Abstract
Celastrol (CEL) was shown to display anti-inflammatory properties, and played an important role in anti-apoptosis. There were inflammation mediated by cytokines and apoptosis distinctly in the progression of acute lung injury (ALI) burn-induced. This study was conducted to explore the role of CEL in ALI induced by burns. In order to induce burn injury, rats were exposed to a 92°C water bath for 18 seconds. After burn experiment, the Burn + Celastrol group received CEL, and vehicle (DMSO) was used to treat the rats from Burn + Vehicle group. And the Sham + Burn group received no treatment. Vascular protein leakage was detected by Evans blue (EB) concentration to evaluate the lung microvascular permeability. Then wet-to-dry lung weight ratio (W/D), and hematoxylin and eosin staining (H&E) were measured respectively to investigate interstitial edema, neutrophil recruitment, and histopathological changes in lung tissues burn-induced ALI. To explore the mechanism of action of CEL, we assessed levels of inflammatory cytokines by ELISA assay, TUNEL staining and western blotting. Then we detected apoptosis-related factors, including the amount of apoptotic cells, caspase-3 activity, and Bax or Bcl-xl, respectively. The pulmonary microvascular hyperpermeability, histopathological characteristics, and a high W/D were attenuated by CEL for burn-injury rats. The concentration of cytokines burn-induced ALI from tissues and serum were decreased by CEL. Furthermore, after CEL treatment, TUNEL-positive cells, the protein level of Bax and caspase-3 activity reduced, and the level of Bcl-xl in protein increased. In conclusion, in lung injury burn-induced, CEL has a positive effect on anti-inflammation and anti-apoptosis. Thus, CEL could be as a latent for the cure of ALI burn-induced.
Keywords: Celastrol, expansive burn, acute lung injury, apoptosis, inflammatory
Introduction
Burn injury is a kind of common tissue damage caused by high temperature, chemicals or electricity. And lung injury is a main visceral complication caused by burn [1]. Acute lung injury (ALI) following severe burns remains a prominent source of morbidity and mortality among critically ill patients [2]. The primary pathological mechanisms underlying ALI are vascular endothelial and alveolar epithelial cell damages, which result in the destruction of blood-alveolar barrier. This destruction yields pulmonary edema, intrapulmonary hemorrhage, and severely impaired gas exchange [3]. Although the precise mechanisms of the development of ALI severe burn-induced remain unclear, inflammatory response accounts for major reasons. Literatures show that inflammatory responses following burn insult are associated with robust release of pro-inflammatory cytokines and activation of sympathetic inflammatory signaling pathways [2]. Inflammasomes are pattern recognition receptors, and associated with a great diversity of immune and cell apoptosis pathways. Among them, NLRP3 inflammasome is one of the most widely studied as it is related to many different kinds of diseases [4]. NLRP3, a complex adaptor protein, is composed of apoptosis-associated speck-like protein (ASC), and the serine protease caspase-1 (Casp1) [4]. In addition, NLRP3 regulates the maturity and release of IL-1β and IL-6; targeting of the NLRP3 inflammasome can play a protective role in paraquat and lipopolysaccharide (LPS)-induced ALI [1].
It was reported that the Chinese herb Thunder God Vine (Tripterygium wilfordii Hook F.) suppressed inflammatory and effectively treated many immune-related diseases [5]. CEL is a main element extracted from Thunder of God Vine. CEL showed anti-inflammation in inflammatory including rheumatoid arthritis and Crohn’s disease, by suppressing NF-κB activity and regulating the levels of pro-inflammatory cytokines [6]. CEL also was reported to suppress the expression of TNF-alpha and IL-1β from lung macrophages induced by LPS [7]. The above researches indicate that CEL may be able to treat lung injury.
In light of these findings, the underlying role of CEL in ALI remains unclear. According to the previous studies of CEL in inflammation, this study was to assess the effects of CEL against ALI burn-induced in rats.
