Abstract
It has been proven that NEAT1 as a long non-coding RNA (lncRNA) is highly expressed in bladder cancer (BC). Nevertheless, the oncogenic roles of NEAT1 in BC remain largely unknown. In the present study, we observed that the RNA level of NEAT1.1, one RNA variant of NEAT1, was reduced in cisplatin-sensitive T24 cells compared to cisplatin-resistant T24 (T24R) cells after both treated with cisplatin modulated through Wnt/β-catenin signaling pathway using RNA-seq. Furthermore, NEAT1.1 was knocked down within T24R cells and caused a phenotype of the compromised cell growth, invasion and enhanced apoptosis upon cisplatin treatment compared to untreated T24R cells. Finally, c-MYC, OCT4 and p53 were determined to contribute to the transcriptional regulation of NEAT1.1 under cisplatin using ChIP assay. Taken together, our results suggest that NEAT1.1 blocking can promote the effect of cisplatin for BC treatment.
Keywords: lncRNA, NEAT1, bladder cancer, cisplatin
Introduction
Bladder cancer (BC), one of the most common urogenital tumors, has high incidence, prevalence, recurrence, and mortality [1]. Multiple factors are involved in etiology of BC, including genetic, epigenetic, and environmental factors. To date, chemotherapy is still extensively applied for BC treatment in the clinic. Cisplatin is the most widely used of all current chemotherapy regimens including M-VAP (Methotrexate, vincristine, adriamycin, cisplatin), GC (gemcitabine, cisplatin) and MVP (Methotrexate, vincristine, cisplatin). However, cisplatin resistance and relapse as a common drawback to its clinical effectiveness has attracted attention in recent decades, and understanding the issue of how the drug resistance arises in long term usage may help researchers design new protocol to overcome cisplatin resistance.
Currently, long non-coding RNAs (lncRNAs) have attracted attention as one of the epigenetic regulatory factors and potential therapeutic targets for multiple cancers. However, the complicated regulatory network of lncRNAs for tumorigenesis is not fully understood. Recent studies revealed that lncRNA nuclear paraspeckle assembly transcript 1 (NEAT1) played an important role in multiple cancers, including liver [2], lung [3], glioma [4], and gastric cancers [5]. NEAT1 was found to exert as an oncogenic factor to accelerate tumor cell invasion and proliferation by regulation of various targeted miRNAs in different cancers. Nevertheless, NEAT1 was reported to be associated with cisplatin resistance but in contrast, improves cisplatin sensitivity in lung cancer [6] and cholangiocarcinoma [7] but contributes to the cisplatin resistance in osteosarcoma [8] and liver cancer [9]. Furthermore, few studies focused on the different roles between two splicing variants of NEAT1 in cancers, and the underlying mechanism of NEAT1 was never elucidated in BC [10].
In this study, we characterized the RNA profiling of T24 BC cells with cisplatin sensitivity and resistance, and focused on NEAT1.1, one RNA variant of NEAT1 to try to figure out the roles in tumor cell growth, migration, invasion of BC and the effect modulated by cisplatin. Our study may help provide a potential therapeutic target for BC treatment.
Materials and methods
Cell culture
BC T24, 253J, Biu-87 cells were obtained (ATCC, USA) and cultured in RPMI-1640 medium with 10% FBS (Thermo Fisher Scientific, USA) at the incubator with 37°C, 5% CO2 and 100% humidity. Cisplatin-resistant cell lines were prepared by stepwise increments of exposure to cisplatin as previously described [11]. In brief, T24 cells were treated with final concentration 2 μM of cisplatin (Sinopharm Chemical Reagent, China) for 2 h and changed with fresh medium to be restored, and then gradually increased as 4, 8, 16, 32, 64, 128 and 256 μM in order. Cells becoming resistant at 256 uM of cisplatin (T24R) were used for next experiments. NEAT1.1 was cloned from T24 cells and inserted into CMV500 (#33362, Addgene, USA). NEAT1.1 RNAi oligonucleotides (5’-TGGCTAGCTCAGGGCTTCAG-3’) were obtained (GeneChem, China) and transfected into cells using lipofectamine 3000 (Thermo Fisher Scientific, USA) for 72 h.
CCK-8 assay
100 μl T24 and T24R cells were cultured in 96 well plate with 80% density, and treated with 64, 128 and 256 μM cisplatin for 24 h, followed by adding 10 μl CCK-8 solution (Solarbio, China) to incubate additional 1 h. Absorption values of 450 nm were detected using Multiskan FC microplate reader (Thermo Fisher Scientific, USA) and established the regression equation. IC50 of T24 and T24R were calculated from theregression equation.
RNA-seq
Total RNA of T24R cells was extracted using Trizol (Thermo Fisher Scientific, USA) according to the manufacturer’s protocol. 5 μg of RNA in each group were used for library preparation by NEB Next Ultra Directional RNA Library Prep Kit for Illumina (NEB, USA) following manufacturer’s recommendations and were sequenced on an Illumina Hiseq platform. The raw data was trimmed adaptors and filter out low quality reads using Trimmomatic [12], and checked the quality of clean reads using Fastqc [13]. Next, clean reads were aligned to the latest human genome assembly hg38 using Hisat2 [14]. The transcripts were assembled and estimated the expression levels by FPKM values using the StringTie algorithm with default parameters [15]. Differential mRNA and lncRNA expression among the groups were evaluated using a R package Ballgown [16], and computed the significance of differences by the Benjamini & Hochberg (BH) p-value adjustment method. Gene annotation is described by Ensembl genome browser database (http://www.ensembl.org/index.html). The R package ClusterProfiler was used to annotate the differential genes with gene ontology (GO) terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways [17]. Raw data was submitted to ArrayExpress with the accession number of E-MTAB-7437.
