Abstract
Yes-associated protein 1 (YAP1) plays important roles in facilitating cell proliferation and decreasing apoptosis and is related to gastric cancer. Abnormal down-regulation of miR-141 is associated with gastric cancer pathogenesis, suggesting a potentially tumor suppressor role. Bioinformatics analysis found complementary binding sites between miR-141 and YP1. This study investigated the role of miR-141 in mediating YAP1 expression and biological behavior of gastric cancer cells. Gastric cancer tissues were collected using normal mucosal tissues as the control. qRT-PCR compared expression of miR-141 and YAP1 mRNA, and western blot quantified YAP1 protein expression. Spearman approach analyzed the correlation between miR-141 and YAP1 mRNA in cancer tissues. Dual luciferase reporter gene assay confirmed the targeted regulation between miR-141 and YAP1. Using GES-1 cell as the control, miR-141 and YAP1 expression were measured in gastric cancer cell lines SGC7901 and MGC03. Those cells were transfected with miR-141 mimic in the presence or absence of miR-YAP1 mimic followed by flow cytometry for apoptosis and EdU staining for proliferation. Cancer tissues had decreased miR-141 and higher YAP1 expression, which was associated with TNM stage. YAP1 mRNA and miR-141 were positively correlated (r=-0.623, P<0.001). Dual luciferase assay demonstrated targeted regulation between miR-141 and YAP1. Comparing to GES-1 cells, SGC7901 and MGC803 cells had decreased miR-141 and increased YAP1 expression. Transfection of miR-141 mimic inhibited YAP1 expression or cell proliferation and facilitated apoptosis. However, overexpression of YAP1 decreased the effect of miR-141 mimic on cell proliferation and apoptosis. miR-141 down-regulation and YAP1 up-regulation are correlated with gastric cancer pathogenesis. miR-141 targets and inhibits YAP1 expression, to suppress gastric cancer cell proliferation and induce apoptosis.
Keywords: Gastric cancer, miR-141, YAP1, cell proliferation, apoptosis
Introduction
Gastric cancer (GC) is one commonly occurred malignant tumor in digestive tract and is the fourth popular cancer with second high mortality. GC is characterized by high malignancy, rapid progression, unfavorable survival and prognosis and high mortality [1-3].
Yes-associated protein 1 (YAP1) is the major effector and target protein of canonical Hippo-YAP signal pathway. It can regulate target gene expression via nuclear translocation for transcriptional co-activation, thus facilitating occurrence of various tumors [4-6]. Previous studies showed significantly enhanced expression and functional activity of YAP1 in GC tissues, and its correlation with tumor invasion, progression and prognosis, indicating its oncogenic role in occurrence and progression of GC. MicroRNA (miR) is one type of endogenous non-coding small RNA molecules in eukaryotes, and can interact with 3’-untranslated region (3’-UTR) of target gene mRNA via complementary binding manner, to regulate target gene expression by degrading mRNA or suppressing mRNA translation, thus modulating various biological processes including cell survival, proliferation, apoptosis and migration. Abnormal expression or function of miRs has attracted various research interests in onset and occurrence of various tumors including lung cancer, breast cancer, gall bladder cancer and ovarian carcinoma [7-10]. Studies have found significantly decreased miR-141 expression in GC tumor tissues or cell lines [11,12], suggesting its potential role asa tumor suppressor for GC. Online analysis from microRNA.org showed the existence of complementary binding sites between miR-141 and YAP1 mRNA, suggesting potential regulation between these two molecules. This study thus analyzed the expression profile of miR-141 and YAP1 in GC patient tumor tissues for revealing the relationship between miR-141 and YAP1 expression, and investigated the role of miR-141 in modulating YAP1 expression and in affecting GC cell proliferation or apoptosis using an in vitro cell model.
Materials and methods
Major reagents and materials
Normal human gastric mucosal epithelial cell line GES-1, GC cell lines SGC7901, MGC-803, and HEK293T were purchased from Shanghai Cell Bank, Chinese Academy of Sciences. RPMI1640 medium was purchased from Gibco (US). Fetal bovine serum (FBS) was purchased from Gemini Bio-Products (US). qRT-PCR SuperMix was purchased from TransGen Biotech (China). pcDNA3.1 was purchased from Thermo Fisher Scientific (US). Trizol and Lipofectamine 2000 were purchased from Invitrogen (US). miR-NC and miR-141 mimic were purchased from RioBio (China). EdU cell proliferation flow cytometry kit was purchased from Sigma (US). Rabbit anti-human polyclonal YAP1, β-catenin primary antibody, and HRP conjugated secondary antibody were purchased from Abcam (US). pGL3 plasmid and luciferase activity assay kit Dual-Glo Luciferase Assay System were purchased from Promega (US). RIPA lysis buffer, BeyoECL Plus chemiluminescent reagent and FITC Annexin V/PI apoptosis kit were purchased from Beyotime (China). PBS was purchased from Sangon (China). PBST was purchased from Duoji Bio (China). PVDF membrane was purchased from Jinsui Biotech (China). Other common reagents were purchased from Guoao Bio (China).
