Abstract
In this randomized, double-blind, placebo-controlled, crossover pilot trial, we evaluated the effects of 7-day H2 inhalation on exercise performance outcomes and serum hormonal and inflammation profiles in a cohort of young men and women. All participants (age 22.9 ± 1.5 years; body mass index 23.4 ± 2.5 kg m-2; 10 women and 10 men) were allocated to receive either gaseous hydrogen (4%) or placebo (room air) by 20-min once-per-day inhalation for 7 days, with a wash-out period of 7 days to prevent the residual effects of interventions across study periods. The primary treatment outcome was the change in running time-to-exhaustion in the incremental maximal test from baseline to day 7. Additionally, assessment of other exercise performance endpoints and clinical chemistry biomarkers was performed at baseline and at 7 days after each intervention. The trial was registered at ClinicalTrials.gov (ID NCT03846141). Breathing 4% hydrogen for 20 min per day resulted in increased peak running velocity (by up to 4.2%) as compared to air inhalation (P = 0.05). Hydrogen inhalation resulted in a notable drop in serum insulin-like growth factor 1 (IGF-1) by 48.2 ng/mL at follow-up (95% confidence interval [CI]: from -186.7 to 89.3) (P < 0.05), while IGF-1 levels were elevated by 59.3 ng/mL after placebo intervention (95% CI; from -110.7 to 229.5) (P < 0.05). Inhalational hydrogen appears to show ergogenic properties in healthy men and women. Gaseous H2 should be further evaluated for its efficacy and safety in an athletic environment.
Keywords: Hydrogen, Running to exhaustion, Insulin, IGF-1, Ergogenic
INTRODUCTION
The use of medical gasses has been recently described as an emerging exotic strategy in the exercise physiology and sports medicine community [1], with a few unconventional medical gasses (such as NO, Xe, O3) put forward as performance-enhancing agents. Among others, molecular hydrogen (H2) appears as an innovative compound that might be applicable among athletes. Usually administered in the form of a dietary supplement, either as hydrogen-rich water or hydrogen-producing tablets, H2 appears to positively affect exercise capacity in both animal studies [2–4] and human trials [5–8]. This might be due to its antioxidant and anti-inflammatory properties [9] that perhaps reduce exercise-induced inflammation and oxidative stress or through alteration of anabolic hormones production by signal modulation [10,11]. For instance, Aoki and co-workers [5] reported that hydration with 1.5 L/day of hydrogen-rich water (0.92–1.02 mM of hydrogen) significantly reduced blood lactate levels and improved exercise-induced decline of muscle function in male soccer players. Buffering capacity of hydrogen-rich water (1.1 mM) during exercise-induced acidosis has also been demonstrated after both continuous [6] and progressive running-to-exhaustion exercise [7]. In addition, two weeks of hydrogen-rich water intake (2 L per day, 0.45 mM of free hydrogen) helped to maintain peak power output in repetitive sprints to exhaustion over 30 minutes in male cyclists [8]. Although the above preliminary studies provided initial evidence about the performance-enhancing capacity of hydrogen-rich water, it remains an open question whether the favourable effects originate from H2 itself or perhaps from magnesium, a conventional source of hydrogen in hydrogen-rich water. Specifically, the apparent buffering capacity of hydrogen-rich water might be due to various pH buffers (e.g. bicarbonate, metallic magnesium) found in liquid hydrogen products used previously [6,7] rather than to H2 gas, which is known not to influence pH. Applying pure hydrogen gas, instead of magnesium-based hydrogen formulations, might, therefore, help to better reveal the authentic ergogenic potential of H2. Moreover, using inhalation as a parenteral route of H2 administration could emphasize the systemic action of hydrogen, including the possible impact on insulin and ghrelin secretion [10]. Drinking hydrogen-rich water appears to alter plasma glucose and insulin levels, an effect likely mediated by enhanced expression of fibroblast growth factor 21 (FGF21), a metabolic hormone that improves insulin sensitivity and glucose clearance [10]. The possible augmented insulin response driven by hydrogen inhalation might promote energy utilization and performance during exercise [12], thus fostering H2 as an insulin secretion stimulator in an athletic environment. Furthermore, recent evidence suggests that H2 has therapeutic value for diseases that involve inflammation [13,14], thus raising the possibility of its use in the athletic environment by counterbalancing biomarkers of exercise-induced inflammation and damage (e.g. creatine kinase, myoglobin, ferritin, C-reactive protein). In this randomized controlled preliminary trial, we evaluated the effects of 7-day H2 inhalation on exercise performance outcomes and serum hormonal and inflammation profiles in a cohort of young active men and women. We hypothesized that gaseous H2 would improve cardiorespiratory and muscular performance, and stimulate insulin secretion, along with attenuation of the inflammatory response. This appears to be the first clinical study where H2 inhalation was used for athletic performance.