Methylthiazoletetrazolium (MTT) assay
Cells were sub-cultured in 96-well plates with density of 1×104 cells/well, and treated with gradient concentration of MTT (Sinopharm Chemical Reagent, China) (0.1, 0.2, 0.4, 0.8 and 1.6 mg/ml) for 4-hour incubation, and washed by PBS twice, and incubated 10 minutes with 200 μl DMSO in dark, followed by detection of the absorbance value at 570 nm by a microplate reader (BioTek, USA). Each concentration was used in three individual experiments and we calculated the IC50 to assess cell proliferation.
Flow cytometric assay
Cells attached on 6-well plates were digested by trypsin, washed by PBS, placed into single cell suspension and fixed by 70% ethanol at 4°C overnight. The pellets dissolved in 100 μl were incubated with 100 μg/ml RNaseA, 10 μg/ml propidium iodide and 10 μg/ml Annexin-V (Abcam, USA) for 20 minutes on ice in dark, and detected the apoptotic cells by FL1 and FL2 channels. The populations of dead cells, live cells and apoptotic cells were observed and analyzed in the first, third and fourth quadrants respectively.
Transwell assay
1×105 cells were cultured within 200 μl suspension in upper and 800 μl fresh medium in lower transwell chamber (Corning, USA) on 24-well plates for 24 hours. The cells at the lower chamber were cross-linked by 1% paraformaldehyde for 10 minutes and stained by 0.5% crystal violet (Sinopharm Chemical Reagent, China) for 5 minutes. The stained cells were counted under a light microscope to evaluate the cell invasion.
Wound healing assay
T24 and T24R Cells were sub-cultured in 6 well plate with 80% density and gently scratch using yellow tips. After 12, 24 and 36 h cell culture, the scratch was captured, and we measured the dynamic change of width and analyzed the cell migration by Image J.
Real-time PCR
First-Strand Synthesis System for reverse transcription (Invitrogen) was used to synthesize cDNA from 1.5 μg total RNA according to the oligo (dT) version of the protocol. Real-time PCR was performed using CFX Fast real-time PCR system (Bio-Rad). The following cycle parameters were used in this study: 94°C 20 s, and 60°C 30 s, 72°C 30 s for a total of 45 cycles. The Ct values were harvested and analyzed by delta-delta methods. The relative mRNA levels of certain genes were normalized by GAPDH. All primer sequences used in this study were listed in Table 1.
Table 1.
Primers used in this study
| Symbol | Sequence | Tm (°C) |
|---|---|---|
| NEAT1.1 | CACAAATTTTCTTCCACTTC | 58 |
| GGCCTTAGCTGAGGTGGCAGG | ||
| NEAT1.2 | CACAAATTTTCTTCCACTTC | 60 |
| ATAAACAGTCTATTAACACAT | ||
| GAPDH | GGAGCGAGATCCCTCCAAAAT | 60 |
| GGCTGTTGTCATACTTCTCATGG | ||
| NEAT1 promoter -600~-450 | GTTAACCAGGGAGAGGTT | 55 |
| TGGACCGTGTAGCGGGC | ||
| NEAT1 promoter -250~-100 | ATATCTTGGTTTTACATT | 57 |
| CGGGCGCTTCAGGGGC |
Western blot assay
The T24 and T24R cells were harvested and placed in cold radioimmunoprecipitation assay buffer containing freshly prepared 2 mM PMSF (Beyotime Bio, China). Tissue blocks were ground on ice for 30 minutes, and centrifuged at 13000×g for 30 minutes at 4°C. Before loading onto a 12% sodium dodecyl sulfate-polyacrylamide gel, equal amounts of protein were boiled with 10× loading buffer for 5 minutes. Electrophoresis was performed at 80 V for 30 minutes and 120 V for 120 minutes. Separated proteins were transferred onto a polyvinylidene difluoride membrane at 120 V for 120 minutes. Membranes were blocked with 5% non-fat dry milk overnight at 4°C, and then incubated with primary antibody, rabbit polyclonal anti-c-MYC (1:1000; Aigma), anti-OCT4 (1:1000; Abcam), anti-C/EBPβ (1:2000; Abcam) and anti-p53 (1:2000; Abcam) overnight at 4°C. Equal loading of protein was confirmed by subsequent GAPDH immunoblots (1:5000; Sigma). Immunodetection was performed by electrochemiluminescence (Pierce, Rockford, IL, USA) after incubation with horseradish peroxidase-conjugated goat anti-rabbit or rabbit anti-mouse (1:5000; Jackson ImmunoResearch, USA) antibody for 1 hour at 37°C, and the X-ray films were finally photographed.
