Abstract
Homo sapiens metastasis associated lung adenocarcinoma transcript 1 (LncRNA MALAT1) plays an important role in many types of cancer, but its role in human lung adenocarcinoma (LAC) is still unclear. In this paper, we found that LncRNA MALAT1 had high expression in human LAC tissues (vs. paracancerous normal tissue) and human lung adenocarcinoma cells (vs. human normal lung tissue cells). The expression of lncRNA MALAT1 was significantly associated with human lung adenocarcinoma tumor size, lymph node metastasis, and TNM staging, and was negatively correlated with miR-429 expression in lung adenocarcinoma tissues. In vitro, LncRNA MALAT1 could block human LAC cells in the G1 phase to inhibit proliferation by reducing the expression of cyclin D1 protein. LncRNA MALAT1 could inhibit the invasion and migration of human LAC cells by decreasing the expression of MMP-9 and vimentin and increasing the expression of E-cadherin. We also found that Malat1 functions as a competing endogenous RNA (ceRNA) for miR-429 and directly suppressed the expression of RhoA protein. RhoA knockout and transfection of miR-429-mimic could play the same function which is to decrease the expression of cyclin D1, MMP-9, and vimentin proteins and increased E-cadherin protein expression. These results suggested that LncRNA Malat1 could promote the proliferation and EMT of human lung adenocarcinoma cells by competing with RhoA for binding to miR-429.
Keywords: LncRNA, MALAT1, miR-429, RhoA
Introduction
Since the 1980s, lung cancer has gradually taken on the highest rates of morbidity and mortality in the world, and its incidence and death rate have been rising year by year. According to the 2016 American Cancer Society survey statistics, the average mortality rate of lung cancer in male and female patients was 27% and 26%, respectively [1]. The incidence and mortality of lung cancer in China alsoranked first among all cancers, and became the deadliest cancer [2,3]. Among lung cancer patients, small cell lung cancer (SCLC) accounted for about 15%, and non-small cell lung cancer (NSCLC) accounted for about 80%, among which lung adenocarcinoma accounted for the largest proportion.
Long non-coding RNA (lncRNA) plays an important role in the development of various cancers, and it had become a new hot spot in tumor research due to its significant tumor tissue specificity and its potential for application in tumor diagnosis and prognosis prediction [4,5]. lncRNA is a type of RNA that is over 200 bp in length, has no or little open reading frame, and can rarely encode a protein. It is usually composed of multiple exons spliced, transcribed by RNA polymerase II, with a histone modification similar to the encoded protein [6,7]. In recent years, more and more lncRNAs have been found closely related to the development of tumors [6,8], and studies have found that lncRNAs can be used as promoters of certain tumors [9,10].
Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), also named as nuclear-enriched autosomal transcript 2 (NEAT2), is an important member of the lncRNA family and was discovered in NSCLC tissue at 2003 [9]. Many studies have found that MALAT1 was abnormally expressed in multiple tumor tissues [11,12]. Previous researchers found that MALAT1 could promote the proliferation, metastasis and invasion of tumor cells [12,13] by specific recruitment of SR protein family members [14,15], involved in epigenetic regulation [16,17] and regulating the cell cycle [18,19]. In this paper, we present a novel molecular mechanism that MALAT1 promoted human lung adenocarcinoma cell proliferation and EMT, namely LncRNA MALAT1 could promote the proliferation and EMT of human lung adenocarcinoma cells by competing with RhoA for binding to miR-429.
Materials and methods
Patient and tissue specimens
Thirty-nine cases of lung adenocarcinoma tumor tissues and normal tissues (>5 cm away from tumor tissue) were collected at General Hospital of Guangzhou Military Command of PLA. 22 males and 17 females, aged 45-78 years. Patients with distant metastases of cancer cells and any treatment before surgery were excluded. All clinical specimens were removed by surgery, and parts of the tissue were frozen and stored in liquid nitrogen. The remaining tissues were fixed in formalin and paraffin sections were prepared. Consent from all patients was obtained, and the study methodologies met the standard set by the Declaration of Helsinki and were approved by the Ethics committee of General Hospital of Guangzhou Military Command of PLA.
Cells and cell culture
Human normal lung epithelial cells (BEAS-2B) (CRL-9609, ATCC, VA, USA), human bronchial epithelioid cells (HBE) (FS-0400, Shanghai Fusheng Industrial Co., Ltd., Shanghai, China), human alveolar epithelial cells (HPAEpiC) (NCI-H1650, Shanghai North Connaught Biological Technology Co., Ltd., Shanghai, China); A549 (CRM-CRL-185, ATCC, VA, USA), H1299 (CRL-5803, ATCC, VA, USA), SPC-A-1 (SX-0879, Shanghai Sixin Biotechnology Co., Ltd., Shanghai, China) and PG49 (Y-02117, Shanghai Fuheng Biological Technology Co., Ltd., Shanghai, China) were human lung adenocarcinoma cells. All normal lung cells and lung adenocarcinoma cells were cultured with DMEM medium (12491-15, Thermofisher, CA, USA) to which was added 10% of fetal bovine serum (10100-147, Thermofisher, CA, USA) and 1% penicillin-streptomycin (15640055, Thermofisher, CA, USA).
Cell transfection
si-NC, si-MALAT1-1, si-MALAT1-2, WT-MALAT1, MUT-MALAT1, WT-RhoA and MUT-RhoA were designed and synthesized by Shenggong Bioengineering Co., Ltd. (Shanghai, China) si-NC, si-MALAT1-1 or si-MALAT1-2 were directly transferred into cells by Lipofectamine™ 2000 transfection reagent (11668019, Invitrogen, CA, USA), but WT-MALAT1, MUT-MALAT1, WT-RhoA or MUT-RhoA were first integrated into the pmirGLO plasmid (49380, Addgene, CA, USA) and then transferred to cells.
