Abstract
Protein tyrosine kinase 7 (PTK7) is a catalytically inactive receptor tyrosine kinase that is involved in development and tumorigenesis. PTK7 expression and its functional roles have been investigated in several human cancers, although controversial results have been obtained. In this study, we investigated the expression of PTK7 protein in invasive ductal breast cancer tissues, and analyzed its relationship with clinicopathologic parameters. Seventy-nine consecutive invasive breast cancer tissues were included in the study, and PTK7 protein was detected in invasive ductal breast cancers and normal breast epithelial cells by immunohistochemistry. Positive staining was noted in all normal breast epithelial cells and differential expression was observed in breast carcinomas. Thirty-eight of 79 samples (48.1%) were negative or stained weakly for PTK7 (-), 19 (24.1%) showed moderate staining (+), and 22 (27.8%) were strongly stained (++). PTK7 expression was negatively associated with tumor grade (P=0.025, r=-0.251), tumor-node-metastasis stage (P=0.004, r=-0.317), lymph node metastasis (P=0.002, r=-0.351), human epidermal growth factor receptor 2 expression (P=0.029, r=-0.245), and Ki67 expression (P=0.004, r=-0.317), and positively associated with estrogen receptor (ER) expression (P=0.037, r=0.235). No significant relationship was found between PTK7 expression and patient age or progesterone receptor (PR) expression. Our data indicated that PTK7 protein was down-regulated in breast cancer cells compared with healthy epithelial cells, and that PTK7 may be a tumor suppressor gene in breast cancer. Future studies should explore the molecular mechanisms underlying the down-regulation and functional roles of PTK7 in breast cancer.
Keywords: PKT7, tyrosine kinase receptor, breast cancer
Introduction
Protein tyrosine kinase 7 (PTK7) was originally identified as an mRNA expressed in human colon carcinomas [1]. It is classified as a pseudokinase, which is a highly evolutionarily conserved cell surface planar cell polarity receptor belonging to the receptor tyrosine kinase family [2]. PTK7 expression has been investigated in several human cancers, and was shown to be upregulated in colorectal cancer [3,4], esophageal squamous cell carcinoma [5], and intrahepatic cholangiocarcinoma [6]. Moreover, Tian et al. documented higher PTK7 mRNA expression in colorectal cancer than non-tumorous mucosa [4].
PTK7 expression has been correlated with tumor differentiation, lymph node metastasis, distant metastasis, and tumor-node-metastasis (TNM) stage in colorectal cancer. Additionally, a significant correlation between PTK7 overexpression and favorable survival was observed in colorectal cancer patients. Jin et al. explored the underlying mechanism of PTK7 in intrahepatic cholangiocarcinoma by suppressing its expression in cell lines [6]. PTK7 knockdown with small interfering RNA in cells expressing high levels of PTK7 impaired invasion, migration, and DNA synthesis, and also induced cell apoptosis and decreased phospho-RhoA expression. Moreover, PTK7 protein expression was detected in 75.9% of intrahepatic cholangiocarcinoma samples, compared with 6.8% of healthy bile ducts, suggesting that PTK7 has an oncogenic role in tumorigenesis.
However, PTK7 also has a tumor suppressive role in some cancer types. PTK7 expression was tested in a panel of human melanoma cell lines at defined stages of progression, together with 10 melanoma biopsies [7], and shown to be quantifiable in healthy melanocytes. These levels were significantly higher than the trace amounts detected in primary melanomas and metastatic melanoma cell lines. Moreover, PTK7 expression was only detected only in 3/4 early melanomas, and in 2/10 metastatic melanoma biopsy samples. These results suggested a tendency toward the loss of PTK7 expression with increasing tumorigenicity of melanoma cell lines.
Kim et al. detected PTK7 downregulation at both the mRNA and protein level in lung squamous cell carcinoma [8], and found that PTK7 overexpression inhibited cell proliferation, invasion, and migration. PTK7 downregulation was also observed in clear renal cell carcinoma compared with normal renal tissues [9]. We previously measured PTK7 expression in epithelial ovarian cancer, and found that PTK7 was expressed in 92.8% of normal fallopian tubes and in 45.1% of epithelial ovarian tumors [10]. PTK7 expression was significantly associated with clinical stage and metastasis in ovarian borderline serous tumors, and associated with clinical stage, World Health Organization grading, and M.D. Anderson Cancer Center grading in ovarian serous carcinoma. Survival analysis showed that patients with negative PTK7 expression had a poorer outcome than those with positive expression, indicating that PTK7 may be a tumor suppressor in ovarian serous carcinoma.
