Abstract
EphB2, a receptor tyrosine kinase for ephrin ligands, is overexpressed in various cancers and plays an important role in tumor progression. EPHB2 promotes endothelial-mesenchymal transition (EMT) and elicits associated pathologic characteristics of glioblastoma multiforme (GBM) such as invasion and migration. However, the mechanisms of the EPHB2 regulatory network in glioma remain enigmatic. Here, we report that EPHB2 is epigenetically overexpressed in hypoxia, a condition highly prevalent in malignancy. Furthermore, HIF-2α is required for EPHB2 stabilization by hypoxia. Lastly, we discovered that the overexpression of EPHB2 promotes GBM invasion by the phosphorylation of paxillin in hypoxia. These findings establish the HIF-2α-EPHB2-paxillin axis as a regulatory mechanism of epithelial-mesenchymal transition.
Keywords: Hypoxia, EPHB2, paxillin, invasion, glioblastoma
Introduction
Glioblastoma multiforme (GBM) is characterized by rapid and invasive growth throughout the brain. Despite recent advances in multimodal treatment approaches including surgery, radiotherapy and chemotherapy, the median overall survival of patients with glioblastoma is still poor, i.e. in the range of 16 months in selected clinical trials [1], and 11 months in other population-based studies [2]. The extremely poor prognosis of patients with gliomas is largely due to the epithelial-mesenchymal transition (EMT) and the high tendency of tumor invasiveness that results in severe structural and functional damage to the surrounding brain tissue, leading to incomplete surgical resection, and a high frequency of tumor recurrence [3].
Eph receptors are the largest family of receptor tyrosine kinases and have vital functions during development and cellular homeostasis, including cell adhesion, migration, and axon guidance [4-7]. Eph receptors and their cognate ligands, Ephrins, have been found to be aberrantly expressed in many cancers, including GBM [8]. Family members implicated in gene deregulation and function in GBM include EphA2, EphA7, EphB2, and ephrin-A5 [9-11]. EPHB2 appears to play a critical role in EMT in glioma [10-12]. However, the mechanisms of the EPHB2 regulatory network in glioma in response to tumor microenvironment remain unclear.
Cellular responses to hypoxia are commonly regulated by the hypoxia-inducible factor (HIF) family of transcriptional factors [13,14]. Intratumoral hypoxia is a common feature of solid malignancies, including sarcomas. The hypoxia-inducible factor 1α (HIF1α), along with HIF2α, is a requisite transcription factor for cellular adaptation to hypoxia, and both strongly influence the metastatic potential of tumor cells [15]. Here we have explored the epithelial-mesenchymal transition functions of EPHB2, and whether there is a correlation between EPHB2 expression and hypoxia, a condition that is permissive and prevalent in GBM.
We identified that EPHB2 is stabilized by HIF2α. Furthermore, we discovered that the overexpression of EPHB2 promotes GBM invasion by the phosphorylation of paxillin.
Materials and methods
Cell lines and reagents
The human GBM cell lines U87, A172, LN229, SW1783 were purchased from the American Type Culture Collection. The human GBM cell lines U251 and T98 were obtained from RIKEN BioResource Center (Tsukuba, Japan). To maintain the authenticity of the cell lines, frozen stocks were prepared from the initial stocks, and every 3 months, a new frozen stock was used for the experiments. Before each experiment, GBM cells were cultured in DMEM, supplemented with 10% FBS, sodium pyruvate, nonessential amino acids, L-glutamine, and a vitamin solution. NHAs were purchased from Lonza (Walkersville, MD, USA) and maintained following the manufacturer’s instructions. Cell lines were maintained at 37°C in a humidified atmosphere with 5% CO2. For the hypoxia treatment, the cells were first maintained in a regular normoxic incubator for around 12 h until the cells attached to the flasks. Following this, the flasks were transferred to the tri-gas incubator (Sanyo MCO 18M, from Sanyo E&E Europe BV, Etten-Leur, The Netherlands) filled with 1% O2, 5% CO2 and 94% N2, at 98% humidity and 37°C.
