Abstract
Background:
Task-specific focal dystonias selectively affect movements during the production of highly learned and complex motor behaviors. Manifestation of some task-specific focal dystonias, such as musician’s dystonia, has been associated with excessive practice and overuse, whereas the etiology of others remains largely unknown.
Objectives:
In this study, we aimed to examine the neural correlates of task-specific dystonias in order to determine their disorder-specific pathophysiological traits.
Methods:
Using multimodal neuroimaging analyses of resting-state functional connectivity, voxel-based morphometry and tract-based spatial statistics, we examined functional and structural abnormalities that are both common to and distinct between four different forms of task-specific focal dystonias.
Results:
Compared to the normal state, all task-specific focal dystonias were characterized by abnormal recruitment of parietal and premotor cortices that are necessary for both modality-specific and heteromodal control of the sensorimotor network. Contrasting the laryngeal and hand forms of focal dystonia revealed distinct patterns of sensorimotor integration and planning, again involving parietal cortex in addition to inferior frontal gyrus and anterior insula. On the other hand, musician’s dystonia compared to nonmusician’s dystonia was shaped by alterations in primary and secondary sensorimotor cortices together with middle frontal gyrus, pointing to impairments of sensorimotor guidance and executive control.
Conclusion:
Collectively, this study outlines a specialized footprint of functional and structural alterations in different forms of task-specific focal dystonia, all of which also share a common pathophysiological framework involving premotor-parietal aberrations.
Keywords: functional connectivity, gray matter volume, musician’s focal hand dystonia, singer’s laryngeal dystonia, spasmodic dysphonia, white matter integrity, writer’s cramp
Dystonia is a neurological disorder causing abnormal recurrent movements and fixed postures in affected individuals. One of the forms of dystonia, isolated task-specific focal dystonia (TSFD), selectively impairs the fine control of skilled and goal-oriented motor behaviors, such as writing and speaking. As in the case with other focal dystonias, symptomatology of TSFD is well described, but its exact causes and pathophysiological mechanisms remain less clear.
Recent neuroimaging studies have shown that, compared to other focal dystonias (e.g., blepharospasm and cervical dystonia), TSFD patients present not only with abnormalities in the basal ganglia, cerebellum, and primary sensorimotor cortex, but also in higher-order motor and associative cortical regions.1,2 Overall greater involvement of cortical alterations in TSFD is thought to reflect impairments of highly learned, complex sequences of voluntary motor actions, whereas other dystonias are characterized by loss of control of more stereotyped movements (e.g., eye blinking, neck posture).
Previous studies have also suggested that manifestation of dystonic symptoms in the specific body region might be influenced by distinct brain alterations. For example, patients with focal hand dystonia, writer’s cramp (WC), were found to exhibit decreased connectivity of the hand region of primary sensorimotor cortex accompanied by decreased dorsal premotor and superior parietal connectivity and increased putaminal connectivity.3–5 In a separate study, patients with spasmodic dysphonia (SD) were characterized by decreased connectivity of the laryngeal region of primary sensorimotor cortex, again along with decreased premotor and putaminal connectivity but increased inferior parietal connectivity.6,7 Further analysis of large-scale neural networks identified TSFD-specific pathophysiological traits in global brain organization, including altered information transfer through a group of highly influential sensorimotor brain regions, hubs, that form an abnormal dystonic network kernel.8
In this study, we aimed to investigate functional and structural abnormalities across different TSFD forms compared to age- and sex-matched healthy controls as well as between TSFD patients within the same experimental setting in order to detail the contribution of the affected body region and affected skilled motor behavior to the disorder pathophysiology. We utilized multivariate probabilistic independent component analysis (ICA)9 of resting-state functional MRI (fMRI) to zoom into the regional alterations of the arguably most impaired sensorimotor network in dystonia as well as voxel-based morphometry (VBM) and tract-based spatial statistics (TBSS) to identify associated alterations of gray matter volume and white matter tract integrity, respectively, depending on the affected body region (i.e., hand or larynx) or affected motor behavior (writing/speaking or playing a musical instrument/singing). We hypothesized that different forms of TSFD will share common structural alterations in those cortical regions that control the fundamental aspects of sensorimotor processing and integration, fitting an overall characteristic feature of common dystonia pathophysiology. We further hypothesized that differences in symptom manifestation would be related to an increased propensity of network components for sensorimotor processing and motor control, whereas differences between affected behaviors would be linked to abnormal functional specializations of sensorimotor planning and executive processes.
Patients and Methods
Subjects
A total of 63 subjects participates in the study, including 47 TSFD patients and 16 healthy controls (see detailed demographics in Table 1). All participants, including patients and healthy controls, were right-handed as determined by the Edinburgh Handedness Inventory and had no past or present history of neurological (other than isolated TSFD in patients), psychiatric, or laryngeal problems. This was established based on the history and physical examination as well as neurological and laryngeal evaluations. WC and musician’s focal hand dystonia (MFHD) were focal to the right hand and task specific to writing and playing a musical instrument, respectively; SD patients had the focal adductor type, which was task specific to speaking, and singer’s laryngeal dystonia (SLD) was specific to singing. All patients with MFHD and SLD were professional musicians (Table 1). Healthy musicians were healthy professionally trained individuals; healthy nonmusicians had no formal musical training. There were no statistically significant differences in age (P ≥ 0.45), gender (P ≥ 0.71), years of musical practice (P ≥ 0.17), dystonia duration (P ≥ 0.46), or age at the onset of dystonia (P ≥ 0.40), as applicable, between the examined groups. Thus, all comparisons were tightly matched by age, sex, level of musical training, instrument, handedness, genetic and cognitive status, language, and duration and onset of dystonia, whenever applicable. A neuroradiological examination confirmed normal gross brain anatomy in all participants. Patients receiving botulinum toxin injections for symptom management participated in the study at least 3 months after they received their last injection to ensure that they were fully symptomatic at the time of participation.
