Abstract
Heat shock protein 90 (HSP90), a molecular chaperone, plays critical roles in cellular protection against various stressful stimuli and in the regulation of cellular growth and apoptosis. HSP90 has four human isoforms; HSP90α, HSP90β, glucose related protein 94 (GRP94), and tumor necrosis factor (TNF) receptor-associated protein 1 (TRAP1). We evaluated the differential expression of these HSP90 isoforms in colorectal cancer (CRC) and correlated their expression levels with clinicopathological factors and patient survival rates. We performed immunohistochemical staining for HSP90α, HSP90β, GRP94, and TRAP1 in 129 CRC tumor samples and found that HSP90α expression was significantly associated with advanced pT stage (P = 0.011) and shorter recurrence-free survival (RFS) (P = 0.010), whereas GRP94 expression was correlated with low grade (P = 0.029) and better RFS (P < 0.001). HSP90β and TRAP1 had no prognostic impact, although HSP90β expression was positively correlated with tumor size (P = 0.008). Based on our results, HSP90α and GRP94 are potential prognostic biomarkers of CRC. In addition, the differences in expression and functional activities among four HSP90 isoforms imply that isoform selectivity should be seriously considered when HSP90 inhibitors are studied or adopted for the treatment of CRC.
Keywords: Colorectal cancer, HSP90, GRP94, TRAP1, immunohistochemistry, prognosis
Introduction
Colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer-related deaths worldwide [1]. Over the last few decades, the incidence and mortality of CRC in Korea have gradually increased [2]. Although the widespread use of advanced screening techniques has enabled early detection and treatment of the disease, there are still a considerable number of patients suffering from unresectable metastatic CRC. Several treatment regimens are available for patients with metastatic CRC, including conventional chemotherapies with 5-fluorouracil-based regimens, irinotecan, oxaliplatin, and targeted therapies such as anti-epidermal growth factor receptor (EGFR) treatment with cetuximab or panitumumab and anti-vascular endothelial growth factor (VEGF) treatment with bevacizumab [3]. Unfortunately, metastatic CRC becomes unresponsive to these medical therapies in the end. Currently, there are no effective treatments for refractory CRC patients, and thus, novel therapeutic approaches are necessary to improve the clinical outcomes of these patients.
Heat shock proteins (HSPs) are highly conserved proteins found in all cell types, and are induced by diverse environmental and pathophysiological stressors [4,5]. HSPs function as molecular chaperones that fold and stabilize newly translated or denatured proteins to maintain cellular integrity and to protect cells from stressful conditions [5,6]. The client proteins of HSPs include several oncogenic signal transduction molecules involved in the regulation of cellular growth and apoptosis [7]. HSPs are frequently expressed at high levels in various cancers and are related to tumor growth, differentiation, resistance to treatment, and prognosis [8,9]. Therefore, suppression of HSPs by their specific inhibitors has been attracting much attention as a novel therapeutic approach for cancer patients [10-12].
HSPs are classified into several families according to their molecular mass [10]. Ninety kilodalton HSP (HSP90) is a representative family that is the most widely studied target for cancer therapy and includes several human isoforms such as the cytoplasmic isoforms HSP90α (inducible) and HSP90β (constitutional), an endoplasmic reticulum isoform, glucose related protein 94 (GRP94), and a mitochondrial isoform, tumor necrosis factor (TNF) receptor-associated protein 1 (TRAP1) [10]. The relative importance of these four HSP90 isoforms for cancer development and treatment remains poorly understood. With regards to CRC, some previous studies have showed the relationship between the expression of an individual HSP90 isoform in tumor tissues and clinicopathologic factors [13-20], but there is no comprehensive study incorporating the differential expression of four HSP90 isoforms simultaneously.
In this study, we investigated the differential expression of HSP90 isoforms in CRC and the correlation of their expression levels with various clinicopathological factors, including overall patient survival. This allowed us to assess the potential of HSP90 expression to be a predictive biomarker for isoform-specific HSP90 inhibitor therapy or as a prognostic biomarker of CRC.
Materials and methods
Patients and tissue samples
One hundred and twenty nine patients who underwent CRC resection at Samsung Changwon Hospital from January 2007 to December 2009, were included in this study. No patients had neoadjuvant chemotherapy. Demographic and clinicopathological data were obtained from medical and pathological reports. The pathological stage was determined according to the 7th edition of the American Joint Committee on Cancer TNM staging system [21]. Follow-up data were included the period from CRC resection until September 2017 or until death or loss to follow-up.
