Abstract
Aims
To study the prevalence of unknown atrial fibrillation (AF) in a high-risk, 75/76-year-old, population using N-terminal B-type natriuretic peptide (NT-proBNP) and handheld electrocardiogram (ECG) recordings in a stepwise screening procedure.
Methods and results
The STROKESTOP II study is a population-based cohort study in which all 75/76-year-old in the Stockholm region (n = 28 712) were randomized 1:1 to be invited to an AF screening programme or to serve as the control group. Participants without known AF had NT-proBNP analysed and were stratified into low-risk (NT-proBNP <125 ng/L) and high-risk (NT-proBNP ≥125 ng/L) groups. The high-risk group was offered extended ECG-screening, whereas the low-risk group performed only one single-lead ECG recording. In total, 6868 individuals accepted the screening invitation of which 6315 (91.9%) did not have previously known AF. New AF was detected in 2.6% [95% confidence interval (CI) 2.2–3.0] of all participants without previous AF. In the high-risk group (n = 3766/6315, 59.6%), AF was diagnosed in 4.4% (95% CI 3.7–5.1) of the participants. Out of these, 18% had AF on their index-ECG. In the low-risk group, one participant was diagnosed with AF on index-ECG. The screening procedure resulted in an increase in known prevalence from 8.1% to 10.5% among participants. Oral anticoagulant treatment was initiated in 94.5% of the participants with newly diagnosed AF.
Conclusion
N-terminal B-type natriuretic peptide-stratified systematic screening for AF identified 4.4% of the high-risk participants with new AF. Oral anticoagulant treatment initiation was well accepted in the group diagnosed with new AF.
Keywords: Atrial fibrillation, N-terminal B-type natriuretic peptide, Screening, Stroke, Oral anticoagulants
What’s new?
N-terminal B-type natriuretic peptide-stratified systematic screening for atrial fibrillation (AF) identified high-risk individuals with untreated AF.
N-terminal B-type natriuretic peptide had better AF prediction performance than the CHA2DS2-VASc score.
The implementation of an automated electrocardiogram (ECG) interpretation algorithm reduced ECG-related workload within the screening process.
Introduction
Atrial fibrillation (AF) is an important health problem that regardless of symptoms increases stroke risk.1 A considerable share of patients have no symptoms from AF2 and this poses a major difficulty when detecting new AF. Oral anticoagulant (OAC) treatment reduces stroke-risk by 60–70% in individuals at increased risk.3
Since AF is a common, chronic and progressive and commonly asymptomatic disease, with increased risk of serious but treatable complications it meets most of the World Health Organization’s criteria for population screening as defined by the Wilson and Jungner in 1968.4 However, there is yet no data on the long-term influence on hard endpoints from AF screening. The yield and feasibility of using intermittent handheld electrocardiogram (ECG) recordings in population-based AF screening has previously been reported,5 where screening with handheld ECG increased the prevalence of known AF by ∼30%.
N-terminal B-type natriuretic peptide (NT-proBNP) levels are elevated in patients with AF, and previous studies have shown that NT-proBNP elevation can predict development of AF.6 In patients with known AF, NT-proBNP levels are associated with risk of stroke.7
A previous study showed that using NT-proBNP 125 ng/L as cut-off showed a negative predictive value of 92%. Using this cut-off suggested that 35% fewer participants would have to undergo ECG monitoring,8 while possibly still identifying those at highest risk for stroke. The STROKESTOP II trial was designed with the aim to study if NT-proBNP stratified AF screening will reduce stroke in the intervention group. The study design has previously been published.9
The aim of this analysis from the interventional arm of the STROKESTOP II trial is to report data regarding AF detection and prediction using NT-proBNP levels and initiation and 1-year adherence to OAC treatment. ClinicalTrials.gov identifier: NCT02743416.
Methods
Study population and invitation procedure
The study design has been previously reported.9 In brief, all 75/76-year-old individuals (n = 28 712) residing in the Stockholm region were identified using their personal identification number by Statistics Sweden. A stratified, gender- and aged-based 1:1 randomization provided a control and intervention group (n = 14 356). No information or intervention was provided to the control group. After control of vital status, the intervention group was invited to screening via mail with a maximum of two reminders for non-responders. There were no inclusion criteria other than year of birth and residence in the Stockholm region for those in the intervention group and no exclusion criteria.
Screening protocol
Invitees in the intervention group were invited to the closest of three screening sites. All participants received oral and written information and signed informed consent documents. Participants were asked to self-report their medical history regarding prior AF diagnosis, OAC treatment, thromboembolic risk factors according to CHA2DS2-VASc, pacemaker treatment, palpitation symptoms, weight, and height. No further examination was performed in participants with known AF.
Using point-of-care analysis (Cobash 232, Roche diagnostics, Rotkreutz, Switzerland), NT-proBNP was analysed from venous blood samples in participants without known AF. Depending on NT-proBNP results participants were stratified into low-risk (NT-proBNP <125 ng/L) and high-risk (NT-proBNP ≥125 ng/L) groups. These cut-off values were predefined based on previous findings.8
All participants without known AF recorded an index-ECG consisting of a 30-s ECG using a handheld one-lead device (Zenicor 2 device, Zenicor Medical Systems, Stockholm, Sweden) regardless of NT-proBNP value. If AF was detected, participants were referred to a cardiologist. If index-ECG showed sinus rhythm, participants in the high-risk group were offered 2-week intermittent ambulatory handheld ECG recordings using the Zenicor II device and were instructed to perform ECG recordings four times daily, at morning, noon, afternoon, and evening. Participants in the low-risk group underwent no further investigation if their index ECG revealed sinus rhythm.
