Abstract
Species of flies in the genus Drosophila differ dramatically in their preferences for mates, but little is known about the genetic or neurological underpinnings of this evolution. Recent advances have been made to our understanding of one case: pheromone preference evolution between the species D. melanogaster and D. simulans. Males of both species are very sensitive to the pheromone 7,11-HD that is present only on the cuticle of female D. melanogaster. In one species this cue activates courtship, and in the other it represses it. This change in valence was recently shown to result from the modification of central processing neurons, rather than changes in peripherally expressed receptors, but nothing is known about the genetic changes that are responsible. In the current study, we show that a 1.35 Mb locus on the X chromosome has a major effect on male 7,11-HD preference. Unfortunately, when this locus is divided, the effect is largely lost. We instead attempt to filter the 159 genes within this region using our newfound understanding of the neuronal underpinnings of this phenotype to identify and test candidate genes. We present the results of these tests, and discuss the difficulty of identifying the genetic architecture of behavioral traits and the potential of connecting these genetic changes to the neuronal modifications that elicit different behaviors.
Keywords: male mate choice, pheromones, courtship behavior, reproductive isolation, behavioral genetics
Understanding the proximate mechanisms of phenotypic divergence has long been a goal of evolutionary biologists (Stern and Orgogozo 2008; Stern et al. 2009). Advances in genome sequencing have led to a recent boom in genotype-phenotype association studies, the large majority being for morphological traits (Martin and Orgogozo 2013). These findings have resulted in a better understanding of the molecular underpinnings of morphological evolution, allowing us to observe general patterns about the types of genetic changes commonly associated with phenotypic change, the number of loci involved, and the general size of their effects (Orgogozo and Martin 2013; Rebeiz and Williams 2017; Kittelmann et al. 2017). Comparatively, we have fewer studies of the proximate mechanisms underlying behavioral divergence with which to draw broad conclusions. This is unfortunate, because behaviors are important phenotypes, particularly with respect to speciation and biodiversity. For example, differences in host, habitat, and mating behaviors can form strong reproductive barriers between species (Coyne and Orr 1997, 2004).
Behaviors holistically involve the detection of stimuli via the peripheral nervous system, sensory integration via central nervous system processing, and the coordinated production of a behavioral output. It is therefore surprising that a large proportion of genes known to cause behavioral divergence between species affect sensory perception at the periphery, rather than the other molecular determinants of behavior (Leary et al. 2012; Cande et al. 2013; McBride et al. 2014; Auer et al. 2019). This could indicate that changes at the periphery, mainly in membrane-bound stimulus-detecting receptors, are favored targets of selection because they can have drastic effects on specific phenotypes while minimizing pleiotropic effects (McBride 2007). It is, however, difficult to generalize from so few examples. Moreover, it is quite possible that this pattern is due mainly to ascertainment bias (Rockman 2012). Indeed, many case studies that have successfully mapped causal genes explaining behavioral divergence have used a candidate-gene approach targeting sensory receptors, so it is plausible that other types of changes are more common but have been overlooked. For example, recent studies that take whole-genome approaches have identified important variants in genes affecting behavior that act at the synapse (Kocher et al. 2018), or as hormonal neuromodulators (Bendesky et al. 2017). To make generalized predictions about the types of changes underlying behavioral divergence, we need more studies of the genetic basis of behavior that take unbiased approaches, preferably in systems with multiple comparable cases of evolution in similar behaviors (i.e., “metamodel systems” sensu Kopp 2009). With an active research community, many genetic tools, and an easily manipulated life-history, the Drosophila species group provides an excellent opportunity to make further progress, particularly because closely related species differ dramatically in many behaviors.
Courtship preference is one behavior that varies dramatically among Drosophila species. In Drosophila, males and females express a blend of cuticular hydrocarbons (CHCs), many of which act as gustatory pheromones (Pardy et al. 2018). These CHCs are variable between species (Jallon and David 1987), and can act as sex pheromones (Ferveur and Sureau 1996) and species identification signals (Billeter et al. 2009). For example, D. melanogaster females predominantly express 7,11-heptacosadiene (7,11-HD), while females in the closely-related species, D. simulans, primarily express 7-tricosene (7T). The male responses to these pheromones differ dramatically between species: D. melanogaster males willingly court conspecific females, but the presence of 7,11-HD on the D. melanogaster female cuticle suppresses courtship by D. simulans males (Billeter et al. 2009; Clowney et al. 2015; Seeholzer et al. 2018). Male pheromone preference, therefore, constitutes an early barrier to interspecific mating (Shahandeh et al. 2018).
Both D. melanogaster and D. simulans males detect 7,11-HD via gustatory receptor neurons in the forelegs that express the ion channel ppk23 (Lu et al. 2012, 2014; Thistle et al. 2012; Toda et al. 2012). From there, the signal is propagated through two clusters of neurons (vAB3 and maL neurons) that simultaneously excite and inhibit the P1 central courtship neurons (Clowney et al. 2015). These P1 central courtship neurons act as command neurons, essentially like an on/off switch for male courtship (Auer and Benton 2016). A recent study found that the evolution of the interactions among these neurons in the central nervous system causes the difference in 7,11-HD preference, rather than the evolution of the peripheral nervous system. (Seeholzer et al. 2018). In D. melanogaster, 7,11-HD promotes courtship because the excitation of P1 neurons is greater than the inhibition; in D. simulans, the opposite seems to be the case (Seeholzer et al. 2018). However, the molecular changes underlying these differences in neuronal interactions remain unknown. This detailed, if still incomplete, understanding of the cellular basis of behavioral evolution presents an excellent opportunity to map the causal genes and link evolution in behavior at the genetic, cellular, and organismal levels.
Here we present the results of a genotype-phenotype association study where we make considerable progress toward identifying the loci underlying shifts in 7,11-HD preference behavior between D. simulans and D. melanogaster. First, we confirm a previous result demonstrating that a portion of the male preference phenotype maps to the X chromosome (Kawanishi and Watanabe 1981). We then show that the preference for 7,11-HD can be recovered in hybrids with a single 1.35 Mb region of the D. melanogaster X chromosome. We additionally present the results of two attempts to map this region to a causal gene; both a fine-mapping and candidate gene approach were unsuccessful. Nonetheless, our findings have identified a fraction of the D. melanogaster genome containing loci for further functional investigation.
Methods
Fly stocks and maintenance
We maintained all fly strains in 25 mm diameter vials on standard cornmeal/molasses/yeast medium at 25° under a 12 h:12 h light/dark cycle. Under these conditions, we established non-overlapping two-week lifecycles. Every 14 days, we transferred all of the emerged male and female adult flies into vials containing fresh food, where they were allowed to oviposit for 1–3 days before being discarded. To test for species differences in male preference, we used two D. simulans strains: simC167.4 (obtained from the UC San Diego Drosophila Stock Center; Stock #: 14021-0251.99) and Lhr (Watanabe 1979; Brideau et al. 2006). We used four D. melanogaster strains: Canton-S, DGRP-380 (Mackay et al. 2012), C(1)DX-LHM and LHM (Rice et al. 2005). To screen portions of the X chromosome, we created duplication hybrids (see below) using a total of 22 Dp(1;Y) strains listed in Table 1 (Cook et al. 2010).
Table 1. DP(1;Y) D. melanogaster stocks. 1A. The 16 Y-linked X duplication strains used to create duplication hybrids and the reported breakpoints of their Y-linked X duplication segment (if multiple segments, base pairs are indicated for each). Ranges are shown for breakpoints where precise estimation is not available. 1B. The 6 Y-linked X duplication strains used to create duplication hybrids overlapping the region covered by BSC100 and the reported breakpoints of their Y-linked X duplication segment. Note, all translocations contain a basal segment of the X chromosome (X:1;X:493529), and many also contain a region from the end of the X chromosome (e.g.: X:21572099-22456281). Coordinates of translocated segments are from D. melanogaster genome release 6 (dos Santos et al., 2015).
| (A) Duplication | DP(X:Start;X:Finish) |
|---|---|
| BSC297 | X:1;X:1947870-2009846 |
| BSC74 | X:1922620-2009846;X:3689139 & X:1;X:493529 |
| BSC158 | X:3842645-3949866;X:4931440-4931826 & X:1;X:493529 |
| BSC277 | X:4919037-5430041;X:6695007 & X:1;X:523278 |
| BSC33 | X:8129732-8192725;X:9030055 & X:1;X:493529 |
| BSC170 | X:8589125-8688160;X:9686653 & X:1;X:493529 |
| BSC58 | X:9355691-9500067;X:10744934 & X:1;X:493529 |
| BSC264 | X:11136887-11168928;X:11453958 & X:21572099-22456281;h28-h29 & X:1;X:493529 |
| BSC100 | X:11453063-11557017;X:12903175 & X:21572099-22456281;h28-h29 & X:1;X:493529 |
| BSC267 | X:13762300-13830297;X:15498953 & X:1;X:493529 |
| BSC266 | X:15484325-15569994;X:16091666 & X:1;X:493529 |
| BSC228 | X:15982454-16005803;X:16655817 & X:1;X:493529 |
| BSC200 | X:16533041-16610393;X:17682814 & X:1;X:493529 |
| BSC68 | X:17698398-17842622;X:18506941 & X:1;X:493529 |
| BSC129 | X:18506719-18632082;X:19887155 & X:1;X:493529 |
| BSC276 | X:20393272-20429518;h28-h29 & X:1;X:361245-493529 |
| (B) Duplication | DP(X:Start;X:Finish) |
| BSC47 | X:11325824-11349993;X:12007087 & X:21318903-21382540;h28-h29 & X:1;X:493529 |
| BSC49 | X:11409964-11453063;X:12007087 & X:1;X:493529 |
| BSC54 | X:11580188-11706436;X:12007087 & X:21572099-22456281;h28-h29 & X:1;X:493529 |
| BSC101 | X:11557017-11580188;X:12903175 & X:1;X:493529 |
| BSC315 | X:11839318-11881910;X:13284291 & X:1;X:493529 |
| BSC317 | X:12006937-12107815;X:13284291 & X:1;X:493529 |
Hybrid crosses
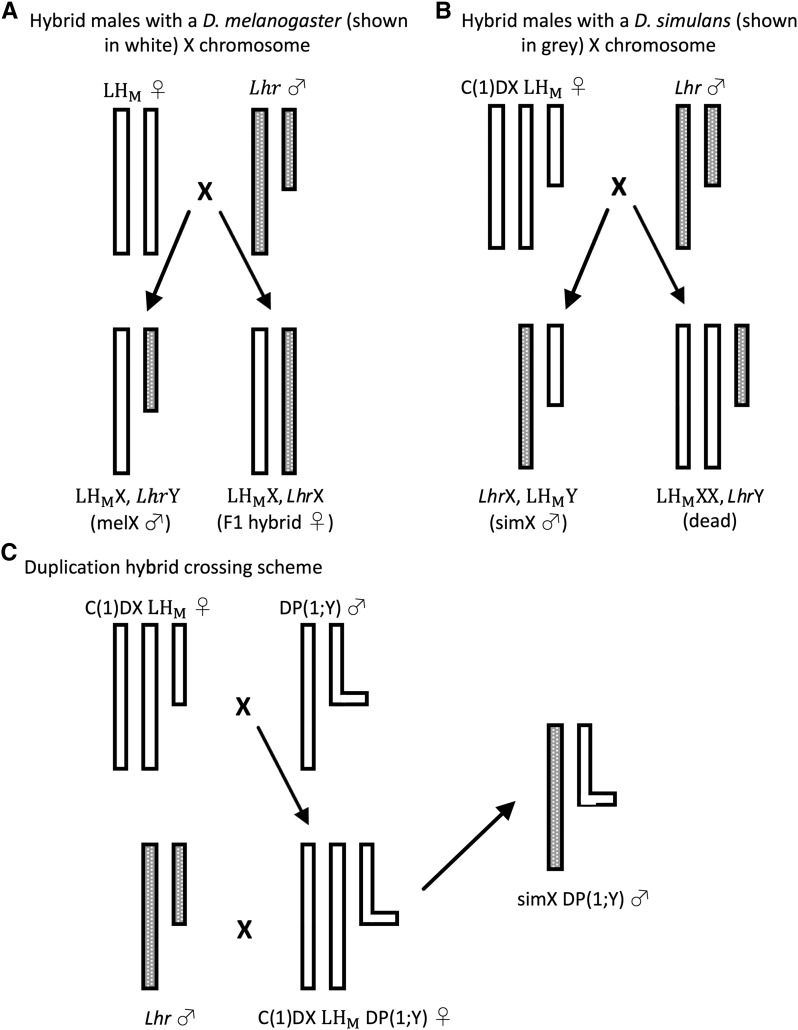
To confirm the role of the X chromosome in male mate discrimination, we needed to make F1 hybrid males by crossing D. melanogaster females to D. simulans males (hybrid offspring would then have a D. melanogaster X) and by the reciprocal cross (hybrids would have the same autosomal genotype, but the D. simulans X). D. melanogaster/D. simulans hybrid males normally die during development, while hybrid females are infertile (Watanabe 1979). The Lhr strain of D. simulans rescues male viability, allowing us to collect living male and female hybrid offspring (Figure 1A). However, crossing females from this D. simulans hybrid male rescue strain to D. melanogaster males never yielded offspring, probably because of very strong pre-mating isolation. Instead, we used genetic tools (see below) to make these same genotypes while only crossing D. melanogaster females to D. simulans males. In this crossing direction, we found hybrids could be made using the following steps. First, we collected 20 D. simulans males as virgins and aged them for 7-12 days. We collected 10 D. melanogaster females as very young virgins, just 2-4 hr after eclosion. We immediately combined 10 very young D. melanogaster virgins with 20 aged D. simulans males in a vial with food media. We pushed a foam plug into the vial, leaving only 1-2 cm of space above the food surface. We held these hybrid cross vials in this manner for 2-3 days before transferring the flies to a new vial with fresh food to oviposit.