Chromatin immunoprecipitation (ChIP) assay
The ChIP assay was performed as described [18]. In brief, 10% whole cell lysates were saved as input after genomic DNA was broken into 200-500 bp by sonication. 1 μg antibodies of c-MYC, OCT4 and p53 were incubated with the rest of the lysate overnight, followed by 2 h protein-A beads incubation at 4°C for target protein pull down. Primers were designed to encompass ~150 bp around the target regions (-600~-450 and -250~-100) of NEAT1 promoter. Their sequences were listed in Table 1.
Statistical analysis
The results were presented as the mean ± SD. The significance of difference among the groups was assessed by Student’s t-test. All analysis was processed by SPSS 20 software. p value <0.05 was considered significant.
Results
Identification of cisplatin-resistant T24 cells
Initially, we established the cisplatin-resistant T24 cells (T24R) by stepwise increments of exposure to cisplatin. T24 and T24R were exposed under gradient concentrations of cisplatin for 24 h and tested the IC50 by CCK-8 assay, and we observed that the IC50 value of T24R was significantly higher than T24 (358.33±36.72 μM vs. 112.36±14.87 μM, t=33.65, P<0.05) (Figure 1A). Furthermore, the cell cycle pattern of T24 and T24R were compared and observed that the T24R cells were more at the stage of G0/G1, while less at the stage of G2/M compared to T24 (Figure 1B). Taken together, our results determined that the cisplatin-resistant T24 BC cell subline was successfully established.
Figure 1.
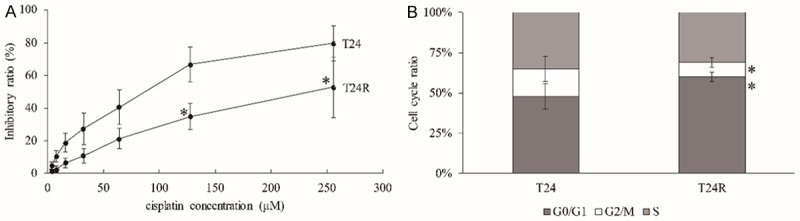
Identification of cisplatin-resistant T24 cells. Cell viability (A) and cell cycle (B) of T24R and T24 cells after cisplatin treatment. All data are presented as the mean ± standard error of five individual experiments. “*” p value less than 0.05 vs. T24.
Characterization of RNA profiling of T24R cells
Next, we further characterized the difference of RNA profiling between T24R and T24 cells. Deep sequencing of mRNA libraries generated total 180.3 M reads of T24 and 192.7 M reads of T24R groups. Approximate 67.51% reads were uniquely mapped and 18.32% reads were mismatches to the human genome 38 (Ensemble Genomes release 92) using HISAT2, while only 0.35% of all reads were mapped to rRNAs, which indicated a high quality of our RNA libraries preparation without poly-A selection (Table 2). StringTie was used to quantify the gene expression with FPKM distribution (Figure 2A), and presented the differentially expressed genes among the three groups. We observed that plenty of genes changed their transcriptional levels (Figure 2B). 346 mRNA and 120 lncRNA were up-regulated (fold change >2, p value <0.05), while 55 mRNA and 36 lncRNA were down-regulated (fold change <0.5, p value <0.05) in T24R cells compared to T24 cells (Table 3). Furthermore, the associated function enrichments and pathways involved in differentially expressed genes including cell morphogenesis regulation, response to drugs, ureteric bud development (biological process), ion channel complex, transcription factor complex, β-catenin destruction complex (cellular component), transcriptional regulation, ion channel activity regulation (molecular function) as well as Wnt, HIF-1, AMPK and Rap1 signaling pathways (KEGG pathway) (Figure 2C, 2D). Here, we noticed that the lncRNA NEAT1 was highly expressed in T24R cells (fold change=36.8, P=0.0001) compared to T24 cells. Taken together, our results determined that NEAT1 as one of the novel candidate genes displayed aberrantly high expression in T24R cells.
Table 2.
Summary of RNA-seq data
| Sample names | T24_1 | T24_2 | T24_3 | T24R_1 | T24R_2 | T24R_3 |
|---|---|---|---|---|---|---|
| Raw reads | 58933548 | 59871070 | 61514088 | 63778852 | 64235706 | 64668936 |
| Total raw bases | 8840032200 | 8980660500 | 9227113200 | 9566827800 | 9635355900 | 9700940400 |
| Clean reads | 56587510 | 57490828 | 59066942 | 61842520 | 61678722 | 62707156 |
| Total clean bases | 8310853554 | 8443586068 | 8675127770 | 914634152 | 9058309704 | 9274120003 |
| Mapped reads | 47318925 | 48083736 | 50725437 | 54740709 | 51597349 | 55502209 |
| Mapped ratio | 83.62% | 83.64% | 82.46% | 88.52% | 83.66% | 88.51% |
| Uniquely mapped reads | 36411305 | 36993627 | 38460380 | 46228373 | 39711626 | 46846663 |
| Uniquely mapped ratio | 64.35% | 64.35% | 62.52% | 74.75% | 64.38% | 74.71% |
| Mismatch ratio | 21.08% | 20.75% | 20.75% | 13.32% | 20.7% | 13.32% |
| rRNA ratio | 0.34% | 0.34% | 0.34% | 0.38% | 0.34% | 0.38% |
Figure 2.