RNA extraction and qPCR analysis
The total RNA from the cell lines was isolated using TRIzol Reagent (Invitrogen) following the manufacturer’s protocol. qPCR was performed using an Applied Biosystems 7900 Sequence Detection system. First-strand cDNA were synthesized using the PrimerScriptTM RT Master Mix (TaKaRa). The primer pairs of EPHB2 were 5’-AGAAACGCTAATGGACTCCACT-3’ and 5’-GTGCGGATCGTGTTCATGTT-3’. The primer pairs of DLK1 were 5’-CTTTCGGCCACAGCACCTAT-3’ and 5’-TGTCATCCTCGCAGAATCCAT-3’. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA was also amplified in the same PCR reactions as an internal control using the primers 5’-TGCACCACCAACTGCTTAGC-3’ and 5’-GGCATGGACTGTGGTCATGAG-3’. Relative gene expression was calculated using the 2-ΔΔCt method.
Invasion assay
Invasion was examined using 24-well BD Matrigel invasion chambers (BD Biosciences) according to the manufacturer’s instructions. 2×104 cells were seeded in the upper well of the invasion chamber in DMEM without serum. The lower chamber contained DMEM supplemented with 10% FBS to stimulate invasion. After incubation for 24 h, the non-invading cells were removed from the top well with a cotton swab while the bottom cells were fixed with 100% methanol, and stained with 0.1% crystal violet, and photographed in three independent 10× magnification fields.
Wound healing assay
About 3×105 cells were seeded in 6-well dishes and an incision was made in the central area of the confluent culture to create an artificial wound. Images of the wound area were captured on a bright field microscope equipped with differential interference contrast (Leica, Wetzlar, Germany) at 24 h after injury.
Westernblot
Harvested cells were homogenized with a lysis buffer and cell lysates were centrifuged at 4°C for 15 minutes at 10,000 rpm. Then the supernatant was placed in fresh tubes and quantified using the Bradford protein assay. 50 mg of proteins underwent electrophoresis on a 12% SDS-polyacrylamide gel and were transferred to a PVDF membrane (Bio-RAD, Hercules, CA, USA) by electroblotting. The membranes were blocked for 2 h with 5% nonfat milk and then incubated at room temperature with primary antibodies, followed by incubating them with a horseradish peroxidase-conjugated secondary antibody. The bound antibodies were detected using the enhanced chemiluminescence method (Amersham Biosciences, Uppsala, Sweden). β-actin was used as an internal control. The following antibodies were used: HIF1α [1:1000, Abcam (ab2185), Cambridge, UK], HIF2α [1:500, abcam (ab199)] EPHB2 [1:200, abcam (ab5418)], N-cadherin [1:1000, abcam (ab18203)], E-cadherin [1:500, abcam (ab1416)], fibronectin [1:500, abcam (ab2413)], FAK [1:1000, abcam (ab40794)], paxillin [1:1000, abcam (ab32084)], anti-phospho-focal adhesion kinase (FAK) (Y576/577) [1:5000, abcam (ab76244)], (ab50581), anti-phosphopaxillin (Y118) [1:1000, abcam (ab75740)] and β-actin [1:500, abcam (ab8227)].
Luciferase reporter assay
Luciferase activity was detected using the dual-luciferase reporter assay (Promega, Madison, WI, USA) according to the manufacturer’s instructions. The cells plated in a 96-well plate were co-transfected with 150 ng of the expression plasmids, 45 ng of the promoter reporter plasmids and 5 ng of the pRL-TK (Renila luciferase) plasmids using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. After 6 h of transfection, the cells were washed and replaced in a fresh medium. The dual-luciferase reporter assay was performed after 48 h and the luciferase activity was normalized to the Renilla luciferase activity.
Statistical analysis
The data were analyzed via a one-way analysis of variance (ANOVA) for multiple group comparisons and two tailed Student’s t-tests for 2-group comparisons, using the SPSS 13.0 software package. Graphing was processed using GraphPad Prism 6.0 software. Differences were considered statistically significant at P<0.05.
