.8 | F | x |
| 6 | 37 | 19 | F | x |
| 7 | 38 | 19.9 | M | x |
| 8 | 38 | 24.8 | F | x |
| 9 | 39 | 22.1 | M | x |
| 10 | 39 | 23.4 | M | x |
| Mean | 35.1 | 21.9 | ||
| SD | 3.6 | 1.9 | ||
| SEM | 1.1 | 0.6 | ||
| Decile 40-49 |
||||
| 11 | 40 | 23.2 | F | Single duct with PanIN1a |
| 12 | 41 | 22.9 | F | Several small foci of PanIN1a and ADM |
| 13 | 46 | 24.6 | F | x |
| 14 | 46 | 24.4 | M | Single duct with PanIN1a in small focus of ADM |
| 15 | 43 | 23.5 | F | x |
| 16 | 47 | 25.1 | F | x |
| Mean | 43.8 | 24.0 | ||
| SD | 2.9 | 0.9 | ||
| SEM | 1.2 | 0.4 | ||
| Decile 50-59 |
||||
| 17 | 50 | 23.4 | M | x |
| 18 | 52 | 23.6 | F | One small focus with PanIN1a-b and ADM |
| 19 | 53 | 22.6 | F | x |
| 20 | 53 | 23.5 | M | x |
| 21 | 52 | 16.8 | F | x |
| 22 | 52 | 17.9 | M | x |
| 23 | 53 | 17.8 | M | x |
| 24 | 55 | 23 | M | x |
| 25 | 57 | 15.1 | F | x |
| 26 | 57 | 22.4 | M | x |
| 53.4 | 20.6 | |||
| 2.3 | 3.3 | |||
| 0.7 | 1.0 | |||
| Decile 60-69 |
||||
| 27 | 60 | 20.8 | M | x |
| 28 | 60 | 21.7 | M | x |
| 29 | 60 | 22.4 | M | x |
| 30 | 61 | 16.2 | M | x |
| 31 | 62 | 14.5 | F | x |
| 32 | 63 | 20.3 | M | Single duct with PanIN1a, single duct with PanIN1b |
| 33 | 63 | 23.4 | M | x |
| 34 | 64 | 23.3 | M | x |
| 35 | 65 | 20 | F | Several small ducts with PanIN1a |
| 36 | 68 | 24.5 | M | x |
| Mean | 62.6 | 20.7 | ||
| SD | 2.6 | 3.2 | ||
| SEM | 0.8 | 1.0 | ||
| Decile 70-79 |
||||
| 37 | 73 | 15.2 | F | Three ducts with PanIN1a |
| 38 | 73 | 20.6 | F | Multiple foci of ADM |
| 39 | 74 | 18.7 | M | x |
| 40 | 75 | 20.8 | F | x |
| 41 | 75 | 23 | M | x |
| 42 | 75 | 23.5 | F | Single duct with PanIN1a |
| 43 | 76 | 15.1 | F | x |
| 44 | 77 | 24.7 | M | x |
| 45 | 78 | 21.6 | F | One focus of PanIN1a in ducts |
| 46 | 79 | 18.6 | F | Single duct with PanIN1a |
| Mean | 75.5 | 20.2 | ||
| SD | 2.0 | 3.3 | ||
| SEM | 0.6 | 1.0 | ||
| Decile 80-89 |
||||
| 47 | 80 | 22.9 | F | One focus of ADM and PanIN1a in ducts |
| 48 | 81 | 24 | M | x |
| 49 | 83 | 21.9 | F | Multiple foci of ADM |
| 50 | 84 | 21.4 | F | x |
| 51 | 86 | 23.1 | M | Multiple foci of ADM |
| 52 | 86 | 24.3 | M | x |
| 53 | 88 | 20.1 | F | One focus of ADM, multiple small ducts with PanIN1a |
| 54 | 89 | 18 | F | Single duct with PanIN1a |
| 55 | 89 | 23.2 | F | Multiple ducts with PanIN1a, multiple areas of ADM |
| 56 | 86 | 16 | F | Several small ducts with PanIN1a |
| Mean | 85.2 | 21.5 | ||
| SD | 3.2 | 2.7 | ||
| SEM | 1.0 | 0.9 | ||
| Decile 90+ |
||||
| 57 | 90 | 17.6 | F | x |
| 58 | 90 | 18.5 | F | One focus of ADM |
| 59 | 90 | 23 | F | x |
| 60 | 91 | 16.2 | M | Multiple ducts with PanIN1a-b lesions |
| 61 | 92 | 18.4 | M | Several foci of ADM |
| 62 | 93 | 23.2 | F | x |
| 63 | 97 | 19.6 | F | x |
| 64 | 100 | 23.6 | F | Multiple ducts with PanIN1a-b; single duct with PanIN2; several foci of ADM |
| 65 | 100 | 24.9 | F | PanIN1a-b lesions in 2 larger ducts; several foci of ADM |
| 66 | 103 | 19.3 | F | Several larger ducts with PanIN1a lesions |
| Mean | 94.6 | 20.4 | ||
| SD | 4.9 | 3.0 | ||
| SEM | 1.6 | 0.9 | ||
x, no dysplasia; PanIN, Pancreatic intraepithelial neoplasia; ADM, acinar-to-ductal metaplasia
Permission for the study was obtained from the University of California, Los Angeles (UCLA), and Mayo Clinic Institutional Review Boards. Potential cases were first identified by retrospective analysis of the Mayo Clinic autopsy database. To be included, cases were required to have had 1) a full autopsy within 24 h of death; 2) pancreatic tissue stored that was of adequate size and quality; 3) no history of diabetes, pancreatitis, or pancreatic surgery; and 4) no use of glucocorticoids. Cases were excluded if pancreatic tissue had undergone autolysis. Preference was given to cases where the final illness was relatively short term (for example, trauma or a sudden vascular event), so as to minimize the confounding effects of a prolonged final illness on the nutritional status of the individual and any effects this may have had on islet morphology. Case subjects were identified based on these preferences at the Mayo Clinic, and the sections of selected case subjects were obtained and made available to UCLA investigators in a manner coded to conceal the personal identity of the subjects. The blood glucose value was obtained from