Abstract
Aim: This study aimed to investigate the effect of lycopene on LPS-induced liver injury in mice and its mechanisms. Methods: Male C57bl/6 mice were randomly assigned to three groups: sham control group (S-C), LPS control group (L-C), lycopene treatment group (L-T). The mice from the L-T were treated with lycopene for 2 weeks, and the remaining mice with solvent. Afterwards, the mice from the L-C and the L-T received an intraperitoneal injection of LPS (20 mg/kg, dissolved in sterile saline), and the S-C mice were injected with sterile saline. Serum levels of alanine transaminase (ALT) and aspartate aminotransferase (AST) were determined for analysis of liver function. Levels of inflammatory cytokines including tumor necrosis factor (TNF)-α and interleukin (IL)-6, malondialdehyde (MDA) content, and the activity of superoxide dismutase (SOD), were detected in serum. Liver tissues were operated for morphologic analysis and determination of protein by western blot. Results: Pretreatment with lycopene significantly decreased levels of ALT, AST, and TNF-α and IL-6, reduced MDA content, and increased activity of SOD in serum compared with the L-C mice. Lycopene increased expression of nuclear factor-erythroid 2 related factor 2 (Nrf2), and reduced expression of cyclooxygenase (COX)-2, and phosphorylation of nuclear factor-kappa B (NF-κB) and extracellular regulated protein kinases 1/2 (ERK1/2). Conclusion: The results showed that lycopene attenuates LPS-induced liver injury by reducing NF-κB/COX-2 signaling by upregulation of Nrf2/HO-1 activation.
Keywords: Lycopene, LPS, liver injury
Introduction
Sepsis is a severe clinical inflammatory response syndrome with high mortality [1]. Infection caused by Gram-negative bacteria is a leading cause of sepsis. Various studies showed that lipopolysaccharide (LPS), an important component in cell wall of Gram-negative bacteria, triggers release of pro-inflammatory cytokines and reactive oxygen species (ROS) which damage to multiple tissues and organs including liver, heart, and kidney [2,3]. Liver diseases are increasingly becoming a worldwide problem, and there are still few effective treatments for severe hepatic failure. Increasing evidence confirmed that LPS plays a critical role in the development and progression of hepatic injury [4-6]. Clinical and animal experiments suggested that level of LPS in plasma is associated with fibrosis and cirrhosis [7,8]. Inflammatory mediators such as TNF-α and IL-6 induced by LPS contributes to liver injury by depleting intracellular antioxidants, and causing lipid peroxidation and oxidative damage [9,10]. Antioxidants such as vitamin E and tocopherols have been reported to play an important role in protection from oxidative damage [11].
Lycopene, a natural lipophilic carotenoid with no provitamin A activity, is synthesized in tomatoes. Increasing evidence showed that lycopene and its metabolites exhibit various important biological functions [12-14]. Lycopene possesses many conjugated double bonds, so its ability to quench singlet oxygen is about 10 times as much as that of vitamin E [15,16]. Lipophilic lycopene affects lipid metabolism and protects lipid from peroxidation [17-20]. Further, lycopene reduces DNA oxidative damage induced by ROS, and protects endothelial function from oxidative stress [21-23]. Animal studies showed that lycopene reduces synthesis of C-reactive protein (CRP) secreted by the liver [24,25], and inflammation [26,27].
Therefore, this study aimed to investigate the effect of lycopene on LPS-induced liver injury and the relevant mechanisms involving oxidative stress and pro-inflammation.
Materials and methods
Materials
Lipopolysaccharide (LPS) was purchased from Sigma (St. Louis, USA). Elisa mice interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) kits were purchased from Hefei Bomei Biotechnology CO., LTD (Hefei China). SOD and MDA commercially available kits were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Primary polyclonal antibodies β-actin, HO-1, TNF-α, IL-6, COX-2, NF-κB, p-NF-κB, ERK1/2, p-ERK1/2 and Nrf2 were purchased from Bio Basic Inc., Canada.
Animals
C57bl/6 mice (20±2 g) were obtained from the Animal Experimental Center in Wannan Medical College. Animal experiments obeyed Chinese Community Guidelines for the use of experimental animals. The mice were raised with a standard animal facility for acclimatization. After 1 weeks, the animals were randomly assigned to three groups (10 mice per group): sham control group (S-C), LPS control group (L-C), lycopene treatment group (L-T). The mice from lycopene treatment group received lycopene treatment by oral administration for 2 weeks, and the others were treated with solvent. Then, the L-C and L-T mice were intraperitoneally injected with LPS (20 mg·kg-1) dissolved in sterile saline, and the S-C mice received sterile saline. After 6 hours, animals were anesthetized with sodium pentobarbital (50 mg/kg); the livers were collected, stored in liquid nitrogen and partially fixed with 4% neutral formalin. Blood samples were obtained for biochemical analyses.
Estimation of liver function
To estimate the effect of Lycopene on liver function, aspartate transaminase (AST) and alanine transaminase (ALT) in plasma were detected by an automated biochemical analyzer.