Materials and methods
Animal model and grouping
This study was approved by Ethics Committee of Gansu Provincial Hospital. Healthy adult male Sprague-Dawley rats (200-250 g) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd (Shanghai, China) and adapted themselves to the new environment for a week before experiment. All the rats were randomly divided into 3 groups: a Sham Burn group, a Burn + Vehicle group, and a Burn + Celastrol group. Rats were narcotized (1% pentobarbital sodium 5 mL/kg) 24 h before burn experiment, and their dorsal fur was clipped using an electric clipper. Animals were subsequently placed into a template constructed to estimate 30% of their total body surface area (TBSA) and subjected to full-thickness burn injury via exposure to 92°C water for 18 seconds [2]. After burn injury, rats in Burn + Celastrol group were intraperitoneally injected with 4 mg/kg CEL, whereas rats in Burn + Vehicle group were treated with vehicle control (1 mL/kg DMSO) immediately. Later, rats were revived with 10 mL/kg hypodermic injection of normal salt solution to posterior limb [8]. The Sham Burn group rats were subjected to an identical procedure; however, they were immersed in room temperature water for 18 seconds, and no fluid resuscitation was performed.
Sample collection
Rats were sacrificed under anesthesia at 24 h after burn experiment. Blood samples were taken from aorta ventralis centrifuged at 1500 g for 10 minutes; serum was collected and stored at -80°C until needed. Animals’ sternums were cut open at the midline, and trachea and lungs were exposed. The lung tissues were collected and divided into three parts for western blotting, H&E staining and TUNEL analysis.
H&E staining
The lung tissue samples were obtained from the different groups at 24 h after burn injury, and were first cut into 0.5 cm thick pieces and fastened in 4% paraformaldehyde. To evaluate the quantification of lungs injury, lung tissues pieces were stained with hematoxylin and eosin (H&E), and 6 fields were randomly selected from each group and scored in a blinded manner by two individuals, as previously described [9].
Lung microvascular permeability assay
In the permeability assay, rats were exposed to anesthesia and were injected intravenously with EB (Sigma-Aldrich, St. Louis, USA) at a dose of 20 mg/kg. Thirty minutes later, euthanasia for rats was conducted and lung tissues were washed with the normal saline solution. When the pulmonary intravascular content was removed and lung tissue was weighed. Then, lung tissues were incubated in N, N-dimethylformamide (Sigma) for 48 h at room temperature. Finally, the absorbance values were detected at 620 nm using a microplate reader (SpectraMax M5; Molecular Devices, Sunnyvale, California, USA), and the amount was expressed as micrograms per 100 mg dry tissue.
Wet lung/dry lung weight ratio
Lungs were removed, weighed and subsequently dried in an oven at 80°C for 48 h. Then, dry weight of lung tissues was measured and we calculated the wet-to-dry lung weight ratio (W/D).
Measurement of inflammatory mediators
The concentrations of inflammatory cytokines in the lung or serum were measured using commercial enzyme-linked immunosorbent assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Then the optical density (OD) values were read at a wavelength of 450 nm and the results were showed as micrograms per milligram tissue or pictograms per milliliter serum.
TUNEL assay
Lung tissues paraffin-embedded were deparaffinized twice with xylene for 5 minutes each time and then were hydrated with a gradient of ethanol from 100% to 70%. After washed three times with PBS, slides were incubated with proteinase K for 30 min. Then, slides were washed again based on the previous method, fixed with formaldehyde and blocked. Finally, slides were stained using TUNEL kit (Roche Applied Science, Indianapolis, USA), and detected by a fluorescence microscope.
Caspase-3 activity measurement
Lung tissues were homogenized with lysis buffer at 10%. Then, lung homogenates were put on ice to allow the tissue to lyse fully, centrifuged and the supernatant was collected. The concentration of supernatant protein was detected and then the caspase-3 activity of the same amount protein from different groups was measured with the caspase-3 assay kit (Sigma-Aldrich, USA).
