Abstract
Peroxisome proliferator-activated receptor γ coactivator 1α (PGC1α) encoded by the PPARGC1A gene is a vital regulator of glucose and fatty acid oxidation, mitochondrial biogenesis, and skeletal muscle fibre conversion. Several studies have investigated the association between PPARGC1A Gly482Ser polymorphism and athletic performance in humans. However, the results were contradictory. In the present study, two meta-analyses were performed to assess the association between the Gly482Ser polymorphism and endurance or power athletic performance to resolve this inconsistency. Ten articles were identified, including a total of 3,708 athletes and 6,228 controls. Higher frequencies of the Gly/Gly genotype (OR, 1.26; 95% CI, 1.11–1.42) and the Gly allele (OR, 1.29; 95% CI, 1.09–1.52) were observed in Caucasian endurance athletes. Furthermore, higher incidences of the Gly/Gly genotype (OR, 1.30; 95% CI, 1.16–1.46) and the Gly allele (OR, 1.22; 95% CI, 1.12–1.33) were observed in power athletes compared to controls. This finding demonstrates that the Gly/Gly genotype and the Gly allele of the PPARGC1A Gly482Ser polymorphism may facilitate athletic performance regardless of the type of sport, as well as providing solid evidence to support the possible influence of genetic factors on human athletic performance.
Keywords: Meta-analysis, PPARGC1A, Polymorphism, Endurance, Power, Athletic performance
INTRODUCTION
Human athletic performance is a multifactorial trait determined by the interaction of genetic and environmental factors. It is estimated that around 66% of the variance in athletic status could be explained by genetic factors [1]. The remaining variance is dependent on environmental factors, such as physical training, nutrition, and technological support. With the development of molecular research in sport, at least 155 genetic variants have been found to be associated with athletic performance, with the angiotensin converting enzyme (ACE) gene I/D and the alpha actinin-3 (ACTN3) gene R577X polymorphisms having been the most extensively studied [2–4]. However, partly owing to the small sample size of studies, a considerable number of these proposed associations have not been consistently replicated in independent investigations by different teams of researchers [5].
PPARGC1A has been suggested to be associated with athletic performance because of its role in a wide variety of biological responses [6, 7]. It encodes peroxisome proliferator-activated receptor γ coactivator 1α (PGC1α), a transcriptional coactivator of the peroxisome proliferator-activated receptor (PPAR) family. PGC1α regulates the expression of several key genes involved in glucose and fatty acid oxidation [8, 9]. It is also a key stimulator of mitochondrial biogenesis by activating transcription of the nuclear respiratory factors NRF1 and NRF2, inducing expression of mitochondrial transcription factor A (TFAM) [10]. PGC1α is also important for skeletal muscle fibre conversion. Over-expression of PPARGC1A leads to the conversion of fast-twitch type IIb muscle fibres to type IIa and slow-twitch type I fibres by 20% and 10%, respectively [11]. Furthermore, PPARGC1A expression correlates with both short-term exercise and endurance training in rodents and humans [12–14].
The PPARGC1A gene is located on chromosome 4 (4p15.2). The Gly482Ser (rs8192678) polymorphism is the most frequently analyzed of all the gene variations that have been discovered. The polymorphism has been reported to be associated with type 2 diabetes, obesity and elevated blood pressure [15–17]. In three case-control studies, a significantly lower frequency of the Ser allele has been reported in elite endurance athletes compared with sedentary controls [18–20]. However, several other studies have failed to replicate the same association [21–26]. Furthermore, two studies observed a higher frequency of the Gly/Gly genotype in power athletes [20, 24]. Therefore, no definitive conclusions have been drawn about the relationship between the PPARGC1A Gly482Ser polymorphism and athletic performance. Tharabenjasin et al. recently reported the results of a meta-analysis about the association of the PPARGC1A Gly428Ser polymorphism with athletic ability and sports performance [27].
The aim of this study is to summarize the association between the PPARGC1A Gly482Ser polymorphism and athletic performance by conducting meta-analyses, which might provide a more definitive answer compared with individual research reports.
MATERIALS AND METHODS
Literature identification
All procedures involved in the meta-analyses were carried out in accordance with the PRISMA guidelines [28]. A comprehensive literature search was performed using the PubMed and Web of Science databases, from inception to September 2018. The combination of the following keywords was used: “PPARGC1A or PGC1α”, “polymorphisms”, “rs8192678” and “sports”. No language limitations or publication restrictions were applied to the search strategy.
Inclusion and exclusion criteria
Studies that reported the distribution of PPARGC1A polymorphism among both athletes and sedentary controls were considered. If the same data were presented in multiple studies, the highest quality study was included. Exclusion criteria were: (i) review articles or conference literatures; (ii) studies involving animal experiments, or the target population was not athletes; (iii) articles did not provide sufficient original data; (iv) genotype distribution deviated from Hardy–Weinberg equilibrium (HWE) in the control group; (v) studies only concerning mixed endurance-power type of sports, such as football.
Quality assessment
The Newcastle-Ottawa Scale (NOS) was used to evaluate the methodological quality of the included studies by two reviewers independently [29]. Each study was assessed and scored based on three aspects: case and control selection, comparability, and exposure. NOS score ranges from 0 to 9 stars. Studies that scored seven or more stars were considered to be of high quality.
Statistical analysis
Hardy–Weinberg equilibrium was examined in controls for each study by Pearson’s chi-squared test. Heterogeneity across the studies was assessed by the I square statistic (I 2), with I 2 < 50% indicating reduced statistical difference [30]. A fixed-effects model was used in cases of low statistical heterogeneity, otherwise a random-effects model was applied [31, 32]. The association between polymorphism and athletic performance was estimated by calculating the odds ratio (OR) with corresponding 95% confidence interval (95% CI), comparing athletes and controls. Potential publication bias was examined by Begg’s and Egger’s tests and funnel plots [33, 34]. Sensitivity analysis was also conducted to examine the stability of the overall results by sequential exclusion of one study at a time. All statistical analyses were conducted with STATA software (version 15, StataCorp, College Station, Texas).