RhoA knockout
CRISPR-Case9 technique was used to knockout the gene of RhoA in A549. The primer sequence of gRNA in lentiCRISPRv2 vector (98291, Addgene, CA, USA) was designed as shown in Table 1. The lentiCRISPRv2 plasmid harboring the gDNA sequence and Cas9 gene, psPAX2 plasmid (12260, Addgene, CA, USA) and pMD2.G plasmid (12259, Addgene, CA, USA) were co-transfected into HEK-293T cells by HG-Trans293TM transfection reagent (TG-10014-1, Healthgene, NingPo, China). Virus-containing supernatants were collected 72 h after transfection and were added into A549 cells with 8 ug/mL ploybrene (sc-134220, SANTA CRUZ, MA, USA), and puromycin (A1113802, ThermoFisher, CA, USA) was used to select puromycin resistant cells.
Table 1.
siRNA, oligo and PCR primer sequences
| Name | Sequence (5’-3’) |
|---|---|
| si-MALAT1-1 | F: CACAGGGAAAGCGAGTGGTTGGTAA |
| R: TTACCAACCACTCGCTTTCCCTGTG | |
| si-MALAT1-2 | F: GAGGUGUAAAGGGAUUUAUTT |
| R: AUAAAUCCCUUUACACCUCTT | |
| MALAT1 | F: ATGCGAGTTGTTCTCCGTCT |
| R: TATCTGCGGTTTCCTCAAGC | |
| miR-429 | F: ACACTCCAGCTGGGTAATACTGTCTGGTAA |
| R: TGGTGTCGTGGAGTCG | |
| RhoA gRNA | F: CACCGGCTCGAGCCGGCACAGGTCG |
| R: AAACCGACCTGTGCCGGCTCGAGCC | |
| U6 | F: AUAAAUCCCUUUACACCUCTT |
| R: AAUAAAUCCCUUUACACCUCTT | |
| GAPDH | F: GATGAACCTAAGCTGGGACCC |
| R: TGTGAACGGATTTGGCCGTA |
Flow cytometric analysis
We collected cells that haD been treated in different ways and added 70% pre-cooled ethanol (pre-chilled PBS and water-free configuration) at 4 degrees overnight, then washed with PBS, and stained with PI. MACSQuant® Analyzer 10 Flow cytometry (Miltenyi Biotec, German) was used to detect cell cycle.
Real-time fluorescence quantitative PCR
Trizol was used to extract the total RNA of the tissue or cells. The extracted RNA was reverse transcribed into cDNA by using Prime Script™ RT Master Mix reverse transcription kit (RR036B, Takara, Beijing, China). PCR parameters set: 37°C/60 minutes, 85°C/5 seconds. 20 μl Real-time fluorescence quantitative PCR (RT-qPCR) system was prepared according to the SYBR Green qPCR Master Mix kit instructions (638320, TakaRa, Beijing, China) and amplified using ABI 7500 fluorescence quantitative PCR instrument (Applied Biosystems, Maryland, USAA). PCR parameters set: 95°C/30 s, [90°C/5 s, 65°C/30 s] -40 cycles.
Western blot
Tissue or cell lysates were separated by SDS-page and then transferred to PVDF membrane. Primary antibodis were selected as follows: anti-cyclin D1 (ab134175, 1:5000), or anti-MMP-9 (ab73734, 1:1000), or anti-Vimentin (ab8978, 1:500), or anti-E-Cadherin (ab76055, 1:500), or anti-RhoA (ab187027, 1:2000), or anti-GAPDH (ab9484, 1:3000). Secondary antibody was selected as follows: goat anti-rabbit (ab150077, 1:1000), or goat anti-rat (ab150117, 1:1000). Primary antibody was incubated overnight at 4°C and second antibody was incubated for 1 hour at room temperature.
Transwell
A transwell experiment was used to determine migration and invasion ability of cells. Matrigel (BD354248, Becton Dickinson, USA) stored at -20°C was thawed on ice overnight at 2-8°C. 100 μl of Matrigel was pipetted into ice-cold 300 μl serum-free medium with ice-cold pipette and mixed well. 25 μl of the above diluted Matrigel was added to the Transwell plate upper chamber (Costar, USA) and the entire polycarbonate film was coated at 37°C for 30 min to polymerize Matrigel into gel. Cells were digested with trypsin (Gbico, USA), washed with PBS and resuspended in serum-free DMEM medium (D6046, Sigma Aldrich, USA). The cell density was adjusted to 0.5 × 106 cells/ml and then cells were added to 24-well Transwell upper chamber (Corning, USA). Media containing 20% FBS (Hyclone, USA) was added into Transwell lower chamber and Transwell was incubated at 37°C for 24 h; Transwell was taken out and medium was removed, washed twice with PBS, added to methanol, and dried after fixed for 30 minutes. After membrane was dried, it was stained with crystal violet for 20 minutes, and the relative migration was determined by measuring the absorbance at 595 nm.
Cell scratch test
5 × 105 cells were placed in a 6-well plate with 2 ml/well. A scratch was obtained as far as possible perpendicular to the back of horizontal line by using tips against ruler, and tips should be vertical and cannot be tilted. Cells were washed with PBS for three times and scratched cells were removed, and serum-free DMEM medium was added. Cells were cultured at 37°C in 5% CO2 incubator for 24 h, and pictures were taken.