Participants with the following findings were also offered further investigations:
N-terminal B-type natriuretic peptide ≥900 ng/L and without previously known heart failure were referred to a cardiologist in addition to the ECG screening.
Individuals with known AF without OAC treatment were referred to cardiologist for assessment.
In case of new AF diagnosis during the screening procedure, participants were referred to a cardiologist for a standard follow-up during which OAC treatment was initiated unless contraindicated.
Diagnostic modalities
The Zenicor II ECG device10 records a 30-s ECG in lead I and automatically transmits the encrypted recording to a password protected database. The Zenicor device has been extensively validated and used in several previous AF screening studies.11
A computerized algorithm was used to identify ECG recordings with sinus rhythm or minor artefacts.12 These were not manually interpreted systematically, only a random sample was scrutinized by cardiologists. All ECGs identified by the algorithm as abnormal were manually interpreted by specially trained nurses, and all pathological ECGs were scrutinized by a cardiologist. Participants with insufficient signal quality (less than 50% of recordings interpretable) on handheld ECG or possible positive finding like atrial flutter were offered a 5-day Holter recording. Participants with episodes of irregular supraventricular tachycardia—consisting of at least 5 b.p.m. with a heart rate of at least 100 b.p.m. suggestive of AF but with a duration less than 30 s—were followed-up in a sub-study using extended ECG recording with a continuous event recorder (R-test 4 evolution, Novacor, Rueil-Malmaison, France) for 14 days. The medical records of participants with pacemaker treatment were studied for atrial high rate episodes with a duration of at least 6 min and available device electrograms.
Outcome measures
Atrial fibrillation was defined as at least one episode of completely irregular rhythm with no organized or regular atrial activity and a duration of 30 s on one-lead ECG. Participants with AF diagnosed using handheld ECG did not undergo any additional ECG investigation. Initiation of OAC treatment was defined as an issued prescription after a cardiologist visit and adherence to treatment at 1-year follow-up.
Statistical methods
Baseline characteristics including age, sex, height, weight, and previous medical history according to CHA2DS2-VASc were summarized using frequencies for categorical variables and means with standard deviation (SD) for continuous variables. For CHA2DS2-VASc and NT-proBNP, both mean (SD) and median with 25th and 75th percentiles were calculated. The following tests were used for differences among groups: the χ2 test was used for categorical variables, Student’s t-test was used for height and weight, and Mann–Whitney U test was used for CHA2DS2-VASc and NT-proBNP, which showed a skewed distribution. The relations between clinical variables and logarithmically transformed NT-proBNP with new AF were investigated in the group with NT-proBNP ≥ 125 ng/L using multivariable logistic regression. A P-value of <0.05 was regarded as significant. Analyses were performed using STATA/MP 15.1 (StataCorp, College Station, TX, USA).
Ethics
The study complies with the Declaration of Helsinki, and the protocol was approved by the regional ethics committee in Stockholm (DNR 2015/2079-31/1). Written informed consent was obtained from all participants in the screening programme.
Results
Participation and patient characteristics
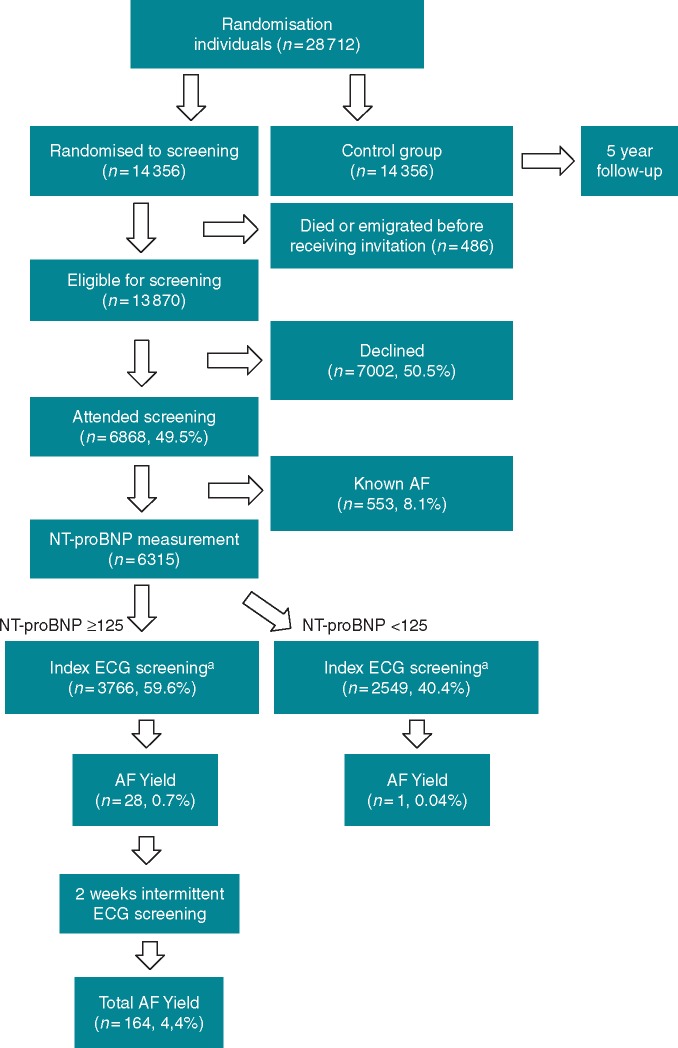
The number of inhabitants identified as eligible for participation in this screening study was 14 356. Excluded from analysis were 486 individuals that had died or migrated before receiving an invitation. In all, 6868 (6868/13870, 49.5%) individuals accepted the invitation. The cumulative response to the three invitations was 37%, 47%, and 49.5%. The consent to participate was later withdrawn by 27 individuals. The study flow chart is shown in Figure 1.
