Abstract
Celastrol is a traditional Chinese medicine, that is derived from Tripterygium wilfordii. It has been proposed to offer anti-tumor potential. MicroRNAs also play important roles in tumorigenesis. However, the anti-tumor mechanism of Celastrol and whether miRNAs are involved in the process are still unknown. In the present study, MTT assay was used to test the IC50 of Celastrol and cell viability. PCNA, PI3K, Akt, GSK3β, phosphorylated Akt and GSK3β were measured by western blotting. Flow cytometry was introduced to detect the apoptosis. We found Celastrol inhibited colon cancer cell viability in a dose-dependent manner companied with PCNA downregulation. Apoptosis was induced by Celastrol. After Celastrol treatment, BCL-2 expression decreased while BAX increased and the Caspase-3 activity was induced. Simultaneously, miR-21 expression was reduced in Celastrol-treated colon cancer cells. miR-21 mimic overexpression could enhance the cell viability, inhibit the apoptosis, decrease BCL-2 expression, increase BAX and induce Caspase-3 activity to some extent which were reversed by Celastrol. In addition, the PI3K/AKT/GSK-3β pathway was activated by miR-21 mimic but partially arrested by extra-adding Celastrol. Thus, Celastrol may inhibit colon cancer cell proliferation by negatively regulating miR-21 and the PI3K/AKT/GSK-3β pathway.
Keywords: Celastrol, proliferation, miR-21, Akt, colon cancer
Introduction
Colon cancer, also known as colorectal cancer and bowel cancer, is a malignant tumor arising from the inner wall of the large intestine (colon) or rectum that has the ability to invade to other parts of the body. According the 2015 data released from Chinese National cancer center, both the incidence and mortality of colon cancer are fifth among all types of cancer [1]. Every year, there is 376,300 new colon cancer cases, and 191,000 die because of colon cancer in China. Thus, developing more efficient or complementary treatment is urgently needed in the clinic. For decades, traditional Chinese medicine drew plenty of attention for oncotherapy [2]. Especially some natural products are effective chemotherapy agents [3].
Celastrol is a traditional Chinese medicine, that is a pentacyclic triterpene monomer extracted from Tripterygium wilfordii [4]. Previous studies suggested that Celastrol has anti-bacteria, anti-inflammation [5], anti-virus functions [6] and has been used for the treatment of rheumatism [7], blood disease [8]. Recently, it has been reported that Celastrol exhibits anti-tumor effects [9,10]. But the underlying molecular mechanism in oncotherapy is still enigmatic.
MicroRNAs (miRNAs) are a cluster of small non-coding RNAs, 18-22 nucleotides in length [11]. miRNAs play pivotal roles in various cell processes including proliferation, development, differentiation, senescence, apoptosis and so on, mainly through post-transcriptional regulation of gene expression [12]. Disrupted expression of miRNAs is thought to be associated with various types of cancer such as lung cancer, breast cancer, liver cancer, stomach cancer, and colon cancer [13]. Many miRNAs are involved in colon cancer, including miR-204 [14], miR-155 [15], miR-191 [16], and miR-21 [17,18]. Some miRNAs are downregulated in tumors and act as tumor suppressor, while some miRNAs are upregulated and function as onco-miRNAs. For example, miR-21 is upregulated in colon cancers but the relationship between miR-21 and colon cancer is still not totally defined.
The phosphoinositide 3-kinases (PI3Ks) are a superfamily of lipid enzymes on the plasma membrane. In past years, studies suggested that PI3Ks play significant roles in various cellular processes such as metabolism, inflammation, survival, and cancer progression [19]. AKT is one of the major downstream targets of PI3K. Once PI3K undergoes activation, AKT is translocated to the inner membrane and is phosphorylated by PDK1. GSK-3 is a primary target of Akt and has numerous cellular effects such as cell cycle progression, and cell survival [20]. In brief, the PI3K/AKT/GSK-3β pathway is involved in many cell processes including tumor progression. Most important is that PI3K/AKT/GSK-3β pathway prevents the differentiation of colon cancer [21]. However, whether this pathway is involved in the Celastrol treatment is still unclear.
