Abstract
Objective: MAPK kinase 1 (MEK1) plays an important role in regulating cell proliferation and apoptosis through activation of the ERK/MAPK signaling pathway. It was found that the expression of miR-195 in bladder cancer was abnormally decreased, suggesting that miR-195 may affect the development of bladder cancer. In this study, we examined the expression of miR-195 and MEK1 in bladder cancer tissues and analyzed the relationship between miR-195 and MEK1 in cell proliferation and apoptosis in bladder cancer cells. Patients and methods: The expression of MEK1 in bladder cancer tissues was detected by western blot, and the expression levels of miR-195 and MEK1 mRNA were detected by qRT-PCR. Log Rank test was used to compare the survival and prognosis of patients with low and high expression of miR-195 and MEK1 by using the median expression of miR-195 and MEK1. Bioinformatics analysis and double luciferase reporter gene test were used to verify the relationship between miR-195 and MEK1. Bladder cancer BIU-87 and 5637 cells were cultured in vitro and divided into two groups: miR-NC group and miR-195 mimic group. The expression of MEK1 and p-MEK1 protein was detected by western blot, apoptosis was detected by flow cytometry, and cell proliferation was detected by EdU staining. Results: Compared with normal bladder tissue, expression of miR-195 in bladder cancer tissue was significantly decreased, while the expression of MEK1 mRNA and protein was significantly increased. The prognosis of patients with low expression of miR-195 was worse than those with high expression of miR-195. The prognosis of patients with low expression of MEK1 was better than those with high expression of MEK1. Bioinformatics analysis showed that there was a target complementary binding site between miR-195 and MEK1. Double luciferase reporter gene experiments confirmed that there was a target regulatory relationship between miR-195 and MEK1. miR-195 mimic transfection could significantly down-regulate the expression of MEK1 and p-MEK1 proteins in BIU-87 and 5637 cells, weaken cell proliferation, and increase cell apoptosis. Conclusion: Overexpression of miR-195 can inhibit the proliferation of bladder cancer cells by inhibiting MEK1, which provides further evidence for developing therapy against bladder cancer.
Keywords: miR-195, MEK1, bladder cancer, proliferation, apoptosis, prognosis
Introduction
Bladder cancer (BC) is one of the most common malignant tumors in the urogenital system. It ranks as one of the top ten malignant tumors in the world. The incidence of BC is listed as the ninth most common malignant tumor.
MAPK kinase 1 phosphorylates ERK proteins, and further activates the extracellular signal regulated kinases (ERK)/mitogen activated protein kinase (MAPK) signaling pathway [2,3]. Studies have shown that enhanced expression and functional activity of MEK1 are associated with bladder cancer [4]. It was also found that the expression of miR-195 in bladder cancer tissue was significantly decreased, suggesting that miR-195 may play an oncogene role in the pathogenesis of bladder cancer [5,6]. In this study, we aimed to explore the relationship between miR-195 and MEK1 in the prognosis of bladder cancer patients.
Materials and methods
Main reagents and materials
Human bladder cancer cell line BIU-87, 5637 and human normal bladder epithelial cell SV-HUC-1 were purchased from Beijing North NAH, China. DMEM medium and FBS were purchased from Gibco, USA. Lip 2000 was purchased from Invitrogen, USA. QuantiTect SYBR Green RT-PCR Kit was purchased from Qiagen, Germany. miR-NC, miR-195 mimic was purchased from Guangzhou Rubo biology, China. Rabbit anti human MEK1, p-MEK1, and beta -actin antibodies were purchased from Abcam, USA. HRP was purchased from Shanghai biotech industry, China. RIPA protein extract and Annexin V/PI apoptosis reagents were purchased from Biyun Tian, Jiangsu, China. Dual-Luciferase Reporter Assay System, pGL3-promoter plasmids were purchased from Promega, USA. EdU Flow Cytometry Kit was purchased from Sigma, USA.
Clinical data
From June 2014 to August 2015, 80 patients with metastatic bladder cancer underwent resection in our hospital, including 50 males and 30 females, with an average age of 59.7 (+ 10.6 years). Another 30 cases of normal bladder mucosa were collected as controls. All samples were informed and agreed by the hospital ethics committee.
