Abstract
Objective: To examine the expression level of B7-H6 in chronic myeloid leukemia (CML) patients and to explore its clinical significance. Methods: Two hundred twenty-eight CML patients were included and peripheral blood (PB) and bone marrow (BM) mononuclear cells were collected for B7-H6 mRNA expression analyses by quantitative real time polymerase chain reaction (PCR). Results: The expression of B7-H6 mRNA was successfully detected in all PB and BM samples. According to the clinical characteristics of CML patients, no difference was found in the B7-H6 level of PBMCs. However, a significantly decreased B7-H6 level was noted in BM samples from CML with BCR-ABL1/ABL > 0.1% (10 copies of BCR-ABL1/10000 copies of ABL) compared to ≤ 0.1% (P < 0.0001), and a negative correlation was found between the expression level of B7-H6 in BM and the number of BCR-ABL1/ABL transcripts (r = -0.26, P = 0.0057). A significant difference in PFS was observed between patients with high expression level of B7-H6 and low expression level in BM (χ2 = 12.53, P = 0.0004). Conclusion: The expression of the B7-H6 gene in CML is correlated with BCR-ABL1 copy number and responsiveness to treatment, and monitoring of B7-H6 expression may be used to predict CML prognosis, progression, and treatment efficacy evaluation.
Keywords: Chronic myeloid leukemia, B7-H6, BCR-ABL1, real-time quantitative PCR, complete cytogenetic response
Introduction
Chronic myeloid leukemia (CML) is a malignant myeloproliferative disease, characterized by clonal expansion and accumulation of bone marrow cells [1]. Recent application of tyrosine kinase inhibitor (TKI) in clinical practice has resulted in transition of malignant CML into a controllable disease, and an estimated more than 80% 10-year overall survival has been achieved. However, the prognosis of CML is still poor due to the rapid malignant progression of the disease despite of TKI treatment. In addition, adverse reactions associated with long-term TKI treatment, including toxicity and acquired resistance, could also deteriorate in CML patients [2,3]. Therefore, seeking an effective disease biomarker to monitor disease progression and treatment response is urgently needed in current clinical practice.
B7-H6, a newly discovered member of the B7 family, encodes a 454-aa-long type I trans-membrane protein, which is selectively expressed on certain tumor cells such as leukemia, lymphoma, and gastric carcinoma [4]. It is reported that B7-H6 can bind with NKp30 on the surface of natural killer cells and initiates NKp30-dependent innate immune responses [5,6]. Although B7-H6 exists extensively on the leukemia cell surface, its exact role in different subtype of leukemia is largely unknown.
In the present study, we aimed to examine the expression level of B7-H6 in peripheral blood (PB) and bone marrow mononuclear cells (BMMCs) of CML patients and to explore its possible clinical significance.
Materials and methods
CML patients
A total of 120 PBMCs and 108 BMMCs samples of CML patients between November 2014 and June 2017 were obtained from the tissue bank of the Department of Hematology of The First Affiliated Hospital of Soochow University. The clinical data of all enrolled CML patients were obtained from the patient database of the First Affiliated Hospital of Soochow University.
PBMCs samples were derived from 81 male patients and 39 females with a median age of 41 yrs (5-72 yrs), including 7 cases of accelerated phase (AP), 9 cases of blast phase (BP), and 104 cases of chronic phase (CP). BMMC samples were derived from 78 male patients and 30 females with a median age of 41 years (7-88 yrs), including 5 cases of accelerated phase (AP), 14 cases of blast phase (BP) and 89 cases of chronic phase (CP) (See Supplemental Data).
This study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University, adhered to the guidelines of the Declaration of Helsinki and written informed consents were obtained from all included patients.
