Abstract
Background
Interleukin-18 (IL-18) is involved in endothelial activation and dysfunction, and in the pathogenesis and severity of acute graft-versus-host disease (aGVHD). Its relevance for patient outcome after allogeneic stem cell transplantation (alloSCT) has not yet been comprehensively addressed.
Methods
Pre-transplant serum levels of free IL-18 were retrospectively assessed in a cohort of 589 patients (training cohort). Results were validated in 688 patients allografted in a different centre. The primary endpoint was overall survival (OS). Secondary endpoints included incidences of non-relapse mortality (NRM), relapse, and aGVHD.
Findings
In the training cohort, higher pre-transplant levels of free IL-18 were significantly associated with worse OS (hazard ratio [HR] per 1-log2 increase, 1.25, P = 0.008) in multivariable models. This was due to a higher hazard of NRM (HR per 1-log2 increase, 1.39, P = 0.001), rather than relapse. The associations of pre-transplant free IL-18 with higher NRM (HR per 1-log2 increase, 1.24, P = 0.02) and shorter OS (HR per 1-log2 increase, 1.22, P = 0.006) were confirmed in the validation cohort. In both cohorts, the correlations of higher pre-transplant free IL-18 serum levels with increased NRM and worse OS were mainly driven by fatal infectious complications. No associations with incidence of aGVHD were observed.
Interpretation
Higher pre-transplant levels of free IL-18 were associated with non-relapse and overall mortality after alloSCT. Our results may provide a rationale for prospective studies evaluating IL-18 status and inhibition of IL-18 activity in patients undergoing allografting.
Keywords: Interleukin-18, Allogeneic stem cell transplantation, Outcome, Mortality
Research in context
Evidence before this study
Treatment-associated mortality still poses a major challenge for successful allogeneic stem cell transplantation (alloSCT). Acute graft-versus-host disease (aGVHD), infectious complications and other inflammatory responses are potentially fatal complications of allografted patients and major contributors to overall post-transplant mortality. Interleukin-18 (IL-18) is a pleiotropic, pro-inflammatory cytokine implicated in endothelial activation and dysfunction, and in the pathogenesis and severity of aGVHD. We searched PubMed with the terms “interleukin-18″, “interleukin-18 binding protein” “acute graft-versus-host disease”, “endothelium”, “stem cell transplantation”, “mortality”, “outcome”, and “survival”, for all articles in English published between Jan 1, 1980, and December 31, 2018. The search retrieved more than 20 studies on IL-18 in the field of alloSCT. However, most of the data on IL-18 were derived from experiments using murine transplantation models. The few clinical studies on IL-18 in allografted patients were restricted by the small numbers of individuals investigated (<40) in most series and the focus on total IL-18 rather than free IL-18. The search did not identify articles that evaluated the relevance of free IL-18 for post-transplant outcome.
Added value of this study
The present study is the first to investigate the clinical associations and address the prognostic significance of IL-18 in the setting of alloSCT. Higher levels of pre-transplant free IL-18 were associated with increased non-relapse and overall mortality. These findings, obtained on a training set of patients, were substantiated by an independent validation cohort also suggesting prognostic value in terms of overall post-transplant outcome. No associations of pre-transplant free IL-18 with incidence of aGVHD were observed.
Implications of all the available evidence
Considering recent novel and successful IL-18-neutralizing approaches in autoimmune disorders, our results provides a rationale for the design of interventional clinical studies evaluating IL-18 status and inhibition thereof in patients undergoing alloSCT.
1. Introduction
Allogeneic stem cell transplantation (alloSCT) is a highly effective treatment for a variety of haematologic malignancies, but non-relapse mortality (NRM) remains a major challenge. In addition to acute graft-versus-host disease (GVHD), severe infections and vascular events, both of which involve dysfunction of the endothelial system, may contribute to NRM.
Several studies, including our own, have focused on endothelial markers that correlate with NRM and GVHD-related mortality in order to understand why escalating immunosuppressive therapy is ineffective in a fraction of patients with acute GVHD [1], [2], [3], [4]. Consequently, we put forward the hypothesis of “endothelial vulnerability” in the setting of alloSCT [3], suggesting that pre-existing endothelial distress can deteriorate outcome after a subsequent severe endothelial challenge such as acute GVHD, without necessarily being associated with a higher incidence of GVHD.