In the present study, we attempted to elucidate whether and how Celastrol contributes to colon cancer. We first confirmed the IC50 of Celastrol in colon cancer cell line HCT-116 and revealed that Celastrol could inhibit the cell viability and induced apoptosis. Then we found that miR-21 was negatively regulated by Celastrol. Finally, we demonstrated that Celastrol suppressed cell viability and enhanced apoptosis via both the miR-21 and PI3K/AKT/GSK-3β pathway. Our study shed a light on the understanding of Celastrol in colon cancer treatment.
Materials and methods
Cell culture and treatment
Colon cancer cell line HCT-116 was obtained from RiboBio Co. (Guangzhou, China). Cells were cultured in DMEM (Dulbecco’s Modified Eagle’s Medium, Gibco, USA) media with 10% fetal bovine serum (FBS; HyClone, Logan, UT, USA), 1% penicillin and streptomycin (Sigma-Aldrich, St. Louis, MO, USA) at 37°C with 5% of CO2. Medium was changed every 2 days.
Celastrol was purchased from Sigma-Aldrich (St. Louis, MO, USA) and was dissolved into DMSO to a final concentration of 50 mM. HCT-116 was treated with Celastrol under different concentrations for 24 hrs, 48 hrs and 72 hrs to detected the IC50.
Transient transfection
NC mimic and miR-21 mimic were purchased from GenePharma Co., Ltd. (Shanghai, China). All oligos were transfected into HCT-116 cells using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from HCT-116 cells using TRIzol (Invitrogen) and RNA concentration was detected by NanoDropND-1000 spectrophotometer. The TaqMan miRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) was applied to reverse-transcribe into cDNAs. miR-21 expression was determined using SYBR® Green (Promega, Madison, WI, USA) according to the manufacturer’s protocol. U6 small nuclear RNA (snRNA) was used as a reference gene for miR-21. Fluorescence was detected in iQTM5 Multicolor Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). The relative expression of miR-21 was calculated by the 2-ΔΔCt method. Primer sequences: miRNA-21, forward 5’-GCCCGCTAGCTTATCAGACTGATG-3’ and reverse 5’-GCCCGCTAGCTTATCAGACTGATG-3’; and U6 forward 5’-GTTGACATCCGTAAAGACC-3’ and reverse 5’-GGAGCCAGGGCAGTAA-3’.
Western blot
Cells were washed with Tris-buffered saline (TBS), then we added RIPA buffer and maintained on ice for 30 min accompanied with vortexing 30 s per 10 min. We centrifuged for 15 min at 4°C with 12000 rpm and transferred the supernatant to a new tube for next experiments. The protein concentrations were measured using Nanodrop2000 (Thermo Fisher Scientific, San Jose, CA, USA). Then we added loading buffer to the protein solution and heated on 95°C for 5 min. Proteins (10 ug) were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene fluoride membranes (PVDF, Millipore, Bedford, MA, USA). After blocking with 5% dried skimmed milk in TBS, the membranes were incubated with specific primary antibodies including anti-PCNA, anti-Akt, anti-PI3K, anti-phospho Akt, anti-GSK3β, anti-phospho GSK3β (1:100 solution), and all of these antibodies were purchased from Santa Cruz Biotechnology Inc. and anti-GAPDH antibody (1:2000 dilution, Santa Cruz Biotechnology Inc.) at 4°C overnight. The PVDF membrane was washed five times for 10 min each with Tris-buffered saline with Tween-20 (TBST), followed by incubation with HRP-conjugated anti-mouse IgG secondary antibody (1:2000 dilution, Santa Cruz Biotechnology Inc.) at room temperature for 1 hr. After washing with TBST five times, the protein signals were detected using PierceTM ECL western blotting substrate (Thermo Fisher Scientific).