Cell culture
BIU-87, 5637, SV-HUC-1 cells were all cultured in DMEM medium containing 10% FBS and 1% penicillin and put into DMEM incubator containing 5% CO2 at 37°C. After maturation the cells were subcultured in 1:4 ratio among which with good logarithmic phase growth were selected for experiment.
Double luciferase gene report experiment
Using HEK293T cell genomic mRNA as template, the fragments containing targeted binding sites or their mutant fragments in the 3’-UTR region of MEK1 gene were amplified. The PCR products were harvested by digesting and ligated into pGL3 vector. The DH5a receptor cells were transformed and the positive clones were screened by colony PCR. The plasmids were named pGL3-MEK1-WT, pGL3-MEK1-MUT respectively.
50 ng pGL3-MEK1-WT (or pGL3-MEK1-MUT) and 100 nmol miR-195 mimic (or miR-NC) were co-transfected into HEK293 cells by Lip 2000. After 48 hours of culture, the culture medium was discarded, the rest was washed twice with PBS and were lysed with 100 µL Passive Lysis Buffer for 15 min, then were centrifuged by 1000 rpm for 5 min. After the reaction between 50 L lysate and 50 L luciferase substrate, the luciferase activity was determined immediately, and the reaction was terminated by adding 50 µL Stop&Glo, and the murine kidney luciferase activity was determined immediately. The ratio of luciferase activity to the murine kidney luciferase activity was relative luciferase activity.
Cell transfection and grouping
BIU-87 and 5637 cells were cultured in vitro and divided into two groups: miR-NC transfection group and miR-195 mimic transfection group. The transfection steps were as follows: diluting 10 μL Lip 2000, 50 nmoL miR-NC and 50 nmoL miR-195 mimic respectively with 100 μL serum-free Opti-MEM, incubating at room temperature for 5 minutes, mixing Lip 2000 gently with microRNAs-NC and microRNAs-195 mics, respectively. After incubation at room temperature for 20 minutes, the transfected cells were added to the cell culture medium, and then cultured for 72 hours.
Flow cytometry
After trypsin digestion and collection, the transfected cells were incubated with 10 μM EdU for 2 hours and cultured 48 h. Trypsin digestion and collection were performed and then the cells were washed, fixed, and permeated by centrifugation. We added Alexa Fluor 488 labelled detection reaction solution. Then the cells were incubated at room temperature in the presence of light for 30 minutes. After centrifugation and washing, the cell proliferation was detected by Beckmann Coulter FC500MCL cytosolic assay.
Apoptosis was detected by flow cytometry
After being washed twice with PBS, the cell precipitate was added with 100 μL Binding Buffer. The cell precipitate was added with 5 μL Annexin V-FITC and 5 μL PI. After 20 minutes of light-proof reaction, 400 μL Binding Buffer was added to suspend the cells and the apoptosis was detected by FC500MCL flow cytometry.
Detection of gene expression using qRT-PCR
Trizol was used to extract intracellular RNA, and Q Kit was used to detect RNA gene expression by one-step qRT-PCR. In the qRT-PCR (reaction system of 20 μL), including 10.0 μL 2 × QuantiTect SYBR Green RT-PCR Master Mix, 1.0 μL 0.5 μm/L pre-and post-primers, 2 μg Template RNA, 0.5 μL QuantiTect RT Mix, at last, replenishing ddH2O to 20.0 μL. The reaction conditions of qRT-PCR were 45°C, 5 min, 94°C, 30 s, (95°C, 5 s; 60°0, 30 s) × 40 cycles. The gene expression was detected by Bio-Rad CFX96 real-time quantitative PCR.
Western blot
The protein supernatant was transferred to the new EP tube by centrifugation at 10000 g for 15 min after adding 100 μL protein extract to every 5 mg or 1 million cells. The protein concentration was detected by BCA method. The protein was separated by electrophoresis in SDS-PAGE for 3 h, transferred to PVDF membrane, sealed at room temperature for 60 min with 5% skimmed milk powder, and incubated overnight at 4°C. The dilution ratios of MEK1, p-MEK1, and beta-actin were 1:2000, 1:1000 and 1:10000, respectively. Then the protein was washed three times with PBST, added HRP-labeled antibodies (1:10000 dilution) incubating at room temperature for 1 h, washed three times with PBST and reacted with ECL for 1-3 minutes. At last, the film with the protein was exposed, developed, fixed, scanned and the data were stored.