Interleukin-18 (IL-18) is a pleiotropic, interferon (IFN)γ-inducing, pro-inflammatory and immunoregulatory cytokine, whose maturation and secretion is mediated by the inflammasome [5]. The cytokine is expressed in a variety of cells including monocytes, epithelial and endothelial cells [5,6]. IL-18 is bound by a high affinity IL-18 binding protein (IL-18BP) which in serum is present at a 20-fold molar excess over IL-18 [6]. Only unbound free IL-18 is biologically active and therefore more relevant than total IL-18, an important caveat when interpreting IL-18 concentrations and functions in vivo [5]. IL-18 induces IFNγ in different lymphocytic subsets as well as differentiation of various T cell populations. Thus, IL-18 has been implicated in the pathogenesis and severity of several immune-mediated diseases including acute GVHD [6,7]. A role for IL-18 has been shown also in general inflammatory states, most notably sepsis [8,9], but also in cardiovascular disease [10,11], metabolic syndrome and obesity [12,13].
Depending on the condition, polymorphisms of the IL-18 gene were shown to be associated with both increased and reduced IL-18 serum levels [5,14,15]. In addition, IL-18 can be released from dying endothelium [6], and de-regulation of the IL-18/IL-18BP pathway itself was also shown to cause endothelial cell dysfunction [16]. In one of our previous reports [4], pre-transplant levels of free IL-18 were positively correlated with serum levels of asymmetric dimethylarginine (ADMA). ADMA causes endothelial cell dysfunction and is an established risk factor for cardiovascular complications. In allografted patients, ADMA was associated with NRM particularly after the onset of acute GVHD supporting the concept of “endothelial vulnerability” [3].
In view of these considerations, the present retrospective study sought to investigate the impact of free IL-18 on outcome in allografted patients.
2. Patients and methods
2.1. Patients
Adult patients (age ≥18 years) consecutively allografted between 2004 and 2015 at the University Hospital Heidelberg who had provided permission for sample and data collection and had serum samples available comprised the training cohort (n = 589). The validation cohort consisted of adult patients who underwent allografting at the University Hospital Essen (Germany) between 2009 and 2013 and had pre-transplant serum samples available (n = 688). Only patients who had undergone first alloSCT were included. With regard to the corresponding treatment periods the study covers 70–80% of the overall allograft recipients in both centres. In addition, a total of 43 healthy control subjects (19 males, median age 40 years, interquartile range [IQR] 30–57) were analysed.
Written informed consent according to the Declaration of Helsinki was obtained for all patients and healthy controls, and the local ethics committees had approved the sample and data collection and analysis (reference number 120/2002). Patient and outcome data were obtained from medical records and chart review. Disease stage prior to alloSCT was assessed by applying published criteria [17].
2.2. Assessment of cytokine serum levels and single nucleotide polymorphism (SNP) analyses
Pre-transplant serum samples were collected in gel tubes (S-Monovette® Z-Gel, SARSTEDT AG & Co. KG, Nuembrecht, Germany) before alloSCT and cryopreserved at −80 °C. In the training cohort, post-transplant serum samples were also available. Serum levels of total IL-18, IL-18-binding protein A (IL-18BPa) and free IL-18 as well as C-X-C motif ligand 9 (CXCL9)/monokine induced by gamma-interferon (MIG) and soluble suppression of tumorigenecity 2 (ST2) levels were assessed prior to the start of pre-transplant conditioning in both cohorts. IL-18, IL-18BPa, and free IL-18 were determined on days 0, +3, +4, +7, +8, +12, +21, and +28 after alloSCT in the training cohort. For a detailed description of the methodology and the SNP analyses please refer to the Supplement (section on Methods).
2.3. Statistical analysis
Categorical data of patient characteristics were compared using the χ2 test. Continuous variables were compared applying the Mann-Whitney U test or the Kruskal-Wallis test. Since this is, to our knowledge, the first investigation to systematically explore free IL-18 in the setting of alloSCT in large patient cohorts, a thorough analysis of associations between levels of pre-transplant free IL-18 and patient characteristics including those reflecting performance status, inflammatory states and comorbidities prior to alloSCT (patient age and sex, disease, body-mass index [BMI], levels of C-reactive protein [CRP], Karnofsky performance status [KPS], haematopoietic cell transplantation specific comorbidity index [HCT-CI] and the comorbidities diabetes, cardiovascular disease and infection as defined by Sorror et al. [18]) was conducted in the training cohort.