Figure 1.
Hybrid crossing schemes. Each diagram shows only the sex chromosomes. Y chromosomes are depicted as shorter, while Y chromosomes with X duplications are depicted as an “L”. D. melanogaster (LHM) chromosomes are shown in white, and D. simulans (Lhr) chromosomes are shown in gray. A. To create melX hybrids, we crossed LHM females to Lhr males, resulting in hybrid males with the D. melanogaster X chromosome and F1 hybrid females. B. To create simX hybrids, we crossed C(1)DX-LHM females to Lhr males, resulting in hybrid males with the D. simulans X chromosome. Females of this cross are inviable. C. To create duplication hybrids, we first crossed males from DP(1;Y) strains to C(1)DXLHM females. We took the female offspring of this first cross and crossed them to Lhr males. The male offspring of this second cross inherit the D. simulans X chromosome in addition to a segment of the D. melanogaster X chromosome (depicted by the small horizontal bar) attached to the D. melanogaster Y chromosome.
To create male hybrids with the D. melanogaster X chromosome (melX), we set up the above crosses using the LHM strain of D. melanogaster and the Lhr strain of D. simulans. To create male hybrids with the X chromosome of D. simulans (simX), we set up the same cross using the C(1)DX-LHM strain of D. melanogaster and the Lhr strain of D. simulans. Females of the C(1)DX-LHM strain have a compound X chromosome and a Y chromosome in an LHM autosomal background. The male offspring of this cross inherit their X chromosome from the father, while their Y chromosome and cytoplasm are inherited from the mother (Figure 1B). Thus, simX and melX hybrids only differ in their sex chromosomes. By directly comparing them we can isolate the effect of the sex chromosomes (primarily the X chromosome) on male courtship preference behavior.
Male courtship assays
For all courtship assays, we aged virgin males and females in single-sex vials for 4 days at 25° in densities of 10 and 20, respectively. We gently aspirated a single experimental male into a 25 mm diameter vial with standard cornmeal/molasses/yeast medium 24 hr before observation. On the morning of observation, we aspirated a single female into the vial and pushed a foam plug down into the vial, leaving a space of 1-3 cm above the food surface. This limited space ensures that flies interact within the observation time period. We observed flies for 30 min, collecting minute-by-minute courtship data by manually scoring each pair for three easily observed stages of courtship: singing (single wing extensions and vibration), attempted copulation, and successful copulation. We scored pairs that exhibited multiple stages within a single minute once within that minute. We conducted 2 observations per day at room temperature between the hours of 9 and 11 am (0-2 hr after fly incubator lights turn on). All observers were blind to both male and female genotype (see below).
We tested the male courtship behavior of the following strains: D. melanogaster (LHM, Canton-S, RNAi strains), D. simulans (Lhr and simC167.4), and various melX and simX F1 hybrids. We measured male courtship toward three types of females, D. melanogaster, D. simulans, and F1 hybrid females, using no-choice (single female) assays. All D. simulans female courtship objects were of the simC167.4 strain. Female courtship objects for D. melanogaster were of the DGRP-380 strain when testing males of the LHM, Lhr, melX, simX, and duplication hybrid genotypes (i.e., when testing for an effect of the X chromosome), but we used females from the Canton-S strain with Canton-S males, RNAi males and controls, and gene aberration melX hybrids (see below), because these additional data were collected during a separate follow-up experiment. We made F1 hybrid females using the methods described above (i.e., from a cross between LHM and Lhr; Figure 1A).
Duplication hybrid crosses
Mapping the genes responsible for this evolved behavior is very challenging for several reasons. QTL analysis is not possible using these species, as hybrid males are inviable and hybrid females are infertile. One way around this problem is to use large engineered deletions to make loci hemizygous rather than heterozygous in F1 hybrids, exposing recessive or additive alleles from the D. simulans parent (Moehring and Mackay 2004; Ryder et al. 2004; Laturney and Moehring 2012; Pardy et al. 2018). However, a deletion screen is not possible for an X-linked behavior present only in males because the X is already hemizygous, so large deletions are lethal. Instead, we used a set of transgenic strains (Cook et al. 2010) to create duplication hybrid males – hybrids with a complete D. simulans X chromosome and an additional segment of the D. melanogaster X chromosome translocated to the D. melanogaster Y chromosome (Figure 1C, Table 1A). This method has been used to map hybrid incompatibility loci (Cattani and Presgraves 2012), but to our knowledge has not been used to map differences in morphology or behavior. The primary caveat to this method is the inability to detect D. melanogaster loci that are recessive to their D. simulans counterparts. Because F1 hybrid males are hemizygous, it is impossible to assess dominance a priori. Despite this limitation, the primary advantage to this method is our ability to assay a large portion of the X chromosome (80%) using just 16 DP(1;Y) hybrid strains. To create these hybrids, we crossed D. melanogaster males from a DP(1;Y) strain to D. melanogaster females from the C(1)DX-LHM strain (Figure 1C). We then took the resulting female offspring, which carry a D. melanogaster compound X chromosome and a D. melanogaster Y chromosome that has a translocated segment of the X chromosome, and crossed them to D. simulans Lhr males using the hybrid cross methods described above. The DP(1;Y) Y chromosome is marked with the dominant visible Bar mutation, so inheritance of this chromosome is easy to track. After assaying our original 16 duplication hybrid strains, we tested additional strains with duplications that partially overlap a region we identified in the initial screen in an attempt to fine-map loci within DP(1;Y) segments of interest (Table 1B).
Perfuming D. simulans females with 7,11-HD
Adapting the methods of Thistle et al. (2012), we perfumed D. simulans females with synthetic 7,11-HD to test for a role in courtship behavior for a duplication of interest (BSC100). To perfume females, we placed 20 simC167.4 females into an empty 25 mm diameter vial. We had previously added either 40 μL of ethanol (sham treatment), or 200 or 400 μg of 7,11-HD dissolved in 40 μL ethanol (perfume treatment) to the vial, and allowed the liquid to evaporate. We then vortexed the vials on the highest setting for three 20-second intervals separated by 20 sec of recovery. We allowed females to recover from vortex mixing for 30 min before loading them into courtship vials. We conducted courtship assays as described above. We detected no difference in courtship between our 200 and 400 μg perfuming treatments (χ2= 0.083, df = 1, P = 0.773), so we combined them into a single perfume treatment in our final analysis.
Selecting and testing candidate genes for validation
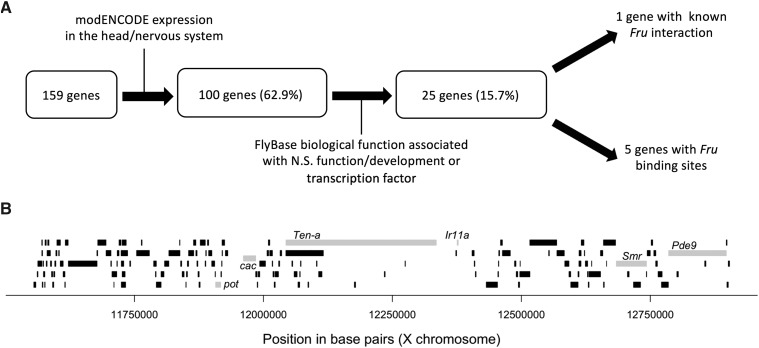
The BSC100 duplication region that has a significant effect on male courtship behavior in a hybrid background (see results) contains 159 genes (Supplemental Material, Table S1). In order to select appropriate candidate genes to test for a role in divergent male courtship behaviors, we used modENCODE expression data (Graveley et al. 2011) to identify genes expressed in the D. melanogaster central nervous system, a justification set by the findings of Seeholzer et al. (2018). We further filtered this list of genes, obtained from FlyBase (dos Santos et al. 2015), for biological function in nervous system development/function, or transcription factor activity to exclude any genes not specific to the nervous system (i.e., cell maintenance loci). Finally, we selected any of the 25 remaining genes that have known fruitless binding sites or interactions, as fruitless is responsible for the male specific wiring of the central nervous system (Manoli et al. 2005; Goto et al. 2011; Vernes 2014). This left us with a list of 6 candidate genes. For 4 of the 6 genes, we were able to procure a non-lethal aberration (Table 2A). We crossed females of these strains to D. simulans Lhr males to create melX hybrids with individual gene knockouts. Two of these knockouts (Smr and pot) are held over a balancer chromosome in females, and thus produce two types of melX hybrid males: those carrying a balancer (intact) X chromosome, and those carrying a defective X-linked allele. Unfortunately, pot defective hybrid males were not viable, and thus could not be observed (Table S3). For Smr, we compared both balancer hybrids and Smr- hybrids paired with D. melanogaster and D. simulans females in the courtship assays described above. For the remaining, unbalanced strains (Ten-a and Pde9), we compared courtship of knockout hybrid males toward D. simulans and D. melanogaster females. Smr and Pde9 hybrids presented an extra challenge, as males have white eyes (Table 2A), and thus, difficulty tracking a female courtship target. To remedy this challenge, we observed these males as above, but in a smaller arena to increase interaction between visually impaired males and females. To modify the courtship arena for these males, we inserted a single piece of plastic vertically into the media, dividing the vial in half, before pushing a foam plug down into the vial, resting 1-2 cm from the food surface.