Statistical analysis
SPSS 18.0 was used for statistical analysis of the data. The measurement data were expressed as mean ± standard deviation. The comparison of measurement data between the two groups was performed by t-test. The comparison of measurement data among multiple groups was performed by one-way ANOVA and Bonferroni method. Mann-Whitney U test was used to compare the miR-195 in bladder tissue of the two groups. Spearman method was used to analyze the correlation among the expression of MEK1 mRNA and the expression of miR-195 and MEK1 mRNA in bladder cancer tissues. Kaplan-Meier method was used to determine the survival curves. Log-rank test was used to compare the survival rates; P<0.05 was considered significant.
Result
Abnormal expression of miR-195 and MEK1 in bladder cancer tissues
The results of qRT-PCR showed that the expression of MEK1 mRNA in bladder cancer tissues was significantly higher than that in normal bladder mucosa tissues (P<0.05) (Figure 1A). The expression of microRNA-195 in bladder cancer tissues was significantly lower than that in normal bladder mucosa tissues (P<0.05) (Figure 1B).
Figure 1.
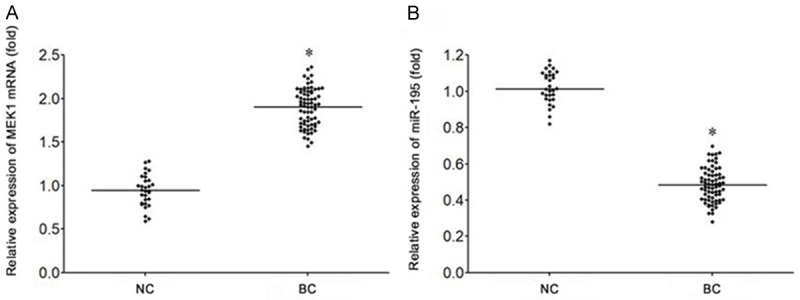
Abnormal expression of miR-195 and MEK1 in bladder cancer tissues. A. qRT-PCR was used to detect the expression of miR-195 in bladder cancer. B. qRT-PCR was used to detect the expression of MEK1 mRNA in bladder cancer. *P<0.05, compared with NC group. NC, normal bladder mucosa tissues. BC, bladder cancer tissues.
The expression of MEK1 protein in bladder cancer tissues increased significantly
Western blot showed that the expression of MEK1 protein in bladder cancer tissues was significantly increased compared to that in normal bladder mucosa tissues (P<0.05) (Figure 2A and 2B). Moreover, compared with bladder cancer of stage I-II, the expression of MEK1 protein in bladder cancer of stage III-IV was significantly elevated (P<0.05) (Figure 2A and 2B).
Figure 2.
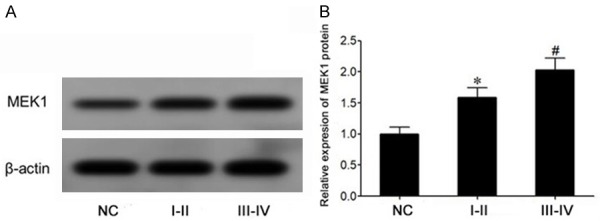
The expression of MEK1 protein in bladder cancer tissue increased significantly. A. Western blot was used to detect the expression of MEK1 protein in bladder cancer. B. The expression of MEK1 protein in bladder cancer tissues was compared and expressed. *P<0.05, compared with NC group. #P<0.05, comparing stage I to stage II of bladder cancer (I-II). III-IV, stage III to stage IV of bladder cancer.