Reverse transcription real time quantitative PCR
RNA was extracted from the above PBMCs and BMMCs using Trizol reagent (Thermo Fisher, CA, USA) and the quality and quantity evaluation was performed using NanoDrop 2000 (Thermo Fisher, CA, USA). cDNA synthesis was then carried out using RevertAid RT Reverse Transcription Kit (Thermo Fisher, CA, USA) according to manufacturer’s instruction, followed by Taqman method and SYBR green method for real time quantitative PCR analysis for BCR-ABL and ABL genes and B7-H6 expression, respectively. For Taqman method, PCR was carried out using an Ex Taq™ HS PCR kit (Takara, Dalian, China) and a MJ Research Opticon TM2 system (MJ Research, Waltham, MA, USA) for evaluation of the expression level of BCR-ABL and ABL. For SYBR green method, PCR was carried out using a SYBR Green PCR Master Mix (Roche, Basel, Switzerland) and a StepOne Plus Real-Time PCR System (Applied Biosystems, foster City, CA, USA) for evaluation of the expression level of B7-H6. Expression of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was assessed simultaneously in all samples as an internal control for mRNA. The actual copy numbers were determined for both the BCR-ABL fusion gene and the ABL gene. The ratio of the BCR-ABL fusion gene and the ABL gene was calculated according to 10 BCR-ABL copies/10000 ABL copies unless noted otherwise. Relative gene expression of B7-H6 was determined by the ΔCT method as follows: ΔCT = CTB7-H6-CTGAPDH. Oligonucleotide sequences specific for B7-H6, GAPDH, BCR-ABL and ABL are listed in Table 1.
Table 1.
Oligonucleotide sequences specific for B7-H6, GAPDH, BCR-ABL and ABL
| Target gene | Primer sequence | |
|---|---|---|
| B7H6 | Forward | 5’-GACCTGGAGCCATTGTGTCT-3’ |
| Reverse | 5’-AAGCTGGACTGTTCCCTGTG-3’ | |
| GAPDH | Forward | 5’-GCAAATTCCATGGCACCGT-3’ |
| Reverse | 5’-TCGCCCCACTTGATTTTGG-3’ | |
| BCR-ABL | Forward | 5’-AGCATTCCGTCTGACCATCA-3’ |
| Reverse | 5’-ACTCAGACCCTGAGGCTCAAAG-3’ | |
| Probe | FAM-AAGCCCTTCAGCGGCCAGTAGCAT-TAMRA | |
| ABL | Forward | 5’-GATACGAAGGGAGGGTGTACCA-3’ |
| Reverse | 5’-CTCGGCCAGGGTGTTGAA-3’ | |
| Probe | FAM-TGCTTCTGATGGCAAGCTCTACGTCTCCT-TAMRA |
Positive control plasmid construction
The fragment of the ABL gene was amplified, purified and cloned into PUCm-T vector, and then transformed and screen to obtain PUCm-ABL-T plasmid. After DNA quantification by NanoDrop 2000, the molecular copy number was calculated according to molecular weight and Avogadro constant (6.023 × 1023 molecules/mol), and diluted for 10 times with 109 copies/μL to establish a positive template gradient as reference for comparison. All the positive controls were frozen at -20°C for further experiments.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 6 (GraphPad Software, Inc., San Diego, CA, USA). The expression levels of B7-H6 were expressed as Mean ± Standard deviation (SD). The categorical data were expressed as number and percentage. Kruskal-Wallis H test and Mann-Whitney U test were respectively employed for the multiple groups and between groups comparison. Spearman rank test was used for correlation analysis. Moreover, the expression levels of B7-H6 in BMMCs samples were divided into high and low expression groups according to the mean value of the ΔCT in the setting of Progression-free survival (PFS)-calculation. PFS is defined as the time elapsed from initial diagnosis to disease progression, PFS and hazard ratio (HR) with two-sided 95% confidence interval (CI) of CML patients with high and low B7-H6 expression were calculated by Kaplan-Meier method and compared by Log-rank test. P < 0.05 indicates statistically significance.
Results
Difference of expression of B7-H6 in PBMCs according to clinical features of CML patients
No difference was found in B7-H6 level of PBMCs according to the clinical characteristics of CML patients including sex, age, phase, BCR-ABL1/ABL fusion gene number, additional chromosome abnormality, ABL kinase region, with or without chemotherapy, transplantation, TKI medication, and presence or absence of CCR or MMR (Table 2).
Table 2.
