Abstract
miR-135b is expressed abnormally in various tumors and plays an important role in the occurrence and development of tumors bydifferent pathways. The role of miR-135b in osteosarcoma (OS) and its mechanisms were uncertain. The study aimed to clarify the role of miR-135b in osteosarcoma (OS) cells and explore the effect of miR-135b inhibitor on the biological characteristics of OS cells. Firstly, Compared with adjacent bone tissues, the expression of miR-135b was increased and inversely correlated with potential target-PPM1A mRNA, while the expression of PPM1A was decreased in OS tissues. Dual luciferase reporter assay was used to verify that PPM1A was a target gene of miR-135b and RT-PCR and western blot were used to detect that miR-135b negatively regulated expression of PPM1A in MG63 and U2OS cell lines. After transfection, miR-135b inhibitor significantly inhibited cell proliferation and invasion both in MG63 and U2OS cells and blocked cell cycle in the G2/M phase and induced cells apoptosis, while PPM1A knockdown abolished the inhibition of miR-135b inhibitor on the proliferation and invasion of OS cells. In conclusion, miR-135b is up-regulated in OS cells and down regulating miR-135b expression could inhibit the proliferation and invasion of OS cells by up-regulating PPM1A. miR-135b might be a new therapeutic target of OS.
Keywords: miR-135b, protein phosphatase Mg2+/Mn2+ dependent 1A, osteosarcoma, proliferation, invasion
Introduction
Osteosarcoma (OS) is a common primary malignant bone tumor in orthopedics, with the characteristics of high morbidity, rapid progression, high degree of malignity, and high distant metastasis and recurrence in children [1]. Surgery combined with chemoradiotherapy is the main treatment, but the long-term survival rate is poor, with 5-year survival rates for patients with OS only about 60% [2]. Therefore, it is significant to elucidate the molecular mechanism of OS and explore the novel available markers for the diagnosis and treatment of OS.
In recent years, evidence showed that the miRNAs could function as tumor promoters or suppressors, which might be molecular targets for diagnosis and treatment of various cancers. The aberrant expression of miRNAs had been found to be associated with the occurrence and development of cancers, such as miR-143, miR-199a-3p and miR-21 were all involved in the proliferation and invasion of OS by regulating different target genes or signaling pathways [3-5]. Several reports suggested that miR-135b was expressed abnormally in various tumors such as breast cancer, colon cancer and cervical cancer, and played an important role in the occurrence and development of tumors by different pathways [6-8]. Studies hadshown that miR-135b was up-regulated in OS specimens, promoting OS cell proliferation, invasion and migration [9], but other studies indicated miR-135b was down-regulated in OS tissues and markedly suppressed OS cell proliferation, migration, and invasion [10]. Bioinformatics analysis had previously predicted that the protein phosphatase Mg2+/Mn2+ dependent 1A (PPM1A) as a member of serine/threonine phosphatase family, might be a direct target of miR-135b. However, we was still unclear about the regulatory effect of miR-135b on PPM1A and the role of miR-135b in OS and its mechanisms. Based on existing studies, this study aimed to explore the role of miR-135b in the progression of OS and its regulation of PPM1A, clarifying its possible mechanism.
Materials and methods
Clinical specimens
OS tissues were collected from 25 cases of OS patients at the orthopedics department of the First Affiliated Hospital of Zhengzhou University from June, 2016 to June, 2017, including 14 male and 11 female and the ages were 6-50 years old. All of the patients had no other tumors except for OS, and did not received radiotherapy or chemotherapy before surgery. The adjacent morphologically normal bone tissues were selected as control. The study was confirmed by each of the patients and family and approved by the ethics committee of the First Affiliated Hospital of Zhengzhou University.
Cell culture
Human OS cell lines MG63 and U2OS were purchased from Shanghai cell bank, Chinese Academy of Sciences. PRMI-1640 was purchased from Gibco, American. MG63 and U2OS were cultured in RPMI-1640 supplemented with 10% fetal bovine serum (FBS), 100 U/m penicillin and 100 ug/ml streptomycin (Thermo) in a cell incubator with an atmosphere of 5% CO2 at 37°C. The cells were subcultured when 80% fused.
Luciferase reporter assay
Based on the sequence of PPM1A gene and psiCHECKTM-2, the wild and mutant primers with Xho I/Not I sites were designed (synthesized by Tsingke Biological Technology, Wuhan). The fragments wild type and mutant 3’UTR with miR-135b binding site of PPM1A were amplified for further cloning: wild type plasmid psiCHECK-PPM1A-3’UTR-WT and mutant plasmid psiCHECK-PPM1A-3’UTR-Mu. MG63 and U2OS cells were seeded in 24-well plates and cotransfected with psiCHECK-PPM1A-3’UTR-WT or psiCHECK-PPM1A-3’UTR-Mu and miR-135b inhibitor or miR-135b negative control (miR-NC). After 48 h, the luciferase activities were determined using dual luciferase report assay system detection kit (Promega, USA).