MTT assay
We inoculated 2 × 103 cells/well in a 96-well culture plate containing the indicator medium (DMEM medium plus 10% FBs). We evaluated the viability of PG49 and A549 cells at five time points (on days 1, 2, 3, 4 and 5), and MTT was used to quantify mitochondrial dehydrogenase activity. In short, after 4 hours MTT (10 μl, 10 mg/ml) was added to the cells and incubated, cell supernatant was removed and then 100 ul DMSO was added. After 0.5 hour, optical density (OD) using a plate reader (ELx808 Bio-Tek Instruments, City, ST, USA) to detect.
Statistical analysis
Data are presented in (mean ± standard deviation) and analyzed by SPSS 20.0. Student’s t-test or chi-square test was used to compare differences between two groups. Kaplan-Meier analysis was used to generate the survival plots and the significance of the differences was assessed by log-rank test. P<0.05 indicates a significant difference.
Results
LncRNA MALAT1 upregulated in LAC tissue and cell
RT-qPCR was used to measure the expression of LncRNA MALAT1, and as shown in Figure 1. LncRNA MALAT1 had high expression in human LAC tissues (vs. paracancerous normal tissues, t=16.387, P<0.001) and human LAC cells (H1299, SPC-A-1, PG49 and A549 vs. HBE, BEAS-2B and HPAEpiC).
Figure 1.
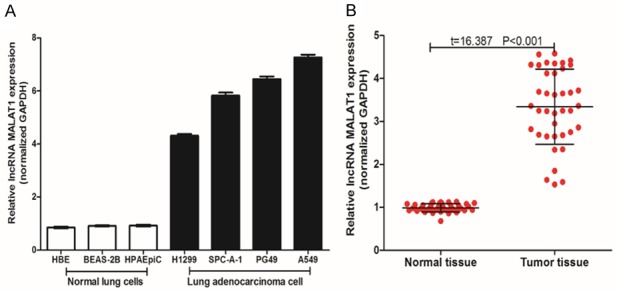
LncRNA MALAT1 is highly expressed in LAC tissue and cells. A. The expression of lncRNA MALAT1 in human normal lung cells and lung adenocarcinoma cells (three independent experiments); B. The expression of lncRNA MALAT1 lung adenocarcinoma tissue and normal tissue (n=39).
39 LAC patients were divided into two groups depending on the ratio of the expression of LncRNA MALAT1 in LAC tissues to that in normal tissues: 19 cases for low expression of MALAT1 (the ratio < the median value of 39 LAC patients) and 20 cases for high expression of MALAT1 (the ratio ≥ the median value of 39 LAC patients), as shown in Table 2. We found the expression of MALAT1 was not associated with age or sex in patients with LAC, but was significantly associated with T staging, N staging, and TNM staging in LAC patients. In addition, the 2-year survival of patients with low expression of LncRNA MALAT1 LAC was significantly higher than that of LncRNA MALAT1 high expression in lung adenocarcinoma (Long rank =4.773, P=0.0289) (Figure 2).
Table 2.
Correlation of MALAT1 expression and clinicopathologic features in 39 cases of LAC patients (n)
| Variable | Case | IncRNA MALAT1 | χ2 | P | |
|---|---|---|---|---|---|
|
| |||||
| Low expression | High expression | ||||
| Gender | |||||
| Male | 22 | 12 | 10 | 0.686 | 0.408 |
| Female | 17 | 7 | 10 | ||
| Age/year | |||||
| <60 | 25 | 14 | 11 | 1.478 | 0.224 |
| ≥60 | 14 | 5 | 9 | ||
| TNM stage | |||||
| I | 15 | 11 | 4 | 9.234 | 0.010 |
| II | 10 | 5 | 5 | ||
| III | 14 | 3 | 11 | ||
| T stage | |||||
| T1-T2 | 18 | 14 | 4 | 11.299 | 0.001 |
| T3-T4 | 21 | 5 | 16 | ||
| N stage | |||||
| N0 | 15 | 11 | 4 | 5.912 | 0.015 |
| N1-N3 | 24 | 8 | 16 | ||
Figure 2.
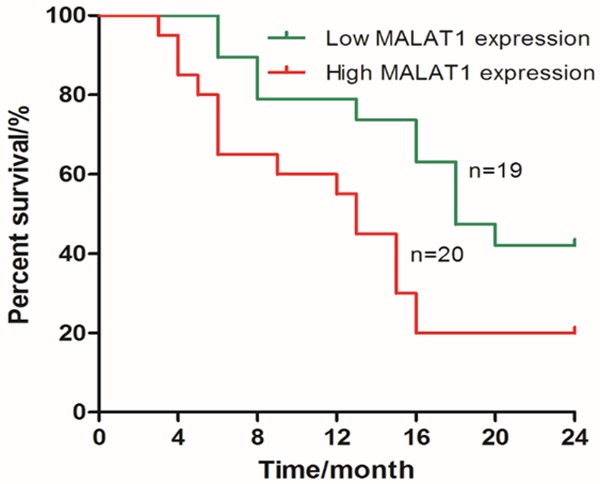
High MALAT1 expression reduced 2-year overall survival in patients with lung adenocarcinoma.