Abbreviations
- ANOVA
Analysis of variance
- CI
Confidence interval
- CRP
C-reactive protein
- ESR
Erythrocyte sedimentation rate
- FGF21
Fibroblast growth factor 21
- H2
Molecular hydrogen
- IGF-1
Insulin-like growth factor 1
- MVIS
Maximal voluntary isometric strength
- VO2max
Maximal oxygen uptake
MATERIALS AND METHODS
Participants
Twenty healthy, physically active young volunteers (age 22.9 ± 1.5 years; body mass index 23.4 ± 2.5 kg/m2; 10 women and 10 men) signed informed consent to voluntarily participate in this randomized, double-blind, placebo-controlled, crossover pilot trial, with all procedures approved by the local Institutional Review Board at the University of Novi Sad in accordance with the Declaration of Helsinki. All participants had no history of H2 supplementation (or other performance-enhancing dietary supplements or drugs) within the 4 weeks before the study commenced, and no acute or chronic disorders and diseases, as evaluated by the pre-participation health check. Participants were asked to maintain their usual diets and physical activity levels during the study.
Experimental intervention
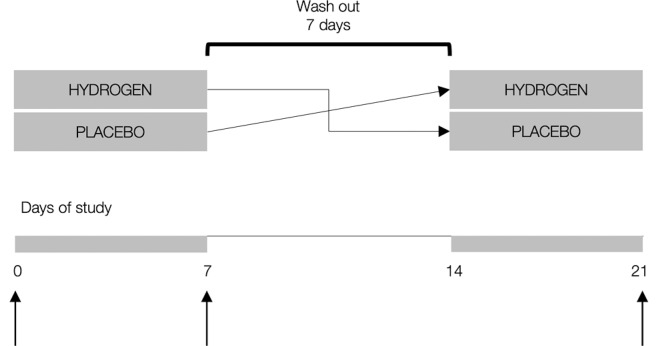
All participants were allocated to receive either gaseous hydrogen (4%) or placebo (room air) by 20-min once-per-day inhalation for 7 days, with a wash-out period of 7 days to prevent the residual effects of interventions across study periods. The concentration of H2 used and the duration of an inhalational session (20 min) were chosen as a method that gave a favourable effect in a previous human study [15]. Previous studies suggested that continuous inhalation of H2 gas requires ~ 10 min to reach equilibrium in the tissue and blood [16]. Gaseous hydrogen was provided via a gas mask by biological gas supplying apparatus (MIZ Company Ltd, Kanagawa, Japan), with the H2 flow rate approximately constant (~ 45 mL/min) and diluted by ambient air. Day-to-day H2 inhalation was supervised by the study investigators throughout the trial. Placebo gas was identical in appearance to hydrogen. The inhalation was administered at the same time of day (08:00–09:00), ~ 60 min before breakfast, with all participants receiving the intervention simultaneously using multiple machines. The primary treatment outcome was the change in running time-to-exhaustion in the incremental test (see below) from baseline to day 7. Additionally, assessment of other exercise performance endpoints and clinical chemistry biomarkers was performed at baseline and at 7 days after each intervention (Figure 1).
FIG. 1.
