Abstract
Recently, miRNA-23 has been illustrated to play an important role in causing myocardial ischemia/reperfusion injury (MIRI), indicated that inhibition of miR-23 could protect the cardiomyocyte from MIRI. However, the underlying mechanism of miR-23 inhibition in alleviating the reperfusion-induced myocardial damage is unclear. Recognizing that the bone marrow mesenchymal stem cells (BMSCs) have the potential for pluripotent differentiation into myocardial cells, we therefore hypothesis that the BMSCs are involved in the process of miR-23 alleviating IRI. For verification, the BMSCs was established firstly and confirmed by the immunofluorescence assay and flow cytometry analysis. As results revealed that BMSCs were positive for CD44 which was known for BMSC markers, and negative expression for CD45, indicating that the BMSCs was successfully established in our work. Subsequently, we have investigated the effect of miR-23 on the expression of hyaluronan synthase-2 (Has2), a critical gene during heart morphogenesis. Results obtained by the Western-blot and qRT-PCR assay displayed that the levels of Has2 in the BMSCs treated by miR-23 inhibitor was significantly up-regulated than that of control group. Furthermore, the effect of miR-23 on promoting the transformation of BMSCs into myocardial cells was investigated. As demonstrated by the results that the expression level of the cardiac markers in BMSCs transfected with miR-23 inhibitor was remarkably elevated, indicating that inhibition of miR-23 exactly facilitated to the transformation of BMSCs into myocardial cells. The underlying mechanisms experiments showed that the Wnt1, TCF4, and the β-catenin could be significantly elevated by treating with miR-23 inhibitor, suggesting that the activation of Wnt pathway has played a significant role in that process. Finally, the in vivo IRI antagonism effect of miR-23 inhibition was studied and results displayed that the myocardium lesions of these IR rats could be significantly recovered by treating with miR-23 inhibitor.
Keywords: miRNA-23, ischemia/reperfusion injury, bone marrow mesenchymal stem cells, hyaluronan synthase-2, Wnt pathway
Introduction
Ischemia/reperfusion injury (IRI), represents one of the dominant factors that contributes significantly to postoperative mortality and morbidity, and is the consequence of vessel occlusion followed by multiple stresses during the restoration of blood flow to the tissue [1]. Increasing evidence demonstrated that IRI is a complex pathophysiologic event that causes a serious health problem by deterioration of heart function, and limits the benefits of reperfusion after acute myocardial infarction [2]. Moreover, a previously reported study has exhibited that myocardial ischemia reperfusion injury (MIRI) resulted in over 50% of the MI size among the experiment MI animal models [3]. Recognizing that the histopathology changes leaded by reperfusion are the main causes of cardiac myocyte death, prevention or reversion of this change might, therefore, represent a promising strategy for alleviation of MIRI [4].
Micro ribonucleic acids (microRNAs, miRs) are endogenous short non-coding RNAs. They have important regulatory functions in a wide range of biological processes, including regulating cell proliferation, apoptosis, and aging [5]. In addition, they are also involved in the development of the heart and the regeneration of the myocardium [6]. Recently, emerging evidence showed that miRNAs were closely associated with stem cell differentiation. It has been reported that overexpression of miR-21 significantly decreased infarct size after acute myocardial infarction [7]. Furthermore, recent studies have reported that miR-23 promotes apoptosis induced by oxidative stress or ischemic/reperfusion, suggesting that inhibition of miR-23 could protect the oxidative stress-induced cardiomyocyte [8]. However, whether miR-23 plays essential roles during the reperfusion-mediated cardioprotection and was involved in inducing differentiation of bone marrow mesenchymal stem cells into cardiomyocytes is unclear.
Bone marrow mesenchymal stem cells (BMSCs) are a type of bone marrow-derived, non-hematopoietic stem cell that has the potential for self-renewal and pluripotent differentiation [9]. The unraveling of many of their exceptional characteristics and the encouraging preclinical and clinical data indicates that MSCs will provide a revolutionary advantage in the therapeutic intervention of various diseases including MIRI [10]. As demonstrated, the differentiation of BMSCs into myocardial cells was supposed to closely relevant to the activation of the Wnt signaling pathway [11]. In human embryonic stem cells, activating the wnt/β-catenin increases the protein levels of Cx43 and Nkx2.5, which are required for cardiac differentiation. In mouse embryonic stem cells, activating the wnt/β-catenin by wnt3a induces cardiac differentiation, whereas inhibiting the pathway reduces the cardiac differentiation as indicated by measuring cardiac-specific genes, such as β-myosin heavy chain (b-MHC), cardiac troponin T (cTnT), Nkx2.5, and sarcomeric myosin heavy chain (sMHC) [12].
Has2 (hyaluronic acid synthase 2) is one of the causes of downregulation of multiple pathways of bone morphogenetic protein (BMP) that initiates EC formation from human to zebrafish [13]. The importance of regulating Has2 expression in the endocardium has been exemplified by the observation that in Has2 deficient mice the cardiac jelly does not expand and ECs fail to form [14]. However, the detailed linkage between the Has2 and protecting myocardial cells and the effect of miR-23 in regulating Has2 expression still remains unclear. Based on the factors above, we hypothesize that the effect of miR-23 inhibitin against myocardial ischemia reperfusion injury is mainly via regulating the expression of Has2 and promoting the differentiation of BMSCs into myocardial cells. For verification, a series of in vivo and in vitro experiments have been conducted and our primary goal is to provide an innovative strategy for prevention of IRI.