Cell transfection and grouping
MG63 and U2OS cells were trypsinized and seeded into 6-well plates the day before transfection to ensure 70% cell fusion. The transfection was conducted according to the Lipofectamine 2000 (Invitrogen) instructions and the cells were divided into blank control group (no treatment), miR-NC group, miR-135b inhibitor group, siRNA-NC group and siRNA-PPM1A group. miRNA-135b negative control or inhibitor (final concentration was 100 nM., Ribo Bio, Guangdong) was transfected into miR-NC group or miR-135b inhibitor group. siRNA-PPM1A-negative control or siRNA-PPM1A (Boster, Wuhan) and miR-135b inhibitor were cotransfected in siRNA-NC group or siRNA-PPM1A group. 24 h post-transfection, follow-up experiments were performed.
Real-time PCR (RT-PCR)
The expression of miR-135b and PPM1A in tissues and cells were detected by quantitative real-time PCR. Total RNA was extracted from tissues or cells by TRIzol reagent (Invitrogen) and cDNA was generated with the Reverse Transcription Kit (Takara) following the manufacturer’s instructions. The sequence-specific primers were synthesized from Sangon Biotech, Shanghai (Table 1). The qRT-PCR analysis was carried out using SYBR Green PCR kit (Takara). U6snRNA and β-actin were used as an internal control to calcz by 2-ΔΔCt. RT-PCR was repeated three times.
Table 1.
Primer sequences for RT-PCR
| Gene | Primer sequences (5’-3’) | |
|---|---|---|
| miR-135b | Forward | 5’-ACACTCCAGCTGGGTATGGCTTTTCATTCCT-3’ |
| Reverse | 5’-CAGTGCGTGTCGTGGAGT-3’ | |
| U6 | Forward | 5’-CTCGCTTCGGCAGCACA-3’ |
| Reverse | 5’-AACGCTTCACGAATTTGCGT-3’ | |
| PPM1A | Forward | 5’-GTGCAGATAGAAGTGGGTCAAC-3’ |
| Reverse | 5’-CTTTCTCCAGCGGATTACTTGG-3’ | |
| β-actin | Forward | 5’-CACGAAACTACCTTCAACTCCAT-3’ |
| Reverse | 5’-ATCTTGATCTTCATTGTGCTGGG-3’ | |
Western blot
Total protein was extracted from cells by protein extraction kit (Beyotime), and the protein concentration was determined by BCA protein assay kit (Beyotime). SDS-PAGE electrophoresis was performed and blocked with 5% milk for 1 h after transferring. The primary antibodies (Abcam, British) were incubated as follows: anti-PPM1A (1:1000), anti-Ki67 (1:3000), anti-P53 (1:1000), anti-P21 (1:1000) and anti-β-actin (1:1000), 4°C overnight. After being washed, HPR-goat anti-rabbit or mouse IgG (1:2000, Abcam, British) was incubated at room temperature for 1 h. BeyoECL Plus was added into the membrane to detected the protein bands and the image analysis were performed using Image J to calculate the relative expression of proteins with the β-actin as the internal reference.
Cell counting Kit-8 (CCK-8) assays
The proliferation of OS cells was detected by CCK-8 (Solarbio) according to the manufacturer’s instructions. The transfected MG63 and U2OS cells were seeded into 96-well plates and cultured for 24, 48, 72 and 96 h. 10 µl CCK-8 solution was added into each well, incubated for 2 h, and the absorbance at 450 nm was measured on the multifunctional enzyme label to calculate the cell proliferation.
Transwell assays
The invasion and migration of OS cells were detected by cell invasion assay kit (BD Biosciences), The cells were suspended in RPMI-1640 medium (no FBS) at 1 × 105 cells/ml and 200 µl cell suspension was added into the upper chamber coated with matrix. The lower chamber was supplied with 500 µl RPMI-1640 medium containing 10% FBS. The cells were incubated at 37°C and 5% CO2 incubator for 24 h. The cells in upper chamber were removed with a cotton swab and the cells that had migrated to the other side of the membrane were fixed with methanol and stained with Giemsa for 15 min. The numbers of invaded cells from three randomly selected fields were counted using microscope and the average number was calculated.
Flow cytometric analysis
Cell cycle and apoptosis were assessed using flow cytometry according to the cell cycle and apoptosis analysis kit instructions. After transfection for 24 h, 1 × 105 cells were collected by 1000 rpm, centrifuged for 5 min and washed with PBS. The cells were fixed with 70% ethanol at 4°C overnight. After washing, the cells were re-suspended in 500 µl staining buffer. We added 2 µl RNase A and 10 µl PI and incubated for 30 min at 37°C in the dark. The cell cycles were detected by flow cytometry.
The transfected cells were collected and washed with PBS to formulate a cell suspension. Adding 5 µl Annexin V and 5 µl PI in 100 µl cells incubated for 15 min in the dark, the apoptosis rate was measured by flow cytometry.