Study protocol. Vertical arrows indicate sampling intervals for primary and secondary outcomes.
Study design
The study was conducted in the FSPE Applied Bioenergetics Laboratory at the University of Novi Sad from February 2018 to April 2018, with the trial registered at ClinicalTrials.gov (ID NCT03846141). Laboratory assessments were carried out between 08:00 and 12:00 after an overnight fast and no exhaustive exercise over the previous 24 h. Before testing exercise performance outcomes, participants first provided a blood sample at rest from a median cubital vein into an evacuated test tube while seated. The venous blood was immediately centrifuged within the next 10 min at 3000 g, with serum separated and analyzed for ghrelin and insulin-like growth factor 1 (IGF-1) using commercial ELISA kits on an automated analyzer (ChemWell 2910, AWARENESS Technology Inc., Palm City, FL). Insulin and ferritin were analyzed using chemiluminescence immunoassays (ADVIA Centaur XP, Siemens Healthcare GmbH, Erlangen, Germany). Myoglobin was analyzed with solid-phase enzyme immunoassay (AIA-360, Tosoh Bioscience, San Francisco, CA), while serum creatine kinase (CK) and C-reactive protein (CRP) were measured by standard enzymatic methods with an automatic analyzer (Hitachi 912, Tokyo, Japan). Erythrocyte sedimentation rate (ESR) was measured with the reference Westergren technique. Blood lactates were measured by the enzymatic-colorimetric method (Accu-Trend, Hoffmann-La Roche Ltd., Basel, Switzerland). After blood chemistry analyses, participants performed a series of different exercise tests. First, maximal voluntary isometric strength (MVIS) of forearm muscles was evaluated with a hydraulic hand dynamometer (Jamar J00105, Lafayette Instrument Company, Lafayette, IN), and MVIS of torso and leg muscles with a Back-Leg-Chest dynamometer (Baseline 12-0403, Fabrication Enterprises Inc., White Plains, NY). Second, muscular endurance in the upper body was assessed through the gender-specific YMCA Bench Press Test [17]. Finally, cardiorespiratory endurance was evaluated by a maximal incremental running test on an institutional treadmill (3-min warm-up walk at 6 km/h followed by running at 8 km/h with a progressive workload increment rate of 1.5 km/h every 60 s until exhaustion). Gas exchange data were collected throughout the test using a breath-by-breath metabolic system (Quark CPET, COSMED, Rome, Italy). The test was finished when participants were too physically tired to continue running, and additional criteria for the maximal test were met (e.g. rise in oxygen uptake satisfied a plateau representing less than 2 mL/kg/min to the next level, respiratory exchange ratio ≥ 1.10 and peak heart rate ≥ 95% of age-predicted maximal heart rate). Participants were also instructed to report on adverse effects of H2 intervention through an open-ended questionnaire for self-assessment of side effects during the study. All participants were familiarized with testing procedures and were assessed on the same day with the tests performed in the same order.
Statistical analyses
The appropriate sample size (n = 20) was calculated using power analysis (effect size 0.3, alpha error probability 0.05, power 0.80) for the primary treatment outcome (G-Power 3, Heinrich Heine University Düsseldorf, Germany). Two-way mixed model analysis of variance (ANOVA) with repeated measures was used to establish whether any significant differences existed between participants’ responses over time of intervention (0 vs. 7 days). When non-homogeneous variances were identified, values were compared using Friedman’s 2-way ANOVA by ranks. Identification and removal of outliers were conducted according to the interquartile range method. The significance level was set at P ≤ 0.05.