Materials and methods
Animals and cell culture
The Sprague-Dawley rats (male, 3-4 weeks, ~ 200 g) were obtained from the BK Lab Animal Ltd. (Shanghai, China) and maintained in a specific pathogen-free laboratory with free access to sufficient food and water. Of great importance, all the animal experiments here were performed in accordance with the Guide for the Care and Use of Laboratory Animals, approved by the Animal Care and Use Committee.
The bone marrow mesenchymal stem cells (BMSCs) were achieved using a previously reported method with a slight modification [10]. In brief, the SD rats were euthanized and immersed in 75% medical alcohol to achieve the bilateral femurs through rapidly removing the soft tissues that was attached tightly to the femur. Then, the obtained femurs were transferred to an ultra-clean bench followed by excision of the metaphysis. Subsequently, the marrow contents were flushed out with the PBS (pH 7.4) and collected using the method of centrifugation (400 g for 4 min). To obtain the BMSCs, the collected cell pellets were subjected to resuspension by 5 mL minimum essential medium (MEM)-α and cultured under 37°C.
Characterization of BMSCs by immunocytochemistry
The BMSCs at logarithmic phase were washed twice with PBS and fixed by 4% paraformaldehyde for 10 min. Subsequently, the cells were permeabilized using 0.1% triton X-100 and blocked by the blocking solution which containing 2% bovine serum albumin, 0.2% goat serum, and 0.1% Tween 20. Thereafter, the BMSCs subjected to incubation with primary antibodies (1:50 dilution) against rat CD44 (BD Biosciences, San Jose, CA, USA) and CD34 (Santa Cruz Biotechnology Inc., Dallas, TX, USA), respectively. After an overnight incubation, the primary antibodies were removed and the cells were incubated with secondary antibodies that conjugated to Alexa Fluor 546 (1:200 dilution) for 1 hour under room temperature. Finally, the cells were washed twice using the PBS and stained by 4’,6-diamidino-2-phenylindole (DAPI) before observing the fluorescent signal under a fluorescent microscope (TE2000 Nikon, Japan). Moreover, the expressions of CD44 and CD34 on BMSCs were further quantitatively analyzed using the Flow Cytometry analysis.
Real-time PCR
The expression levels of various genes were evaluated by the RT-PCR according to the manufacturer’s instructions. In brief, the PCR products were firstly amplified from the obtained cDNA samples using the TaqMan MicroRNA Assays kit together with the TaqMan Universal PCR Master Mix (Applied Biosystems). Then the relative quantitation of gene expression was measured using the comparative Ct (threshold cycle) method with arithmetic formulae (2-ΔΔCt). Notably, the annealing temperature was set at 90°C and the circulation coefficient was set at 40. All experiments were repeated three times independently. The specific primer sequences used in this study were as follows: Has2: F, gaaaagggtcctggtgagacggatgag; R, ttcaccatctccacagatgaggcagg; and GAPDH: F, gaccacagtccatgccatca; R, gtcaaaggtggaggagtggg.
Western immunoblot experiment
To determine the expressions of various proteins on BMSCs, western immunoblot experiments were performed. For cellular assays, the BMSCs that have been received with various treatment strategies were trypsinized by 0.25% trypsin-EDTA followed by centrifugation for 5 min. Then, the total protein samples were extracted using the M-PER Mammalian Protein Extraction Reagent and quantitatively determined through the BCA protein assay. Subsequently, the protein samples were separated by SDS-polyacrylamide gel electrophoresis (PAGE) with electrophoresis and transferred to a nitrocellulose membrane. Thereafter, a blocking solution (5% BSA in a Tris-buffered Tween 20 solution) was introduced to pre-block the samples followed by incubation with various primary antibodies, including anti-Has2, anti-Gata-4, anti-Nkx2-5, anti-MHC, anti-cTnl, anti-Wnt1, and anti-TCF4 antibodies (1:1000; Sakura, Torrance, CA, USA). After an overnight incubation at 4°C under gentle agitation, the primary antibodies were removed and replaced with goat anti-rabbit IgG secondary antibody (1:5000; Abcam, UK). Two hours later, the bands of interest were examined using the FluorChem R multiplex imaging system (ProteinSimple, USA).
Histopathologic evaluation
Mice were randomly divided into three groups as follows: Healthy control group (treated by saline), I/R group (I/R mice without any treatment), miR-23 inhibitor group (I/R mice treated by miR-23 inhibitor), sh-Has2 group (I/R mice treated by sh-Has2), and inhibitor + sh-Has2 group (I/R mice treated by inhibitor + sh-Has2). For histopathologic evaluation, organs including liver, lung, kidney and colon of mice were obtained and fixed in 4% paraformaldehyde for overnight. After that, all tissues were embedded in paraffin and preserved under -20°C before the tissues were sectioned for 5 μm. Finally, H&E staining was conducted in accordance with the manufacturer’s guideline and the results were analyzed using a light microscope (BX51; Olympus, Tokyo, Japan).