Tissue microarrays and immunohistochemistry
Representative areas of tumors were marked on hematoxylin and eosin-stained slides and used for tissue microarrays (TMAs). Two tissue cores per tumor with a diameter of 2 mm were taken from donor paraffin blocks and put in blank recipient paraffin blocks. TMA blocks were sectioned at 4 μm for immunohistochemical staining using a Ventana Benchmark XT (Roche-Ventana, Tucson, AZ, USA). All sections were deparaffinized and subjected to pretreatment with CC1 (Roche-Ventana) for 60 min at 100°C. Sections were washed with reaction buffer followed by incubation with primary antibodies for 32 or 60 min at 37°C. Primary antibodies were against HSP90α (clone D7a, 1:100, Abcam, Cambridge, UK), HSP90β (clone E296, 1:50, Epitomics, Burlingame, CA, USA), GRP94 (clone EPR3988, 1:100, Epitomics, Burlingame, CA, USA), and TRAP1 (clone EPR5381, 1:100, Epitomics, Burlingame, CA, USA). An UltraView Universal DAB kit (Roche-Ventana) was used according to the manufacturer’s recommendations to detect primary antibodies, which was followed by counterstaining with hematoxylin (Roche-Ventana). For the negative control, buffer was used instead of the primary antibody.
Assessment of immunohistochemical results
Immunostained slides were evaluated by two independent pathologists (HWL and EHL) without clinicopathological information. Discrepant cases were reviewed on a multihead microscope until consensus was reached. For each HSP90 isoform, cases were considered positive when 10% or more of the tumor cells expressed the protein. The staining intensity of the positive cases was scored as 1 (weak), 2 (moderate), or 3 (strong).
Statistical analysis
Statistical analyses were performed with SPSS Ver. 18 (SPSS Inc., Chicago, IL, USA). To evaluate the relationships between the expression of HSP90 isoforms and clinicopathological parameters, we used Fisher’s exact test for categorical variables or the Mann-Whitney test for ordinal variables. The Impact of parameters on overall survival (OS) and recurrence-free survival (RFS) were analyzed by the Kaplan-Meier method, and differences were compared using the log-rank test. Multivariate analyses for OS and RFS used the Cox proportional hazards model. A P < 0.05 was considered statistically significant.
Results
Clinicopathological characteristics
The 129 patients with CRC included in this study consisted of 76 men and 53 women. At the time of diagnosis, the median patient age was 68 years (range 24-93 years). Regarding tumor location, 30 (23%) were found in the cecum and ascending colon, 11 (9%) in the transverse colon, 8 (6%) in the descending colon, 45 (35%) in the sigmoid colon, and 35 (27%) in the rectum. The median tumor size was 4.0 cm (range 1.6-9.0 cm). Thirty tumors (23%) were well differentiated, 91 (71%) were moderately differentiated, and 8 (6%) were poorly differentiated. Pathologic T stage was T1 (pT1) for 2 tumors (2%), pT2 for 17 (13%), pT3 for 85 (66%), and pT4 for 25 (19%). Lymphovascular invasion and nodal metastasis were detected in 29 (22%) and 64 cases (50%), respectively. Distant metastasis was present at initial diagnosis in 20 (16%) patients. Fifteen tumors (12%) were stage I, 45 (35%) were stage II, 49 (38%) were stage III, and 20 (16%) were stage IV. These clinicopathological characteristics are summarized in Table 1.
Table 1.