Clinical information
A total of 42 GC patients who received treatment in The Third Affiliated Hospital of Anhui Medical University (Hefei, Anhui, China) from September 2017 to January 2018 were recruited in this study. Tissue samples were collected during the surgery for GC and were confirmed by tissue pathology examination. There were 11, 16 and 15 cases for TNM stage I, stage II and stage III, respectively. Another cohort of 12 samples of gastric mucosal tissues collected from endoscope examination in The People’s Hospital of Guangxi Zhuang Autonomous Region (Nanning, Guangxi, China) were recruited as the control group.
This study was approved by ethics committee in The Third Affiliated Hospital of Anhui Medical University (Hefei, Anhui, China) and all the enrolled objects had signed informed consent.
Cell culture
SGC7901, MGC-803 and GES-1 cells were all incubated in RPMI1640 medium containing 10% FBS and 1% penicillin-streptomycin, in a 37°C incubator with 5% CO2 (model HERA cell 240i, Thermo, US). Cells were passed at 1:4 ratio, and those cells at log-growth phase were used for experiments.
Dual luciferase reporter gene assay
Cellular mRNA was extracted from HEK293T cells using Trizol kit following the manual instruction. Using mRNA of HEK293T cells as the template, full length or mutant form of 3’-UTR of YAP1 gene was sub-cloned into pGL3 plasmid for transforming DH5α competent cells. Sequencing was performed to screen out plasmid with correct insertion and these were named as pGL3-YAP1-WT or pGL3-YAP1-MUT. Lipofectamine 2000 was used to co-transfect pGL3-YAP1-WT (or pGL3-YAP1-MUT) and miR-141 mimic (or miR-NC) into HEK293T cells. After 48 h transfection, Dual-Glo Luciferase Assay System was used to measure relative luciferase activity.
YAP1 overexpression plasmid construction
YAP1 CDS region was amplified using genomic DNA of SGC7901 cells followed by digestion and connection into pcDNA3.1 vector, which was then transfected into JM109 cell and positive colone was selected and confirmed by gene sequencing, which was then named as pcDNA3.1-YAP1. pcDNA3.1-Blank was used as a control.
Cell transfection and grouping
Cultured SGC7901 and MGC-803 cells were divided into three transfection groups: miR-NC+pcDNA3.1-Blank transfection group and miR-141 mimic+pcDNA3.1-Blank transfection group and miR-141 mimic+pcDNA3.1-YAP1 transfection group.
Flow cytometry for cell proliferation
Cells from all transfection groups were digested by trypsin and were collected. Cells were re-suspended in RPMI 1640 complete medium containing 10% FBS. After incubation in 10 μM EdU for 2 h at 37°C, cells were seeded into culture plate for 48 h continuous incubation. Cells were then digested by trypsin and were collected, followed by centrifugation in PBS and washing. Cells were fixed in paraformaldehyde, centrifugated in PBS for washing, and were permeabilized by adding 100 μL buffer. 500 μL reaction buffer was added for 30 min dark incubation at room temperature. 3 mL permeabilization buffer was added for centrifugation and washing. 500 μL wash buffer was added for resuspension of cells, and proliferation was quantified on FC500MCL flow cytometry.
Flow cytometry for cell apoptosis
Transfected cells were digested by trypsin and collected. Following the manual instruction, 100 μL Annexin V Binding Buffer was added for resuspending cells. 5 μL FITC Annexin V and 10 μL PI were sequentially added. After 15 min room temperature incubation, 400 μL Annexin V Binding Buffer was added for measuring cell apoptosis on FC500MCL flow cytometry.
qRT-PCR for measuring gene expression
TransScript Green One-Step qRT-PCR SuperMix was used to measure relative expression level of target genes using RNA extracted by Trizol reagent. In a 20 μL reaction system, one added 1 μg template RNA, 0.2 μM forward and reverse primer, 10 μL 2XTransStar Tip Green qPCR SuperMix, 0.4 μL One-Step RT Enzyme Mix, 0.4 μL Passive Reference Dye II, and RNase-free water top to 20 μL. qRT-PCR conditions were: 45°C for 5 min and 94°C for 30 s, and 40 cycles each consisting of 94°C for 5 s and 60°C for 30 s. Gene expression was measured on a Bio-Rad CFX96 real-time fluorescent quantitative PCR cycler.