The expression of miR-195 and MEK1 is related to the prognosis of patients with bladder cancer
According to the median expression of microRNA-195 and MEK1, the patients with bladder cancer were divided into 4 groups: miR-195 high expression group, miR-195 low expression group, MEK1 high expression group and MEK1 low expression group. The relationship between the expression of microRNA-195 and MEK1 and survival and prognosis was further analyzed. Survival curve analysis showed that the survival and prognosis of those with high miR-195 expression were favorable compared to those with low miR-195 expression (Log-rank test χ2=5.137, P=0.023) (Figure 3B), while the survival and prognosis of patients with low expression of MEK1 mRNA was poor in comparison to those with low expression of MEK1 mRNA (Log-rank test χ2=4.413, P=0.042) (Figure 3A).
Figure 3.
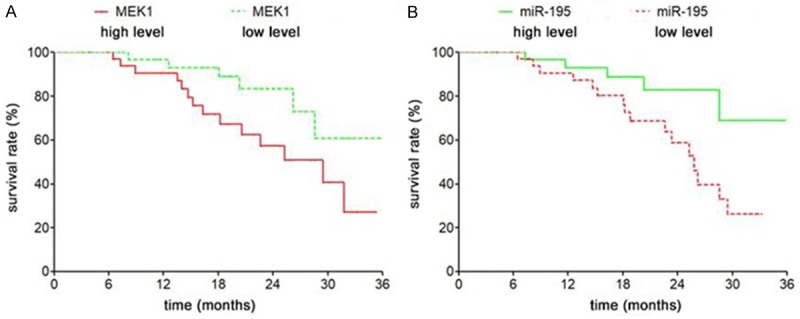
The expression of miR-195 and MEK1 is related to the prognosis of bladder cancer patients. A. The survival curves of MEK1 mRNA high expression and low expression were compared. B. The survival rates of miR-195 with high expression and low expression were compared.
There is a target regulatory relationship between miR-195 and MEK1
The predicted results from the microRNA.org website showed that there was a targeted complementary binding site between miR-195 and 3’-UTR of MEK1 mRNA (Figure 4A). The data of double luciferase gene report validated that transfection of miR-195 mimic significantly decreased the activity of relative luciferase in HEK293T cells transfected with pGL3-MEK1-WT, but did not affect the activity of relative luciferase in HEK293T cells transfected with pGL3-MEK1-MUT (P<0.05) (Figure 4B), suggesting that there was a targeted regulation relationship between miR-195 and MEK1 mRNA.
Figure 4.
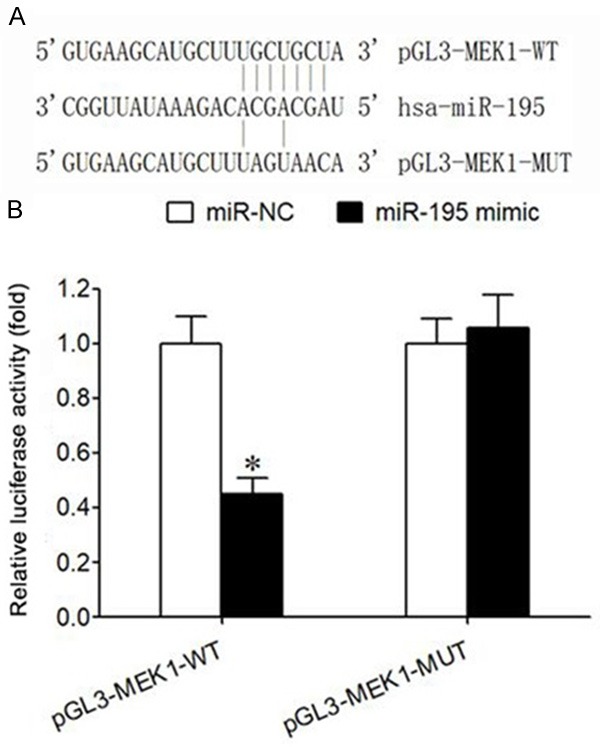
Relationship between miR-195 and MEK1. A. There is a binding site between miR-195 and the 3’-UTR of MEK1 mRNA. B. Double luciferase gene experiment report; Note: *P<0.05, compared with miR-NC group.