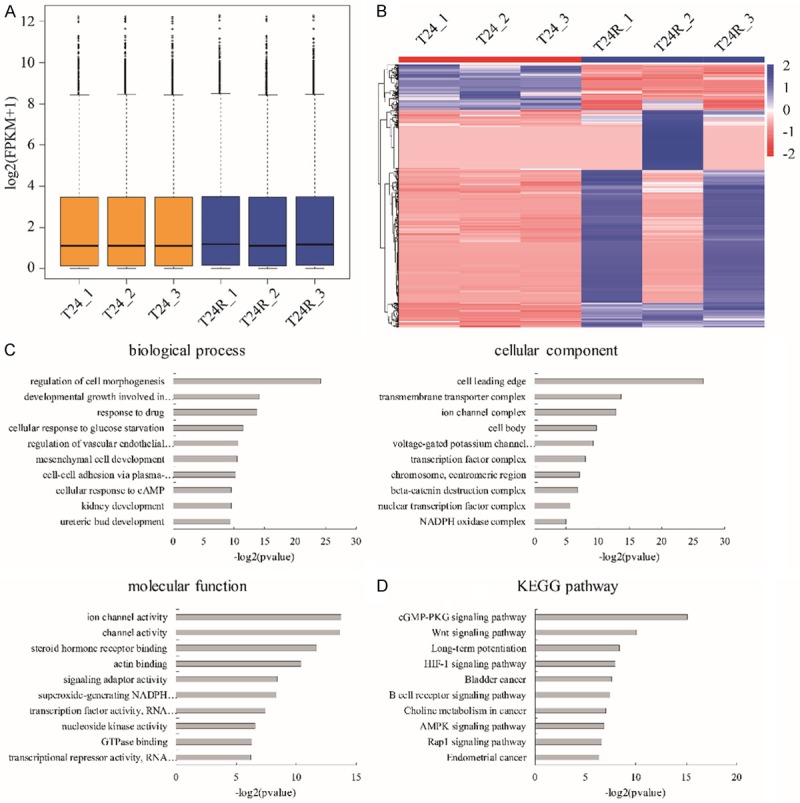
RNA profiling of cisplatin-resistant T24 cells. (A) The FPKM distribution of RNA-seq data. (B) Heatmap of genes with differential expression between T24R and T24 cells. Color bars above the heatmap represent sample groups: red is for up-regulated genes and blue is for down-regulated genes. Gene ontology analysis including biological process, cellular component and molecular function (C) and KEGG analysis (D) of the top 10 function enrichments or pathways associated with these differentially expressed genes of T24R vs. T24 cells.
Table 3.
The top 20 up- and down-regulated RNAs between T24R and T24 cells
| Gene name | T24_1 | T24_2 | T24_3 | T24R_1 | T24R_2 | T24R_3 | Fold change | P value | |
|---|---|---|---|---|---|---|---|---|---|
| TSIX | 0 | 0 | 0.001145 | 44.3223 | 0 | 44.4631 | 2851.674 | 0.043527 | UP |
| CALCB | 0 | 0 | 0 | 5.48965 | 0 | 5.6515 | 372.3717 | 0.046705 | UP |
| INA | 0 | 0 | 0 | 3.40445 | 0 | 3.4335 | 228.9317 | 0.044256 | UP |
| AC011479.1 | 0 | 0 | 0 | 2.09401 | 2.46234 | 1.48416 | 202.3503 | 0.001455 | UP |
| CDX2 | 0 | 0 | 0 | 2.84571 | 0 | 2.96137 | 194.5693 | 0.048734 | UP |
| HAND1 | 0 | 0 | 0 | 2.54288 | 0 | 2.23333 | 160.207 | 0.029952 | UP |
| MTATP6P29 | 0 | 0 | 0 | 2.08806 | 0 | 1.57415 | 123.0737 | 0.026104 | UP |
| IRS4 | 0.143147 | 0.196057 | 0.15983 | 32.0636 | 0.130196 | 32.3778 | 122.1124 | 0.045718 | UP |
| SMOC1 | 0 | 0 | 0.023121 | 2.68218 | 0.022096 | 2.70471 | 102.3886 | 0.048069 | UP |
| PAX3 | 0 | 0 | 0 | 1.61395 | 0 | 1.29241 | 97.87867 | 0.026044 | UP |
| LBX1-AS1 | 0 | 0 | 0 | 1.4822 | 0 | 1.29934 | 93.718 | 0.031974 | UP |
| WDR38 | 0 | 0.047591 | 0.057662 | 5.83899 | 0.055105 | 5.32661 | 83.18267 | 0.037259 | UP |
| AL121900.2 | 0 | 0 | 0 | 0 | 2.20274 | 0 | 74.42467 | 0.006024 | UP |
| SLC4A5 | 0 | 0 | 0 | 1.09691 | 0 | 1.10524 | 74.405 | 0.042567 | UP |
| FOXB1 | 0 | 0 | 0 | 1.08689 | 0 | 1.11149 | 74.27933 | 0.047311 | UP |
| FENDRR | 0 | 0 | 0 | 1.22109 | 0 | 0.945853 | 73.23143 | 0.027006 | UP |
| IRF8 | 0 | 0 | 0 | 0.92567 | 0.024952 | 0.979648 | 65.34233 | 0.018378 | UP |
| RENBP | 0 | 0 | 0 | 1.10646 | 0 | 0.784627 | 64.03623 | 0.037669 | UP |
| CDR1 | 0 | 0 | 0.014682 | 1.2849 | 0.051334 | 1.32987 | 60.33982 | 0.03778 | UP |
| FIBIN | 0 | 0 | 0 | 0.848011 | 0 | 0.826709 | 56.824 | 0.038981 | UP |
| AL358332.1 | 0.174681 | 0.086032 | 0.167478 | 0 | 0 | 0 | 0.065475 | 0.028332 | DOWN |
| HIF1A-AS2 | 0.123822 | 0.152257 | 0.152246 | 0 | 0 | 0 | 0.065456 | 0.001169 | DOWN |
| KRTAP3-1 | 0.148837 | 0.146606 | 0.142699 | 0 | 0 | 0 | 0.064083 | 7.35E-06 | DOWN |
| RSL24D1P8 | 0.180292 | 0.17759 | 0.086141 | 0 | 0 | 0 | 0.063288 | 0.027062 | DOWN |