Statistical analysis
All data were analyzed by SPSS 19.0. Measurement data sare shown as mean ± SD. Data comparison among multiple groups was performed with single factor analysis of variance, and the comparison between two groups was performed by LSD-t test; The correlations between miR-135b and PPM1A mRNA were analyzed by Pearson. P < 0.05 represented a significant difference.
Results
The expression of miR-135b and PPM1A mRNA in tissues
As shown in Figure 1, compared with the adjacent normal bone tissues, the expression of miR-135b was increased while that of PPM1A mRNA was decreased in OS tissues. The expression level of miR-135b was negatively correlated with PPM1A mRNA. Thus it was speculated that miR-135b was involved in the occurrence and development of OS and regulated the expression of PPM1A.
Figure 1.
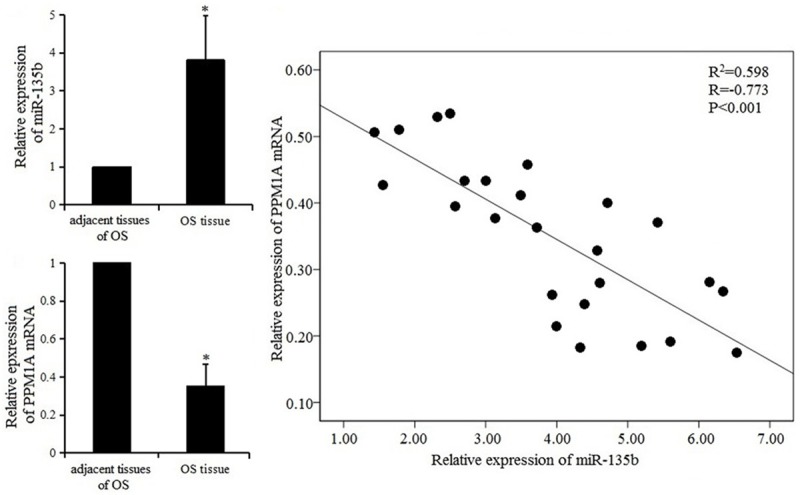
Expression of miR-135b and PPM1A mRNA in OS tissues and correlation analysis.
The expression of miR-135b and PPM1A in OS cells
The expression of miR-135b and PPM1A detected by RT-PCR and western blot are shown in Figure 2. The expression of miR-135b in the miR-135b inhibitor group was lower, while the expression of PPM1A mRNA and protein were significantly higher than that in the miR-NC group and blank control group (P < 0.05). The results suggested that miR-135b inhibitor could up-regulate PPM1A mRNA and protein in MG63 and U2OS cell lines.
Figure 2.
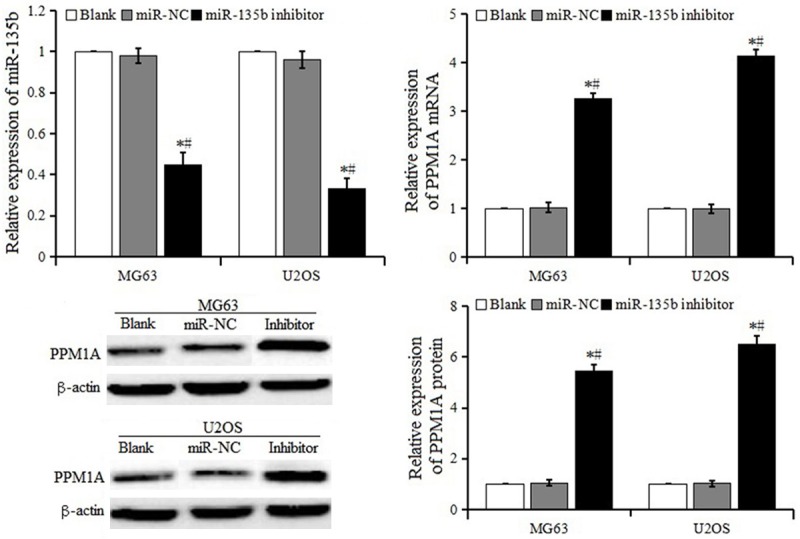
Expression of miR-135b and PPM1A in OS cells after transfection.
miR-135b targets to PPM1A
To verify whether PPM1A was a direct target of miR-135b, the dual luciferase reporter assay system was used to detect the luciferase activity. As shown in Figure 3, after being cotransfected with psiCHECK-PPM1A-3’UTR-WT vector, the luciferase activity in the miR-135b inhibitor group was higher than that in the miR-NC group. There was no difference between the miR-135b inhibitor group and miR-NC group with the psiCHECK-PPM1A-3’UTR-Mu vector transfection. The results indicated that miR-135b negatively regulated PPM1A expression by direct binding to the PPM1A 3’UTR region both in MG63 and U2OS cell lines.
Figure 3.