Determination of inflammatory cytokines
Levels of TNF-α, and IL-6 were determined by mice TNF-α and IL-6 specific ELISA kits according to the instructions. Their levels were expressed as ng/L, respectively.
Change of antioxidation
Activity of antioxidases such as superoxide dismutase (SOD), and level of malondialdehyde (MDA) were measured by commercially available kits for assessment of antioxidant effects.
Morphological analysis
Livers fixed in formalin were dehydrated, and then embedded in paraffin. Embedded livers were cut into 5-μm sections, and mounted on glass slides. Sections were stained with hematoxylin and eosin. Morphological examination was performed under a light microscope at magnifications of 400×.
Western blot
Livers were dissected out, homogenized and lysed in lysis buffer (50 mmol/L HEPES, 2 mmol/L EDTA, 100 mmol/L Na4P2O7, 100 mmol/L NaF, and 1% Triton X-100) with 0.2 mmol/L PMSF for 10 min. Homogenates were centrifuged at 13,000 g at 4°C. for 15 min. Equal amounts of protein were separated by SDS-PAGE, and then electrophoretically transferred to nitrocellulose membranes. Subsequently, the membranes were incubated with primary rabbit antibodies including β-actin (1:1000), HO-1 (1:1000), TNF-α, IL-6, p-ERK1/2, ERK, COX-2, NFκB, p-NFκB, and Nrf 2 (Bio Basic Inc., Canada) dissolved in TBS-T (10 mmol/L Tis-HCl, 150 mmol/L NaCl, and 1% Tween 20) containing 5% nonfat milk overnight at 4°C, respectively. After incubated with goat anti-rabbit secondary antibody, the membranes were rinsed, and then used to detect the immunoreactive bands by visualization with DAB (Bio Basic Inc., Canada).
Statistics
Data were expressed as mean ± standard deviation (SD). Statistical analysis was performed by SPSS16.0. Statistical difference was analyzed by Tukey’s test for unpaired data and one-way Analysis of Variance (ANOVA), followed by Bonferroni’s post-test. A value of P<0.05 was considered significant.
Results
Lycopene attenuates LPS-induced liver injury
Serum levels of AST and ALT were determined for assessment of liver injury. Levels of AST and ALT in the L-C mice were significantly increased compared with the S-C (P<0.01) (Figure 1). Preconditioning of lycopene decreased levels of AST and ALT (P<0.01) (Figure 1).
Figure 1.
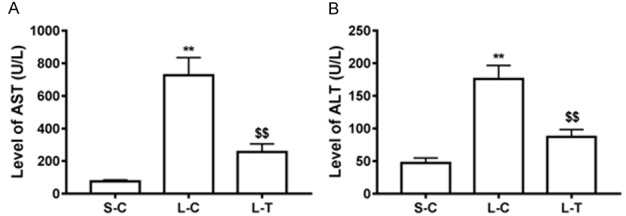
Effect of lycopene on liver function. Activity of AST (A) and ALT (B) in serum was measured. Levels of AST (A) and ALT (B) are shown as means and standard deviation. **P<0.01 vs. S-C; $$P<0.01 vs. L-C.
To further investigate LPS-induced liver injury, morphology of liver tissues was observed. Liver histological sections of the L-C mice showed the infiltration of inflammatory cells, and necrosis of liver cells (Figure 2). Lycopene treatment improved architecture of tissue, and reduced congestion in the L-T mice. Fewer inflammatory cells and intact lobular structure were observed in the L-T mice (Figure 2).
Figure 2.
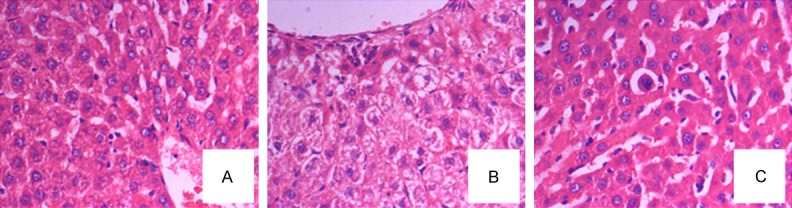
Observation of morphology of liver tissues. A. Sham control mice; B. LPS control mice; C. treated mice with lycopene.
Effect of lycopene on oxidative stress
To assess the effect of lycopene on oxidative stress, we first determined level of MDA, and activity of SOD in serum. Level of MDA was increased in the L-C mice compared with the S-C (P<0.01) (Figure 3), while activity of SOD was reduced in the L-C mice (P<0.01) (Figure 3). However, administration of lycopene prior to PLS decreased level of MDA, and increased activity of SOD compared to LPS alone (P<0.01) (Figure 3). Further, we measured expression of antioxidase such as HO-1, and observed that expression of HO-1 was significantly increased in the L-T mice compared with the L-C (P<0.01) (Figure 3).
Figure 3.