Table 2. A. A list of the available Bloomington Drosophila Stock Center gene aberration strains used to test candidate genes. B. A list of UAS-hairpin RNAi and pan-neuronal Gal4 drivers used to knockdown expression of genes when aberrations were available, or when aberrations were male lethal.
| (A) BDSC # | Gene target | genotype |
|---|---|---|
| 57114 | papillote (pot) | y[1] w[*] pot[D] P{ry[+t7.2]=neoFRT}19A/FM7c, P{w[+mC]=GAL4-Kr.C}DC1, P{w[+mC]=UAS-GFP.S65T}DC5, sn[+] |
| 13116 | Smrtr (Smr) | w[1118] P{w[+mGT]=GT1}Smr[BG01648]/FM7a |
| 42195 | Phosphodiesterase 9 (Pde9) | y[1] w[*]; Mi{y[+mDint2]=MIC}Pde9[MI06972] |
| 35826 | Tenascin-a (Ten-a) | Ten-a[cbd-KS171] introgressed into a CantonS background |
| (B) BDSC # | Gene target | genotype |
| 27244 | cacophony (cac) | y[1] v[1]; P{y[+t7.7] v[+t1.8]=TRiP.JF02572}attP2 |
| 61898 | Ionotropic receptor 11a (Ir11a) | y[1] v[1]; P{y[+t7.7] v[+t1.8]=TRiP.HMJ23453}attP40 |
| 8765 | elav-Gal4 | P{w[+mC]=GAL4-elav.L}2/CyO |
Because the two remaining genes were either lethal in males (cacophony), or had no aberration available at all (Ir11a), we knocked down expression in D. melanogaster flies using RNAi under the control of the Gal4/UAS system (Perkins et al. 2015). For RNAi knock-down strains, we compared progeny of RNAi lines crossed with the pan-neuronal elav-Gal4 stock (Table 2B). The elav-Gal4 insertion is maintained in heterozygotes over a balancer chromosome. Thus, this cross yields RNAi males, which express hairpin RNAi for the target gene in all of their neurons, and control males, which encode for, but do not express the hairpin RNAi because they lack Gal4 expression in neurons. Control males express a dominant visible marker contained on the balancer chromosome they inherit instead of the Gal4 insertion. In this way, for males paired with both D. melanogaster and D. simulans females, we compare the courtship of males where expression of the gene of interest is reduced in neurons, to males of the same background without reduced expression in the nervous system. While knocking out (or down) expression of these 6 candidate genes allows us to compare the specific effects of loss of function of D. melanogaster alleles to functioning alleles in their respective backgrounds (hybrid for knock-outs, D. melanogaster for RNAi knockdown), it is not immediately clear what phenotype to expect during these tests. This presents another challenge in interpreting these results (see Discussion).
Data analysis
From the minute-by-minute courtship data, for each male-female combination (see below), we collected binomial data (court/did not court) to determine the courtship frequency (CF) of male genotypes. We only considered males that spent 10% or more of the total assay time (i.e., > or = 3 min) in one of the three courtship stages as successfully displaying courtship. For male genotypes, unless otherwise stated, we used Fisher’s exact test to compare the proportion of males that courted a given female type, as well as to compare courtship between male genotypes for the same female type, followed by posthoc analysis with sequential Bonferroni tests (Holm 1979). For instances where we used multiple strains of the same species, we used a consensus p-value (Rice 1990), which tests the combined effect of independent tests of the same hypothesis, to determine overall species patterns.
For each male that displayed courtship toward a female target, we also calculated the total percent of assay time (30 min) that a male spent courting as a proxy for male courtship effort (CE). For males that mated with females, we calculated CE as the percent of time a male spent courting from the start of the assay until the time of copulation. Unlike with CF, we used no minimum threshold for CE. CE is representative of male investment in any given female, another indication of male choice (Edward and Chapman 2011). Because the courtship effort distributions are highly skewed, we report the median values for comparison across strains. For each male genotype, we compared courtship effort between female genotypes using the Mann-Whitney U-test followed by posthoc analysis with sequential Bonferroni tests (Holm 1979).
Data availability
Tables 1 and 2 describe all of the Drosophila reagents we used. Table S1 describes the 159 genes identified within the candidate X chromosome region. The raw courtship data has been uploaded to figshare. Table S2 contains the sample size, courtship frequency, and median courtship effort for each male-female pairing we observed. Table S3 shows the male and female offspring count for crosses of one candidate gene knockout hybrid cross, papillote. Supplemental material available at figshare: https://doi.org/10.25387/g3.10284269.
Results
A significant conspecific courtship preference between D. melanogaster and D. simulans
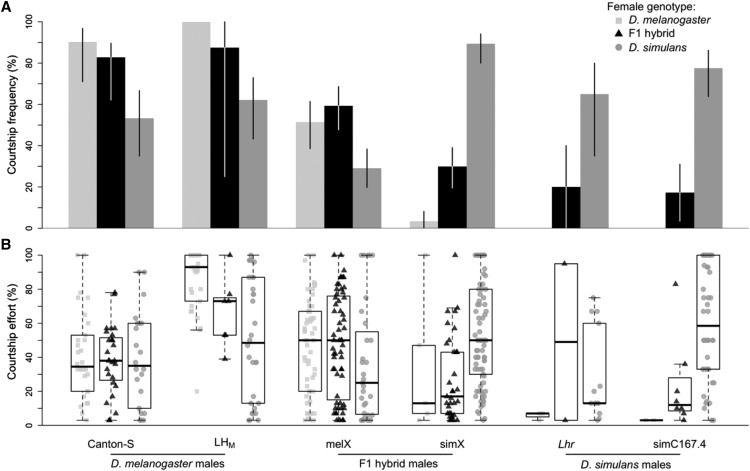
For both D. melanogaster strains that we assayed, we detected significantly higher courtship frequencies when males were paired with females of their own species (Figure 2A, Table S2). Both LHM and Canton-S courted D. melanogaster females significantly more frequently than D. simulans females (LHM: P = 0.0003; Canton-S: P = 0.0032). Likewise, both D. simulans strains displayed higher courtship frequencies with D. simulans females than D. melanogaster females (Lhr: P = 5.34E-09; simC167.4: P = 5.47E-12). There were no differences in the courtship frequencies among strains of the same species (P = 1 for both D. melanogaster and D. simulans). Unsurprisingly, when we calculated a consensus p-value for the two D. melanogaster strains and the two D. simulans strains, conspecific courtship preferences remained highly significant (P = 1.61E-5 and P <1.00E-25, respectively), suggesting this is indeed a species-level, rather than strain-specific difference.
Figure 2.
Courtship behaviors of D. melanogaster males, D. simulans males, and their hybrids. A. Courtship frequencies are shown for two strains of D. melanogaster (Canton-S and LHM), two strains of D. simulans (Lhr and simC167.4), and their reciprocal hybrids (melX and simX) toward three female types: D. melanogaster (light gray), F1 hybrids (black), and D. simulans (dark gray). Whiskers represent 95% bias corrected and accelerated bootstrapped confidence intervals. B. Courtship effort is shown for the same male and female combinations (light gray squares for D. melanogaster females, black triangles for F1 females, and dark gray circles for D. simulans females). Courtship effort is calculated as the percent of time that males spent courting (only males that courted were included in these calculations). Boxplots show the median (bold black line), interquartile range (box) and full extent of the data excluding outliers (whiskers).
We also observed each D. melanogaster and D. simulans strain with F1 hybrid females (Figure 2A, Table S2). These females still produce 7,11-HD, due to a single functioning copy of desatF (Shirangi et al. 2009), and concordantly, D. melanogaster and D. simulans males court them similarly to D. melanogaster females. We found that our D. melanogaster strains were just as likely to court F1 hybrid females as they were to court D. melanogaster (P = 0.6486 for LHM, and P = 6724 for Canton-S), and these comparisons remained non-significant when we calculated a consensus p-value (P = 0.7980). Conversely, for one of our D. simulans strains, simC167.4, we found that males court F1 hybrid females significantly less often than D. simulans females (P = 5.59E-07). Lhr males had a much lower courtship frequency with F1 hybrid females compared to D. simulans females (20% vs. 65%, respectively), but this difference was not significant, likely due to small sample size (P = 0.1003 and N = 10). Supporting this, a consensus p-value for both D. simulans strains found that D. simulans overall had a significantly higher courtship frequency with D. simulans females than with F1 females (P = 9.93E-07). For both D. simulans strains, we detected no difference in courtship frequency toward F1 and D. melanogaster females, as all had relatively low courtship frequencies (for Lhr P = 0.1230, and for simC167.4 P = 0.2089; and consensus P = 0.1197).
We find less striking differences when we calculate the courtship effort of those males that did court (Figure 2B, Table S2). For example, once they began courting, Canton-S males courted all three female types with equal vigor (P = 1 for all comparisons). LHM males, however, courted D. melanogaster with much higher vigor than D. simulans females (P = 0.0011). Thus, unlike courtship frequency, courtship effort appears to have strain-specific effects within the D. melanogaster strains we surveyed. For D. simulans, we detected a nearly significant increase in courtship effort for simC167.4 males that courted D. simulans females compared to males that courted F1 females (P = 0.0506). We are unable to detect significant differences in courtship effort for Lhr males among any comparisons, or for simC167.4 male comparisons involving D. melanogaster females, because so few males courted D. melanogaster females (and F1 females for Lhr).
The X chromosome partially explains differences in courtship behavior
Qualitatively, the behavior of hybrid males largely replicates the behavior of the X chromosome donating parent (Figure 2, Table S2). Like males of the D. melanogaster parent strain (LHM), melX hybrid males court both D. melanogaster and F1 hybrid females significantly more often than D. simulans females (D. melanogaster: P = 0.0052, F1: P = 0.0005 Figure 2A). Also like the LHM parent strain, melX males court F1 hybrid females and D. melanogaster females at similar frequencies (P = 0.6724). Quantitatively, however, the behavior of melX males does not entirely replicate that of the D. melanogaster parent strain. For instance, although still significantly higher than when paired with D. simulans females, melX males court both D. melanogaster females and F1 females at significantly lower frequencies than LHM (P = 9.92E-07 and P = 0.0022, respectively). With respect to courtship effort, melX males court each female indistinguishably (all P >0.8541, Figure 2B).