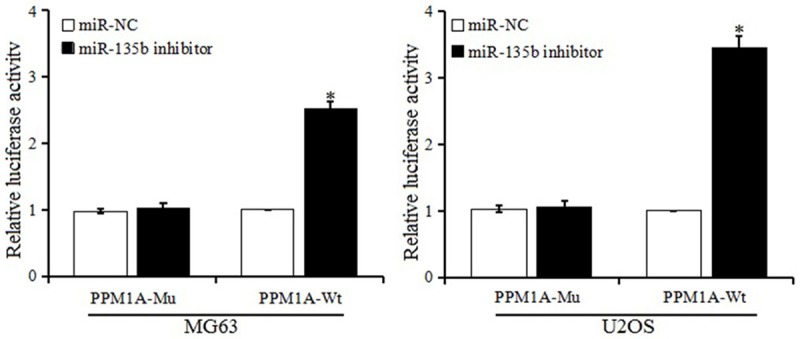
Detection of relative activity of luciferase.
Effect of miR-135b inhibitor on proliferation in MG63 and U2OS cells
CCK-8 assay was conducted to evaluate the proliferation in MG63 and U2OS cells after transfection with miR-135b inhibitor or NC. As shown in Figure 4, the miR-135b inhibitor significantly inhibited the proliferation of MG63 and U2OS cells, compared to the miR-NC group and blank control group. The expression of proliferation marker Ki-67 in the miR-135b inhibitor group was significantly lower than the miR-NC group and blank control group. The results showed that down-regulated miR-135b expression might inhibit the proliferation of MG63 and U2OS cells.
Figure 4.
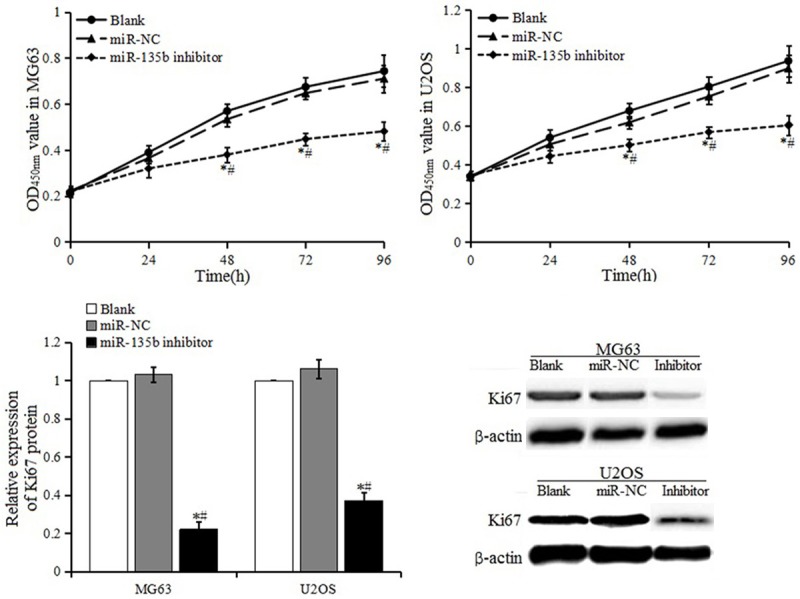
Cell proliferation activity and expression of protein Ki-67.
Effect of miR-135b inhibitor on cell cycle
As Figure 5 shows, compared with blank control group and miR-NC group, the proportion of cells in S phase decreased and the proportion of cells in G2/M phase increased in the miR-135b inhibitor group, and the difference was significant. WB detection showed that the expression of P53 and P21 in miR-135b inhibitor group were increased, and were significantly higher than that in the blank control group and miR-NC group. It was implied that miR-135b inhibitor arrested cells in the G2/M phase where apoptosis may be induced.
Figure 5.
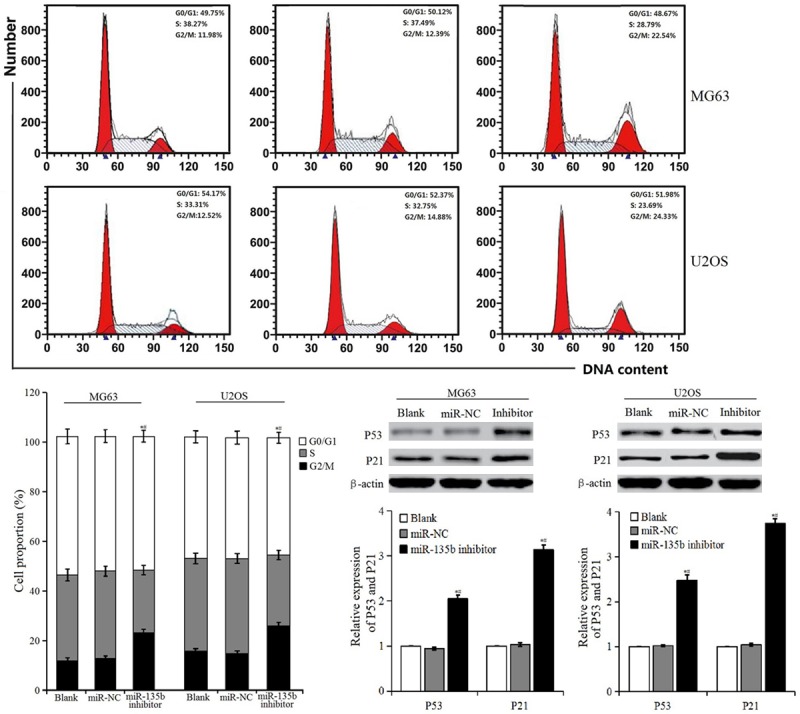
Cell cycle distribution and expression of P53 and P21 proteins.