TABLE 1.
Participant demographics
| N (a total of 63 participants) | TSFD vs. Healthy Controls | Laryngeal Dystonia vs. Focal Hand Dystonia | Nonmusician’s Dystonia vs. Musician’s Dystonia | |||
|---|---|---|---|---|---|---|
| Group composition | 16 TSFD vs. 16 HC | 16 SD/SLD vs. 16 WC/MFHD | 16 SD/WC vs. 16 MFHD/SLD | |||
| 4 SD | 8 HC | 8 SD | 8 WC | 8 SD | 8 SLD | |
| 4 SLD | 8 MHC | 8 SLD | 8 MFHD | 8 WC | 8 MFHD | |
| 4 WC | ||||||
| 4 MFHD | ||||||
| Sex (F/M) | 8/8 | 7/9 | 8/8 | 8/8 | 5/11 | 4/12 |
| Age (years; mean ± standard deviation) | 45.3 ± 10.8 | 43.9 ± 11.9 | 54.4 ± 9.9 | 53.3 ± 10.3 | 54.8 ± 9.8 | 52.2 ± 9.8 |
| Handedness | Right | |||||
| Language | Monolingual native English | |||||
| Cognitive status | Mini-Mental State Examination ≥27 points | |||||
| Genetic status | Negative for DYT1, DYT6, DYT4, DYT25, arylsulfatase G; no familial history of dystonia | |||||
| Years of musical training | 23.3 ± 9.8 | 28.1 ± 8.2 | 24.0 ± 13.5 | 35.5 ± 9.2 | n/a | 30.7 ± 11.4 |
| Instrument (for professional musicians) | 2 keyboard | 3 keyboard | 16 voice | 8 keyboard | n/a | 2 keyboard |
| 2 strings | 2 strings | 8 strings | 3 strings | |||
| 4 voice | 2 percussion | 3 percussion | ||||
| 1 voice | 8 voice | |||||
| Duration of dystonia (years; mean ± standard deviation) | 12.7 ± 10.6 | n/a | 16.4 ± 8.4 | 13.9 ± 10.5 | 15.1 ± 10.3 | 12.9 ± 9.1 |
| Age of onset (years; mean ± standard deviation) | 32.3 ± 8.0 | n/a | 37.0 ± 10.1 | 40.0 ± 9.5 | 40.4 ± 8.7 | 37.3 ± 14.2 |
HC, healthy controls; MHC, professional musician healthy controls; SD, spasmodic dysphonia confined to laryngeal muscles only and specific to speaking; SLD, singer’s laryngeal dystonia confined to laryngeal muscles only and specific to singing; WC, writer’s cramp confined to the right hand only and specific to writing; MFHD, musician’s focal hand dystonia confined to the right hand only and specific to playing a musical instrument; F, female; M, male.
The subjects were assigned to five experimental groups to perform three pair-wise comparisons:
To characterize shared neural alterations across different forms of TSFD, we compared a group of 16 TSFD (composed of 4 patients per each TSFD form with WC, MFHD, SD, and SLD; 45.3 ± 10.8 years old; 8 females/8 males) to a group of 16 age- and sex-matched healthy subjects (composed of 8 nonmusicians and 8 professional musicians, 43.9 ± 11.9 years old; 7 females/9 males).
To assess functional and structural alterations depending on the TSFD-affected body region (i.e., hand or larynx), we compared 16 patients with focal hand dystonia (composed of 8 MFHD and 8 WC; 53.3 ± 10.3 years old; 8 females/8 males) to 16 patients with focal laryngeal dystonia (composed of 8 SD and 8 SLD;54.4 ± 9.9 years old; 8 females/8 males).
To examine brain abnormalities associated with the affected motor behavior, we contrasted 16 patients with musician’s dystonia (composed of 8 MFHD and 8 SLD; 52.2 ± 9.8 years old; 4 females/12 males) to 16 patients with nonmusician’s dystonia (composed of 8 WC and 8 SD; 54.8 ± 9.8 years old; 5 females/11 males).
Written informed consent was obtained from all subjects before study participation, which was approved by the Institutional Review Board of the Massachusetts Eye and Ear, Harvard Medical School.
Data Acquisition
Brain images were acquired on a 3 T Siemens Skyra scanner (Siemens, Erlangen, Germany) equipped with a 32-channel head coil. During resting-state fMRI, subjects were instructed to rest with their eyes closed without specific thoughts in an environment with dimmed lights. To minimize head motion, the subject’s head was cushioned within the coil, and all were continuously monitored for movements while scanning. No subject reported falling asleep in the scanner. A total of 300 resting-state volumes were acquired using a gradient-weighted echo-planar-imaging pulse sequence (repetition time [TR] = 1 s, echo time [TE] = 30 ms, flip angle = 90 degrees, field of view [FOV] = 240 mm, voxel size = 2.2 mm3, 70 slices).