RESULTS
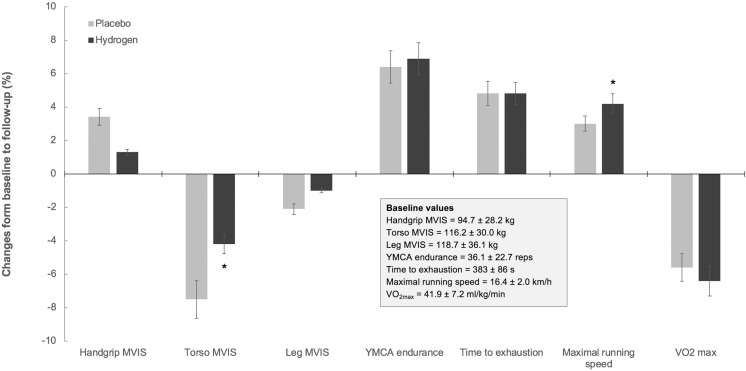
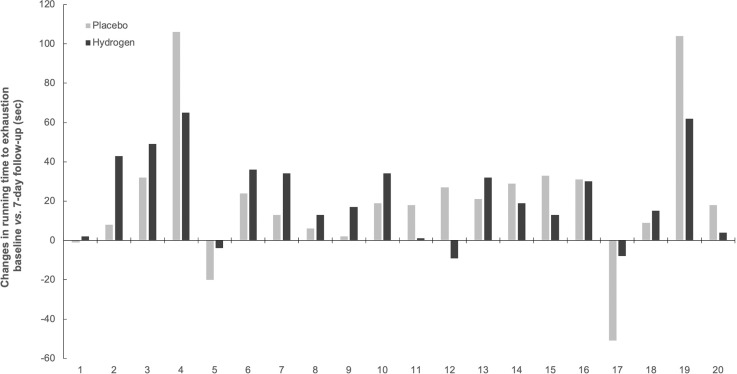
All participants completed the study, with no men or women reporting any adverse events of molecular hydrogen or placebo inhalation. No significant differences were found in most biomarkers’ responses between two interventions (P > 0.05), except for serum IGF-1, CRP, and ferritin (Table 1). Hydrogen inhalation resulted in a notable drop in serum IGF-1 for 48.2 ng/mL at follow-up (95% confidence interval [CI]: from -186.7 to 89.3), while IGF-1 levels were elevated by 59.3 ng/mL after placebo intervention (95% CI; from -110.7 to 229.5). Baseline CRP levels were decreased by 1.0 mg/L (95% CI; 0.6–1.4) and by 0.7 mg/L (95% CI; 0.3–1.2) after hydrogen and placebo inhalation at 7-day follow up, respectively. Hydrogen also induced a more powerful drop in serum ferritin at follow-up, as compared to placebo (6.4 μg/L vs. 5.6 μg/L; P ≤ 0.05). Breathing H2 was superior to placebo to increase peak running velocity during a maximal incremental running test (by up to 4.2%), also to attenuate a drop in MVIS of torso muscles at 7-day follow-up (Figure 2). No inter-group differences were observed in terms of handgrip and leg MVIS, muscular endurance during repetitive bench press exercise, or running time-to-exhaustion and maximal oxygen consumption. Individual changes in the primary outcome (running time-to-exhaustion) between trials are presented in Figure 3, with 12 out of 20 participants (60%) having performed better (or less inferior) after H2 inhalation. Nevertheless, an effect size analysis for the primary outcome measure change revealed a small effect size for time vs. intervention interaction (95% CI; from 71 to 183 s after H2 intervention, and from 66 to 174 s after placebo intervention, η2 = 0.002). Also, resting blood pressure and heart rate remained unaffected by either intervention (not presented here).
TABLE 1.
Changes in biochemical markers during the study. Values are mean ± SD.