3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2htetrazolium bromide (MTT) assay
Cell viability was measured with MTT assay kit (Sigma-Aldrich) according to the manufacturer’s protocol. In brief, cells (2×103) were seeded into 96-well plates (Corning Costar, Corning, NY, USA). Then we added 30 µL serum-free media with MTT solution into each well and incubated for 4 h at 37°C. After discarding the media, 150 ul MTT solvent (4 mM HCl, 0.1% NP40 in isopropanol) was added into each well and then incubated for 3 h at 37°C. Then the absorbance (A) value of each well was measured at OD = 450 nm using the Microplate Reader (MG LABTECH, Durham, NC, USA).
Cell apoptosis
Cell apoptosis was determined using Annexin V-FITC apoptosis detection kit (Beyotime, Shanghai, China). Cell was cultured in DMEM with 10% FBS for 48 h. Then cells (1×106) were collected and washed with phosphate-buffered saline (PBS). After re-suspending in Annexin V-FITC binding solution, cells were stained with Annexin V-FITC and propidium iodide. Finally, cell apoptosis was detected using flow cytometry (BD Biosciences, San Jose, CA, USA).
Statistical analysis
The data are presented as mean ± SD (standard deviation) from three biological replicated experiments. The analysis of results apre shown and plotted using GraphPad Prism 7.0 (GraphPad Software, San Diego, CA, USA). All group comparisons were carried out using the Student t-test. The p values less than 0.05 was regarded as statistically significant.
Results
Celastrol inhibits HCT-116 proliferation in a dose-dependent manner
To test the cytotoxicity of Celatrol on the proliferation of HCT-116, the cells were seeded into 96-well plates and treated with eight different concentrations of Celatrol for 24 hrs, 48 hrs, and 72 hrs, and then MTT assay was used to measure the cell viability. As shown in Figure 1A, we found a relationship between drug concentration and cell survival rate. Then we calculated that the IC50 s for 24 h, 48 h, 72 h were 5.56 μM, 4.10 μM and 3.20 μM individually. Thus, we chose 4 μM for the next experiments and treated the HCT-116 cells with 4 μM Celastrol for 24 hrs, 48 hrs and 72 hrs and the MTT results showed that cell viability was significant impaired by Celastrol (Figure 1B).
Figure 1.

Celastrol inhibited HCT-116 proliferation in a dose-dependent manner. A. HCT-116 colon cell lines were treated with different concentrations of Celastrol for 24 hrs, 48 hrs and 72 hrs. B. HCT-116 cells were treated with 4 μM Celastrol for 3 days, and the cell viablity was detected on 24 h, 48 h and 72 h. *Means P<0.05. C. PCNA expression was detected by western blotting and gray scale was analyzed. Data are expressed as means ± SD.
PCNA is an auxiliary protein for DNA polymerase that expresses maximally in S phase and has been widely used as an index of the proliferative activity in many cancers [22]. We checked the PCNA expression after Celastrol treatment and the western blotting data implicated that Celastrol may inhibit the PCNA expression significantly (Figure 1C).
Celastrol induces HCT-116 apoptosis
Cell viability decrease may be caused by proliferation arrest or apoptosis. Thus, we checked the apoptosis status after Celastrol treatment. As Figure 2A shows, the proportion of Annexin V-positive cells increased approximately 3.5 fold from about 10% to more than 35%.
Figure 2.

Celastrol induced HCT-116 apoptosis. A. Apoptosis was analyzed by flow cytometry with PI and Annexin V staining. Annexin V-positive cells were supposed to be apoptotic. B. Western blotting analyses of Bax, Bcl-2 and cleaved caspase-3 levels in HCT-116 with or without celastrol administration. GAPDH was used as loading control. *Means P<0.05. Data are expressed as means ± SD.