Western blot analysis
Protein concentrations were measured using BCA kit (Pierce Biotechnology, Rockford, IL, USA) at first. 15 µg protein was separated with 10% sodium dodecyl sulfate polyacrylamide gels and blotted onto polyvinylidene fluoride membranes. Transferred blots were blocked with 5% fat-free milk powder in TBS at room temperature for 2 h. Blots were then incubated over-night at 4°C with the primary antibodies against Bcl-xl, Bax, NLRP3, ASC, Caspase-1 p20 (Abcam, Cambridge, UK) and β-actin (Tianjin Sungene Biotech Co., Ltd., Tianjin, China), washed, and probed again with species-specific secondary antibodies (Earth, UK) coupled with horseradish peroxidase. And protein expression level was detected using an enhanced chemiluminescence reagent.
Statistical analysis
All data was analyzed with SPSS 16.0 and GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA, USA). The results were showed as the mean ± SD. Difference analysis between groups was conducted with the paired t-test and Student’s t-test. Difference was considered to be significant when P < 0.05.
Results
Celastrol treatment attenuated burn-induced lung injury
For the Burn + Vehicle group, the lung cavity was narrowed and there was interstitial edema, congestion, a good deal of inflammatory cells and more alveolar exudate in the lung tissues. After CEL treatment, we found these symptoms were relieved visibly (Figure 1A). Histopathological scores were calculated and showed a significant increase in post-burn rats (Sham Burn group vs Burn + Vehicle group, P < 0.01) (Figure 1B). Treatment of CEL significantly reduced the score (Burn + Celastrol group vs Burn + Vehicle group: P < 0.01). Similarly, we found that EB in the lung was distinctly increased in post-burn rats (Sham Burn group vs Burn + Vehicle group: P < 0.01) while the EB from Burn + Celastrol group was significantly decreased (Burn + Celastrol group vs Burn + Vehicle group: P < 0.01) (Figure 1C). In addition, W/D from different groups were detected and analyzed at 24 h post-burn and the result was similar to those of EB (Figure 1D).
Figure 1.
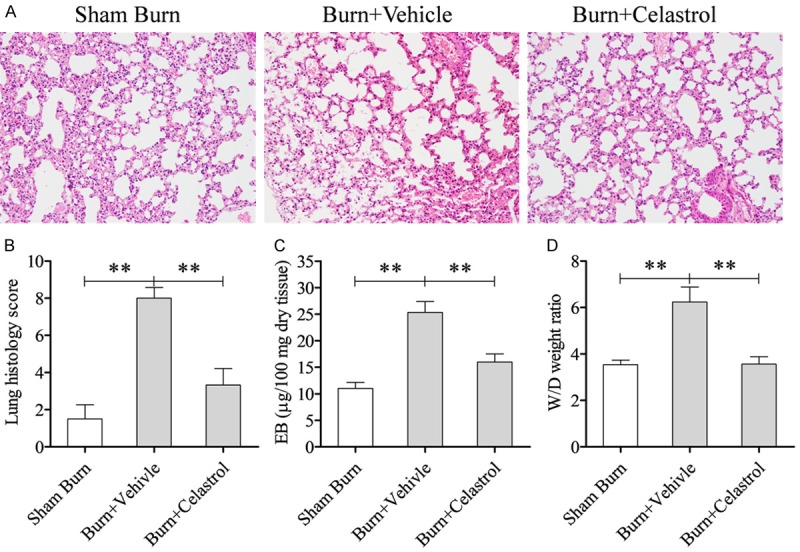
Celastrol attenuated burn-induced lung injury. (A) H&E staining showing the morphological changes of the lung tissue from rats receiving severe burn injury at 24 h post-burn, Vehicle = DMSO solution. And the lung histology score was showed in (B). (C) Showed the influence of Celastrol on pulmonary microvascular permeability and edema. Evans blue (EB) was extracted from the lungs obtained from each group at 24 h post-burn and measured. (D) Wet-to-dry lung weight ratio was showed (W/D). **P < 0.01; *P < 0.05.
Celastrol treatment attenuated local and systemic levels of inflammatory mediators induced by burn injury
For the burn rats, TNF-α, IL-1β, and IL-6 levels in the lung tissues were dramatically higher than those in sham group (Sham Burn group vs Burn + Vehicle group: P < 0.01), and these levels were significantly decreased in the CEL treatment group (Burn + Celastrol group vs Burn + Vehicle group: P < 0.01; Figure 2A-C). Similar to their levels in lung tissues, the levels of TNF-α, IL-1β, and IL-6 in the serum were significantly higher than those in Sham Burn group (Sham Burn group vs Burn + Vehicle group: P < 0.01) while the levels were obviously reduced by CEL treatment (Burn + Celastrol group vs Burn + Vehicle group: P < 0.01; Figure 2D-F).
