Abstract
Background: The upregulation of long non-coding RNA SPRY4 intronic transcript 1 (lncRNA SPRY4-IT1) has been observed in breast cancer (BC). However, there is no previous study of the relationship between SPRY4-IT1 and patient prognosis in BC. This study investigated the prognostic value of SPRY4-IT1 in BC patients. Methods: The relative expression levels of SPRY4-IT1 were detected by RT-qPCR in 102 paired BC tissues and adjacent noncancerous tissues. The association of SPRY4-IT1 expression with clinicopathological features and prognosis was statistically analyzed. Results: The findings revealed that the SPRY4-IT1 expression was significantly upregulated in clinical BC tissues compared to adjacent normal tissues (P < 0.001). Furthermore, the SPRY4-IT1 level was significantly associated with tumor size (P = 0.009), TNM stage (P = 0.0008) and lymph node metastasis (P = 0.01). Using a Kaplan-Meier analysis, it was shown that patients with high SPRY4-IT1 expression had a significantly poor overall survival (OS) rate (P = 0.0056) and a disease-free survival (DFS) rate (P = 0.0001). Moreover, multivariate Cox analysis revealed that SPRY4-IT1 expression was an independent poor prognostic factor for both OS (P = 0.024) and DFS (P = 0.025) in BC patients. Conclusions: SPRY4-IT1 expression is an independent prognostic factor for patients with BC and may serve as a potential biomarker to predict prognosis in BC patients.
Keywords: SPRY4-IT1, breast cancer, prognostic marker
Introduction
Breast cancer (BC) is the most commonly diagnosed cancer in women and has become the second leading cause of cancer death among women worldwide [1]. During recent decades, remarkable progress has been made, and the disease can be treated by means of surgery, endocrine, cytotoxic, or targeted therapies. The prognosis of the patients with distal metastasis remains poor. Despite the great developments in medical science’s understanding of breast cancer, the complex molecular mechanisms in BC pathogenesis remain largely unclear. Thus, new biomarkers are needed to improve the detection and prognostic outcomes of BC.
Long noncoding RNAs (lncRNAs) are defined as RNA molecules with more than 200 nucleotides in length not translated into protein. Recently, emerging evidence has demonstrated the characterization of lncRNAs as a critical component in cancer biology [2,3]. lncRNAs can regulate the expression of downstream genes through transcriptional and posttranscriptional regulation, and the dysregulation of lncRNAs contributes to cancer initiation, progression, and metastasis [4]. Given that many lncRNAs are expressed through tissue and cancer type restriction, specific lncRNAs are now more likely to be translated into clinical applications for diagnosis, prognosis or predicting the response to treatment [5,6].
In this study, we focused on lncRNA SPRY4 intronic transcript 1 (SPRY4-IT1), which is located within an intron of the SPRY4 gene. SPRY4-IT1 was previously reported to be up-regulated in melanoma [7], gastric cancer [8], breast cancer [9], esophageal squamous cell carcinoma [10] and colorectal cancer [11]. However, whether SPRY4-IT1 was associated with the prognosis of BC patients remains unknown. Herein, we assessed the expression profile of SPRY4-IT1 in BC tissues. Next, we investigated its associations with clinical features and prognosis to identify its clinical value in BC.
Patients and methods
Patients
This study included 102 female patients with breast invasive ductal carcinoma who underwent surgical treatment at our hospital between January 2008 and December 2010. Primary cancer tissues and paired adjacent noncancerous tissues were snap-frozen in liquid nitrogen immediately after resection and then stored at -80°C for RNA extraction. The range of the patients’ ages at the time of diagnosis was 30-70 years (median, 50 years). All diagnoses were confirmed by postoperative pathological examinations. Complete clinical data was available for each patient. Data from the patients treated without preoperative therapy were used for the analysis of prognosis. This study was conducted according to the principles of the Helsinki Declaration. This study was approved by the Hospital’s Ethics Committee, and informed consent was obtained from each patient.