BCL-2 plays an important role in promoting cellular survival and inhibiting the actions of pro-apoptotic proteins such as BAX which may initiate apoptosis in a Caspase 3-dependent manner. We used western blot to check their expression. As data show, BCL-2 was down-regulated after Celastrol treatment accompanied by increased BAX, and cleaved-caspase 3 expression (Figure 2B).
miR-21 is inhibited by Celastrol
To further explore the apoptotic effect of Celastrol in Colon cancer cells, miR-21 expression was measured by qRT-PCR. After Celastrol treatment, miR-21 expression was decreased significantly about two-thirds (Figure 3A). To better understand the relationship between Celastrol and miR-21, we overexpressed miR-21 mimic in HCT-116 (Figure 3B) and treated the transfected cells with Celastrol. As the figure shows, the Celastrol inhibited miR-21 expression distinctly (Figure 3C).
Figure 3.
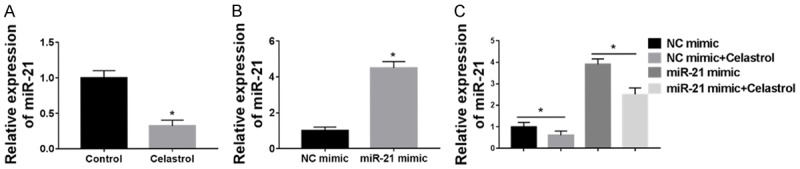
miR-21 was inhibited by Celastrol. A. qRT-PCR was used to detect the miR-21 expression with or without Celastrol treatment. B. miR-21 mimic was transfected into the HCT-116 colon cancer cell line and the overexpression efficiency was detected by qRT-PCR. C. miR-21 expression was detected with or without Celastrol treatment. Data are expressed as means ± SD.
Celastrol inhibited cell viability and induced apoptosis by regulating miR-21
Considering that Celastrol may negatively regulate miR-21 expression and Celastrol could inhibit cell viability and induce apoptosis, we examined the role of miR-21 plays in the cell processes. As expected, miR-21 overexpression enhanced the cell viability significantly which could be strongly reversed by Celastrol (Figure 4A).
Figure 4.
Celastrol inhibited cell viability and induced apoptosis by regulating miR-21. HCT-116 cells were divided into four groups, NC mimic, NC mimic + 4 μM Celastrol, miR-21 mimic, miR-21 mimic + 4 μM Celastrol groups. A. Cell viability was detected by MTT assay. B. PCNA expression was measured by western blotting and grey scale was analyzed. C. Cells were stained with PI and FITC-Annexin V and apoptosis was analyzed by flow cytometry. D. Western blotting analyses of BCL-2, Bax, and cleaved caspase-3 levels in each group. *Means P<0.05. Data are expressed as means ± SD.
PCNA expression was upregulated in the miR-21 mimic group and down-regulated after Celastrol treatment in the HCT-116 transfected miR-21 mimic (Figure 4B). Additionally, we checked the apoptotic status. As in Figure 2, Celastrol could induce HCT-116 apoptosis significant, but miR-21 mimic attenuated the Annexin V-positive cell proportion. However, treatment with both miR-21 and Celastrol significantly promoted apoptosis compared with the miR-21 mimic group (Figure 4C). In addition, we investigated the expression of BCL-2, BAX, and cleaved-Caspase 3 and the western blotting data demonstrated that miR-21 increased BCL-2 expression, and inhibited BAX expression and Caspase-3 activity while these phenomena could be reversed by Celastrol treatment (Figure 4D). In conclusion, Celastrol inhibited cell viability and induced apoptosis by negatively regulating miR-21 expression.
Celastrol partially arrests PI3K/AKT/GSK-3β pathway activation by miR-21
The PI3K/Akt/GSK3β pathway has been proposed to play a pivotal role in cell survival by stimulating cell proliferation and inhibiting cell apoptosis. Thus, we examined the pathway in the colon cancer cells treated with Celastrol. As the Figure 5 shows, Celastrol could inhibit PI3K expression, while miR-21 mimic induced PI3K expression; and Celastrol could reverse the PI3K expression induced by miR-21 overexpression.