Figure 1.
Study flow chart. aRegardless of NT-proBNP level participants without known AF recorded a 30-s ECG using a handheld one-lead device (Zenicor II device, Zenicor Medical Systems, Stockholm, Sweden). AF, atrial fibrillation; ECG, electrocardiogram; NT-proBNP, N-terminal B-type natriuretic peptide.
Baseline characteristics for participants in the low-risk group and the high-risk group are shown in Table 1. Heart failure, hypertension, vascular disease, female gender, CHA2DS2-VASc, palpitations, and OAC use were more common in the high-risk group than in the low-risk group.
Table 1.
Baseline characteristics at study entry in all participants, low- and high-risk groups
| All participants | Low-risk group NT-proBNP < 125 ng/L (n = 2549) | High-risk group NT-proBNP ≥ 125 ng/L (n = 3766) | P-value low risk vs. high risk | |
|---|---|---|---|---|
| Congestive heart failure, n (%) | 168 (2.4) | 9 (0.4) | 69 (1.8) | <0.001 |
| Hypertension, n (%) | 3548 (51.7) | 1251 (49.1) | 1975 (52.4) | 0.009 |
| Diabetes mellitus, n (%) | 785 (11.4) | 280 (11.0) | 412 (10.9) | |
| Prior stroke/TIA, n (%) | 556 (8.1) | 180 (7.1) | 278 (7.4) | |
| Vascular disease, n (%) | 474 (6.9) | 95 (3.7) | 297 (7.9) | <0.001 |
| Female gender, n (%) | 3708 (54.0) | 1260 (49.4) | 2247 (59.7) | <0.001 |
| CHA2DS2-VASc | ||||
| Mean ± SD | 3.4 ± 1.0 | 3.3 ± 1.0 | 3.5 ± 1.0 | <0.001 |
| Median (IQR) | 3 (1) | 3 (1) | 3 (1) | |
| OAC treatment, n (%) | 602 (8.8) | 27 (1.1) | 76 (2.0) | 0.003 |
| Systolic BP, mean ± SD | 138 ± 17.2 | 140 ± 19.1 | <0.001 | |
| Diastolic BP, mean ± SD | 82 ± 9.6 | 81 ± 10.7 | <0.001 | |
| Height (cm), mean ± SD | ||||
| All | 170 ± 9.0 | 170 ± 9.1 | ||
| Women | 164 ± 6.1 | 164 ± 6.1 | ||
| Men | 177 ± 6.4 | 178 ± 6.6 | <0.001 | |
| Weight (kg), mean ± SD | ||||
| All | 76 ± 13.1 | 73 ± 13.7 | <0.001 | |
| Women | 70 ± 12.0 | 68 ± 12.2 | <0.001 | |
| Men | 81 ± 11.5 | 81 ± 12.0 | ||
| BMI (kg/m2), mean ± SD | ||||
| All | 26 ± 3.8 | 25 ± 4.0 | <0.001 | |
| Women | 26 ± 4.2 | 25 ± 4.4 | <0.001 | |
| Men | 26 ± 3.3 | 26 ± 3.4 | ||
BMI, body mass index; BP, blood pressure; IQR, interquartile range; NT-proBNP, N-terminal B-type natriuretic peptide; OAC, oral anticoagulation; SD, standard deviation; TIA, transient ischaemic attack.
Electrocardiogram recordings
Compliance to ECG recordings was high with a mean of 49 recordings of the 56 (87.5%) instructed in high-risk participants. Only 137 participants of 3766 (3.6%) recorded less than 50% of the stipulated amount of ECG recordings. The total number of ECG recordings was 187 353. Of all the ECG recordings, 22 729 (12.1%) were interpreted as abnormal by the algorithm. Extended Holter monitoring was performed in 81 (2.2%) of the participants due to poor signal quality. No sustained ventricular arrhythmias were recorded on handheld ECG.
N-terminal B-type natriuretic peptide
N-terminal B-type natriuretic peptide was analysed in 6315 participant. Values ≥125 ng/L were found in 3766 (59.6%) of the participants. N-terminal B-type natriuretic peptide values ≥900 ng/L were found in 102 (1.6%) participants without previously known heart failure.