RNA Extraction and qRT-PCR
Total RNA was extracted using the Trizol reagent (Invitrogen, Carlsbad, CA, USA) and followed by cDNA synthesis using the SuperScript first-strand synthesis system (Invitrogen, Carlsbad, CA, USA). To evaluate SPRY4-IT1 expression levels, we used quantitative real time polymerase chain reaction (qRT-PCR) with 2× TaqMan Premix Ex Taq (Takara, Japan), using an ABI7900 system (Applied Biosystems, USA). Dissociation curve analysis was used to evaluate the PCR products. GAPDH was used as an internal control. The comparative 2-ΔΔCt method was used for relative quantification and statistical analysis. The primers of GAPDH and SPRY4-IT1 were synthesized by Sangon Biotech (Shanghai, China). The sequences of the primers that were used were as follows: for SPRY4-IT1, 5’-ATCCGAAGCGCAGACACAATTCA-3’ (forward) and 5’-CCTCGATGTAGTCTATGTCATAGGA-3’ (reverse); For GAPDH, 5’-ATTCAACGGCACAGTCAAGG-3’ and 5’-GCAGAAGGGGCGGAGATGA-3’.
Statistical analysis
All statistical analyses were performed using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). The χ2 test was used to analyze the relationship between SPRY4-IT1 expression and clinicopathological characteristics. Disease free survival (DFS) and overall survival (OS) curves were performed by the Kaplan-Meier survival plot using Graph Pad Prism 5 (Graphpad Software Company, USA). Univariate analysis and multivariate analysis were performed using the Cox proportional hazard regression model. A p value less than 0.05 was considered statistically significant. All of the experiments were repeated at least three times.
Results
SPRY4-IT1 is highly expressed in BC tissues
We first examined the expression of SPRY4-IT1 by qRT-PCR in 102 pairs of BC tissues and non-cancerous adjacent tissues. As shown in Figure 1, the results showed that the relative expression of SPRY4-IT1 was significantly higher in BC tissues than in normal breast tissues (P < 0.001).
Figure 1.
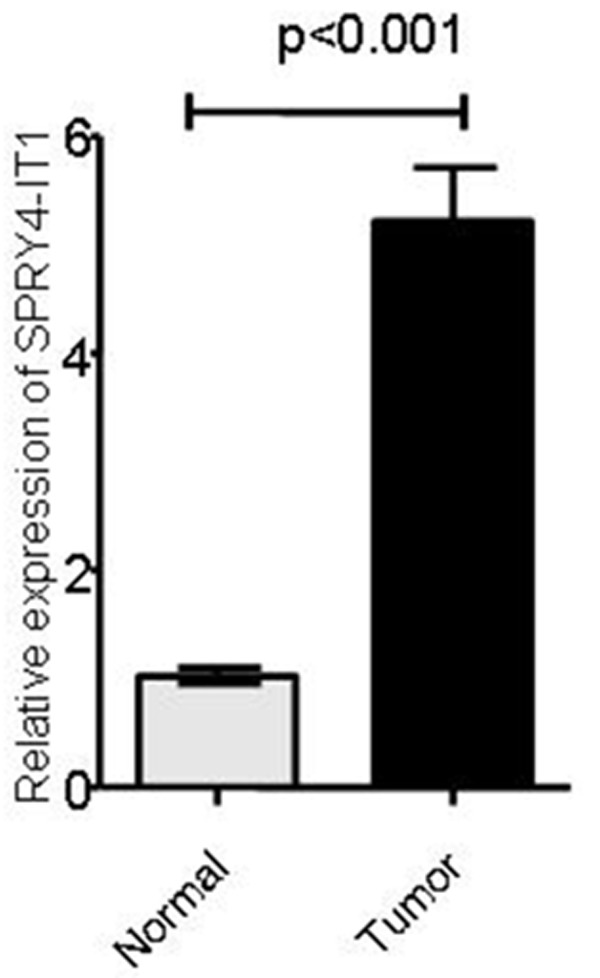
Relative expression of SPRY4-IT1 in 102 BC patients. The up-regulated level of SPRY4-IT1 was measured in BC tissues compared with adjacent normal tissues.
The relationship between SPRY4-IT1 expression and the clinicopathologic features of BC
Next, the correlations between SPRY4-IT1 expression level and the clinicopathological features were evaluated. Patients with BC were divided into high and low expression groups by the mean expression level of SPRY4-IT1. The clinical relevance is summarized in Table 1. The results showed that high SPRY4-IT1 expression significantly correlated with larger tumor size (P = 0.009), advanced TNM stage (P = 0.0008) and lymph node metastasis (P = 0.01). However, there was no obvious correlation between the level of SPRY4-IT1 expression in BC tissues and other clinicopathological features such as age, menopausal status, grade, ER, PR, HER-2 (all P > 0.05, Table 1).
Table 1.