RESULTS
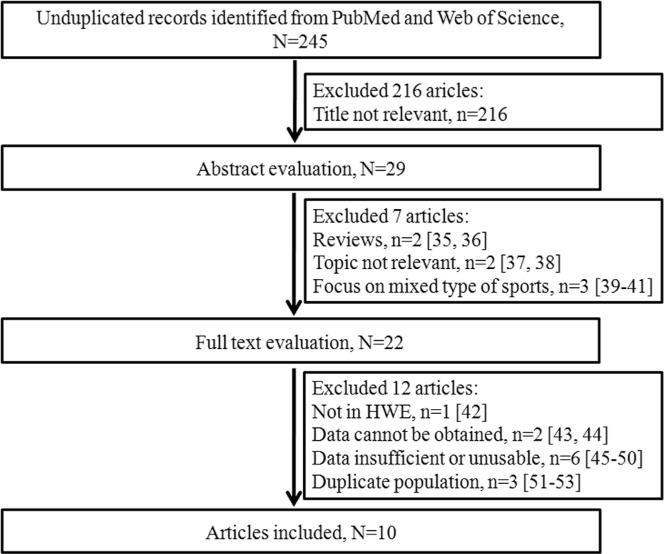
The initial search of electronic databases identified 245 unduplicated articles. As shown in Figure 1, after excluding articles whose titles were not relevant, 29 abstracts were retrieved for the next step. After abstract evaluation, 22 articles were included in a more detailed full text evaluation [35–41]. Then 12 articles were excluded [42–53]. Ultimately 10 articles were included in this study (Fig. 1).
FIG. 1.
Flow diagram of literature search and screen.
The 10 studies involved a total of 3,708 athletes and 6,228 controls. The athletes were divided into endurance-type and power-type groups in accordance with their sporting discipline [4]. The endurance group included athletes who participated in marathon, biathlon, long-distance swimming, pentathlon, rowing, long-distance running, road cycling, cross-country skiing, long-distance track and field athletics, triathlon, race walking and mountain biking. The power group included athletes involved in sprinting, weightlifting, short-distance track and field athletics, powerlifting, kayaking, judo, wrestling, boxing, fencing, short-distance swimming, alpine skiing, artistic gymnastics, and throwing and jumping events. It should be pointed out that no clear-cut distinction can be drawn between endurance and power sports. There are elements of power in the endurance sports mentioned, as there are endurance elements in power sports. All the power athletes were from Caucasian populations. The data for athletes who participated in mixed-type sports were not extracted from the studies included.
For all articles, the following data were extracted from original publications: first author and year of publication, country of the study, total number of athletes and controls, type of athletes and controls, race of participants, and genotype and allele frequencies among athletes and controls and for each of the subgroups (Table 1 and Table 2). The results of HWE tests demonstrated that the genotype distributions in controls were all in HWE (all P > 0.05). And according to the quality criteria, the NOS score for all articles is greater than or equal to 7, except for one article [23]. Two meta-analyses were carried out with the endurance group and power group.
TABLE 1.
Summary of primary data for association between PPARGC1A Gly482Ser polymorphism and endurance performance.
| Authors | Year | Country | Ethnicity | Group | Number (N) | Genotype (N) | MAF | PHWE | NOS Score | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gly/Gly | Gly/Ser | Ser/Ser | |||||||||
| Lucia et al. | 2005 | Spain | Caucasian | Case Control | 104 | 52 | 43 | 9 | 0.293 | 1.0000 | 7 |
| 100 | 36 | 48 | 16 | 0.400 | |||||||
| Eynon et al. | 2010 | Israeli | Caucasian | Case Control | 74 | 37 | 37 | 0 | 0.250 | 0.9529 | 7 |
| 240 | 79 | 117 | 44 | 0.427 | |||||||
| Muniesa et al. | 2010 | Spanish | Caucasian | Case Control | 141 | 65 | 52 | 24 | 0.355 | 0.2261 | 7 |
| 123 | 47 | 63 | 13 | 0.362 | |||||||
| Ginevičienė et al. | 2011 | Lithuanian | Caucasian | Case Control | 77 | 40 | 33 | 4 | 0.266 | 0.5177 | 9 |
| 250 | 129 | 104 | 17 | 0.276 | |||||||
| Maciejewska et al. | 2012 | Polish | Caucasian | Case Control | 84 | 46 | 34 | 4 | 0.250 | 0.8938 | 8 |
| 684 | 280 | 314 | 90 | 0.361 | |||||||
| Russian | Caucasian | Case Control | 548 | 273 | 239 | 36 | 0.284 | 0.6651 | |||
| 1132 | 489 | 505 | 138 | 0.345 | |||||||
| He et al. | 2015 | Chinese | Asian | Case Control | 235 | 73 | 115 | 47 | 0.445 | 0.6321 | 7 |
| 504 | 156 | 244 | 104 | 0.448 | |||||||
| Yvert et al. | 2016 | Japanese | Asian | Case Control | 175 | 45 | 87 | 43 | 0.494 | 0.8741 | 8 |
| 649 | 191 | 324 | 134 | 0.456 | |||||||
| Peplonska et al. | 2017 | Polish | Caucasian | Case Control | 225 | 102 | 105 | 18 | 0.313 | 0.0871 | 6 |
| 451 | 199 | 213 | 39 | 0.323 | |||||||
| Guilherme et al. | 2018 | Brazilian | Caucasian | Case Control | 316 | 153 | 140 | 23 | 0.294 | 0.6187 | 7 |
| 893 | 428 | 385 | 80 | 0.305 | |||||||
MAF = minor allele frequency, P HWE = P value for Hardy–Weinberg equilibrium of controls, NOS = Newcastle-Ottawa Scale.