the most recent ambulatory overnight-fasted value in the Mayo Medical Center clinical record, not from the final in-patient glucose values, which are subject to confounding factors such as pre-mortem stress and intravenous glucose administration. The characteristics of the cases are summarized in Table 1. Seventy lean, non-diabetic subjects were identified, spanning the adult age range of 30 to 103 years at the time of death. A lean adult was defined by a BMI of less than 25 kg/m2. Four of the cases were excluded (one because of a diagnosis of AIDS, one because of a BMI >25 kg/m2, one because of poor tissue quality, and one because the same tissue had been allocated two different codes), leaving 66 cases in total. Study size was determined by the number of autopsy cases from which pancreatic sections were available that met study criteria. A summary of the decile case demographics, including average fasting plasma glucose for each decile, is presented in Table 2.
Table 2.
Summary of decile case demographics included in this study.
| Age groups | n (M/F) | Age (years) | BMI (kg/m2) | FPG (mg/dl)* |
|---|---|---|---|---|
| 30-39 years | 10 (6/4) | 35.1 ± 1.1 | 21.9 ± 0.6 | 85 ± 3 |
| 40-49 years | 6 (1/5) | 43.8 ± 1.2 | 24.0 ± 0.4 | 90 ± 3 |
| 50-59 years | 10 (6/4) | 53.4 ± 0.7 | 20.6 ± 1.0 | 88 ± 3 |
| 60-69 years | 10 (8/2) | 62.6 ± 0.8 | 20.7 ± 1.0 | 89 ± 2 |
| 70-79 years | 10 (3/7) | 75.5 ± 0.6 | 20.2 ± 1.0 | 90 ± 5 |
| 80-89 years | 10 (3/7) | 85.2 ± 1.0 | 21.5 ± 0.9 | 95 ± 3 |
| 90+ years | 10 (2/8) | 94.6 ± 1.6 | 20.4 ± 0.9 | 95 ± 3 |
Values are means ± SEM.
FPG had been measured within one year prior to death in 46 subjects.
Pancreatic tissue processing.
At autopsy, the sample of pancreas was collected from the tail and, together with a sample of spleen, fixed in formaldehyde and embedded in paraffin for subsequent analysis. 5μm sections were stained for glucagon (Diaminobenzidine peroxidase staining) and hematoxylin for light microcopy. The primary antibody was glucagon rabbit (Immunostar, Hudson, WI, Cat. No. 20076, RRID: AB_572241) at a dilution of 1:1000. The secondary antibody was biotin donkey anti-rabbit at a dilution of 1:100 (Jackson ImmunoResearch Laboratories, West Grove, PA, Cat. No. 711-166-152, RRID: AB_2313568). For DAB staining, a DAB peroxidase substrate kit was used. The primary glucagon antibody used here was thoroughly validated, as described in our prior publication 17.
Staining Protocol.
After rehydration, the tissue was blocked with 3% H2O2/10% Methanol for 30 minutes at room temperature followed by blocking with Tris B for one hour at room temperature. Next, the tissue was incubated with Glucagon Rabbit antibody (1:1000) in Tris B overnight at +4°C. The next day, the sections were incubated in biotin donkey anti-rabbit (1:100) in Tris B for 1 hour at room temperature. Next ABC Peroxidase solution (VECTASTATIN ABC Peroxidase PK-4000, Vector Laboratories Inc., Burlingame, CA) was added for 1 hour at room temperature. Then, DAB solution was added for about 1-2 minutes per section under the microscope to ensure proper staining of tissue. After washing with water, sections were counterstained in 5 times H2O-diluted Harris Hematoxylin (Thermo Fisher Scientific, Waltham, MA) for 15 seconds and cover slipped with Permount (Thermo Fisher Scientific, Waltham, MA).
Morphometric analysis.
To quantify the pancreatic fractional alpha-cell area, first the entire pancreatic section for each case was imaged at 40X magnification (4X objective) using an inverted Olympus microscope (Olympus Inc., Center Valley, PA) and digitally stored. The fractional alpha-cell area was then quantified by determining the ratio of alpha-cell area to total pancreas parenchymal area using Image Pro Plus software (Media Cybernetics, Silver Springs, MD). A.S.M.M and M.C. independently evaluated the pancreatic fractional alpha-cell area and, if the result between investigators varied by 20 % or more, both investigators performed the analyses again. This occurred in 6 cases and, in all 6 cases, with re-analysis the resulting variance was less than 20%. In those 6 cases, the mean of the two second evaluations was then used in the final analysis. The mean of the measurements by the two investigators was used in the calculations.