Figure 2.
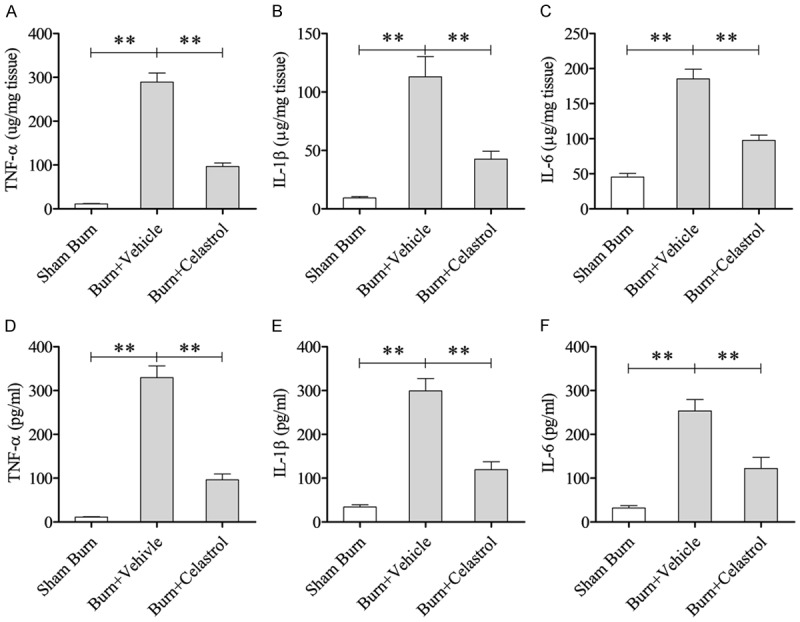
Effect of Celastrol on local and systemic inflammatory mediators after burn injury. The protein levels of TNF-α (A), IL-1β (B), and IL-6 (C) in the lung tissues were determined at 24 h post-burn. (B) The circulating levels of TNF-α (D), IL-1β (E), and IL-6 (F) in the serum were measured at 24 h after burn. **P < 0.01; *P < 0.05.
Celastrol treatment prevented lung cell apoptosis in burn-injury rats
According to Figure 3A-B, apoptotic cells from Burn + Vehicle group went up significantly compared with the Sham Burn group (Sham Burn group vs Burn + Vehicle group P < 0.01); these were significantly reduced by CEL treatment (Burn + Celastrol group vs Burn + Vehicle group: P < 0.01). Furthermore, we found the protein level of Bax was up-regulated and Bcl-xl was down-regulated by burn injury, while the protein expression of Bax and Bcl-xl decreased and increased by CEL treatment, respectively (Figure 3C). Caspases play a decisive role in cellular apoptosis [1]. After detection, we found that caspase-3 activity was observably increased in the lung tissues from rats after burn injury, which was prevented by CEL treatment (Sham Burn group vs Burn + Vehicle group: P < 0.01; Burn + Vehicle group vs Burn + Celastrol group: P < 0.01, Figure 3D).
Figure 3.
Celastrol treatment prevented lung cell apoptosis in burn-injury rats. A. Terminal deoxyribonucleotidyl transferase-mediated deoxyuridine 5-triphosphate-digoxigenin nick end labeling staining images shown at 200 magnification. B. Representation of terminal deoxyribonucleotidyl transferasemediated deoxyuridine 5-triphosphate-digoxigenin nick end labeling (TUNEL)-positive cells averaged over 10 microscopic fields per animal. C. Expression of Bcl-xl and Bax in the lung tissues. D. Caspase-3 activity in the lung tissues was determined. Data are presented as the mean ± SD. *P < 0.05; **P < 0.01.