Western blot
RIPA approach was used to extract total proteins, which were quantified by BCA method. 40 μg protein samples were separated in SDS-PAGE (10% separating gel and 4% condensing gel), and were transferred to PVDF membrane (250 mA, 100 min). The membrane was blocked in PBST containing 5% skim milk powder for 60 min. Primary antibody (anti-YAP1 at 1:2000 dilution, and anti-β-actin at 1:5000 dilution) was added for 4°C overnight incubation. On the next day, the membrane was washed in PBST for three times, and HRP conjugated secondary antibody (1:10000 dilution) was added for 60 min room temperature incubation. The membrane was then washed in PBST for three times. BeyoECL Plus working solution A and B were mixed with equal volume and was added onto the membrane for development in dark for 2~3 min. The membrane was exposed, and the film was scanned for data analysis.
Statistical analysis
SPSS 18.0 was used for statistical analysis. Measurement data are presented as mean ± standard deviation (SD). Student t-test was used for comparison of measurement data between two groups. One-way analysis of variance (ANOVA) was used for comparison of measurement data among multiple groups, followed by Bonferroni post-hoc comparison. Mann-Whitney U comparison was used to compare miR-141 and YAP1 mRNA in tissues. Spearman approach was used to analyze the correlation between miR-41 and YAP1 mRNA expression in GC tissues. Statistical significance was set at P<0.05.
Results
Abnormal expression of miR-141 and YAP1 in GC tissues
qRT-PCR results showed significantly decreased miR-141 expression in TNM stage II GC tissues comparing to controlled tissues, and TNM stage IV GC tissues had further decreased miR-141 expression than TNM stage III tissues. No significant difference was found in miR-141 expression between TNM stage II and stage III GC tissues (Figure 1A). qRT-PCR results also showed markedly increased YAP1 mRNA expression in GC tissues comparing to normal gastric mucosal tissues. TNM stage III tissues had significantly higher YAP1 than those in TNM stage II tumor tissues, while TNM stage IV showed similar YAP1 mRNA expression with those in stage III (Figure 1B). Spearman rank correlation analysis showed significantly negative correlation between miR-141 and YAP1 mRNA expression in GC tissues (r=-0.623, P<0.001, Figure 1C). Western blot results showed significantly higher YAP1 protein expression than normal group, and even higher YAP1 protein expression in tumors with more advanced TNM stage (Figure 1D).
Figure 1.
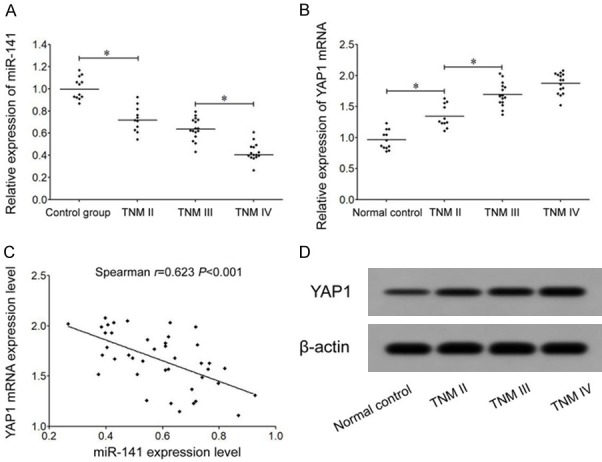
Abnormal expression of miR-141 and YAP1 in GC tissues. A. qRT-PCR assay for miR-141 expression in GC tissues. B. qRT-PCR for YAP1 mRNA expression in GC tissues. C. Spearman analysis for the correlation between miR-141 and YAP1 mRNA expression. D. Western blot for YAP1 protein expression in GC tissues. *, P<0.05 compared between two groups.
miR-141 down-regulation and YAP1 up-regulation in GC cells
qRT-PCR results showed that, compared to normal gastric mucosal epithelial cell cline GES-1, GC cell lines SGC7901 and MGC803 showed significantly lower miR-141 (Figure 2A) and higher YAP1 mRNA level (Figure 2B). Western blot results showed that compared to GES-1 cell, GC cell lines SGC7901 and MGC803 had elevated YAP1 protein expression (Figure 2C).
Figure 2.