Proportion of patients with high HSP90 isoform expression according to clinicopathological characteristics of patients with colorectal cancer
| Variables | No. of cases | HSP90α | HSP90β | GRP94 | TRAP1 | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| No. (%) | P | No. (%) | P | No. (%) | P | No. (%) | P | ||
| Total | 129 | 99 (77) | 68 (53) | 90 (70) | 83 (64) | ||||
| Age (years) | |||||||||
| < 70 | 71 | 56 (79) | 0.538 | 37 (52) | 1.000 | 46 (65) | 0.184 | 47 (66) | 0.713 |
| ≥ 70 | 58 | 43 (74) | 31 (53) | 44 (76) | 36 (62) | ||||
| Sex | |||||||||
| Male | 76 | 61 (80) | 0.293 | 36 (47) | 0.156 | 52 (68) | 0.846 | 51 (67) | 0.460 |
| Female | 53 | 38 (72) | 32 (60) | 38 (72) | 32 (60) | ||||
| Location | |||||||||
| Right | 30 | 25 (83) | 0.393 | 15 (50) | 0.814 | 20 (67) | 0.952 | 19 (63) | 0.128 |
| Transverse | 11 | 7 (64) | 5 (46) | 8 (73) | 4 (36) | ||||
| Left | 88 | 67 (76) | 48 (55) | 62 (71) | 60 (68) | ||||
| Differentiation | |||||||||
| Well | 30 | 23 (77) | 1.000 | 14 (47) | 0.389 | 26 (87) | 0.029 | 22 (74) | 0.105 |
| Moderately | 91 | 70 (77) | 49 (54) | 59 (65) | 58 (63) | ||||
| Poorly | 8 | 6 (75) | 5 (63) | 5 (63) | 3 (38) | ||||
| Size (cm) | |||||||||
| < 4 | 66 | 47 (71) | 0.148 | 27 (41) | 0.008 | 46 (70) | 1.000 | 40 (61) | 0.462 |
| ≥ 4 | 63 | 52 (83) | 41 (66) | 44 (70) | 43 (68) | ||||
| Pathological T stage | |||||||||
| pT1 | 2 | 2 (100) | 0.011 | 1 (50) | 0.712 | 1 (50) | 0.254 | 1 (50) | 0.138 |
| pT2 | 17 | 8 (47) | 9 (53) | 13 (77) | 12 (71) | ||||
| pT3 | 85 | 67 (79) | 46 (54) | 60 (71) | 58 (68) | ||||
| pT4 | 25 | 22 (88) | 12 (48) | 16 (64) | 12 (48) | ||||
| Pathological N stage | |||||||||
| pN0 | 65 | 46 (71) | 0.288 | 33 (51) | 0.871 | 46 (71) | 0.914 | 41 (63) | 0.753 |
| pN1 | 43 | 38 (88) | 25 (58) | 29 (67) | 28 (65) | ||||
| pN2 | 21 | 15 (71) | 10 (48) | 15 (71) | 14 (67) | ||||
| Distant metastasis | |||||||||
| No metastasis | 109 | 82 (75) | 0.405 | 58 (53) | 0.812 | 76 (70) | 1.000 | 71 (65) | 0.800 |
| Metastasis | 20 | 17 (85) | 10 (10) | 14 (70) | 12 (60) | ||||
| TNM stage | |||||||||
| I | 15 | 8 (53) | 0.073 | 7 (47) | 0.856 | 11 (73) | 0.721 | 10 (67) | 0.861 |
| II | 45 | 35 (78) | 24 (53) | 32 (71) | 27 (60) | ||||
| III | 49 | 39 (80) | 27 (55) | 33 (67) | 34 (69) | ||||
| IV | 20 | 17 (85) | 10 (50) | 14 (70) | 12 (60) | ||||
| Lymphovascular invasion | |||||||||
| Negative | 100 | 77 (77) | 1.000 | 55 (55) | 0.400 | 72 (72) | 0.360 | 62 (62) | 0.381 |
| Positive | 29 | 22 (76) | 13 (45) | 18 (62) | 21 (72) | ||||
| HSP90α | |||||||||
| Low | 30 | 12 (40) | 0.144 | 27 (90) | 0.006 | 23 (77) | 0.130 | ||
| High | 99 | 56 (57) | 63 (64) | 60 (61) | |||||
| HSP90β | |||||||||
| Low | 61 | 43 (71) | 0.144 | 41 (46) | 0.570 | 34 (56) | 0.066 | ||
| High | 68 | 56 (82) | 49 (54) | 49 (72) | |||||
| GRP94 | |||||||||
| Low | 39 | 36 (92) | 0.006 | 19 (49) | 0.570 | 19 (49) | 0.017 | ||
| High | 90 | 63 (70) | 49 (54) | 64 (71) | |||||
| TRAP1 | |||||||||
| Low | 46 | 39 (85) | 0.130 | 19 (41) | 0.066 | 26 (30) | 0.017 | ||
| High | 83 | 60 (72) | 49 (59) | 64 (71) | |||||
Expression and correlation of HSP90 isoforms
All four HSP90 isoforms were expressed in the cytoplasm of CRC tumor cells with high positive rates and a wide range of staining intensities (Figure 1). Of the 129 tumors, positivity was seen in 110 (85%) for HSP90α, 129 (100%) for HSP90β, 112 (87%) for GRP94, and 128 (99%) for TRAP1. Expression was moderate or strong, namely high expression, in 99 (77%) for HSP90α, 68 (53%) for HSP90β, 90 (70%) for GRP94, and 83 (64%) for TRAP1. There was a negative correlation between HSP90α and GRP94 expression (P = 0.006), whereas a positive correlation was seen between GRP94 and TRAP1 expression (P = 0.017). HSP90β had no statistically significant correlation with the other HSP90 isoforms (Table 1).
Figure 1.