| SLC38A8 | 0.150917 | 0.175791 | 0.144694 | 0 | 0 | 0 | 0.059832 | 0.002592 | DOWN |
| AL133368.1 | 0.217221 | 0.106983 | 0.208264 | 0 | 0 | 0 | 0.053336 | 0.027518 | DOWN |
| AC007547.2 | 0.205469 | 0.20239 | 0.196996 | 0 | 0 | 0 | 0.047255 | 6.63E-06 | DOWN |
| AL138999.1 | 0.265286 | 0.17382 | 0.169188 | 0 | 0 | 0 | 0.047 | 0.011463 | DOWN |
| STK19B | 0.218092 | 0.214823 | 0.209098 | 0 | 0 | 0 | 0.044642 | 6.53E-06 | DOWN |
| Z98742.3 | 0.26125 | 0.257334 | 0.250476 | 0.005615 | 0 | 0 | 0.044571 | 8.44E-06 | DOWN |
| AC009127.2 | 0.219377 | 0.21609 | 0.210331 | 0 | 0 | 0 | 0.044392 | 6.52E-06 | DOWN |
| AL513331.1 | 0.168504 | 0.331958 | 0.161555 | 0 | 0 | 0 | 0.043352 | 0.039337 | DOWN |
| SNRPFP1 | 0.170737 | 0.337508 | 0.164817 | 0 | 0 | 0 | 0.04267 | 0.039199 | DOWN |
| AL109811.4 | 0.188 | 0.319967 | 0.221454 | 0 | 0 | 0 | 0.039504 | 0.015707 | DOWN |
| APOC3 | 0.146373 | 0.288358 | 0.312313 | 0 | 0 | 0 | 0.038608 | 0.025139 | DOWN |
| HMGN2P24 | 0.161921 | 0.318989 | 0.310488 | 0 | 0 | 0 | 0.036523 | 0.023345 | DOWN |
| AC004241.2 | 0.394257 | 0.196135 | 0.374816 | 0 | 0 | 0 | 0.030144 | 0.023448 | DOWN |
| AL162713.1 | 0.328441 | 0.323519 | 0.314896 | 0 | 0 | 0 | 0.030095 | 5.78E-06 | DOWN |
| PCDHGB4 | 0.83584 | 0.614691 | 0.669538 | 0 | 0 | 0 | 0.013953 | 0.002149 | DOWN |
| AC024075.1 | 2.96262 | 2.82808 | 2.94387 | 0 | 0 | 0 | 0.003423 | 2.41E-06 | DOWN |
NEAT1.1 as a cisplatin negatively responder for tumor malignancy in BC cells
Since we found that NEAT1 was highly expressed in T24R compared to T24 upon cisplatin treatment from RNA-seq data, which transcript isoform of NEAT1 needed to be further determined. Hence, we conducted qPCR to measure the transcriptional levels of two variants of NEAT1 in T24, T24R, Biu-87 and 253J BC cells and observed that NEAT1.1 was all down-regulated in cisplatin treated BC cells, but robustly expressed in T24R with or without cisplatin treatment. NEAT1.1 was obviously higher in T24R than T24 and other BC cells without cisplatin treatment. NEAT1.2 showed no significant change no matter in cisplatin resistance or treatment (Figure 3A). Furthermore, we knockdown NEAT1.1 (Figure 3B) and observed that T24R cells with NEAT1.1 silencing displayed a declining cell proliferation (Figure 3C) and increasing cell apoptosis compared to control after cisplatin treatment by MTT and flow cytometric assay (Figure 3D). Moreover, we also found that NEAT1.1 silencing could suppress the migration ability of cells by transwell and wound healing assay (Figure 3E, 3F). Taken together, our results presented that NEAT1.1 negatively responded to cisplatin in regular BC cells but displayed persistently high expression in cisplatin resistant cells.
Figure 3.