LncRNA MALAT1 knockdown suppressed the proliferation of LAC cells
To evaluate the effect of LncRNA MALAT1 on LAC cell proliferation, we inhibited MALAT1 in human lung adenocarcinoma cells PG49 and A549 by si-MALAT1-1 or si-MALAT1-2, and RT-qPCR was used to confirm this, as shown in Figure 3A. The cell growth of PG49 and A549 were monitored by MTT assays after LncRNA MALAT1 knockdown in PG49 and A549, and we found that (Figure 3B, 3C) the proliferation of PG49 and A549 after LncRNA MALAT1 knockdown were significantly suppressed (P<0.05).
Figure 3.
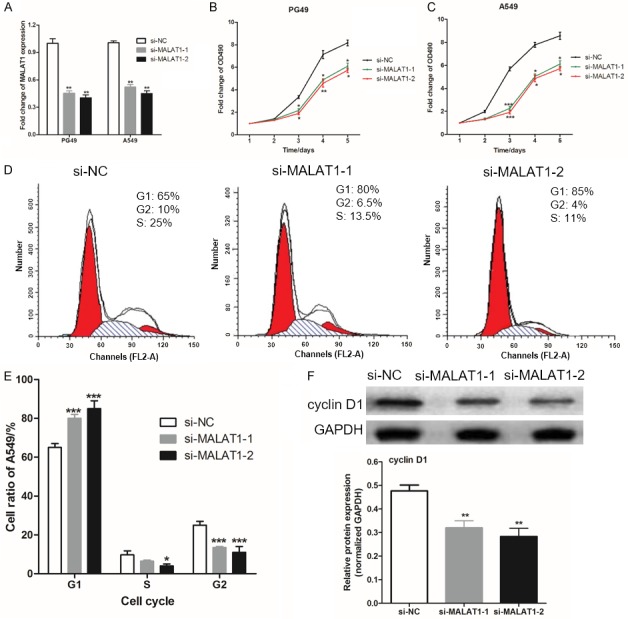
Changes toproliferation and cell cycle after lncRNA MALAT1 knockdown in LAC cells. A. RT-qPCR was used to detect the expression of MALAT1 in LAC cells; B, C. The cell growth of PG49 and A549 were monitored by MTT assays; D, E. cell cycle of A549 was detected by flow cytometry; F. Western blotting was used to measure the protein expression of cyclin D1 in A549. There were three independent repeats per experiment. Compared with the si-NC, *was P<0.05, **was P<0.01 and ***was P<0.001.
Further, we also found that MALAT1 knockdown could increase the proportion of G1 A549 cells, but reduce the proportion of A549 cells in S phase and G2 phase (Figure 3D, 3E). The cyclin D1 protein is a protein encoded by the CCND1 gene, also known as the G1/S-specific cyclin D1, which plays an important positive regulatory role in the cell cycle across R-dots. However, we found that expression of cyclin D1 protein in A549 cells was significantly reduced after MALAT1 inhibition in PG49 and A549 by si-MALAT1-1 or si-MALAT1-2 (P<0.05). This indicates that LncRNA MALAT1 knockdown suppressed the proliferation of LAC cells
LncRNA MALAT1 knockdown suppressed the EMT of LAC cells
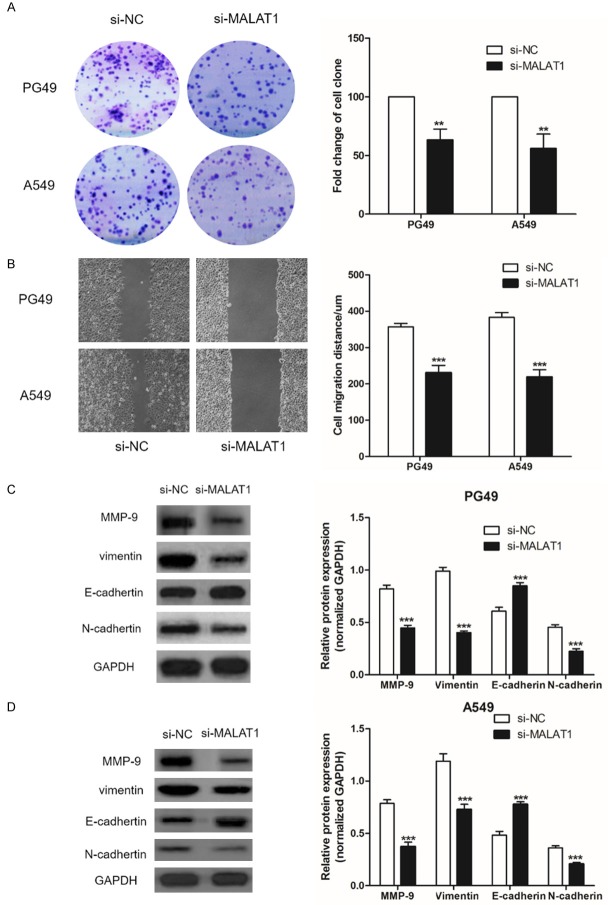
Transwell test was used to evaluate the invasive ability of LAC cells and cell scratch test was used for migration. We found (Figure 4A, 4B) that the invasion and migration of PG49 and A549 were significantly suppressed after LncRNA MALAT1 knockdown. Further, as shown in Figure 4C, 4D, LncRNA MALAT1 knockdown could reduce the expression of MMP-9, vimentin and N-cadherin, and upgrade the expression of E-cadherin.
Figure 4.
LncRNA MALAT1 knockdown suppressed the EMT of LAC cells. A. Transwell assay was used to evaluate the invasion of PG49 and A549; B. Cell scratch test was used to evaluate the migration of PG49 and A549; C, D. Western blotting was used to measure the protein expression of EMT in PG49 and A549. Three independent repeats per experiment. Compared with the si-NC, *was P<0.05, **was P<0.01 and ***was P<0.001.