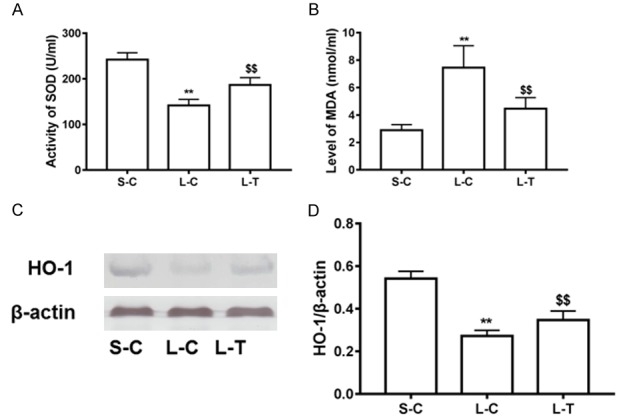
Improvement of lycopene on antioxidant effect. Activity of SOD (A) and content of MDA (B) were determined. Protein expression of HO-1 (C) was evaluated in liver tissues with western blot. Relative Level of HO-1 (D) was expressed as means and standard deviation. **P<0.01 vs. S-C; $$P<0.01 vs. L-C.
Lycopene decreases levels of pro-inflammatory cytokines
Pro-inflammatory cytokines play a pivotal role in LPS-induced liver injury, thus we investigated the effect of lycopene on production of pro-inflammatory cytokines. The results showed that levels of pro-inflammatory cytokines such as TNF-α and IL-6 in serum were significantly increased in the L-C mice compared with the S-C (P<0.01) (Figure 4). Pretreatment of lycopene reduced the increase in levels of TNF-α and IL-6 (P<0.01) (Figure 4). Second, expression of TNF-α and IL-6 in liver tissues was significantly decreased in the L-T mice compared with the L-C (P<0.01) (Figure 4).
Figure 4.
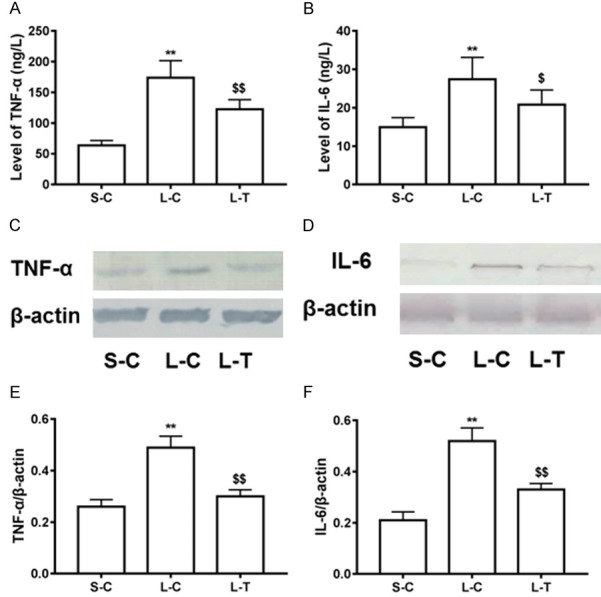
Effect of lycopene on inflammatory cytokines. Levels of TNF-α (A) and IL-6 (B) in serum were measured. Protein expression of TNF-α (C) and IL-6 (D) were determined in liver tissues by western blot. Relative levels of TNF-α (E) and IL-6 (F) were expressed as means and standard deviation. **P<0.01 vs. S-C; $P<0.05 and $$P<0.01 vs. L-C.
Effect of lycopene on p-ERK1/2, COX-2, p-NFκB, and Nrf 2 protein expression
To further explore the mechanism of lycopene treatment in LPS-induced liver injury, we determined expression of COX-2 and Nrf 2 protein, and phosphorylated levels of NF-κB and ERK1/2. The result showed that expression of COX-2, and phosphorylated levels of NF-κB and ERK1/2 were increased in the L-C mice compared with the S-C (P<0.01) (Figure 5), while expression of Nrf 2 was reduced (P<0.01) (Figure 5). Pretreatment by lycopene increased expression of Nrf 2, and decreased expression of COX2 and phosphorylated levels of NF-κB and ERK1/2 (P<0.01) (Figure 5).
Figure 5.
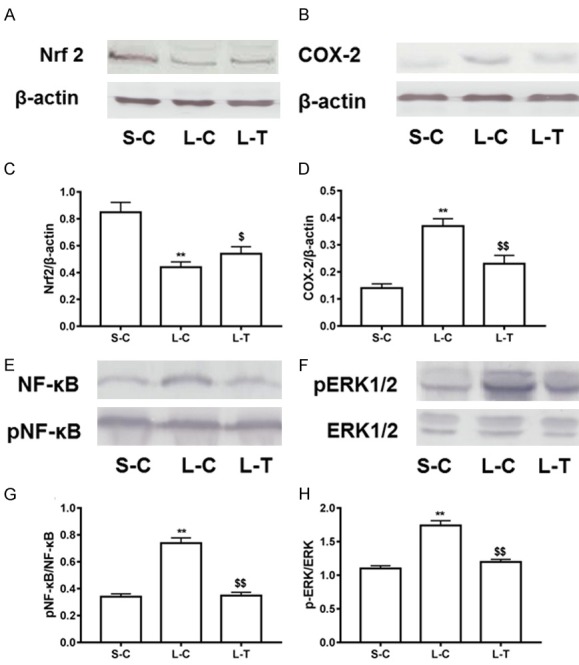
Regulation of lycopene on inflammation and antioxidant signaling. Protein levels of Nrf 2 (A) and COX-2 (B), and phosphorylated levels of NF-κB (E) and ERK (F) were detected in liver tissues by western blot. Relative expression levels Nrf 2 (C) and COX-2 (D), and relative phosphorylation levels of NF-κB (G) and ERK (H) were expressed as means and standard deviation. **P<0.01 vs. S-C; $$P<0.01 vs. L-C.