Correlation between SPRY4-IT1 expression and clinicopathological features in patients with breast cancer
| SPRY4-IT1 expression | P Value | ||
|---|---|---|---|
|
| |||
| Low (n = 50) | High (n = 52) | ||
| Age | 0.237 | ||
| ≤ 50 | 23 | 30 | |
| > 50 | 27 | 22 | |
| Menopausal status | 0.698 | ||
| Premenopausal | 25 | 24 | |
| Postmenopausal | 25 | 28 | |
| Tumor size | 0.009 | ||
| ≤ 2 cm | 27 | 15 | |
| > 2 cm | 23 | 37 | |
| Lymph node status | 0.01 | ||
| Negative | 30 | 18 | |
| Positive | 20 | 34 | |
| Grade | 0.672 | ||
| I-II | 21 | 24 | |
| III | 29 | 28 | |
| ER status | 0.237 | ||
| Negative | 23 | 30 | |
| Positive | 27 | 22 | |
| PR | 0.239 | ||
| Negative | 24 | 31 | |
| Positive | 26 | 21 | |
| HER-2/neu status | 0.538 | ||
| Negative | 29 | 27 | |
| Positive | 21 | 25 | |
| Clinical stage | 0.0008 | ||
| I-II | 40 | 25 | |
| III | 10 | 27 | |
The prognostic value of SPRY4-IT1 expression in BC
In order to assess the prognostic value of SPRY4-IT1 expression for BC, we analyzed the prognostic value of SPRY4-IT1 expression levels in 102 BC patients using a Kaplan-Meier analysis and the log-rank test. The association between SPRY4-IT1 expression status and OS and DFS in BC patients was studied using the log-rank test. As shown in Figure 2, patients with high SPRY4-IT1 expression had dramatically poor OS (Figure 2A, P = 0.0056) and DFS (Figure 2B, P = 0.0001) than those with low SPRY4-IT1 expression. Furthermore, SPRY4-IT1 expression was associated with both OS and PFS in a univariate Cox proportional hazards regression analysis (Tables 2, 3). Finally, a multivariate survival analysis based on the Cox proportional hazards model showed that SPRY4-IT1 expression was an independent poor prognostic factor for both OS (HR, 7.812, 95% CI, 1.311-46.545; P = 0.024, Table 2) and DFS (HR, 4.322, 95% CI, 1.205-15.500; P = 0.025, Table 3) in BC patients. All of these data demonstrate that SPRY4-IT1 is an independent prognostic factor and that high SPRY4-IT1 expression was correlated with unfavorable survival in BC patients.
Figure 2.
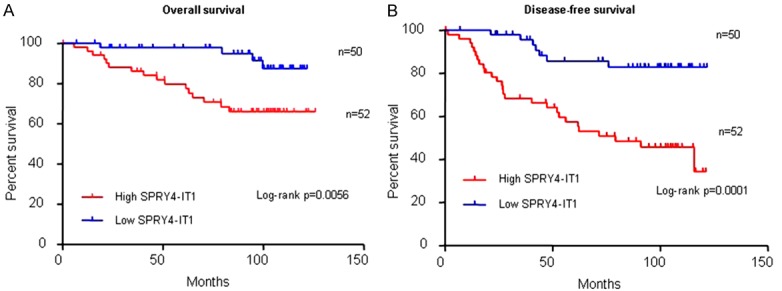
Kaplan-Meier survival curves stratified by SPRY4-IT1 expression levels in breast cancer patients. Patients in the high SPRY4-IT1 group showed decreased overall survival (OS, A) and disease-free survival (DFS, B), as compared with the low SPRY4-IT1 group, P = 0.0056 and P = 0.0001, respectively. The p value was calculated using the log-rank test.
Table 2.