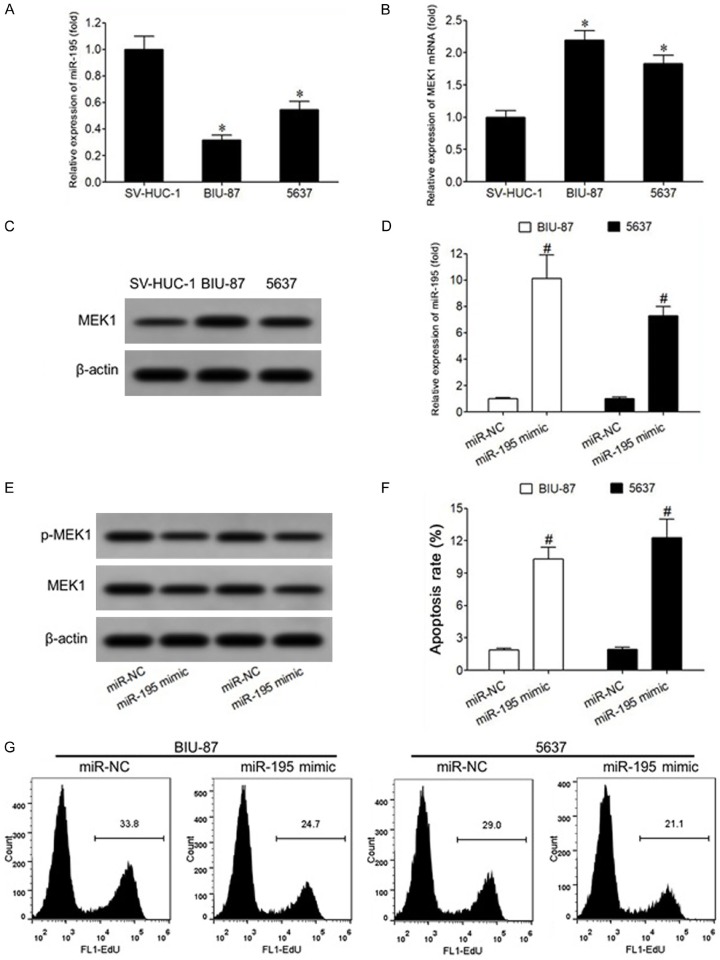
Overexpression of miR-195 can downregulate MEK1 expression, inhibit proliferation of bladder cancer cells, and induce apoptosis
Compared with that in normal bladder epithelial cells SV-HUC-1, the expression of miR-195 in bladder cancer BIU-87 and 5637 cells was significantly decreased (P<0.05) (Figure 5A), while the expression of MEK1 mRNA was significantly increased (P<0.05) (Figure 5B). Western blot showed similar alterations at protein level (Figure 5C). The results of qRT-PCR showed that the transfection of miR-195 mimic in BIU-87 and 5637 cells increased significantly the level of miR-195 (Figure 5D), and significantly decreased the expression of MEK1 and p-MEK1 protein (P<0.05) (Figure 5E). Flow cytometry showed that transfection of miR-195 mimic into bladder cancer BIU-87 and 5637 cells also significantly increased the apoptosis rate (P<0.05) (Figure 5F). The results of EdU staining showed that the positive rate of EdU in BIU-87 and 5637 cells was significantly lower and the proliferation of cells was significantly inhibited (P<0.05) (Figure 5G).
Figure 5.
Overexpression of miR-195 can downregulate MEK1 expression, inhibit bladder cancer cell proliferation, and induce apoptosis. A. qRT-PCR was used to detect the expression of miR-195 in bladder cancer cells. B. qRT-PCR was used to detect the expression of MEK1 mRNA in bladder cancer cells. C. Western blot was used to detect the expression of MEK1 protein in bladder cancer cells. D. qRT-PCR was used to detect miR-195 expression in bladder cancer cells of each transfection group. E. Western blot was used to detect the protein expression of bladder cancer cells in each transfection group. F. Flow cytometry was used to detect the apoptosis rate of bladder cancer cells in each transfection group. G. Flow cytometry was used to detect the proliferation ability of bladder cancer cells in each transfection group. *P<0.05, compared with SV-HUC-1 cells. #P<0.05, compared with miR-NC.