TABLE 2.
Summary of primary data for association between PPARGC1A Gly482Ser polymorphism and power performance.
| Authors | Year | Country | Ethnicity | Group | Number (N) | Genotype (N) | MAF | PHWE | NOS Score | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gly/Gly | Gly/Ser | Ser/Ser | |||||||||
| Eynon et al. | 2010 | Israeli | Caucasian | Case Control | 81 | 35 | 36 | 10 | 0.346 | 0.9529 | 7 |
| 240 | 79 | 117 | 44 | 0.427 | |||||||
| Ginevičienė et al. | 2011 | Lithuanian | Caucasian | Case Control | 51 | 29 | 21 | 1 | 0.225 | 0.5177 | 9 |
| 250 | 129 | 104 | 17 | 0.276 | |||||||
| Maciejewska et al. | 2012 | Polish | Caucasian | Case Control | 210 | 118 | 79 | 13 | 0.25 | 0.8938 | 8 |
| 684 | 280 | 314 | 90 | 0.361 | |||||||
| Russian | Caucasian | Case Control | 724 | 329 | 322 | 73 | 0.323 | 0.6651 | |||
| 1132 | 489 | 505 | 138 | 0.345 | |||||||
| Gineviciene et al. | 2016 | Russian | Caucasian | Case Control | 114 | 62 | 35 | 17 | 0.303 | 0.7450 | 8 |
| 947 | 424 | 416 | 107 | 0.333 | |||||||
| Lithuanian | Caucasian | Case Control | 47 | 24 | 22 | 1 | 0.255 | 0.4860 | |||
| 255 | 132 | 106 | 17 | 0.275 | |||||||
| Peplonska et al. | 2017 | Polish | Caucasian | Case Control | 188 | 97 | 73 | 18 | 0.290 | 0.0871 | 6 |
| 451 | 199 | 213 | 39 | 0.323 | |||||||
| Guilherme et al. | 2018 | Brazilian | Caucasian | Case Control | 314 | 173 | 116 | 25 | 0.264 | 0.6187 | 7 |
| 893 | 428 | 385 | 80 | 0.305 | |||||||
MAF = minor allele frequency, P HWE = P value for Hardy–Weinberg equilibrium of controls, NOS = Newcastle-Ottawa Scale.
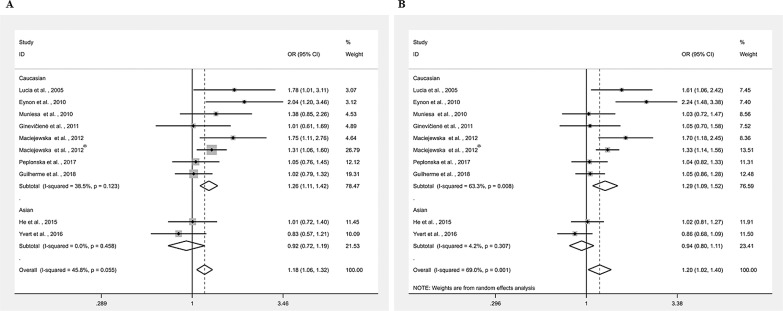
As shown in Fig. 2A, a higher frequency of the Gly/Gly genotype was observed in endurance athletes compared to controls in Caucasian populations. The combined OR for the Gly/Gly genotype compared to the Gly/Ser + Ser/Ser genotype was 1.26 (95% CI, 1.11–1.42). The degree of heterogeneity across the studies was moderate (I 2 = 38.5%). There was no significance observed in Asian endurance athletes (OR, 0.92; 95% CI, 0.72–1.19). A higher frequency of the Gly allele (OR, 1.29; 95% CI, 1.09–1.52) was also observed in Caucasian endurance athletes, but not in Asian counterparts (OR, 0.94; 95% CI, 0.80–1.11; Fig. 2B).
FIG. 2.
Meta-analysis of the association between endurance performance and PPARGC1A Gly482Ser polymorphism. (A) Gly/Gly vs. Gly/Ser+Ser/Ser; (B) (Allele Gly vs. Ser). CI= confidence interval; OR= odds ratio. *Different study population from the same article.
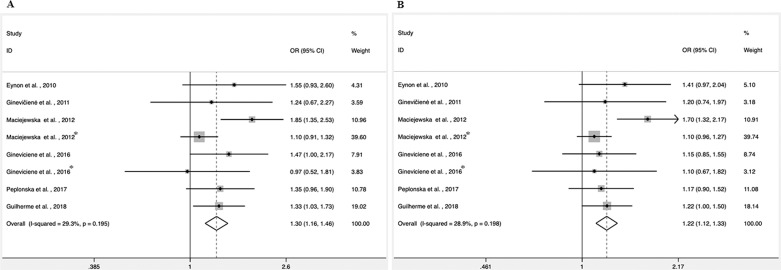
Fig. 3 shows the results of the overall associations between the PPARGC1A Gly482Ser polymorphism and power performance. A significant correlation was found for the Gly/Gly genotype in athletes compared to controls (OR, 1.30; 95% CI, 1.16–1.46; Fig. 3A). The degree of heterogeneity across studies was low (I 2 = 29.3%). A higher frequency of the Gly allele was also observed in power athletes (OR, 1.22; 95% CI, 1.12–1.33; Fig. 3B). Here, the degree of heterogeneity across studies was also low (I 2 = 28.9%).
FIG. 3.