Discussion
In present study, we investigated the effect of lycopene on LPS-induced liver injury. Our results that lycopene attenuated LPS-induced acute liver injury by reducing oxidative stress and inflammatory response. Further, lycopene increased expression of Nrf2, and decreased expression of COX-2 and phosphorylation of NF-κB and ERK1/2.
Sepsis resulting from infection is a leading cause of mortality. Liver damage is a contributor of mortality caused by sepsis. LPS is found to play an important role in the pathogenesis of infection [28], and the study confirmed that a small dose of LPS led to fatal liver damage in mice [29]. LPS result in tissue injury by excessive inflammation, the elevation of oxidative stress and mitochondrial impairment [30]. Küpffer cells activated by LPS by binding with Toll-like receptor 4 (TLR-4) can excessively release pro-inflammatory cytokines and generate a tremendous amount of ROS, which triggers apoptosis of liver cells, even necrosis [31]. Therefore, oxidative stress and inflammation are involved in LPS-induced liver injury [2]. Some studies showed that various antioxidants can prevent LPS-induced liver injury and oxidative stress [2,32-34].
Oxidative stress features an imbalance between oxidants and antioxidants such as excessive consumption of antioxidants and overproduction of ROS [35]. Superoxide, one of the most common ROS, is transformed to hydrogen peroxide catalyzed by SOD, then further to hydrogen peroxide to water by peroxidases such as catalase and glutathione peroxidases [36]. Depletion of antioxidant enzyme such as SOD, catalase and glutathione peroxidase results in overproduction of ROS including superoxide, which damage macromolecules and increase lipid peroxide [37].
Evidence suggested that lycopene supplements elevated activity of the antioxidant enzymes, decreased lipid peroxidation, and attenuated hepatic steatosis [38,39]. Further, lycopene and its metabolite regulated transcription systems and cell signaling pathways [40]. In present study, our results showed that injection of LPS increased levels of AST and ALT (markers of liver injury) in serum, and the infiltration of inflammatory cells was observed in section of liver tissues. Consistent with previous study, LPS treatment decreased activity of SOD, and increased content of MDA. Lycopene pretreatment increased activity of SOD, and decreased content of MDA in serum. Further, lycopene increased expression of antioxidant enzymes such as HO-1 in liver.
Excessive release of pro-inflammatory cytokines is implicated in various acute and chronic disease such as trauma, sepsis, and chronic vascular disease [41]. Pro-inflammatory cytokines is involved in pathogenesis of acute and chronic liver injury [5]. In nonalcoholic liver disease, pro-inflammatory cytokines induced by LPS aggravated liver injury by accelerating apoptosis of liver cells, and was closely associated with severity of liver injury [42]. Our study suggested that lycopene pretreatment decreased levels of serum TNF-α and IL-6, and reduced expression of TNF-α and IL-6 in liver. Previous study confirmed that lycopene attenuated inflammation, and reduced secretion of C-reactive protein (CRP) [24,25].
It is well known that inflammatory response and oxidative stress are closely associated. It has been reported that ROS resulting from oxidative stress regulated inflammatory cytokines by NFκB signaling [43]. NF-κB is a transcription factor which can be activated under oxidative stress status. Activated NF-κB regulated the encoding of various genes such as inflammatory cytokines, and chemokine through upregulation of COX-2, which lead to inflammation [44,45]. It has been reported that LPS regulates inflammatory response by activating NF-κB [46]. Mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) is involved in LPS-induced inflammation [47]. ERK activated by phosphorylation mediates translocation of NF-κB to nuclear, and COX-2 [48,49].
Cyclooxygenase 2 (COX2) is critical for regulation of inflammation signaling, and regarded as the target of drugs. COX-2 can catalyze synthesis of prostaglandins [50,51], which play a vital role in various physiological processes [52]. Some work suggests that prostaglandins are involved in generation of inflammatory cytokines [53]. HO-1, an antioxidant enzyme, can decompose heme into biliverdin, carbon monoxide (CO), and free iron. Catalytic products of HO-1 such as biliverdin and CO have been demonstrated to possess antioxidation [54,55], and CO can reduce inflammatory response and apoptosis of cells [56,57]. Further study showed that increased expression of HO-1 attenuates oxidative stress damage to cells and tissue [58-60]. It has been reported that CO exerts anti-inflammatory effect by modulating inflammatory signaling including NK-κB, and attenuates LPS-induced inflammatory response through reduction of NK-κB [61,62].