BCA protein assay
To quantitatively determine the density of cardiac marker including Mb, CK-MB, and cTn on the nanoparticle surface, a BCA protein assay was performed. Generally, after depicting the standard curve using BSA as standards, 20 ml of samples, along with 160 ml of BCA Protein Assay Reagent were added in the 96-well plate, then incubated at 37°C for 1 h. Thereafter, the absorbance at 562 nm was measured via a microplate reader (Thermo Multiskan MK3, USA).
Statistical analysis
One-way ANOVA analysis was employed for comparison and the data analysis was conducted using the SPSS software. The Shapiro-Wilks test and the Levene’s test were applied to check the assumptions of normality and the equal variance, respectively. Of great importance, the experiments performed here were repeated at least three times and P values smaller than 0.05 were considered significant.
Results
The established BMSCs were high positive for CD44 molecules
In general, the CD44 and CD34 belong to the characteristic molecule markers that presented for BMSCs and previous studies have demonstrated that BMSCs express CD44 at the high level and CD11b, CD34 and CD45 at the low levels [15]. In this case, we determined the expression of CD44 and CD34 on cells to investigate whether the BMSCs was successfully established here. As illustrated in Figure 1A, the established cell models displayed a stronger red fluorescent signal compared with other groups, while undetectable fluorescent signal was observed for CD34. The flow cytometric analysis (Figure 1B) further revealed that the established BMSCs were positive for CD44 with a value of 96%, while the hematopoietic marker CD34 was low in expression (the percent of positive CD34 was only ~ 2%). Taking these results together, we can draw the conclusion that the BMSCs have been successfully established in the present work.
Figure 1.
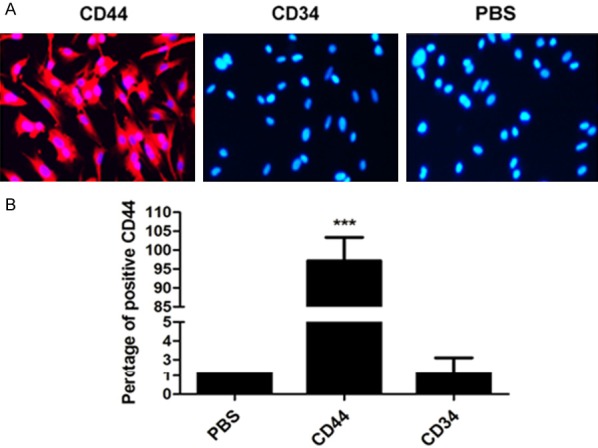
Immunostaining of BMSCs on the basis of surface marker expression. Cells were randomly divided into three groups (n = 3): Control group, CD44 positive group, and the CD34 negative group. A. Qualitative analysis of the expression levels of CD44 and CD34 under an inverted fluorescence microscope. The cell group treated with PBS acted as the control. B. Quantitative analysis of cell surface markers’ expression. Nuclei were stained with DAPI while the MSC markers were visualized by red fluorescence signal. Abbreviation: DAPI, 4’,6-diamidino-2-phenylindole. ***P < 0.01 compared to the control cells.
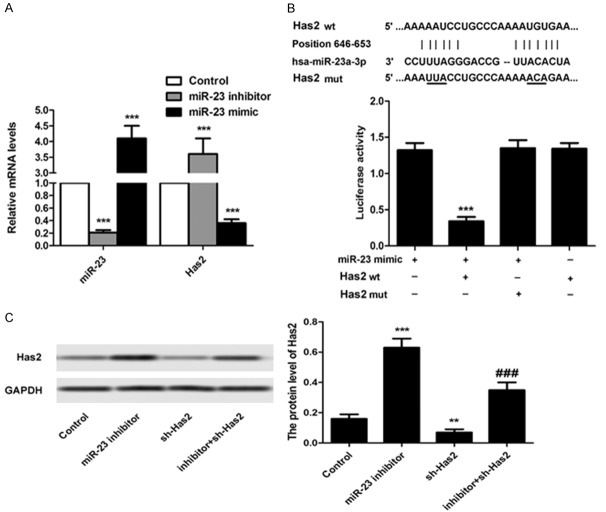
The expression of Has2 is significantly down-regulated by miR-23
The effect of miR-23 on regulating the expression level of Has2 in BMSCs was investigated using the qRT-PCR assays and western immunoblot experiment. For the qRT-PCR assays, the BMSCs were randomly divided into three groups: control group (without any treatment), miR-23 inhibitor group (cells transfected with miR-23 inhibitor/sh-Has2), and miR-23 mimic group (cells transfected with miR-23 mimic). As shown in Figure 2A, the levels of miR-23 in BMSCs could be significantly decreased by treating the cells with miR-23 inhibitor while not the miR-23 mimic. However, the down-regulation of miR-23 expression in BMSCs resulted in an obvious elevation of Has2 level in these cells, indicating that the miR-23 expression has a serious negative effect on the expression of Has2 in BMSCs. For further confirmation, the western immunoblot experiment was also performed with the BMSCs were randomly divided into four groups: control group (without any treatment), miR-23 inhibitor group (cells transfected with miR-23 inhibitor), sh-Has2 group (cells transfected with sh-Has2), and inhibitor + sh-Has2 group (cells transfected with inhibitor + sh-Has2). As displayed in Figure 2C, cells treated by miR-23 inhibitor exhibited the strongest signal of Has2 when compared to other groups. Moreover, the down-regulation of Has2 expression by sh-Has2 could be signally reversed by transfection of BMSCs with miR-23 inhibitor. All these results suggesting that the expression of Has2 could be significantly down-regulated by miR-23 and inhibition of miR-23 contribute dramatically to the expression of Has2 in BMSCs.