Figure 5.

Celastrol partially arrests the PI3K/AKT/GSK-3β pathway activated by miR-21. HCT-116 cells were divided into four groups: NC mimic, NC mimic + 4 μM Celastrol, miR-21 mimic, miR-21 mimic + 4 μM Celastrol groups. PI3K, AKT, phosphorylated AKT, GSK-3β and phosphorylated GSK-3β expression were detected by western blotting and normalized by GAPDH. *Means P<0.05. Data are expressed as means ± SD.
Akt and GSK3β are downstream targets of PI3K which may affect their phosphorylation. Therefore, we further detected the activity of Akt and GSK3β. Akt and GSK3β phosphorylation were decreased after Celastrol treatment while miR-21 mimic increased their phosphorylation level. However, when HCT-116 cells were treated both with Celastrol and miR-21 mimic, the phosphorylation of Akt and GSK3β decreased compared with those cells transfected with miR-21 mimic only (Figure 5). Taken together, the results demonstrated that Celastrol may partially arrest the PI3K/AKT/GSK-3β pathway which is activated by miR-21 mimic in HCT-116 cells.
Discussion
Colon cancer is one of the most common cancers, and causes many deaths. The conventional treatment of colon cancer includes surgery, radiation, chemotherapy and targeted therapy. As a complementary therapy, traditional Chinese medical is in desperate need [23]. Traditional Chinese medical is a style of traditional medicine which was founded more than 2,500 years in China and includes various forms of herbal medicine, acupuncture, massage, and dietary therapy. Among these, herbal medicine has contributed much to colon cancer treatment [24], and now more novel plant ingredients with anti-tumor activities have been found.
Celastrol, as one herbal agent, is a pentacyclic triterpene monomer extracted from Tripterygium wilfordii. Celastrol has antioxidative, anti-inflammatory, and anti-tumor effects and has been used to treat rheumatoid arthritis, bruises, and additional diseases. Previous studies demonstrated that Celastrol is an effective and potent herbal agent in treating ovarian carcinoma cells in vivo [4]. It also significantly inhibited the proliferation and induced apoptosis in gastric cancer cells [25,26] and prostate cancer cells [27].
MicroRNAs (miRNAs) are a type of non-coding small RNA, which are expressed significantly in cancer cells compared with normal tissues. Impaired miRNA expression is related to tumorigenesis, prognosis, and treatment. miR-21 was proposed to play some key roles in cancer such as breast cancer [28], liver cancer [29], cervical cancer [30], and colon cancer [31]. In our present study, we found that miR-21 expression was inhibited in Celastrol-treated colon cells.
PI3K belongs to the lipid kinase family and is involved in cell survival and proliferation. As an important downstream target of PI3K, Akt activation is closely related to tumor development. GSK-3β was one of the first identified substrates of Akt, phosphorylation by which inhibits GSK3 activity. However, GSK3 activity may suppresses cell proliferation and survival [20]. As enzymes involved in cell survival, the PI3K/AKT/GSK-3β pathway provides important signaling for tumor cell proliferation [32].
In our present studies, we found that Celastrol inhibited colon cancer proliferation and induced cell apoptosis. The detailed mechanism of Celastrol in apoptosis is that Celastrol may inhibit the expression of BCL-2 and elevate the BAX, and cleaved-caspase 3. Furthermore, Celastrol may downregulate the miR-21 expression, and partially inhibit the PI3K/AKT/GSK-3β pathway.
In conclusion, we elucidated that Celastrol inhibited colon cancer cell viability and induced cell apoptosis by negatively regulating miR-21 expression and working through the PI3K/AKT/GSK-3β pathway. The results demonstrated that Celastrol may be a potential herbal candidate in anti-tumor treatment.