LncRNA MALAT1 interacted with miR-429 in LAC cells
RT-qPCR was used to detect the expression of miR-429, and as shown in Figure 4A, 4B, miR-429 had low expression in human LAC tissues (vs. paracancerous normal tissues, t=125.837, P<0.001) and human LAC cells (H1299, SPC-A-1, PG49 and A549 vs. HBE, BEAS-2B and HPAEpiC). Pearson’s correlation was used to analyze the correlation of LncRNA MALAT1 and miR-429 in 39 LAC tumor tissues, and we found that there was a negative correlation (r=-0.7144, P<0.001) between LncRNA MALAT1 and miR-429.
We searched for binding sites for miR-429 and LncRNA MALAT1 from the starbase database. To confirm that miR-429 can regulate MALAT1 expression by binding to the MALAT1 3’-UTR end, we used the luciferase gene reporter system. Results showed that transfection of miR-429-mimics significantly decreased WT type 3’-UTR luciferase activity (P<0.001) in A549, but did not work in MUT. Further, as shown in Figure 5F, LncRNA MALAT1 knockdown could reduce the expression of miR-429. The above results suggest that miR-429 and LncRNA MALAT1 can inhibit each other’s expression in lung adenocarcinoma cells.
Figure 5.
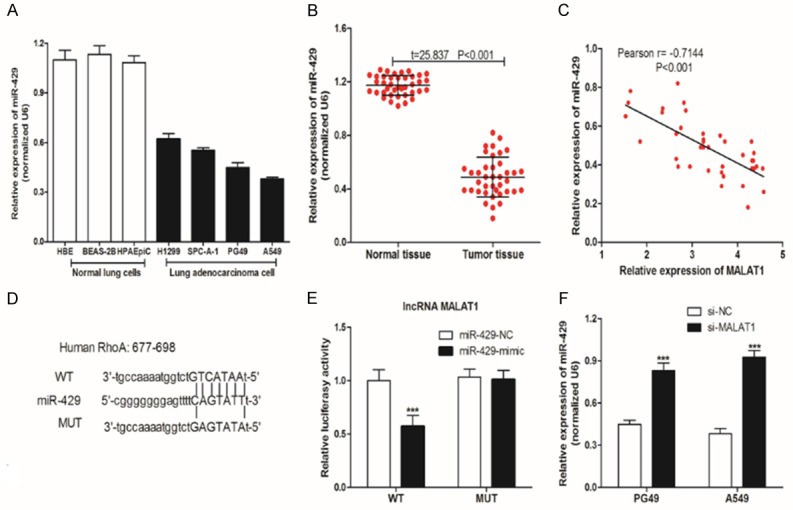
LncRNA MALAT1 and miR-429 are mutually regulated in LAC cells. A, B. RT-qPCR was used to measure the expression of miR-429 in normal lung cells, LAC cells, normal tissues, and LAC tumor tissues; C. Pearson’s correlation between Malat1 and miR-429. D. WT-MALAT1 3’UTR luciferase reporter vector, and a MUT-MALAT1 3’UTR luciferase reporter vector with mutations on miR-429 binding sites of the MALAT1 3’UTR was constructed; E. WT-MALAT1/MUT-MALAT1 and miR-429-NC/miR-429-mimic were transfected into A549, and luciferase activity was detected; F. RT-qPCR was used to measure the expression of miR-429 in PG49/A549 cells after LncRNA MALAT1 knockdown in LAC cells. Three independent repeats per experiment. Compared with the si-NC or miR-429-NC, ***was P<0.001.
LncRNA MALAT1 regulated RhoA expression through binding miR-429
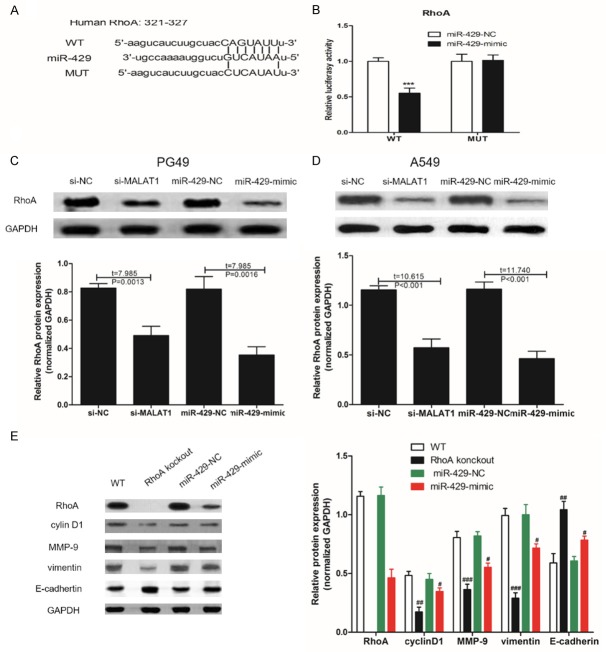
We predict the target genes of miR-429 through bioinformatics, and found that there is a sequence complementary to miR-429 at the 3’-UTR end of RhoA mRNA (Figure 6A). Luciferase gene reporter system was also used to confirm that miR-429 can regulate MALAT1 expression by binding to the RhoA 3’-UTR end. We found that transfection of miR-429-mimics significantly decreased WT type 3’-UTR luciferase activity (P<0.001) in A549, but did not work in MUT. This suggested that miR-429 targets inhibition of RhoA expression in A549 cells. In addition, our study also found that the transfer of si-MALAT1 or miR-429-mimic into LAC cells could also achieve the purpose of inhibiting the expression of RhoA protein. In A549 cells, RhoA knockout could significantly inhibit the expression of cyclin D1, MMP-9, and vimentin proteins, and increase the expression of E-cadherin protein. Transferring miR-429-mimic into A549 could also achieve the same effect. The above studies indicated that MALAT1 could compete with RhoA to bind miR-429 in LAC cells, thereby inhibiting the inhibitory effect of miR-429 on RhoA protein expression.