Celastrol inhibits the activation of NLRP3 inflammasome
To determine whether CEL successfully decreased the NLRP3 activity, the expressions of NLRP3, ASC and Caspase-1 p20 at the protein level were measured by western blotting. A study showed that the NLRP3 inflammasome was significantly activated at 24 h following burn injury; therefore, we selected samples 24 h post-burn for study [5]. The group treatment with CEL significantly inhibited NLRP3 inflammasome signaling NLRP3, ASC and Caspase-1 p20 protein expression (Figure 4A). Densitometric analysis results of triplicate western blots were shown in Figure 4B-D.
Figure 4.
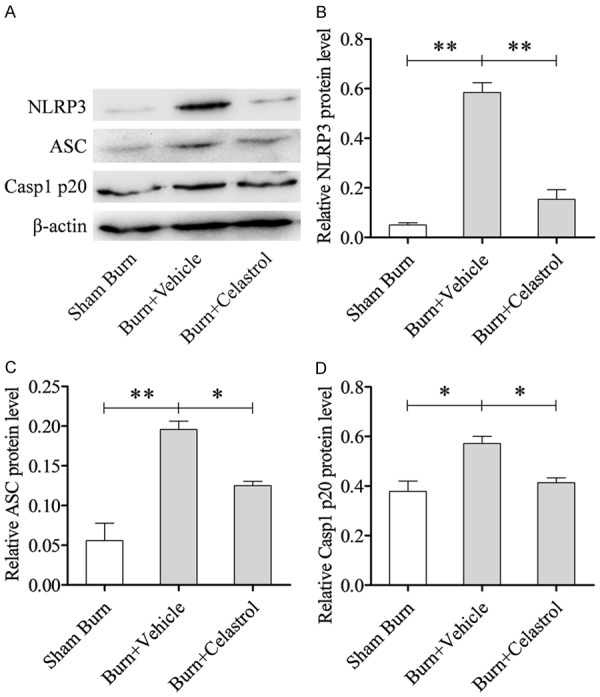
Celastrol inhibited NLRP3 inflammasome. A. Representative western blot results of NLRP3, ASC and Casp1 p20 in the lung tissues; B-D. Densitometric analysis results of triplicate western blots. **P < 0.01; *P < 0.05.
Discussion
CEL has a positive effect on inhibiting inflammation [7], and has been further extensively studied as a target therapy method for inflammatory diseases [6]. Der Sarkissian [10] suggested that it was confirmed that CEL may serve as a new type effective cardio-protective drug to treat myocardial infarction. Cheng et al. [11] also demonstrated that CEL improved cardiac dysfunction caused by myocardial fibrosis. In the present study, these aforementioned histopathological changes in lung injury by burns would be significantly alleviated by the administration of CEL. Furthermore, the results of EB and W/D indicated that CEL effectively relieved both hyper permeability and pneumonedema.
If the pro-inflammatory factors are released abnormally, the body’s immune system will disorder. We found that the level of cytokine factors in tissues or blood was increased after burn-induced injury in the present study. In a mouse model of allergic asthma, it was reported that CEL could inhibit inflammatory via the NF-κB pathway, which decreased the expression of inflammatory cytokines at mRNA and protein expression level [12]. Similarly, the level of pro-inflammatory cytokines was suppressed in CEL-treatment burn injury group, confirming that CEL can effectively reduce the levels of inflammatory cytokines from tissues or blood and help to cure. And our results were consistent with some other studies which indicated that CEL suppressed cytokines release [13,14]. Therefore, CEL could be helpful in improving burn injury indirectly.
It was reported that the secretion of IL-1β could up-regulate the primary function of NLRP3 inflammasome and activate the innate immune system. NLRP3 inflammasome activation positively regulates the cleavage and activation of Casp1, promoting the maturation of effector pro-inflammatory cytokines, such as pro-interleukin (IL)-1β and pro-IL-18 [15-17]. Based on western-blot, we observed that the protein levels of NLRP3, ASC and caspase-1 p20 increased in ALI burn-induced. CEL administration inhibited the NLRP3, and significantly attenuated ALI, which indicated that CEL may inhibit NLRP3 inflammasome and protect lung tissues in the setting of burn-induced ALI.