Univariate and multivariate Cox regression analyses of overall survival in breast cancer patients
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (years), ≤ 50 versus > 50 | 0.906 (0.165-4.985) | 0.910 | ||
| Menopausal status, Premenopausal versus Postmenopausal | 0.755 (0.127-4.482) | 0.757 | ||
| Tumor size (cm), ≤ 2 versus > 2 | 2.893 (1.060-7.898) | 0.038 | 1.341 (0.3385.318) | 0.676 |
| Lymph node status, negative versus positive | 4.235 (1.405-12.766) | 0.010 | 0.920 (0.171-4.943) | 0.922 |
| Grade I-II versus III | 0.302 (0.061-1.490) | 0.141 | ||
| ER status, negative versus positive | 0.261 (0.066-0.702) | 0.011 | 0.415 (0.052-3.299) | 0.406 |
| PR status, negative versus positive | 0.168 (0.046-0.618) | 0.007 | 0.210 (0.024-1.805) | 0.155 |
| HER-2/neu status, negative versus positive | 6.187 (2.169-17.649) | 0.001 | 2.036 (0.546-7.594) | 0.290 |
| TNM stage, I-II versus III | 2.963 (1.711-5.130) | 0.000 | 2.532 (1.059-6.053) | 0.037 |
| SPRY4-IT1 expression, low versus high | 12.706 (2.764-58.415) | 0.001 | 7.812 (1.31146.545) | 0.024 |
Table 3.
Univariate and multivariate Cox regression analyses of disease-free survival in breast cancer patients
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | P value | HR (95%CI) | P value | |
| Age (years), ≤ 50 versus > 50 | 0.807 (0.200-3.259) | 0.764 | ||
| Menopausal status, Premenopausal versus Postmenopausal | 2.007 (0.531-7.582) | 0.304 | ||
| Tumor size (cm), ≤ 2 versus > 2 | 3.102 (1.313-7.330) | 0.010 | 1.919 (0.576-6.393) | 0.288 |
| Lymph node status, negative versus positive | 3.804 (1.565-9.243) | 0.003 | 0.813 (0.196-3.367) | 0.775 |
| Grade I-II versus III | 1.120 (0.312-4.022) | 0.862 | ||
| ER status, negative versus positive | 0.524 (0.223-1.228) | 0.137 | ||
| PR status, negative versus positive | 0.422 (0.175-1.018) | 0.055 | ||
| HER-2/neu status, negative versus positive | 5.602 (2.191-14.326) | 0.000 | 3.117 (0.894-10.874) | 0.075 |
| TNM stage, I-II versus III | 3.787 (2.109-6.802) | 0.000 | 2.481 (1.194-5.159) | 0.015 |
| SPRY4-IT1 expression, low versus high | 10.500 (3.591-30.698) | 0.000 | 4.322 (1.205-15.500) | 0.025 |
Discussion
The prevention and therapy of breast cancer remain a major public health concern. More and more studies have recognized that the appropriate therapies require effective biomarkers to guide them. Recently, a growing number of papers have reported that changes in expression of lncRNAs can be used as robust and important biomarkers for cancer risk, diagnosis, and prognosis [4-6]. Based on previous findings, our attention then focused on SPRY4-IT1 [9].
SPRY4-IT1 (GenBank accession ID AK024556), a 708 bp lncRNA, was first discovered by Khaitan and colleagues in 2011 [7]. SPRY4-IT1 is derived from an intron of the SPRY4 gene residing on chromosome 5q31.3, which encodes an endogenous inhibitor of the receptor-transduced mitogen-activated protein kinase pathway. Computational prediction suggested the secondary structure of SPRY4-IT1 might contain several long hairpins. This lncRNA was originally reported to be highly upregulated in melanoma where it played an oncogenic role [7]. Since its discovery, a growing number of studies have reported the aberrant expression of SPRY4-IT1 in other cancer types, such as lung [12], breast [9], gastric [8], esophageal [10], prostate cancers [13], liver cancer [14], glioma [15], gallbladder cancer [16], renal cancer [17], and bladder cancer [18]. Shi and colleagues showed that SPRY4-IT1 expression was significantly higher in breast cancer tissues as compared with normal tissues [9]. Increased SPRY4-IT1 expression was associated with larger tumor size and a more aggressive pathological stage. Further experiments demonstrated that the knockdown of SPRY4-IT1 significantly inhibited cell proliferation and induced apoptosis in breast cancer cell lines. The enforced expression of SPRY4-IT1 can promote cell proliferation by upregulating the cell proliferation marker MKI67 and the minichromosomal maintenance genes [MCM2, MCM3, MCM4 and MCM5] (MCM2-5). Overexpression of SPRY4-IT1 can also upregulate the anti-apoptotic gene X-linked inhibitor of apoptosis protein (XIAP) and the baculoviral IAP repeat-containing 7 (livin) and downregulate the tumor suppressor dipeptidyl peptidase-IV (DPPIV), thus resulting in apoptosis [19]. Moreover, suppression of SPRY4-IT1 may increase the apoptosis rate through altering lipin 2 in lipid metabolism [20]. In addition, SPRY4-IT1 can increase the migration ability by facilitating the EMT process [10]. Generally, SPRY4-IT1 can promote cancer progression by affecting the phenotypes of cancer cells and molecular pathways [21].