Results
EPHB2 is upregulated under hypoxia in GBM cells
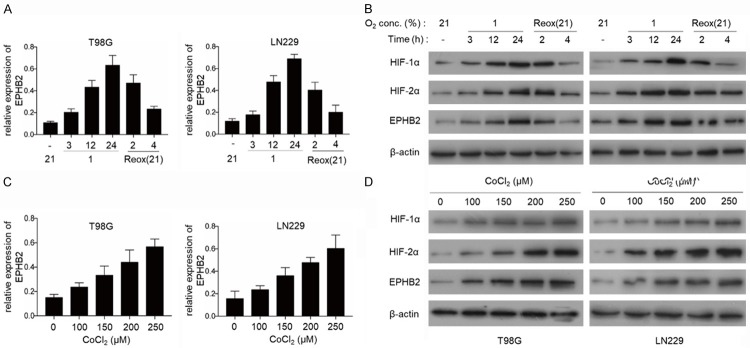
To determine whether EPHB2 could be induced by hypoxia, the GBM cell lines, T98G and LN229 were exposed to hypoxic conditions (1% O2) for up to 24 h. We found that the expression of EPHB2 was significantly increased at the mRNA levels in the GBM cell lines under hypoxic conditions and reduced on reoxygenation (Figure 1A). The protein levels of HIF-1α and HIF-2α increased rapidly in these cell lines (Figure 1B). We also observed the expression levels of HIF-1α, HIF2α and EPHB2 after the GBM cells were treated with cobalt chloride (CoCl2), a known HIF activator. The levels of HIF-1α, HIF-2α and EPHB2 were upregulated in a dose-dependent manner by CoCl2 in T98G and LN229 cells (Figure 1C and 1D). These results suggest that hypoxia induces EPHB2 expression in the GBM cells and that HIF-1α or HIF-2α may be involved in the upregulation of EPHB2 under hypoxia.
Figure 1.
EPHB2 is up-regulated under hypoxia in GBM cells. A. Real-time PCR analysis of EPHB2 mRNA levels in GBM cell lines, exposed to hypoxic conditions (1% O2) for up to 24 h and on reoxygenation (Reox) at 21% O2 for 2 h and 4 h. Data are presented as the mean ± S.E.M. of three independent experiments. B. The expression of EPHB2, HIF-1α and HIF-2α was quantified by western blot under hypoxia from 0 to 24 h and on reoxygenation (Reox) at 21% O2 for 2 h and 4 h. C. The mRNA levels of EPHB2 were detected by real-time PCR after treatment with CoCl2 for 24 h. Data are represented as the mean ± S.E.M. of three independent experiments. D. Western blot analysis of EPHB2, HIF-1α and HIF-2α levels after treatment with CoCl2 for 24 h.
HIF-2α is required for EPHB2 stabilization by hypoxia
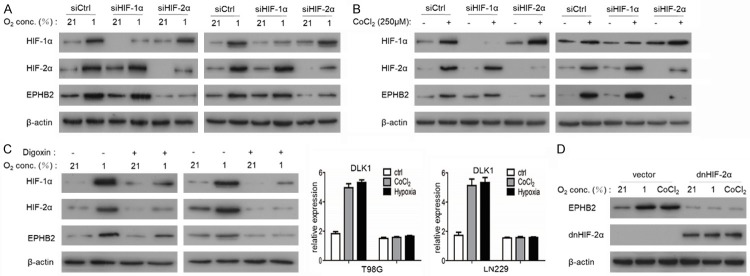
To further investigate the potential role of HIF-1α or HIF-2α in regulating EPHB2 expression, we knocked down the expression of HIF-1α or HIF-2α in both T98G and LN229 cells with siRNA. Under hypoxic conditions (1% O2), the silencing of HIF-2α significantly downregulated EPHB2, whereas the silencing of HIF-1α had no significant effect on EPHB2 protein expression (Figure 2A). Following treatment with CoCl2 (Figure 2B) that mimics hypoxic conditions and digoxin (Figure 2C), HIF-1α and HIF2α inhibitors, suggest that its transcriptional activity is required for EPHB2 regulation by hypoxia. The expression of a dominant-negative HIF-2α mutant also abrogated the increase of EPHB2 expression after hypoxia treatment (Figure 2D) Together these results suggest the requirement of HIF-2α and its transactivation potential in regulating EPHB2 abundance.
Figure 2.
HIF2α is instrumental for the induction of EPHB2. A. Effects of 1% O2 on EPHB2 expression in T98 and LN229 cells in the presence of siRNA against HIF-1α and HIF-2α were analyzed by western blot. B. Effects of the hypoxia mimetics CoCl2 (250 μM) on EPHB2 expression in T98 and LN229 cells in the presence of siRNA against HIF-1α and HIF-2α were analyzed by western blot. C. Western blots showing the effect of digoxin on the expression of the indicated proteins in hypoxia exposed T98 and LN229 cells. D. Over-expression of dominant negative HIF-2α (dnHIF-2α). The expression of EPHB2 was examined using a western blot assay.