Discussion
Bladder cancer is insidious without obvious clinical symptoms in the early stage, but the disease progresses rapidly, and is prone to invasion and metastasis [7-9]. Once pelvic lymph node metastasis or distant metastasis occurs, the 5-year survival rate of patients with bladder cancer is usually less than 20% [10]. ERK/MAPK signal transduction pathway is widely expressed in many tissues and cells, and can regulate cell proliferation, cycle, apoptosis, migration and invasion and other biological processes [11,12]. Excessive activation of ERK/MAPK signaling pathway can lead to abnormal cell proliferation, apoptosis and differentiation, and is closely related to the occurrence, progression and metastasis of oral cancer, esophageal cancer, lung cancer, and other tumors [13-15]. MEK1 is a tyrosine/threonine (Tyr/Thr) dual-specific protein kinase that phosphorylates ERK protein specifically, thereby activating the ERK/MAPK signaling pathway [3,16].
MicroRNA is an endogenous non-coding small RNA molecule in eukaryotes. It can regulate the expression of target genes by degrading mRNA or inhibiting mRNA translation through complementary pairing with the 3’-untranslated region (3’-UTR) of target gene mRNA. It participates in cell survival, proliferation, apoptosis, migration, and other biological processes. Extensive attention has been paid to the role of the abnormal expression and dysfunction of microRNA in the occurrence, progression and prognosis of tumors [17,18].
Abnormal expression of miR-195 is associated with the occurrence and progression of cervical cancer [19], breast cancer [20], and prostate cancer [21]. Studies have shown that [5,6], the expression of miR-195 in bladder cancer tissue significantly decreased, suggesting that miR-195 may play an oncogenic role in the pathogenesis of bladder cancer. In this study, we found that compared with normal bladder tissues, the expression of miR-195 in bladder cancer tissues was significantly reduced, the expression of MEK1 mRNA and protein was significantly increased, and expression of MEK1 protein was even elevated at the late clinical stage. Our result thus indicated that the decreased expression of miR-195 played an important role in increasing the expression of MEK1 and participated in the pathogenesis of bladder cancer, and its expression affected the survival and prognosis of patients with bladder cancer.
In the study of the relationship between miR-195 and bladder cancer, the expression of miR-195 in bladder cancer T24 cells was significantly lower than that in adjacent tissues, while the expression of miR-195 in bladder cancer CDC42 cells was abnormally increased [5]. It is also demonstrated that the expression of miR-195 in bladder cancer tissue was significantly lower than that in normal bladder mucosal epithelium [22]. The expression of miR-195 in bladder cancer tissue was significantly lower than that in normal bladder mucosa tissue in sequencing results [23]. Compared with normal urethral epithelial cells HUC, the expression of miR-195 in bladder cancer T24 cells was significantly decreased, and the expression of GLUT3 gene was significantly increased [24]. These results suggest that miR-195 plays an inhibitory role in bladder cancer, and the decrease of its expression is related to bladder cancer, similar to the results observed in this study.
In vitro assay suggested that the abnormalities of miR-195 and MEK1 were related to the malignant biological characteristics of bladder cancer cells. Therefore, this study further explores whether miR-195 plays a role in regulating the expression of MEK1 and affecting the biological effects of bladder cancer cells. We observed that the expression of MEK1 in bladder cancer BIU-87 and 5637 cells was significantly down-regulated by transfection of miR-195 mimic, and the expression of p-MEK1 was also significantly down-regulated. The down-regulated expression of p-MEK1 might be due to the down-regulated expression of MEK1. Transfection of miR-195 mimic significantly inhibited the proliferation and apoptosis of bladder cancer cells.
In a study of the relationship between miR-195 and the biological effects of bladder cancer cells, bladder cancer T24 cells transfected miR-195 mimic could inhibit the expression of CDC42 gene, inhibit the proliferation of T24 cells and weaken their malignant biological characteristics [5]. Overexpression of miR-195 in bladder cancer T24 cells could inhibit the proliferation, cloning and apoptosis of bladder cancer cells by regulating the expression of the target gene GLUT3 [24], which confirms that miR-195 can attenuate the malignant biological effects of bladder cancer cells. Consistently, we unraveled the role of miR-195 in up-regulating the expression of MEK1 and promoting the pathogenesis of bladder cancer, which highlights the potential benefits of the anti-bladder cancer treatment in clinical practice.