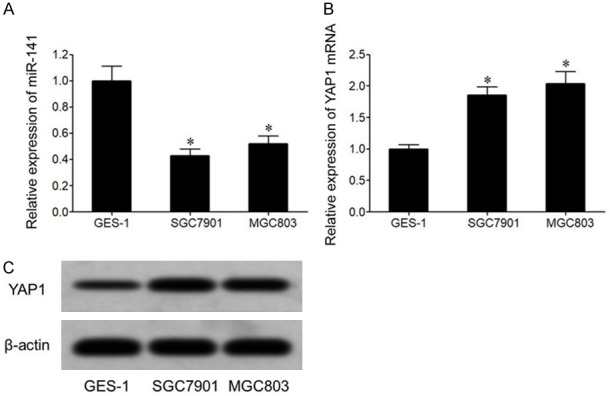
miR-141 down-regulation and YAP1 up-regulation in GC cells. A. qRT-PCR for miR-141 expression in GC cells. B. qRT-PCR for YAP1 mRNA expression in GC cells. C. YAP1 protein expression in GC cells. *, P<0.05 compared to GES-1 cells.
Targeted regulation between miR-141 and YAP1
Online prediction of microRNA.org showed the existence of complementary binding sites between miR-141 and 3’-UTR of YAP1 mRNA (Figure 3A). Dual luciferase gene reporter assay showed that transfection of miR-141 mimic significantly decreased relative luciferase activity in HEK293T cells transfected with pGL3-YAP1-WT plasmid but did not affect relative luciferase activity in HEK293T cells transfected with pGL3-YAP1-MUT plasmid (Figure 3B). These results showed a targeted regulatory relationship between miR-141 and YAP1 mRNA.
Figure 3.
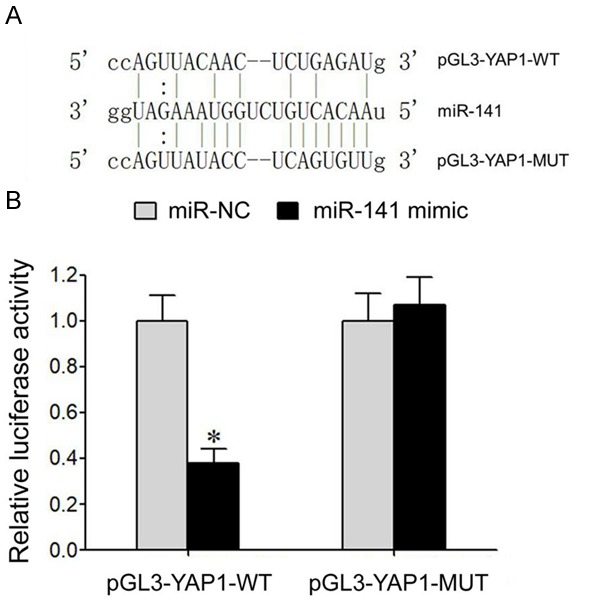
Targeted regulation between miR-141 and YAP1 mRNA. A. Binding sites between miR-141 and 3’-UTR of YAP1 mRNA. B. Dual luciferase gene reporter assay. *, P<0.05 compared to miR-NC group.
Overexpression of miR-141 inhibits YAP1 expression or GC cell proliferation and facilitates cell apoptosis
qRT-PCR results showed that miR-141 mimic transfection significantly suppressed YAP1 mRNA expression in SGC7901 and MGC803 cells, and overexpression of YAP1 significantly upregulated YAP1 mRNA expression (Figure 4A). Western blot results showed that miR-141 mimic transfected SGC7901 and MGC803 cells showed remarkably depressed YAP1 protein expression and YAP1 overexpression significantly increased YAP1 protein expression (Figure 4B). Flow cytometry results showed that transfection of miR-141 mimic remarkably increased apoptosis of SGC7901 and MGC803 cells (Figure 4C) but suppressed proliferation potency (Figure 4D). However, YAP1 overexpression decreased the effect of miR-141 mimic on cell proliferation and apoptosis.
Figure 4.