Meta-analysis of the association between power performance and PPARGC1A Gly482Ser polymorphism. (A) Gly/Gly vs. Gly/Ser+Ser/Ser; (B) (Allele Gly vs. Ser). CI= confidence interval; OR= odds ratio. *Different study population from the same article.
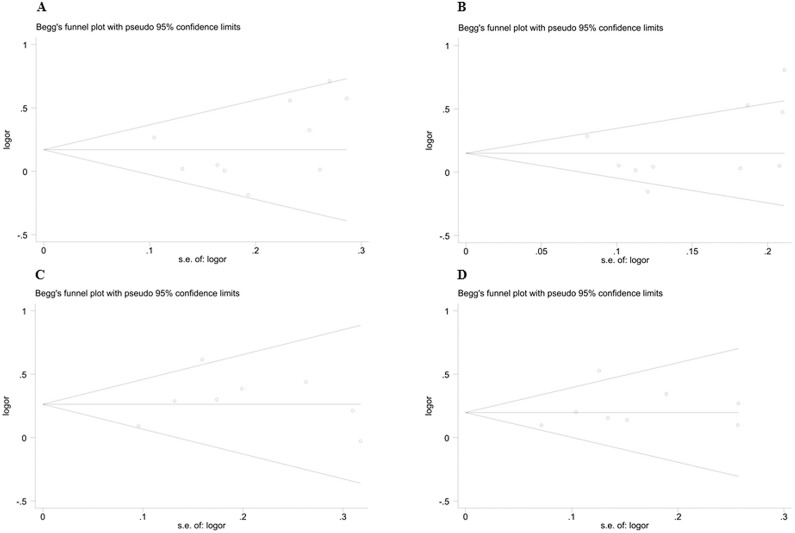
Publication bias was assessed by Begg’s and Egger’s tests and funnel plots. There was no obvious asymmetry in the Begg’s funnel plot (Figure 4). The results of Begg’s test (Gly/Gly vs. Gly/Ser+Ser/Ser for endurance performance: P = 0.269; allele Gly vs. Ser for endurance performance: P = 0.066; Gly/Gly vs. Gly/Ser+Ser/Ser for power performance: P = 1.0; allele Gly vs. Ser for power performance: P = 1.0) and Egger’s test (Gly/Gly vs. Gly/Ser+Ser/Ser for endurance performance: P = 0.093; allele Gly vs. Ser for endurance performance: P = 0.200; Gly/Gly vs. Gly/Ser+Ser/Ser for power performance: P = 0.481; allele Gly vs. Ser for power performance: P = 0.525) also suggested no statistically significant publication bias.
FIG. 4.
Begg’s funnel plot for eligible studies of association between PPARGC1A Gly482Ser polymorphism and athletic performance. (A) Homozygotes Gly/Gly vs. Gly/Ser+Ser/Ser for endurance performance; (B) Allele Gly vs. Ser for endurance performance; (C) Homozygotes Gly/Gly vs. Gly/Ser+Ser/Ser for power performance; (D) Allele Gly vs. Ser for power performance. OR= odds ratio.
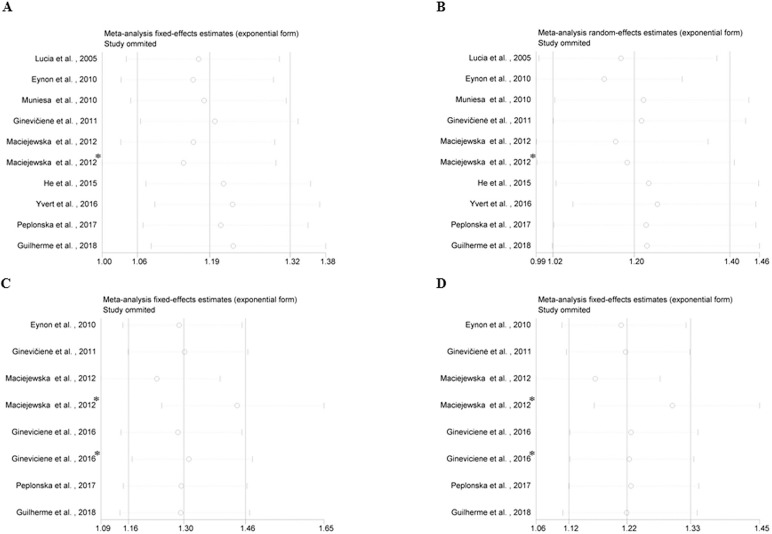
Sensitivity analysis was performed to evaluate the effect of each included study on the overall results. One study was excluded each time. Then pooled ORs were recomputed and compared with the overall OR. Significant associations between the PPARGC1A Gly allele and endurance performance were not observed after excluding the Maciejewska et al. article [20] (Fig. 5A; Fig. 5B). It indicated that the results were unstable. Moreover, the overall results of the associations between PPARGC1A Gly482Ser polymorphism and power performance were rather stable (Fig. 5C; Fig. 5D).
FIG. 5.
Sensitivity analysis of the association PPARGC1A Gly482Ser polymorphism and athletic performance. (A) Homozygotes Gly/Gly vs. Gly/Ser+Ser/Ser for endurance performance; (B) Allele Gly vs. Ser for endurance performance; (C) Homozygotes Gly/Gly vs. Gly/Ser+Ser/Ser for power performance; (D) Allele Gly vs. Ser for power performance. *Different study population from the same article.
DISCUSSION
PPARGC1A encodes a key regulator of cellular energy metabolism. This study estimated the association of human athletic performance with PPARGC1A Gly482Ser polymorphism by means of meta-analysis. The main finding of the current study is that higher frequencies of the Gly/Gly genotype 1.26 (95% CI, 1.11–1.42) and the Gly allele (OR, 1.29; 95% CI, 1.09–1.52) were observed in Caucasian endurance athletes, but not in Asian counterparts. Furthermore, higher incidences of the Gly/Gly genotype (OR, 1.30; 95% CI, 1.16–1.46) and the Gly allele (OR, 1.22; 95% CI, 1.12–1.33) were observed in power athletes compared to controls.