Conclusion
In conclusion, overexpression of miR-195 suppresses the proliferation of bladder cancer cells by the inhibition of MEK1.
Acknowledgements
This work was supported by grant from the Fudan University outstanding talent plan.
Disclosure of conflict of interest
None.
References
- 1.Latini DM, Lerner SP, Wade SW, Lee DW, Quale DZ. Bladder cancer detection, treatment and outcomes: opportunities and challenges. Urology. 2010;75:334–339. doi: 10.1016/j.urology.2009.09.051. [DOI] [PubMed] [Google Scholar]
- 2.Guegan JP, Fremin C, Baffet G. The MAPK MEK1/2-ERK1/2 pathway and its implication in hepatocyte cell cycle control. Int J Hepatol. 2012;2012:328372. doi: 10.1155/2012/328372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimoni-Sebag A, Lebenthal-Loinger I, Zender L, Karni R. RRM1 domain of the splicing oncoprotein SRSF1 is required for MEK1-MAPK-ERK activation and cellular transformation. Carcinogenesis. 2013;34:2498–2504. doi: 10.1093/carcin/bgt247. [DOI] [PubMed] [Google Scholar]
- 4.Hirata H, Hinoda Y, Ueno K, Shahryari V, Tabatabai ZL, Dahiya R. MicroRNA-1826 targets VEGFC, beta-catenin (CTNNB1) and MEK1 (MAP2K1) in human bladder cancer. Carcinogenesis. 2012;33:41–48. doi: 10.1093/carcin/bgr239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao C, Qi L, Chen M, Liu L, Yan W, Tong S, Zu X. microRNA-195 inhibits cell proliferation in bladder cancer via inhibition of cell division control protein 42 homolog/signal transducer and activator of transcription-3 signaling. Exp Ther Med. 2015;10:1103–1108. doi: 10.3892/etm.2015.2633. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Lin Y, Wu J, Chen H, Mao Y, Liu Y, Mao Q, Yang K, Zheng X, Xie L. Cyclin-dependent kinase 4 is a novel target in micoRNA-195-mediated cell cycle arrest in bladder cancer cells. FEBS Lett. 2012;586:442–447. doi: 10.1016/j.febslet.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 7.Draeger DL, Sievert KD, Hakenberg OW. Psychosocial distress in bladder cancer stratified by gender, age, treatment, and tumour stage. Urol Int. 2018;101:31–37. doi: 10.1159/000489502. [DOI] [PubMed] [Google Scholar]
- 8.Mason SJ, Downing A, Wright P, Hounsome L, Bottomley SE, Corner J, Richards M, Catto JW, Glaser AW. Health-related quality of life after treatment for bladder cancer in england. Br J Cancer. 2018;118:1518–1528. doi: 10.1038/s41416-018-0084-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.PDQ Cancer Information Summaries. Bethesda (MD): National Cancer Institute (US); 2002. Bladder cancer treatment (PDQ®): health professional version. [Google Scholar]
- 10.Rose TL, Deal AM, Nielsen ME, Smith AB, Milowsky MI. Sex disparities in use of chemotherapy and survival in patients with advanced bladder cancer. Cancer. 2016;122:2012–2020. doi: 10.1002/cncr.30029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchegger K, Silva R, Lopez J, Ili C, Araya JC, Leal P, Brebi P, Riquelme I, Roa JC. The ERK/MAPK pathway is overexpressed and activated in gallbladder cancer. Pathol Res Pract. 2017;213:476–482. doi: 10.1016/j.prp.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 12.Liao T, Wen D, Ma B, Hu JQ, Qu N, Shi RL, Liu L, Guan Q, Li DS, Ji QH. Yes-associated protein 1 promotes papillary thyroid cancer cell proliferation by activating the ERK/MAPK signaling pathway. Oncotarget. 