To date, PTK7 expression and its role in breast cancer have not been well documented [11,12]. Therefore, the present study aimed to explore PTK7 expression in a set of consecutive invasive ductal breast carcinoma tissues, and to analyze the relationship between PTK7 expression and clinicopathologic data.
Materials and methods
Tissue samples
Breast cancer tissues were retrospectively collected from the Pathology Department of Nantong Women and Children Health Care Hospital, China. Seventy-nine consecutive invasive breast cancer tissues were included in this project, and patients were diagnosed between 2015 and 2016 at the Nantong Women and Children Health Care Hospital. All patients were diagnosed with invasive ductal breast cancer, and received surgical intervention as their primary treatment without adjuvant chemotherapy before the operation. Clinicopathogic data are detailed in Table 1. The study was approved by the Regional Committee for Medical Research Ethics of Nantong Women and Children Health Care Hospital, China.
Table 1.
Relationship between PTK7 expression and clinicopathologicdatain invasive ductal breast carcinoma
| Parameters | No. | PTK7 protein expression | P value | rs | ||
|---|---|---|---|---|---|---|
|
| ||||||
| - | + | ++ | ||||
| 79 | 38 (48.1%) | 19 (24.1%) | 22 (27.8%) | |||
| Age (years) | ||||||
| >50 | 47 | 25 (53.2%) | 11 (23.4%) | 11 (23.4%) | 0.232 | 0.136 |
| ≤50 | 32 | 13 (40.6%) | 8 (25.0%) | 11 (34.4%) | ||
| Grade | ||||||
| 1/2 | 54 | 22 (40.7%) | 13 (24.1%) | 19 (35.2%) | 0.025 | -0.251 |
| 3 | 25 | 16 (64.0%) | 6 (24.0%) | 3 (12.0%) | ||
| TNM stage | ||||||
| 1 | 19 | 3 (15.8%) | 6 (31.6%) | 10 (52.6%) | 0.004 | -0.317 |
| 2 | 40 | 23 (57.5%) | 9 (22.5%) | 8 (20.0%) | ||
| 3/4 | 20 | 12 (60.0%) | 4 (20.0%) | 4 (20.0%) | ||
| Lymph node metastasis | ||||||
| No | 32 | 9 (28.1%) | 9 (28.1%) | 14 (43.8%) | 0.002 | -0.351 |
| Yes | 47 | 29 (61.7%) | 10 (21.3%) | 8 (17.0%) | ||
| Molecular subtype | ||||||
| A+B | 43 | 16 (37.2%) | 14 (32.6%) | 13 (30.2%) | 0.195 | -0.147 |
| HER2 | 21 | 14 (66.7%) | 3 (14.3%) | 4 (19.0%) | ||
| Triple | 15 | 8 (53.3%) | 2 (13.3%) | 5 (33.3%) | ||
| ER expression | ||||||
| - | 38 | 24 (63.2%) | 5 (13.2%) | 9 (23.7%) | 0.037 | 0.235 |
| + | 41 | 14 (34.1%) | 14 (34.1%) | 13 (31.7%) | ||
| PR expression | ||||||
| - | 33 | 15 (45.5%) | 7 (21.2%) | 11 (33.3%) | 0.508 | -0.076 |
| + | 46 | 23 (50.0%) | 12 (26.1%) | 11 (23.9%) | ||
| HER2 expression | ||||||
| 0/1+/2+ | 59 | 24 (40.7%) | 16 (27.1%) | 19 (32.2%) | 0.029 | -0.245 |
| 3+ | 20 | 14 (70.0%) | 3 (15.0%) | 3 (15.0%) | ||
| Ki67 expression | ||||||
| <20% | 49 | 17 (34.7%) | 15 (30.6%) | 17 (34.7%) | 0.004 | -0.317 |
| ≥20% | 30 | 21 (70.0%) | 4 (13.3%) | 5 (16.7%) | ||
Immunohistochemical analysis
Immunohistochemical staining was performed on 4 µm-thick formalin-fixed, paraffin-embedded tissue sections as previously described [10]. Briefly, each section was deparaffinized and rehydrated, then autoclaved in 10 mM citrate buffer at 120°C for 2 min for antigen retrieval. Endogenous peroxidase was quenched with 3% H2O2 for 10 min. The following primary antibodies were incubated at 4°C overnight: rabbit polyclonal anti-PTK7 antibody (Abgent, San Diego, CA; 1:600), monoclonal antibodies for estrogen receptor (ER) (Dako, Carpenteria, CA), progesterone receptor (PR) (Dako, Carpenteria, CA), human epidermal growth factor receptor (HER)2 (Dako), and Ki67 (abcam, Cambridge, UK). Secondary antibodies and the color development kit were purchased from Dako (Dako REAL Envision Detection System, Dako). Phosphate-buffered saline was used instead of primary antibody as a negative control for PTK7 detection, and healthy fallopian tube tissue was used as a positive control.