Nrf2 is a vital transcription factor which mediates expression of various antioxidases including HO-1 [63,64]. Under oxidative stress, activated Nrf2 translocates into the nucleus, and modulates expression of antioxidant-related genes at transcriptional level [65]. Nrf2 plays a vital role in antioxidant defense systems by reducing inflammation and oxidative stress [66]. In this study, our data showed that lycopene pretreatment increased Nrf2 expression, and decreased expression of COX-2, and phosphorylation of ERK and NF-κB. These findings suggested that the protective effect of lycopene against LPS-induced liver injury may be associated with suppression of NF-κB/COX-2 by Nrf2/HO-1 activation.
In conclusion, our results suggest that lycopene protected liver against LPS-induced injury. Lycopene exerted its beneficial effect by reducing oxidative stress and inflammation damage, which may be associated with upregulation of Nrf2/HO-1 signaling pathway, and inhibition of LPS-activated inflammatory signaling (NF-κB/COX-2). Although the exact mechanisms called for further elaboration, our results support a preventive effect of lycopene in acute liver injury.
Acknowledgements
This work was supported by The Natural Science Foundation of Wannan Medical College (No. WK201603).
Disclosure of conflict of interest
None.
References
- 1.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 2.Ajuwon OR, Oguntibeju OO, Marnewick JL. Amelioration of lipopolysaccharide-induced liver injury by aqueous rooibos (Aspalathus linearis) extract via inhibition of pro-inflammatory cytokines and oxidative stress. BMC Complement Altern Med. 2014;14:392. doi: 10.1186/1472-6882-14-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayraktar O, Tekin N, Aydin O, Akyuz F, Musmul A, Burukoglu D. Effects of S-allyl cysteine on lung and liver tissue in a rat model of lipopolysaccharide-induced sepsis. Naunyn Schmiedebergs Arch Pharmacol. 2015;388:327–335. doi: 10.1007/s00210-014-1076-z. [DOI] [PubMed] [Google Scholar]
- 4.Soares JB, Pimentel-Nunes P, Roncon-Albuquerque R, Leite-Moreira A. The role of lipopolysaccharide/toll-like receptor 4 signaling in chronic liver diseases. Hepatol Int. 2010;4:659–672. doi: 10.1007/s12072-010-9219-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nolan JP. The role of intestinal endotoxin in liver injury: a long and evolving history. Hepatology. 2010;52:1829–1835. doi: 10.1002/hep.23917. [DOI] [PubMed] [Google Scholar]
- 6.Gao LN, Cui YL, Wang QS, Wang SX. Amelioration of Danhong injection on the lipopolysaccharide-stimulated systemic acute inflammatory reaction via multi-target strategy. J Ethnopharmacol. 2013;149:772–782. doi: 10.1016/j.jep.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 7.Lin RS, Lee FY, Lee SD, Tsai YT, Lin HC, Lu RH, Hsu WC, Huang CC, Wang SS, Lo KJ. Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J Hepatol. 1995;22:165–172. doi: 10.1016/0168-8278(95)80424-2. [DOI] [PubMed] [Google Scholar]
- 8.Chan CC, Hwang SJ, Lee FY, Wang SS, Chang FY, Li CP, Chu CJ, Lu RH, Lee SD. Prognostic value of plasma endotoxin levels in patients with cirrhosis. Scand J Gastroenterol. 1997;32:942–946. doi: 10.3109/00365529709011206. [DOI] [PubMed] [Google Scholar]
- 9.Sun S, Zhang H, Xue B, Wu Y, Wang J, Yin Z, Luo L. Protective effect of glutathione against lipopolysaccharide-induced inflammation and mortality in rats. Inflamm Res. 2006;55:504–510. doi: 10.1007/s00011-006-6037-7. [DOI] [PubMed] [Google Scholar]
- 10.Sebai H, Ben-Attia M, Sani M, Aouani E, Ghanem-Boughanmi N. Protective effect of resveratrol on acute endotoxemia-induced nephrotoxicity in rat through nitric oxide independent mechanism. Free Radic Res. 2008;42:913–920. doi: 10.1080/10715760802555577. [DOI] [PubMed] [Google Scholar]
- 11.Sattler W, Christison J, Stocker R. Cholesterylester hydroperoxide reducing activity associated with isolated high- and low-density lipoproteins. Free Radic Biol Med. 1995;18:421–429. doi: 10.1016/0891-5849(94)00170-o. [DOI] [PubMed] [Google Scholar]
- 12.Wang XD. Lycopene metabolism and its biological significance. Am J Clin Nutr. 2012;96:1214S–1222S. doi: 10.3945/ajcn.111.032359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lian F, Smith DE, Ernst H, Russell RM, Wang XD. Apo-10’-lycopenoic acid inhibits lung cancer cell growth in vitro, and suppresses lung tumorigenesis in the A/J mouse model in vivo. Carcinogenesis. 2007;28:1567–1574. doi: 10.1093/carcin/bgm076. [DOI] [PubMed] [Google Scholar]