Difference in the expression level of B7-H6 in peripheral blood according to clinical features of CML patients
| Clinical parameters | Patient No. (%) | B7-H6 level | P-value |
|---|---|---|---|
| Sex | 0.904 | ||
| Male | 81 (67.5%) | 9.11 ± 2.15 | |
| Female | 39 (32.5%) | 8.87 ± 1.66 | |
| Age | 0.317 | ||
| ≤ 41 yrs | 64 (53.3%) | 8.96 ± 2.22 | |
| > 41 yrs | 56 (46.7%) | 9.12 ± 1.74 | |
| Phases | 0.655 | ||
| Acceleration phase | 7 (5.8%) | 9.44 ± 1.59 | |
| Blast phase | 9 (7.5%) | 9.21 ± 3.77 | |
| Chronic phase | 104 (86.7%) | 8.99 ± 1.84 | |
| BCR-ABL1/ABL | 0.494 | ||
| ≤ 10 copies/10000 copies | 60 (50.0%) | 8.99 ± 2.07 | |
| > 10 copies/10000 copies | 60 (50.0%) | 9.08 ± 1.95 | |
| Additional chromosome abnormalities | 0.115 | ||
| Yes | 9 (7.5%) | 8.43 ± 1.54 | |
| No | 18 (15%) | 9.95 ± 2.01 | |
| ABL kinase domain mutation | 0.637 | ||
| Yes | 15 (12.5%) | 9.55 ± 2.86 | |
| No | 49 (40.8%) | 9.08 ± 2.14 | |
| Hematopoietic stem cell transplantation | 0.195 | ||
| Yes | 15 (12.5%) | 10.25 ± 3.47 | |
| No | 105 (87.5%) | 8.86 ± 1.65 | |
| Chemotherapy | 0.232 | ||
| Yes | 3 (2.5%) | 10.61 ± 2.69 | |
| No | 117 (97.5%) | 8.99 ± 1.98 | |
| TKI medication | 0.735 | ||
| First generation | 21 (17.5%) | 8.63 ± 1.50 | |
| Second generation | 35 (29.2%) | 8.87 ± 2.46 | |
| Complete cytogenetic response (CCR) | 0.120 | ||
| Yes | 65 (54.2%) | 8.86 ± 2.04 | |
| No | 17 (14.2%) | 9.51 ± 2.21 |
Patient no. was obtained from available data, and expressed as number and percentages, whereas the expression of B7-H6 was expressed as mean ± standard deviation. TKI: Tyrosine kinase inhibitors.
Difference of the expression of B7-H6 in BMMCs according to clinical features of CML patients
Patients with BCR-ABL1/ABL ≤ 0.1% or with complete cytogenetic response (CCR) after TKI treatment respectively had significantly higher B7-H6 level in BMMCs than those with BCR-ABL1/ABL > 0.1% (P < 0.0001) or without CCR (P = 0.001) (Table 3). However, no difference was found in B7-H6 level in BMMCs based on other clinical measures including sex, age, phase, additional chromosome abnormality, ABL kinase region, with or without chemotherapy, transplantation, and TKI medication (Table 3).
Table 3.