Pancreas parenchymal volume.
To establish alpha-cell mass, the pancreas parenchymal volume was computed for each individual by using the equations derived by Saisho as developed in a large cohort of individuals undergoing pancreas CT scans 15. For subjects less than 60 years of age, the equation for calculating pancreatic parenchymal volume is y = 34.6 + 0.55x where x is the BMI; for subjects 60 years or older, the equation for calculating pancreatic parenchymal volume is y = 71.7 - 0.45x where x is age in years. In short, pancreas parenchymal volume in humans remains relatively constant from 20 to 60 years of age, after which it progressively declines. The alpha-cell mass in each individual was calculated as the product of the fractional alpha-cell area and the computed pancreas parenchymal weight (assuming 1 g of weight per 1 cm3 pancreas volume).
Glucagon immunoreactive cells in and around pancreatic ducts and pancreatic duct glands.
It has been proposed that glucagon positive cells in and around ducts may be newly forming from non-endocrine precursors and may increase with pancreatic dysplasia. Since pancreas undergoes some dysplastic change with age, we quantified the presence of glucagon immunoreactive cells in and around ducts. For a cell to be considered a glucagon positive cell in a pancreatic duct or PDG, it had to be located in the epithelial lining with a visible nucleus that was encircled at least 50% by glucagon positive cytoplasm. The numbers of glucagon positive cells present were quantified for all large ducts and pancreatic duct glands (PDGs) in each section. All duct cells contained within the large duct and pancreatic duct gland compartment were counted on each section of pancreas. The total number of duct cells counted for each decile is as follows: Age 30-39 years, Mean 1,236 cells (Range 44-6,934); Age 40-49 years, Mean 904 cells (Range 84-5,168); Age 50-59 years, Mean 648 cells (Range 104-2,401); Age 60-69 years, Mean 643 cells (Range 83-2,859); Age 70-79 years, Mean 717 cells (Range 67-3,547); Age 80-89 years, Mean 613 cells (Range 189-1,109); Age 90+ years, Mean 2,204 cells (Range 108-7,471).
Fat deposition in pancreas across human lifespan.
To determine the area of pancreas occupied by fat, 6 randomly selected cases from each of three deciles (3rd, 6th and 9th) were analyzed. The area of pancreas occupied by fat was determined for each case along with the total area of the pancreas using Image J software. Thereby, the percentage area occupied by fat (relative to total pancreas area) was calculated for the selected cases from each decile.
Alpha cell distribution in islets in human lifespan.
To determine the distribution of alpha cells within islets in human pancreas, pancreatic sections from 6 randomly selected subjects from each of three deciles (3rd, 6th and 9th) were analyzed. In total, 842 islets were analyzed. The number of alpha cells in the periphery of an islet (alpha cells present in the islet mantle) and those in the central region of an islet (alpha cells present in the islet core) were counted manually. The area of each islet was measured using Image J software. The density of alpha cells, in both the islet mantle and core, was calculated using the alpha cell number and islet area data.
Statistical Analysis.
Data are presented as mean ± SEM. Statistical comparisons were carried out using the Student t test or one-way ANOVA, with a p value of < 0.05 taken as significant. A simple regression was carried out for the correlation analysis.
RESULTS
Distribution of glucagon immunoreactivity in pancreatic sections.
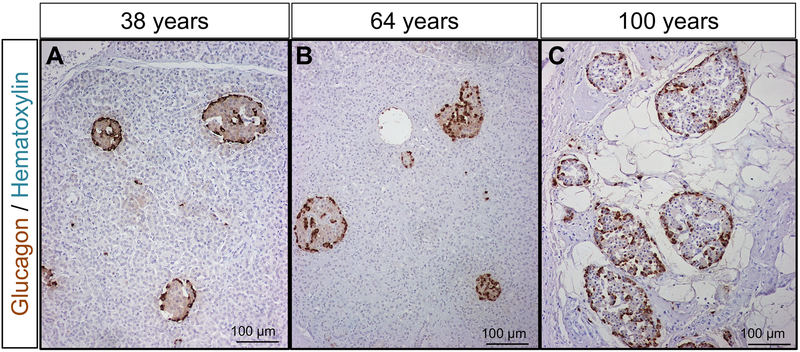
As expected, alpha-cell immunoreactivity in pancreas sections was largely confined to Islets of Langerhans. As has been previously described in humans, alpha cells are not solely confined to the periphery of the islet as in rodents. Rather, the alpha cells are distributed throughout the islet 18. This pattern of glucagon staining was unchanged throughout adult life (Fig. 1A-C).