- 14.Chung J, Koo K, Lian F, Hu KQ, Ernst H, Wang XD. Apo-10’-lycopenoic acid, a lycopene metabolite, increases sirtuin 1 mRNA and protein levels and decreases hepatic fat accumulation in ob/ob mice. J Nutr. 2012;142:405–410. doi: 10.3945/jn.111.150052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Mascio P, Kaiser S, Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch Biochem Biophys. 1989;274:532–538. doi: 10.1016/0003-9861(89)90467-0. [DOI] [PubMed] [Google Scholar]
- 16.Khachik F, Carvalho L, Bernstein PS, Muir GJ, Zhao DY, Katz NB. Chemistry, distribution, and metabolism of tomato carotenoids and their impact on human health. Exp Biol Med (Maywood) 2002;227:845–851. doi: 10.1177/153537020222701002. [DOI] [PubMed] [Google Scholar]
- 17.Palozza P, Catalano A, Simone RE, Mele MC, Cittadini A. Effect of lycopene and tomato products on cholesterol metabolism. Ann Nutr Metab. 2012;61:126–134. doi: 10.1159/000342077. [DOI] [PubMed] [Google Scholar]
- 18.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 19.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Visioli F, Riso P, Grande S, Galli C, Porrini M. Protective activity of tomato products on in vivo markers of lipid oxidation. Eur J Nutr. 2003;42:201–206. doi: 10.1007/s00394-003-0415-5. [DOI] [PubMed] [Google Scholar]
- 21.Devaraj S, Mathur S, Basu A, Aung HH, Vasu VT, Meyers S, Jialal I. A dose-response study on the effects of purified lycopene supplementation on biomarkers of oxidative stress. J Am Coll Nutr. 2008;27:267–273. doi: 10.1080/07315724.2008.10719699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porrini M, Riso P, Brusamolino A, Berti C, Guarnieri S, Visioli F. Daily intake of a formulated tomato drink affects carotenoid plasma and lymphocyte concentrations and improves cellular antioxidant protection. Br J Nutr. 2005;93:93–99. doi: 10.1079/bjn20041315. [DOI] [PubMed] [Google Scholar]
- 23.Kim JY, Paik JK, Kim OY, Park HW, Lee JH, Jang Y. Effects of lycopene supplementation on oxidative stress and markers of endothelial function in healthy men. Atherosclerosis. 2011;215:189–195. doi: 10.1016/j.atherosclerosis.2010.11.036. [DOI] [PubMed] [Google Scholar]
- 24.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC Jr, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the centers for disease control and prevention and the American heart association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 25.Myers GL, Christenson RH, Cushman M, Ballantyne CM, Cooper GR, Pfeiffer CM, Grundy SM, Labarthe DR, Levy D, Rifai N, Wilson PW. National academy of clinical biochemistry laboratory medicine practice guidelines: emerging biomarkers for primary prevention of cardiovascular disease. Clin Chem. 2009;55:378–384. doi: 10.1373/clinchem.2008.115899. [DOI] [PubMed] [Google Scholar]
- 26.Armoza A, Haim Y, Bashiri A, Wolak T, Paran E. Tomato extract and the carotenoids lycopene and lutein improve endothelial function and attenuate inflammatory NF-kappaB signaling in endothelial cells. J Hypertens. 2013;31:521–529. doi: 10.1097/HJH.0b013e32835c1d01. discussion 529. [DOI] [PubMed] [Google Scholar]
- 27.Gouranton E, Thabuis C, Riollet C, Malezet-Desmoulins C, El Yazidi C, Amiot MJ, Borel P, Landrier JF. Lycopene inhibits proinflammatory cytokine and chemokine expression in adipose tissue. J Nutr Biochem. 2011;22:642–648. doi: 10.1016/j.jnutbio.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 28.Westphal M, Stubbe H, Sielenkamper AW, Borgulya R, Van Aken H, Ball C, Bone HG. Terlipressin dose response in healthy and endotoxemic sheep: impact on cardiopulmonary performance and global oxygen transport. Intensive Care Med. 2003;29:301–308. doi: 10.1007/s00134-002-1546-5. [DOI] [PubMed] [Google Scholar]
- 29.Ferluga J, Allison AC. Role of mononuclear infiltrating cells in pathogenesis of hepatitis. Lancet. 1978;2:610–611. doi: 10.1016/s0140-6736(78)92828-3. [DOI] [PubMed] [Google Scholar]