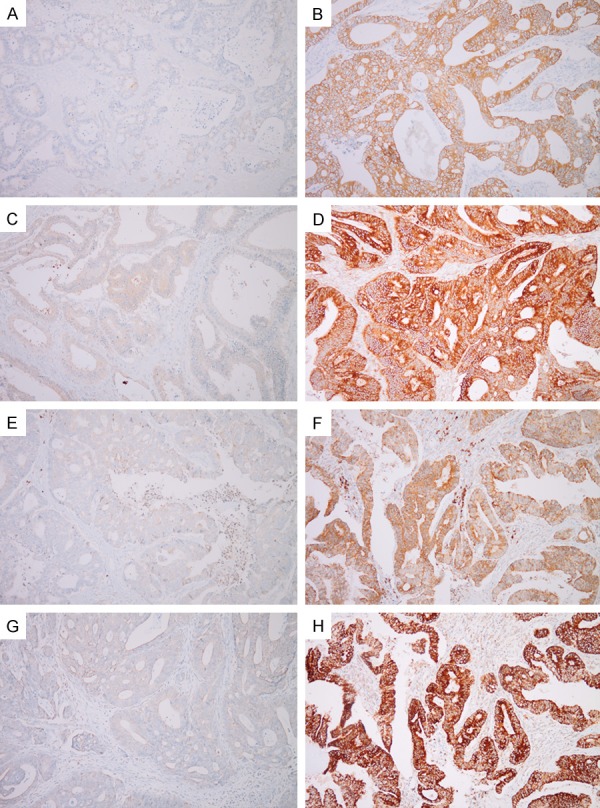
Immunohistochemical staining of HSP90 isoforms in colorectal cancers. Low (A) and high (B) expression of HSP90α, low (C) and high (D) expression of HSP90β, low (E) and high (F) expression of GRP94, and low (G) and high (H) expression of TRAP1.
Correlation between HSP90 isoforms and clinicopathological factors
Expression levels of HSP90 isoforms were compared with clinicopathological factors (Table 1). Of the isoforms, HSP90α was expressed more highly in CRCs with advanced pT and TNM stages than those with early pT and TNM stages (P = 0.011 and P = 0.073, respectively). HSP90β expression was higher in CRCs with a diameter of 4 cm or larger than those with a diameter smaller than 4 cm (P = 0.008). Well-differentiated CRCs showed a high expression of GRP94 more frequently than poorly-differentiated tumors (P = 0.029). TRAP1 expression was not significantly associated with clinicopathological factors.
Survival analysis
The median follow-up period was 38 months (range 1-96 months). During follow-up, 48 (37%) of the 129 patients died and 36 (28%) developed recurrent disease. In univariate analysis, the HSP90 isoforms did not show a significant impact on OS. For all patients, the HSP90α high-expression group had a tendency towards shorter RFS, but this was not statistically significant (Figure 2A; P = 0.075). When stratified by patient age at diagnosis, high expression of HSP90α was significantly associated with worse RFS in CRC patients younger than 70 years old (Figure 2B; P = 0.010). CRC patients with high expression of GRP94 had longer RFS (Figure 2C; P < 0.001). When HSP90α and GRP94 expression results were combined, CRC patients with high HSP90α and low GRP94 expression had the worst RFS (Figure 2D; P = 0.001). Both HSP90β and TRAP1 had no significant association with RFS. Of the measured clinicopathological variables, higher pT, pN, or TNM stage (P = 0.045, P= 0.010, and P = 0.025, respectively), and lymphovascular invasion (P < 0.001) were significantly correlated with shorter RFS. Multivariate Cox regression analysis including GRP94 and clinicopathological variables that were significantly associated with prognosis in univariate analysis showed that GRP94 was an independent prognostic factor for RFS (Table 2).
Figure 2.