Atrial fibrillation detection
All but one of the participants with new AF were in the high-risk group, resulting in a detection rate of 164/3766 [4.4%, 95% confidence interval (CI) 3.7–5.1] in the high-risk group. In total, new AF was detected in 165/6315 (2.6%, 95% CI 2.2–3.0) participants without previously known AF.
A previous diagnosis of AF was present in 553 patients (8.1%). Baseline characteristics for participants with known AF, new AF, and no AF are shown in Table 2.
Table 2.
Baseline characteristics at study entry in the groups with known AF, new AF, and no AF
| Known AF (n = 553) | P-value known vs. new AF | New AF (n = 165) | P-value new vs. no AF | No AF (n = 6150) | |
|---|---|---|---|---|---|
| Congestive heart failure, n (%) | 90 (16.2) | <0.001 | 7 (4.2) | <0.001 | 71 (1.2) |
| Hypertension, n (%) | 322 (58.2) | 90 (54.5) | 3136 (51.0) | ||
| Diabetes mellitus, n (%) | 93 (16.8) | 0.015 | 15 (9.1) | 677 (11.0) | |
| Prior stroke/TIA, n (%) | 98 (17.7) | 0.001 | 11 (6.7) | 447 (7.3) | |
| Vascular disease, n (%) | 82 (14.8) | 0.012 | 12 (7.3) | 380 (6.2) | |
| Female gender, n (%) | 201 (36.3) | 0.012 | 78 (47.3) | 0.038 | 3429 (55.7) |
| CHA2DS2-VASc | |||||
| Mean ± SD | 3.8 ± 1.3 | <0.001 | 3.4 ± 1.1 | 3.4 ± 1.0 | |
| Median (IQR) | 4 (2) | 3 (1) | 3 (1) | ||
| OAC treatment, n (%) | 499 (90.2) | <0.001 | 4 (2.4) | 99 (1.6) | |
| Systolic BP, mean ± SD | 136 ± 19.2 | 0.039 | 139 ± 18.3 | ||
| Diastolic BP, mean ± SD | 81 ± 11.5 | 81 ± 10.2 | |||
| NT-proBNP | |||||
| Mean ± SD | 657 ± 843 | <0.001 | 212 ± 317 | ||
| Median (IQR) | 325 (495) | 149 (173) | |||
| Height (cm), mean ± SD | |||||
| All | 172 ± 9.1 | 0.005 | 170 ± 9. | ||
| Women | 165 ± 6.4 | 0.036 | 164 ± 6.1 | ||
| Men | 178 ± 6.8 | 0.050 | 177 ± 6.5 | ||
| Weight (kg), mean ± SD | |||||
| All | 77 ± 14.9 | 0.005 | 74 ± 13.5 | ||
| Women | 71 ± 15.7 | 0.036 | 69 ± 12.1 | ||
| Men | 82 ± 12.3 | 81 ± 11.8 | |||
| BMI (kg/m2), mean ± SD | |||||
| All | 25.9 ± 4.5 | 25.6 ± 3.9 | |||
| Women | 26.2 ± 5.5 | 0.018 | 25.4 ± 4.3 | ||
| Men | 25.7 ± 3.4 | 25.8 ± 3.4 | |||
AF, atrial fibrillation; BP, blood pressure; BMI, body mass index; IQR, interquartile range; NT-proBNP, N-terminal B-type natriuretic peptide; OAC, oral anticoagulation; SD, standard deviation; TIA, transient ischaemic attack.
In 29 (29/165, 18%) participants, a new diagnosis of AF was made on the index-ECG. In addition, 136 more cases were identified during extended ECG screening.
The sensitivity and specificity for NT-proBNP 125 ng/L as cut-off for the index-ECG screening and for other clinically relevant cut-points recommended for heart failure diagnostics in acute and non-acute settings13,14 are shown in Table 3.
Table 3.
Sensitivity and specificity for detecting AF on index-ECG for NT-proBNP levels 125 ng/L, 300 ng/L, 450 ng/L, 900 ng/L, and 1800 ng/L
| Level of NT-proBNP cut-point (ng/L) | Sensitivity (%) | Specificity (%) |
|---|---|---|
| 125 | 97 | 41 |
| 300 | 86 | 80 |
| 450 | 86 | 91 |
| 900 | 59 | 98 |
| 1800 | 34 | 100 |
AF, atrial fibrillation; ECG, electrocardiogram; NT-proBNP, N-terminal B-type natriuretic peptide.
The addition of screening four times a day compared to twice daily yielded 17 participants who had AF registered exclusively during mid-day recordings, i.e. at 9 am–6 pm. Extended ECG investigation was performed in 263 participants due to poor signal quality, possible atrial flutter, or shorter bursts of possible AF on handheld ECG. New AF was diagnosed in 30 (30/263, 11.4%) out of those cases. Scrutinizing the medical records of participants with pacemakers did not reveal any new or untreated AF. In total, the screening procedure resulted in an almost 30% increase in AF prevalence among participants, or an absolute increase in AF prevalence from 8.1% to 10.5%.