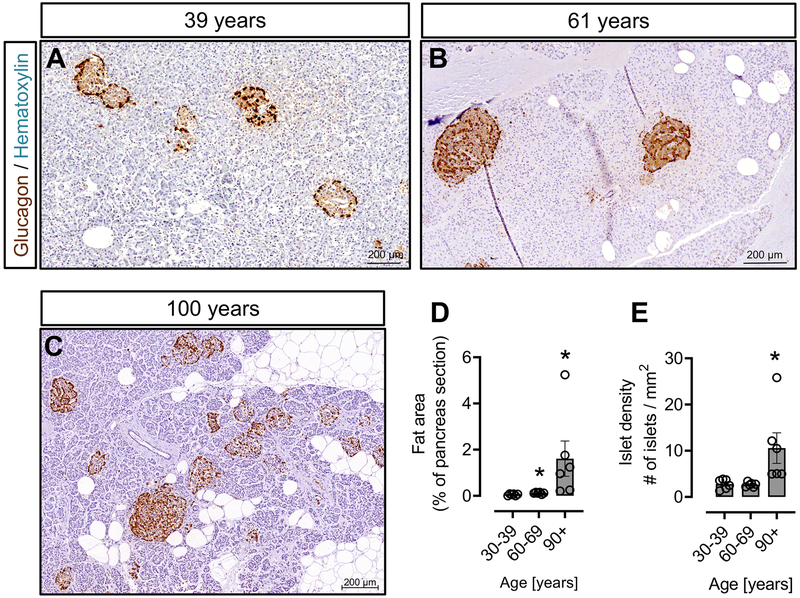
Figure 1. Histology of the exocrine pancreas and the distribution of alpha cells in islets across the adult human lifespan.
Representative sections of human pancreas stained for glucagon (DAB) and counterstained with hematoxylin from subjects who died at ages encompassing the adult human lifespan [38 years (A), 64 years (B) and 100 years (C)]. Of note, glucagon-positive cells are not confined to the periphery of the islet, but are distributed throughout the islet, and this distribution does not change with age. However, due to atrophy of the exocrine pancreas, the density of islets is increased from ~70 years onwards; this results in an increase in glucagon area % in human pancreas from subjects aged ~70 years or more, though alpha-cell mass remains constant. Scale bars, 100μm.
Consistent with prior reports, from age ~60 years, there was an appreciable increase in pancreatic fibrosis coincident with the presumptive acinar atrophy. Pancreatic dysplasia was minimal in this cohort but did tend to increase with age.
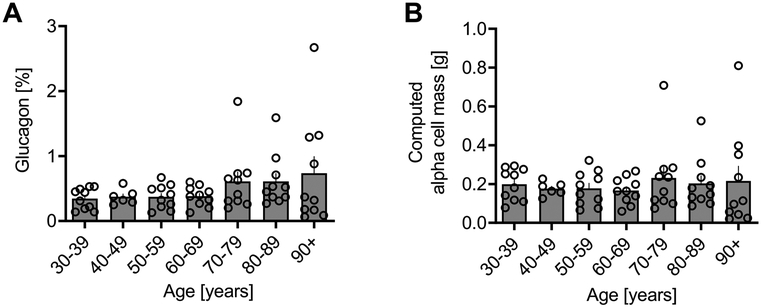
Pancreatic alpha cell fractional area.
The pancreatic fractional alpha cell area was relatively constant at ~0.35% from age 30-60 years, and then progressively increased thereafter to ~0.73% by age 100 years (Fig. 2A). The computed alpha cell mass remained remarkably constant at ~190 mg throughout adult life (Fig. 2B), the increase in pancreatic fractional alpha cell area from age ~60 years coinciding with the decline in pancreas mass. Computed alpha cell mass also remained constant between genders throughout the human lifespan (Supplementary Fig. 1).
Figure 2. Alpha cell area% and computed alpha cell mass according to age in lean non-diabetic subjects.
Glucagon area %, shown as individual data with mean bar graphs (A), and computed alpha cell mass data also shown as individual data with mean bar graphs (B). Glucagon area % remained constant from the 30s decile through the 60s decile, after which glucagon area % increased due to the replacement of exocrine pancreas by fibrous tissue. By contrast, alpha-cell mass remained constant throughout the adult human lifespan.
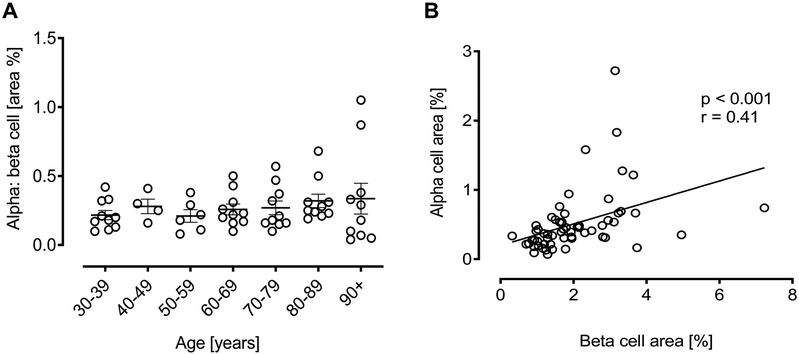
Alpha: Beta Cell Ratio.
The ratio of alpha to beta cell fractional area remained constant throughout the adult human lifespan (Fig. 3A) and so, as expected, the pancreatic fractional area occupied by alpha or beta cells was also positively correlated (Fig. 3B) (p < 0.001, r = 0.41).
Figure 3. Ratio of alpha to beta fractional area according to age in lean non-diabetic subjects.
The ratio of alpha to beta cell fractional area in each decile group (A). No change was seen in this ratio with advancing age. The positive correlation of pancreatic fractional area occupied by alpha or beta cells (B).