Figure 6.
miR-429 inhibits RhoA expression in LAC cells. A. WT-RhoA 3’UTR luciferase reporter vector, and a MUT-RhoA 3’UTR luciferase reporter vector with mutations on miR-429 binding sites of the RhoA 3’UTR was constructed; B. WT-RhoA/MUT-RhoA and miR-429-NC/miR-429-mimic were transfected into A549, and luciferase activity was detected; C, D. Western blotting was used to measure the protein expression of RhoA in PG49 and A549; E. Western blotting was used to measure the protein expression in WT-A549, RhoA knockout-A549 and A549 cells that miR-429-NC/miR-429-mimic were transfected into. Three independent repeats per experiment. Compared with the si-NC or miR-429-NC, ***was P<0.001. Compared with the WT group, #was P<0.05, ##was P<0.01 and ###was P<0.001.
Discussion
Previous research has shown that the expression of MALAT1 was high in liver cancer [20], thyroid cancer [21], lung cancer [22], bladder cancer [23] and colorectal cancer [24]. The high expression of MALAT1 could play a important role in promoting the proliferation and EMT of cancer cells, but its specific molecular mechanisms vary. In this paper, we found that LncRNA MALAT1 had high expression in human LAC tissues and cells. The expression of lncRNA MALAT1 was significantly associated with human lung adenocarcinoma tumor size, lymph node metastasis, TNM staging, and 2-year survival. Although some studies have partially revealed the molecular mechanism of MALAT1 promoting NSCLC tumor cell proliferation and EMT, such as regulation of splicing [14,15], participation in epigenetic regulation [16,17] and involvement in cell cycle regulation [18,19], we believed these are just the tip of the iceberg.
In this paper, we found that miR-429 could inhibit the expression of MALAT1 through binding the 3’-UTR of MALAT1. microRNA is a non-coding, single-stranded RNA encoded by 20-25 ribonucleotides that can regulate cell proliferation, differentiation, apoptosis, metabolism, and invasion/migration through regulating the gene translation and transcription of its target gene [25,26]. It is not only a biomarker for the diagnosis of multiple diseases, but also plays an important role in the development of the disease [27].
In recent years, several studies have shown [28,29] that the development of non-small cell lung cancer is related to the abnormal expression of miRNA. miR-429 is a member of the miR-200 family, located on chromosome 1 p36.33. It is expressed abnormally in many tumors and involved in biologic behaviors such as proliferation, apoptosis, invasion, and migration of tumors. miR-429 had low expressed in breast cancer [30], gastric cancer [31], colorectal cancer [32], and played a important role as a tumor suppressor gene. We found that miR-429 had low expression in human LAC tissues and human LAC cells, and its expression level in human LAC tissues was negatively correlated with LncRNA MALAT1. There were two very important interaction mechanisms between LncRNA and miRNA. One was that lncRNA could act as a “molecular sponge” to block post-transcriptional inhibitory effects of miRNAs on downstream target genes, restoring the function of target genes, and these lncRNAs are called competitive endogenous RNA (ceRNA) [33]. The other is that LncRNAs and miRNAs could compete with target genes to reduce the inhibitory effect of miRNAs on target genes and increase their stability [33]. This suggests that the high expression of lncRNA MALAT1 in human lung adenocarcinoma may inhibit the ability of miR-429 to affect its tumor suppressor genes through binding to miR-429.
The luciferase reporter system showed that miR-429 inhibits the expression of RhoA protein in LAC cells. Rho protein is a GTP-binding protein with a relative molecular mass of between 20-25 kD and has GTP protease activity, also known as Rho GTPase. RhoA is an important member of Rho GTPase. Recently, studies had shown that RhoA was highly expressed in many malignant tumors such as breast cancer and pancreatic cancer, and high expression promotes the proliferation and migration of cancer cells and the clinicopathologic features of postoperative patients [34,35].
In lung cancer, Suczuki et al. [36] found ANLN could participate in phosphoinositide 3-kinase/AKT pathway through activation of RhoA, and promote the proliferation, invasion, and migration of lung cancer cells. The same oncogene function of RhoA protein was also found by Touge et al. [37]. Can high expression of lncRNA MALAT1 in lung adenocarcinoma reduces miR-429 inhibition of RhoA protein expression through competitive binding to miR-429, thereby promoting LAC cell proliferation and EMT? I believe that this study has answered the above question.
In this paper, first, as shown in Figures 3 and 4, MALAT1 knockdown not only inhibited LAC cell proliferation by decreasing cyclin D1 expression, but also inhibited LAC cell invasion and migration by inhibiting MMP-9 and vimentin and enhancing E-cadherin protein expression. Second, miR-429 inhibited the expression of MALAT1 and RhoA in LAC cells, (Figures 5D, 5E and 6A, 6B), and the up-regulation of miR-429 and MALAT1 knockdown could achieve the same effect of inhibiting RhoA protein expression.