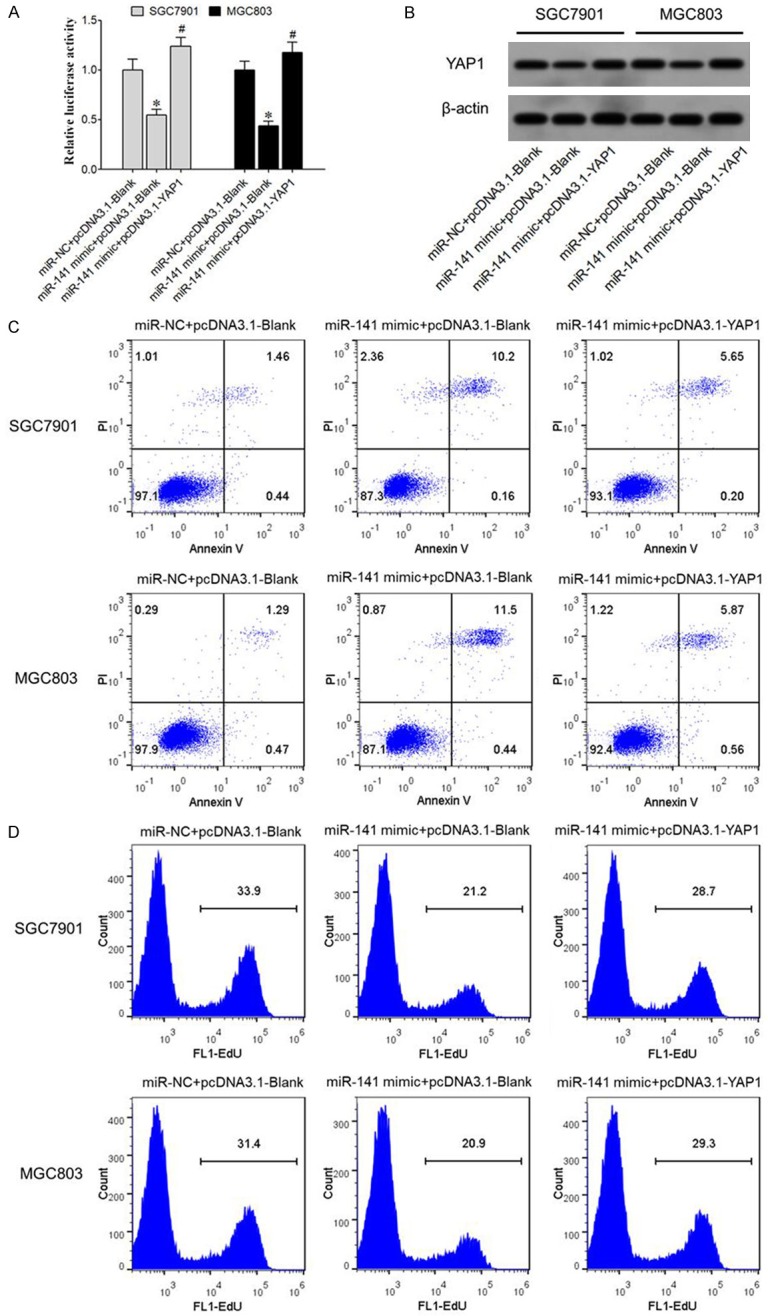
Over-expression of miR-141 inhibits YAP1 expression or GC cell proliferation and facilitates cell apoptosis. A. qRT-PCR for YAP1 mRNA expression; B. Western blot for YAP1 protein expression; C. Flow cytometry for cell apoptosis; D. Flow cytometry for cell proliferation. *, P<0.05 compared to miR-NC group.
Discussion
GC has insidious pathogenesis, as patients do not present prominent comfortability or symptoms at early stage. When having clinical symptoms and receiving medical examination, most patients are already at advanced stage with high malignancy and lower sensitivity for chemo- or radio-therapy, plus a higher incidence of distal metastasis, all of which contribute to higher treatment difficulty, lower efficiency, decreased survival rate, and unfavorable prognosis [1-3]. Therefore, the early detection of pathogenic molecules during GC progression is of critical importance for investigating GC pathogenesis mechanism, improving treatment efficiency and improving prognosis.
YAP1 gene localizes to human chromosome 11q13 and encodes one protein with 65 KD size [13]. YAP1 can specifically recognize and bind with nuclear transcription factors containing PPXY sequence via its WW structural domain, thus modulating transcription and expression of various genes, facilitating cell proliferation, inhibiting apoptosis, inducing contact disinhibition, and accelerating malignant transformation [13,14]. Previous studies have shown abnormal elevation of YAP1 expression in various tumors including prostate cancer [15], pancreatic carcinoma [16], and gallbladder cancer [17]. It is known that YAP1 expression and functional activity in GC tumor tissues are potentiated, and are related with tumor invasion, progression, and prognosis [18,19], indicating the oncogenic role of YAP1 in onset and progression of GC. miR-141 is one widely studied microRNA molecule, and is correlated with pathogenesis, progression and prognosis of various tumors including prostate cancer, colon cancer, and breast cancer [20-22]. Studies have shown very decreased miR-141 expression in GC tumor tissues and cells [11,12], suggesting that miR-141 might be a tumor suppressor in GC onset. Bioinformatics analysis showed the existence of complementary binding sites between miR-141 and YAP1 mRNA, suggesting potentially regulatory effects. This study thus investigated if miR-141 played a role in mediating YAP1 expression and affecting GC cell proliferation or apoptosis.