Diffusion-weighted images (DWIs) were acquired with anterior-posterior and posterior-anterior phase encoding directions, each with 64 noncollinear directions and six nondiffusion images (b0; TR = 3880 ms, TE = 90 ms, flip angle = 80 degrees, FOV = 240 mm, voxel size =2.0 mm3, b = 1000 s/mm2, 69 slices).
High-resolution T1-weighted images were collected using a three-dimensional magnetization prepared rapid acquisition gradient echo sequence with an inversion recovery (3D-MP2RAGE: TR = 4000 ms, TE = 1.9 ms, TI1/TI2 = 633/1860 ms, flip angle = 4 degrees, FOV = 186 × 162 mm, 224 slices, voxel size = 1.0 mm3, number of averages = 2).
Resting-State fMRI and Probabilistic Independent Component Analysis
Preprocessing of resting-state fMRI data was performed using FSL10 and AFNI11 software following a standard pipeline, as described earlier.6,12,13 Each time-series was first truncated by removing the first four volumes to account for potential T1 stabilization effects, slice-time corrected, and high-pass filtered at 0.01 Hz. An affine preregistration to the corresponding anatomical image was performed using a single-band reference image as a template for motion correction. The images were normalized to the Montreal Neurological Institute (MNI) space, and the final registration was performed using a non-linear elastic cost function. All time-series were inspected for motion artifacts and corrected for potential motion or physiological noise effects using a linear regression based on white matter (WM) and cerebrospinal fluid (CSF) mean signals and six motion parameters. WM and CSF covariates were calculated by segmenting the high-resolution T1-weighted image using SPM 12 software14, which were applied to each time series at 90% of tissue probability. Nuisance regressors were extracted by masking and then averaging all voxels across time-series. All images were smoothed using a 5-mm full width at half maximum Gaussian kernel and mean intensity normalized for the subsequent group analysis.
Multivariate probabilistic ICA was performed using the temporal concatenation approach in the FSL’s MELODIC (Multivariate Exploratory Linear Optimized Decomposition into Independent Components) toolbox.6,9 Preprocessed four-dimensional time-series across all subjects were concatenated and decomposed to determine the common group-averaged spatially independent components (ICs). The ICs were visual inspected for their spatial distribution in order to extract the sensorimotor components9,15 as a network most significantly implied in dystonia pathophysiology.3,6,8,16–18 The group-averaged sensorimotor ICs were concatenated and used in a dual regression to generate subject-specific spatial maps and associated time-series.6,19,20 For each group comparison, independent two-tailed t-tests were performed on the individual Z-score maps derived from dual regression to determine between-group differences at a family-wise error (FWE)-corrected P ≤ 0.01 with a voxel-wise P ≤ 0.001 using spatial autocorrelation function.
VBM
VBM analysis was performed using the CAT12 toolbox of SPM12 software. T1-weighted images were normalized and segmented into gray and white matter tissues using standard SPM tissue probability maps. Gray matter probability maps were registered to the SPM standard template in the MNI space using a diffeomorphic nonlinear registration (DARTEL). Image quality was performed by visual inspection and using the quality check modules of the CAT12 toolbox. The resultant images were smoothed using a 6-mm Gaussian kernel. For each comparison, voxel-wise group-level statistics were conducted using two-sample t-tests with subject’s age, sex, and total intracranial volume as nuisance covariates. Statistical maps were corrected at a cluster-wise P ≤ 0.01 with a voxel-wise threshold of P ≤ 0.001.
TBSS
DWI data were preprocessed using the FSL DTI toolbox to correct the motion and eddy current distortions. Fractional anisotropy (FA) maps were created and registered through linear and nonlinear transformations to the MNI space in AFNI software.21 Following the FSL TBSS pipeline,22 a white matter skeleton was created for each image with an FA threshold of 0.2. For each group comparison, voxel-wise group-level statistics were conducted using a two-sample t-test with subject’s age and sex as nuisance covariates. The statistical maps were corrected at a cluster-wise P ≤ 0.01 with a voxelwise threshold of P ≤ 0.001.
Results
Functional and Structural Alterations in TSFD Versus Healthy Controls
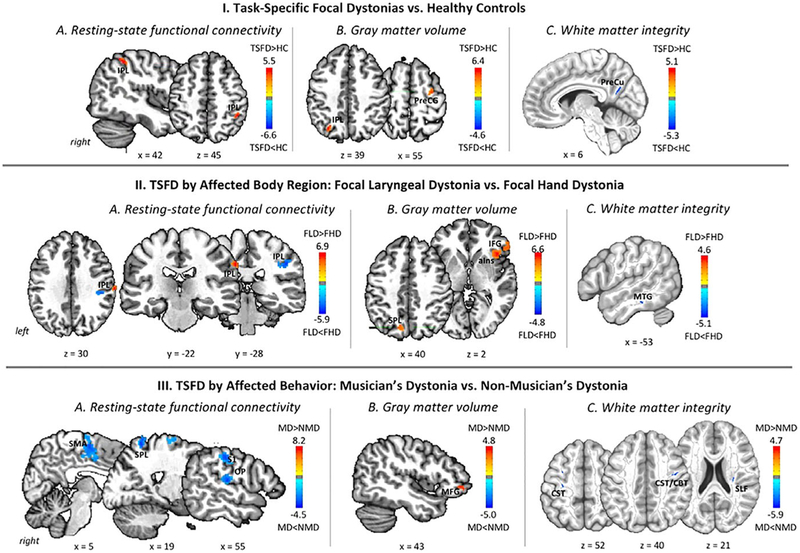
As hypothesized, both common and distinct alterations of functional and structural integrity were found across different forms of TSFD. Compared to healthy controls, all TSFD patients showed increased functional connectivity in the right inferior parietal lobule (areas PGa/hIP2/hIP3; Fig. 1-IA; Table 2). Common increases in gray matter volume were found in the right premotor cortex (area 6) and left inferior parietal lobule (areas PGa/hIP3; Fig. 1-IB; Table 2). In addition, all TSFD patients showed decreased fractional anisotropy in white matter underlying the right precuneus (Fig. 1-IC; Table 2). These findings suggest that the overall dystonic brain organization across different TSFD forms may be shaped by abnormal functional and structural consolidation of the network’s cortical components, which are primarily involved in sensorimotor processing and integration.