| Baseline | At 7 days | ||
|---|---|---|---|
| Placebo | H2 | ||
| Insulin (IU/mL) | 5.3 ± 1.6 | 4.6 ± 1.6 | 4.7 ± 1.5 |
| Ghrelin (ng/mL) | 9.1 ± 4.3 | 15.7 ± 4.8 | 13.4 ± 5.0 |
| IGF-1 (ng/mL) | 513.1 ± 235.3 | 572.5 ± 293.1 | 464.9 ± 192.2 * |
| Creatine kinase (U/L) | 214.0 ± 125.8 | 229.5 ± 125.1 | 220.4 ± 130.7 |
| Myoglobin (ng/mL) | 36.6 ± 12.8 | 42.1 ± 15.1 | 41.3 ± 15.5 |
| C-reactive protein (mg/L) | 1.4 ± 0.8 | 0.7 ± 0.6 | 0.4 ± 0.5 * |
| Ferritin (μg/L) | 31.8 ± 19.7 | 26.2 ± 22.0 | 25.4 ± 19.2 * |
| ESR (mm/1 h) | 4.6 ± 3.0 | 4.1 ± 2.3 | 4.4 ± 2.5 |
| Lactate (mmol/L) | 2.0 ± 0.7 | 1.8 ± 0.4 | 1.9 ± 0.5 |
Abbreviations: IGF-1 – insulin-like growth factor 1, ESR – erythrocyte sedimentation rate.
indicates a significant difference (P ≤ 0.05) for time vs. trial interaction between placebo and H2 intervention.
FIG. 2.
Changes in exercise performance outcomes at baseline vs. follow-up (7 days). Values are presented as mean percentage changes, with error bars representing SD. Asterisk (*) indicates a significant difference between trials at P ≤ 0.05. MVIS – maximal voluntary isometric strength, VO2max – maximal oxygen uptake.
FIG. 3.
Individual changes in primary treatment outcome (running time-to-exhaustion) between trials.
DISCUSSION
This first-in-humans randomized controlled pilot trial provided preliminary evidence that short-term hydrogen inhalation is superior to placebo (room air) in improving exercise performance in healthy men and women, with ergogenic effects of hydrogen accompanied by notable changes in selected hormonal and inflammatory biomarkers at follow-up. However, inhalational H2 demonstrated no significant effect on insulin and ghrelin secretion. In addition, hydrogen inhalation caused no adverse events during our trial, implying low risk of this route of H2 administration for a short-term experimental period.
Hydrogen administration as a performance-enhancing intervention in humans dates back to 2012. A Japanese group was first to demonstrate that H2 dispensed before exercise during 7 days reduced blood lactate levels and improved the exercise-induced decline of muscle function in male soccer players subjected to strenuous exercise [5]. Peak torque and muscle activity throughout 100 repetitions of maximal isokinetic knee extension appear to be less attenuated after hydrogen intervention, as compared to placebo. Similar results were found in a recent study [8], with 2-week hydrogen intake maintaining peak power output during repetitive sprints to exhaustion in trained male cyclists. Our study confirmed the above results, with inhalational hydrogen increasing peak running velocity during exhaustive exercise and attenuating the drop in maximal isometric strength of the upper body muscles. Although this level of enhancement for peak running velocity (4.2%) could be considered trivial, it might be relevant for competing athletes, particularly due to the fact that the effects were noted after such short-term administration. A combination of a nominal increase in time-to-exhaustion and notable improvement of maximal running speed after inhaling H2 (although VO2max tended to drop at follow-up) perhaps suggests better anaerobic capacity at the end of exercise. Hence, H2 inhalation might be a suitable performance-enhancing strategy for sporting activities characterized by a high anaerobic contribution, including middle- and long-distance events, team games or martial arts. While previous studies used magnesium-based hydrogen [18], with magnesium possibly contributing to the ergogenic properties of the formulation by itself, the present study confirmed beneficial effects of pure hydrogen gas per se.