The specificity of the PTK7 antibody was tested by a peptide absorption assay. The PTK7 antibody (N-term) blocking peptide (Abgent) was used to block PTK7 antibody binding to the PTK7 protein in tumor cells. The PTK7 antibody and peptide were added into antibody dilution buffer at 1:20 mol, incubated at 4°C overnight with rotation, then used as the primary antibody. To further test the specificity of the PTK7 antibody, fresh breast cancer tissues were collected for western blotting, then homogenized in T-PER Tissue Protein Extraction Reagent (Thermo Fisher, Rockford, IL) at 1:20 (w/v). The lysate was centrifuged at 10,000 g for 5 min to pellet tissue debris, and western blotting was performed using standard protocols with an antibody against PTK7.
Immunohistochemistry scoring and quantification
PTK7 protein expression was semi-quantitatively assessed. Tissue specimens were assigned one of four scores according to the intensity of antibody staining (0, none; 1, weakly positive/pale yellow; 2, moderately positive/medium brown-yellow; and 3, strongly positive/dark brown) (Figure 1). The extent of staining was graded according to the percentage of stained tumor cells, and was defined as follows: 0 for complete absence of staining; 1 for <25%; 2 for 25%-50%, and 3 for >50% positively stained cells. The staining intensity and staining extent values were added together and used to define the expression status as follows: 0-2, negative (-); 3-4, weakly positive (+); and 5-6, strongly positive (++) [13] (Figures 2, 3 and 4). ER and PR expression was determined by estimating the percentage of positively-stained nuclei as follows: <1% negative (0/1+), 1%-9% low positive (2+), and ≥10% (3+). HER2 immunostaining was scored as previously described for gastric cancer [14] as follows: 0 (negative), no reactivity or <10%; 1+ (negative), faint with partial membrane staining ≥10%; 2+ (positive) for weak-to-moderate with complete or basolateral staining >10%; and 3+ (positive) for moderate-to-strong with complete or basolateral staining ≥10%. Ki67-positive tumor cells demonstrated punctate yellow-brown nuclear staining. High Ki67 expression was defined as ≥50% positive tumor cell staining [15]. Immunostained specimens were evaluated independently by two pathologists, and any discrepancies were resolved by consensus after further evaluation.
Figure 1.
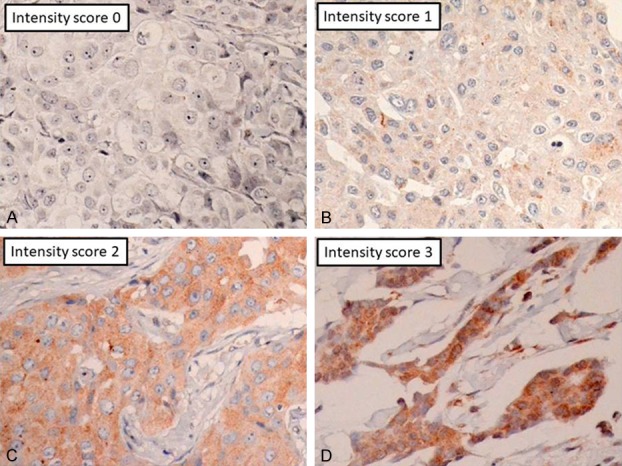
Representative examples of the intensity of PTK7 antibody staining. A. Score 0; B. Score 1; C. Score 2; D. Score 3.