To date, the results of individual studies on the associations between the PPARGC1A Gly482Ser polymorphism and athletic performance have been discrepant. Lucia et al. first detected a significantly lower frequency of the Ser allele in Spanish endurance athletes [18]. A later study supported this association [19, 20], although there were also some exceptions [21–26]. One of the greatest limitations of these case–control association studies is their small sample size, which often leads to statistical insignificance and results in controversial conclusions. The current meta-analyses overcame this limitation by combining the findings from 10 studies. The analyses involved 3,708 athletes and 6,228 controls. The results revealed that higher frequencies of the Gly/Gly genotype and the Gly allele were observed in Caucasian endurance and power athletes. Thus, the study provides solid evidence for an association between PPARGC1A polymorphism and athletic performance.
It is interesting to note that higher frequencies of the Gly/Gly genotype and the Gly allele of the PPARGC1A Gly482Ser polymorphism were found in both endurance athletes and power athletes from Caucasian populations. Endurance sports are generally considered to mainly use the aerobic energy system to produce energy, while power sports rely mostly on anaerobic metabolism as the energy source [54]. However, they are not totally distinct entities. Modern endurance sports also require very powerful muscle contractions at competitively critical stages [55], while the contribution of the aerobic energy system to some kinds of speed/power sports is considerable [56]. A previous study demonstrated that the Ser allele is associated with lower expression of PPARGC1A [57]. Several studies have shown the effect of Gly482Ser polymorphism on the functional activity of PGC1α, but the results are controversial. Choi et al. firstly suggested that the PGC1α 482Gly variant had impaired co-activator activity on the TFAM promoter [58]. In contrast, Okauchi et al. reported no difference in activity between the variants when activating the adiponectin promoter [59]. A study performed by Michael et al. demonstrated that PGC1α could bind to and co-activate the muscle-selective transcription factor (MEF) 2C, then increased the expression of glucose transporter 4 (GLUT4) [60]. Also, the change from Gly to Ser at position 482 in PGC1α decreased its binding interaction with MEF2C [61]. Thus, the decreased interaction might impair the GLUT4 insulin-stimulated glucose uptake, which would then affect glycogen synthesis and the subsequent synthesis of fatty acids. Finally, the PGC1α 482Ser variant might weaken the efficiency of aerobic metabolism. Moreover, the Pgc1α/Mef2c complex could bind to the Ppargc1a promoter and activate it [62]. So the decreased interaction between PGC1α/MEF2C might decrease the expression of PPARGC1A itself. Therefore, the Gly allele of the Gly482Ser polymorphism may facilitate athletic performance through increasing the expression of PPARGC1A and enhancing the efficiency of aerobic metabolism.
Although the current study was about a similar topic as a recently published meta-analysis [27], this study contributes to the PPARGC1A research in athletic performance. First, the present study corrects the mistakes that appeared in the Tharabenjasin et al. study. This study excluded articles that there were duplicate genotype data of athletes or controls. For example, genotype data of 1132 Russian controls and partial Russian athletes were duplicated between the Ahmetov et al. article and Maciejewska et al. article [20, 52]. Duplicate data may affect overall results, especially when it comes to large samples. Thus only the Maciejewska et al. article was included in this study. By contrast, both articles were included in the Tharabenjasin et al. study. Second, the results of this study indicated that endurance-type and power-type sports might have more in common than was generally believed regarding the genetic background, especially when a specific gene polymorphism was taken into account.
Several limitations should be considered in interpreting the results of this study. First, owing to the inconsistent definition of endurance events among some studies, phenotypic heterogeneity cannot be completely avoided. Second, owing to the different standards of elite, sub-elite and non-elite athletes, the present study did not consider the potential confounding effects of performance levels. In addition, Begg’s and Egger’s tests as well as funnel plots were used to assess publication bias in this study, whereas such tests have low power when applied to studies whose number is < 10 [63]. Finally, because not all the relevant data could be obtained from the included studies, further detailed sub-analysis was limited. For example, if the athletes could have been subdivided into male and female groups, more comprehensive results could be presented.
CONCLUSIONS
In conclusion, the current meta-analyses based on 10 studies revealed that higher frequencies of the Gly/Gly genotype and the Gly allele of the PPARGC1A Gly482Ser polymorphism were observed in Caucasian endurance athletes, and the Gly/Gly genotype and the Gly allele were significantly associated with power athletes compared to controls. The results demonstrate that the Gly/Gly genotype and the Gly allele of the PPARGC1A Gly482Ser polymorphism may facilitate athletic performance regardless of the type of sport. This finding also provides solid evidence to support the possible influence of genetic factors on human athletic performance.
Conflict of interest declaration
The authors have no conflict of interests.