Although the exact mechanism of H2 action remains to be elucidated, the acute ergogenic effects of hydrogen might be due to its strong antioxidative power and buffering capacity that could counterbalance exercise-induced changes in metabolism [19]. Furthermore, hydrogen appears to stimulate many signalling pathways and expression of genes that alter mitochondrial bioenergetics and hormone secretion [20,21], which in turn might have a steady effect on exercise performance. In the present study, we demonstrated an effect of hydrogen on insulin-like growth factor-1, an anabolic hormone that acts as a primary mediator of the effects of growth hormone. Serum IGF-1 appeared to drop by ~ 10% after 7-day hydrogen inhalation while serum IGF-1 remained high after placebo intervention, implying a possible down-regulation link between exogenous H2 and the anabolic response. This disagrees with a recent paper that reported an up-regulating effect of hydrogen-rich water on the growth hormone-IGF-1 axis, with an effect mediated by ghrelin, a peptide hormone produced predominantly in the gut [22]. We found no notable differences in serum ghrelin response after hydrogen gas or placebo in our pilot trial. This suggests that inhalational and oral hydrogen might have different effects on the ghrelin-growth-hormone-IGF-1 axis, with ghrelin-mediated effects perhaps playing a minor or irrelevant role during short-term hydrogen inhalation. A drop in IGF-1 driven by hydrogen inhalation could be beneficial among athletes, particularly those who strive for lower body mass, since a decreased level of IGF-1 seems to be associated with reduced weight and fat mass in an active population [23]. H2 inhalation might, therefore, be recognized as a novel short-term strategy to manage weight, yet more studies are needed to confirm this presumption. We also found that H2 inhalation reduces levels of serum ferritin and CRP, both non-specific biomarkers of inflammation. This corroborates previous findings from animal and human studies about anti-inflammatory effects of H2 [24,25], with hydrogen gas possibly exerting a regulatory role in the release of pro- and anti-inflammatory cytokines mediated by haem oxygenase-1 expression and activation [26]. In the context of the athletic environment, which is often characterized by low-grade systemic inflammation [27], inhalational hydrogen thus may contribute to the more favourable internal milieu and perhaps act as a protective compound [28] and an alternative to non-steroidal anti-inflammatory agents. On the other hand, regular exercise induces inflammation to promote repair, remodelling and signalling in the body, with this hormetic response considered beneficial to achieve abiding muscle overcompensation and adaptation [29]. In the present study, we found that non-specific biomarkers of inflammation were reduced after short-term H2 inhalation, yet whether hydrogen affects long-term hormesis remains unknown at the moment. A recent study [30] proposed that H2 may act as an exercise mimetic and redox adaptogen, potentiating the benefits from regular exercise (accompanied by low-grade inflammation), and reducing the adverse effects of harmful exercise (high-grade inflammation).
Although our pilot study provided early evidence about the beneficial effects of short-term hydrogen inhalation for athletic performance, several limitations must be considered when the study findings are interpreted. We recruited only physically active young healthy volunteers; it remains unknown how breathing H2 affects elite and sub-elite athletes or the active population of advanced age. Here, we evaluated both men and women, yet the small sample size limited subgroup analyses that might reveal possible gender-specific effects of gaseous hydrogen. Even though we asked participants to maintain their usual diets and physical activity levels during the study, a lack of strict control of subject compliance with dietary and exercise regime calls into question a possible role of these confounding variables for changes in study outcomes. The short duration of H2 treatment perhaps restricted the scope of our trial to acute responses while long-term intervention might reveal alternative or opposing effects of inhalational hydrogen. With a limited number of clinical tests employed, a course of hydrogen action could not be reliably determined. Therefore, more studies are highly warranted to identify the exact mechanism underlying the ergogenic effects of inhalational hydrogen, using randomized-controlled design for long-term and well-powered trials that include advanced physiological, metabolic and genomic profiling. Also, legislative advocacy is needed to address regulatory issues related to this route of H2 administration. While the World Anti-Doping Agency forbids the use of specific medical gases (such as argon and xenon) [1], it permits the use of inhalational oxygen, while other therapeutic gases (including molecular hydrogen) are currently not controlled.
CONCLUSIONS
In conclusion, breathing 4% gaseous hydrogen for 20 min/day for 7 days resulted in increased peak running velocity and attenuated the drop in maximal isometric strength of trunk muscles in a cohort of healthy, physically active young men and women. This was accompanied by hydrogen-driven changes in serum levels of insulin-like growth hormone-1, ferritin and C-reactive protein at follow-up, as compared to room air inhalation. Inhalational hydrogen should be further evaluated for its efficacy and safety in an athletic environment.