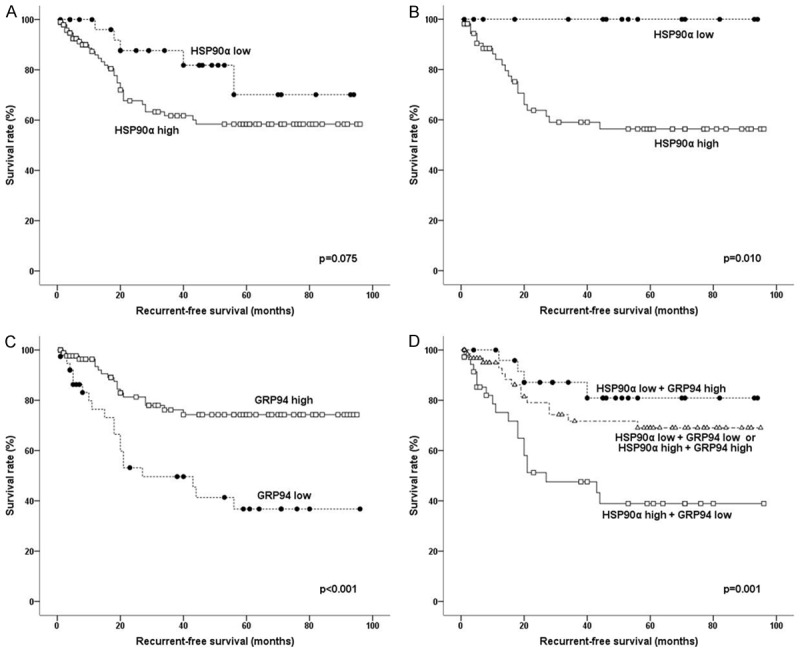
Survival analysis using the Kaplan-Meier method: Recurrence-free survival (RFS) according to the expression levels of HSP90α in all patients (A) and in patients younger than 70 years old (B). RFS according to the expression levels of GRP94 (C) and the combined expression levels of HSP90α and GRP94 (D) in all patients.
Table 2.
Multivariate analysis for recurrence-free survival in 129 patients with colorectal cancer
| Factors | HR | 95% CI of HR | P |
|---|---|---|---|
| GRP94 | |||
| Low | 2.62 | 1.33-5.14 | 0.005 |
| High | 1.00 | ||
| pT stage | |||
| pT4 | 1.72 | 0.77-3.85 | 0.190 |
| pT1-3 | 1.00 | ||
| Lymph node metastasis | |||
| Present | 1.65 | 0.77-3.53 | 0.201 |
| Absent | 1.00 | ||
| Lymphovascular invasion | |||
| Present | 2.36 | 1.13-4.91 | 0.022 |
| Absent | 1.00 |
CI, confidence interval; HR, hazard ratio.
Discussion
The major functions of HSP90 as a molecular chaperone are to facilitate the appropriate folding of unfolded or misfolded proteins and to prevent their aggregation and malfunction. These functions contribute to the crucial roles of HSP90 in the protection and maintenance of cellular viability against environmentally and pathophysiologically stressful stimuli [5,6]. In addition, HSP90 is involved in the regulation of cell signaling, protein trafficking, and apoptosis [7,22]. On the basis of these functions, cancer cells are considered to be more dependent on HSP90 than normal cells. Transformed cells are faced with oncogenic stresses caused by overexpressed abnormal oncoproteins and higher metabolic requirements; high HSP90 expression thus promotes the growth and survival of tumors [8-10]. Previous studies have shown that the overexpression of HSP90 isoforms is involved in oncogenesis and is associated with tumor aggressiveness and poor prognoses in patients with various types of cancers, including colon, stomach, breast, and lung cancer [15,23-26].
With regards to CRC, there have been many studies that have investigated the expression of HSP90 isoforms. These studies focused on the association between only one or two of the HSP90 isoforms and clinicopathologic factors [13-20]. However, differences in expression among four HSP90 isoforms in the same tumor cohort have not been reported yet. The relative expression and functional importance of HSP90 isoforms could be different according to cell type and cellular microenvironment, and this could influence potential treatment regimens with HSP90 inhibitors, especially isoform-specific inhibitors. Therefore, it is worth evaluating the expression of four HSP90 isoforms simultaneously in the same tumor tissue.
In our study, CRCs with advanced pT and TNM stages had higher expression of HSP90α than those at an early stage of cancer progression. Based on this result, two hypotheses could be considered. First, HSP90α could directly or indirectly encourage tumor growth and stromal invasion. Second, advanced stage tumors could be exposed to more stressful conditions and require higher expression of molecular chaperones than early stage tumors. HSP90α might function as a major molecular chaperone in advanced CRCs. These hypotheses are supported by previous studies. Chen et al. [27] and Nagaraju [28] demonstrated that HSP90α enhanced CRC cell migration and invasion. Moreover, HSP90α overexpression was associated with advanced tumor stage in CRC and gastric cancer [15,23]. We found that high HSP90α expression was significantly correlated with worse RFS in CRC patients younger than 70 years old when adjusted for age. There are only a limited number of studies on the relationship between HSP90α expression and CRC patient survival. Drecoll et al. [14] showed that high HSP90 levels could predict prolonged survival of colon cancer patients. However, this result is exceptional and inconsistent with ours. One potential reason for this discrepancy is that they used an anti-HSP90 primary antibody, which could detect both HSP90α and HSP90β at once, and not a specific anti-HSP90α antibody. In our study, HSP90β did not have any prognostic significance. More validation studies with a specific anti-HSP90α antibody are needed to determine the exact impact of HSP90α on the prognosis of CRC patients.