Effect of miR-135b inhibitor on apoptosis
The apoptosis rates of MG63 and U2OS cells in miR-135b inhibitor group both were significantly higher than those in the miR-NC group and blank control group, while there was no difference in apoptosis rate between the miR-NC group and blank control group (in Figure 6; Table 2). It was suggested that miR-135b inhibitor promoted apoptosis of MG63 and U2OS cells.
Figure 6.
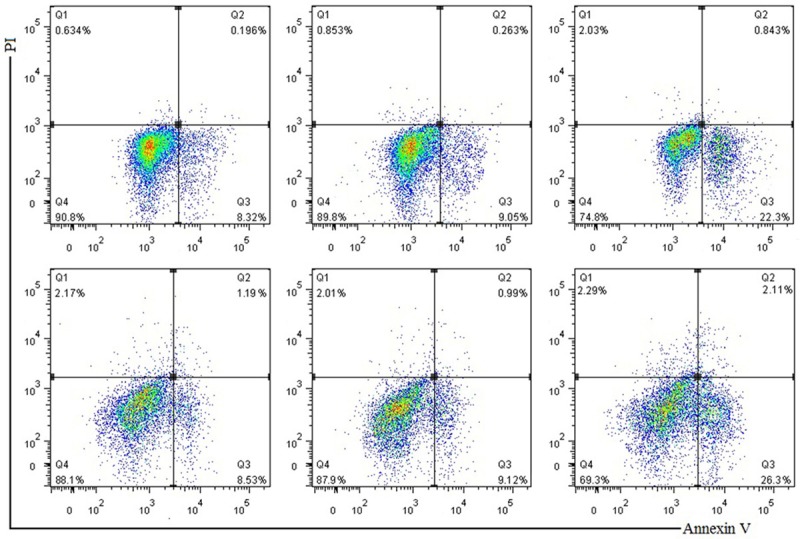
Apoptotic rate of each group by flow cytometry.
Table 2.
The apoptotic rate in each group (%)
| Apoptosis rate (%) | |||
|---|---|---|---|
|
| |||
| Blank Control | miR-NC | miR-135b inhibitor | |
| MG63 | 8.01±1.56 | 8.37±1.62 | 22.5±2.56*,# |
| U2OS | 8.71±1.89 | 9.21±1.83 | 25.7±3.47*,# |
P < 0.05, compared with blank control group;
P < 0.05, compared with miR-NC group.
Effect of miR-135b inhibitor on cell invasion
The results of transwell assay are shown in Figure 7. The migration and invasion of MG63 and U2OS cells in the miR-135b inhibitor group was reduced, which was significantly different from blank control group and miR-NC group (P < 0.05), suggesting that miR-135b inhibitor suppressed the migration and invasion of MG63 and U2OS cells.
Figure 7.
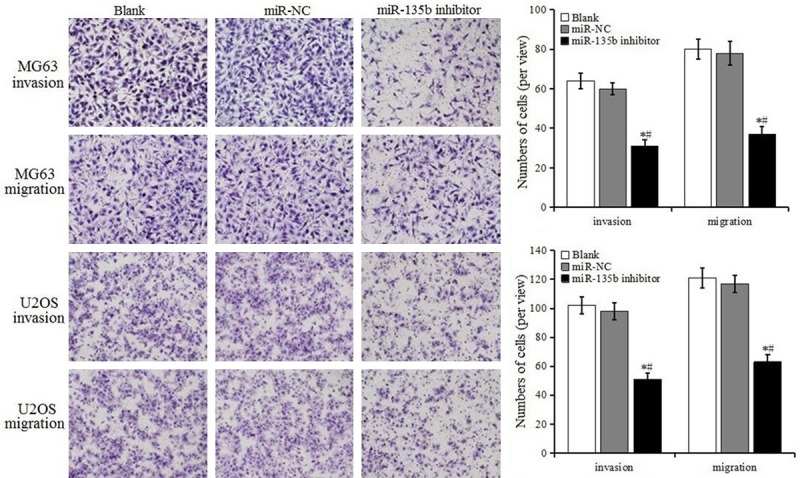
Detection of cell invasion and migration by transwell assays.
Effect of PPM1A knockdown on cell proliferation and invasion
We further explored the possibility that miR-135b inhibitor inhibited OS cell proliferation and invasion and induced apoptosis by mediating PPM1A. Cell proliferation and invasion were detected in the MG63 and U2OS cells that were cotransfected with miR-135b inhibitor and PPM1A siRNA. As shown in Figure 8, in cells cotransfected with miR-135b inhibitor and PPM1A siRNA, the proliferation and invasion ability were enhanced, significantly different from the siRNA-NC group. This meant that PPM1A knockdown reversed miR-135b inhibitor-induced proliferation and invasion inhibition in MG63 and U2OS cells. These results demonstrated that miR-135b inhibitor inhibited the proliferation and invasion, and induced apoptosis of OS cells by up-regulating PPM1A expression.