The behavior of simX males more closely reproduces that of the D. simulans parent strain (Lhr). Like Lhr, simX males court D. simulans females at much higher frequencies than D. melanogaster females (P = 1.32E-15, Figure 2A) and F1 females (P = 4.03E-14). Unlike the Lhr parent strain, simX males court F1 females significantly more frequently than D. melanogaster females (P = 1.57E-05). Courtship toward D. melanogaster was still too rare to detect differences in courtship effort compared to D. simulans or F1 females (both P = 1, Figure 2B), but simX males courted D. simulans with significantly higher effort than F1 females (P = 0.0005, Figure 2B). Quantitatively, simX males behave very similarly to their D. simulans parents. simX males court D. melanogaster and F1 females with frequencies equivalent to that of Lhr males (P = 0.5876 and P = 0.7190, respectively), but they court D. simulans females at a higher frequency (P = 0.0373). We suspect this latter result is a byproduct of increased heterozygosity relative to the inbred Lhr parent strain.
A single region of the D. melanogaster X chromosome changes simX hybrid courtship behavior
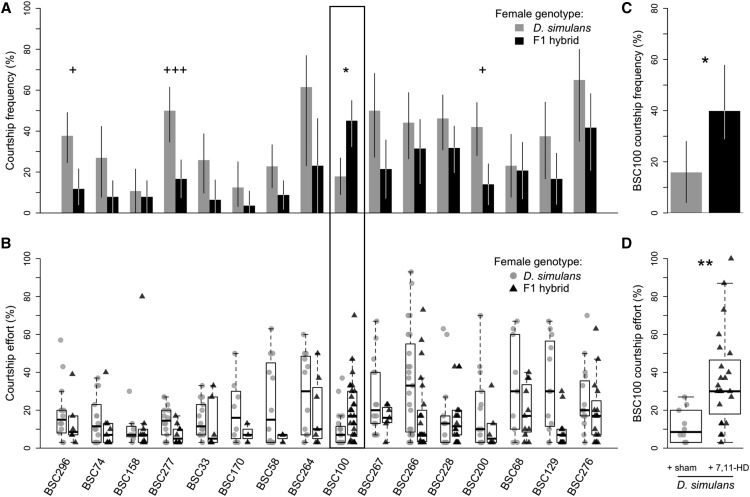
To test specific regions of the X chromosome for their role in courtship preference differences, we measured the courtship behavior of 16 duplication hybrids (Table 1A, Figure 1C). These duplication hybrids are simX hybrids made heterozygous for one stretch of the D. melanogaster X chromosome. We observed 15 of these strains with D. melanogaster females, and interestingly, none displayed courtship (N = 6-20 for each, N = 179 total, Table S2). All 16 of the duplication hybrid strains did court both D. simulans and F1 females, however. We detected significant variation in the amount of courtship these lines displayed to both female courtship targets (χ2= 55.36, df = 15, p-value = 1.55e-06 for D. simulans females, N = 709 total; and χ2= 66.58, df = 15, p-value = 1.80e-08 for F1 females, N = 759 total).
On average, the duplication hybrid lines had significantly higher courtship frequencies toward D. simulans females than toward F1 females (average CF = 35.86% for D. simulans females, average CF = 19.30% for F1 females, P = 5.43E-08, N = 16). This pattern is consistent with what we see for simX hybrids without X-linked duplications, which similarly courted D. simulans females more often than F1 females. However, courtship frequencies with both females are significantly higher for simX males compared to duplication hybrids (average simX CF with F1 females = 30%, Student’s t = -3.3664, df = 15, P = 0.0042; average simX CF with D. simulans females = 89%, Student’s t = -12.891, df = 15, P = 1.614e-09). In total, 15 of the 16 duplication hybrid genotypes courted D. simulans with higher frequency than F1 females (Figure 3A, Table S2); three of these lines showed a significant preference for D. simulans females after correction for multiple tests (P = 0.0415 for BSC296, P = 0.0061 for BSC277, and P = 0.0437, for BSC200). Only one duplication hybrid strain, BSC100, courted F1 hybrids with higher frequency than D. simulans hybrids (P = 0.0136). In general, the duplication hybrid strains courted D. simulans females with greater effort than F1 hybrid females (grand median CE = 17% for D. simulans females, and the grand median CE = 10% for F1 females, P = 8.78E-05). Again, BSC100 duplication hybrids were the only hybrids to display higher courtship effort toward F1 females (CE = 30%) than toward D. simulans females (CE = 13.33%), although this difference was not significant after correcting for multiple comparisons (P = 0.0976, Figure 3B, Table S4).
Figure 3.
Courtship behaviors of duplication hybrid males. A. The courtship frequencies for each of the 16 duplication hybrids when paired with both D. simulans females (gray) and F1 females (black). B. The courtship effort for each of the 16 duplication hybrids when paired with D. simulans females (gray circles) and F1 females (black triangles). BSC100, the only duplication to display greater courtship frequency and effort toward F1 hybrids over D. simulans females is enclosed within the black box. C. The frequency of BSC100 males that courted sham-perfumed D. simulans females (gray), and D. simulans females perfumed with synthetic 7,11-HD (black). D. The courtship effort of BSC100 males that courted sham-perfumed D. simulans females (gray circles), and D. simulans females perfumed with synthetic 7,11-HD (black triangles). For all, symbols denote degree of significance after correction for multiple comparisons (* = P <0.05, ** = P <0.01, and *** = P <0.001). Asterisks mark duplication hybrid lines that court F1 hybrid females significantly more. Plus signs mark duplication hybrid lines that court D. simulans females more. For A. and C., whiskers represent 95% bias corrected and accelerated bootstrapped confidence intervals. For B. and D., boxplots show the median (bold black line), interquartile range (box) and full extent of the data excluding outliers (whiskers).
BSC100 duplication hybrid males prefer females with the cuticular hydrocarbon 7,11-HD
To verify that CHCs are involved in the courtship behaviors that BSC100 duplication hybrid males exhibited, we perfumed D. simulans females with 7,11-HD. When we observed BSC100 duplication hybrid males with perfumed D. simulans females, we saw a significant increase in courtship frequency relative to sham-perfumed D. simulans females (P = 0.0400, Figure 3C, Table S2). BSC100 hybrid courtship frequency increased from 16% with D. simulans sham-perfumed females, to 40% with D. simulans females perfumed with 7,11-HD. Note, these courtship frequencies are similar to those seen when BSC100 hybrid males were paired with D. simulans females (CF = 17.91%, P = 1) and F1 females (CF = 45.07%, P = 0.8603), respectively. Again, the courtship effort data supports this conclusion (Figure 3D). We observed significantly higher courtship effort toward D. simulans females perfumed with 7,11-HD (CE = 30%) when compared to sham-perfumed D. simulans females (CE = 3%, P = 0.0014). Encouragingly, the courtship effort we observed toward 7,11-HD-perfumed D. simulans females is even higher than that of BSC100 hybrid male courtship effort toward F1 females (P = 0.0062). Additionally, BSC100 hybrid males court sham-perfumed D. simulans and non-manipulated D. simulans with equal effort (P = 0.4451).
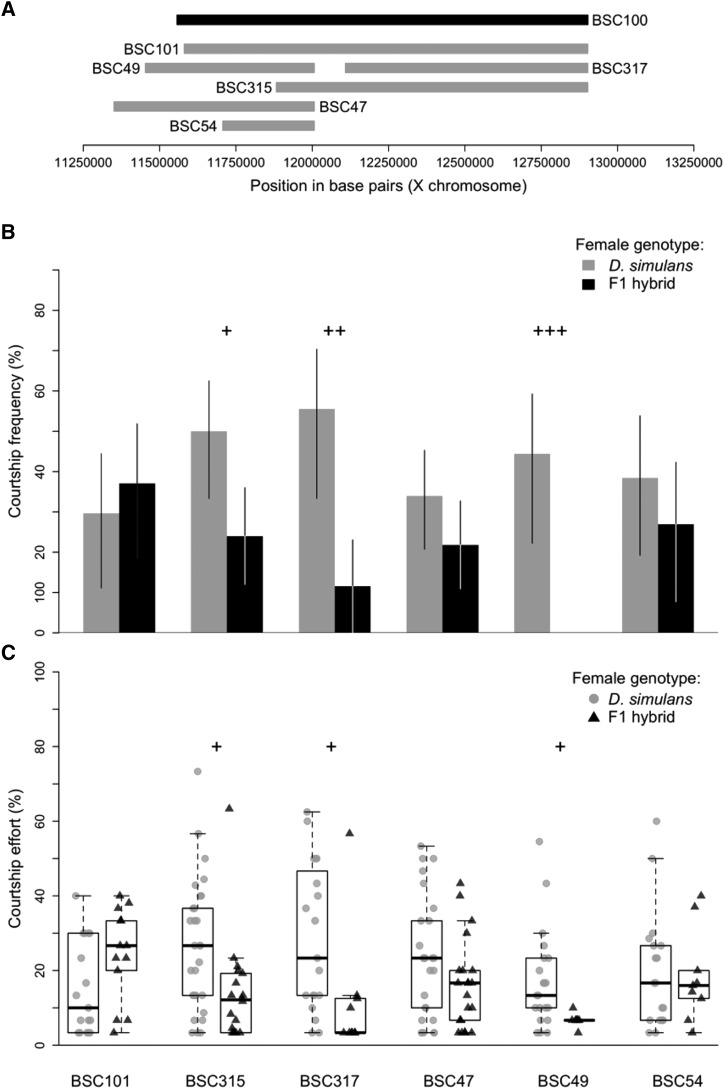
Fine-mapping the BSC100 region of the X chromosome
The BSC100 X chromosome segment spans ∼1.35 Mb, from position 11,557,017 to 12,903,175 of the D. melanogaster X chromosome. This locus contains 159 genes (Table S1). To further refine this region, we measured the courtship behavior of 6 additional duplication hybrids heterozygous for various sections of the X chromosome within our region of interest (Figure 4A, Table 1B). The 6 overlapping duplication hybrids had an average courtship frequency of 42% with D. simulans and 20.22% with F1 females, comparable to the original 16 duplication hybrids. Five of the strains (BSC47, BSC49, BSC54, BSC315, and BSC317) had higher courtship frequencies with D. simulans females than with F1 females (Figure 4B, Table S2), with three (BSC49, BSC315, and BSC317) being significant (P = 0.0002, P = 0.0462, and P = 0.0057 respectively). Overall, the overlapping duplication hybrid strains also displayed significantly higher courtship effort toward D. simulans females (CE = 20%) than toward F1 females (CE = 12.5%, P = 0.0029). The same three strains (BSC49, BSC315, and BSC317) that courted D. simulans females with higher frequencies than F1 females also displayed higher courtship effort toward D. simulans females (P = 0.0460, P = 0.0408, and P = 0.0408, respectively).
Figure 4.
Courtship behaviors of BSC100 overlapping duplication hybrid males. A. The physical positions of the original duplication hybrid (BSC100, black), and the six partially overlapping strains we assayed to fine-map the region (gray). B. The courtship frequencies for each of the 6 overlapping duplication hybrids when paired with both D. simulans females (gray) and F1 females (black). Whiskers represent 95% bias corrected and accelerated bootstrapped confidence intervals. C. The courtship effort for each of the 6 overlapping duplication hybrids when paired with D. simulans females (gray circles) and F1 females black triangles). Boxplots show the median (bold black line), interquartile range (box) and full extent of the data excluding outliers (whiskers). For all, plus signs mark duplication hybrid lines that court D. simulans females more, and denote degree of significance after correction for multiple comparisons (+ = P <0.05, ++ = P <0.01, and +++ = P <0.001).