Difference of the expression level of B7-H6 in bone marrow according to clinical features of CML patients
| Clinical parameters | Patient No. (%) | B7-H6 level | P-value |
|---|---|---|---|
| Sex | 0.310 | ||
| Male | 78 (72.2%) | 8.07 ± 3.17 | |
| Female | 30 (27.8%) | 8.81 ± 1.74 | |
| Age | 0.208 | ||
| ≤ 41 yrs | 55 (50.9%) | 7.94 ± 3.07 | |
| > 41 yrs | 53 (49.1%) | 8.62 ± 2.60 | |
| Phase | 0.597 | ||
| Acceleration phase | 5 (4.6%) | 8.96 ± 3.82 | |
| Blast phase | 14 (13.0%) | 7.35 ± 3.83 | |
| Chronic phase | 89 (82.4%) | 8.38 ± 2.63 | |
| BCR-ABL1/ABL | < 0.0001**** | ||
| ≤ 10 copies/10000 copies | 52 (48.1%) | 9.34 ± 2.82 | |
| > 10 copies/10000 copies | 56 (51.9%) | 7.28 ± 2.52 | |
| Additional chromosome abnormalities | 0.339 | ||
| Yes | 18 (16.7%) | 7.38 ± 2.88 | |
| No | 19 (17.6%) | 8.66 ± 2.53 | |
| ABL kinase domain mutation | 0.610 | ||
| Yes | 8 (7.4%) | 9.34 ± 3.55 | |
| No | 37 (34.3%) | 8.37 ± 2.88 | |
| Hematopoietic stem cell transplantation | 0.948 | ||
| Yes | 9 (8.3%) | 8.07 ± 5.16 | |
| No | 99 (91.7%) | 8.29 ± 2.59 | |
| Chemotherapy | 0.411 | ||
| Yes | 10 (9.3%) | 8.52 ± 3.22 | |
| No | 98 (90.7%) | 8.25 ± 2.83 | |
| TKI medication | 0.672 | ||
| First generation | 15 (13.9%) | 9.13 ± 2.24 | |
| Second generation | 13 (12.0%) | 8.88 ± 2.15 | |
| Complete cytogenetic response (CCR) | 0.001** | ||
| Yes | 53 (49.1%) | 9.09 ± 2.75 | |
| No | 9 (8.3%) | 5.74 ± 2.67 |
Patient no. was obtained from available data, and expressed as number and percentages, whereas the expression of B7-H6 was expressed as mean ± standard deviation. TKI: Tyrosine kinase inhibitors.
P < 0.01;
P < 0.0001.
Correlation analysis between the expression level of B7-H6 gene in BMMCs and BCR-ABL1/ABL transcripts
We also performed correlation analyses between the expression level of B7-H6 in BMMCs and BCR-ABL1/ABL transcript s. Negative correlations were found between the expression level of B7-H6 and BCR-ABL1/ABL ratio (r = -0.26, P = 0.0057) (Figure 1).
Figure 1.
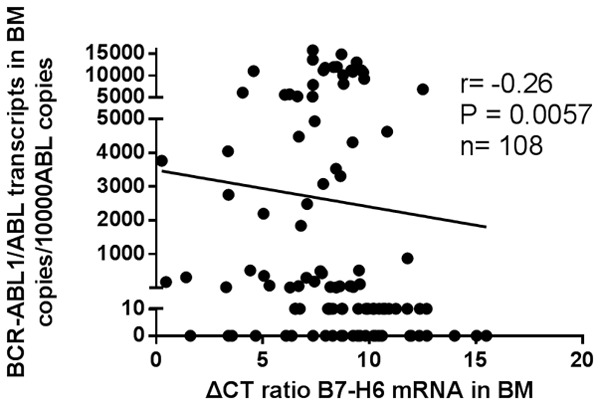
Spearman rank correlation analysis of the relationship between the expression level of B7-H6 gene in BMMNCs and BCR-ABL1/ABL transcripts (r = -0.26, P = 0.0057).
Survival analysis
A significant difference in PFS was observed between patients with high expression level of B7-H6 and patients with low level in BMMCs (χ2 = 12.53, P = 0.0004). B7-H6-high group (n = 36; median PFS, not achieved; 8-year PFS, 92.3%), B7-H6-low group (n = 23; median PFS, 81 months; 8-year PFS, 41.8%). Patients with high B7-H6 level had significantly longer PFS than those with low level (Hazard Ratio: 0.06 vs 15.58, 95% CI: 0.03-0.37 vs 2.68-30.23) (Figure 2).
Figure 2.
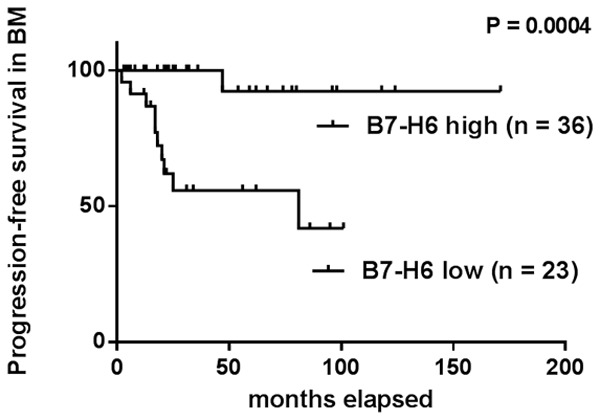
Kaplan-Meier curves for progression-free survival (PFS) of CML patients in BM according to B7-H6 expression levels. B7-H6-high group (n = 36; median PFS: not achieved; 8-year PFS: 92.3%; Hazard Ratio: 0.06; 95% CI = 0.03-0.37), B7-H6-low group (n = 23; median PFS, 81 months; 8-year PFS: 41.8%; Hazard Ratio: 15.58; 95% CI = 2.68-30.23) (P = 0.0004).