Disclosure of conflict of interest
None.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y, Jiong-Hui LI, Bi L. Combination of traditional Chinese medicine cancer chemotherapy damage repair clinical observation. Chinese Journal of Basic Medicine in Traditional Chinese Medicine. 2012;6:115–117. [Google Scholar]
- 3.Efferth T, Li PC, Konkimalla VS, Kaina B. From traditional Chinese medicine to rational cancer therapy. Trends Mol Med. 2007;13:353–361. doi: 10.1016/j.molmed.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H, Li J, Li G, Wang S. Effects of celastrol on enhancing apoptosis of ovarian cancer cells via the downregulation of microRNA-21 and the suppression of the PI3K/Akt-NF-κB signaling pathway in an in vitro model of ovarian carcinoma. Mol Med Rep. 2016;14:5363–5368. doi: 10.3892/mmr.2016.5894. [DOI] [PubMed] [Google Scholar]
- 5.Allison AC, Cacabelos R, Lombardi VR, Alvarez XA, Vigo C. Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:1341–1357. doi: 10.1016/s0278-5846(01)00192-0. [DOI] [PubMed] [Google Scholar]
- 6.Narayan V, Ravindra KC, Chiaro C, Cary D, Aggarwal BB, Henderson AJ, Prabhu KS. Celastrol inhibits tat-mediated human immunodeficiency virus (HIV) transcription and replication. J Mol Biol. 2011;410:972–983. doi: 10.1016/j.jmb.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cascão R, Vidal B, Fonseca JE, Moita LF. Gambogic acid and celastrol are two powerful anti-inflammatory drugs in arthritis. Ann Rheum Dis. 2011;70:A169. [Google Scholar]
- 8.Kusy S, Ghosn EE, Herzenberg LA, Contag CH. Development of B cells and erythrocytes is specifically impaired by the drug celastrol in mice. PLoS One. 2012;7:e35733. doi: 10.1371/journal.pone.0035733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mou H, Zheng Y, Zhao P, Bao H, Fang W, Xu N. Celastrol induces apoptosis in non-small-cell lung cancer a549 cells through activation of mitochondria- and fas/fasL-mediated pathways. Toxicol Vitro. 2011;25:1027–1032. doi: 10.1016/j.tiv.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 10.Kim Y, Kang H, Jang SW, Ko J. Celastrol inhibits breast cancer cell invasion via suppression of NF-ĸB-mediated matrix metalloproteinase-9 expression. Cell Physiol Biochem. 2011;28:175–184. doi: 10.1159/000331729. [DOI] [PubMed] [Google Scholar]
- 11.Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Rådmark O, Kim S. The nuclear RNase III drosha initiates microRNA processing. Nature. 2012;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 12.Bushati N, Cohen SM. microRNA Functions. Annu Rev Cell Dev Biol. 2007;23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- 13.Santulli G. microRNA: cancer. from molecular biology to clinical practice. Anticancer Res. 2016:2047–2048. [Google Scholar]
- 14.Wu K, He Y, Li G, Peng J. Expression and proliferative regulation of miR-204 related to mitochondrial transcription factor A in colon cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 2015;18:1041–6. [PubMed] [Google Scholar]
- 15.Al-Haidari A, Algaber A, Madhi R, Syk I, Thorlacius H. MiR-155-5p controls colon cancer cell migration via post-transcriptional regulation of human antigen R (HuR) Cancer Lett. 2018;421:145–151. doi: 10.1016/j.canlet.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 16.Zhang XF, Li KK, Gao L, Li SZ, Chen K, Zhang JB, Wang D, Tu RF, Zhang JX, Tao KX. miR-191 promotes tumorigenesis of human colorectal cancer through targeting C/EBPβ. Oncotarget. 2015;6:4144–4158. doi: 10.18632/oncotarget.2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng J, Lei W, Fu JC, Zhang L, Li JH, Xiong JP. Targeting miR-21 enhances the sensitivity of human colon cancer HT-29 cells to chemoradiotherapy in vitro. Biochem Biophysical Res Commun. 2014;443:789–795. doi: 10.1016/j.bbrc.2013.11.064. [DOI] [PubMed] [Google Scholar]