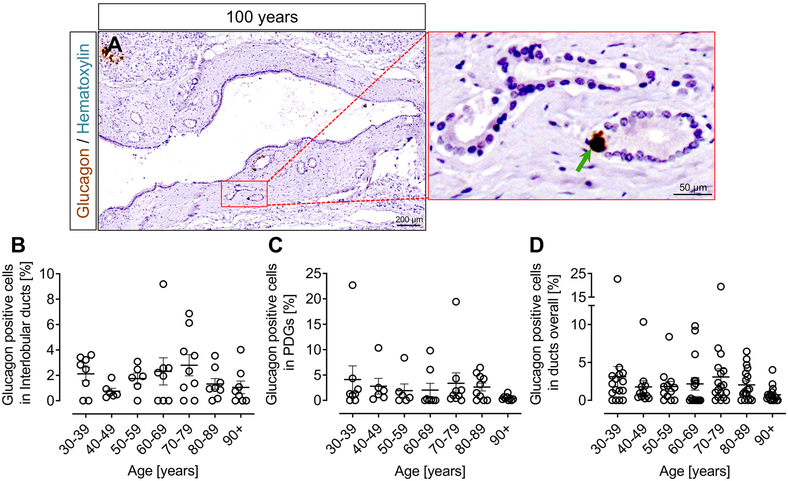
Glucagon positive cells in ducts.
Glucagon positive cells were occasionally present in and around ducts (Fig. 4A) in pancreas sections from across the whole age spectrum, with no appreciable change with age. The mean and range of percentage glucagon positive cells in interlobular ducts (Fig. 4B) and pancreatic duct glands (PDGs) (Fig. 4C) combined for each decile of adult life was as follows: Age 30-39 years, Mean 3.52% (Range 0.85-11.75%); Age 40-49 years, Mean 1.23% (Range 0.25-3.74%); Age 50-59 years, Mean 2.09% (Range 0.85-4.38%); Age 60-69 years, Mean 2.69% (Range 1.45-7.38%); Age 70-79 years, Mean 2.88% (Range 0.17-10.03%); Age 80-89 years, Mean 2.60% (Range 0.22-6.45%); Age 90+ years, Mean 0.90% (Range 0.38-1.79%) (Figure 4D). There was no change in the frequency of glucagon positive cells in ducts (either interlobular or PDGs) with age (ANOVA).
Figure 4. Glucagon positive cells in ducts according to age in lean non-diabetic subjects.
Representative sections of human pancreas stained for glucagon (DAB) and counterstained with hematoxylin to show glucagon expression in ducts in human (100 years) pancreas (A). Inset, higher power area of glucagon staining in pancreatic duct glands (PDGs) indicated by red square in the lower power image. The green arrow indicates glucagon immunoreactivity in a PDG. The percentage of glucagon positive cells in large ducts (B), PDGs (C) and in the combined ductal epithelial compartment (D). The percentage of glucagon positive cells present within the ductal epithelial compartment was unchanged across the adult lifespan. Scale bars, 200μm for lower power image and 50μm for higher power image.
Changes of exocrine pancreas and islet density with advancing age.
Human pancreas undergoes morphological changes with age, mainly due to fibrosis or fat deposition in the exocrine pancreas 19. We measured the area occupied by fat in the pancreas sections of subjects from 3 deciles [3rd decile (age range 30-39 years), 6th decile (age range 60-69 years) and 9th decile (age range 90+ years)]. Our data confirmed that the exocrine pancreas undergoes atrophy with increasing age and that the fat content increases with advancing age (Fig. 5A-C). The mean fat area (% of total pancreas area) of the 9th decile age group was increased compared to that of the 6th or 3rd decile age groups (0.05 ± 0.01 vs 1.6 ± 0.8, % fat area of total pancreas area, 3rd decile age group vs 9th decile age group, p<0.05; 0.11 ± 0.02 vs 1.6 ± 0.8, % fat area of total pancreas area, 6th decile age group vs 9th decile age group, p<0.05). The % fat area of the 6th decile age group was also higher compared to 3rd decile age group (0.05 ± 0.01 vs 0.11 ± 0.02, % fat area (of total pancreas area), 3rd decile age group vs 6th decile age group vs, p<0.05) (Fig. 5D), thus demonstrating an increasing trend towards fat accumulation in the exocrine pancreas with aging.
Figure 5. Pancreas area occupied by fat and islet density across the human lifespan.
Representative sections of human pancreas stained for glucagon (DAB) and counterstained with hematoxylin to show the glucagon immunoreactivity, distribution of fat and density of islets from subjects who died at ages encompassing the adult human lifespan [39 years (A), 61 years (B) and 100 years (C)]. A significant increase in fat area was found in the 6th decile group (age range 60-69 years) and, even more so, in the 9th decile group (age range 90+ years) compared with the 3rd decile group (age range 30-39 years) (D). A significant increase in islet density was found in the 9th decile group (age range 90+ years) compared to both the 3rd decile group (age range 30-39 years) and the 6th decile group (age range 60-69 years) (E). Even though pancreatic atrophy is apparent from age 60, there was no change in islet density in the 6th decile group (age range 60-69 years) compared to 3rd decile group. *, p < 0.05, N = 6 cases per decile group. Scale bars, 200μm.