Figure 2.
The expression of Has2 is signifcantly down-regulated by miR-23. For evaluation of the effect of miR-23 on the levels of Has2 in BMSCs, the cells were randomly grouped (n = 4) and treated by miR-23 inhibitor, miR-23 mimic, shRNA-Has2, and the shRNA-Has2 + miR-23, respectively. A. RT-PCR analysis of miR-23 and Has2 in BMSCs after receiving of miR-23 inhibitor and miR-23 mimic, respectively. B. Detection of luciferase activity was conducted using the ELISA according to the manufacturer’s protocol. C. Western blot was applied to investigate the expression of Has2 in BMSCs after receiving of different treatment strategies. The left represents the qualitative images and the right represents the semi-quantitative analysis of the expression of Has2 in BMSCs post western blot. **P < 0.05, ***P < 0.01 compared to the cells treated by miR-23. ###P < 0.01 compared to the control cells.
Inhibition of miR-23 promotes the transformation of bone marrow mesenchymal stem cells into cardiomyocytes
For verification of whether inhibition of miR-23 could promote the transformation of bone marrow mesenchymal stem cells into cardiomyocytes, various markers of cardiomyocytes including the GATA-4 [16], Nkx2-5 [17], MHC [18] and cTnI [19] were examined. As demonstrated in Figure 3, post treatment of miR-23 inhibitor, the levels of GATA-4, Nkx2-5, MHC, and cTnI were markedly elevated when compared to the control. However, the expression these genes dramatically declined post transfection cells with sh-Has2. Of great importance, the inhibition effect of sh-Has2 could be relieved to a certain extent by co-treating with the miR-23 inhibitor. For quantitative investigation, the qRT-PCR experiments were further performed. As shown that the expression level of these cardiac markers in BMSCs transfected with sh-Has2 was remarkably reduced with a knockdown efficiency of about 60%. However, the expression of these genes in the cells treated by miR-23 inhibitor was significantly upregulated.
Figure 3.
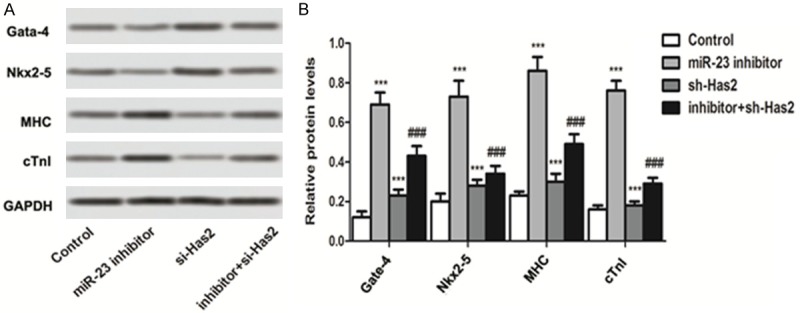
Inhibition of miR-23 promotes the transformation of bone marrow mesenchymal stem cells into cardiomyocytes. A. Western blot was applied to investigate the expression of cardiomyocyte markers after receiving of different treatment strategies. Post treatment of miR-23 inhibitor, the levels of GATA-4, Nkx2-5, MHC, and cTnI were markedly elevated when compared to the control. B. Semi-quantitative analysis of the expression of GATA-4, Nkx2-5, MHC, and cTnI for the western blot. ***P < 0.01 compared to the control group. ###P < 0.01 compared to the control cells.
miR-23 inhibition promotes the activation of Wnt pathway
In the present study, to verify whether Wnt signaling pathway was involved in the promotion of BMSCs differentiation into myocardial cell, the Wnt1, TCF4 and β-catenin, which are the specific markers of Wnt signaling pathway, were determined. As results depicted in Figure 4 show, Wnt1, TCF4, and the β-catenin displayed the lowest levels in the cells transfected with sh-Has2, indicating that the Wnt/β-catenin pathway was remarkably suppressed. However, the expressions of those genes in BMSCs could be significantly elevated by treating with miR-23 inhibitor. Taking these results together, transfection of BMSCs with miR-23 inhibitor could promote the differentiation of these cells into myocardial cells via the activation of Wnt pathway.
Figure 4.