Discussion
B7-H6, a novel molecule of the B7 family, can bind its receptor NKp30 to exert anti-tumor effects by helping NK cells to recognize abnormal cells [7]. Studies have shown that the expression of B7-H6 is restricted to tumor cells, and not expressed on normal hematopoietic cells of healthy individuals or in a steady state. However, B7-H6 expression can be induced under the stimulation of pro-inflammatory cytokines [8,9].
Chronic myeloid leukemia (CML) is caused by the uncontrolled proliferation of bone marrow myeloid progenitor cells harboring BCR-ABL1 oncoprotein [10]. Studies have shown that increased expression of BCR-ABL1 could lead to a progression of CML from chronic phase to acute phase or to an explosive crisis [11]. Currently, quantification of BCR-ABL transcripts has proven to be the most sensitive method available for monitoring the progression of CML [12]. Our study found that the expression level of B7-H6 in BMMCs in patients with BCR-ABL1/ABL > 0.1% was significantly lower than that of patients with BCR-ABL1/ABL ≤ 0.1%. We further analyzed the correlation between the expression level of B7-H6 gene and the relative amount of BCR-ABL1 transcripts in BMMCs and confirmed a negative correlation between them. Moreover, the results of survival analysis also shown that CML patients with high B7-H6 expression level had longer progression-free survival. Christiansson et al. previously showed that upregulated B7-H1 in myeloid cells from CML patients served as one of the mechanisms of immune escape from immunotherapy in combination with tyrosine kinase inhibitors. Moreover, enhanced B7-H1 and B7-DC expression was found in bone marrow derived CD34+ cells from myelodysplastic syndrome patients treated with hypomethylating agents [13,14]. These data together with ours suggested that enhanced B7 family molecules expression might serve as protective indicators predicting prognosis of CML patients in clinical practices. Decreased expression level of B7-H6 in BMMCs implied poor prognosis or malignant transform in CML.
Complete Cytogenetic Response (CCR) is the gold standard during TKI treatment and should be maintained in a long-term manner during the course of CML [12]. Our study found that the expression level of B7-H6 in BMMCs from CML patients who achieved CCR after TKI treatment was higher than in those without CCR, supporting our conclusion that enhanced B7-H6 expression might be used as a protective indicator in CML patients.
However, we found no significant difference in the expression level of B7-H6 according to BCR-ABL1/ABL ratio and CCR status in PBMCs from CML patients. We attributed this result to the cell differentiation status. B7-H6 is considered to be related to the differentiation status of cancer tissues, and upregulated B7-H6 expression is significantly associated with high differentiation [15,16]. Our results also showed that the relative expression of B7-H6 mRNA in PBMCs was higher than that in BMMCs (9.03 ± 2.00 vs 8.27 ± 2.85) and the results were the same in PBMCs and BMMCs of the same patient. (8.79 ± 1.79 vs 7.21 ± 4.74) (Figures 3, 4).
Figure 3.
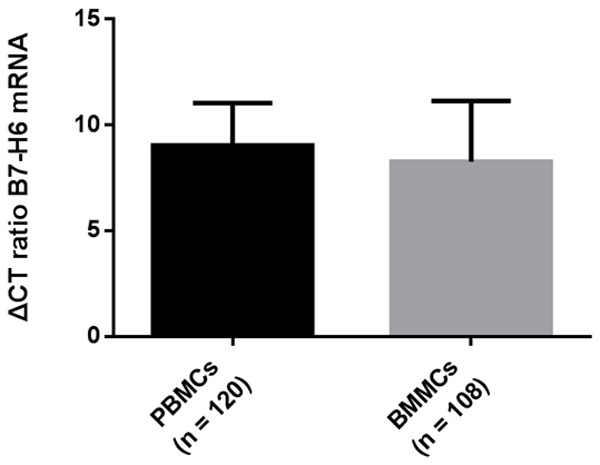
The relative expression of B7-H6 mRNA in PBMCs was higher than that in BMMCs. B7-H6 expression levels in PBMCs vs BMMCs (9.03 ± 2.00 vs 8.27 ± 2.85).