Apoptosis has been shown to be one of the pathogenesis of lung injury [18]. Based on the apoptosis of alveolar cells and accumulation of inflammatory cells and cytokines in lung, there is a vital connection between apoptosis and lung injury, and finally lung injury further deteriorates into ALI [19,20]. When there are more apoptotic cells in the lungs, the inflammation in the lungs will be more serious [21]. Our research showed that apoptotic cells from CEL treatment group were less than those from burn injury group, indicating that CEL may play a protective role in lung tissues via reducing lung cells apoptosis.
At the molecular level, apoptosis-related genes, including anti-apoptotic Bcl-xl, pro-apoptotic Bax and caspase-3, were extensively studied [22-24]. Caspase-3, is recognized to be the most essential of the executioner caspases [25]. Thus, caspase-3 activity was measured in this study and the results showed that CEL impeded the up-regulation of Bax and caspase-3 activity by burn injury, and down-regulation of Bcl-xl, which suggested that CEL may inhibit cell apoptosis in burn-induced rats and indirectly treat ALI.
Conclusion
In summary, this study demonstrated that CEL inhibited inflammasome mediators, the activation of NLRP3 inflammasome and reduced lung cell apoptosis in ALI burn-induced. This inhibition could effectively reduce inflammatory responses and the lung cell apoptosis burn-induced to protect lung tissue in acute lung injury.
Disclosure of conflict of interest
None.
References
- 1.Li T, Cai S, Zeng Z, Zhang J, Gao Y, Wang X, Chen Z. Protective effect of polydatin against burn-induced lung injury in rats. Respir Care. 2014;59:1412–1421. doi: 10.4187/respcare.02831. [DOI] [PubMed] [Google Scholar]
- 2.Han S, Cai W, Yang X, Jia Y, Zheng Z, Wang H, Li J, Li Y, Gao J, Fan L, Hu D. ROS-mediated NLRP3 Inflammasome activity is essential for burn-induced acute lung injury. Mediators Inflamm. 2015;2015:720457. doi: 10.1155/2015/720457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wree A, Eguchi A, McGeough MD, Pena CA, Johnson CD, Canbay A, Hoffman HM, Feldstein AE. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology. 2014;59:898–910. doi: 10.1002/hep.26592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sang X, Chen Y, Chen W, Xie J, Meng G, Zhong J, Li T, Lu A. Celastrol specifically inhibits the activation of NLRP3 inflammasome. Sci China Life Sci. 2018;61:355–357. doi: 10.1007/s11427-017-9048-8. [DOI] [PubMed] [Google Scholar]
- 6.Wei Y, Wang Y. Celastrol attenuates impairments associated with lipopolysaccharide-induced acute respiratory distress syndrome (ARDS) in rats. J Immunotoxicol. 2017;14:228–234. doi: 10.1080/1547691X.2017.1394933. [DOI] [PubMed] [Google Scholar]
- 7.Allison AC, Cacabelos R, Lombardi VR, Alvarez XA, Vigo C. Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:1341–1357. doi: 10.1016/s0278-5846(01)00192-0. [DOI] [PubMed] [Google Scholar]
- 8.Şehirli AÖ, Satılmış B, Tetik Ş, Çetinel Ş, Yeğen B, Aykaç A, Şener G. Protective effect of betaine against burn-induced pulmonary injury in rats. Ulus Travma Acil Cerrahi Derg. 2016;22:417–422. doi: 10.5505/tjtes.2015.60137. [DOI] [PubMed] [Google Scholar]