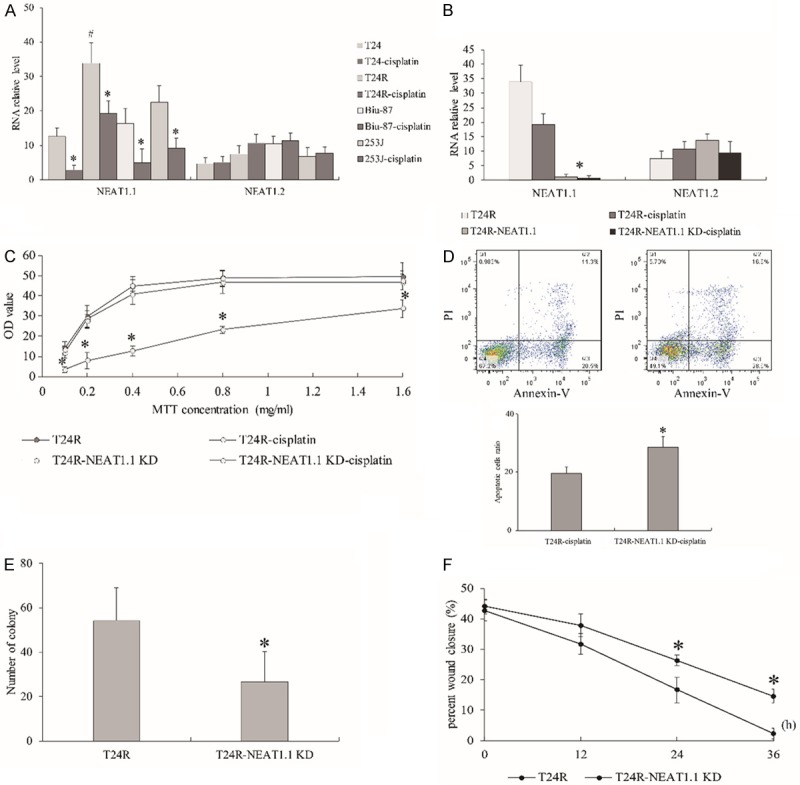
The effect of NEAT1.1 knockdown in BC cells. (A) The expression of NEAT1.1 and NEAT1.2 in BC cells with or without cisplatin treatment. (B) The expression of NEAT1.1 and NEAT1.2 in BC cells with NEAT1.1 knockdown. The assays of MTT (C), flow cytometry (D), transwell (E) and wound healing (F) in T24R cells with NEAT1.1 knockdown. In flow cytometric assay, the population of dead cells, live cells and apoptotic cells were observed in the first, third, and fourth quadrants respectively. All data are presented as the mean ± standard error of three individual experiments. “*” p value less than 0.05 vs. control. “#” p value less than 0.05 vs. T24, Biu-87 and 253J.
Transcriptional activation of NEAT1 modulated by multiple transcription factors in T24R cells
A previous study reported that c-MYC [19], C/EBPβ [20], OCT4 [21], and p53 [22] could interact with the NEAT1 promoter region to regulate NEAT1 transcription verified by ChIP assay. To investigate the regulatory role of NEAT1 transcription affected by cisplatin resistance, these four transcription factors were employed to investigate the effect upon cisplatin in T24R cells. We observed that c-MYC, OCT4, and p53 were all up-regulated in T24R compared to T24 cells (Figure 4A), which was consistent with RNA-seq data. Furthermore, ChIP-qPCR assay was conducted to investigate the enrichment of these transcription factors on the promoter of NEAT1. We observed that these three transcription factors displayed no significant difference of enrichment without cisplatin between T24 and T24R cells, but all showed stronger affinities with the promoter of NEAT1 (-600~-450 and -250~-100) upon cisplatin treatment in T24R cells compared to T24 cells (Figure 4B, 4C). Collectively, our data determined that the aberrant roles of oncogenic transcription factors such as c-MYC, OCT4 and p53 regulated the transcriptional activity of NEAT1 in cisplatin-resistant BC cells.
Figure 4.
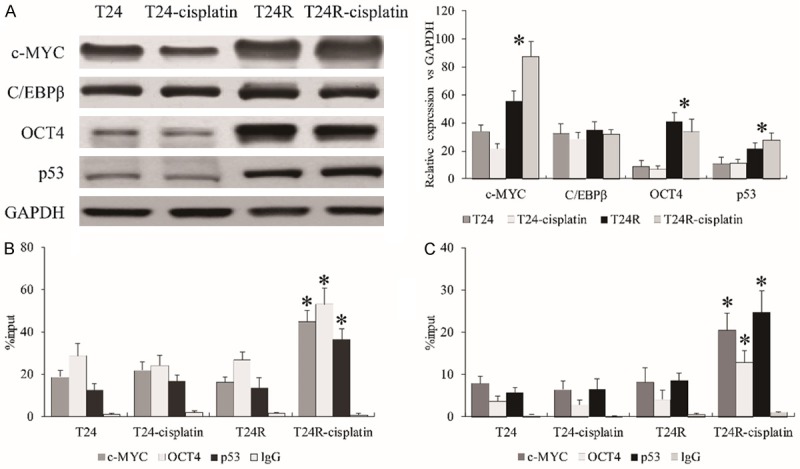
The transcription activity of NEAT1 regulated by c-MYC, OCT4 and p53. (A) The expression of c-MYC, OCT4, and p53 in T24R cells with cisplatin treatment. The enrichments of c-MYC, OCT4, and p53 on the promoter of NEAT1 at -600~-450 (B) and -250~-100 (C). All data are presented as the mean ± standard error of three individual experiments. “*” p value less than 0.05 vs. T24R.