Larger CRC tumors had significantly higher HSP90β expression than smaller ones. Although there have been no published data on the association between HSP90 expression and tumor size in CRC, some studies have reported that HSP90 expression was positively correlated with tumor size in advanced gastric cancer and gastrointestinal stromal tumors [23,29]. These results imply that HSP90β could contribute to tumor growth or be increasingly expressed in larger tumors due to their higher metabolic stresses. HSP90β overexpression has been associated with worse patient survival in lung cancer [26,30]. However, we could not find any prognostic significance for HSP90β in ours and other CRC studies.
It has been suggested that GRP94 is involved in cancer progression by chaperoning its oncogenic client proteins or regulating tumor-associated macrophages [31,32], and that elevated expression of GRP94 correlates with higher tumor grade, advanced stage, and poor outcome in a variety of cancers [17,24,31]. In contrast with these previous results, we found that well-differentiated CRCs showed higher GRP94 expression than moderately- to poorly-differentiated ones, and that CRC patients with high GRP94 expression had significantly better RFS than those with low GRP94 expression. The discrepancy could be explained by two conflicting functions of GRP94, in that it can promote tumor progression and cause an anti-tumor immune response depending on the cellular conditions or microenvironment [31,32]. In addition, Slotta-Huspenina et al. [33] demonstrated that esophageal adenocarcinomas with high GRP94 expression were more sensitive to chemotherapy than those with low GRP94 expression, although they could not determine the exact mechanism. The prolonged RFS of CRC patients with high GRP94 expression in our study might result from higher sensitivity to chemotherapy. Further investigations are warranted to elucidate the role of GRP94 on CRC patient survival and to identify its mechanism of action.
Recent studies uncovered that TRAP1 overexpression was associated with advanced stage and poor outcome in CRC patients [18-20]. In contrast, we could not find any correlation between TRAP1 expression and clinicopathological factors and patient survival. However, there were differences in the TRAP1 antibody used for immunostaining, as well as the interpretation method for the immunohistochemical results between the previous studies and our current study. Thus, the clinicopathological significance of TRAP1 suggested in previous studies should be further verified with a standardized method.
In this study, we evaluated the association among four HSP90 isoforms. There was a negative correlation between HSP90α and GRP94, while GRP94 was positively correlated with TRAP1. Taken together with other results, we speculate that HSP90α plays the most important role among the four HSP90 isoforms in the carcinogenesis of CRC, whereas GRP94 could have anti-tumor effects. Down-regulation of GRP94 could promote tumor progression and predict poor prognosis in CRC. Before the present study, the differential expression of all four HSP90 isoforms in the same CRC tumor tissues has not been reported. Therefore, validation of our results is required.
Several HSP90 inhibitors have been studied as novel therapeutic agents, and some have been evaluated in clinical trials for different types of cancers [10-12]. Our study showed that a high proportion of CRCs had increased expression of HSP90 isoforms. This suggests that HSP90 isoforms could be therapeutic targets in CRC. Furthermore, based on our results, HSP90α-specific inhibitors might be more effective against CRC and have less adverse side effects than inhibitors of other isoforms or pan-HSP90 inhibitors. The impact of isoform selectivity on the treatment index and toxic effects of HSP90 inhibitors should be explored in future studies.
In conclusion, this study is the first to investigate the differential expression of four HSP90 isoforms and the correlation of their expression levels with various clinicopathological factors and patient survival in CRC. HSP90α expression was significantly associated with advanced pT stage and shorter RFS, whereas GRP94 expression was correlated with low grade and better RFS. HSP90β and TRAP1 had no prognostic impact although HSP90β expression was positively correlated with tumor size. These differences in expression and functional activities among four HSP90 isoforms indicate that isoform selectivity should be seriously considered when HSP90 inhibitors are studied or adopted for the treatment of CRC. HSP90α and GRP94 could be prognostic biomarkers in CRC. Our study is retrospective, includes a relatively small number of cases, lacks molecular validation, and contains contradictory results to those found in previous studies. Thus, large-scale, prospective studies with standardized methods and molecular validation are needed to verify our results.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2017R1C1B5018270).