- 30.Lowes DA, Webster NR, Murphy MP, Galley HF. Antioxidants that protect mitochondria reduce interleukin-6 and oxidative stress, improve mitochondrial function, and reduce biochemical markers of organ dysfunction in a rat model of acute sepsis. Br J Anaesth. 2013;110:472–480. doi: 10.1093/bja/aes577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Gao LN, Cui YL, Jiang HL. Protective effect of danhong injection on acute hepatic failure induced by lipopolysaccharide and d-galactosamine in mice. Evid Based Complement Alternat Med. 2014;2014:153902. doi: 10.1155/2014/153902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park EJ, Cheenpracha S, Chang LC, Pezzuto JM. Suppression of cyclooxygenase-2 and inducible nitric oxide synthase expression by epimuqubilin A via IKK/IkappaB/NF-kappaB pathways in lipopolysaccharide-stimulated RAW 264.7 cells. Phytochem Lett. 2011;4:426–431. doi: 10.1016/j.phytol.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozkaya MO, Naziroglu M. Multivitamin and mineral supplementation modulates oxidative stress and antioxidant vitamin levels in serum and follicular fluid of women undergoing in vitro fertilization. Fertil Steril. 2010;94:2465–2466. doi: 10.1016/j.fertnstert.2010.01.066. [DOI] [PubMed] [Google Scholar]
- 34.Kao ES, Hsu JD, Wang CJ, Yang SH, Cheng SY, Lee HJ. Polyphenols extracted from Hibiscus sabdariffa L. inhibited lipopolysaccharide-induced inflammation by improving antioxidative conditions and regulating cyclooxygenase-2 expression. Biosci Biotechnol Biochem. 2009;73:385–390. doi: 10.1271/bbb.80639. [DOI] [PubMed] [Google Scholar]
- 35.Klein T, Neuhaus K, Reutter F, Nusing RM. Generation of 8-epi-prostaglandin F(2alpha) in isolated rat kidney glomeruli by a radical-independent mechanism. Br J Pharmacol. 2001;133:643–650. doi: 10.1038/sj.bjp.0704111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown KK, Cox AG, Hampton MB. Mitochondrial respiratory chain involvement in peroxiredoxin 3 oxidation by phenethyl isothiocyanate and auranofin. FEBS Lett. 2010;584:1257–1262. doi: 10.1016/j.febslet.2010.02.042. [DOI] [PubMed] [Google Scholar]
- 37.Sewerynek E, Melchiorri D, Chen L, Reiter RJ. Melatonin reduces both basal and bacterial lipopolysaccharide-induced lipid peroxidation in vitro. Free Radic Biol Med. 1995;19:903–909. doi: 10.1016/0891-5849(95)00101-3. [DOI] [PubMed] [Google Scholar]
- 38.Ahn J, Lee H, Jung CH, Ha T. Lycopene inhibits hepatic steatosis via microRNA-21-induced downregulation of fatty acid-binding protein 7 in mice fed a high-fat diet. Mol Nutr Food Res. 2012;56:1665–1674. doi: 10.1002/mnfr.201200182. [DOI] [PubMed] [Google Scholar]
- 39.Choi SK, Seo JS. Lycopene supplementation suppresses oxidative stress induced by a high fat diet in gerbils. Nutr Res Pract. 2013;7:26–33. doi: 10.4162/nrp.2013.7.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharoni Y, Linnewiel-Hermoni K, Zango G, Khanin M, Salman H, Veprik A, Danilenko M, Levy J. The role of lycopene and its derivatives in the regulation of transcription systems: implications for cancer prevention. Am J Clin Nutr. 2012;96:1173S–1178S. doi: 10.3945/ajcn.112.034645. [DOI] [PubMed] [Google Scholar]
- 41.Popa C, Netea MG, van Riel PL, van der Meer JW, Stalenhoef AF. The role of TNF-alpha in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J Lipid Res. 2007;48:751–762. doi: 10.1194/jlr.R600021-JLR200. [DOI] [PubMed] [Google Scholar]
- 42.Fukui H. Relation of endotoxin, endotoxin binding proteins and macrophages to severe alcoholic liver injury and multiple organ failure. Alcohol Clin Exp Res. 2005;29:172S–179S. doi: 10.1097/01.alc.0000189278.30237.e9. [DOI] [PubMed] [Google Scholar]
- 43.Naik E, Dixit VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med. 2011;208:417–420. doi: 10.1084/jem.20110367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 45.Nakao S, Ogata Y, Shimizu-Sasaki E, Yamazaki M, Furuyama S, Sugiya H. Activation of NFkappaB is necessary for IL-1beta-induced cyclooxygenase-2 (COX-2) expression in human gingival fibroblasts. Mol Cell Biochem. 2000;209:113–118. doi: 10.1023/a:1007155525020. [DOI] [PubMed] [Google Scholar]
- 46.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 47.Jang SI, Kim HJ, Kim YJ, Jeong SI, You YO. Tanshinone IIA inhibits LPS-induced NF-kappaB activation in RAW 264.7 cells: possible involvement of the NIK-IKK, ERK1/2, p38 and JNK pathways. Eur J Pharmacol. 2006;542:1–7. doi: 10.1016/j.ejphar.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 48.Kregel KC, Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol. 2007;292:R18–36. doi: 10.1152/ajpregu.00327.2006. [DOI] [PubMed] [Google Scholar]