A single strain, BSC101, displayed a different pattern than the others. Interestingly, this duplication segment spans 98% of the region covered by BSC100, while the other duplications cover various smaller portions of the region, ranging from 22 to 76% coverage (Figure 4A). BSC101 had a higher courtship frequency when paired with F1 females (CF = 37.04%) than when paired with D. simulans females (CF = 29.63%), though not significantly so (P = 1 after correcting for multiple comparisons). However, BSC101 duplication hybrids also did not differ from BSC100 duplication hybrids with respect to courtship frequency toward either D. simulans or F1 females (P = 0.2663 and P = 0.5028, respectively). Likewise, BSC101 duplication hybrids were the only strain to display higher effort when paired with F1 females (CE = 26.67%) than when paired with D. simulans females (CE = 10%), though not significantly so (P = 0.2067). These numbers are also comparable to the courtship effort of BSC100 males toward each female type.
Testing candidate genes
The BSC100 region is known to contain 159 genes (Table S1). To reduce this list to a testable number, we focused on genes with neurological functions and fruitless binding sites (see Methods). This produced 6 candidate genes: papillote (pot), cacophony (cac), Tenascin-a (Ten-a), Smrtr (Smr), Ionotropic receptor 11a (Ir11a), and Phosphodiesterase 9 (Pde9, Figure 5). We used loss-of-function mutations or RNAi to investigate 5 of these 6 genes further (unfortunately, pot loss-of-function hybrid males were inviable).
Figure 5.
The 159 genes uncovered by BSC100. A. A pipeline for identifying relevant candidate genes among the 159 covered by BSC100. We expect genes to be expressed in the central nervous system and to have nervous system functions (or act as transcription factors for loci with such functions). Because fruitless is important to wiring the male nervous system, we also looked for specific genes that interact with Fru as particularly strong candidates. B. A schematic of the 159 genes. The 6 genes that meet our filtering criteria are relatively evenly distributed across the region and shown in gray.
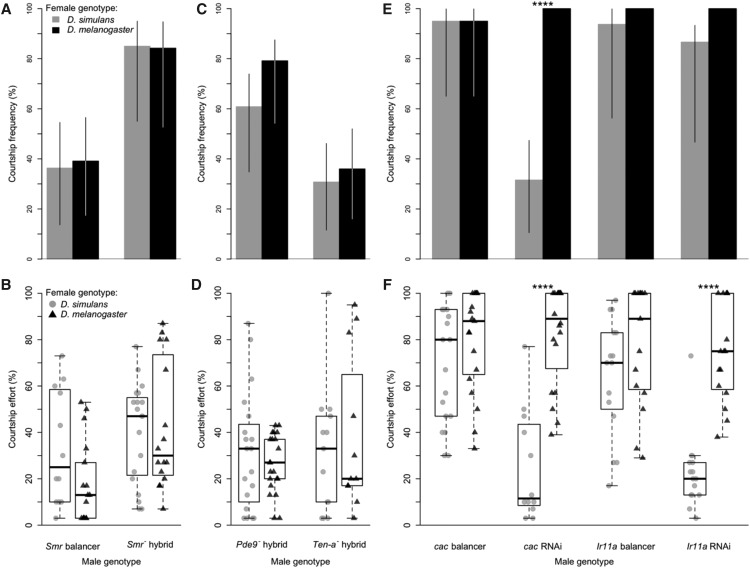
For Smr, the hybrid cross between the D. melanogaster knockout strain produced balancer hybrid (melX males with the X chromosome intact) and Smr- hybrid males (melX males with the X chromosome lacking a functional Smr gene). Smr balancer hybrids courted D. simulans and D. melanogaster at similar frequencies (P = 1, Figure 6A, Table S2), and with similar efforts (P = 0.1136, Figure 6B). The same is true for Smr- hybrid males (P = 1 for both CF and CE). However, these males courted both females at significantly higher frequencies than balancer hybrids (P = 0.0073 for D. simulans, and P = 0.0135 for D. melanogaster). Despite quantitative differences, both Smr- and balancer hybrid males show no preference for D. melanogaster or D. simulans females. Thus, there is no clear effect of the loss of Smr on male preference when compared to intact balancer hybrid males.
Figure 6.
The courtship behaviors of RNAi knockdown and D. melanogaster X chromosome hybrids with individual gene knockouts for males paired with D. simulans females (gray) and D. melanogaster females (black). A. The courtship frequency of Smr knockout hybrid males (Smr- hybrid) is compared to Smr X chromosome balancer males (Smr balancer). B. The courtship effort of Smr knockout hybrid males (Smr- hybrid) is compared to Smr X chromosome balancer males (Smr balancer). C. The courtship frequency for Pde9- and Ten-a- knockout hybrid males. D. The courtship effort for Pde9 and Ten-a knockout hybrid males. E. The courtship frequency of RNAi knockdown and balancer genotypes for two genes, cac and Ir11a. F. The courtship effort of RNAi knockdown and balancer genotypes for two genes, cac and Ir11a. For A., C., and E., whiskers represent 95% bias corrected and accelerated bootstrapped confidence intervals. For B., D., and F., boxplots show the median (bold black line), interquartile range (box) and full extent of the data excluding outliers (whiskers). Asterisks denote degree of significance after correction for multiple comparisons (**** = P <0.0001).
In contrast, Ten-a and Pde9 are not held over a balancer, so hybrid crosses yield only knockout males. Ten-a- hybrids court both D. simulans and D. melanogaster at equal frequencies (P = 1), and with similar effort (P = 1). Pde9- hybrids court D. melanogaster at non-significantly higher frequencies than D. simulans females (P = 0.2124), and court both females with equal effort (P = 1). In contrast, melX males with functioning copies of these genes court D. melanogaster females more frequently (and with non-significantly higher courtship effort). Although these knockout hybrids differ in some aspects from melX males, it is difficult to discern whether these differences are due to the different D. melanogaster strains with which these hybrids were made (see Discussion) or to the loss-of-function mutations they harbor.
For cac and Ir11a, we compared the behavior of D. melanogaster RNAi knockdown flies (Figure 6E,F) to their siblings lacking knockdown (balancer). For Ir11a, we found that balancer males court D. simulans and D. melanogaster indiscriminately in terms of courtship frequency (P = 1) and courtship effort (P = 1). We found the same pattern for cac balancer males (P = 1 for both CF and CE). While Ir11a RNAi males also courted D. melanogaster and D. simulans females at equal frequencies (P = 0.9032), cac RNAi males had significantly lower courtship frequencies with D. simulans females than with D. melanogaster females (P = 1.34E-05). Interestingly, both Ir11a and cac RNAi males displayed reduced effort toward D. simulans females compared to D. melanogaster females (P = 8.13E-06 and P = 1.76E-05, respectively).
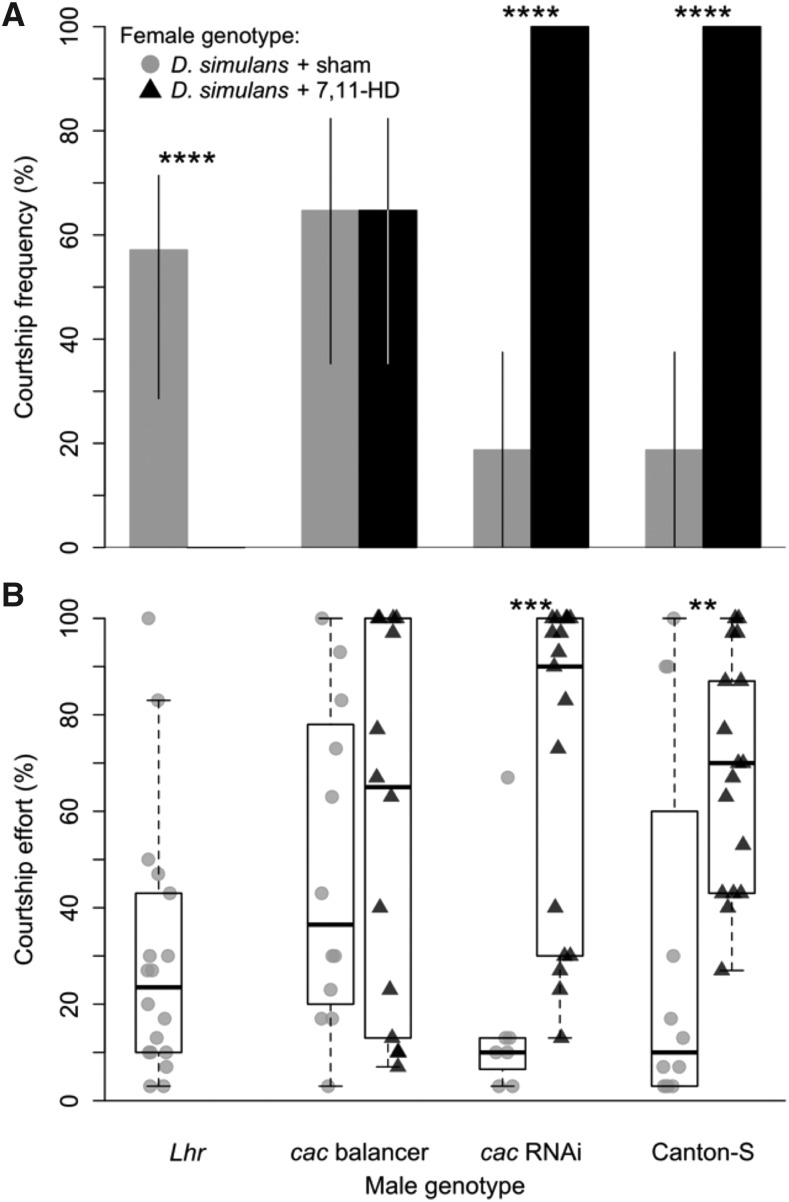
To test if the reduction in courtship frequency and effort of cac RNAi males with D. simulans female is driven by the absence of 7,11-HD on the D. simulans cuticle, we also observed cac RNAi and balancer males with sham- and 7,11-HD-perfumed D. simulans (Figure 7A,B). We found that 7,11-HD does indeed cause this effect: balancer hybrids court sham-perfumed and 7,11-HD-perfumed D. simulans with equal frequencies (P = 1), and there was no significant difference in courtship effort (P = 0.5876). In contrast, cac RNAi hybrids court 7,11-HD-perfumed D. simulans at significantly higher frequency and effort than sham-perfumed females (P = 3.91E-06 for CF and P = 0.0027 for CE). Thus, cac RNAi hybrids require 7,11-HD to stimulate high amounts of courtship.