FIG. 1.
Common and distinct functional and structural neural abnormalities in TSFD. Resting-state functional connectivity (A), gray matter volume (B), and white matter integrity (C) are depicted across all examined forms of task-specific focal dystonia compared to healthy controls (I); between task-specific focal dystonias contingent upon the affected body region in focal laryngeal dystonia compared to focal hand dystonia (II); and between task-specific focal dystonias based on the affected motor behavior in musician’s dystonia compared to nonmusician’s dystonia (III). The colors bar indicates the t statistics; the corresponding cluster peaks and P values are provided in Table 2. HC, healthy controls; FLD, focal laryngeal dystonia; FHD, focal hand dystonia; MD, musician’s dystonia; NMD, nonmusician’s dystonia; IPL, inferior parietal lobule; PreCG, precentral gyrus; PreCu, precuneus; IFG, inferior frontal gyrus; aIns, anterior insula; MGH, middle temporal gyrus; SMA, supplementary motor area; SPL, superior parietal lobule; S1, primary somatosensory cortex; OP, parietal operculum; MFG, middle frontal gyrus; CST, corticospinal tract; CBT, corticobulbar tract; SLF, superior longitudinal fasciculus.
TABLE 2.
Functional and structural brain abnormalities in TSFD
| I. All TSFD Patients vs. Healthy Controls | ||||
|---|---|---|---|---|
| Region | Cluster t-Score | Coordinates x y z | Cluster P Value | |
| Resting-state connectivity | R inferior parietal lobule (area PGa/hIP2/hIP3) | 5.3 | 44 −47 55 | 0.0004 |
| Gray matter volume | L inferior parietal lobule (area PGa/hIP3) | 6.4 | −29 −68 50 | 0.001 |
| R precentral gyrus (area 6) | 4.9 | 31 −9 64 | 0.003 | |
| White matter integrity | R white matter underlying precuneus | −4.8 | 8 −62 27 | 0.001 |
| II. Focal Hand Dystonia (WC and MFHD) vs. Laryngeal Dystonia (SD and SLD) | ||||
| Region | Cluster t-Score | Coordinates x y z | Cluster P Value | |
| Resting-state connectivity | R inferior parietal lobule (area PFt) | 6.9 | 63 −28 40 | 0.00003 |
| −5.9 | 44 −35 39 | 0.0001 | ||
| Gray matter volume | R inferior frontal gyrus (area 45)/anterior insula | 6.6 | 41 17 2 | 0.0007 |
| L superior parietal lobule (area 7A/7p) | 5.2 | −14 −71 50 | 0.003 | |
| White matter integrity | L white matter underlying middle temporal gyrus | −5.0 | −53 −33 −18 | 0.0004 |
| III. Musician’s Dystonia (MFHD and SLD) vs. Nonmusician’s Dystonia (WC and SD) | ||||
| Region | Cluster t-Score | Coordinates x y z | Cluster P Value | |
| Resting-state connectivity | R superior parietal lobule (area 5 L) | −7.3 | 19 −55 72 | 0.0002 |
| R supplementary motor area (area 6) | −6.8 | 6 −11 62 | 0.0003 | |
| R primary somatosensory cortex/parietal operculum/inferior parietal lobule (area 2/OP1/PFt/PFop) | −8.2 | 59 −27 19 | 0.0004 | |
| Gray matter volume | R middle frontal gyrus (area 10) | 4.5 | 47 44 −6 | 0.004 |
| White matter integrity | L corticospinal tract | 5.7 | −30 −17 52 | 0.0004 |
| R corticospinal/corticobulbar tract | 4.7 | 41 −5 39 | 0.003 | |
| R superior longitudinal fasciculus | 5.1 | 30 −19 21 | 0.0007 | |
Statistically significant differences (FWE-corrected P ≤ 0.05 with vowel-wise threshold P ≤ 0.001 and cluster-wise threshold P ≤ 0.01) in resting-state functional connectivity, gray matter volume, and white matter fractional anisotropy in (I) the composite TSFD patient group vs. healthy controls; (II) focal laryngeal dystonia (SD and SLD) vs. focal hand dystonia (WC and MFHD), and (III) musician’s dystonia (MFHD and SLD) vs. nonmusician dystonia (SD and WC).