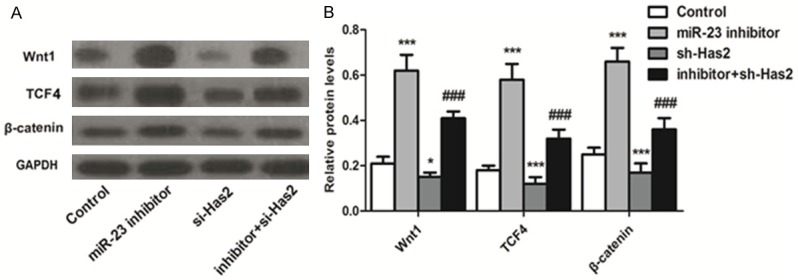
Inhibition of miR-23 promotes cell differentiation-related pathway-Wnt signaling pathway activation. To verify whether Wnt signaling pathway was involved in the promotion of BMSCs differentiation into myocardial cell, the Wnt1, TCF4 and β-catenin, that are the specific markers of Wnt signaling pathway, were determined by western blot. A. Qualitative evaluation of the levels of Wnt1, TCF4 and β-catenin in BMSCs post various treatments. B. Quantitative evaluation of the levels of Wnt1, TCF4, and β-catenin in BMSCs post various treatments. *P < 0.1, ***P < 0.01 compared to the control group. ###P < 0.01 compared to the control cells.
There were an opposite expressions of miR-23 and Has-2 in myocardial tissue of myocardial reperfusion mice
To determine the expression levels of miR-23 and Has-2 myocardial tissue of myocardial reperfusion mice, the RT-PCR experiments were conducted. As results in Figure 5 indicated, the expression of miR-23 was markedly higher than that of the control group. However, the level of Has-2 in myocardial tissue of myocardial reperfusion mice was absolutely opposite. As exhibited in the results, the expression of Has-2 was significantly lower than that of the control group.
Figure 5.
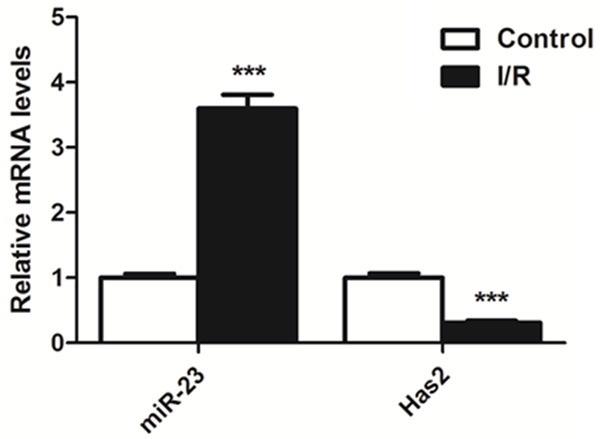
Expression of miR-23 and Has-2 in myocardial tissue of myocardial reperfusion mice. The RT-PCR experiments indicated that the expression of miR-23 was markedly higher than that of control group. However, the expression level of Has-2 was significantly lower than that of control group. ***P < 0.01 compared to the control group.
miR-23 inhibition contributed significantly to the protection of myocardial lesions
To evaluate the effect of miR-23 inhibition on alleviating the myocardial ischemia reperfusion injury, the IR animal models were euthanized followed by H&E staining analysis of heart. For comparison, normal mice that did not receive any treatment were also subjected to the histopathologic analysis. As shown in Figure 6A, compared to the normal group which was not treated by any anticancer agents, the IR rats and the rat treated by sh-Has2 displayed the obvious myocardiallesions such as blurry cardiac muscle layers, disruption of glands and exfoliation of the superfcial epithelium. Of great importance, the myocardium lesions of these IR rats could be significantly recovered by treating with miR-23 inhibitor.
Figure 6.
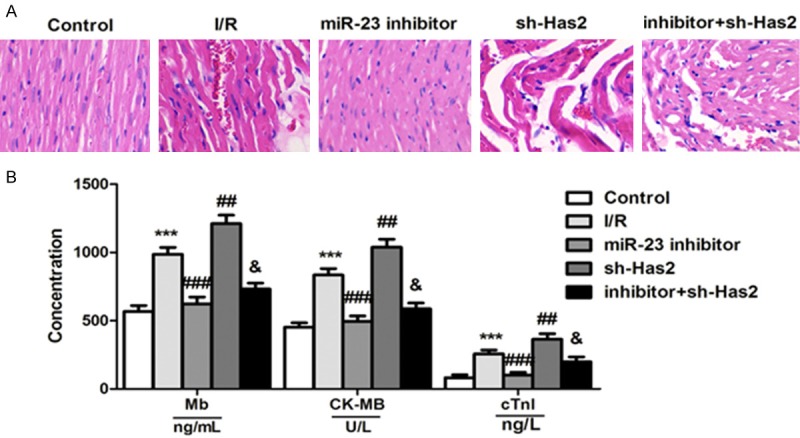
Inhibition of miR-23 has an obvious myocardial protection in mice with myocardial reperfusion injury. A. Histopathologic analysis of organs post treatment of Salid demonstrated that the myocardium lesions of these IR rats could be significantly recovered by treating with miR-23 inhibitor. B. The BCA protein assay was applied to determine the concentration of Mb, CK-MB, and cTn in the heart tissues of IR rats post receiving with various treatments. ***P < 0.01 compared to the control cells. ##P < 0.05, ###P < 0.01 compared to the control cells.