- 18.Chen XY, Zhang J, Hou LD, Zhang R, Chen W, Fan HN, Huang YX, Liu H, Zhu JS. Upregulation of PD-L1 predicts poor prognosis and is associated with miR-191-5p dysregulation in colon adenocarcinoma. Int J Immunopathol Pharmacol. 2018;32:2058738418790318. doi: 10.1177/2058738418790318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11:329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 20.Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Pandurangan AK. Potential targets for prevention of colorectal cancer: a focus on PI3K/Akt/mTOR and Wnt pathways. Asian Pac J Cancer Prev. 2013;14:2201–2205. doi: 10.7314/apjcp.2013.14.4.2201. [DOI] [PubMed] [Google Scholar]
- 22.Kelman Z. PCNA: structure, functions and interactions. Oncogene. 1997;14:629–640. doi: 10.1038/sj.onc.1200886. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Z, Yuanyuan GU, Han Y, Zhong L, Tian J, Zhou Z. Position thinking and research in treatment of cancer of traditional chinese medicine. Liaoning Journal of Traditional Chinese Medicine. 2018 [Epub ahead of print] [Google Scholar]
- 24.McCulloch M, Broffman M, van der Laan M, Hubbard A, Kushi L, Abrams DI, Gao J, Colford JM Jr. Colon cancer survival with herbal medicine and vitamins combined with standard therapy in a whole-systems approach: ten-year follow-up data analyzed with marginal structural models and propensity score methods. Integr Cancer Ther. 2011;10:240–259. doi: 10.1177/1534735411406539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sha M, Ye J, Zhang LX, Luan ZY, Chen YB, Huang JX. Celastrol induces apoptosis of gastric cancer cells by miR-21 inhibiting PI3K/Akt-NF-κB signaling pathway. Pharmacol. 2014;93:39–46. doi: 10.1159/000357683. [DOI] [PubMed] [Google Scholar]
- 26.Lee HW, Jang KS, Choi HJ, Jo A, Cheong JH, Chun KH. Celastrol inhibits gastric cancer growth by induction of apoptosis and autophagy. BMB Rep. 2014;47:697–702. doi: 10.5483/BMBRep.2014.47.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolfram J, Suri K, Huang Y, Molinaro R, Borsoi C, Scott B, Boom K, Paolino D, Fresta M, Wang J. Evaluation of anticancer activity of celastrol liposomes in prostate cancer cells. J Microencapsul. 2014;31:501–507. doi: 10.3109/02652048.2013.879932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frankel LB, Christoffersen NR, Jacobsen A, Lindow M, Krogh A, Lund AH. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J Biol Chem. 2008;283:1026–1033. doi: 10.1074/jbc.M707224200. [DOI] [PubMed] [Google Scholar]
- 29.Zhu M, Wang N, Tsao G, Feng Y. miR-21 and miR-23a were altered by coptidis rhizoma aqueous extract in human liver cancer cells. Exp Ther Med. 2011;2:27–32. doi: 10.3892/etm.2010.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Georgios D, Corrie SR, Feng Q, Janice M, Joshua S, Hawes SE, Kiviat NB. Expression of Mir-21 and Mir-143 in cervical specimens ranging from histologically normal through to invasive cervical cancer. PLoS One. 2011;6:e28423. doi: 10.1371/journal.pone.0028423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kjaerfrifeldt S, Hansen TF, Nielsen BS, Joergensen S, Lindebjerg J, Soerensen FB, Christensen RD, Jakobsen A. The prognostic importance of miR-21 in stage II colon cancer: a population-based study. Br J Cancer. 2012;107:1169–1174. doi: 10.1038/bjc.2012.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: Its functions and alterations in human cancer. Apoptosis. 2004;9:667–676. doi: 10.1023/B:APPT.0000045801.15585.dd. [DOI] [PubMed] [Google Scholar]