Differences in Functional and Structural Organization Between Different TSFD Form Based on the Affected Body Region
A comparison of patients with focal hand dystonia (including WC and MFHD) to patients with focal laryngeal dystonia (including SD and SLD) found distinct regions of abnormal right inferior parietal (area PFt) functional connectivity, with each region of alterations characterizing each TSFD group (Fig. 1-IIA; Table 2). Gray matter volumetric differences depending on the affected body region (larynx > hand) were found in the right inferior frontal gyrus (area 45) and adjoining anterior insula, as well as in the left superior parietal lobule (areas 7A/7P; Fig. 1-IIB; Table 2). In addition, white matter integrity was distinctly affected in the left middle temporal gyrus in patients with focal laryngeal dystonia compared to focal hand dystonia (Fig. 1-IIC; Table 2).
Differences in Functional and Structural Organization Between Different TSFD Form Based on the Affected Behavior
When comparing patients with musician’s dystonia (including MFHD and SLD) to patients with nonmusician’s dystonia (including WC and SD), we found that the former group exhibited significantly reduced functional connectivity in the right primary somatosensory cortex (area 2), parietal operculum (area OP1), inferior parietal lobule (areas PFt/PFop), superior parietal lobule (area 5 L), and supplementary motor area (area 6; Fig. 1-IIIA; Table 2). Structurally, gray matter volumetric increases in musician’s dystonia were found in the right middle frontal gyrus (area 10; Fig. 1-IIIB; Table 2). Decreases in white matter integrity in musician’s dystonia encompassed the right superior longitudinal fasciculus as well as the right corticospinal/corticobulbar tract, underlying the laryngeal representation within the ventral region of precentral gyrus, and the left corticospinal tract, underlying the hand representation within the precentral gyrus (Fig. 1-IIIC; Table 2). Collectively, these findings point to significantly weaker embedding of motor guidance and planning loops in patients with musician’s dystonia.
Discussion
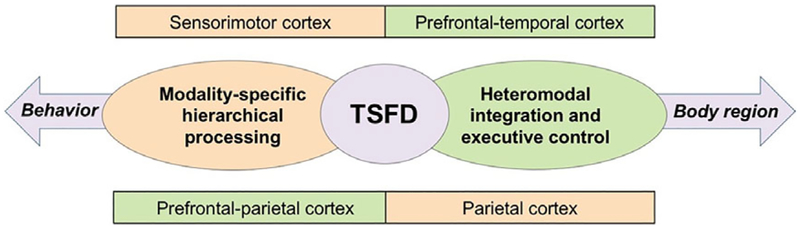
The performance of complex learned sequences for the fulfillment of motor behaviors typically requires the coordination between primary sensorimotor and heteromodal association areas in frontal, parietal, and temporal regions. It is therefore not surprising that greater alterations of cortical than subcortical structures highlighted the common pathophysiology of different TSFD forms (Fig. 2).
FIG. 2.
Schematic representation of main findings and their interpretation. Diagram depicts a visual summary of functional and structural abnormalities in TSFDs.
Most prominently, TSFDs, compared to the normal state, were characterized by abnormal integration of inferior/superior parietal cortex, which was found to exhibit both functional and structural alterations across all patient groups. The parietal cortex is a critical relay station, which provides a sensorimotor interface for the control of higher-order, multimodal integration processes that are necessary to inform and guide movement execution.23–26 The right inferior parietal cortex, in particular, has been associated with supramodal spatial processing and perception of sound movements.27,28 The emergence of specialized abnormalities in these parietal areas, along with volumetric changes in the premotor cortex, across all examined TSFD forms points to a potential pathological breakdown of the mechanisms underlying hierarchical processing and sensorimotor integration leading to dystonic movement execution. Stratifying patients on the basis of the affected body region further showed that the pathophysiological complexity of each TSFD phenotype may be accounted for by different, albeit spatially neighboring, functional and structural aberrations of the parietal cortex. These areas have been previously shown to be altered in focal dystonias,5,8,29–32 linked to their polygenic risk,20 and considered as one of the objective diagnostic biomarkers of this disorder.33
An additional involvement of volumetric increases in the inferior frontal cortex and anterior insula and decreases in white matter integrity underlying middle temporal gyrus in patients with different laryngeal forms of TSFD pointed to their shared pathophysiology that is also distinct when compared to the focal hand forms of TSFD. These structures are known to contribute to the important aspects of speech comprehension, motor planning and coordination, articulatory modulations, and pitch and tone control during singing.12,34–41 Functional and structural alterations in the inferior frontal gyrus and anterior insula are in line with previous neuroimaging studies in SD, which identified abnormal relationships between inferior frontal volume and activity during symptomatic task production as well as between insular volume and the disorder severity.42,43 Furthermore, a spatial disorganization of anterior insular structural connectivity with the inferior frontal gyrus was recently demonstrated in SD patients compared to healthy individuals.40 Given that the neural circuits responsible for the control of speaking and singing potentially overlap,36 our findings suggest that these regional alterations, too, reflect a commonly affected voice-specialized modality across different forms of laryngeal dystonia. Clinically, these findings support evidence showing that the phenomenology of laryngeal TSFDs is often interlocked: the majority of SD patients report their singing being affected similar to speaking, whereas SLD patients gradually develop SD symptoms.44,45
Looking into the TSFD pathophysiology from another angle, certain aspects of dystonia-associated brain alterations may also be contingent upon the affected motor behavior, such as in musician’s versus nonmusician’s dystonia. Musical training requires enhanced integration across sensorimotor and auditory processing systems, leading to substantial cortical reorganization and plasticity of brain regions (within the normal ranges) that support the acquisition and performance of complex motor skills.46–50 Attention processes may also be important, particularly for sight reading, a skill that all musicians need to fully master in order to perform at a professional level.50 In focal dystonias, extensive training has been associated with maladaptive plasticity and underpinning disorganization of motor and somatosensory representations, abnormal coupling between sensory input and motor output regions, and altered integration of sensory feedback and executive control.5,8,51–58 In line with this, musician TSFDs exhibited deficits of functional connectivity in the primary somatosensory cortex, parietal operculum, and supplementary motor area, the regions needed to process sensorimotor information for orchestrated execution of skilled movements. In addition, significant gray matter volumetric increases in musician’s dystonia compared to nonmusician’s dystonia were observed in the middle frontal gyrus, which points to TSFD form-specific alterations of the executive control over the capacity to learn, remember, and coordinate the correct sequences of complex motor tasks. On the other hand, white matter changes were localized to the corticospinal and corticobulbar tracts that underlie the motocortical representations of the hand and larynx, respectively, suggesting particular vulnerability of these white matter pathways to extensive and strenuous motor training in musician’s dystonia.