Figure 4.
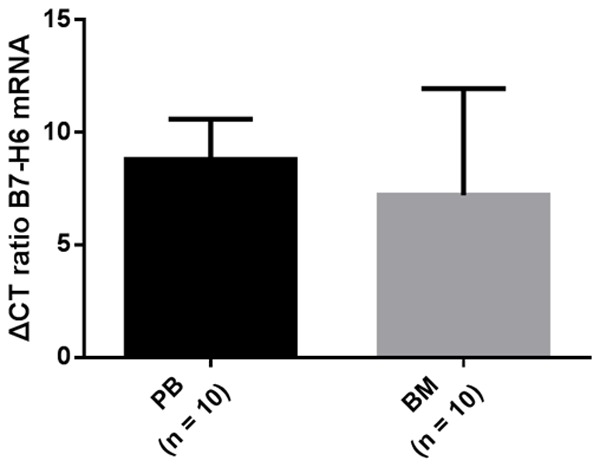
The relative expression of B7-H6 mRNA in PBMCs of the same individual was higher than that in BMMCs. B7-H6 expression levels in PBMCs vs BMMCs (8.79 ± 1.79 vs 7.21 ± 4.74).
Although BCR-ABL transcripts by reverse transcription real time quantitative PCR is the gold standard strategy to evaluate response to TKI therapy, there are some shortcomings for RT-PCR to detect BCR-ABL transcripts [17]. Above all, using RT-qPCR to detect CML patients with low-copy number transcripts is limited [18]. Recently, several studies point that only 40% of CML patients without detecting BCR-ABL transcripts after treatment with tyrosine kinase inhibitors therapy, and did not relapse after drug discontinuation, whereas 60% of patients relapsed [19,20]. According to previous descriptions, calibration errors between targets and standards and ABL as the control gene gives a spuriously high result at high transcript levels contribute to the uncertainty in this method [21,22]. Finally, each laboratory’s BCR-ABL assay has own characteristics including accuracy, precision, analytic sensitivity and specificity, and reportable range, which leads to differences in measuring BCR-ABL transcripts between laboratories. So a standardized international scale should be established, but lack of flexibility to improve individual laboratory testing will be the main limitation [23]. However, detection of B7-H6 mRNA by real-time quantitative PCR was not restricted by low-copy number transcripts or control genes and there was little difference in each laboratory. It is easier and more convenient to use real-time quantitative PCR to detect the expression level of B7-H6 in bone marrow as an adjunct to monitor CML progression and predict prognosis.
Our study also has some limitations: First, the number of included patients was relatively small, which may result in relative high deviation. Second, we only employed real-time quantitative PCR here to analyze the mRNA level of B7-H6, but the protein level was not confirmed due to the lacking of protein samples. Third, the emergence of TKI makes the 10-year overall survival rate of CML patients exceed 80% [2]. Our survival analysis did not include overall survival because all patients were still alive when conducting this study. Furthermore, confirmation of the expression profiles of B7-H6 and the accurate location of B7-H6 in the cell subpopulation could not be achieved. Taken together, further study with a larger number of CML patients with similar clinical features and available of the sufficient amount of bone marrow cells and PBMCs might be analyzed to confirm the results obtained here in the near future.
In summary, our study demonstrated for the first time the clinical significance of B7-H6 expression in chronic myeloid leukemia. By analyses of the B7-H6 expression in PBMCs and BMMCs of CML patients and exploration its possible clinical significance, we found that a decreased level of B7-H6 is an unfavorable indicator of CML development and is inversely related to the copy number of the fusion gene BCR-ABL1 transcript, suggesting that the detection of B7-H6 expression levels in BM may be further employed to assist CML progression monitoring and prognosis prediction in clinical practice.