Figure 8.
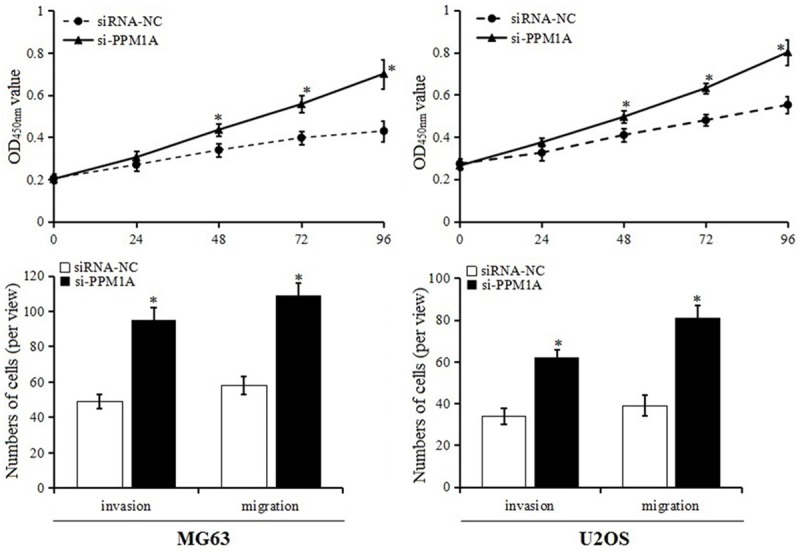
Cell proliferation and invasion after PPM1A knockdown.
Discussion
Research on the molecular mechanism of osteoscarcoma (OS) has found that many miRNAs play a key role in the proliferation and invasion of OS, such as miR-335 and miR-101 which can inhibit the invasion and metastasis of OS by directly inhibiting the expression of target gene ROCK1 [11,12]. miR-143 was expressed lower in OS cell lines and could inhibit Bcl-2 expression, inducing apoptosis and suppressing cell migration and invasion in OS cells. Other research indicated that down-regulation of miR-143 was correlated with the lung metastasis of human OS cells by promoting cellular invasion, probably through MMP-13 up-regulation [4,13]. miR-21 was also found significantly over-expressed in OS tissues and it had a key role in regulating invasion and migration of OS, likely through regulating RECK [14]. As more miRNAs relevant to OS were found, it stimulated exploration of the pathogenesis and therapy of OS.
It also was confirmed that miR-135b played an important role in human physiologic and pathologic processes: miR-135b could directly regulate the bone marrow mesenchymal stem cells to inhibit the formation of osteoblasts; and miR-135b is up-regulated in multiple tumors, such as colon cancer, breast cancer and lung cancer, to promote tumorigenesis. It was speculated that miR-135b might be an oncogenic miRNA [3,15,16]. Fan et al. [17] suggested that miR-135b expression down-regulated Ppm1e to activate AMPK signaling, which protects osteoblastic cells from dexamethasone. Wu et al. [18] found that miR-135b was up-regulated in colorectal cancer tissues, and miR-135b inhibitor transfected cells markedly reduced cell migration and invasion abilities through regulating MTSS1 expression. A study from Yue et al. [19] found that miR-135b was involved in the occurrence and development of endometrial carcinoma partially by regulating its target gene FOXO1. Arigoni et al. [20] observed that miR-135b was up-regulated in basal or normal-like human breast cancers, and correlated with patient survival and early metastasis. Lin et al. [21] demonstrated that miR-135b as an oncogenic miRNA, was up-regulated in high invasive lung cancer cells CL1-5, and enhanced cell invasive and migratory ability in vitro as well as the orthotopic tumor growth and lung metastasis activity in vivo. Recently, Jin et al. [22] had identified a novel role for miR-135b in OS: miR-135b had an activating effect on both Wnt/β-catenin and Notch signaling. Up-regulating miR-135b could promote cancer stem cell traits, lung metastasis, and tumor recurrence in OS. In order to explore the role and mechanism of miR-135b in OS, the expression of miR-135b and its target gene PPM1A were detected in our study. The results showed that the expression of miR-135b was up-regulated and PPM1A was down-regulated in OS tissues, which was a significant difference with adjacent bone tissues. There was a negative correlation between miR-135b and PPM1A mRNA, suggesting miR-135b plays an important role in the occurrence and development of OS and correlates with the expression of PPM1A.