Use and initiation of oral anticoagulants
In the 165 participants with new AF, 156/165 (94.5%) were initiated on OAC treatment. A majority, 499 out of 553 (90.2%) of those with previously known AF were on OAC treatment. Of those not on OAC, 10 were not interested in referral for cardiologist assessment, but in those referred, 31/44 (70.5%) were initiated on OAC treatment. At 1-year follow-up, 96% of the patients initiated on OAC treatment were still adherent to the treatment. A detailed description of the 32 participants not receiving OAC treatment is found in a Supplementary material online, Table S1. In total, 219/6868 (3.2%) participants were diagnosed with new or previously untreated AF and thus eligible for OAC therapy.
None of the patients were referred for electrical cardioversion nor ablation after their initial cardiologist assessment. Pacemaker was implanted in six patients due to higher atrioventricular-block and two were referred for left atrial appendage occlusion because of OAC contraindication. Thus, only eight participants (0.1%) underwent invasive downstream therapy due to the screening procedure.
Risk factors—prediction of atrial fibrillation
The 29 participants diagnosed with new AF on their index-ECG had significantly higher NT-proBNP than those diagnosed with new AF during the following 2 weeks, with a median NT-proBNP levels of 1308 ng/L [interquartile range (IQR) 663–2180 ng/L] and 305 ng/L (IQR 199–522 ng/L), respectively, Figure 2.
Figure 2.
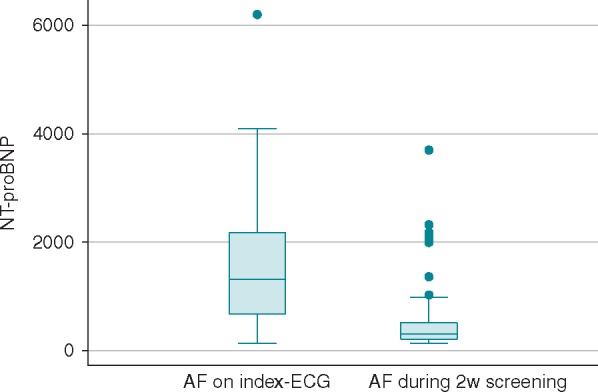
NT-proBNP levels in participants with new AF on index-ECG vs. 2 weeks screening. The 29 participants diagnosed with new AF on the index-ECG had a median NT-proBNP level of 1308 ng/L (IQR 663–2180 ng/L) and those with new AF during the following 2 weeks 305 ng/L (IQR 199–522 ng/L). AF, atrial fibrillation; ECG, electrocardiogram; IQR, interquartile range; NT-proBNP, N-terminal B-type natriuretic peptide.
The burden of AF represented by the number of AF episodes during the screening period did not have a linear association with NT-proBNP levels. In the highest quartile of NT-proBNP (NT-proBNP >353 ng/L), 25/945 (2.6%) participants had AF on the index-ECG and an additional 51/945 (5.4%) had AF diagnosed during prolonged screening. Of those diagnosed with AF on the index-ECG 25/29 (86%) were in the highest NT-proBNP quartile as well as 51/136 (38%) participants with AF diagnosed during screening. Figure 3 shows the predicted probability of having a new AF for each NT-proBNP quartile in the high-risk group.
Figure 3.
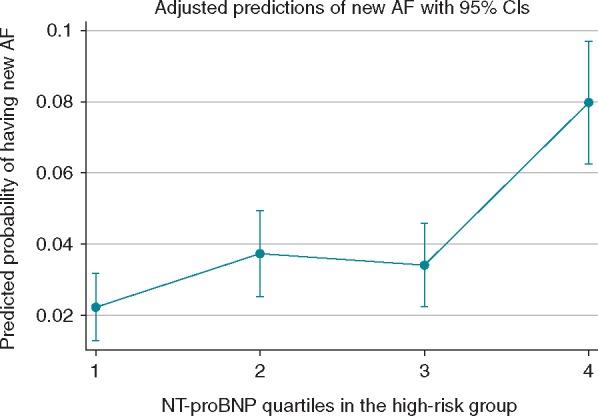
Predicted probability of new AF diagnosis in relation to NT-proBNP quartiles in the high-risk group. 1, quartile 1 (NT-proBNP 125–171 ng/L); 2, quartile 2 (NT-proBNP 171–236 ng/L); 3, quartile 3 (NT-proBNP 237–352 ng/L); and 4, quartile 4 (NT-proBNP 353–9000 ng/L). AF, atrial fibrillation; NT-proBNP, N-terminal B-type natriuretic peptide.
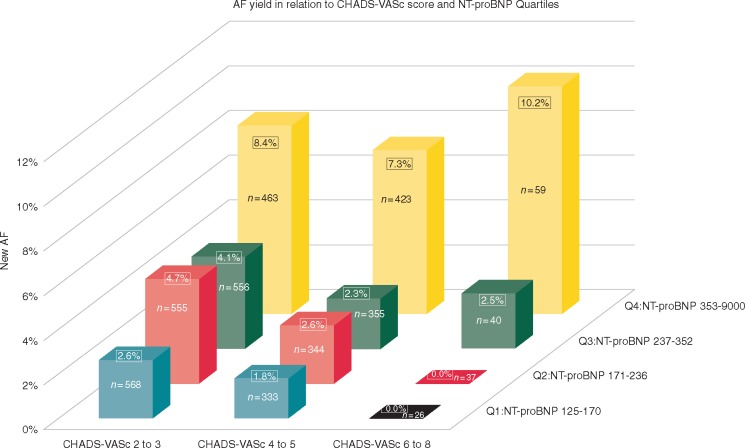
The distribution of new AF in relation to NT-proBNP levels and CHA2DS2-VASc scores is depicted in Figure 4. The interquartile odds ratio from NT-proBNP quartile 1 to 4 was 4.19 (95% CI 2.54–6.93).