REFERENCES
- 1.De Moor MH, Spector TD, Cherkas LF, Falchi M, Hottenga JJ, Boomsma DI, De Geus EJ. Genome-wide linkage scan for athlete status in 700 British female DZ twin pairs. Twin Res Hum Genet. 2007;10:812–20. doi: 10.1375/twin.10.6.812. [DOI] [PubMed] [Google Scholar]
- 2.Ahmetov II, Egorova ES, Gabdrakhmanova LJ, Fedotovskaya ON. Genes and Athletic Performance: An Update. Med Sport Sci. 2016;61:41–54. doi: 10.1159/000445240. [DOI] [PubMed] [Google Scholar]
- 3.Pitsiladis Y, Wang G, Wolfarth B, Scott R, Fuku N, Mikami E, He Z, Fiuza-Luces C, Eynon N, Lucia A. Genomics of elite sporting performance: what little we know and necessary advances. Br J Sports Med. 2013;47:550–5. doi: 10.1136/bjsports-2013-092400. [DOI] [PubMed] [Google Scholar]
- 4.Ma F, Yang Y, Li X, Zhou F, Gao C, Li M, Gao L. The association of sport performance with ACE and ACTN3 genetic polymorphisms: a systematic review and meta-analysis. PLoS One. 2013;8:e54685. doi: 10.1371/journal.pone.0054685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouchard C. Overcoming barriers to progress in exercise genomics. Exerc Sport Sci Rev. 2011;39:212–7. doi: 10.1097/JES.0b013e31822643f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang H, Ward WF. PGC-1alpha: a key regulator of energy metabolism. Adv Physiol Educ. 2006;30:145–51. doi: 10.1152/advan.00052.2006. [DOI] [PubMed] [Google Scholar]
- 7.Cheng CF, Ku HC, Lin H. PGC-1α as a Pivotal Factor in Lipid and Metabolic Regulation. Int J Mol Sci. 2018;19:3447. doi: 10.3390/ijms19113447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413:131–8. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- 9.Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–83. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- 10.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–24. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 11.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 12.Russell AP, Feilchenfeldt J, Schreiber S, Praz M, Crettenand A, Gobelet C, Meier CA, Bell DR, Kralli A, Giacobino JP, Dériaz O. Endurance training in humans leads to fibre type-specific increases in levels of peroxisome proliferator-activated receptor-gamma coactivator-1 and peroxisome proliferator-activated receptor-alpha in skeletal muscle. Diabetes. 2003;52:2874–81. doi: 10.2337/diabetes.52.12.2874. [DOI] [PubMed] [Google Scholar]
- 13.Norrbom J, Sundberg CJ, Ameln H, Kraus WE, Jansson E, Gustafsson T. PGC-1alpha mRNA expression is influenced by metabolic perturbation in exercising human skeletal muscle. J Appl Physiol (1985) 2004;96:189–94. doi: 10.1152/japplphysiol.00765.2003. [DOI] [PubMed] [Google Scholar]
- 14.Terada S, Tabata I. Effects of acute bouts of running and swimming exercise on PGC-1alpha protein expression in rat epitrochlearis and soleus muscle. Am J Physiol Endocrinol Metab. 2004;286:E208–16. doi: 10.1152/ajpendo.00051.2003. [DOI] [PubMed] [Google Scholar]
- 15.Ridderstråle M, Johansson LE, Rastam L, Lindblad U. Increased risk of obesity associated with the variant allele of the PPARGC1A Gly482Ser polymorphism in physically inactive elderly men. Diabetologia. 2006;49:496–500. doi: 10.1007/s00125-005-0129-8. [DOI] [PubMed] [Google Scholar]
- 16.Barroso I, Luan J, Sandhu MS, Franks PW, Crowley V, Schafer AJ, O’Rahilly S, Wareham NJ. Meta-analysis of the Gly482Ser variant in PPARGC1A in type 2 diabetes and related phenotypes. Diabetologia. 2006;49:501–5. doi: 10.1007/s00125-005-0130-2. [DOI] [PubMed] [Google Scholar]
- 17.Vimaleswaran KS, Luan J, Andersen G, Muller YL, Wheeler E, Brito EC, O’Rahilly S, Pedersen O, Baier LJ, Knowler WC, Barroso I, Wareham NJ, Loos RJ, Franks PW. The Gly482Ser genotype at the PPARGC1A gene and elevated blood pressure: a meta-analysis involving 13,949 individuals. J Appl Physiol (1985) 2008;105:1352–8. doi: 10.1152/japplphysiol.90423.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lucia A, Gómez-Gallego F, Barroso I, Rabadán M, Bandrés F, San Juan AF, Chicharro JL, Ekelund U, Brage S, Earnest CP, Wareham NJ, Franks PW. PPARGC1A genotype (Gly482Ser) predicts exceptional endurance capacity in European men. J Appl Physiol (1985) 2005;99:344–8. doi: 10.1152/japplphysiol.00037.2005. [DOI] [PubMed] [Google Scholar]
- 19.Eynon N, Meckel Y, Sagiv M, Yamin C, Amir R, Sagiv M, Goldhammer E, Duarte JA, Oliveira J. Do PPARGC1A and PPARalpha polymorphisms influence sprint or endurance phenotypes? Scand J Med Sci Sports. 2010;20:e145–50. doi: 10.1111/j.1600-0838.2009.00930.x. [DOI] [PubMed] [Google Scholar]
- 20.Maciejewska A, Sawczuk M, Cieszczyk P, Mozhayskaya IA, Ahmetov II. The PPARGC1A gene Gly482Ser in Polish and Russian athletes. J Sports Sci. 2012;30:101–13. doi: 10.1080/02640414.2011.623709. [DOI] [PubMed] [Google Scholar]
- 21.Muniesa CA, González-Freire M, Santiago C, Lao JI, Buxens A, Rubio JC, Martín MA, Arenas J, Gomez-Gallego F, Lucia A. World-class performance in lightweight rowing: is it genetically influenced? A comparison with cyclists, runners and non-athletes. Br J Sports Med. 2010;44:898–901. doi: 10.1136/bjsm.2008.051680. [DOI] [PubMed] [Google Scholar]