Dystonia has been traditionally considered a basal ganglia disorder with prominent cerebellar involvement.59,60 Accordingly, numerous previous neuroimaging studies, including ours, have demonstrated a range of functional and structural changes in these structures across different forms of dystonia.61–63 This is, however, in contrast to the present study, which found no significant differences in the basal ganglia and cerebellum. This might be attributed to some methodological issues, such as patient selection and grouping as well as higher statistical thresholding of the resultant data compared to the other studies using similar methodological approaches.63 An alternative possibility is a somatotopically distinct distribution of alterations in the basal ganglia and cerebellum that are not being captured in the group assessment across different forms of TSFD.
Another sticking feature of TSFD alterations was their predominant right-hemispheric distribution. This is an interesting finding because of a known left-hemispheric dominance for manual tasks and speech production in right-handed individuals. That said, the role of the right hemisphere has been emerging in the literature. Recent neuroimaging studies in focal dystonias have reported alterations in right frontoparietal regions and a characteristic loss of hemispheric asymmetry in the distribution of large-scale neural communities.32,33,61,64 Together with the present findings, right-hemispheric involvement points to abnormal interhemispheric integration and may represent an endophenotypic trait specific to TSFDs.
In summary, analysis of functional and structural brain organization showed that TSFD patients, in contrast to healthy individuals, were characterized by abnormal participation of parietal and premotor cortices that are necessary for both modality-specific and heteromodal control of the sensorimotor network. Contrasting the laryngeal and hand forms of TSFD revealed differences in sensorimotor integration and planning, again involving the parietal cortex along with the inferior frontal gyrus and anterior insula. On the other hand, musician’s dystonia was characterized by impairments of sensorimotor guidance and executive control compared to nonmusician’s dystonia. Taken together, these findings suggest that different TSFD forms are likely shaped by a specialized footprint of functional and structural alterations, while sharing a common pathophysiological framework.
Acknowledgments:
We thank Andrew Blitzer, MD, DDS, for patient referrals and Azadeh Hamzehei Sichani, MS, for her help with subject recruitment and imaging data acquisition.
Funding agencies: This study was funded by the R01NS088160 grant to K.S. from the National Institute of Neurological Disorders and Stroke, National Institutes of Health.
Footnotes
Relevant conflicts of interest/financial disclosures: Nothing to report.
Full financial disclosures and author roles may be found in the online version of this article.
References
- 1.Battistella G, Termsarasab P, Ramdhani RA, Fuertinger S, Simonyan K. Isolated focal dystonia as a disorder of large-scale functional networks. Cereb Cortex 2017;27:1203–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramdhani RA, Kumar V, Velickovic M, Frucht SJ, Tagliati M, Simonyan K. What’s special about task in dystonia? A voxel-based morphometry and diffusion weighted imaging study. Mov Disord 2014;29:1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohammadi B, Kollewe K, Samii A, Beckmann CF, Dengler R, Munte TF. Changes in resting-state brain networks in writer’s cramp. Hum Brain Mapp 2012;33:840–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delnooz CC, Helmich RC, Medendorp WP, Van de Warrenburg BP, Toni I. Writer’s cramp: increased dorsal premotor activity during intended writing. Hum Brain Mapp 2013;34:613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delnooz CC, Helmich RC, Toni I, van de Warrenburg BP. Reduced parietal connectivity with a premotor writing area in writer’s cramp. Mov Disord 2012;27:1425–1431. [DOI] [PubMed] [Google Scholar]
- 6.Battistella G, Fuertinger S, Fleysher L, Ozelius LJ, Simonyan K. Cortical sensorimotor alterations classify clinical phenotype and putative genotype of spasmodic dysphonia. Eur J Neurol 2016;23:1517–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Putzel GG, Battistella G, Rumbach A, Ozelius L, Sabuncu MR, Simonyan K. Polygenic risk of spasmodic dysphonia is associated with vulnerable sensorimotor connectivity. Cereb Cortex 2018;28:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuertinger S, Simonyan K. Task-specificity in focal dystonia is shaped by aberrant diversity of a functional network kernel. Mov Disord 2018;33:1918–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging 2004;23:137–152. [DOI] [PubMed] [Google Scholar]
- 10.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23(Suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- 11.Cox RW. AFNI: software for analysis and visualisation of functional magnetic resonance neuroimages. Comput Biomed Res 1996; 29:162–173. [DOI] [PubMed] [Google Scholar]