Results of this study showed significantly decreased miR-141 expression in GC tumor tissues comparing to normal gastric mucosal tissues. With more advanced TNM stage, the expression of miR-141 was further decreased, accompanied by elevated YAP1 mRNA and protein expression which was associated with TNM stage. Results of in vitro cultured cells showed significantly lower miR-141 expression in SGC7901 and MGC803 cells comparing to normal gastric mucosal epithelial cells GES-1, plus higher YAP1 expression. Results indicated miR-141 down-regulation might play a role in elevating YAP1 expression and facilitating GC onset. Dual luciferase gene reporter assay showed that transfection of miR-141 significantly decreased relative luciferase activity in HEK293T cells with pGL3-YAP1-WT transfection, indicating targeted regulatory relationship between miR-141 and YAP1-mRNA. Correlation analysis showed a significantly negative relationship between miR-141 and YAP1 expression level. In previous studies of the relationship between miR-141 and GC, Huang et al showed that comparing to tumor adjacent tissues, GC tumor tissues had significantly lower miR-141 expression by 50%, and those with relatively lower miR-141 expression had prominently lower survival rate than those patients having higher miR-141 expression [23]. Chen et al showed that compared to normal gastric tissues, GC tissues had abnormally decreased miR-141 expression, and compared to GES-1 cells, GC cells SGC7901, NUGC-3 and MKN45 cells also presented with significantly decreased miR-141 expression [11]. Lu et al found that, compared to normal gastric mucosal tissues, GC tissues showed abnormally decreased miR-141 expression, which was associated with cell differentiation grade, lymph node metastasis, distal metastasis and TNM stage [12]. Zhou et al found significantly lower miR-141 expression in GC tumor tissues than adjacent tissues, and GC cell lines MKN45 and SGC7901 all had much lower miR-141 expression than GES-1 cells [24]. All these results supported a possible role of miR-141 as the suppressor factor for GC, while miR-141 down-regulation is the adverse factor for GC pathogenesis, supporting results from our study. In a study of the correlation between YAP1 and GC, Yu et al showed that compared to normal gastric tissues, GC tissues had significantly higher YAP1 expression, which was associated with depth of tumor invasion and lymph node metastasis, as those patients with relatively lower YAP1 expression presented significantly lower survival rate than those with higher YAP1 expression (HR=1.48, P<0.00033) [18]. Deng et al found that YAP1 up-regulation was associated with GC pathogenesis, and over-expression of YAP1 can facilitate proliferation and invasion of GC cells SGC7901 [25]. Sun et al found that compared to tumor adjacent tissues, GC tissues had significantly higher YAP1 expression, which was related with lymph node metastasis, distal metastasis and TNM stage, and comparing to GES-1 cells, GC cell lines BGC823 and SGC7901 showed significantly elevated YAP1 expression [19], which can be supported by results from this study.
Due to the regulatory relationship between miR-141 and YAP1, and the correlation between abnormal expression of those two genes and GC onset, we thus further investigated the role of miR-141 and YAP1 in affecting GC cell proliferation and apoptosis in this study. Results showed that over-expression of miR-141 can markedly weaken proliferation potency of cells and enhance apoptosis by inhibiting intracellular YAP1 expression, suggesting that miR-141 could exert anti-tumor effects via targeting YAP1. In a study between miR-141 and GC cell biology , Huang et al found that over-expression of miR-141 could inhibit GC cell proliferation and clonal formation, induce cell cycle arrest, and weaken in vivo growth and tumorigenicity of GC cells in animal models via modulating IGF1R expression [23]. Chen et al further found that over-expression of miR-141 could suppress proliferation of in vitro cultured GC cells SGC7901 and MKN45 and weaken migration or invasion potency of cells, and the tumor suppressor effect of miR-141 is mainly achieved via targeted inhibition on HDGF expression [11]. Zhou et al found that elevated miR-141 expression in GC cell lines MKN45 and SGC701 can target and inhibit E2F3 gene expression, inhibit tumor cell proliferation, and induce cell cycle arrest and cell apoptosis [24]. Du et al found the targeted regulation between miR-141 and ZEB2 gene in GC cells SGC7901 and HGC27, and elevation of miR-141 markedly inhibited migration potency of GC cells [26]. Zuo et al found that elevation of miR-141 expression could significantly inhibit target gene ZAT expression and inhibit GC cell proliferation, migration or invasion [27]. These studies suggest a role of miR-141 in alleviating malignant biological properties of GC cells, similar to our studies.
Currently although various studies have supported the role of miR-141 in GC onset, no relationship of miR-141 for regulating YAP1 in GC pathogenesis has been reported. This study for the first time made the connection between miR-141 and YAP1, revealing the role of miR-141 in targeting YAP1 expression and affecting GC cell proliferation and apoptosis. However, the biological effect of miR-141-YAP1 regulatory axis in vivo has not been illustrated, and further animal studies plus clinical trials are required for substantiation.