Figure 2.
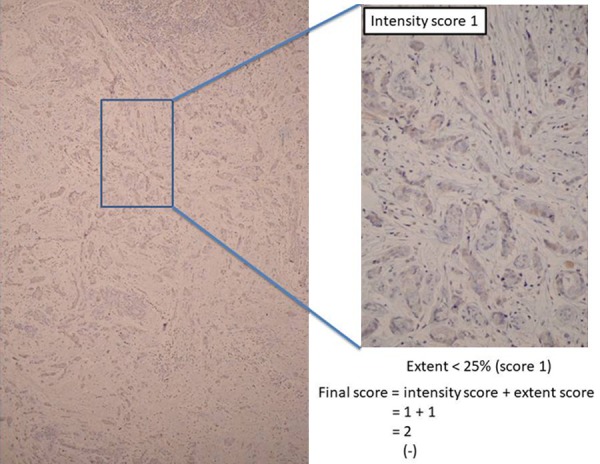
Representative example of final scores obtained by adding staining intensity and staining extent values together. Final score = intensity score (1) + extent score (1) =2; PTK7 expression (-).
Figure 3.
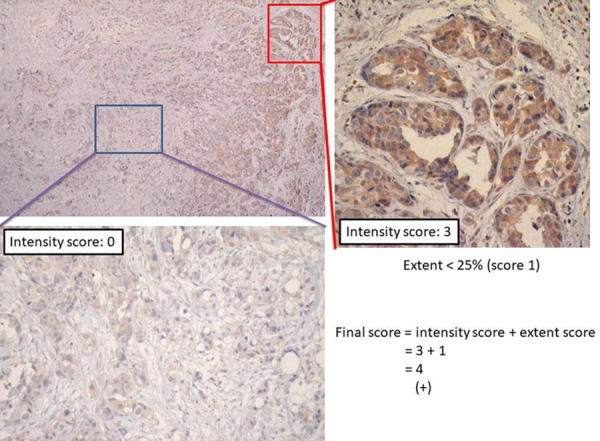
Representative example of final scores obtained by adding staining intensity and staining extent values together. Final score = intensity score (3) + extent score (1) =4; PTK7 expression (+).
Figure 4.
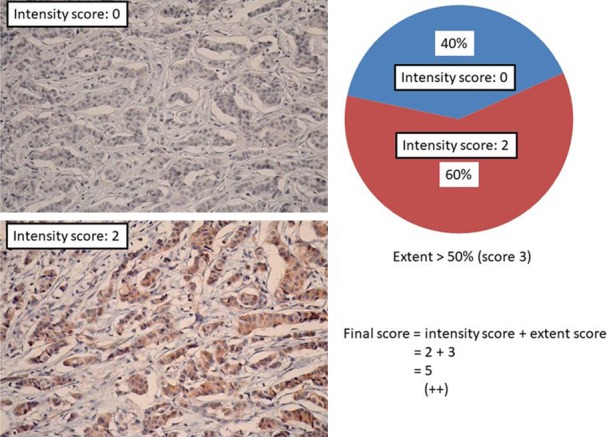
Representative example of final scores obtained by adding staining intensity and staining extent values together. Final score = intensity score (2) + extent score (3) =5; PTK7 expression (++).
Statistical analysis
Statistical analysis was performed using SPSS version 17.0 for Windows (SPSS Inc., Chicago, IL). The Spearman test was used to analyze possible associations between PTK7 expression and clinicopathologic parameters. A P value <0.05 was considered significant.
Results
Antibody specificity test
The specificity of the PTK7 antibody was tested by a peptide absorption assay. As shown in Figure 5A and 5B, positive staining of PTK7 was observed in breast cancer, but this was prevented after inhibition with the blocking peptide. Western blotting analyses of breast cancer tissues revealed that PTK7 protein was differentially detected in breast cancer tissues (Figure 5C). The molecular weight of the PTK7 protein was 11.8 kDa, which is consistent with the expected value.
Figure 5.