In the present work, we first determined the expression levels of SPRY4-IT1 in BC and matched normal tissues and observed a significantly greater increased expression of SPRY4-IT1 in the BC tissues than in the adjacent normal breast tissues. We further found the expression level of SPRY4-IT1 was significantly associated with tumor size, TNM stage and lymph node metastasis, suggesting SPRY4-IT1 contributed to progression of BC. In addition, the Kaplan-Meier analysis demonstrated that a high SPRY4-IT1 expression level was associated with poorer OS and DFS. Based on these data, we performed a multivariate analysis, and the results indicated that SPRY4-IT1 was an independent prognostic factor for predicting the OS and DFS of BC patients. Similar results were also found in other types of cancer, and a higher expression of SPRY4-IT1 predicted poor prognosis in many cancers, such as gastric cancer [8], esophageal squamous cell carcinoma [10], colorectal cancer [11], non-small cell lung cancer [12], and clear cell renal cell carcinoma [17]. On the basis of the previous studies, we hypothesized that the contribution of high SPRY4-IT1 expression to the poor prognosis of BC patients might be caused by its facilitative efficiency on BC cell proliferation and metastasis.
This study has several limitations: (1) the expression of SPRY4-IT1 was only analyzed in tissues, and we will collect serum samples of breast cancer to further confirm our findings. (2) This study lacked an independent cohort to identify the predictive value of the SPRY4-IT1 signature. Thus, the findings of this study should be further confirmed for a larger sample in a multicenter, randomized, controlled, and prospective study.
Conclusions
In conclusion, this study indicates that SPRY4-IT1 expression is significantly upregulated in BC tissues compared with corresponding normal breast tissues, and its expression is significantly associated with the tumor size, TNM stage, and lymph node metastasis of BC patients. Moreover, increased expression of SPRY4-IT1 is predictive of a worse prognosis in BC patients. All the evidence above suggests that SPRY4-IT1 is a novel molecule correlated with BC progression, and it may serve as a potential biomarker to predict prognosis in BC patients.
Acknowledgements
This work was supported by grants from the Science and Technology Project of Wenzhou (Y20170187).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Maruyama R, Shipitsin M, Choudhury S, Wu Z, Protopopov A, Yao J, Lo PK, Bessarabova M, Ishkin A, Nikolsky Y, Liu XS, Sukumar S, Polyak K. Altered antisense-to-sense transcript ratios in breast cancer. Proc Natl Acad Sci U S A. 2012;109:2820–2824. doi: 10.1073/pnas.1010559107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perez DS, Hoage TR, Pritchett JR, Ducharme-Smith AL, Halling ML, Ganapathiraju SC, Streng PS, Smith DI. Long, abundantly expressed non-coding transcripts are altered in cancer. Hum Mol Genet. 2008;17:642–655. doi: 10.1093/hmg/ddm336. [DOI] [PubMed] [Google Scholar]
- 4.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, Wang Y, Brzoska P, Kong B, Li R, West RB, van de Vijver MJ, Sukumar S, Chang HY. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miao Z, Ding J, Chen B, Yang Y, Chen Y. HOTAIR overexpression correlated with worse survival in patients with solid tumors. Minerva Med. 2016;107:392–400. [PubMed] [Google Scholar]
- 6.Yang Y, Qian J, Xiang Y, Chen Y, Qu J. The prognostic value of long noncoding RNA HOTTIP on clinical outcomes in breast cancer. Oncotarget. 2017;8:6833–6844. doi: 10.18632/oncotarget.14304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khaitan D, Dinger ME, Mazar J, Crawford J, Smith MA, Mattick JS, Perera RJ. The melanoma-upregulated long noncoding RNA SPRY4-IT1 modulates apoptosis and invasion. Cancer Res. 2011;71:3852–3862. doi: 10.1158/0008-5472.CAN-10-4460. [DOI] [PubMed] [Google Scholar]