- 12.Fuertinger S, Horwitz B, Simonyan K. The functional connectome of speech control. PLoS Biol 2015;13:e1002209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Termsarasab P, Ramdhani RA, Battistella G, et al. Neural correlates of abnormal sensory discrimination in laryngeal dystonia. Neuro-Image Clin 2016;10:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashburner J, Friston KJ. Unified segmentation. Neuroimage 2005; 26:839–851. [DOI] [PubMed] [Google Scholar]
- 15.van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol 2010;20:519–534. [DOI] [PubMed] [Google Scholar]
- 16.Dresel C, Li Y, Wilzeck V, Castrop F, Zimmer C, Haslinger B. Multiple changes of functional connectivity between sensorimotor areas in focal hand dystonia. J Neurol Neurosurg Psychiatry 2014;85: 1245–1252. [DOI] [PubMed] [Google Scholar]
- 17.Hinkley LB, Sekihara K, Owen JP, Westlake KP, Byl NN, Nagarajan SS. Complex-value coherence mapping reveals novel abnormal resting-state functional connectivity networks in task-specific focal hand dystonia. Front Neurol 2013;4:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuertinger S, Simonyan K. Connectome-wide phenotypical and genotypical associations in focal dystonia. J Neurosci 2017;37: 7438–7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filippini N, MacIntosh BJ, Hough MG, et al. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A 2009;106:7209–7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Putzel GG, Battistella G, Rumbach AF, Ozelius LJ, Sabuncu MR, Simonyan K. Polygenic risk of spasmodic dysphonia is associated with vulnerable sensorimotor connectivity. Cereb Cortex 2018;28: 158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor PA, Saad ZS. FATCAT: (an efficient) functional and tracto-graphic connectivity analysis toolbox. Brain Connect 2013;3: 523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006;31:1487–1505. [DOI] [PubMed] [Google Scholar]
- 23.Culham JC, Valyear KF. Human parietal cortex in action. Curr Opin Neurobiol 2006;16:205–212. [DOI] [PubMed] [Google Scholar]
- 24.Sereno MI, Huang RS. Multisensory maps in parietal cortex. Curr Opin Neurobiol 2014;24:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gottlieb J. From thought to action: the parietal cortex as a bridge between perception, action, and cognition. Neuron 2007;53:9–16. [DOI] [PubMed] [Google Scholar]
- 26.Gottlieb J, Balan P, Oristaglio J, Suzuki M. Parietal control of attentional guidance: the significance of sensory, motivational and motor factors. Neurobiol Learn Mem 2009;91:121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bushara KO, Weeks RA, Ishii K, et al. Modality-specific frontal and parietal areas for auditory and visual spatial localization in humans. Nat Neurosci 1999;2:759–766. [DOI] [PubMed] [Google Scholar]
- 28.Griffiths TD, Rees G, Rees A, et al. Right parietal cortex is involved in the perception of sound movement in humans. Nat Neurosci 1998;1:74–79. [DOI] [PubMed] [Google Scholar]
- 29.Delmaire C, Vidailhet M, Elbaz A, et al. Structural abnormalities in the cerebellum and sensorimotor circuit in writer’s cramp. Neurology 2007;69:376–380. [DOI] [PubMed] [Google Scholar]
- 30.Gallea C, Horovitz SG, Ali Najee-Ullah M, Hallett M. Impairment of a parieto-premotor network specialized for handwriting in writer’s cramp. Hum Brain Mapp 2016;37:4363–4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pirio Richardson S, Beck S, Bliem B, Hallett M. Abnormal dorsal premotor-motor inhibition in writer’s cramp. Mov Disord 2014;29: 797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Battistella G, Termsarasab P, Ramdhani RA, Fuertinger S, Simonyan K. Isolated focal dystonia as a disorder of large-scale functional networks. Cereb Cortex 2017;27:1203–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Battistella G, Fuertinger S, Fleysher L, Ozelius LJ, Simonyan K. Cortical sensorimotor alterations classify clinical phenotype and putative genotype of spasmodic dysphonia. Eur J Neurol 2016;23: 1517–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann N Y Acad Sci 2010;1191:62–88. [DOI] [PubMed] [Google Scholar]
- 35.Riecker A, Mathiak K, Wildgruber D, et al. fMRI reveals two distinct cerebral networks subserving speech motor control. Neurology 2005;64:700–706. [DOI] [PubMed] [Google Scholar]
- 36.Callan DE, Tsytsarev V, Hanakawa T, et al. Song and speech: brain regions involved with perception and covert production. Neuroimage 2006;31:1327–1342. [DOI] [PubMed] [Google Scholar]
- 37.Ackermann H, Riecker A. The contribution(s) of the insula to speech production: a review of the clinical and functional imaging literature. Brain Struct Funct 2010;214:419–433. [DOI] [PubMed] [Google Scholar]
- 38.Flinker A, Korzeniewska A, Shestyuk AY, et al. Redefining the role of Broca’s area in speech. Proc Natl Acad Sci U S A 2015;112: 2871–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dronkers NF. A new brain region for coordinating speech articulation. Nature 1996;384:159–161. [DOI] [PubMed] [Google Scholar]