Conclusion
Down-regulation of miR-141 and up-regulation of YAP1 are correlated with GC pathogenesis. miR-141 can inhibit GC cell proliferation and induce cell apoptosis by targeted inhibition of YAP1 expression.
Disclosure of conflict of interest
None.
References
- 1.Habibi D, Rafiei M, Chehrei A, Shayan Z, Tafaqodi S. Comparison of survival models for analyzing prognostic factors in gastric cancer patients. Asian Pac J Cancer Prev. 2018;19:749–753. doi: 10.22034/APJCP.2018.19.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orman S, Cayci HM. Gastric cancer: factors affecting survival. Acta Chir Belg. 2018:1–7. doi: 10.1080/00015458.2018.1453437. [DOI] [PubMed] [Google Scholar]
- 3.Goshayeshi L, Hoseini B, Yousefli Z, Khooie A, Etminani K, Esmaeilzadeh A, Golabpour A. Predictive model for survival in patients with gastric cancer. Electron Physician. 2017;9:6035–6042. doi: 10.19082/6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Zhou M, Wei H, Zhou H, He J, Lu Y, Wang D, Chen B, Zeng J, Peng W, Du F, Gong A, Xu M. Furin promotes epithelial-mesenchymal transition in pancreatic cancer cells via Hippo-YAP pathway. Int J Oncol. 2017;50:1352–1362. doi: 10.3892/ijo.2017.3896. [DOI] [PubMed] [Google Scholar]
- 5.He C, Mao D, Hua G, Lv X, Chen X, Angeletti PC, Dong J, Remmenga SW, Rodabaugh KJ, Zhou J, Lambert PF, Yang P, Davis JS, Wang C. The Hippo/YAP pathway interacts with EGFR signaling and HPV oncoproteins to regulate cervical cancer progression. EMBO Mol Med. 2015;7:1426–49. doi: 10.15252/emmm.201404976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma Y, Yang Y, Wang F, Wei Q, Qin H. Hippo-YAP signaling pathway: a new paradigm for cancer therapy. Int J Cancer. 2015;137:2275–2286. doi: 10.1002/ijc.29073. [DOI] [PubMed] [Google Scholar]
- 7.Lv X, Li CY, Han P, Xu XY. MicroRNA-520a-3p inhibits cell growth and metastasis of non-small cell lung cancer through PI3K/AKT/mTOR signaling pathway. Eur Rev Med Pharmacol Sci. 2018;22:2321–2327. doi: 10.26355/eurrev_201804_14822. [DOI] [PubMed] [Google Scholar]
- 8.Zhao R, Liu Q, Lou C. MicroRNA-299-3p regulates proliferation, migration and invasion of human ovarian cancer cells by modulating the expression of OCT4. Arch Biochem Biophys. 2018;651:21–27. doi: 10.1016/j.abb.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Pardini B, Cordero F, Naccarati A, Viberti C, Birolo G, Oderda M, Di Gaetano C, Arigoni M, Martina F, Calogero RA, Sacerdote C, Gontero P, Vineis P, Matullo G. microRNA profiles in urine by next-generation sequencing can stratify bladder cancer subtypes. Oncotarget. 2018;9:20658–20669. doi: 10.18632/oncotarget.25057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, Yang L, Guo X, Jin G, Wang Q, Lv D, Liu J, Chen Q, Song Q, Li B. Sevoflurane suppresses proliferation by upregulating microRNA-203 in breast cancer cells. Mol Med Rep. 2018;18:455–460. doi: 10.3892/mmr.2018.8949. [DOI] [PubMed] [Google Scholar]
- 11.Chen B, Huang T, Jiang J, Lv L, Li H, Xia S. miR-141 suppresses proliferation and motility of gastric cancer cells by targeting HDGF. Mol Cell Biochem. 2014;388:211–8. doi: 10.1007/s11010-013-1912-3. [DOI] [PubMed] [Google Scholar]
- 12.Lu YB, Hu JJ, Sun WJ, Duan XH, Chen X. Prognostic value of miR-141 downregulation in gastric cancer. Genet Mol Res. 2015;14:17305–11. doi: 10.4238/2015.December.16.31. [DOI] [PubMed] [Google Scholar]