EPHB2 increased invasion capability under hypoxic conditions in GBM cell lines
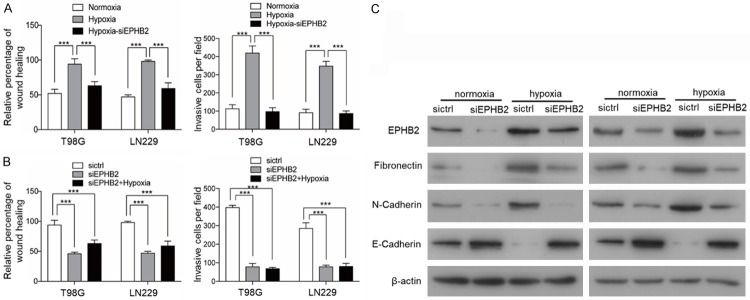
It has reported that EPHB2 is associated with tumor invasion capability in human cancers including GBM [11,12]. HIF-2α has a key role in promoting the invasion of GBM [16]. Therefore, it is likely that EPHB2 can regulate glioma cell invasion under hypoxic conditions. We next performed in vitro experiments in T98G and LN229 cells. Exposure of T98G and LN229 cells to hypoxia (1% O2) for 72 h resulted in a marked difference in their invasion capability compared to normoxia (20% O2). The invasion potential of T98G and LN229 cells was tested using wound healing and transwell assays. Hypoxia-exposed cells demonstrated an increased invasion capability in comparison to cells cultured under normoxic conditions. To further investigate the role of EPHB2 we silenced the expression of EPHB2 with siRNAs. The exposure of siEPHB2 transfected T98G and LN229 cells to hypoxia prevented an increased invasion capability under hypoxic conditions (Figure 3A). Significant reduction in the invasive potential was also observed in T98G and LN229 cells under hypoxia following the silencing of EPHB2 (Figure 3B). These results indicate that EPHB2 is a crucial mediator of the hypoxia-induced, HIF2α-dependent invasive phenotype of these GBM cells. Western blotting confirmed the inhibition of EPHB2-induction by hypoxia in the specific EPHB2 siRNA-transfected T98G and LN229 cells lines together with an E-cadherin accumulation and absence of Fibronectin and N-cadherin accumulation (Figure 3C).
Figure 3.
EPHB2 mediates hypoxia-induced EMT. A, B. The quantification of a transwell invasion assay is shown, and the quantification of the wound healing assay is shown. *P<0.001. C. Western blot analyses showing effective downregulation of Fibronectin and N-cadherin in T98 and LN229 cells following siEPHB2-mediated gene silencing and exposure to hypoxia when compared to control scramble siCtrl. The effects on EPHB2 and E-cadherin expression were also investigated.
Overexpression of EPHB2 by HIF-2α promotes GBM invasion via the phosphorylation of paxillin
It has been reported that the phosphorylation levels of FAK and paxillin were significantly decreased in EphB2 knockdown in cholangiocarcinoma [17]. To evaluate whether the decrease in EphB2 upon siRNA treatment in T98G cells affects the activity of signaling molecules with known involvement in cell invasion, the phosphorylation level of FAK (Y576/577) and paxillin (Y118) was determined. Western blot analyses demonstrated that phosphorylation levels of FAK and paxillin were significantly decreased in EphB2 knockdown cells under hypoxia conditions (Figure 4A) compared with control cells. A similar effect of EphB2 knockdown was also observed in the LN229 cell line (Figure 4A). Following treatment with PF-00562271, which selectively suppresses the phosphorylation level of FAK and paxillin (Figure 4B). A significant reduction in the invasive potential was also observed in T98G and LN229 cells (Figure 4C).
Figure 4.