- 40.Battistella G, Kumar V, Simonyan K. Connectivity profiles of the insular network for speech control in healthy individuals and patients with spasmodic dysphonia. Brain Struct Funct 2018;223: 2489–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gajardo-Vidal A, Lorca-Puls DL, Hope TMH, et al. How right hemisphere damage after stroke can impair speech comprehension. Brain 2018;141:3389–3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simonyan K, Ludlow CL. Abnormal structure-function relationship in spasmodic dysphonia. Cereb Cortex 2012;22:417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bianchi S, Battistella G, Huddlestone H, et al. Phenotype- and genotype-specific structural alterations in spasmodic dysphonia. Mov Disord 2017;32:560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chitkara A, Meyer T, Keidar A, Blitzer A. Singer’s dystonia: first report of a variant of spasmodic dysphonia. Ann Otol Rhinol Laryngol 2006;115:89–92. [DOI] [PubMed] [Google Scholar]
- 45.Guiry S, Worthley A, Simonyan K. A separation of innate and learned vocal behaviors defines the symptomatology of spasmodic dysphonia. Laryngoscope 2018. December 24. doi: 10.1002/lary.27617. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amunts K, Schlaug G, Jancke L, et al. Motor cortex and hand motor skills: structural compliance in the human brain. Hum Brain Mapp 1997;5:206–215. [DOI] [PubMed] [Google Scholar]
- 47.Vaquero L, Hartmann K, Ripolles P, et al. Structural neuroplasticity in expert pianists depends on the age of musical training onset. Neuroimage 2016;126:106–119. [DOI] [PubMed] [Google Scholar]
- 48.Bailey JA, Zatorre RJ, Penhune VB. Early musical training is linked to gray matter structure in the ventral premotor cortex and auditory-motor rhythm synchronization performance. J Cogn Neurosci 2014;26:755–767. [DOI] [PubMed] [Google Scholar]
- 49.Luo C, Guo ZW, Lai YX, et al. Musical training induces functional plasticity in perceptual and motor networks: insights from resting-state FMRI. PLoS One 2012;7:e36568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Altenmuller E. Focal dystonia: advances in brain imaging and understanding of fine motor control in musicians. Hand Clin 2003;19: 523–538, xi. [DOI] [PubMed] [Google Scholar]
- 51.Byl NN, McKenzie A, Nagarajan SS. Differences in somatosensory hand organization in a healthy flutist and a flutist with focal hand dystonia: a case report. J Hand Ther 2000;13:302–309. [DOI] [PubMed] [Google Scholar]
- 52.McKenzie AL, Nagarajan SS, Roberts TP, Merzenich MM, Byl NN. Somatosensory representation of the digits and clinical performance in patients with focal hand dystonia. Am J Phys Med Rehabil 2003; 82:737–749. [DOI] [PubMed] [Google Scholar]
- 53.Quartarone A, Bagnato S, Rizzo V, et al. Abnormal associative plasticity of the human motor cortex in writer’s cramp. Brain 2003;126 (Pt 12):2586–2596. [DOI] [PubMed] [Google Scholar]
- 54.Quartarone A, Pisani A. Abnormal plasticity in dystonia: disruption of synaptic homeostasis. Neurobiol Dis 2011;42:162–170. [DOI] [PubMed] [Google Scholar]
- 55.Quartarone A, Rizzo V, Bagnato S, et al. Homeostatic-like plasticity of the primary motor hand area is impaired in focal hand dystonia. Brain 2005;128(Pt 8):1943–1950. [DOI] [PubMed] [Google Scholar]
- 56.Rosenkranz K, Williamon A, Butler K, Cordivari C, Lees AJ, Rothwell JC. Pathophysiological differences between musician’s dystonia and writer’s cramp. Brain 2005;128(Pt 4):918–931. [DOI] [PubMed] [Google Scholar]
- 57.Sampaio-Baptista C, Khrapitchev AA, Foxley S, et al. Motor skill learning induces changes in white matter microstructure and myeli-nation. J Neurosci 2013;33:19499–19503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci 2009;12:1370–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marsden CD, Obeso JA, Zarranz JJ, Lang AE. The anatomical basis of symptomatic hemidystonia. Brain 1985;108(Pt 2):463–483. [DOI] [PubMed] [Google Scholar]
- 60.Jinnah HA, Hess EJ. A new twist on the anatomy of dystonia: the basal ganglia and the cerebellum? Neurology 2006;67:1740–1741. [DOI] [PubMed] [Google Scholar]
- 61.Zoons E, Booij J, Nederveen AJ, Dijk JM, Tijssen MA. Structural, functional and molecular imaging of the brain in primary focal dystonia—a review. Neuroimage 2011;56:1011–1020. [DOI] [PubMed] [Google Scholar]
- 62.Lehericy S, Tijssen MA, Vidailhet M, Kaji R, Meunier S. The anatomical basis of dystonia: current view using neuroimaging. Mov Disord 2013;28:944–957. [DOI] [PubMed] [Google Scholar]
- 63.Ramdhani RA, Simonyan K. Primary dystonia: conceptualizing the disorder through a structural brain imaging lens. Tremor Other Hyperkinet Mov (N Y) 2013;3:tre-03-152-3638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haslinger B, Erhard P, Dresel C, Castrop F, Roettinger M, Ceballos-Baumann AO. “Silent event-related” fMRI reveals reduced sensorimotor activation in laryngeal dystonia. Neurology 2005;65:1562–1569. [DOI] [PubMed] [Google Scholar]