Figure 7.
cac RNAi male behavior is driven by 7,11-HD. A. The courtship frequency of RNAi knock-down and balancer genotypes for cac, Lhr, and Canton-S for males paired with sham-perfumed D. simulans females (gray bars) and 7,11-HD-perfumed D. simulans females (black bars). Whiskers represent 95% bias corrected and accelerated bootstrapped confidence intervals. B. The courtship effort of RNAi knockdown and balancer genotypes for cac, Lhr, and Canton-S for males paired with sham-perfumed D. simulans females (gray circles) and 7,11-HD-perfumed D. simulans females (black triangles). Boxplots show the median (bold black line), interquartile range (box) and full extent of the data excluding outliers (whiskers). For all, asterisks denote degree of significance after correction for multiple comparisons (** = P <0.01, and *** = P <0.001, **** = P <0.0001).
Discussion
An important difference in male courtship preference between D. melanogaster and D. simulans partially maps to the X chromosome
Our observation of two D. simulans and two D. melanogaster strains confirms a previously described species difference in male courtship preference, where each species dramatically prefers their own females (Manning 1959). While we did observe some variation in the quantitative amount of courtship among our lines (Figure 2A), the valence of male preference was always consistent within species. Because we also used females from two different lines, this variation in male behavior could be due to individual variation in female CHC quantity (Pardy et al. 2018), variation in other female traits, and/or variation in male preferences (Pischedda et al. 2014). Our results demonstrate that male courtship preferences act as a large reproductive barrier for D. simulans and D. melanogaster, as has been shown between D. simulans and D. sechellia (Shahandeh et al. 2018). A detailed understanding of the genetic basis of this preference would prove illuminating, not only with respect to the evolution of behavior, but also with respect to the evolution of reproductively isolating barriers.
The courtship data we collected using reciprocal D. melanogaster/D. simulans hybrids (melX and simX males) created in a homogenous background and controlled for cytoplasmic inheritance also confirms the significant role of the X chromosome in male courtship preference differences between these species (Kawanishi and Watanabe 1981). Though we didn’t strictly control for an effect of the Y chromosome, it is unlikely to explain our results because hybrids with the D. simulans Y behave more like D. melanogaster, and hybrids with the D. melanogaster Y behave more like D. simulans. Because we only used reciprocal hybrids to demonstrate the X-effect, we cannot rule out the potential of transgressive autosomal effects that cannot be detected in a hybrid background (Mittleman et al. 2017). Indeed, although the behavior of our hybrids qualitatively mirrors that of the X-donating parent, quantitative differences in both courtship frequency and effort, particularly between LHM and melX hybrids, suggest an additional role of autosomal loci (likely D. simulans dominant, as simX males behave both qualitatively and quantitatively similar to D. simulans males). These results mirror findings from a QTL study mapping male courtship frequency differences among D. simulans and D. sechellia, another species where females express 7,11-HD and males are stimulated to court by it (M.P. Shahandeh & T.L. Turner, in prep). In this case, the D. simulans X chromosome contributes to 7,11-HD aversion, and autosomal D. sechellia loci contribute to 7,11-HD attraction. However, in D. simulans-D. sechellia reciprocal hybrid males, the effects of the autosomal loci are far greater than the X chromosome, as both hybrids behave more similarly to D. simulans males. It remains to be seen whether the same loci affect 7,11-HD response between these species, but the effects/interactions of loci are undoubtedly different.
A single segment of the D. melanogaster X chromosome has a large effect on male pheromone preference
We expect all DP(1;Y) hybrid strains to behave like the simX hybrid strain, unless the duplicated D. melanogaster X chromosome segment harbors dominant or additive male preference loci, in which case they should behave more like melX hybrids. When paired with D. melanogaster females, however, we observed no courtship from the 15 DP(1;Y) hybrid strains we observed, which all behaved like simX males in this respect (the 16th unobserved duplication hybrid strain’s CF and CE for D. simulans and F1 hybrids also follows this trend). This result is consistent with several possibilities, including: (1) the loci for male courtship preference reside in the 20% of the X chromosome not covered by the duplication hybrid strains, (2) D. melanogaster courtship preference alleles are recessive to, or epistatic with, D. simulans alleles, (3) the presence of Y-linked X translocations disrupts male behavior in general by making regions of the X chromosome heterozygous, and/or (4) the D. melanogaster allele is not expressed properly due to the genomic environment of the translocation. This last possibility is perhaps unlikely, as the duplications have been shown to rescue the loss of 94% of X-linked mutations (Cook et al. 2010).
Similar to our duplication hybrids, we never observed any of our D. simulans strains courting D. melanogaster females, but each courted F1 females at low levels (18% of all D. simulans males courted F1 females), suggesting they may display intermediate female cues. However, D. simulans males still strongly preferred D. simulans females over F1 hybrid females, likely due to the presence of 7,11-HD on the F1 female cuticle (Coyne 1996), which suppresses courtship in D. simulans males (Billeter et al. 2009). This is also likely the reason that D. melanogaster males court F1 females comparably to D. melanogaster females. As with the male preference difference between D. melanogaster and D. simulans females, the preference difference between D. simulans and F1 hybrid females still maps to the X chromosome: simX males behave like D. simulans males – courting F1 females infrequently, and significantly less frequently and with less effort, than D. simulans females (Figure 2). Likewise, melX males behave like D. melanogaster, courting F1 females with a similar frequency as D. melanogaster females, and significantly more frequently than D. simulans females. Because the overall preference patterns between D. melanogaster and D. simulans were replicated when we compared melX and simX male courtship toward F1 and D. simulans females, we additionally observed each of the 16 DP(1;Y) hybrid strains with F1 hybrid females. Again, we expect the courtship preferences of all DP(1;Y) hybrid strains to resemble the simX hybrid strain unless the duplicated D. melanogaster X chromosome segment harbors male preference loci, in which case we expect them to court F1 hybrid females at higher frequencies than D. simulans females, as we see for melX males.
When we observed hybrid males from the 16 DP(1;Y) genotypes, we found that some males from every strain courted F1 females. Most genotypes courted F1s at low levels, as we saw for simX hybrids. BSC100 hybrids were the only duplication hybrids that displayed higher courtship frequency and effort with F1 hybrids than with D. simulans females, replicating the pattern seen in melX hybrid males. However, like simX hybrids, BSC100 hybrids showed no courtship toward D. melanogaster females. The fact that BSC100 hybrid males prefer F1 females to D. simulans females, but are still unwilling to court D. melanogaster females, suggests that the D. melanogaster variants at this locus are insufficient to completely mask the effects of the D. simulans X genome, which is also present in the BSC100 hybrid. We hypothesize that the greater courtship we see toward F1 hybrid females is not seen toward D. melanogaster females because the multiple, partially redundant cues that influence male courtship in Drosophila are more intermediate in F1 females (Arbuthnott et al. 2017). If there are multiple male preferences and female cues that have evolved, the BSC100 duplication may recover the D. melanogaster preference toward one signal, but still be insufficient to activate the P1 courtship command neurons because of inhibition on other sensory channels by the D. simulans genome (Clowney et al. 2015).
This hypothesis is supported by our perfuming data. Although past work has shown that the male preference difference between D. melanogaster and D. simulans is primarily dictated by female pheromones (Manning 1959) – specifically 7,11-HD (Billeter et al. 2009) – we could not be sure that that BSC100 hybrid males were responding to this cue, especially because they were unwilling to court D. melanogaster females that also express this pheromone. However, we found that BSC100 hybrid males significantly preferred D. simulans females perfumed with 7,11-HD to sham-perfumed females, and courted them at a frequency and effort comparable to what we saw with F1 hybrid females, which also have 7,11-HD on their cuticle. These results confirm the role of this X region in 7,11-HD response. Taken together, our findings demonstrate that a single 1.35 Mb segment of the X chromosome has a specific effect on the evolved 7,11-HD preference differences between D. simulans and D. melanogaster.
Subdividing this region for fine-mapping results in the loss of the significant preference difference
In order to further fine map the X chromosome region duplicated in BSC100 hybrids, we created 6 additional hybrid genotypes with partially overlapping duplicated segments (Table 1B). When we observed these overlapping duplication hybrid strains, none showed the same pattern we observed for BSC100. Five of these had higher courtship frequencies with D. simulans females than with F1 females, just like simX males, although two of these differences were not significant. One strain, BSC101, did have a marginally higher CF and CE with F1 females than D. simulans females, albeit non-significantly. This segment has the largest overlap, covering 98% of the segment in BSC100 hybrids (Figure 3C). We hypothesize 2 potential explanations for the loss of significant preference when this region was subdivided.
The genetic architecture of male courtship preference within this region is polygenic. It is possible that male courtship preference differences are polygenic— even if these genes are constrained within a single 1.35 Mb segment. In this case, subdivision of this locus may reduce the behavioral effect if these loci are additive, or result in its loss altogether if these loci have epistatic interactions. This possibility fits somewhat with the pattern we observe with our overlapping duplications: hybrids with the smaller overlapping segments have lost the phenotype entirely, while the largest overlap appears to, at least partially, reproduce the phenotype. The simplest model that fits this scenario consists of at least two interacting loci, at either end of BSC100, such that all loci are never captured by any of the overlapping duplication hybrid strains. BSC101 overlaps 98% of BSC100. If this is indeed the case, then at least one locus must reside within the 2% not covered by BSC101. This type of genetic architecture is not uncommon. Many loci contributing to a single phenotype constrained within a single region have similarly been discovered for morphological traits in Drosophila and other organisms (Fanara et al. 2002; Harbison et al. 2004; Miller et al. 2014; Peichel and Marques 2017).
Hybrid males heterozygous for regions of the X chromosome behave differently than typical hybrids. Overall, we observed reduced levels of courtship among our duplication hybrid strains compared to simX or melX hybrids, suggesting that the duplication hybrids behave differently from typical hybrids. The consistent reduction in courtship by duplication hybrids also reduces our statistical ability to detect a significant effect. We feel that abnormal behavior of duplication hybrids – particularly those carrying smaller subdivisions of the initial 16 duplication segments, is the most likely explanation for why our attempts to fine-map the BSC100 region were unsuccessful.
Duplication hybrid males may behave differently for a variety of reasons; the most likely are those that stem from the Y-translocated X-duplication segments themselves. Males made heterozygous for regions of the X chromosome that are typically hemizygous may have abnormal behavior due to epistatic interactions between X chromosome loci. In the melX and simX hybrids, D. melanogaster and D. simulans X loci are not present in the same genetic background, but they are in duplication hybrids. These loci may interact in unpredictable, non-additive ways, having unforeseen effects on behavior. Alternatively, genes translocated from the X to the Y chromosome may have altered expression patterns due to their new genomic environment, producing a similar effect. Smaller segments, like those we used for fine-mapping, may be more susceptible to this problem, as genes contained within smaller translocated segments are more likely to be surrounded by a foreign genomic environment.
The panel of Y-linked X duplications we used to create duplication hybrids was also created using irradiation (Cook et al. 2010). In fact, each breakpoint was induced by irradiating males, originally creating strains that contained large subdivisions of the X chromosome, like BSC100. Further subdivision of these regions (to create strains like BSC101 and all of the smaller subdivisions that we used for fine-mapping) required additional irradiation. This additional irradiation likely introduced new mutations to the genetic background of these flies, making comparisons between BSC100 and its subdividing strains, like BSC101, imperfect.