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013, CA. Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Lim D, Ha M, Song I. Trends in major cancer mortality in Korea, 1983-2012, with a joinpoint analysis. Cancer Epidemiol. 2015;39:939–946. doi: 10.1016/j.canep.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 3.Cercek A, Shia J, Gollub M, Chou JF, Capanu M, Raasch P, Reidy-Lagunes D, Proia DA, Vakiani E, Solit DB, Saltz LB. Ganetespib, a novel Hsp90 inhibitor in patients with KRAS mutated and wild type, refractory metastatic colorectal cancer. Clin Colorectal Cancer. 2014;13:207–212. doi: 10.1016/j.clcc.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlesinger MJ. Heat shock proteins. J Biol Chem. 1990;265:12111–12114. [PubMed] [Google Scholar]
- 5.Hendrick JP, Hartl FU. Molecular chaperone functions of heat-shock proteins. Annu Rev Biochem. 1993;62:349–384. doi: 10.1146/annurev.bi.62.070193.002025. [DOI] [PubMed] [Google Scholar]
- 6.Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 7.Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 8.Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci. 2006;31:164–172. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Khalil AA, Kabapy NF, Deraz SF, Smith C. Heat shock proteins in oncology: diagnostic biomarkers or therapeutic targets? Biochim Biophys Acta. 2011;1816:89–104. doi: 10.1016/j.bbcan.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Carbonero R, Carnero A, Paz-Ares L. Inhibition of HSP90 molecular chaperones: moving into the clinic. Lancet Oncol. 2013;14:e358–e369. doi: 10.1016/S1470-2045(13)70169-4. [DOI] [PubMed] [Google Scholar]
- 11.Chatterjee S, Burns TF. Targeting heat shock proteins in cancer: a promising therapeutic approach. Int J Mol Sci. 2017;18:1978. doi: 10.3390/ijms18091978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boroumand N, Saghi H, Avan A, Bahreyni A, Ryzhikov M, Khazaei M, Hassanian SM. Therapeutic potency of heat-shock protein-90 pharmacological inhibitors in the treatment of gastrointestinal cancer, current status and perspectives. J Pharm Pharmacol. 2018;70:151–158. doi: 10.1111/jphp.12824. [DOI] [PubMed] [Google Scholar]
- 13.Rappa F, Sciume C, Lo Bello M, Bavisotto CC, Marino Gammazza A, Barone R, Campanella C, David S, Carini F, Zarcone F, Rizzuto S, Lena A, Tomasello G, Uzzo ML, Spatola GF, Bonaventura G, Leone A, Gerbino A, Cappello F, Bucchieri F, Zummo G, Farina F. Comparative analysis of Hsp10 and Hsp90 expression in healthy mucosa and adenocarcinoma of the large bowel. Anticancer Res. 2014;34:4153–4159. [PubMed] [Google Scholar]
- 14.Drecoll E, Nitsche U, Bauer K, Berezowska S, Slotta-Huspenina J, Rosenberg R, Langer R. Expression analysis of heat shock protein 90 (HSP90) and Her2 in colon carcinoma. Int J Colorectal Dis. 2014;29:663–671. doi: 10.1007/s00384-014-1857-3. [DOI] [PubMed] [Google Scholar]
- 15.Milicevic Z, Bogojevic D, Mihailovic M, Petrovic M, Krivokapic Z. Molecular characterization of hsp90 isoforms in colorectal cancer cells and its association with tumour progression. Int J Oncol. 2008;32:1169–1178. [PubMed] [Google Scholar]
- 16.Takahashi H, Wang JP, Zheng HC, Masuda S, Takano Y. Overexpression of GRP78 and GRP94 is involved in colorectal carcinogenesis. Histol Histopathol. 2011;26:663–671. doi: 10.14670/HH-26.663. [DOI] [PubMed] [Google Scholar]
- 17.Wang XP, Qiu FR, Liu GZ, Chen RF. Correlation between clinicopathology and expression of heat shock protein 70 and glucose-regulated protein 94 in human colonic adenocarcinoma. World J Gastroenterol. 2005;11:1056–1059. doi: 10.3748/wjg.v11.i7.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pak MG, Koh HJ, Roh MS. Clinicopathologic significance of TRAP1 expression in colorectal cancer: a large scale study of human colorectal adenocarcinoma tissues. Diagn Pathol. 2017;12:6. doi: 10.1186/s13000-017-0598-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maddalena F, Simeon V, Vita G, Bochicchio A, Possidente L, Sisinni L, Lettini G, Condelli V, Matassa DS, Li Bergolis V, Fersini A, Romito S, Aieta M, Ambrosi A, Esposito F, Landriscina M. TRAP1 protein signature predicts outcome in human metastatic colorectal carcinoma. Oncotarget. 2017;8:21229–21240. doi: 10.18632/oncotarget.15070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao JY, Song BR, Peng JJ, Lu YM. Correlation between mitochondrial TRAP-1 expression and lymph node metastasis in colorectal cancer. World J Gastroenterol. 2012;18:5965–5971. doi: 10.3748/wjg.v18.i41.5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obrocea FL, Sajin M, Marinescu EC, Stoica D. Colorectal cancer and the 7th revision of the TNM staging system: review of changes and suggestions for uniform pathologic reporting. Rom J Morphol Embryol. 2011;52:537–544. [PubMed] [Google Scholar]