Thirdly, up-regulation of miR-429 and RhoA knockout both reduced cyclin D1, MMP-9, and vimentin, and enhanced E-cadherin protein expression (Figure 6E). Summarizing these three conclusions, we found that LncRNA Malat1 could promote the proliferation and EMT of human lung adenocarcinoma cells by competing with RhoA for binding to miR-429.
Conclusion
LncRNA MALAT1 is highly expressed in human lung adenocarcinoma tissues and human lung adenocarcinoma cells, and high expression of lncRNA MALAT1 can reduce miR-429’s inhibition of RhoA expression by binding to miR-429, thereby promoting human lung adenocarcinoma cell proliferation, invasion, and migration.
Disclosure of conflict of interest
None.
References
- 1.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–89. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Chen WQ, Li H, Sun KX, Zheng RS, Zhang SW, Zeng HM, Zou XN, Gu XY, He J. Report of cancer incidence and mortality in China, 2014. China Cancer. 2016;40:5. doi: 10.3760/cma.j.issn.0253-3766.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Clark MB, Johnston RL, Inostrozaponta M, Fox AH, Fortini E, Moscato P, Dinger ME, Mattick JS. Genome-wide analysis of long noncoding RNA stability. Genome Res. 2012;22:885–898. doi: 10.1101/gr.131037.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tani H, Mizutani R, Salam KA, Tano K, Ijiri K, Wakamatsu A, Isogai T, Suzuki Y, Akimitsu N. Genome-wide determination of RNA stability reveals hundreds of short-lived noncoding transcripts in mammals. Genome Res. 2012;22:947–956. doi: 10.1101/gr.130559.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhan A, Soleimani M, Mandal SS. Long noncoding rna and cancer: a new paradigm. Cancer Res. 2017;77:3965. doi: 10.1158/0008-5472.CAN-16-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hon CC, Ramilowski JA, Harshbarger J, Bertin N, Rackham OJ, Gough J, Denisenko E, Schmeier S, Poulsen TM, Severin J. An atlas of human long non-coding RNAs with accurate 5’ ends. Nature. 2017;543:199. doi: 10.1038/nature21374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandra Gupta S, Nandan Tripathi Y. Potential of long non-coding RNAs in cancer patients: from biomarkers to therapeutic targets. Int J Cancer. 2017;140:1955–1967. doi: 10.1002/ijc.30546. [DOI] [PubMed] [Google Scholar]
- 9.Rao AKDM, Rajkumar T, Mani S. Perspectives of long non-coding RNAs in cancer. Mol Biol Rep. 2017;44:1–16. doi: 10.1007/s11033-017-4103-6. [DOI] [PubMed] [Google Scholar]
- 10.Liu L, Cui S, Wan T, Li X, Tian W, Zhang R, Luo L, Shi Y. Long non-coding RNA HOTAIR acts as a competing endogenous RNA to promote glioma progression by sponging miR-126-5p. J Cell Physiol. 2018;233:6822–6831. doi: 10.1002/jcp.26432. [DOI] [PubMed] [Google Scholar]
- 11.Zheng HT, Shi DB, Wang YW, Li XX, Xu Y, Tripathi P, Gu WL, Cai GX, Cai SJ. High expression of lncRNA MALAT1 suggests a biomarker of poor prognosis in colorectal cancer. Int J Clin Exp Pathol. 2014;7:3174–81. [PMC free article] [PubMed] [Google Scholar]
- 12.Yang L, Bai HS, Deng Y, Fan L. High MALAT1 expression predicts a poor prognosis of cervical cancer and promotes cancer cell growth and invasion. Eur Rev Med Pharmacol Sci. 2015;19:3187–3193. [PubMed] [Google Scholar]
- 13.Zhang Y, Dai Q, Zeng F, Liu H. MALAT1 promotes the proliferation and metastasis of osteosarcoma cells by activating the Rac1/JNK pathway via targeting MiR-509. Oncol Res. 2018 doi: 10.3727/096504017X14957939026111. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Tripathi V, Ellis JD, Shen Z, Song DY, Pan Q, Watt AT, Freier SM, Bennett CF, Sharma A, Bubulya PA. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell. 2010;39:925–938. doi: 10.1016/j.molcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su L, Liu B, Zhu Z, Wang J, Chen X, Li P, Cai Q, Yu B, Wu W. MALAT1 promotes cell proliferation in gastric cancer by recruiting SF2/ASF. Biomed Pharmacother. 2014;68:557–564. doi: 10.1016/j.biopha.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Bantignies F, Roure V, Comet I, Leblanc B, Schuettengruber B, Bonnet J, Tixier V, Mas A, Cavalli G. Polycomb-dependent regulatory contacts between distant hox loci in drosophila. Cell. 2011;144:214–226. doi: 10.1016/j.cell.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 17.Bernstein E, Duncan EM, Masui O, Gil J, Heard E, Allis CD. Mouse polycomb proteins bind differentially to methylated histone H3 and RNA and are enriched in facultative heterochromatin. Mol Cell Biol. 2006;26:2560–2569. doi: 10.1128/MCB.26.7.2560-2569.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tripathi V, Shen Z, Chakraborty A, Giri S, Freier SM, Wu X, Zhang Y, Gorospe M, Prasanth SG, Lal A, Prasanth KV. Long noncoding RNA MALAT1 controls cell cycle progression by regulating the expression of oncogenic transcription factor B-MYB. PLoS Genet. 2013;9:e1003368. doi: 10.1371/journal.pgen.1003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang F, Yi F, Han X, Du Q, Liang Z. MALAT-1 interacts with hnRNP C in cell cycle regulation. FEBS Lett. 2013;587:3175–3181. doi: 10.1016/j.febslet.2013.07.048. [DOI] [PubMed] [Google Scholar]