Finally, these duplication segments are marked with a dominant visible eye mutation, Bar, that substantially reduces the shape of the eye to a small sliver in males. These males likely have restricted fields of vision, and may have difficulty tracking females in the courtship arena, as has been shown for mutations affecting eye pigmentation (Connolly and Cook 1973; Spiess and Schwer 1978). Indeed, our data collectors noted during courtship observations that males often seemed to lose track of the females they were courting, and courtship would cease. This, too, likely contributed to the reduced courtship frequency and effort we observed, but is constant across all duplication hybrids. Regardless of the cause(s) of atypical behavior in our duplication hybrids, our failure to fine-map the BSC100 region must be considered in light of the above caveats.
Testing five candidate genes yields inconclusive results
We were able to test five candidate genes we identified within the BSC100 region, either through the use of gene aberrations or RNAi knockdown. Qualitatively, our experiments using an Smr gene aberration suggest that the loss of Smr expression in a melX hybrid background has no effect on courtship behavior, as Smr- hybrids behaved like Smr balancer hybrids in that they court both D. simulans and D. melanogaster females at equal frequency and with equal effort. Quantitatively, however, Smr- hybrids showed higher overall courtship frequencies and efforts toward both females compared to balancer hybrids. This result suggests that males harboring an X chromosome balancer are less vigorous, and may behave atypically due to the presences of large inversions on a hemizygous sex chromosome. Thus, balancer hybrids are not an ideal comparison.
The results of our comparisons of Ten-a- and Pde9- hybrids are congruent with that of Smr- hybrids. Ten-a- and Pde9- hybrids also court both female types with equal frequency and effort. There are, however, quantitative differences in the courtship frequencies of each hybrid, suggesting that D. melanogaster genetic background also influences male behavior (each has the same D. simulans background), making comparisons between strains imperfect.
Nonetheless, in courting indiscriminately, all three of these strains display a different overall pattern of courtship than intact melX males, which court D. melanogaster females at significantly higher frequencies than D. simulans females (melX males also display non-significantly higher efforts with D. melanogaster females). This may simply be because melX males were created using a different D. melanogaster background (LHM), and other D. melanogaster backgrounds may not discriminate as strongly (consistent with the Smr balancer hybrid data and RNAi/balancer data discussed below). In the case of Ten-a- hybrids, the D. melanogaster background is Canton-S, which also courts D. melanogaster more frequently than D. simulans females, and does not differ from LHM in this respect (Figure 2A). Thus, it may also be because each of these genes plays a small roll in reducing male preference for D. melanogaster females, and additively produce a much larger effect, like that seen with BSC100 hybrids.
The results of our RNAi knockdown screen did identify one gene that, when knocked down, significantly changed male behavior: cac. Although there was a general effect of RNAi knockdown on courtship effort overall, only cac RNAi males showed reduced courtship frequency toward D. simulans females, while Ir11a RNAi males and both cac and Ir11a balancer males displayed high courtship frequencies to both species. Further, we’ve shown that this effect is driven by the lack of 7,11-HD on the D. simulans female cuticle, because when we add 7,11-HD synthetically, cac RNAi male courtship frequency and effort with D. simulans females return to high levels. This result is, at first, not entirely intuitive; the loss of expression of a D. melanogaster allele makes males behave comparatively more like D. melanogaster. We take this result to imply that cac, a calcium channel subunit expressed in neurons, is important to general signal transduction in the male CNS. Thus, the loss of cac expression results in difficulty activating P1 courtship neurons in the absence of multiple attractive stimuli, like 7,11-HD, that activate different pathways converging on P1 courtship neurons (Clowney et al. 2015). If anything, this result suggests that cac is neither necessary nor sufficient for 7,11-HD response.
Ultimately, these results are difficult to interpret, because it is unclear what phenotypes to expect from a hemizygous male harboring a gene disruption. The only case where this test should yield a clear result is where the difference in behavior is attributable to a loss of function or expression in D. simulans. However, differences in phenotype can also be due to coding differences among genes, or differences in the amount, location, or timing of gene expression. In these cases, it is unclear what phenotypic change to expect from males completely lacking a gene altogether. While comparing gene aberrations from a D. simulans male may provide the reciprocal test (if phenotypic change is due to a loss in D. melanogaster), this test is still subject to all of the same problems discussed above. Additionally, it would require significant time and effort to create these aberrations, as none are currently available in D. simulans strains. This difficulty is specific to mapping male phenotypes on the X chromosome, as quantitative complementation (Turner 2014) and reciprocal hemizygosity tests are not possible (Stern 2014).
It is important to note that we only tested five candidate genes. We selected these genes because they met specific criteria (see methods) that we believed made them likely candidates, and because screening 159 knockouts is too large an undertaking (we observed nearly 500 pairs of courting flies to test our 5 candidates). It is quite possible, then, that the gene(s) responsible are among those that did not meet our strict criteria. For instance, perhaps the gene(s) are expressed in the developing larvae, rather than the adult CNS. Alternatively, perhaps the gene(s) are not specific to the nervous system, and instead have a more general function. Finally, the gene(s) may not directly interact with Fru, and instead act downstream or independently of Fru.
Conclusions
Our results demonstrate that male courtship preference differences between D. melanogaster and D. simulans is at least partially explained by 1.35 Mb region of the X chromosome. We further show that this region responds to the presence of the D. melanogaster cuticular hydrocarbon pheromone 7,11-HD. Unfortunately, attempts to fine-map this region were unsuccessful using the DP(1;Y) hybrid method and a candidate gene approach. Because hybrid offspring of these species are sterile, we cannot pursue other avenues to map the causal loci, such as QTL mapping. Similarly, because males are hemizygous for the X chromosome, we cannot use large engineered chromosomal deletions (as in Laturney & Moehring 2012; Pardy et al., 2018).
Still, our findings contribute to our understanding of the 7,11-HD preference phenotype. Although the neuronal circuitry required for 7,11-HD response in D. melanogaster has been known for some time (Clowney et al. 2015), it was just recently found that the same circuitry detects and responds to 7,11-HD in D. simulans (Seeholzer et al. 2018). While the anatomy of this circuit has remained constant during the divergence of these species, the valence of male response has undoubtedly changed – in large part due to changes in the interactions between these neurons, rather than their physical connections. It is still unclear what genetic changes are required to modify the interactions of these neurons, but our results provide a narrowed region of the genome with which to identify and continue to test candidates. Our results also highlight the difficulty of dissecting such a complex phenotype using a purely mapping approach. It is our hope that these data, when paired with functional dissection of the nervous system, can contribute to the identification of alleles explaining behavioral evolution. This is a necessary goal if we wish to understand the common patterns of genetic change underlying behavioral divergence.
Acknowledgments
We are grateful to Veronica Cochrane, Cameryn Brock, Susanne Tilk, Wesley Cochrane, Maddie Evancie, Devon Cooper, and Eamon Winden for their hours spent observing Drosophila courtship. We also thank the undergraduate students of the MCDB161L M’13 course at UCSB. This program was supported in part by a grant to the University of California, Santa Barbara from the Howard Hughes Medical Institute through the Science Education Program. This work would not have been possible without the community-supported resources available at the Cornell Drosophila Stock Center (CSBR 1820594), the Bloomington Drosophila stock center (NIH P400D018537), or FlyBase (flybase.org; Gramates et al., 2017). This work was funded by the National Institutes of Health (R01 GM098614).
Footnotes
Supplemental material available at figshare: https://doi.org/10.25387/g3.10284269.
Communicating editor: A. Wong
Literature cited
- Arbuthnott D., Fedina T. Y., Pletcher S. D., and Promislow D. E. L., 2017. Mate choice in fruit flies is rational and adaptive. Nat. Commun. 8: 13953 10.1038/ncomms13953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer T. O., and Benton R., 2016. Sexual circuitry in Drosophila. Curr. Opin. Neurobiol. 38: 18–26. 10.1016/j.conb.2016.01.004 [DOI] [PubMed] [Google Scholar]
- Auer T. O., Khallaf M. A., Silbering A. F., Zappia G., Ellis K. et al. , 2019. The making of an olfactory specialist. bioRxiv 10.1101/546507 [DOI] [Google Scholar]
- Bendesky A., Kwon Y. M., Lassance J. M., Lewarch C. L., Yao S. et al. , 2017. The genetic basis of parental care evolution in monogamous mice. Nature 544: 434–439. 10.1038/nature22074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter J.-C., Atallah J., Krupp J. J., Millar J. G., and Levine J. D., 2009. Specialized cells tag sexual and species identity in Drosophila melanogaster. Nature 461: 987–991. 10.1038/nature08495 [DOI] [PubMed] [Google Scholar]
- Brideau N. J., Flores H. A., Wang J., Maheshwari S., Wang X. et al. , 2006. Two Dobzhansky-Muller Genes interact to cause hybrid lethality in Drosophila. Science 314: 1292–1295. 10.1126/science.1133953 [DOI] [PubMed] [Google Scholar]
- Cande J., Prud’homme B., and Gompel N., 2013. Smells like evolution: The role of chemoreceptor evolution in behavioral change. Curr. Opin. Neurobiol. 23: 152–158. 10.1016/j.conb.2012.07.008 [DOI] [PubMed] [Google Scholar]
- Cattani V. M., and Presgraves D. C., 2012. Incompatibility between X chromosome factor and pericentric heterochromatic region causes lethality in hybrids between drosophila melanogaster and its sibling species. Genetics 191: 549–559. 10.1534/genetics.112.139683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowney E. J., Iguchi S., Bussell J. J., Scheer E., and Ruta V., 2015. Multimodal chemosensory circuits controlling male courtship in Drosophila. Neuron 87: 1036–1049. 10.1016/j.neuron.2015.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly K., and Cook R., 1973. Rejection responses by female Drosophila melanogaster - their ontogeny, causality and effects upon behavior of the courting male. Behaviour 44: 142–166. 10.1163/156853973X00364 [DOI] [Google Scholar]
- Cook R. K., Deal M. E., Deal J. A., Garton R. D., Brown C. A. et al. , 2010. A new resource for characterizing X-linked genes in Drosophila melanogaster: Systematic coverage and subdivision of the X chromosome with nested, Y-linked duplications. Genetics 186: 1095–1109. 10.1534/genetics.110.123265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne J. A., 1996. Genetics of differences in pheromonal hydrocarbons between Drosophila melanogaster and D. simulans. Genet. Anal. 143: 353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne J. A., and Orr H. A., 1997. Patterns of Speciation in Drosophila Revisited. Evolution. 51: 295–303. [DOI] [PubMed] [Google Scholar]