- 22.Neckers L, Workman P. Hsp90 molecular chaperone inhibitors: are we there yet? Clin Cancer Res. 2012;18:64–76. doi: 10.1158/1078-0432.CCR-11-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Cui S, Zhang X, Wu Y, Tang H. High expression of heat shock protein 90 is associated with tumor aggressiveness and poor prognosis in patients with advanced gastric cancer. PLoS One. 2013;8:e62876. doi: 10.1371/journal.pone.0062876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng HC, Takahashi H, Li XH, Hara T, Masuda S, Guan YF, Takano Y. Overexpression of GRP78 and GRP94 are markers for aggressive behavior and poor prognosis in gastric carcinomas. Hum Pathol. 2008;39:1042–1049. doi: 10.1016/j.humpath.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Diehl MC, Idowu MO, Kimmelshue K, York TP, Elmore LW, Holt SE. Elevated expression of nuclear Hsp90 in invasive breast tumors. Cancer Biol Ther. 2009;8:1952–1961. doi: 10.4161/cbt.8.20.9639. [DOI] [PubMed] [Google Scholar]
- 26.Biaoxue R, Xiling J, Shuanying Y, Wei Z, Xiguang C, Jinsui W, Min Z. Upregulation of Hsp90-beta and annexin A1 correlates with poor survival and lymphatic metastasis in lung cancer patients. J Exp Clin Cancer Res. 2012;31:70. doi: 10.1186/1756-9966-31-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen WS, Chen CC, Chen LL, Lee CC, Huang TS. Secreted heat shock protein 90α (HSP90α) induces nuclear factor-κB-mediated TCF12 protein expression to down-regulate E-cadherin and to enhance colorectal cancer cell migration and invasion. J Biol Chem. 2013;288:9001–9010. doi: 10.1074/jbc.M112.437897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagaraju GP, Long TE, Park W, Landry JC, Taliaferro-Smith L, Farris AB, Diaz R, El-Rayes BF. Heat shock protein 90 promotes epithelial to mesenchymal transition, invasion, and migration in colorectal cancer. Mol Carcinog. 2015;54:1147–1158. doi: 10.1002/mc.22185. [DOI] [PubMed] [Google Scholar]
- 29.Li CF, Huang WW, Wu JM, Yu SC, Hu TH, Uen YH, Tian YF, Lin CN, Lu D, Fang FM, Huang HY. Heat shock protein 90 overexpression independently predicts inferior disease-free survival with differential expression of the alpha and beta isoforms in gastrointestinal stromal tumors. Clin Cancer Res. 2008;14:7822–7831. doi: 10.1158/1078-0432.CCR-08-1369. [DOI] [PubMed] [Google Scholar]
- 30.Kim SH, Ji JH, Park KT, Lee JH, Kang KW, Park JH, Hwang SW, Lee EH, Cho YJ, Jeong YY, Kim HC, Lee JD, Jang I, Lee JS, Lee HW, Lee GW. High-level expression of Hsp90β is associated with poor survival in resectable non-small-cell lung cancer patients. Histopathology. 2015;67:509–519. doi: 10.1111/his.12675. [DOI] [PubMed] [Google Scholar]
- 31.Ansa-Addo EA, Thaxton J, Hong F, Wu BX, Zhang Y, Fugle CW, Metelli A, Riesenberg B, Williams K, Gewirth DT, Chiosis G, Liu B, Li Z. Clients and oncogenic roles of molecular chaperone gp96/grp94. Curr Top Med Chem. 2016;16:2765–2778. doi: 10.2174/1568026616666160413141613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu BX, Hong F, Zhang Y, Ansa-Addo E, Li Z. GRP94/gp96 in cancer: biology, structure, immunology, and drug development. Adv Cancer Res. 2016;129:165–190. doi: 10.1016/bs.acr.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Slotta-Huspenina J, Wolff C, Drecoll E, Feith M, Bettstetter M, Malinowsky K, Bauer L, Becker K, Ott K, Höfler H, Becker KF, Langer R. A specific expression profile of heat-shock proteins and glucose-regulated proteins is associated with response to neoadjuvant chemotherapy in oesophageal adenocarcinomas. Br J Cancer. 2013;109:370–378. doi: 10.1038/bjc.2013.319. [DOI] [PMC free article] [PubMed] [Google Scholar]