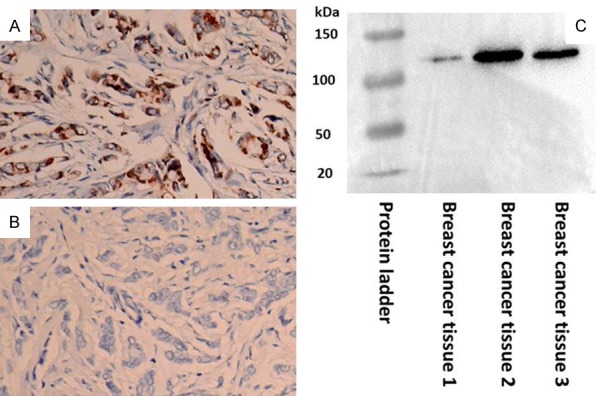
Specificity of the PTK7 antibody was tested by peptide absorption and western blotting. Peptide absorption test. A. Positive staining of PTK7 in breast cancer. B. Negative staining of the same slide after the PTK7 antibody was blocked by a blocking peptide (magnification ×400). C. Western blot analyses detected differential PTK7 protein expression in breast cancer samples as a single band (11.8 kDa).
PTK7 expression in breast carcinoma
Diffuse PTK7 staining and granular particles were detected in the cytoplasm of breast carcinoma cells, and were considered to represent positive antigen staining of the Golgi apparatus (Figure 6). Positive staining was noted in all healthy breast epithelial cells (Figure 6A), but was different in breast carcinoma cells, ranging from negative (Figure 6B), to weak (Figure 6C), and strongly positive (Figure 6D). As shown in Table 1, 38 out of 79 samples (48.1%) were negatively or weakly stained (-), 19 (24.1%) were moderately stained (+), and 22 (27.8%) were strongly stained (++).
Figure 6.
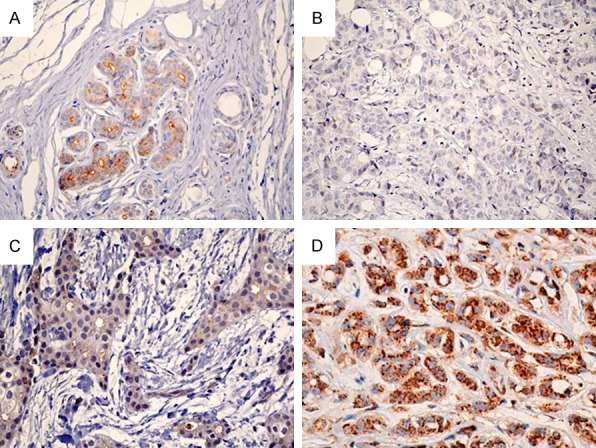
Immunohistochemistry of PTK7 protein in invasive ductal breast carcinoma. A. Strongly positive staining in healthy breast epithelial cells. B. Negative staining in invasive ductal breast carcinoma. C. Weak staining in invasive ductal breast carcinoma. D. Strongly positive staining in invasive ductal breast carcinoma (magnification ×400).
Association of PTK7 expression with clinicopathologic parameters
To better understand the role of PTK7 in breast tumorigenesis, we examined PTK7 protein expression using immunohistochemistry in 79 human invasive ductal carcinomas. PTK7 expression was negatively associated with tumor grade (P=0.025, r=-0.251), tumor-node-metastasis stage (P=0.004, r=-0.317), lymph node metastasis (P=0.002, r=-0.351), human epidermal growth factor receptor 2 expression (P=0.029, r=-0.245), and Ki67 expression (P=0.004, r=-0.317), and positively associated with estrogen receptor (ER) expression (P=0.037, r=0.235) (Figures 7 and 8). No significant relationship was found between PTK7 expression and patient age or PR expression.
Figure 7.