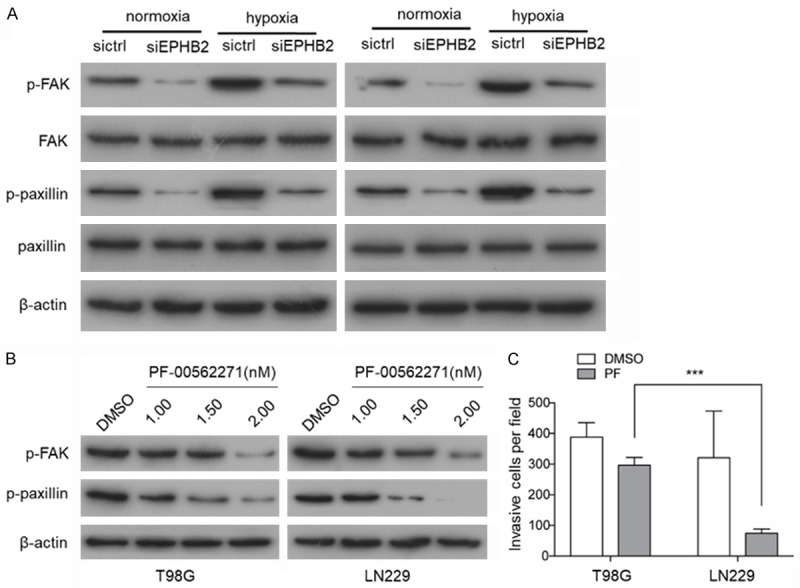
Overexpression of EPHB2 promotes GBM invasion by the phosphorylation of paxillin. A. Western blot analysis of the phosphorylation of paxillin and FAK levels when EPHB2 knocked down in T98 and LN229 cells. B. Western blot analysis of the phosphorylation of paxillin and FAK levels after treatment with PF-00562271 for 24 h. C. The quantification of transwell invasion assay is shown and the quantification of the wound healing assay is shown. *P<0.001.
Discussion
The invasion of malignant glioma cells into regions of the normal brain is a major cause of the dismal prognosis of malignant gliomas, but the underlying mechanisms remain poorly understood [18]. In the present study, we demonstrated two novel findings. One is that the expression of EPHB2 increases under hypoxia when compared to normoxic conditions. Second, we show that these augmentations contribute to the increase of invasion under hypoxia, to induce malignant phenotypes in GBM.
The hypoxic microenvironment within solid tumor masses is one of the characteristics of glioblastoma [19]. Hypoxia is known to induce the epithelial to mesenchymal transition in glioblastoma cells, leading to morphological changes and scattering [20]; Hypoxic regions are frequently found in GBM and the presence of extensive hypoxic areas has been associated with a poorer prognosis in GBM patients, which has been linked to hypoxic cancer cells displaying a more malignant phenotype and being more resistant to chemotherapy and radiation [21-23]. The HIF transcription factors are instrumental for orchestrating adaptive responses to cope with an oxygen shortage, in particular, HIF2α was found to be upregulated in many malignant tumors primarily by hypoxia-mediated protein stabilization [24].
Hypoxia is a strong inducer stimulating an invasive ability in GBM that is associated with upregulation of known mesenchymal markers like fibronectin and N-cadherin, and an elevated invasive potential in vitro [25]. It has been reported that EPHB2 appears to play a critical role in the epithelial-to mesenchymal transition in glioma. In this study, we independently tested the role of HIF2α and EPHB2. We observed an up-regulation of EPHB2 under hypoxic conditions. HIF1α is the most well-studied member of the HIFα family due to its universal pattern of expression, while HIF2α shows a more restricted expression pattern. Notably, HIF2α has been reported to play a crucial role in regulating stemness in GBM [26]. However, we found that hypoxia-induced HIF2α, and not HIF1α, is a key mediator for the overexpression of EPHB2. EPHB2 is known to regulate EMT in epithelial cancers [12] and it appears instrumental in this transition since the siRNA-dependent silencing of EPHB2 prevented the hypoxia enhanced invasive capacity. Moreover, we identified a critical role for EPHB2 in mediating a phosphorylation of paxillin-induced mesenchymal shift in GBM cells. In addition, it was previously shown that EphB2-FAK interactions mediate cell migration in human glioma cells and the suppression of EphB2 expression inhibited cholangiocarcinoma cell migration, likely mediated through a reduction of FAK and paxillin phosphorylation [12]. Nevertheless, a reduced expression of EphB2 that inhibits invasion and metastasis in colorectal tumors was reported [27]. The opposite role of EphB2 might depend on the unique microenvironment in each cancer type. Furthermore, we demonstrated that a decrease in EphB2 GBM cells affected the phosphorylation levels of FAK and paxillin that have been shown to beinvolved in cell invasion. Apparently, some GBM cells are refractory to one mesenchymal-inducing stimulus while being sensitive to others, providing multiple ways for GBM cells to acquire the aggressive invasion ability.