- 13.Jerhammar F, Johansson AC, Ceder R, Welander J, Jansson A, Grafstrom RC, Soderkvist P, Roberg K. YAP1 is a potential biomarker for cetuximab resistance in head and neck cancer. Oral Oncol. 2014;50:832–9. doi: 10.1016/j.oraloncology.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Song S, Honjo S, Jin J, Chang SS, Scott AW, Chen Q, Kalhor N, Correa AM, Hofstetter WL, Albarracin CT, Wu TT, Johnson RL, Hung MC, Ajani JA. The hippo coactivator YAP1 mediates EGFR overexpression and confers chemoresistance in esophageal cancer. Clin Cancer Res. 2015;21:2580–90. doi: 10.1158/1078-0432.CCR-14-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collak FK, Demir U, Ozkanli S, Kurum E, Zerk PE. Increased expression of YAP1 in prostate cancer correlates with extraprostatic extension. Cancer Biol Med. 2017;14:405–413. doi: 10.20892/j.issn.2095-3941.2017.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang H, Xiong G, Shen P, Cao Z, Zheng L, Zhang T, Zhao Y. MicroRNA-1285 inhibits malignant biological behaviors of human pancreatic cancer cells by negative regulation of YAP1. Neoplasma. 2017;64:358–366. doi: 10.4149/neo_2017_306. [DOI] [PubMed] [Google Scholar]
- 17.Li S, Yu Z, Chen SS, Li F, Lei CY, Chen XX, Bao JM, Luo Y, Lin GZ, Pang SY, Tan WL. The YAP1 oncogene contributes to bladder cancer cell proliferation and migration by regulating the H19 long noncoding RNA. Urol Oncol. 2015;33:427, e1–10. doi: 10.1016/j.urolonc.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Yu L, Gao C, Feng B, Wang L, Tian X, Wang H, Ma D. Distinct prognostic values of YAP1 in gastric cancer. Tumour Biol. 2017;39:1010428317695926. doi: 10.1177/1010428317695926. [DOI] [PubMed] [Google Scholar]
- 19.Sun D, Li X, He Y, Li W, Wang Y, Wang H, Jiang S, Xin Y. YAP1 enhances cell proliferation, migration, and invasion of gastric cancer in vitro and in vivo. Oncotarget. 2016;7:81062–81076. doi: 10.18632/oncotarget.13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding L, Yu LL, Han N, Zhang BT. miR-141 promotes colon cancer cell proliferation by inhibiting MAP2K4. Oncol Lett. 2017;13:1665–1671. doi: 10.3892/ol.2017.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li JZ, Li J, Wang HQ, Li X, Wen B, Wang YJ. MiR-141-3p promotes prostate cancer cell proliferation through inhibiting kruppel-like factor-9 expression. Biochem Biophys Res Commun. 2017;482:1381–1386. doi: 10.1016/j.bbrc.2016.12.045. [DOI] [PubMed] [Google Scholar]
- 22.Debeb BG, Lacerda L, Anfossi S, Diagaradjane P, Chu K, Bambhroliya A, Huo L, Wei C, Larson RA, Wolfe AR, Xu W, Smith DL, Li L, Ivan C, Allen PK, Wu W, Calin GA, Krishnamurthy S, Zhang XH, Buchholz TA, Ueno NT, Reuben JM, Woodward WA. miR-141-mediated regulation of brain metastasis from breast cancer. J Natl Cancer Inst. 2016:108. doi: 10.1093/jnci/djw026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang M, Wu L, Qin Y, Li Z, Luo S, Qin H, Yang Y, Chen J. Anti-proliferative role and prognostic implication of miR-141 in gastric cancer. Am J Transl Res. 2016;8:3549–3557. [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou X, Ji G, Ke X, Gu H, Jin W, Zhang G. MiR-141 inhibits gastric cancer proliferation by interacting with long noncoding RNA MEG3 and down-regulating E2F3 expression. Dig Dis Sci. 2015;60:3271–3282. doi: 10.1007/s10620-015-3782-x. [DOI] [PubMed] [Google Scholar]
- 25.Deng J, Lei W, Xiang X, Zhang L, Yu F, Chen J, Feng M, Xiong J. MicroRNA-506 inhibits gastric cancer proliferation and invasion by directly targeting Yap1. Tumour Biol. 2015;36:6823–6831. doi: 10.1007/s13277-015-3364-8. [DOI] [PubMed] [Google Scholar]
- 26.Du Y, Wang L, Wu H, Zhang Y, Wang K, Wu D. MicroRNA-141 inhibits migration of gastric cancer by targeting zinc finger E-box-binding homeobox 2. Mol Med Rep. 2015;12:3416–3422. doi: 10.3892/mmr.2015.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuo QF, Zhang R, Li BS, Zhao YL, Zhuang Y, Yu T, Gong L, Li S, Xiao B, Zou QM. MicroRNA-141 inhibits tumor growth and metastasis in gastric cancer by directly targeting transcriptional co-activator with PDZ-binding motif, TAZ. Cell Death Dis. 2015;6:e1623. doi: 10.1038/cddis.2014.573. [DOI] [PMC free article] [PubMed] [Google Scholar]