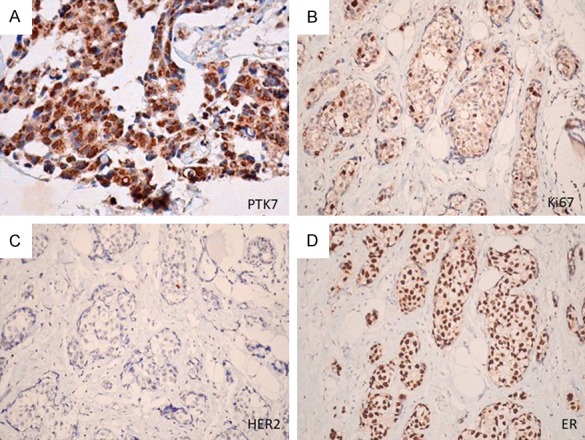
Representative example of positive associations between PTK7 and ER expression, and negative association with HER2 and Ki67 expression. A. Positive staining of PTK7. B. Weak staining of Ki67. C. Negative staining of HER2. D. Positive staining of ER (magnification ×400).
Figure 8.
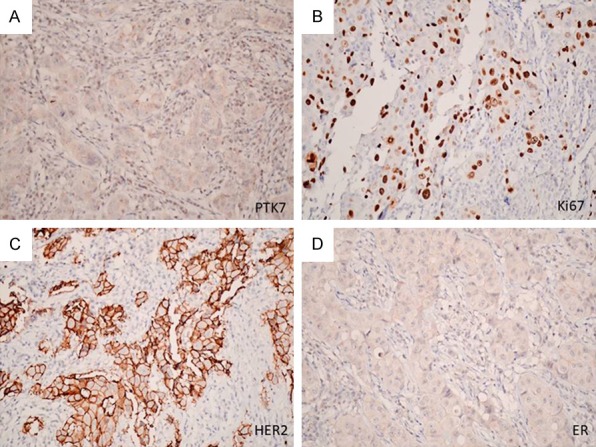
Representative examples of positive association between PTK7 and positive expression, and negative association with HER2 and Ki67 expression. A. Negative staining of PTK7. B. Positive staining of Ki67. C. Positive staining of HER2. D. Negative staining of ER (magnification ×400).
Discussion
Receptor tyrosine kinases including PTK7 have been shown to have various roles in tumorigenesis, including as tumor promoters or tumor suppressors in different tumors or organs. The overexpression of PTK7 has been reported in colorectal cancer, esophageal squamous cell carcinoma, and intraheptic cholangiocarcinoma, while PTK7 downregulation was found in lung cancer, melanoma, renal clear cell carcinoma, and ovarian serous carcinoma.
In this study, we examined PTK7 expression in a set of invasive ductal breast cancer tissues and analyzed its association with clinicopathological parameters. Our data showed that PTK7 protein expression was lost in some invasive ductal breast cancers compared with healthy breast epithelial cells, as reported in several other human cancers [8-10,16]. Downregulation of a gene can be caused by the hypermethylation of CpG islands in the promoter region, microRNA regulation, gene deletions, or mutations [17-20]. We previously reported that the downregulation of receptor tyrosine kinase EphA7 expression in colorectal cancer was caused by hypermethylation of CpG islands in the promoter region [17], so we hypothesize that this is a likely mechanism of PTK7 loss in breast cancer. However, the molecular mechanisms leading to of PTK7 downregulation in breast cancer should be investigated in future studies.
Our data indicated that PTK7 expression was negatively associated with HER2 and Ki67 expression, and positively associated with ER expression. Ataseven et al. previously assessed PTK7 expression in 133 patients with triple negative breast cancer (TNBC) by immunohistochemistry [11]. They found that expression levels were correlated with clinicopathological features and survival, and that PTK7 was expressed in 28.6% of tumors. As shown in Table 1, we observed PTK7 staining in 33.3% of TNBC, which is similar to the results of Atseven et al.
Atseven et al. also found no significant impact of PTK7 expression on disease-free survival (DFS) or overall survival, but detected higher PTK7 expression in smaller tumors (≤2 cm). These data suggested that PTK7 has a tumor suppressor role in TNBC. We were unable to analyze the association of PTK7 with patient survival in our present study because of time restraints. In another study, Atseven et al. detected PTK7 mRNA expression in breast cancers [11], and observed a variation in the effect of PTK7 expression according to whether chemotherapy was administered. A particular benefit from chemotherapy was noted for patients with high lymph node tissue PTK7 mRNA expression.