In conclusion, our study demonstrates, for the first time, the role of EPB2 in promoting glioma invasion mediated by HIF-2α. Hypoxia induces a mesenchymal shift in GBM that is mediated by the HIF-2α-EPHB2-paxillin axis leading to an elevated invasive potential. Moreover, EPHB2 expression correlates with HIF-2α in glioma patients. This novel HIF-2α-EPHB2-paxillin axis provides new insights into the mechanisms underlying glioma invasion, and suggests that targeting HIF-2α-EPHB2-paxillin may be a potential therapeutic strategy for the treatment of patients with malignant gliomas.
Acknowledgements
This study was supported by grants from the Science and Technology Mutual Fund of Guizhou Province (grant No. [2015] 7444).
Disclosure of conflict of interest
None.
References
- 1.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 2.Johnson DR, Sawyer AM, Meyers CA, O’Neill BP, Wefel JS. Early measures of cognitive function predict survival in patients with newly diagnosed glioblastoma. Neuro Oncol. 2012;14:808–816. doi: 10.1093/neuonc/nos082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norden AD, Jan D, Alona M, Karly D, Mary G, M Brenna M, Phuong P, Ainsley R, Santosh K, Wen PY. An exploratory survival analysis of anti-angiogenic therapy for recurrent malignant glioma. J Neurooncol. 2009;92:149–155. doi: 10.1007/s11060-008-9745-8. [DOI] [PubMed] [Google Scholar]
- 4.Holder N, Klein R. Eph receptors and ephrins: effectors of morphogenesis. Development. 1999;126:2033–2044. doi: 10.1242/dev.126.10.2033. [DOI] [PubMed] [Google Scholar]
- 5.Fanny M, Christiane P, Frank D, Renping Z, Jürgen B. Ephrins regulate the formation of terminal axonal arbors during the development of thalamocortical projections. Development. 2002;129:3945–3955. doi: 10.1242/dev.129.16.3945. [DOI] [PubMed] [Google Scholar]
- 6.Wilkinson DG. Eph receptors and ephrins: regulators of guidance and assembly. Int Rev Cytol. 2000;196:177–244. doi: 10.1016/s0074-7696(00)96005-4. [DOI] [PubMed] [Google Scholar]
- 7.O’Leary DD, Wilkinson DG. Eph receptors and ephrins in neural development. Curr Opin Neurobiol. 1999;9:65–73. doi: 10.1016/s0959-4388(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 8.Qin H, Noberini R, Huan X, Shi J, Pasquale EB, Song J. Structural characterization of the EphA4-Ephrin-B2 complex reveals new features enabling eph-ephrin binding promiscuity. J Biol Chem. 2010;285:644–654. doi: 10.1074/jbc.M109.064824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li JJ, Liu DP, Liu GT, Xie D. EphrinA5 acts as a tumor suppressor in glioma by negative regulation of epidermal growth factor receptor. Oncogene. 2009;28:1759–1768. doi: 10.1038/onc.2009.15. [DOI] [PubMed] [Google Scholar]
- 10.Mitsutoshi N, Niska JA, Hisashi M, Mcdonough WS, Jie W, Hiroshi S, Berens ME. The phosphorylation of EphB2 receptor regulates migration and invasion of human glioma cells. Cancer Res. 2004;64:3179. doi: 10.1158/0008-5472.can-03-3667. [DOI] [PubMed] [Google Scholar]
- 11.Nakada M, Niska JA, Tran NL, Mcdonough WS, Berens ME. EphB2/R-ras signaling regulates glioma cell adhesion, growth, and invasion. Am J Pathol. 2005;167:565–576. doi: 10.1016/S0002-9440(10)62998-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang SD, Rath P, Lal B, Richard JP, Li Y, Goodwin CR, Laterra J, Xia S. EphB2 receptor controls proliferation/migration dichotomy of glioblastoma by interacting with focal adhesion kinase. Oncogene. 2012;31:5132–5143. doi: 10.1038/onc.2012.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beasley NJ, Leek R, Alam M, Turley H, Cox GJ, Gatter K, Millard P, Fuggle S, Harris AL. Hypoxia-inducible factors HIF-1alpha and HIF-2alpha in head and neck cancer: relationship to tumor biology and treatment outcome in surgically resected patients. Cancer Res. 2002;62:2493–2497. [PubMed] [Google Scholar]