- 22.Ginevičienė V, Pranckevičienė E, Milašius K, Kučinskas V. Gene variants related to the power performance of the Lithuanian athletes. Cent Eur J Biol. 2011;6:48–57. [Google Scholar]
- 23.Peplonska B, Adamczyk JG, Siewierski M, Safranow K, Maruszak A, Sozanski H, Gajewski AK, Zekanowski C. Genetic variants associated with physical and mental characteristics of the elite athletes in the Polish population. Scand J Med Sci Sports. 2017;27:788–800. doi: 10.1111/sms.12687. [DOI] [PubMed] [Google Scholar]
- 24.Guilherme JPLF, Bertuzzi R, Lima-Silva AE, Pereira ADC, Lancha Junior AH. Analysis of sports-relevant polymorphisms in a large Brazilian cohort of top-level athletes. Ann Hum Genet. 2018;82:254–264. doi: 10.1111/ahg.12248. [DOI] [PubMed] [Google Scholar]
- 25.He ZH, Hu Y, Li YC, Gong LJ, Cieszczyk P, Maciejewska-Karlowska A, Leonska-Duniec A, Muniesa CA, Marín-Peiro M, Santiago C, Garatachea N, Eynon N, Lucia A. PGC-related gene variants and elite endurance athletic status in a Chinese cohort: a functional study. Scand J Med Sci Sports. 2015;25:184–95. doi: 10.1111/sms.12188. [DOI] [PubMed] [Google Scholar]
- 26.Yvert T, Miyamoto-Mikami E, Murakami H, Miyachi M, Kawahara T, Fuku N. Lack of replication of associations between multiple genetic polymorphisms and endurance athlete status in Japanese population. Physiol Rep. 2016;4:e13003. doi: 10.14814/phy2.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tharabenjasin P, Pabalan N, Jarjanazi H. Association of PPARGC1A Gly428Ser (rs8192678) polymorphism with potential for athletic ability and sports performance: A meta-analysis. PLoS One. 2019;14:e0200967. doi: 10.1371/journal.pone.0200967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 30.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MANTEL N, HAENSZEL W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 32.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 33.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 34.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmetov II, Fedotovskaya ON. Current Progress in Sports Genomics. Advances in clinical chemistry. 2015;70:247–314. doi: 10.1016/bs.acc.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Caló MC, Vona G. Gene polymorphisms and elite athletic performance. J Anthropol Sci. 2008;86:113–31. [PubMed] [Google Scholar]
- 37.Gonzalez-Freire M, Santiago C, Verde Z, Lao JI, Oiivan J, Gomez-Gallego F, Lucia A. Unique among unique. Is it genetically determined? British journal of sports medicine. 2009;43:307–9. doi: 10.1136/bjsm.2008.049809. [DOI] [PubMed] [Google Scholar]
- 38.He Z, Hu Y, Feng L, Bao D, Wang L, Li Y, Wang J, Liu G, Xi Y, Wen L, Lucia A. Is there an association between PPARGC1A genotypes and endurance capacity in Chinese men? Scand J Med Sci Sports. 2008;18:195–204. doi: 10.1111/j.1600-0838.2007.00648.x. [DOI] [PubMed] [Google Scholar]
- 39.Egorova ES, Borisova AV, Mustafina LJ, Arkhipova AA, Gabbasov RT, Druzhevskaya AM, Astratenkova IV, Ahmetov II. The polygenic profile of Russian football players. J Sports Sci. 2014;32:1286–93. doi: 10.1080/02640414.2014.898853. [DOI] [PubMed] [Google Scholar]
- 40.Jacob Y, Cripps A, Evans T, Chivers PT, Joyce C, Anderton RS. Identification of genetic markers for skill and athleticism in sub-elite Australian football players: a pilot study. J Sports Med Phys Fitness. 2018;58:241–248. doi: 10.23736/S0022-4707.16.06647-0. [DOI] [PubMed] [Google Scholar]
- 41.Gineviciene V, Jakaitiene A, Tubelis L, Kucinskas V. Variation in the ACE, PPARGC1A and PPARA genes in Lithuanian football players. Eur J Sport Sci. 2014;14:S289–95. doi: 10.1080/17461391.2012.691117. [DOI] [PubMed] [Google Scholar]
- 42.Tural E, Kara N, Agaoglu SA, Elbistan M, Tasmektepligil MY, Imamoglu O. PPAR-α and PPARGC1A gene variants have strong effects on aerobic performance of Turkish elite endurance athletes. Mol Biol Rep. 2014;41:5799–804. doi: 10.1007/s11033-014-3453-6. [DOI] [PubMed] [Google Scholar]
- 43.Akhmetov II, Popov DV, Mozhaĭskaia IA, Missina SS, Astratenkova IV, Vinogradova OL, Rogozkin VA. Association of regulatory genes polymorphisms with aerobic and anaerobic performance of athletes. Ross Fiziol Zh Im I M Sechenova. 2007;93:837–43. [PubMed] [Google Scholar]
- 44.Jin HJ, Hwang IW, Kim KC, Cho HI, Park TH, Shin YA, Lee HS, Hwang JH, Kim AR, Lee KH, Shin YE, Lee JY, Kim JA, Choi EJ, Kim BK, Sim HS, Kim MS, Kim W. Is there a relationship between PPARD T294C/PPARGC1A Gly482Ser variations and physical endurance performance in the Korean population? Genes Genom. 2016;38:389. [Google Scholar]
- 45.Ben-Zaken S, Meckel Y, Nemet D, Eliakim A. Genetic score of power-speed and endurance track and field athletes. Scand J Med Sci Sports. 2015;25:166–74. doi: 10.1111/sms.12141. [DOI] [PubMed] [Google Scholar]