Inhibition of miR-23 has an obviously myocardial protection in mice with myocardial reperfusion injury
Finally, we investigated the effect of miR-23 inhibition on myocardial protection in vivo. For verification, various of cardiac markers including the myoglobin (Mb) [20], CK-MB [21], and troponin (cTn) [22] were determined. We applied the BCA protein assay to evaluate to the concentration of Mb, CK-MB, and cTn, respectively, in the heart tissues of IR rats post receiving with various treatments. As depicted in Figure 6B, post treatment of miR-23 inhibitor, the levels of these diagnostic markers were markedly higher than that of control group. In contrast, the rats given sh-Has2 displayed the highest levels of Mb, CK-MB, and cTn, indicating that the myocardial function of IR rats could be significantly enhanced via inhibition of Has2.
Discussion
Although the timely and effective reperfusion is recommended as the most efficient treatment strategy for lowering ischemic injury and reducing MI size, serious cardiomyocyte death, also known as myocardial reperfusion injury, is an unavoidable issue that should be addressed efficiently [23]. Additionally, reperfusion also contributes significantly to microvascular obstruction, and apoptotic and necrotic damage in cardiomyocytes [24]. BMSCs represent a type of non-hematopoietic stem cell that can be applied in the therapeutic intervention of various diseases for its potential of self-renewal and pluripotent differentiation [9,10]. Importantly, increasing evidence demonstrated that BMSCs can induce to differentiate into myocardial cells activation of Wnt signaling pathway [11,25]. Based on this, the BMSCs were supposed to be a promising target for the alleviation of MIRI.
Recently, miRNAs have been illustrated to play an important role in IRI and inhibition of the miR-23 contributed significantly to the protection of myocardial function from ischemia-reperfusion injury [26,27]. However, the underlying mechanisms of miR-23 in protecting MIRI and the role of such genes in inducing differentiation of bone marrow mesenchymal stem cells into cardiomyocytes remains unclear. Therefore, in the present study, we have elaborately investigated the role of miR-23 in alleviation of MIRI and also the underlying mechanisms.
For experiments, the BMSCs were established firstly using a complicated process. To verify whether the cells were successfully developed, the immunocytochemistry and Flow Cytometry analysis were applied to determine the marker molecules. An immunochemistry assay demonstrated that the established cells in the present study were highly positive for CD44 with a percentage of 96%, while negative for CD55 with a percentage of only 2%.
As demonstrated previously, Has2 is a critical component required during heart morphogenesis and targeted deletion of the hyaluronan synthase-2 (Has2) gene in mice results in an absence of cardiac jelly and endocardial cushions, a loss of vascular integrity, and embryonic death [28]. In this case, we hypothesized that there was a close correlation between the Has2 and the cardiovascular system development. Herein, we have investigated the effect of miR-23 on the expression of Has2 via the WB and RT-PCR experiments. As demonstrated, the expression of Has2 in the BMSCs that were exposed to miR-23 mimic was obviously lower than that of control group. In contrast, the level of Has2 in the group treated by miR-23 inhibitor was significantly up-regulated, confirming that the miR-23 played a significant role in regulating the expression of Has2 in BMSCs.
GATA-4 is a cardiomyocyte-specific zinc finger transcription factor that regulates differentiation, growth, and survival [11]. The cardiac transcription factor Nkx2-5 is an important regulator of ventricular trabeculae and conduction system development. Nkx2-5+/- haploinsufficient mice have an abnormal electrocardiogram, with a prolonged QRS and progressive elongation of the PR interval [29]. Besides, the MHC and cTnI are also the specific markers for myocardium which have been widely used as biomarkers of cardiac infarction and heart failure [18]. In this case, the expressions of GATA-4, Nkx2-5, MHC, and cTnI could be used as the characteristics of cardiomyocytes. In the present study, we demonstrated that the levels of the characteristic molecular markers were markedly elevated after treating the BMSCs by miR-23 inhibitor. In contrast, transfection of cells with sh-Has2 has an obvious negative effect on the expression of those molecules. Such results were further confirmed by quantitative analysis, suggesting that miR-23 inhibition facilitates the differentiation of BMSCs into cardiomyocytes.
As demonstrated previously, the Wnt/β-catenin pathway plays a fundamental role in the regulation of embryonic development and adult homeostasis thorough regulation of numerous cellular processes, such as cell proliferation, differentiation, migration, and apoptosis [30,31]. However, whether it was involved in the process of inducing differentiation of BMSCs into cardiomyocytes remains unclear. We therefore performed a comprehensive experiment to clearly illustrate the underlying mechanism. Results demonstrated that the levels of the Wnt1, TCF4, and the β-catenin in the cells that were transfected with sh-Has2 were dramatically down-regulated, but not in the cells treated by miR-23 inhibitor. Taking these results together, transfection BMSCs with miR-23 inhibitor could promote the differentiation of these cells into myocardial cells via the activation of Wnt pathway.
Given the excellent effect of miR-23 inhibition on inducing differentiation of BMSCs into cardiomyocytes, in vivo efficacy of miR-23 inhibition on alleviating MIRI was finally investigated. As demonstrated by the histopathologic analysis results, the IR rats treated by sh-Has2 displayed the obvious myocardial lesions when compared to the normal group. However, such myocardial lesions of those IR rats could be significantly recovered by treating with miR-23 inhibitor.