We then sought to determine the islet density with advancing age. The average number of islets counted in sections from subjects in the 3rd, 6th and 9th decile groups was 42 ± 3, 40 ± 3 and 58 ± 12, respectively. As a consequence of exocrine tissue atrophy, the density of pancreatic islets increases with advancing age (2.7 ± 0.4 vs 10.5 ± 3.3 islets/mm2, 3rd vs 9th decile, p<0.05; 2.6 ± 0.2 vs 10.5 ± 3.3 islets/mm2, 6th vs 9th decile, p<0.05) (Fig. 5E). Even though a degree of pancreatic atrophy was observed in the of 6th decile group, this was not reflected in an alteration of islet density between the 3rd and 6th decile groups (2.7 ± 0.4 vs 2.6 ± 0.2 islets/mm2, 3rd vs 6th decile, p=0.77) (Fig. 5E).
Distribution of alpha-cells within islets with advancing of age in non-diabetic lean humans.
Human islets have a unique architecture allowing all endocrine cells to be adjacent to blood vessels, with beta-cells tending to be largely in the core region and alpha-cells tending to be largely in the mantle region. Each islet is encompassed by blood vessels that penetrate and branch inside the islets 20.
We investigated the distribution of alpha-cells in islets in the decile groups. Our data demonstrate a complex arrangement of alpha-cells throughout the adult human lifespan. Since the density of islets increased as a consequence of pancreas atrophy with aging, we questioned whether the islets that come to lie adjacent to one another due to loss of exocrine tissue maintain their architectural integrity or whether they coalesce to form larger islets. We reasoned that, if with ageing the islets coalesce with one another, there would be more alpha-cells in the islet core than in the mantle region in the latter deciles. To determine this, we calculated the number of alpha cells in core and mantle regions within islets in pancreas sections of subjects from 3 decile groups (3rd, 6th and 9th). The average number of alpha cells counted in the islet mantle region for the 3rd, 6th and 9th decile groups was 570 ± 30, 497 ± 32 and 687 ± 115, respectively. The average number of alpha cells counted in the islet core region for the 3rd, 6th and 9th decile groups was 768 ± 87, 541 ± 93 and 719 ± 83, respectively. The mean islet area in the 3rd, 6th and 9th decile groups was 0.018 ± 0.001 mm2, 0.013 ± 0.002 mm2 and 0.016 ± 0.001 mm2, respectively.
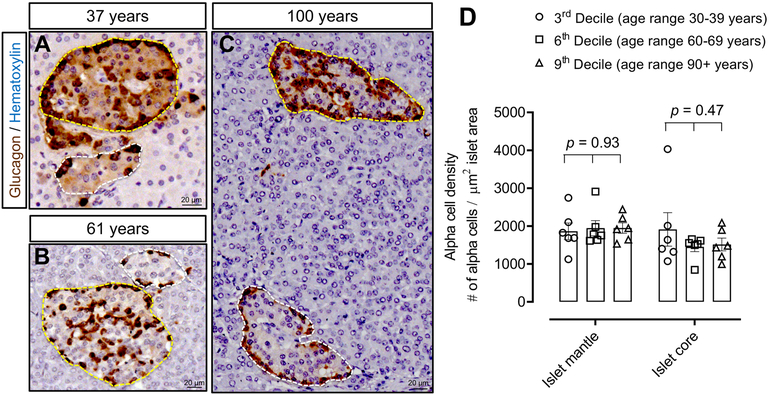
We found a heterogeneous distribution of alpha-cells within islets in all 3 decile groups (Fig. 6A-C) with no alteration in alpha-cell distribution between decile groups in either the islet mantle (1862 ± 220 vs 1945 ± 200 vs 1948 ± 139 alpha-cells in the islet mantle/mm2, 3rd vs 6th vs 9th decile, p=0.93) or the islet core region (1912 ± 443 vs 1449 ± 123 vs 1514 ± 168 alpha-cells in the islet core /mm2, 3rd vs 6th vs 9th decile, p=0.47) (Fig. 6D). These data suggest that human islets maintain their structural integrity and do not merge even under conditions of extreme exocrine atrophy as occurs with advanced age.
Figure 6. Distribution of alpha cells within islets across the human lifespan.
Representative images showing islets with alpha cells distributed in both the mantle and core regions of an islet in human subjects aged 37 years (A), 61 years (B) and 100 years (C). The density of alpha cells in the mantle or core regions did not change with advancing age across the human lifespan (D). Yellow dotted lines encircle islets with alpha cells distributed in both the mantle and core; white dotted lines encircle islets with alpha cells restricted to the mantle. Scale bars, 20μm.
DISCUSSION
While much effort has been dedicated to the study of beta-cell mass in health and disease, less in known about alpha-cell mass. In order to understand whether and how the alpha-cell mass responds to disease states such as type 1 and type 2 diabetes, it is first important to understand if and how alpha-cell mass changes across the human lifespan in health.
With aging, increasing fibrosis in and around islets, as well as between and within the exocrine lobules of the aged pancreas, has been described 19. Fatty replacement increases with age in human pancreas, though the underlying mechanism is not well understood 21. Islets appear generally to be spared from the atrophic process and persist singly or in clusters in the diminishing exocrine compartment 22. Despite the preservation of islets, endocrine cell function may be affected with advancing age. The insulin secretory capacity of beta-cells has been reported to decrease with age 23, 24, for example. Additionally, we considered whether morphological changes in alpha cell distribution occur with aging.