Funding
Study was supported by the Serbian Ministry of Education, Science and Technological Development (# 175037), the Provincial Secretariat for Higher Education and Scientific Research (# 114-451-710), and the Faculty of Sport and Physical Education.
Declaration of interest
The authors report no conflicts of interest associated with this manuscript.
REFERENCES
- 1.Ostojic SM. Medical gases as an emerging topic in sports medicine. Sports Med. 2018;48:2677–2678. doi: 10.1007/s40279-018-0948-7. [DOI] [PubMed] [Google Scholar]
- 2.Tsubone H, Hanafusa M, Endo M, et al. Effect of treadmill exercise and hydrogen-rich water intake on serum oxidative and anti-oxidative metabolites in serum of Thoroughbred horses. J Equine Sci. 2013;24:1–8. doi: 10.1294/jes.24.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ara J, Fadriquela A, Ahmed MF, et al. hydrogen water drinking exerts antifatigue effects in chronic forced swimming mice via antioxidative and anti-inflammatory activities. Biomed Res Int. 2018;2018:2571269. doi: 10.1155/2018/2571269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamazaki M, Kusano K, Ishibashi T, et al. Intravenous infusion of H2-saline suppresses oxidative stress and elevates antioxidant potential in Thoroughbred horses after racing exercise. Sci Rep. 2015;5:15514. doi: 10.1038/srep15514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aoki K, Nakao A, Adachi T, et al. Pilot study: effects of drinking hydrogen-rich water on muscle fatigue caused by acute exercise in elite athletes. Med Gas Res. 2012;2:12. doi: 10.1186/2045-9912-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostojic SM. Serum alkalinization and hydrogen-rich water in healthy men. Mayo Clin Proc. 2012;87:501–502. doi: 10.1016/j.mayocp.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostojic SM, Stojanovic MD. Hydrogen-rich water affected blood alkalinity in physically active men. Res Sports Med. 2014;22:49–60. doi: 10.1080/15438627.2013.852092. [DOI] [PubMed] [Google Scholar]
- 8.Da Ponte A, Giovanelli N, Nigris D, et al. Effects of hydrogen rich water on prolonged intermittent exercise. J Sports Med Phys Fitness. 2018;58:612–621. doi: 10.23736/S0022-4707.17.06883-9. [DOI] [PubMed] [Google Scholar]
- 9.Liu CL, Zhang K, Chen G. Hydrogen therapy: from mechanism to cerebral diseases. Med Gas Res. 2016;6:48–54. doi: 10.4103/2045-9912.179346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamimura N, Nishimaki K, Ohsawa I, et al. Molecular hydrogen improves obesity and diabetes by inducing hepatic FGF21 and stimulating energy metabolism in db/db mice. Obesity. 2011;19:1396–1403. doi: 10.1038/oby.2011.6. [DOI] [PubMed] [Google Scholar]
- 11.Begum R, Bajgai J, Fadriquela A, et al. Molecular hydrogen may enhance the production of testosterone hormone in male infertility through hormone signal modulation and redox balance. Med Hypotheses. 2018;121:6–9. doi: 10.1016/j.mehy.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Fujita S, Rasmussen BB, Cadenas JG, et al. Effect of insulin on human skeletal muscle protein synthesis is modulated by insulin-induced changes in muscle blood flow and amino acid availability. Am J Physiol Endocrinol Metab. 2006;291:E745–754. doi: 10.1152/ajpendo.00271.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noda K, Tanaka Y, Shigemura N, et al. Hydrogen-supplemented drinking water protects cardiac allografts from inflammation-associated deterioration. Transpl Int. 2012;25:1213–1222. doi: 10.1111/j.1432-2277.2012.01542.x. [DOI] [PubMed] [Google Scholar]