Golubkov et al. reported that PTK7 is an important component of the Wnt/planar cell polarity pathway [21]. They found that membrane type-1 matrix metalloproteinase (MT1-MMP) functions as a principal sheddase of PTK, directly cleaving full-length PTK7 to generate an Nterminal PTK7 fragment (sPTK7). The enforced expression of membranous PTK7 in cancer cells leads to reorganization of the actin cytoskeleton and the inhibition of cell invasion. These data suggested that PTK7 acts as a tumor suppressor.
PTK7 exists as six isoforms that are generated by alternative splicing: (http://www.uniprot.org/uniprot/Q13308). In the present study, we detected PTK7 protein using a specific polyclonal antibody for the N-terminal region (amino acids 21-25). However, we were unable to distinguish the expression levels of different PTK7 isoforms and their clinical significance in breast cancer.
In summary, we found that PTK7 protein expression was negatively associated with tumor grade, TNM stage, lymph node metastasis, and HER2 and Ki67 expression, and positively associated with ER expression. Together with the findings of other studies, these results indicate that PTK7 has a tumor suppressor role in breast cancer.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (grant number 81371611) and the National Basic Research Priorities Program 973 Project (grant number 2014CB744504) from the Ministry of Science and Technology of China. We thank Sarah Williams, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn), for editing the English text of a draft of this manuscript.
Disclosure of conflict of interest
None.
References
- 1.Mossie K, Jallal B, Alves F, Sures I, Plowman GD, Ullrich A. Colon carcinoma kinase-4 defines a new subclass of the receptor tyrosine kinase family. Oncogene. 1995;11:2179–2184. [PubMed] [Google Scholar]
- 2.Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–98. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- 3.Lhoumeau AC, Martinez S, Boher JM, Monges G, Castellano R, Goubard A, Doremus M, Poizat F, Lelong B, de Chaisemartin C, Bardin F, Viens P, Raoul JL, Prebet T, Aurrand-Lions M, Borg JP, Goncalves A. Overexpression of the promigratory and prometastatic PTK7 receptor is associated with an adverse clinical outcome in colorectal cancer. PLoS One. 2015;10:e0123768. doi: 10.1371/journal.pone.0123768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian X, Yan L, Zhang D, Guan X, Dong B, Zhao M, Hao C. PTK7 overexpression in colorectal tumors: clinicopathological correlation and prognosis relevance. Oncol Rep. 2016;36:1829–1836. doi: 10.3892/or.2016.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park M, Yoon HJ, Kang MC, Kwon J, Lee HW. PTK7 regulates radioresistance through nuclear factor-kappa B in esophageal squamous cell carcinoma. Tumour Biol. 2016;37:14217–14224. doi: 10.1007/s13277-016-5288-3. [DOI] [PubMed] [Google Scholar]
- 6.Jin J, Ryu HS, Lee KB, Jang JJ. High expression of protein tyrosine kinase 7 significantly associates with invasiveness and poor prognosis in intrahepatic cholangiocarcinoma. PLoS One. 2014;9:e90247. doi: 10.1371/journal.pone.0090247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Easty DJ, Mitchell PJ, Patel K, Florenes VA, Spritz RA, Bennett DC. Loss of expression of receptor tyrosine kinase family genes PTK7 and SEK in metastatic melanoma. Int J Cancer. 1997;71:1061–1065. doi: 10.1002/(sici)1097-0215(19970611)71:6<1061::aid-ijc24>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 8.Kim JH, Kwon J, Lee HW, Kang MC, Yoon HJ, Lee ST, Park JH. Protein tyrosine kinase 7 plays a tumor suppressor role by inhibiting ERK and AKT phosphorylation in lung cancer. Oncol Rep. 2014;31:2708–2712. doi: 10.3892/or.2014.3164. [DOI] [PubMed] [Google Scholar]