Figure 4.
AF screening yield in relation to CHA2DS2-VASc and NT-proBNP quartiles. Interquartile odds ratio from Q1 to Q4, NT-proBNP was 4.19 (95% CI 2.54–6.93). The AF detection yield comparing CHA2DS2-VASc scores within NT-proBNP quartiles was not significant. AF, atrial fibrillation; CI, confidence interval; NT-proBNP, N-terminal B-type natriuretic peptide; Q1, first quartile of NT-proBNP; Q2, second quartile of NT-proBNP; Q3, third quartile of NT-proBNP; Q4, fourth quartile of NT-proBNP.
The strongest predictor in the high-risk group for new AF was NT-proBNP as shown in Table 4.
Table 4.
Multivariable analysis for AF detection in the high-risk group
| Variables | OR (95 % CI) | P-value |
|---|---|---|
| Congestive heart failure (yes) | 0.85 (0.31–2.33) | |
| Hypertension (yes) | 0.97 (0.70–1.36) | |
| Diabetes mellitus (yes) | 0.65 (0.37–1.15) | |
| Prior stroke/TIA (yes) | 0.83 (0.59–1.16) | |
| Vascular disease (yes) | 0.52 (0.27–0.99) | 0.047 |
| Gender (female) | 0.53 (0.38–0.74) | <0.001 |
| BMI (kg/m2) | 1.05 (1.01–1.10) | 0.011 |
| Log NT-proBNP | 3.06 (2.39–3.75) | <0.001 |
The low-risk group was not included in the multivariable analysis, as they only performed an index-ECG and there was no further attempt to diagnose AF.
AF, atrial fibrillation; BMI, body mass index; CI, confidence interval; ECG, electrocardiogram; NT-proBNP, N-terminal B-type natriuretic peptide; OR, odds ratio; TIA, transient ischaemic attack.
Discussion
In this prospective cohort study, we found a high proportion of screening-detected AF in participants with increased NT-proBNP levels. In our analysis, NT-proBNP was the strongest independent predictor for new AF diagnosis. N-terminal B-type natriuretic peptide could hence be useful as a stratifying tool for AF screening.
Using NT-proBNP for risk-stratification led to a similar proportion of new AF being detected with only 59% of the participants recording ECG’s for 2 weeks, as compared to our previous trial, STROKESTOP I,11 in which all the participants underwent the 2-week extended ECG screening procedure. Participants in the high-risk group had significantly higher stroke risk according to the CHA2DS2-VASc score. Initiation of oral anticoagulation treatment was well accepted among participants with newly diagnosed AF.
Participation and patient characteristics
Screening uptake in this study was moderate. Using the experience from our previous trial,15 several measures were taken that aimed to increase uptake. A website (www.strokestop2.se) was launched with general information about AF, information on the screening procedure in the nine most common languages in Sweden and information about the study team. We used three different screening sites in order to shorten travel distance for participants. Despite these measures uptake was lower than we had anticipated although it was significantly higher compared to the uptake within the Stockholm site in STROKESTOP I.15
Only 27 participants (0.4%) withdrew from the current study after the initial visit, indicating that this screening procedure is highly acceptable to participants.
Electrocardiogram recordings
In comparison to our previous AF screening trial, STROKESTOP I,11 we doubled the ECG-recording frequency from twice daily to four times daily. This resulted in 17 cases of new AF that would not have been diagnosed using twice-daily recordings. There was high adherence to the ECG recording protocol, with a mean of 87.5% of the stipulated recordings per participant compared to 75% of the participants in the REHEARSE-AF trial.16 The amount of new AF was also similar in the studies and one might argue that frequent handheld ECG-recordings during a limited time results in higher compliance compared to infrequent long-term handheld ECG-recordings with no difference in AF detection.
The number of participants referred for Holter recording because of poor signal quality was modest and similar to prior studies with the same device. A similarly low proportion of unreadable recordings was reported from the REHEARSE-AF trial.16 Using the validated ECG-interpreting algorithm,12 the ECG-interpreting workload was reduced by over 85% compared to the STROKESTOP I trial.11
N-terminal B-type natriuretic peptide and atrial fibrillation detection
The number of participants who had to undergo intermittent 2-week ECG recordings was reduced by 41% by the use of NT-proBNP pre-test but the overall proportion of newly diagnosed AF was still similar to the STROKESTOP I11 trial, where all participants underwent 2-week intermittent ECG recordings, even when correcting for the 17 cases found during the mid-day recordings. Both studies resulted in an increase of AF prevalence of ∼30%. This agreed with the pilot trial8 preceding this study. Previously undiagnosed AF was found in 2.6% of the participants without previous AF, suggesting that the number needed to screen in this population to diagnose one new case of AF would be 38 (1/0.026). Our findings support prior reports17 on the association between NT-proBNP and incident AF. Although the low-risk group was not investigated with repeated ECG recordings and hence there is risk of undetected AF in that group, the proportion of participants diagnosed with AF on index ECG was markedly higher in the high-risk group, suggesting the discriminative performance of NT-proBNP.