Acknowledgements
This work was supported in part by grants from the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), The National Natural Science Foundation of China (81773356, 81802385, 81673096, 81600565, 81700129 and 81700235), The Natural Science Foundation (BK20171204 and BK20150296) of Jiangsu Province, and The Science and Technology Plan of Suzhou Municipal Government (SYS201704).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Holyoake TL, Vetrie D. The chronic myeloid leukemia stem cell: stemming the tide of persistence. Blood. 2017;129:1595–1606. doi: 10.1182/blood-2016-09-696013. [DOI] [PubMed] [Google Scholar]
- 2.Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP, Baccarani M, Deininger MW, Cervantes F, Fujihara S, Ortmann CE, Menssen HD, Kantarjian H, O’Brien SG, Druker BJ. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376:917–927. doi: 10.1056/NEJMoa1609324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masamoto Y, Kurokawa M. Targeting chronic myeloid leukemia stem cells: can transcriptional program be a druggable target for cancers? Stem Cell Investig. 2018;5:10. doi: 10.21037/sci.2018.03.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandt CS, Baratin M, Yi EC, Kennedy J, Gao Z, Fox B, Haldeman B, Ostrander CD, Kaifu T, Chabannon C, Moretta A, West R, Xu W, Vivier E, Levin SD. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med. 2009;206:1495–1503. doi: 10.1084/jem.20090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joyce MG, Tran P, Zhuravleva MA, Jaw J, Colonna M, Sun PD. Crystal structure of human natural cytotoxicity receptor NKp30 and identification of its ligand binding site. Proc Natl Acad Sci U S A. 2011;108:6223–6228. doi: 10.1073/pnas.1100622108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiegler N, Textor S, Arnold A, Rolle A, Oehme I, Breuhahn K, Moldenhauer G, Witzens-Harig M, Cerwenka A. Downregulation of the activating NKp30 ligand B7-H6 by HDAC inhibitors impairs tumor cell recognition by NK cells. Blood. 2013;122:684–693. doi: 10.1182/blood-2013-02-482513. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Mo J, Jia X, He Y. The B7 family member B7-H6: a new bane of tumor. Pathol Oncol Res. 2018;24:717–721. doi: 10.1007/s12253-017-0357-5. [DOI] [PubMed] [Google Scholar]
- 8.Cao G, Wang J, Zheng X, Wei H, Tian Z, Sun R. Tumor therapeutics work as stress inducers to enhance tumor sensitivity to natural killer (NK) cell cytolysis by up-regulating NKp30 ligand B7-H6. J Biol Chem. 2015;290:29964–29973. doi: 10.1074/jbc.M115.674010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matta J, Baratin M, Chiche L, Forel JM, Cognet C, Thomas G, Farnarier C, Piperoglou C, Papazian L, Chaussabel D, Ugolini S, Vely F, Vivier E. Induction of B7-H6, a ligand for the natural killer cell-activating receptor NKp30, in inflammatory conditions. Blood. 2013;122:394–404. doi: 10.1182/blood-2013-01-481705. [DOI] [PubMed] [Google Scholar]
- 10.El Missiry M, Adnan Awad S, Rajala HL, Al-Samadi A, Ekblom M, Markevan B, Astrand-Grundstrom I, Wold M, Svedahl ER, Juhl BR, Bjerrum OW, Haulin I, Porkka K, Olsson-Stromberg U, Hjorth-Hansen H, Mustjoki S. Assessment of bone marrow lymphocytic status during tyrosine kinase inhibitor therapy and its relation to therapy response in chronic myeloid leukaemia. J Cancer Res Clin Oncol. 2016;142:1041–1050. doi: 10.1007/s00432-015-2101-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaiger A, Henn T, Horth E, Geissler K, Mitterbauer G, Maier-Dobersberger T, Greinix H, Mannhalter C, Haas OA, Lechner K, Lion T. Increase of bcr-abl chimeric mRNA expression in tumor cells of patients with chronic myeloid leukemia precedes disease progression. Blood. 1995;86:2371–2378. [PubMed] [Google Scholar]