- Coyne J. A., and Orr H. A., 2004. Speciation. Sunderland (MA): Sinauer Associates. [Google Scholar]
- Edward D. A., and Chapman T., 2011. The evolution and significance of male mate choice. Trends Ecol. Evol. 26: 647–654. 10.1016/j.tree.2011.07.012 [DOI] [PubMed] [Google Scholar]
- Fanara J. J., Robinson K. O., Rollmann S. M., Anholt R. R. H., and Mackay T. F. C., 2002. Vanaso is a candidate quantitative trait gene for Drosophila olfactory behavior. Genetics 162: 1321–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferveur J. F., and Sureau G., 1996. Simultaneous influence on male courtship of stimulatory and inhibitory pheromones produced by live sex-mosaic Drosophila melanogaster. Proc. Biol. Sci. 263: 967–973. 10.1098/rspb.1996.0143 [DOI] [PubMed] [Google Scholar]
- Goto J., Mikawa Y., Koganezawa M., Ito H., and Yamamoto D., 2011. Sexually dimorphic shaping of interneuron dendrites involves the hunchback transcription Factor. J. Neurosci. 31: 5454–5459. 10.1523/JNEUROSCI.4861-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramates L. S., Marygold S. J., dos Santos G., Urbano J. M., Antonazzo G. et al. , 2017. FlyBase at 25: Looking to the future. Nucleic Acids Res. 45: D663–D671. 10.1093/nar/gkw1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveley B. R., Brooks A. N., Carlson J. W., Duff M. O., Landolin J. M. et al. , 2011. The developmental transcriptome of Drosophila melanogaster. Nature 471: 473–479. 10.1038/nature09715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbison S. T., Yamamoto A. H., Fanara J. J., Norga K. K., and Mackay T. F. C., 2004. Quantitative Trait Loci Affecting Starvation Resistance in Drosophila melanogaster. Genetics 166: 1807–1823. 10.1534/genetics.166.4.1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S., 1979. A Simple Sequentially Rejective Multiple Test Procedure. Scand. J. Stat. Scand. J. Stat. 6: 65–70. [Google Scholar]
- Jallon J.-M., and David J. R., 1987. Variation in cuticular hydrocarbons among the eight species of the Drosophila melanogaster subgroup. Evolution 41: 294–302. [DOI] [PubMed] [Google Scholar]
- Kawanishi M., and Watanabe T. K., 1981. Genes affecting courtship song and mating preference in Drosophila melanogaster, Drosophila simulans and their hybrids. Evolution 35: 1128–1133. 10.1111/j.1558-5646.1981.tb04983.x [DOI] [PubMed] [Google Scholar]
- Kittelmann S., Buffry A. D., Almudi I., Yoth M., Sabaris G. et al. , 2017. Gene regulatory network architecture in different developmental contexts influences the genetic basis of morphological evolution. PLoS Genet. 14: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher S. D., Mallarino R., Rubin B. E. R., Yu D. W., Hoekstra H. E. et al. , 2018. The genetic basis of a social polymorphism in halictid bees. Nat. Commun. 9: 4338 10.1038/s41467-018-06824-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp A., 2009. Metamodels and phylogenetic replication: A systematic approach to the evolution of developmental pathways. Evolution. 63: 2771–2789. 10.1111/j.1558-5646.2009.00761.x [DOI] [PubMed] [Google Scholar]
- Laturney M., and Moehring A. J., 2012. Fine-scale genetic analysis of species-specific female preference in Drosophila simulans. J. Evol. Biol. 25: 1718–1731. 10.1111/j.1420-9101.2012.02550.x [DOI] [PubMed] [Google Scholar]
- Leary G. P., Allen J. E., Bunger P. L., Luginbill J. B., Linn C. E. et al. , 2012. Single mutation to a sex pheromone receptor provides adaptive specificity between closely related moth species. Proc. Natl. Acad. Sci. USA 109: 14081–14086. 10.1073/pnas.1204661109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B., LaMora A., Sun Y., Welsh M. J., and Ben-Shahar Y., 2012. ppk23-Dependent chemosensory functions contribute to courtship behavior in Drosophila melanogaster. PLoS Genet. 8: e1002587 10.1371/journal.pgen.1002587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B., Zelle K. M., Seltzer R., Hefetz A., Ben-Shahar Y. et al. , 2014. Feminization of pheromone-sensing neurons affects mating decisions in Drosophila males. Biol. Open 3: 152–160. 10.1242/bio.20147369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay T. F. C., Richards S., Stone E. A., Barbadilla A., Ayroles J. F. et al. , 2012. The Drosophila melanogaster Genetic Reference Panel. Nature 482: 173–178. 10.1038/nature10811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning A., 1959. The sexual isolation between Drosophila melanogaster and Drosophila simulans. Anim. Behav. 7: 60–65. 10.1016/0003-3472(59)90031-4 [DOI] [Google Scholar]
- Manoli D. S., Foss M., Villella A., Taylor B. J., Hall J. C. et al. , 2005. Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature 436: 395–400. 10.1038/nature03859 [DOI] [PubMed] [Google Scholar]
- Martin A., and Orgogozo V., 2013. The loci of repeated evolution: A catalog of genetic hotspots of phenotypic variation. Evolution. 67: 1235–1250. [DOI] [PubMed] [Google Scholar]
- McBride C. S., 2007. Rapid evolution of smell and taste receptor genes during host specialization in Drosophila sechellia. Proc. Natl. Acad. Sci. USA 104: 4996–5001. 10.1073/pnas.0608424104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride C. S., Baier F., Omondi A. B., Spitzer S. A., Lutomiah J. et al. , 2014. Evolution of mosquito preference for humans linked to an odorant receptor. Nature 515: 222–227. 10.1038/nature13964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. T., Glazer A. M., Summers B. R., Blackman B. K., Norman A. R. et al. , 2014. Modular skeletal evolution in sticklebacks is controlled by additive and clustered quantitative trait loci. Genetics 197: 405–420. 10.1534/genetics.114.162420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittleman B. E., Manzano-Winkler B., Hall J. B., Korunes K. L., and Noor M. A. F., 2017. The large X-effect on secondary sexual characters and the genetics of variation in sex comb tooth number in Drosophila subobscura. Ecol. Evol. 7: 533–540. 10.1002/ece3.2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehring A. J., and Mackay T. F. C., 2004. The Quantitative Genetic Basis of Male Mating Behavior in Drosophila melanogaster. Genetics 167: 1249–1263. 10.1534/genetics.103.024372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardy J. A., Rundle H. D., Bernards M. A., and Moehring A. J., 2018. The genetic basis of female pheromone differences between Drosophila melanogaster and D. simulans. Heredity 122: 93–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peichel C. L., and Marques D. A., 2017. The genetic and molecular architecture of phenotypic diversity in sticklebacks. Philos. Trans. R. Soc. B Biol. Sci. 372: 20150486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins L. A., Holderbaum L., Tao R., Hu Y., Sopko R. et al. , 2015. The transgenic RNAi project at Harvard medical school: Resources and validation. Genetics 201: 843–852. 10.1534/genetics.115.180208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pischedda A., Shahandeh M. P., Cochrane W. G., Cochrane V. A., and Turner T. L., 2014. Natural variation in the strength and direction of male mating preferences for female pheromones in Drosophila melanogaster. PLoS One 9: e87509 10.1371/journal.pone.0087509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeiz M., and Williams T. M., 2017. Using Drosophila pigmentation traits to study the mechanisms of cis-regulatory evolution. Curr. Opin. Insect Sci. 19: 1–7. 10.1016/j.cois.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice W. R., 1990. A Consensus Combined P-Value Test and the Family-Wide Significance of Component Tests. Biometrics 46: 303 10.2307/2531435 [DOI] [Google Scholar]
- Rice W. R., Linder J. E., Friberg U., Lew T. A., Morrow E. H. et al. , 2005. Inter-locus antagonistic coevolution as an engine of speciation: Assessment with hemiclonal analysis. Proc. Natl. Acad. Sci. USA 102: 6527–6534. 10.1073/pnas.0501889102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockman M. V., 2012. The QTN program and the alleles that matter for evolution: All that’s gold does not glitter. Evolution. 66: 1–17. 10.1111/j.1558-5646.2011.01486.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder E., Blows F., Ashburner M., Bautista-Llacer R., Coulson D. et al. , 2004. The DrosDel collection: A set of P-element insertions for generating custom chromosomal aberrations in Drosophila melanogaster. Genetics 167: 797–813. 10.1534/genetics.104.026658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos G., Schroeder A. J., Goodman J. L., Strelets V. B., Crosby M. A. et al. , 2015. FlyBase: Introduction of the Drosophila melanogaster Release 6 reference genome assembly and large-scale migration of genome annotations. Nucleic Acids Res. 43: D690–D697. 10.1093/nar/gku1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeholzer L. F., Seppo M., Stern D. L., and Ruta V., 2018. Evolution of a central neural circuit underlies Drosophila mate preferences. Nature 559: 564–569. 10.1038/s41586-018-0322-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahandeh M. P., Pischedda A., and Turner T. L., 2018. Male mate choice via cuticular hydrocarbon pheromones drives reproductive isolation between Drosophila species. Evolution. 72: 123–135. 10.1111/evo.13389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirangi T. R., Dufour H. D., Williams T. M., and Carroll S. B., 2009. Rapid evolution of sex pheromone-producing enzyme expression in Drosophila. PLoS Biol. 7: e1000168 10.1371/journal.pbio.1000168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess E. B., and Schwer W. A., 1978. Minority mating advantage of certain eye color mutants of Drosophila melanogaster. I. Multiple-choice and single-female tests. Behav. Genet. 8: 155–168. 10.1007/BF01066872 [DOI] [PubMed] [Google Scholar]
- Stern D. L., 2014. Identification of loci that cause phenotypic variation in diverse species with the reciprocal hemizygosity test. Trends Genet. 30: 547–554. 10.1016/j.tig.2014.09.006 [DOI] [PubMed] [Google Scholar]
- Stern D. L., and Orgogozo V., 2008. The loci of evolution: How predictable is genetic evolution? Evolution. 62: 2155–2177. 10.1111/j.1558-5646.2008.00450.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. L., Orgogozo V., Martin A., Hines H. M., Counterman B. A. et al. , 2009. Is genetic evolution predictable? Science 323: 746–751. 10.1126/science.1158997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thistle R., Cameron P., Ghorayshi A., Dennison L., and Scott K., 2012. Contact chemoreceptors mediate male-male repulsion and male-female attraction during Drosophila courtship. Cell 149: 1140–1151. 10.1016/j.cell.2012.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda H., Zhao X., and Dickson B. J., 2012. The Drosophila female aphrodisiac pheromone activates ppk23(+) sensory neurons to elicit male courtship behavior. Cell Reports 1: 599–607. 10.1016/j.celrep.2012.05.007 [DOI] [PubMed] [Google Scholar]
- Turner T. L., 2014. Fine-mapping natural alleles: quantitative complementation to the rescue. Mol. Ecol. 23: 2377–2382. 10.1111/mec.12719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernes S. C., 2014. Genome wide identification of Fruitless targets suggests a role in upregulating genes important for neural circuit formation. Sci. Rep. 4: 4412 10.1038/srep04412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T. K., 1979. A gene that rescues the lethal hybrids between Drosophila melanogaster and D. simulans. Jpn. J. Genet. 54: 325–331. 10.1266/jjg.54.325 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Tables 1 and 2 describe all of the Drosophila reagents we used. Table S1 describes the 159 genes identified within the candidate X chromosome region. The raw courtship data has been uploaded to figshare. Table S2 contains the sample size, courtship frequency, and median courtship effort for each male-female pairing we observed. Table S3 shows the male and female offspring count for crosses of one candidate gene knockout hybrid cross, papillote. Supplemental material available at figshare: https://doi.org/10.25387/g3.10284269.