- 9.Aneja R, Hake PW, Burroughs TJ, Denenberg AG, Wong HR, Zingarelli B. Epigallocatechin, a green tea polyphenol, attenuates myocardial ischemia reperfusion injury in rats. Mol Med. 2004;10:55–62. doi: 10.2119/2004-00032.aneja. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Der Sarkissian S, Cailhier JF, Borie M, Stevens LM, Gaboury L, Mansour S, Hamet P, Noiseux N. Celastrol protects ischaemic myocardium through a heat shock response with up-regulation of haeme oxygenase-1. Br J Pharmacol. 2014;171:5265–5279. doi: 10.1111/bph.12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng M, Wu G, Song Y, Wang L, Tu L, Zhang L, Zhang C. Celastrol-induced suppression of the MiR-21/ERK signalling pathway attenuates cardiac fibrosis and dysfunction. Cell Physiol Biochem. 2016;38:1928–1938. doi: 10.1159/000445554. [DOI] [PubMed] [Google Scholar]
- 12.Kim DY, Park JW, Jeoung D, Ro JY. Celastrol suppresses allergen-induced airway inflammation in a mouse allergic asthma model. Eur J Pharmacol. 2009;612:98–105. doi: 10.1016/j.ejphar.2009.03.078. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Zhang B, Li J, Feng M, Zhang Y, Yao W, Zhang C, Wan L. Celastrol attenuates incision-induced inflammation and pain associated with inhibition of the NF-kappaB signalling pathway via SARM. Life Sci. 2018;205:136–144. doi: 10.1016/j.lfs.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 14.Tong S, Zhang L, Joseph J, Jiang X. Celastrol pretreatment attenuates rat myocardial ischemia/reperfusion injury by inhibiting high mobility group box 1 protein expression via the PI3K/akt pathway. Biochem Biophys Res Commun. 2018;497:843–849. doi: 10.1016/j.bbrc.2018.02.121. [DOI] [PubMed] [Google Scholar]
- 15.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 16.Szabo G, Csak T. Inflammasomes in liver diseases. J Hepatol. 2012;57:642–654. doi: 10.1016/j.jhep.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 17.Gross O, Thomas CJ, Guarda G, Tschopp J. The inflammasome: an integrated view. Immunol Rev. 2011;243:136–151. doi: 10.1111/j.1600-065X.2011.01046.x. [DOI] [PubMed] [Google Scholar]
- 18.Fine A, Janssen-Heininger Y, Soultanakis RP, Swisher SG, Uhal BD. Apoptosis in lung pathophysiology. Am J Physiol Lung Cell Mol Physiol. 2000;279:L423–427. doi: 10.1152/ajplung.2000.279.3.L423. [DOI] [PubMed] [Google Scholar]
- 19.Kitamura Y, Hashimoto S, Mizuta N, Kobayashi A, Kooguchi K, Fujiwara I, Nakajima H. Fas/fasL-dependent apoptosis of alveolar cells after lipopolysaccharide-induced lung injury in mice. Am J Respir Crit Care Med. 2001;163:762–769. doi: 10.1164/ajrccm.163.3.2003065. [DOI] [PubMed] [Google Scholar]
- 20.Matthay MA. Conference summary: acute lung injury. Chest. 1999;116:119S–126S. doi: 10.1378/chest.116.suppl_1.119s. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt EP, Tuder RM. Role of apoptosis in amplifying inflammatory responses in lung diseases. J Cell Death. 2010;2010:41–53. doi: 10.4137/JCD.S5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li L, Wu W, Huang W, Hu G, Yuan W, Li W. NF-kappaB RNAi decreases the bax/bcl-2 ratio and inhibits TNF-alpha-induced apoptosis in human alveolar epithelial cells. Inflamm Res. 2013;62:387–397. doi: 10.1007/s00011-013-0590-7. [DOI] [PubMed] [Google Scholar]
- 23.Du H, Wolf J, Schafer B, Moldoveanu T, Chipuk JE, Kuwana T. BH3 domains other than bim and bid can directly activate bax/bak. J Biol Chem. 2011;286:491–501. doi: 10.1074/jbc.M110.167148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim R, Emi M, Tanabe K. Role of mitochondria as the gardens of cell death. Cancer Chemother Pharmacol. 2006;57:545–553. doi: 10.1007/s00280-005-0111-7. [DOI] [PubMed] [Google Scholar]
- 25.Kaufmann SH, Lee SH, Meng XW, Loegering DA, Kottke TJ, Henzing AJ, Ruchaud S, Samejima K, Earnshaw WC. Apoptosis-associated caspase activation assays. Methods. 2008;44:262–272. doi: 10.1016/j.ymeth.2007.11.005. [DOI] [PubMed] [Google Scholar]