- 46.Grealy R, Herruer J, Smith CL, Hiller D, Haseler LJ, Griffiths LR. Evaluation of a 7–Gene Genetic Profile for Athletic Endurance Phenotype in Ironman Championship Triathletes. PLoS One. 2015;10:e0145171. doi: 10.1371/journal.pone.0145171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eynon N, Ruiz JR, Meckel Y, Morán M, Lucia A. Mitochondrial biogenesis related endurance genotype score and sports performance in athletes. Mitochondrion. 2011;11:64–9. doi: 10.1016/j.mito.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 48.Eynon N, Meckel Y, Alves AJ, Yamin C, Sagiv M, Goldhammer E, Sagiv M. Is there an interaction between PPARD T294C and PPARGC1A Gly482Ser polymorphisms and human endurance performance? Exp Physiol. 2009;94:1147–52. doi: 10.1113/expphysiol.2009.049668. [DOI] [PubMed] [Google Scholar]
- 49.Ruiz JR, Gómez-Gallego F, Santiago C, González-Freire M, Verde Z, Foster C, Lucia A. Is there an optimum endurance polygenic profile? J Physiol. 2009;587:1527–34. doi: 10.1113/jphysiol.2008.166645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsianos GI, Evangelou E, Boot A, Zillikens MC, van Meurs JB, Uitterlinden AG, Ioannidis JP. Associations of polymorphisms of eight muscle- or metabolism-related genes with performance in Mount Olympus marathon runners. J Appl Physiol (1985) 2010;108:567–74. doi: 10.1152/japplphysiol.00780.2009. [DOI] [PubMed] [Google Scholar]
- 51.Maruszak A, Adamczyk JG, Siewierski M, Sozański H, Gajewski A, Żekanowski C. Mitochondrial DNA variation is associated with elite athletic status in the Polish population. Scand J Med Sci Sports. 2014;24:311–8. doi: 10.1111/sms.12012. [DOI] [PubMed] [Google Scholar]
- 52.Ahmetov II, Williams AG, Popov DV, Lyubaeva EV, Hakimullina AM, Fedotovskaya ON, Mozhayskaya IA, Vinogradova OL, Astratenkova IV, Montgomery HE, Rogozkin VA. The combined impact of metabolic gene polymorphisms on elite endurance athlete status and related phenotypes. Hum Genet. 2009;126:751–61. doi: 10.1007/s00439-009-0728-4. [DOI] [PubMed] [Google Scholar]
- 53.Santiago C, Ruiz JR, Muniesa CA, González-Freire M, Gómez-Gallego F, Lucia A. Does the polygenic profile determine the potential for becoming a world-class athlete? Insights from the sport of rowing. Scand J Med Sci Sports. 2010;20:e188–94. doi: 10.1111/j.1600-0838.2009.00943.x. [DOI] [PubMed] [Google Scholar]
- 54.Plowman SA, Smith DL. Exercise physiology for health, fitness, and performance. In: Lupash Emily., editor. Exercise Physiology for Health, Fitness, and Performance. 3rd edition. Lippincott Williams & Wilkins; 2007. p. 61. [Google Scholar]
- 55.Lucia A, San Juan AF, Montilla M, CaNete S, Santalla A, Earnest C, Pérez M. In professional road cyclists, low pedaling cadences are less efficient. Med Sci Sports Exerc. 2004;36:1048–54. doi: 10.1249/01.mss.0000128249.10305.8a. [DOI] [PubMed] [Google Scholar]
- 56.Spencer MR, Gastin PB. Energy system contribution during 200– to 1500–m running in highly trained athletes. Med Sci Sports Exerc. 2001;33:157–62. doi: 10.1097/00005768-200101000-00024. [DOI] [PubMed] [Google Scholar]
- 57.Ling C, Poulsen P, Carlsson E, Ridderstråle M, Almgren P, Wojtaszewski J, Beck-Nielsen H, Groop L, Vaag A. Multiple environmental and genetic factors influence skeletal muscle PGC-1alpha and PGC-1beta gene expression in twins. J Clin Invest. 2004;114:1518–26. doi: 10.1172/JCI21889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choi YS, Hong JM, Lim S, Ko KS, Pak YK. Impaired coactivator activity of the Gly482 variant of peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1alpha) on mitochondrial transcription factor A (Tfam) promoter. Biochem Biophys Res Commun. 2006;344:708–12. doi: 10.1016/j.bbrc.2006.03.193. [DOI] [PubMed] [Google Scholar]
- 59.Okauchi Y, Iwahashi H, Okita K, Yuan M, Matsuda M, Tanaka T, Miyagawa J, Funahashi T, Horikawa Y, Shimomura I, Yamagata K. PGC-1alpha Gly482Ser polymorphism is associated with the plasma adiponectin level in type 2 diabetic men. Endocr J. 2008;55:991–7. doi: 10.1507/endocrj.k08e-070. [DOI] [PubMed] [Google Scholar]
- 60.Michael LF, Wu Z, Cheatham RB, Puigserver P, Adelmant G, Lehman JJ, Kelly DP, Spiegelman BM. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc Natl Acad Sci U S A. 2001;98:3820–5. doi: 10.1073/pnas.061035098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang SL, Lu WS, Yan L, Wu MC, Xu MT, Chen LH, Cheng H. Association between peroxisome proliferator-activated receptor-gamma coactivator-1alpha gene polymorphisms and type 2 diabetes in southern Chinese population: role of altered interaction with myocyte enhancer factor 2C. Chin Med J (Engl) 2007;120:1878–85. [PubMed] [Google Scholar]
- 62.Handschin C, Rhee J, Lin J, Tarr PT, Spiegelman BM. An autoregulatory loop controls peroxisome proliferator-activated receptor gamma coactivator 1alpha expression in muscle. Proc Natl Acad Sci U S A. 2003;100:7111–6. doi: 10.1073/pnas.1232352100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coburn KM, Vevea JL. Publication bias as a function of study characteristics. Psychol Methods. 2015;20:310–30. doi: 10.1037/met0000046. [DOI] [PubMed] [Google Scholar]