Discussion
NEAT1 is an intranuclear lncRNA exerting as a crucial transcriptional regulator for numerous genes involved in multiple diseases including cancer progression. NEAT1 has two transcript isoforms, namely NEAT1.1 and NEAT1.2. The longer transcript variant has an additional triple helical structure at 3’ end. The functions of these two variants were never compared in one study. Limited studies have determined that NEAT1 can affect cisplatin sensitivity in different types of cancers. However, it is uncertain if NEAT1 improves cisplatin sensitivity in lung cancer [6] and cholangiocarcinoma [7] but it contributes to cisplatin resistance in osteosarcoma [8], liver cancer [9]. Moreover, NEAT1.2 is also reported to regulate cisplatin resistance in liver cancer [9]. In our case, we verified that NEAT1.1, not NEAT1.2, does respond upon the effect of cisplatin in BC cells. We also found that in regular BC cells, NEAT1.1 will be reduced after cisplatin adding, which indicates that cisplatin could restrain the transcriptional activity of NEAT1. Correspondingly, knockdown of NEAT1.1 can compromise tumor cell proliferation, invasion, and migration and enhance the tumor apoptosis in cisplatin-resistant BC cells. In general, NEAT1.1 may be a potential downstream target of cisplatin in BC. However, the observations of persistently and aberrantly high expression of NEAT1.1 upon cisplatin treatment in T24R cells suggest that some unknown regulatory factors bridging between cisplatin and NEAT1.1 contribute to receiving the signaling from cisplatin and governing the NEAT1 transcription.
The underlying networks regulated by NEAT1 are extensively studied, including multiple miRNAs and transcription factors [23,24]. However, what regulates NEAT1 transcription is less studied. We noticed that c-MYC, OCT4, C/EBPβ, and p53 were reported to bind at the promoter of NEAT1 and affect its transcription activity verified by ChIP assay. They were also determined to be associated with cisplatin resistance in recent studies [25-28]. Thus, these four transcription factors were considered as the prior targets bridging cisplatin and NEAT1. Our results draw two points. First, the free proteins of c-MYC, OCT4, and p53 are up-regulated in cisplatin-resistant BC cells and second c-MYC, OCT4, and p53 highly concentrate on NEAT1 promoter once cells are stimulated by cisplatin by ChIP-qPCR assay. However, the underlying mechanisms of regulation of NEAT1.1 and NEAT1.2 are not illustrated in this study. We speculate that there are other important enzymes for RNA splicing of NEAT1 in cisplatin-resistant BC cells that need to be further investigated. Thus we reveal that the transcriptional activity of NEAT1 may be regulated by multiple transcription factors upon cisplatin treatment.
Taken together, our data give evidence that NEAT1.1 is harmful for overcoming BC cisplatin resistance, and silencing NEAT1 can enhance the suppression of cell growth, invasion and apoptosis of bladder cancer cells upon cisplatin chemotherapy.
Acknowledgements
This project was funded by the Jiangsu Province Health and Family Planning Commission Scientific Research Project (grant no. Z201529). The experimental protocols were approved by the Institutional Review Board of Taizhou People’s Hospital (Jiangsu, P.R. China; No. 201603).
Disclosure of conflict of interest
None.
References
- 1.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 2.Yang X, Qu S, Wang L, Zhang H, Yang Z, Wang J, Dai B, Tao K, Shang R, Liu Z, Li X, Zhang Z, Xia C, Ma B, Liu W, Li H, Dou K. PTBP3 splicing factor promotes hepatocellular carcinoma by destroying the splicing balance of NEAT1 and pre-miR-612. Oncogene. 2018;37:6399–6413. doi: 10.1038/s41388-018-0416-8. [DOI] [PubMed] [Google Scholar]
- 3.Xiong DD, Li ZY, Liang L, He RQ, Ma FC, Luo DZ, Hu XH, Chen G. The LncRNA NEAT1 accelerates lung adenocarcinoma deterioration and binds to Mir-193a-3p as a competitive endogenous RNA. Cell Physiol Biochem. 2018;48:905–918. doi: 10.1159/000491958. [DOI] [PubMed] [Google Scholar]
- 4.Zhou K, Zhang C, Yao H, Zhang X, Zhou Y, Che Y, Huang Y. Knockdown of long non-coding RNA NEAT1 inhibits glioma cell migration and invasion via modulation of SOX2 targeted by miR-132. Mol Cancer. 2018;17:105. doi: 10.1186/s12943-018-0849-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang CL, Wang D, Yan BZ, Fu JW, Qin L. Long non-coding RNA NEAT1 promotes viability and migration of gastric cancer cell lines through up-regulation of microRNA-17. Eur Rev Med Pharmacol Sci. 2018;22:4128–4137. doi: 10.26355/eurrev_201807_15405. [DOI] [PubMed] [Google Scholar]