2017;8:11719–11728. doi: 10.18632/oncotarget.14319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koyama T, Ogawara K, Kasamatsu A, Okamoto A, Kasama H, Minakawa Y, Shimada K, Yokoe H, Shiiba M, Tanzawa H, Uzawa K. ANGPTL3 is a novel biomarker as it activates ERK/MAPK pathway in oral cancer. Cancer Med. 2015;4:759–769. doi: 10.1002/cam4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Z, Ding L, Hong H, Hoggard J, Lu Q, Chen YH. Claudin-7 inhibits human lung cancer cell migration and invasion through ERK/MAPK signaling pathway. Exp Cell Res. 2011;317:1935–1946. doi: 10.1016/j.yexcr.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng ST, Huo Q, Tuerxun A, Ma WJ, Lv GD, Huang CG, Liu Q, Wang X, Lin RY, Sheyhidin I, Lu XM. The expression and activation of ERK/MAPK pathway in human esophageal cancer cell line EC9706. Mol Biol Rep. 2011;38:865–872. doi: 10.1007/s11033-010-0178-z. [DOI] [PubMed] [Google Scholar]
- 16.Gunawardhana N, Jang S, Choi YH, Hong YA, Jeon YE, Kim A, Su H, Kim JH, Yoo YJ, Merrell DS, Kim J, Cha JH. Helicobacter pylori-Induced HB-EGF upregulates gastrin expression via the EGF receptor, C-Raf, Mek1, and Erk2 in the MAPK pathway. Front Cell Infect Microbiol. 2017;7:541. doi: 10.3389/fcimb.2017.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armstrong CM, Liu C, Lou W, Lombard AP, Evans CP, Gao AC. MicroRNA-181a promotes docetaxel resistance in prostate cancer cells. Prostate. 2017;77:1020–1028. doi: 10.1002/pros.23358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li F, Mahato RI. MicroRNAs and drug resistance in prostate cancers. Mol Pharm. 2014;11:2539–2552. doi: 10.1021/mp500099g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z, Wang H, Wang Z, Cai H. MiR-195 inhibits the proliferation of human cervical cancer cells by directly targeting cyclin D1. Tumour Biol. 2016;37:6457–6463. doi: 10.1007/s13277-015-4540-6. [DOI] [PubMed] [Google Scholar]
- 20.Cecene G, Ak S, Eskiler GG, Demirdogen E, Erturk E, Gokgoz S, Polatkan V, Egeli U, Tunca B, Tezcan G, Topal U, Tolunay S, Tasdelen I. Circulating miR-195 as a therapeutic biomarker in turkish breast cancer patients. Asian Pac J Cancer Prev. 2016;17:4241–4246. [PubMed] [Google Scholar]
- 21.Cai C, Chen QB, Han ZD, Zhang YQ, He HC, Chen JH, Chen YR, Yang SB, Wu YD, Zeng YR, Qin GQ, Liang YX, Dai QS, Jiang FN, Wu SL, Zeng GH, Zhong WD, Wu CL. miR-195 inhibits tumor progression by targeting RPS6KB1 in human prostate cancer. Clin Cancer Res. 2015;21:4922–4934. doi: 10.1158/1078-0432.CCR-15-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Canturk KM, Ozdemir M, Can C, Öner S, Emre R, Aslan H, Cilingir O, Ciftci E, Celayir FM, Aldemir O, Özen M, Artan S. Investigation of key miRNAs and target genes in bladder cancer using miRNA profiling and bioinformatic tools. Mol Biol Rep. 2014;41:8127–8135. doi: 10.1007/s11033-014-3713-5. [DOI] [PubMed] [Google Scholar]
- 23.Han Y, Chen J, Zhao X, Liang C, Wang Y, Sun L, Jiang Z, Zhang Z, Yang R, Chen J, Li Z, Tang A, Li X, Ye J, Guan Z, Gui Y, Cai Z. MicroRNA expression signatures of bladder cancer revealed by deep sequencing. PLoS One. 2011;6:e18286. doi: 10.1371/journal.pone.0018286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fei X, Qi M, Wu B, Song Y, Wang Y, Li T. MicroRNA-195-5p suppresses glucose uptake and proliferation of human bladder cancer T24 cells by regulating GLUT3 expression. FEBS Lett. 2012;586:392–397. doi: 10.1016/j.febslet.2012.01.006. [DOI] [PubMed] [Google Scholar]