- 14.Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–472. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ting W, Gilkes DM, Naoharu T, Lisha X, Weibo L, Bishop CJ, Pallavi C, Green JJ, Semenza GL. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc Natl Acad Sci U S A. 2014;111:E3234. doi: 10.1073/pnas.1410041111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhagat M, Palanichamy JK, Ramalingam P, Mudassir M, Irshad K, Chosdol K, Sarkar C, Seth P, Goswami S, Sinha S. Hif-2α mediates a marked increase in migration and stemness characteristics in a subset of glioma cells under hypoxia by activating an Oct-4/Sox-2- Mena (INV) axis. Int J Biochem Cell Biol. 2016;74:60–71. doi: 10.1016/j.biocel.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 17.Walaiporn K, Anchalee T, Nisana N, Puangrat Y, Narong K, Anucha P, Watcharin L. Increased EphB2 expression predicts cholangiocarcinoma metastasis. Tumor Biol. 2014;35:10031–10041. doi: 10.1007/s13277-014-2295-0. [DOI] [PubMed] [Google Scholar]
- 18.Wong AJ, Bigner SH, Bigner DD, Kinzler KW, Hamilton SR, Vogelstein B. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc Natl Acad Sci U S A. 1987;84:6899–6903. doi: 10.1073/pnas.84.19.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miranda-Gonçalves V, Granja S, Martinho O, Honavar M, Pojo M, Costa BM, Pires MM, Pinheiro C, Cordeiro M, Bebiano G, Costa P, Reis RM, Baltazar F. Hypoxia-mediated upregulation of MCT1 expression supports the glycolytic phenotype of glioblastomas. Oncotarget. 2016;7:46335–46353. doi: 10.18632/oncotarget.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwadate Y. Epithelial-mesenchymal transition in glioblastoma progression. Oncol Lett. 2016;11:1615–1620. doi: 10.3892/ol.2016.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Assi H, Candolfi M, Lowenstein PR, Castro MG. Rodent glioma models: intracranial stereotactic allografts and xenografts. Neuromethods. 2012;77:229–243. doi: 10.1007/7657_2011_33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merighi S, Benini A, Mirandola P, Gessi S, Varani K, Leung E, Maclennan S, Borea PA. Adenosine modulates vascular endothelial growth factor expression via hypoxia-inducible factor-1 in human glioblastoma cells. Biochem Pharmacol. 2006;72:19–31. doi: 10.1016/j.bcp.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh CH, Shyu WC, Chiang CY, Kuo JW, Shen WC, Liu RS. NADPH oxidase subunit 4-mediated reactive oxygen species contribute to cycling hypoxia-promoted tumor progression in glioblastoma multiforme. PLoS One. 2011;6:e23945. doi: 10.1371/journal.pone.0023945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tong WW, Tong GH, Chen XX, Zheng HC, Wang YZ. HIF2α is associated with poor prognosis and affects the expression levels of survivin and cyclin D1 in gastric carcinoma. Int J Oncol. 2015;46:233–242. doi: 10.3892/ijo.2014.2719. [DOI] [PubMed] [Google Scholar]
- 25.Joseph JV, Conroy S, Pavlov K, Sontakke P, Tomar T, Eggens-Meijer E, Balasubramaniyan V, Wagemakers M, den Dunnen WF, Kruyt FA. Hypoxia enhances migration and invasion in glioblastoma by promoting a mesenchymal shift mediated by the HIF1α-ZEB1 axis. Cancer Lett. 2015;359:107–116. doi: 10.1016/j.canlet.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, Shi Q, Cao Y, Lathia J, McLendon RE, Hjelmeland AB, Rich JN. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15:501–513. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo DL, Zhang J, Yuen ST, Tsui WY, Chan AS, Ho C, Ji J, Leung SY, Chen X. Reduced expression of EphB2 that parallels invasion and metastasis in colorectal tumours. Carcinogenesis. 2006;27:454–464. doi: 10.1093/carcin/bgi259. [DOI] [PubMed] [Google Scholar]