- 6.Jiang P, Wu X, Wang X, Huang W, Feng Q. NEAT1 upregulates EGCG-induced CTR1 to enhance cisplatin sensitivity in lung cancer cells. Oncotarget. 2016;7:43337–43351. doi: 10.18632/oncotarget.9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parasramka M, Yan IK, Wang X, Nguyen P, Matsuda A, Maji S, Foye C, Asmann Y, Patel T. BAP1 dependent expression of long non-coding RNA NEAT-1 contributes to sensitivity to gemcitabine in cholangiocarcinoma. Mol Cancer. 2017;16:22. doi: 10.1186/s12943-017-0587-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu Y, Yang Q, Wang L, Wang S, Sun F, Xu D, Jiang J. Knockdown of the oncogene lncRNA NEAT1 restores the availability of miR-34c and improves the sensitivity to cisplatin in osteosarcoma. Biosci Rep. 2018:38. doi: 10.1042/BSR20180375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ru Y, Chen XJ, Guo WZ, Gao SG, Qi YJ, Chen P, Feng XS, Zhang SJ. NEAT1_2-SFPQ axis mediates cisplatin resistance in liver cancer cells in vitro. Onco Targets Ther. 2018;11:5695–5702. doi: 10.2147/OTT.S163774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taheri M, Omrani MD, Ghafouri-Fard S. Long non-coding RNA expression in bladder cancer. Biophys Rev. 2018;10:1205–1213. doi: 10.1007/s12551-017-0379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong JH, Lee E, Hong J, Shin YJ, Ahn H. Antisense Bcl2 oligonucleotide in cisplatin-resistant bladder cancer cell lines. BJU Int. 2002;90:113–117. doi: 10.1046/j.1464-410x.2002.02799.x. [DOI] [PubMed] [Google Scholar]
- 12.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrews S. FastQC a quality control tool for high throughput sequence data. 2013 [Google Scholar]
- 14.Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pertea M, Pertea GM, Antonescu CM, Chang TC, Mendell JT, Salzberg SL. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol. 2015;33:290–295. doi: 10.1038/nbt.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frazee AC, Pertea G, Jaffe AE, Langmead B, Salzberg SL, Leek JT. Ballgown bridges the gap between transcriptome assembly and expression analysis. Nat Biotechnol. 2015;33:243–246. doi: 10.1038/nbt.3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu G, Wang LG, Han Y, He QY. clusterprofiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fei P, Wang W, Kim SH, Wang S, Burns TF, Sax JK, Buzzai M, Dicker DT, McKenna WG, Bernhard EJ, El-Deiry WS. Bnip3L is induced by p53 under hypoxia, and its knockdown promotes tumor growth. Cancer Cell. 2004;6:597–609. doi: 10.1016/j.ccr.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Zeng C, Liu S, Lu S, Yu X, Lai J, Wu Y, Chen S, Wang L, Yu Z, Luo G, Li Y. The c-Myc-regulated lncRNA NEAT1 and paraspeckles modulate imatinib-induced apoptosis in CML cells. Mol Cancer. 2018;17:130. doi: 10.1186/s12943-018-0884-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Fu L, Sun A, Tang D, Xu Y, Li Z, Chen M, Zhang G. C/EBPbeta contributes to transcriptional activation of long non-coding RNA NEAT1 during APL cell differentiation. Biochem Biophys Res Commun. 2018;499:99–104. doi: 10.1016/j.bbrc.2017.10.137. [DOI] [PubMed] [Google Scholar]
- 21.Jen J, Tang YA, Lu YH, Lin CC, Lai WW, Wang YC. Oct4 transcriptionally regulates the expression of long non-coding RNAs NEAT1 and MALAT1 to promote lung cancer progression. Mol Cancer. 2017;16:104. doi: 10.1186/s12943-017-0674-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mello SS, Sinow C, Raj N, Mazur PK, Bieging-Rolett K, Broz DK, Imam JFC, Vogel H, Wood LD, Sage J, Hirose T, Nakagawa S, Rinn J, Attardi LD. Neat1 is a p53-inducible lincRNA essential for transformation suppression. Genes Dev. 2017;31:1095–1108. doi: 10.1101/gad.284661.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li S, Li J, Chen C, Zhang R, Wang K. Pan-cancer analysis of long non-coding RNA NEAT1 in various cancers. Genes Dis. 2018;5:27–35. doi: 10.1016/j.gendis.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu X, Li Z, Zheng H, Chan MT, Wu WK. NEAT1: a novel cancer-related long non-coding RNA. Cell Prolif. 2017:50. doi: 10.1111/cpr.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li QC, Xu H, Wang X, Wang T, Wu J. miR-34a increases cisplatin sensitivity of osteosarcoma cells in vitro through up-regulation of c-Myc and Bim signal. Cancer Biomark. 2017;21:135–144. doi: 10.3233/CBM-170452. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Ma M, Duan X, Zhang H, Yang M. Knockdown of OCT4 may sensitize NSCLC cells to cisplatin. Clin Transl Oncol. 2017;19:587–592. doi: 10.1007/s12094-016-1569-y. [DOI] [PubMed] [Google Scholar]
- 27.Xie X, He G, Siddik ZH. Functional activation of mutant p53 by platinum analogues in cisplatin-resistant cells is dependent on phosphorylation. Mol Cancer Res. 2017;15:328–339. doi: 10.1158/1541-7786.MCR-16-0257-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zong S, Liu T, Wan F, Chen P, Luo P, Xiao H. Endoplasmic reticulum stress is involved in cochlear cell apoptosis in a cisplatin-induced ototoxicity rat model. Audiol Neurootol. 2017;22:160–168. doi: 10.1159/000480346. [DOI] [PubMed] [Google Scholar]