PPM1A (known as PP2CA) is known as a tumor suppressor. As a member of the PPM subfamily of the Ser/Thr phosphatase family, PPM1A participates in the dephosphorylation process in various cell signaling pathways [23]. For example, PPM1A is involved in the regulation of the BMP/Smad1 signaling pathway by catalyzing the dephosphorylation of Smad1 [24]. It also is involved in the regulation of the cell cycle by negatively regulating P38 and JNK MAPK signaling pathway and arrested cells in the G2/M stage, inducing apoptosis by up-regulating endogenous P53 and P21 expression [25]. In the present study, the dual luciferase reporter assay confirmed that PPM1A was a direct target of miR-135b. After transfection with miR-135b inhibitor, the expression of miR-135b was down-regulated and the expression of PPM1A was up-regulated both in MG63 and U2OS cell lines, suggesting miR-135b could negatively regulate the expression of PPM1A. The results also showed that miR-135b inhibitor could inhibit cell proliferation, invasion and migration, and arrest cells in the G2/M stage, inducing apoptosis both in MG63 and U2OS cell lines. This was similar to the results of Pei et al. [26]. Furthermore, knockdown of PPM1A significantly reversed the function of miR-135b inhibitor on proliferation and invasion inhibition in OS cells. The results demonstrated miR-135b inhibitor could inhibit the proliferation and invasion, and induce apoptosis of OS cells by up-regulating PPM1A expression.
Conclusions
miR-135b is up-regulated in OS cells, and promotes the proliferation and invasion of OS cells by regulating PPM1A. miR-135b inhibitor can inhibit the proliferation and invasion of OS cells by up-regulating PPM1A, which might be a new therapeutic target in OS.
Acknowledgements
This work was supported by the Scientific and Technological Research Project of Henan Health and Family Planning Commission, China (No. 201403239).
Disclosure of conflict of interest
None.
References
- 1.Gianferante DM, Mirabello L, Savage SA. Germline and somatic genetics of osteosarcoma-connecting aetiology, biology and therapy. Nat Rev Endocrinol. 2017;13:480. doi: 10.1038/nrendo.2017.16. [DOI] [PubMed] [Google Scholar]
- 2.Wan G, Yang H, Wu D. Repressive effect of miRNA-363 via SOX4 on human osteosarcoma cell growth and apoptosis. Chinese Journal of Pathophysiology. 2017;33:278–283. [Google Scholar]
- 3.Xu XM, Qian JC, Deng ZL, Cai Z, Tang T, Wang P, Zhang KH, Cai JP. Expression of miR-21, miR-31, miR-96 and miR-135b is correlated with the clinical parameters of colorectal cancer. Oncol Lett. 2012;4:339–345. doi: 10.3892/ol.2012.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osaki M, Takeshita F, Sugimoto Y, Kosaka N, Yamamoto Y, Yoshioka Y, Kobayashi E, Yamada T, Kawai A, Inoue T, Ito H, Oshimura M, Ochiya T. MicroRNA-143 regulates human osteosarcoma metastasis by regulating matrix metalloprotease-13 expression. Mol Ther. 2011;19:1123–30. doi: 10.1038/mt.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duan Z, Choy E, Harmon D, Liu X, Susa M, Mankin H, Hornicek F. MicroRNA-199a-3p is downregulated in human osteosarcoma and regulates cell proliferation and migration. Mol Cancer Ther. 2011;10:1337. doi: 10.1158/1535-7163.MCT-11-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leung CO, Deng W, Ye TM, Ngan HY, Tsao SW, Cheung AN, Pang RT, Yeung WS. miR-135a leads to cervical cancer cell transformation through regulation of β-catenin via a SIAH1-dependent ubiquitin proteosomal pathway. Carcinogenesis. 2014;35:1931–1940. doi: 10.1093/carcin/bgu032. [DOI] [PubMed] [Google Scholar]
- 7.Mudduluru G, Lunavat TR, Scharp M, Muppala S, Patil N, Leupold JH, Abba M, Allgayer H. Abstract LB-467: miR-135b and miR-210 induce invasion and distant metastasis by targeting SIAH1 and SETD2 in colorectal cancer. Cancer Res. 2012;72:467. [Google Scholar]
- 8.Chen Y, Zhang J, Wang H, Zhao J, Xu C, Du Y, Luo X, Zheng F, Liu R, Zhang H. miRNA-135a promotes breast cancer cell migration and invasion by targeting HOXA10. BMC Cancer. 2012;12:111. doi: 10.1186/1471-2407-12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu WG, Shang YL, Cong XR, Bian X, Yuan Z. MicroRNA-135b promotes proliferation, invasion and migration of osteosarcoma cells by degrading myocardin. Int J Oncol. 2014;45:2024–2032. doi: 10.3892/ijo.2014.2641. [DOI] [PubMed] [Google Scholar]