N-terminal B-type natriuretic peptide has repeatedly been shown to be one of the strongest predictors for AF development.6 When adding NT-proBNP to the multivariable analyses, heart failure became an insignificant factor for AF prediction. This finding is similar to findings in other studies by Hijazi et al.,18 in which clinical factors such as heart failure, diabetes, hypertension, other cardiovascular diseases, or gender no longer added prognostic value after adding biomarkers to models concerning risk of stroke, such as the age, biomarkers, clinical history (ABC)-stroke risk score.18 Our results extend these findings and highlight the important addition of biomarkers such as NT-proBNP for improved AF screening.
Use and initiation of oral anticoagulants
Among patients diagnosed with new AF, almost 95% accepted initiation of OAC and of those 96% were still adherent to the treatment at 1-year follow-up. This is in accordance with our previous results from AF screening trials.11 This high acceptance was probably due to a predefined care pathway within the trial. Referring the patient outside the screening context has been associated with lower OAC initiation rates.19 A dedicated research team taking responsibility for the entire screening process could of course affect the participants’ willingness to accept OAC therapy. Participants with previously known AF were OAC treated to a higher extent than in our previous trials,5,11 which is in line with considerable increase in OAC use on AF indication in Sweden during the last 5 years.20 Patients with previously known AF who were not on OAC treatment accepted the initiation of OAC in lower amounts. Very few participants were referred for invasive downstream therapies due to screening findings.
Study limitations
This is the first trial evaluating biomarker enrichment in systematic AF screening. Participation in the study was slightly lower than expected, and the mean CHA2DS2-VASc score in the participants was relatively low at 3.4 considering that all participants were awarded 2 points for age alone. In our study, only 2.4% of the participants had previously known heart failure, which suggests that our participants represented a low-risk selection of the population. It is a known problem in medical screening studies that individuals with the highest risk of disease are often those less likely to attend, which could introduce selection bias among participants and possibly lead to fewer AF cases found. The limited participation, in addition to the prespecified age of 75/76 years reduces the generalizability of this study. However, the invitation by mail, and the lack of exclusion criteria in the trial mimics the reality of screening programmes hence our results are likely representative of those that would occur if screening for AF using NT-proBNP enrichment was used in clinical practice.
Medical history was self-reported and collected from a questionnaire without confirmation from medical records. This could have affected validity of medical history data. Regarding predictors for AF, we were restricted to the variables collected within the trial so there is risk for unknown residual confounding.
N-terminal B-type natriuretic peptide level of 125 ng/L as a first-step screening tool is more sensitive than specific, and as such we consider the number of participants (59.6%) having to go on with more intensified screening to be acceptable.
The low-risk group was only screened with one ECG recording and AF might be underdiagnosed in this group but based on current data we presume that those individuals have a lower risk for stroke as they have lower NT-proBNP.7 This hypothesis will be evaluated in our final analysis of the STROKESTOP II study in a 5 years’ time, where we will compare the outcomes in the intervention group to the control group.
Conclusions
N-terminal B-type natriuretic peptide-stratified systematic screening for AF identified 4.4% of the high-risk group with new AF. Oral anticoagulation treatment was well accepted in the group diagnosed with AF. Our results support the use of NT-proBNP-enriched screening for AF detection.
Supplementary Material
Acknowledgements
We thank all the participants in this study, staff at KTA and statistician Henrike Häbel, PhD.
Funding
This work was supported by the Roche diagnostics, the Swedish Heart and Lung Foundation, Carl Bennet AB.
Conflict of interest: K.K.G. reports no disclosures. T.F. has received research grants from Boehringer-Ingelheim. E.S. was supported by the Stockholm County Council (Clinical postdoctoral appointment) and has received lecture fees from Bayer, Bristol-Myers Squibb, Pfizer, Merck-Sharp & Dome, Boehringer-Ingelheim, and Sanofi, as well as research grants from Boehringer-Ingelheim. J.E. was supported by the Stockholm County Council (clinical research appointment) and has received consultancy fees from Sanofi and Pfizer; lecture fees from AstraZeneca, Boehringer-Ingelheim, Medtronic, and Bristol-Myers Squibb; and travel expenses from Boehringer-Ingelheim and Sanofi. F.A.-K. has received lecture fees from Bristol-Myers Squibb, Boehringer-Ingelheim, and Bayer. L.F. received consultancy fees from Sanofi, Bristol-Myers Squibb, Bayer, and Pfizer. V.F. reports lecture fees from Medtronic, MSD, Bayer, and Boehringer-Ingelheim. M.R. reports consultancy and lecture fees from Medtronic, Zenicor, Bayer, Boehringer-Ingelheim, Pfizer, Bristol-Myers Squibb, and Abbott and research grants from Roche Diagnostics, Bristol-Myers Squibb, Sanofi, Boehringer-Ingelheim, and Bayer. Z.H. has received lecture fees from Boehringer-Ingelheim, Roche Diagnostics, Bristol-Myers Squibb, and Pfizer, consulting fees from Merck Sharp & Dohme, Roche Diagnostics, Bristol-Myers Squibb, and Pfizer.