- 14.Hu Z, Wu B, Meng F, et al. Impact of molecular hydrogen treatments on the innate immune activity and survival of zebrafish (Danio rerio) challenged with Aeromonas hydrophila. Fish Shellfish Immunol. 2017;67:554–560. doi: 10.1016/j.fsi.2017.05.066. [DOI] [PubMed] [Google Scholar]
- 15.Korovljev D, Valdemar S, Javorac D, et al. Hydrogen inhalation positively affects cardiometabolic risk factors in men and women aged 65 years or older. Eur Geriatr Med. 2018;9:729–730. doi: 10.1007/s41999-018-0087-6. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson ED. Splanchnic circulation and lymph formation. In: Gregerson H, Lindkaer Jensen S, Moody F, Shokouh-Amiri M, editors. Essentials of Experimental Surgery: Gastroneterology. Boca Raton: CRC Press; 1996. pp. 1–15. [Google Scholar]
- 17.YMCA . YMCA Fitness Testing and Assessment Manual. 4th Ed. Champaign, IL: HK; 2000. [Google Scholar]
- 18.Ohta S. Molecular hydrogen as a preventive and therapeutic medical gas: initiation, development and potential of hydrogen medicine. Pharmacol Ther. 2014;144:1–11. doi: 10.1016/j.pharmthera.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Ostojic SM. Molecular hydrogen in sports medicine: new therapeutic perspectives. Int J Sports Med. 2015;36:273–279. doi: 10.1055/s-0034-1395509. [DOI] [PubMed] [Google Scholar]
- 20.Zheng XF, Sun XJ, Xia ZF. Hydrogen resuscitation, a new cytoprotective approach. Clin Exp Pharmacol Physiol. 2011;38:155–163. doi: 10.1111/j.1440-1681.2011.05479.x. [DOI] [PubMed] [Google Scholar]
- 21.Ostojic SM. Does H2 alter mitochondrial bioenergetics via GHS-R1α activation? Theranostics. 2017;7:1330–1332. doi: 10.7150/thno.18745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCarty MF. Potential ghrelin-mediated benefits and risks of hydrogen water. Med Hypotheses. 2015;84:350–355. doi: 10.1016/j.mehy.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 23.Kim T, Chang JS, Kim H, et al. Intense walking exercise affects serum IGF-1 and IGFBP3. J Lifestyle Med. 2015;5:21–25. doi: 10.15280/jlm.2015.5.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostojic SM, Vukomanovic B, Calleja-Gonzalez J, et al. Effectiveness of oral and topical hydrogen for sports-related soft tissue injuries. Postgrad Med. 2014;126:187–195. doi: 10.3810/pgm.2014.09.2813. [DOI] [PubMed] [Google Scholar]
- 25.Tian R, Hou Z, Hao S, et al. Hydrogen-rich water attenuates brain damage and inflammation after traumatic brain injury in rats. Brain Res. 2016;1637:1–13. doi: 10.1016/j.brainres.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 26.Chen HG, Xie KL, Han HZ, et al. Heme oxygenase-1 mediates the anti-inflammatory effect of molecular hydrogen in LPS-stimulated RAW 264.7 macrophages. Int J Surg. 2013;11:1060–1066. doi: 10.1016/j.ijsu.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Wärnberg J, Cunningham K, Romeo J, et al. Physical activity, exercise and low-grade systemic inflammation. Proc Nutr Soc. 2010;69:400–406. doi: 10.1017/S0029665110001928. [DOI] [PubMed] [Google Scholar]
- 28.Nogueira JE, Passaglia P, Mota CMD, et al. Molecular hydrogen reduces acute exercise-induced inflammatory and oxidative stress status. Free Radic Biol Med. 2018;129:186–193. doi: 10.1016/j.freeradbiomed.2018.09.028. [DOI] [PubMed] [Google Scholar]
- 29.Radak Z, Ishihara K, Tekus E, et al. Exercise, oxidants, and antioxidants change the shape of the bell-shaped hormesis curve. Redox Biol. 2017;12:285–290. doi: 10.1016/j.redox.2017.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LeBaron TW, Laher I, Kura B, et al. Hydrogen gas: from clinical medicine to an emerging ergogenic molecule for sports athletes. Can J Physiol Pharmacol. 2019;97:797–807. doi: 10.1139/cjpp-2019-0067. [DOI] [PubMed] [Google Scholar]