- 49.Jawan B, Kao YH, Goto S, Pan MC, Lin YC, Hsu LW, Nakano T, Lai CY, Sun CK, Cheng YF, Tai MH, Eng HL, Wang CS, Huang CJ, Lin CR, Chen CL. Propofol pretreatment attenuates LPS-induced granulocyte-macrophage colony-stimulating factor production in cultured hepatocytes by suppressing MAPK/ERK activity and NF-kappaB translocation. Toxicol Appl Pharmacol. 2008;229:362–373. doi: 10.1016/j.taap.2008.01.044. [DOI] [PubMed] [Google Scholar]
- 50.Fletcher JR. Eicosanoids. Critical agents in the physiological process and cellular injury. Arch Surg. 1993;128:1192–1196. doi: 10.1001/archsurg.1993.01420230020003. [DOI] [PubMed] [Google Scholar]
- 51.Williams JA, Shacter E. Regulation of macrophage cytokine production by prostaglandin E2. Distinct roles of cyclooxygenase-1 and -2. J Biol Chem. 1997;272:25693–25699. doi: 10.1074/jbc.272.41.25693. [DOI] [PubMed] [Google Scholar]
- 52.Richardson C, Emery P. The clinical implications of inhibition of the inducible form of cyclo-oxygenase. Drug Saf. 1996;15:249–260. doi: 10.2165/00002018-199615040-00003. [DOI] [PubMed] [Google Scholar]
- 53.Rola-Pleszczynski M, Stankova J. Cytokine gene regulation by PGE(2), LTB(4) and PAF. Mediators Inflamm. 1992;1:5–8. doi: 10.1155/S0962935192000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 55.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 56.Wang XM, Kim HP, Nakahira K, Ryter SW, Choi AM. The heme oxygenase-1/carbon monoxide pathway suppresses TLR4 signaling by regulating the interaction of TLR4 with caveolin-1. J Immunol. 2009;182:3809–3818. doi: 10.4049/jimmunol.0712437. [DOI] [PubMed] [Google Scholar]
- 57.Chhikara M, Wang S, Kern SJ, Ferreyra GA, Barb JJ, Munson PJ, Danner RL. Carbon monoxide blocks lipopolysaccharide-induced gene expression by interfering with proximal TLR4 to NF-kappaB signal transduction in human monocytes. PLoS One. 2009;4:e8139. doi: 10.1371/journal.pone.0008139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lancel S, Hassoun SM, Favory R, Decoster B, Motterlini R, Neviere R. Carbon monoxide rescues mice from lethal sepsis by supporting mitochondrial energetic metabolism and activating mitochondrial biogenesis. J Pharmacol Exp Ther. 2009;329:641–648. doi: 10.1124/jpet.108.148049. [DOI] [PubMed] [Google Scholar]
- 59.Morse D, Lin L, Choi AM, Ryter SW. Heme oxygenase-1, a critical arbitrator of cell death pathways in lung injury and disease. Free Radic Biol Med. 2009;47:1–12. doi: 10.1016/j.freeradbiomed.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kapturczak MH, Wasserfall C, Brusko T, Campbell-Thompson M, Ellis TM, Atkinson MA, Agarwal A. Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse. Am J Pathol. 2004;165:1045–1053. doi: 10.1016/S0002-9440(10)63365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morse D, Pischke SE, Zhou Z, Davis RJ, Flavell RA, Loop T, Otterbein SL, Otterbein LE, Choi AM. Suppression of inflammatory cytokine production by carbon monoxide involves the JNK pathway and AP-1. J Biol Chem. 2003;278:36993–36998. doi: 10.1074/jbc.M302942200. [DOI] [PubMed] [Google Scholar]
- 62.Sarady JK, Otterbein SL, Liu F, Otterbein LE, Choi AM. Carbon monoxide modulates endotoxin-induced production of granulocyte macrophage colony-stimulating factor in macrophages. Am J Respir Cell Mol Biol. 2002;27:739–745. doi: 10.1165/rcmb.4816. [DOI] [PubMed] [Google Scholar]
- 63.Choi EM, Suh KS, Kim YJ, Hong SM, Park SY, Chon S. Glabridin alleviates the toxic effects of methylglyoxal on osteoblastic MC3T3-E1 cells by increasing expression of the glyoxalase system and Nrf2/HO-1 signaling and protecting mitochondrial function. J Agric Food Chem. 2016;64:226–235. doi: 10.1021/acs.jafc.5b05157. [DOI] [PubMed] [Google Scholar]
- 64.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Johnson JA, Johnson DA, Kraft AD, Calkins MJ, Jakel RJ, Vargas MR, Chen PC. The Nrf2-ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration. Ann N Y Acad Sci. 2008;1147:61–69. doi: 10.1196/annals.1427.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hwang YP, Jeong HG. Ginsenoside Rb1 protects against 6-hydroxydopamine-induced oxidative stress by increasing heme oxygenase-1 expression through an estrogen receptor-related PI3K/Akt/Nrf2-dependent pathway in human dopaminergic cells. Toxicol Appl Pharmacol. 2010;242:18–28. doi: 10.1016/j.taap.2009.09.009. [DOI] [PubMed] [Google Scholar]