This study was performed as a follow up study to our publication on beta cell mass across the adult human lifespan 16 and demonstrates that alpha-cell mass is well preserved (in contrast to the exocrine pancreas) in humans across the span of adult life and even despite advanced age. These results are strikingly similar to the studies done to establish beta-cell mass where, even with the clear loss of pancreatic acinar tissue occurring with advanced age, beta-cell mass was preserved 25. This finding was subsequently confirmed in a study by Saisho et al where the fractional beta cell area was also relatively constant at ~1.7% from age 30-60 years and gradually increased thereafter to ~2,3% by age 100 years. The computed beta cell mass also remained constant, at ~800 mg, throughout adult life 16. Furthermore, we also determined that islet architecture is maintained, the distribution of alpha cells between the core and mantle regions of the islet being unaffected by the aging process.
Henquin and Rahier studied alpha-cell mass in the same non-diabetic and type 2 diabetic subjects in which they previously reported beta-cell mass, finding no difference in alpha-cell mass in type 2 diabetes compared with control subjects 8. Their study was directed primarily at identifying differences in alpha-cell mass between non-diabetic and type 2 subjects; as such, while their non-diabetic cohort of 52 autopsy subjects did have a wide age spectrum (from 39 to 91 years), half of the subjects were overweight or obese, leaving only 26 lean non-diabetic subjects. By contrast, our study was designed to include only lean subjects distributed in approximately equal numbers in each decile of adult life.
In keeping with other studies aiming to establish beta-cell or alpha-cell mass in humans, there are limitations. Instead of using a direct measurement of pancreas weight in the individuals from whom the pancreas samples were acquired, we have used population-based values attained from CT scans for pancreas volumes 15. We also assumed that the conversion of 1 cm3 of pancreas weighs 1g. Many subjects were examined to establish the population pancreas data, being purposely chosen to include the age and BMI range of the current study subjects. Although the use of population pancreas volumes is clearly a limitation, given the retroperitoneal and locally adherent nature of the human pancreas, measuring the weight of pancreas removed at autopsy is not without error.
Autopsy studies here are confined to the tail of the pancreas, and the changes in pancreas volume are calculated from population values ascertained in a large radiological study, an approach that was validated in nonhuman primates 26. A further limitation of autopsy studies is that changes in alpha-cell mass may occur as a consequence of the final illness or postmortem. To minimize this, we sought to include cases where the course of the final illness was relatively short and the time between death and autopsy was no longer than 12 hours. By definition, autopsy studies are cross-sectional, introducing the possibility of confounding variables.
For example, individuals who live to advanced old age are, by definition, a selected group compared with those who die young so, ideally, a cohort of individuals would be studied throughout life to establish changes in alpha-cell mass with aging.
Despite these limitations, we conclude that alpha-cell mass is robustly preserved in humans despite advanced age. The results of this follow-up study are in keeping with our previously reported data on beta-cell mass across the human lifespan 16. This is in contrast to the exocrine pancreas that undergoes marked atrophy with age. These results are likely generalizable to Caucasian populations but should be cautiously applied with regard to other ethnic groups.
Supplementary Material
Supplementary Figure 1: Comparison of computed alpha cell mass between males (black bars) and females (shaded bars) according to deciles of age. There was no difference in computed alpha cell mass between men and women at any stage of the human lifespan.
ACKNOWLEDGEMENTS
We would like to thank Bonnie Liu from the Larry L. Hillblom Islet Research Center at UCLA for editorial assistance. Funding for these studies was from NIH/NIDDK Grant #DK077967 and the Larry Hillblom Foundation Grant #2014-D-001-NET.
FUNDING
NIH/NIDDK Grant #DK077967 and Larry Hillblom Foundation Grant #2014-D-001-NET to PCB.
Abbreviations:
- BMI
body mass index
- nPOD
Network for Pancreatic Organ Donors with Diabetes
- PDGs
pancreatic duct glands
- T1D
type 1 diabetes
- T2D
type 2 diabetes
Footnotes
DATA AVAILABILITY
Data will be made available upon reasonable request to the corresponding author.
CONFLICT OF INTERESTS
There is no conflict of interests.
FINANCIAL DISCLOSURE
The authors have nothing to disclose.