- 8.Xie M, Nie FQ, Sun M, Xia R, Liu YW, Zhou P, De W, Liu XH. Decreased long noncoding RNA SPRY4-IT1 contributing to gastric cancer cell metastasis partly via affecting epithelial-mesenchymal transition. J Transl Med. 2015;13:250. doi: 10.1186/s12967-015-0595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi Y, Li J, Liu Y, Ding J, Fan Y, Tian Y, Wang L, Lian Y, Wang K, Shu Y. The long noncoding RNA SPRY4-IT1 increases the proliferation of human breast cancer cells by upregulating ZNF703 expression. Mol Cancer. 2015;14:51. doi: 10.1186/s12943-015-0318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui F, Wu D, He X, Wang W, Xi J, Wang M. Long noncoding RNA SPRY4-IT1 promotes esophageal squamous cell carcinoma cell proliferation, invasion, and epithelial-mesenchymal transition. Tumour Biol. 2016;37:10871–10876. doi: 10.1007/s13277-016-4962-9. [DOI] [PubMed] [Google Scholar]
- 11.Tan W, Song ZZ, Xu Q, Qu X, Li Z, Wang Y, Yu Q, Wang S. Up-regulated expression of SPRY4-IT1 predicts poor prognosis in colorectal cancer. Med Sci Monit. 2017;23:309–314. doi: 10.12659/MSM.898369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun M, Liu XH, Lu KH, Nie FQ, Xia R, Kong R, Yang JS, Xu TP, Liu YW, Zou YF, Lu BB, Yin R, Zhang EB, Xu L, De W, Wang ZX. EZH2-mediated epigenetic suppression of long noncoding RNA SPRY4-IT1 promotes NSCLC cell proliferation and metastasis by affecting the epithelial-mesenchymal transition. Cell Death Dis. 2014;5:e1298. doi: 10.1038/cddis.2014.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee B, Mazar J, Aftab MN, Qi F, Shelley J, Li JL, Govindarajan S, Valerio F, Rivera I, Thurn T, Tran TA, Kameh D, Patel V, Perera RJ. Long noncoding RNAs as putative biomarkers for prostate cancer detection. J Mol Diagn. 2014;16:615–626. doi: 10.1016/j.jmoldx.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jing W, Gao S, Zhu M, Luo P, Jing X, Chai H, Tu J. Potential diagnostic value of lncRNA SPRY4-IT1 in hepatocellular carcinoma. Oncol Rep. 2016;36:1085–1092. doi: 10.3892/or.2016.4859. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Y, Wang DL, Pang Q. Long noncoding RNA SPRY4-IT1 is a prognostic factor for poor overall survival and has an oncogenic role in glioma. Eur Rev Med Pharmacol Sci. 2016;20:3035–3039. [PubMed] [Google Scholar]
- 16.Yang L, Cheng X, Ge N, Guo W, Feng F, Wan F. Long non-coding RNA SPRY4-IT1 promotes gallbladder carcinoma progression. Oncotarget. 2017;8:3104–3110. doi: 10.18632/oncotarget.13621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang HM, Yang FQ, Yan Y, Che JP, Zheng JH. High expression of long non-coding RNA SPRY4-IT1 predicts poor prognosis of clear cell renal cell carcinoma. Int J Clin Exp Pathol. 2014;7:5801–5809. [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao XL, Zhao ZH, Xu WC, Hou JQ, Du XY. Increased expression of SPRY4-IT1 predicts poor prognosis and promotes tumor growth and metastasis in bladder cancer. Int J Clin Exp Pathol. 2015;8:1954–1960. [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao W, Mazar J, Lee B, Sawada J, Li JL, Shelley J, Govindarajan S, Towler D, Mattick JS, Komatsu M, Dinger ME, Perera RJ. The long noncoding RNA SPRIGHTLY regulates cell proliferation in primary human melanocytes. J Invest Dermatol. 2016;136:819–828. doi: 10.1016/j.jid.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazar J, Zhao W, Khalil AM, Lee B, Shelley J, Govindarajan SS, Yamamoto F, Ratnam M, Aftab MN, Collins S, Finck BN, Han X, Mattick JS, Dinger ME, Perera RJ. The functional characterization of long noncoding RNA SPRY4-IT1 in human melanoma cells. Oncotarget. 2014;5:8959–8969. doi: 10.18632/oncotarget.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J, Chen Y, Chen Z, He A, Xie H, Zhang Q, Cai Z, Liu Y, Huang W. SPRY4-IT1: a novel oncogenic long non-coding RNA in human cancers. Tumour Biol. 2017;39:1010428317711406. doi: 10.1177/1010428317711406. [DOI] [PubMed] [Google Scholar]