In conclusion, the role of miR-inhibition in protection of cardiomyocytes from MIRI and the underlying mechanisms has been elaborately demonstrated in the present study. Determination of the molecular markers confirmed the successful development of BMSCs and they could be induced to differentiate into myocardial cells by miR-23 inhibition. Studies of the underlying mechanisms exhibited that activation of Wnt signaling pathway plays an essential role in the process above. Moreover, the Has2 was the dominant regulatory protein that is involved in the miR-23 inhibition and protected the ischemia/reperfusion injury via inducing the differentiation of bone marrow mesenchymal stem cells into cardiomyocytes. Overall, this study provides a novel and precise description for the mechanisms of miR-23 inhibition in alleviation of MIRI, which has potential useful value for development of novel strategies for MIRI treatment.
Acknowledgements
The study was supported by a grant from the Guangdong Provincial Department of Science and Technology (grant no. 2017B020247042).
Disclosure of conflict of interest
None.
References
- 1.Li J, Li RJ, Lv GY, Liu HQ. The mechanisms and strategies to protect from hepatic ischemia-reperfusion injury. Eur Rev Med Pharmacol Sci. 2015;19:2036–2047. [PubMed] [Google Scholar]
- 2.Tire Y, Sarkilar G, Esen H, Onoglu R, Uzun ST. The effect of intrathecal sufentanil preconditioning against myocardial ischemia-reperfusion injury. Bratisl Lek Listy. 2018;119:240–244. doi: 10.4149/BLL_2018_045. [DOI] [PubMed] [Google Scholar]
- 3.Frank A, Bonney M, Bonney S, Weitzel L, Koeppen M, Eckle T. Myocardial ischemia reperfusion injury: from basic science to clinical bedside. Semin Cardiothorac Vasc Anesth. 2012;16:123–132. doi: 10.1177/1089253211436350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jennings RB. Historical perspective on the pathology of myocardial ischemia/reperfusion injury. Circ Res. 2013;113:428–438. doi: 10.1161/CIRCRESAHA.113.300987. [DOI] [PubMed] [Google Scholar]
- 5.Francis N, Moore M, Asan SG, Rutter GA, Burns C. Changes in microRNA expression during differentiation of embryonic and induced pluripotent stem cells to definitive endoderm. Gene Expr Patterns. 2015;19:70–82. doi: 10.1016/j.gep.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porrello ER, Johnson BA, Aurora AB, Simpson E, Nam YJ, Matkovich SJ, Dorn GW 2nd, van Rooij E, Olson EN. MiR-15 family regulates postnatal mitotic arrest of cardiomyocytes. Circ Res. 2011;109:670–679. doi: 10.1161/CIRCRESAHA.111.248880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong S, Cheng Y, Yang J, Li J, Liu X, Wang X, Wang D, Krall TJ, Delphin ES, Zhang C. MicroRNA expression signature and the role of microRNA-21 in the early phase of acute myocardial infarction. J Biol Chem. 2009;284:29514–29525. doi: 10.1074/jbc.M109.027896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Aung LH, Long B, Qin D, An S, Li P. miR-23a binds to p53 and enhances its association with miR-128 promoter. Sci Rep. 2015;5:16422. doi: 10.1038/srep16422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pontikoglou C, Deschaseaux F, Sensebé L, Papadaki HA. Bone marrow mesenchymal stem cells: biological properties and their role in hematopoiesis and hematopoietic stem cell transplantation. Stem Cell Rev. 2011;7:569–589. doi: 10.1007/s12015-011-9228-8. [DOI] [PubMed] [Google Scholar]
- 10.Chen Q, Zhou R, Zhang Y, Zhu S, Xiao C, Gong J, Li K, Tang H, Sun C, Zhang J. Bone marrow mesenchymal stromal cells attenuate liver allograft rejection may via upregulation PD-L1 expression through downregulation of miR-17-5p. Transpl Immunol. 2018;51:21–29. doi: 10.1016/j.trim.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Zhang M, Bian YQ, Tao HM, Yang XF, Mu WD. Simvastatin induces osteogenic differentiation of MSCs via Wnt/β-catenin pathway to promote fracture healing. Eur Rev Med Pharmacol Sci. 2018;22:2896–2905. doi: 10.26355/eurrev_201805_14992. [DOI] [PubMed] [Google Scholar]
- 12.Shen X, Pan B, Zhou H, Liu L, Lv T, Zhu J, Huang X, Tian J. Differentiation of mesenchymal stem cells into cardiomyocytes is regulated by miRNA-1-2 via WNT signaling pathway. J Biomed Sci. 2017;24:29. doi: 10.1186/s12929-017-0337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma L, Lu MF, Schwartz RJ, Martin JF. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development. 2005;132:5601–5611. doi: 10.1242/dev.02156. [DOI] [PubMed] [Google Scholar]