- 12.Haznedaroglu IC. Monitoring the response to tyrosine kinase inhibitor (TKI) treatment in chronic myeloid leukemia (CML) Mediterr J Hematol Infect Dis. 2014;6:e2014009. doi: 10.4084/MJHID.2014.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christiansson L, Soderlund S, Svensson E, Mustjoki S, Bengtsson M, Simonsson B, Olsson-Stromberg U, Loskog AS. Increased level of myeloid-derived suppressor cells, programmed death receptor ligand 1/programmed death receptor 1, and soluble CD25 in sokal high risk chronic myeloid leukemia. PLoS One. 2013;8:e55818. doi: 10.1371/journal.pone.0055818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang H, Bueso-Ramos C, DiNardo C, Estecio MR, Davanlou M, Geng QR, Fang Z, Nguyen M, Pierce S, Wei Y, Parmar S, Cortes J, Kantarjian H, Garcia-Manero G. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia. 2014;28:1280–1288. doi: 10.1038/leu.2013.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Zhang G, Qin Y, Bai R, Huang J. B7-H6 expression in non-small cell lung cancers. Int J Clin Exp Pathol. 2014;7:6936–6942. [PMC free article] [PubMed] [Google Scholar]
- 16.Chen XJ, Shen J, Zhang GB, Chen WC. B7-H6 protein expression has no prognostic significance in human gastric carcinoma. Pathol Oncol Res. 2014;20:203–207. doi: 10.1007/s12253-013-9686-1. [DOI] [PubMed] [Google Scholar]
- 17.Alikian M, Whale AS, Akiki S, Piechocki K, Torrado C, Myint T, Cowen S, Griffiths M, Reid AG, Apperley J, White H, Huggett JF, Foroni L. RT-qPCR and RT-digital PCR: a comparison of different platforms for the evaluation of residual disease in chronic myeloid leukemia. Clin Chem. 2017;63:525–531. doi: 10.1373/clinchem.2016.262824. [DOI] [PubMed] [Google Scholar]
- 18.Hughes T, Janssen JW, Morgan G, Martiat P, Saglio G, Pignon JM, Pignatti FP, Mills K, Keating A, Gluckman E, et al. False-positive results with PCR to detect leukaemia-specific transcript. Lancet. 1990;335:1037–1038. doi: 10.1016/0140-6736(90)91102-g. [DOI] [PubMed] [Google Scholar]
- 19.Ross DM, Branford S, Seymour JF, Schwarer AP, Arthur C, Yeung DT, Dang P, Goyne JM, Slader C, Filshie RJ, Mills AK, Melo JV, White DL, Grigg AP, Hughes TP. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood. 2013;122:515–522. doi: 10.1182/blood-2013-02-483750. [DOI] [PubMed] [Google Scholar]
- 20.Mahon FX, Rea D, Guilhot J, Guilhot F, Huguet F, Nicolini F, Legros L, Charbonnier A, Guerci A, Varet B, Etienne G, Reiffers J, Rousselot P. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11:1029–1035. doi: 10.1016/S1470-2045(10)70233-3. [DOI] [PubMed] [Google Scholar]
- 21.Mishra S, Lee Y, Park JW. Direct quantification of trace amounts of a chronic myeloid leukemia (CML) biomarker using locked nucleic acid capture probes. Anal Chem. 2018;90:12824–12831. doi: 10.1021/acs.analchem.8b03350. [DOI] [PubMed] [Google Scholar]
- 22.Gabert J, Beillard E, van der Velden VH, Bi W, Grimwade D, Pallisgaard N, Barbany G, Cazzaniga G, Cayuela JM, Cave H, Pane F, Aerts JL, De Micheli D, Thirion X, Pradel V, Gonzalez M, Viehmann S, Malec M, Saglio G, van Dongen JJ. Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia - a europe against cancer program. Leukemia. 2003;17:2318–2357. doi: 10.1038/sj.leu.2403135. [DOI] [PubMed] [Google Scholar]
- 23.Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J, Baccarani M, Cortes J, Cross NC, Druker BJ, Gabert J, Grimwade D, Hehlmann R, Kamel-Reid S, Lipton JH, Longtine J, Martinelli G, Saglio G, Soverini S, Stock W, Goldman JM. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28–37. doi: 10.1182/blood-2006-01-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