- 10.Liu Z, Zhang G, Li J, Liu J, Lv P. The tumor-suppressive MicroRNA-135b targets c-Myc in osteoscarcoma. PLoS One. 2014;9:e102621. doi: 10.1371/journal.pone.0102621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Rui J, Chao Z, Liu G, Rui G, Han W. MicroRNA-101 inhibits proliferation, migration and invasion in osteosarcoma cells by targeting ROCK1. Am J Cancer Res. 2017;7:88–97. [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Zhao W, Fu Q. miR-335 suppresses migration and invasion by targeting ROCK1 in osteosarcoma cells. Mol Cell Biochem. 2013;384:105–11. doi: 10.1007/s11010-013-1786-4. [DOI] [PubMed] [Google Scholar]
- 13.Li WH, Wu HJ, Li YX, Pan HG, Meng T, Wang X. MicroRNA-143 promotes apoptosis of osteosarcoma cells by caspase-3 activation via targeting Bcl-2. Biomed Pharmacother. 2016;80:8–15. doi: 10.1016/j.biopha.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Wu Z, Yang S, Weng X, Liu X. MicroRNA-21 is involved in osteosarcoma cell invasion and migration. Med Oncol. 2011;28:1469–1474. doi: 10.1007/s12032-010-9563-7. [DOI] [PubMed] [Google Scholar]
- 15.Su W, Mo Y, Wu F, Guo K, Li J, Luo Y, Ye H, Guo H, Li D, Yang Z. miR-135b reverses chemoresistance of non-small cell lung cancer cells by downregulation of FZD1. Biomed Pharmacother. 2016;84:123–129. doi: 10.1016/j.biopha.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 16.Hua K, Jin J, Zhao J, Song J, Song H, Li D, Maskey N, Zhao B, Wu C, Xu H. miR-135b, upregulated in breast cancer, promotes cell growth and disrupts the cell cycle by regulating LATS2. Int J Oncol. 2016;48:1997. doi: 10.3892/ijo.2016.3405. [DOI] [PubMed] [Google Scholar]
- 17.Fan J, Ruan J, Liu W, Zhu L, Zhu X, Yi H, Cui S, Zhao J, Cui Z. miR-135b expression downregulates Ppm1e to activate AMPK signaling and protect osteoblastic cells from dexamethasone. Oncotarget. 2016;7:70613–70622. doi: 10.18632/oncotarget.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu W, Wang Z, Yang P, Yang J, Liang J, Chen Y, Wang H, Wei G, Ye S, Zhou Y. MicroRNA-135b regulates metastasis suppressor 1 expression and promotes migration and invasion in colorectal cancer. Mol Cell Biochem. 2014;388:249–259. doi: 10.1007/s11010-013-1916-z. [DOI] [PubMed] [Google Scholar]
- 19.Yue Z, Shen JJ, Huang QT, Qin YF, Li XN, Liu GB. [MiR-135b promotes proliferation of endometrial carcinoma cells by targeting FOXO1] . Nan Fang Yi Ke Da Xue Xue Bao. 2016;36:675–80. [PubMed] [Google Scholar]
- 20.Arigoni M, Barutello G, Riccardo F, Ercole E, Cantarella D, Orso F, Conti L, Lanzardo S, Taverna D, Merighi I. miR-135b coordinates progression of ErbB2-driven mammary carcinomas through suppression of MID1 and MTCH2. Am J Pathol. 2013;182:2058–2070. doi: 10.1016/j.ajpath.2013.02.046. [DOI] [PubMed] [Google Scholar]
- 21.Lin CW, Lin JC, Chang YL, Chen HY, Pan SH, Hong TM, Yang PC. Abstract 3976: the role of a novel oncomiR, miR-135b, in lung cancer metastasis and clinical application. Cancer Res. 2011;71:3976. [Google Scholar]
- 22.Jin H, Luo S, Wang Y, Liu C, Piao Z, Xu M, Guan W, Li Q, Zou H, Tan QY, Yang ZZ, Wang Y, Wang D, Xu CX. miR-135b stimulates osteosarcoma recurrence and lung metastasis via notch and wnt/β-catenin signaling. Mol Ther Nucleic Acids. 2017;8:111–122. doi: 10.1016/j.omtn.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lv Y. PPM1A act as KAP1/SIRT1 phosphatase to regulate KAP1 and SIRT1. East China Normal University. 2011 [Google Scholar]
- 24.Duan X, Liang YY, Feng XH, Lin X. Protein serine/threonine phosphatase PPM1A dephosphorylates smad1 in the bone morphogenetic protein signaling pathway. J Biol Chem. 2006;281:36526–36532. doi: 10.1074/jbc.M605169200. [DOI] [PubMed] [Google Scholar]
- 25.Zhang B, Zhou Z, Lin H, Lv X, Fu J, Lin P, Zhu C, Wang H. Protein phosphatase 1A (PPM1A) is involved in human cytotrophoblast cell invasion and migration. Histochem Cell Biol. 2009;132:169–79. doi: 10.1007/s00418-009-0601-5. [DOI] [PubMed] [Google Scholar]
- 26.Pei H, Jin Z, Chen S, Sun X, Yu J, Guo W. MiR-135b promotes proliferation and invasion of osteosarcoma cells via targeting FOXO1. Mol Cell Biochem. 2015;400:245–52. doi: 10.1007/s11010-014-2281-2. [DOI] [PubMed] [Google Scholar]