REFERENCES
- 1.Nadal A, Quesada I & Soria B. Homologous and heterologous asynchronicity between identified alpha-, beta- and delta-cells within intact islets of Langerhans in the mouse. J Physiol 1999. 517 (Pt 1) 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quesada I, Todorova MG & Soria B. Different metabolic responses in alpha-, beta-, and delta-cells of the islet of Langerhans monitored by redox confocal microscopy. Biophys J 2006. 90 2641–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunning BE, Foley JE & Ahren B. Alpha cell function in health and disease: influence of glucagon-like peptide-1. Diabetologia 2005. 48 1700–1713. [DOI] [PubMed] [Google Scholar]
- 4.Unger RH & Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet 1975. 1 14–16. [DOI] [PubMed] [Google Scholar]
- 5.Unger RH. Glucagon and insulin: a bihormonal system. Compr Ther 1976. 2 20–26. [PubMed] [Google Scholar]
- 6.Reaven GM, Chen YD, Golay A, Swislocki AL & Jaspan JB. Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1987. 64 106–110. [DOI] [PubMed] [Google Scholar]
- 7.Larsson H & Ahren B. Islet dysfunction in insulin resistance involves impaired insulin secretion and increased glucagon secretion in postmenopausal women with impaired glucose tolerance. Diabetes Care 2000. 23 650–657. [DOI] [PubMed] [Google Scholar]
- 8.Henquin JC & Rahier J. Pancreatic alpha cell mass in European subjects with type 2 diabetes. Diabetologia 2011. 54 1720–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato S, Saisho Y, Inaishi J, Kou K, Murakami R, Yamada T & Itoh H. Effects of Glucocorticoid Treatment on beta- and alpha-Cell Mass in Japanese Adults With and Without Diabetes. Diabetes 2015. 64 2915–2927. [DOI] [PubMed] [Google Scholar]
- 10.Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, Song KH, Yoo SJ, Kang MI, Cha BY, Lee KW et al. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab 2003. 88 2300–2308. [DOI] [PubMed] [Google Scholar]
- 11.Bonnet-Serrano F, Diedisheim M, Mallone R & Larger E. Decreased alpha-cell mass and early structural alterations of the exocrine pancreas in patients with type 1 diabetes: An analysis based on the nPOD repository. PLoS One 2018. 13 e0191528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kou K, Saisho Y, Satoh S, Yamada T & Itoh H. Change in beta-cell mass in Japanese nondiabetic obese individuals. J Clin Endocrinol Metab 2013. 98 3724–3730. [DOI] [PubMed] [Google Scholar]
- 13.Ellenbroek JH, Töns HAM, Hanegraaf MAJ, Rabelink TJ, Engelse MA, Carlotti F & de Koning EJP. Pancreatic α-cell mass in obesity. Diabetes Obes Metab 2017. 19 1810–1813. [DOI] [PubMed] [Google Scholar]
- 14.Stamm BH. Incidence and diagnostic significance of minor pathologic changes in the adult pancreas at autopsy: a systematic study of 112 autopsies in patients without known pancreatic disease. Hum Pathol 1984. 15 677–683. [DOI] [PubMed] [Google Scholar]
- 15.Saisho Y, Butler AE, Meier JJ, Monchamp T, Allen-Auerbach M, Rizza RA & Butler PC. Pancreas volumes in humans from birth to age one hundred taking into account sex, obesity, and presence of type-2 diabetes. Clin Anat 2007. 20 933–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saisho Y, Butler AE, Manesso E, Elashoff D, Rizza RA & Butler PC. beta-cell mass and turnover in humans: effects of obesity and aging. Diabetes Care 2013. 36 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurlo T, Butler PC & Butler AE. Evaluation of immunohistochemical staining for glucagon in human pancreatic tissue. J Histotechnol 2016. 39 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO & Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A 2006. 103 2334–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta R & Ashish Kumar N. Morphology and Aging of the Human Adult Pancreas: An Electron Microscopic Study. Acta Medica Iranica 2018. 56 106–112. [Google Scholar]
- 20.Bosco D, Armanet M, Morel P, Niclauss N, Sgroi A, Muller YD, Giovannoni L, Parnaud G & Berney T. Unique arrangement of alpha- and beta-cells in human islets of Langerhans. Diabetes 2010. 59 1202–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosseel F, Brugman E, Fiey J, Goddeeris T, Bucuk E & Friberg J. Total fat replacement of the pancreas: case report and review of the literature. J Belge Radiol 1996. 79 199–200. [PubMed] [Google Scholar]
- 22.Makay O, Kazimi M, Aydin U, Nart D, Yilmaz F, Zeytunlu M, Goker E & Coker A. Fat replacement of the malignant pancreatic tissue after neoadjuvant therapy. Int J Clin Oncol 2010. 15 88–92. [DOI] [PubMed] [Google Scholar]
- 23.Utzschneider KM, Carr DB, Hull RL, Kodama K, Shofer JB, Retzlaff BM, Knopp RH & Kahn SE. Impact of intra-abdominal fat and age on insulin sensitivity and beta-cell function. Diabetes 2004. 53 2867–2872. [DOI] [PubMed] [Google Scholar]
- 24.Chiu KC, Martinez DS & Chu A. Comparison of the relationship of age and beta cell function in three ethnic groups. Clin Endocrinol (Oxf) 2005. 62 296–302. [DOI] [PubMed] [Google Scholar]
- 25.Rahier J, Guiot Y, Goebbels RM, Sempoux C & Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab 2008. 10 Suppl 4 32–42. [DOI] [PubMed] [Google Scholar]
- 26.Saisho Y, Manesso E, Butler AE, Galasso R, Kavanagh K, Flynn M, Zhang L, Clark P, Gurlo T, Toffolo GM et al. Ongoing beta-cell turnover in adult nonhuman primates is not adaptively increased in streptozotocin-induced diabetes. Diabetes 2011. 60 848–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Comparison of computed alpha cell mass between males (black bars) and females (shaded bars) according to deciles of age. There was no difference in computed alpha cell mass between men and women at any stage of the human lifespan.