- 20.Malakar P, Shilo A, Mogilevsky A, Stein I, Pikarsky E, Nevo Y, Benyamini H, Elgavish S, Zong X, Prasanth KV, Karni R. Long noncoding RNA MALAT1 promotes hepatocellular carcinoma development by SRSF1 upregulation and mTOR activation. Cancer Res. 2017;77:1155–1167. doi: 10.1158/0008-5472.CAN-16-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang JK, Ma L, Song WH, Lu BY, Huang YB, Dong HM, Ma XK, Zhu ZZ, Zhou R. MALAT1 promotes the proliferation and invasion of thyroid cancer cells via regulating the expression of IQGAP1. Biomed Pharmacother. 2016;83:1–7. doi: 10.1016/j.biopha.2016.05.039. [DOI] [PubMed] [Google Scholar]
- 22.Gutschner T, Hämmerle M, Eißmann M, Hsu J, Kim Y, Hung G, Revenko A, Arun G, Stentrup M, Groß M. The non-coding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 2013;73:1180–1189. doi: 10.1158/0008-5472.CAN-12-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang Y, Qi C, Wang Y, Zhou Z, Huang Y, Feng Q. Upregulated MALAT-1 contributes to bladder cancer cell migration by inducing epithelial-to-mesenchymal transition. Mol Biosyst. 2012;8:2289–2294. doi: 10.1039/c2mb25070e. [DOI] [PubMed] [Google Scholar]
- 24.Ji Q, Zhang L, Liu X, Zhou L, Wang W, Han Z, Sui H, Tang Y, Wang Y, Liu N. Long non-coding RNA MALAT1 promotes tumour growth and metastasis in colorectal cancer through binding to SFPQ and releasing oncogene PTBP2 from SFPQ|[sol]|PTBP2 complex. British Journal of Cancer. 2014;111:736. doi: 10.1038/bjc.2014.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu J, Getz G, Miska EA, Alvarezsaavedra E, LACb J, Peck D, Sweetcordero A, Ebert BL, Mak RH, Ferrando AA. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Chen J, Sen S. MicroRNA as biomarkers and diagnostics. J Cell Physiol. 2015;231:25–30. doi: 10.1002/jcp.25056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Xu L, Jiang L. miR-1271 promotes non-small-cell lung cancer cell proliferation and invasion via targeting HOXA5. Biochem Biophys Res Commun. 2015;458:714–719. doi: 10.1016/j.bbrc.2015.02.033. [DOI] [PubMed] [Google Scholar]
- 29.Ye M, Zhang J, Zhang J, Miao Q, Yao L, Zhang J. Curcumin promotes apoptosis by activating the p53-miR-192-5p/215-XIAP pathway in non-small cell lung cancer. Cancer Lett. 2015;357:196–205. doi: 10.1016/j.canlet.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 30.Ye ZB, Ma G, Zhao YH, Xiao Y, Zhan Y, Jing C, Gao K, Liu ZH, Yu SJ. miR-429 inhibits migration and invasion of breast cancer cells in vitro. Int J Oncol. 2015;46:531–538. doi: 10.3892/ijo.2014.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun T, Wang C, Xing J, Wu D. miR-429 modulates the expression of c-myc in human gastric carcinoma cells. Eur J Cancer. 2011;47:2552–2559. doi: 10.1016/j.ejca.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 32.Cristóbal I, Rincón R, Manso R, Caramés C, Aguilera O, Madoz-Gurpide J, Rojo F, García-Foncillas J. Deregulation of miR-200b, miR-200c and miR-429 indicates its potential relevant role in patients with colorectal cancer liver metastasis. J Surg Oncol. 2014;110:484–485. doi: 10.1002/jso.23661. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Li X, Hou Y, Fang N, You J, Zhou Q. The lncRNA XIST exhibits oncogenic properties via regulation of miR-449a and Bcl-2 in human non-small cell lung cancer. This article has been corrected since advanced online publication, and an erratum is also printed in this issue. Acta Pharmacol Sin. 2017;38:371–381. doi: 10.1038/aps.2016.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun B, Nishihira J, Yoshiki T, Kondo M, Sato Y, Sasaki F, Todo S. Macrophage migration inhibitory factor promotes tumor invasion and metastasis via the Rho-dependent pathway. Clin Cancer Res. 2005;11:1050–1058. [PubMed] [Google Scholar]
- 35.Ungefroren H, Witte D, Lehnert H. The role of small GTPases of the Rho/Rac family in TGF-β-induced EMT and cell motility in cancer. Dev Dyn. 2017;247:451–461. doi: 10.1002/dvdy.24505. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki C, Daigo Y, Ishikawa N, Kato T, Hayama S, Ito T, Tsuchiya E, Nakamura Y. ANLN plays a critical role in human lung carcinogenesis through the activation of RHOA and by involvement in the phosphoinositide 3-kinase/AKT pathway. Cancer Res. 2005;65:11314–11325. doi: 10.1158/0008-5472.CAN-05-1507. [DOI] [PubMed] [Google Scholar]
- 37.Touge H, Chikumi H, Igishi T, Kurai J, Makino H, Tamura Y, Takata M, Yoneda K, Nakamoto M, Suyama H, Gutkind JS, Shimizu E. Diverse activation states of RhoA in human lung cancer cells: contribution of G protein coupled receptors. Int J Oncol. 2007;30:709–715. [PubMed] [Google Scholar]