- 9.Behbahani TE, Thierse C, Baumann C, Holl D, Bastian PJ, von Ruecker A, Muller SC, Ellinger J, Hauser S. Tyrosine kinase expression profile in clear cell renal cell carcinoma. World J Urol. 2012;30:559–565. doi: 10.1007/s00345-011-0767-z. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Li G, Yin Y, Wang J, Wang H, Wei W, Guo Q, Ma H, Shi Q, Zhou X, Wang J. PTK7 protein is decreased in epithelial ovarian carcinomas with poor prognosis. Int J Clin Exp Pathol. 2014;7:7881–7889. [PMC free article] [PubMed] [Google Scholar]
- 11.Ataseven B, Gunesch A, Eiermann W, Kates RE, Hogel B, Knyazev P, Ullrich A, Harbeck N. PTK7 as a potential prognostic and predictive marker of response to adjuvant chemotherapy in breast cancer patients, and resistance to anthracycline drugs. Onco Targets Ther. 2014;7:1723–1731. doi: 10.2147/OTT.S62676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ataseven B, Angerer R, Kates R, Gunesch A, Knyazev P, Hogel B, Becker C, Eiermann W, Harbeck N. PTK7 expression in triple-negative breast cancer. Anticancer Res. 2013;33:3759–3763. [PubMed] [Google Scholar]
- 13.Song T, Wang L, Mo Z, Mao L, Ma X, Niu R, Gu K, Yan R, Ma P, Qi Y, Jiao Q. Expression of p-Akt in ovarian serous carcinoma and its association with proliferation and apoptosis. Oncol Lett. 2014;7:59–64. doi: 10.3892/ol.2013.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woo CG, Ho WJ, Park YS, Park SR, Ryu MH, Jung HY, Kang YK. A potential pitfall in evaluating HER2 immunohistochemistry for gastric signet ring cell carcinomas. Pathology. 2017;49:38–43. doi: 10.1016/j.pathol.2016.09.064. [DOI] [PubMed] [Google Scholar]
- 15.Huang G, Chen S, Wang D, Wang R, Lin L, Chen S, Wang L, Huang Q. High Ki67 expression has prognostic value in surgically-resected T3 gastric adenocarcinoma. Clin Lab. 2016;62:141–153. doi: 10.7754/clin.lab.2015.150610. [DOI] [PubMed] [Google Scholar]
- 16.Endoh H, Tomida S, Yatabe Y, Konishi H, Osada H, Tajima K, Kuwano H, Takahashi T, Mitsudomi T. Prognostic model of pulmonary adenocarcinoma by expression profiling of eight genes as determined by quantitative real-time reverse transcriptase polymerase chain reaction. J. Clin. Oncol. 2004;22:811–819. doi: 10.1200/JCO.2004.04.109. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Kataoka H, Suzuki M, Sato N, Nakamura R, Tao H, Maruyama K, Isogaki J, Kanaoka S, Ihara M, Tanaka M, Kanamori M, Nakamura T, Shinmura K, Sugimura H. Downregulation of EphA7 by hypermethylation in colorectal cancer. Oncogene. 2005;24:5637–5647. doi: 10.1038/sj.onc.1208720. [DOI] [PubMed] [Google Scholar]
- 18.Ling Y, Ling X, Fan L, Wang Y, Li Q. Mutation analysis underlying the downregulation of the thyroid hormone receptor beta1 gene in the Chinese breast cancer population. Onco Targets Ther. 2015;8:2967–2972. doi: 10.2147/OTT.S93418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cabrero M, Yu Y, Verma A, Yang H, Colla S, Jia Y, Zheng H, Bohannan Z, Ganan-Gomez I, Futreal A, Takahashi K, Chin L, Kantarjian H, Pellagatti A, Bowman T, Boultwood J, Garcia-Manero G, Wei Y. Downregulation of protection of telomeres 1 expression in myelodysplastic syndromes with 7q deletion. Br J Haematol. 2016;173:161–165. doi: 10.1111/bjh.13574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui H, Zhang S, Zhou H, Guo L. Direct downregulation of B-cell translocation gene 3 by microRNA-93 is required for desensitizing esophageal cancer to radiotherapy. Dig Dis Sci. 2017;62:1995–2003. doi: 10.1007/s10620-017-4579-x. [DOI] [PubMed] [Google Scholar]
- 21.Golubkov VS, Chekanov AV, Cieplak P, Aleshin AE, Chernov AV, Zhu W, Radichev IA, Zhang D, Dong PD, Strongin AY. The Wnt/planar cell polarity protein-tyrosine kinase-7 (PTK7) is a highly efficient proteolytic target of membrane type-1 matrix metalloproteinase: implications in cancer and embryogenesis. J Biol Chem. 2010;285:35740–35749. doi: 10.1074/jbc.M110.165159. [DOI] [PMC free article] [PubMed] [Google Scholar]