References
- 1. Martinez C, Katholing A, Freedman SB.. Adverse prognosis of incidentally detected ambulatory atrial fibrillation. A cohort study. Thromb Haemost 2014;112:276–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Savelieva I, Camm AJ.. Clinical relevance of silent atrial fibrillation: prevalence, prognosis, quality of life, and management. J Interv Card Electrophysiol 2000;4:369–82. [DOI] [PubMed] [Google Scholar]
- 3. Hart RG, Pearce LA, Aguilar MI.. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857–67. [DOI] [PubMed] [Google Scholar]
- 4. Wilson J, Jungner G.. Principles and Practice of Screening for Disease. Geneva: World Health Organization (WHO); 1968. [Google Scholar]
- 5. Engdahl J, Andersson L, Mirskaya M, Rosenqvist M.. Stepwise screening of atrial fibrillation in a 75-year-old population: implications for stroke prevention. Circulation 2013;127:930–7. [DOI] [PubMed] [Google Scholar]
- 6. Patton KK, Ellinor PT, Heckbert SR, Christenson RH, DeFilippi C, Gottdiener JS. et al. N-terminal Pro-B-type natriuretic peptide is a major predictor of the development of atrial fibrillation: the cardiovascular health study. Circulation 2009;120:1768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hijazi Z, Oldgren J, Andersson U, Connolly SJ, Ezekowitz MD, Hohnloser SH. et al. Cardiac biomarkers are associated with an increased risk of stroke and death in patients with atrial fibrillation: a Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) substudy. Circulation 2012;125:1605–16. [DOI] [PubMed] [Google Scholar]
- 8. Svennberg E, Henriksson P, Engdahl J, Hijazi Z, Al-Khalili F, Friberg L. et al. N-terminal pro B-type natriuretic peptide in systematic screening for atrial fibrillation. Heart 2017;103:1271–7. [DOI] [PubMed] [Google Scholar]
- 9. Engdahl J, Svennberg E, Friberg L, Al-Khalili F, Frykman V, Kemp Gudmundsdottir K. et al. Stepwise mass screening for atrial fibrillation using N-terminal pro B-type natriuretic peptide: the STROKESTOP II study design. Europace 2017;19:297–302. [DOI] [PubMed] [Google Scholar]
- 10. Bertsch T, Chapelle JP, Dempfle CE, Giannitsis E, Schwabs M, Zerback R.. Multicentre analytical evaluation of a new point-of-care system for the determination of cardiac and thromboembolic markers. Clin Lab 2010;56:37–49. [PubMed] [Google Scholar]
- 11. Svennberg E, Engdahl J, Al-Khalili F, Friberg L, Frykman V, Rosenqvist M.. Mass screening for untreated atrial fibrillation: the STROKESTOP study. Circulation 2015;131:2176–84. [DOI] [PubMed] [Google Scholar]
- 12. Svennberg E, Stridh M, Engdahl J, Al-Khalili F, Friberg L, Frykman V. et al. Safe automatic one-lead electrocardiogram analysis in screening for atrial fibrillation. Europace 2017;19:1499–53. [DOI] [PubMed] [Google Scholar]
- 13. Januzzi JL, van Kimmenade R, Lainchbury J, Bayes-Genis A, Ordonez-Llanos J, Santalo-Bel M. et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur Heart J 2006;27:330–7. [DOI] [PubMed] [Google Scholar]
- 14. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ. et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 15. Engdahl J, Holmen A, Svennberg E, Friberg L, Frykman-Kull V, Al-Khalili F. et al. Geographic and socio-demographic differences in uptake of population-based screening for atrial fibrillation: the STROKESTOP I study. Int J Cardiol 2016;222:430–5. [DOI] [PubMed] [Google Scholar]
- 16. Halcox JPJ, Wareham K, Cardew A, Gilmore M, Barry JP, Phillips C. et al. Assessment of remote heart rhythm sampling using the AliveCor heart monitor to screen for atrial fibrillation: the REHEARSE-AF study. Circulation 2017;136:1784–94. [DOI] [PubMed] [Google Scholar]
- 17. Seegers J, Zabel M, Gruter T, Ammermann A, Weber-Kruger M, Edelmann F. et al. Natriuretic peptides for the detection of paroxysmal atrial fibrillation. Open Heart 2015;2:e000182.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hijazi Z, Lindback J, Alexander JH, Hanna M, Held C, Hylek EM. et al. The ABC (age, biomarkers, clinical history) stroke risk score: a biomarker-based risk score for predicting stroke in atrial fibrillation. Eur Heart J 2016;37:1582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sandhu RK, Dolovich L, Deif B, Barake W, Agarwal G, Grinvalds A. et al. High prevalence of modifiable stroke risk factors identified in a pharmacy-based screening programme. Open Heart 2016;3:e000515.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.SKL. Antikoagulantia vid förmaksflimmer och riskfaktorer Sveriges Kommuner och Landsting; 2018. https://vardenisiffror.se/indikator? datefrom=2014-01-01&metadatameasure=e33263cc-0d3a-4370-846a-d9a442e14d1c&relatedmeasuresbyid=riksstroke&units=se (12 April 2018, date last accessed).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.