- 14.Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A Jr, Kubalak S, Klewer SE, McDonald JA. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ladak A, Olson J, Tredget EE, Gordon T. Differentiation of mesenchymal stem cells to support peripheral nerve regeneration in a rat model. Exp Neurol. 2011;228:242–252. doi: 10.1016/j.expneurol.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Zuo S, He Z, Yang Y, Pasha Z, Wang Y, Xu M. Paracrine factors released by GATA-4 overexpressed mesenchymal stem cells increase angiogenesis and cell survival. Am J Physiol Heart Circ Physiol. 2010;299:H1772–81. doi: 10.1152/ajpheart.00557.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jay PY, Berul CI, Tanaka M, Ishii M, Kurachi Y, Izumo S. Cardiac conduction and arrhythmia: insights from Nkx2.5 mutations in mouse and humans. Novartis Found Symp. 2003;250:227–238. [PubMed] [Google Scholar]
- 18.Wang EH, Santos L, Li XY, Tran A, Kim SSY, Woo K, Shapiro J, McElwee KJ. Alopecia areata is associated with increased expression of heart disease biomarker cardiac Troponin I. Acta Derm Venereol. 2018;29:776–782. doi: 10.2340/00015555-2964. [DOI] [PubMed] [Google Scholar]
- 19.Alsaad AMS. Dasatinib induces gene expression of CYP1A1, CYP1B1, and cardiac hypertrophy markers (BNP, β-MHC) in rat cardiomyocyte H9C2 cells. Toxicol Mech Methods. 2018;28:678–684. doi: 10.1080/15376516.2018.1497746. [DOI] [PubMed] [Google Scholar]
- 20.Santotoribio JD, León-Justel A, Guerrero JM. Determination of serum myoglobin and troponin T for early diagnosis of acute myocardial infarction. Rev Clin Esp. 2009;209:152–153. doi: 10.1016/s0014-2565(09)70885-9. [DOI] [PubMed] [Google Scholar]
- 21.Stone MJ, Waterman MR, Harimoto D, Murray G, Willson N, Platt MR, Blomqvist G, Willerson JT. Serum myoglobin level as diagnostic test in patients with acute myocardial infarction. Br Heart J. 1977;39:375–380. doi: 10.1136/hrt.39.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okuda S, Sufu-Shimizu Y, Kato T, Fukuda M, Nishimura S, Oda T, Kobayashi S, Yamamoto T, Morimoto S, Yano M. CaMKII-mediated phosphorylation of RyR2 plays a crucial role in aberrant Ca2+ release as an arrhythmogenic substrate in cardiac troponin T-related familial hypertrophic cardiomyopathy. Biochem Biophys Res Commun. 2018;49:1250–1256. doi: 10.1016/j.bbrc.2018.01.181. [DOI] [PubMed] [Google Scholar]
- 23.Yuan X, Xiang Y, Zhu N, Zhao X, Ye S, Zhong P, Zeng C. Salvianolic acid A protects against myocardial ischemia/reperfusion injury by reducing platelet activation and inflammation. Exp Ther Med. 2017;14:961–966. doi: 10.3892/etm.2017.4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SM, Hutchinson M, Saint DA. The role of Toll-like receptor 4 (TLR4) in cardiac ischaemic-reperfusion injury, cardioprotection and preconditioning. Clin Exp Pharmacol Physiol. 2016;43:864–871. doi: 10.1111/1440-1681.12602. [DOI] [PubMed] [Google Scholar]
- 25.Dai F, Du P, Chang Y, Ji E, Xu Y, Wei C, Li J. Downregulation of MiR-199b-5p inducing differentiation of bone-marrow mesenchymal stem cells (BMSCs) toward cardiomyocyte-like cells via HSF1/HSP70 pathway. Med Sci Monit. 2018;24:2700–2710. doi: 10.12659/MSM.907441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan ZX, Yang J. The role of microRNAs in regulating myocardial ischemia reperfusion injury. Saudi Med J. 2015;36:787–793. doi: 10.15537/smj.2015.7.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kou Y, Zheng WT, Zhang YR. Inhibition of miR-23 protects myocardial function from ischemia-reperfusion injury through restoration of glutamine metabolism. Eur Rev Med Pharmacol Sci. 2016;20:4286–4293. [PubMed] [Google Scholar]
- 28.Tien JY, Spicer AP. Three vertebrate hyaluronan synthases are expressed during mouse development in distinct spatial and temporal patterns. Dev Dyn. 2005;233:130–141. doi: 10.1002/dvdy.20328. [DOI] [PubMed] [Google Scholar]
- 29.Jay PY, Berul CI, Tanaka M, Ishii M, Kurachi Y, Izumo S. Cardiac conduction and arrhythmia: insights from Nkx2.5 mutations in mouse and humans. Novartis Found Symp. 2003;250:227–238. [PubMed] [Google Scholar]
- 30.Garcin CL, Habib SJ. A comparative perspective on wnt/β-catenin signalling in cell fate determination. Results Probl Cell Differ. 2017;61:323–350. doi: 10.1007/978-3-319-53150-2_15. [DOI] [PubMed] [Google Scholar]
- 31.Tian A, Benchabane H, Wang Z, Ahmed Y. Regulation of stem cell proliferation and cell fate specification by wingless/Wnt signaling gradients enriched at adult intestinal compartment boundaries. PLoS Genet. 2016;12:e1005822. doi: 10.1371/journal.pgen.1005822. [DOI] [PMC free article] [PubMed] [Google Scholar]