Overall survival (OS), time to relapse, and NRM (death in the absence of prior relapse), as ascertained by transplant centres through regular patient follow-up, were calculated from the date of alloSCT to the appropriate endpoint. Since acute GVHD and its treatment are major contributors to post-transplant mortality, OS and NRM were also assessed after acute GVHD (i.e. from the date of onset of acute GVHD). NRM and recurrence of the underlying malignancy were considered as competing events. For the incidence of acute GVHD, chronic GVHD, and veno-occlusive disease (VOD) the competing events were relapse and death in remission without acute GVHD. Patients who received second alloSCT (<3% for both cohorts) were censored at the time of second transplant.
Due to the lack of an established reference interval of free IL-18, the associations of pre-transplant free IL-18 with post-transplant outcome were analysed considering free IL-18 a continuous variable. Since free IL-18 serum levels did not follow a normal distribution, data was log2-transformed. The effects of pre-transplant free IL-18 as continuous variable (per 1-log2 increase, i.e. doubling of serum pre-transplant free IL-18) on OS, relapse, and NRM were analysed by Cox regression models. Cox proportional hazards regression modelling was used for OS; relapse and NRM were analysed by cause-specific Cox models.
Since the proportional hazards assumption for pre-transplant free IL-18 was violated for OS after the 5th post-transplant year, the data set was restricted to the first 5-year epoch post-transplant. Since mortality following the onset of acute GVHD is most pronounced in the first year after alloSCT the observation period for the associations of pre-transplant free IL-18 with OS and NRM after acute GVHD was confined to the first year after onset of acute GVHD. To illustrate the continuous effects on the different endpoints, patients were grouped according to the quartiles of pre-transplant free IL-18. The predictive performance of pre-transplant free IL-18 within the first 5 years post-transplant was assessed by calculating the prediction error (integrated Brier score) and the concordance index [19,20].
In order to provide guidance for possible interventions in the setting of alloSCT, an optimal cut-off determination with respect to NRM was performed in the training set.
For a detailed description of the validation method and further statistical methods used for analysis, and the reasons for using the cause-specific Cox regression please see Supplement (section on Methods). All statistical tests were two-sided. Hazard ratios (HR) were estimated with 95% confidence interval (CI). P values <0.05 were considered statistically significant.
3. Results
3.1. Patient characteristics, incidence of acute GVHD, and serum levels of total IL-18, IL-18BPa and free IL-18
Patient, disease and transplant characteristics of the training and the validation cohort and the respective incidences of acute GVHD on day+100 post-transplant are given in Table 1. The estimated median follow-up time was 65.9 months (95% CI 50.1–86.7, range 0.1–159.4) in the training cohort and 32.6 months (95% CI 21.6–49.0, range 0.1–110.4) in the validation cohort.
Table 1.
Patient, disease and transplant characteristics of the training and validation cohorts.
| Training cohort | Validation cohort | P | |
|---|---|---|---|
| (n = 589) | (n = 688) | ||
| Parameter | |||
| Age [years] at alloSCT (median, IQR) | 54 (45–61) | 54 (44–61) | 0.67 |
| Patient sex, n (%) | 0.003 | ||
| Female | 227 (39) | 322 (47) | |
| Male | 362 (61) | 366 (53) | |
| Disease stage before alloSCTa, n (%) | <0.001 | ||
| Early/intermediate | 366 (62) | 517 (75) | |
| Late | 223 (38) | 171 (25) | |
| Diagnosis, n (%) | 0.001 | ||
| AML | 227 (39) | 355 (52) | |
| MDS/MPN | 94 (16) | 135 (20) | |
| ALL/Lymphoma | 200 (34) | 172 (25) | |
| MM | 68 (12) | 26 (4) | |
| Conditioningb, n (%) | 0.53 | ||
| RIC | 475 (81) | 544 (79) | |
| MAC | 114 (19) | 144 (21) | |
| Donor, n (%) | 0.18 | ||
| RD | 144 (24) | 192 (28) | |
| MUD | 303 (51) | 319 (46) | |
| MMUD | 142 (24) | 177 (26) | |
| Donor sex, n (%) | 0.44 | ||
| Female | 189 (32) | 236 (34) | |
| Male | 400 (68) | 452 (66) | |
| ATG treatment, n (%) | <0.001 | ||
| No | 176 (30) | 298 (43) | |
| Yes | 413 (70) | 390 (57) | |
| Pre-transplant free IL-18, pg/mL (median, IQR) | 470 (326–703) | 415 (284–607) | <0.001 |
| Cumulative incidence of acute GVHD on day +100 post-transplant,% (95% CI) | |||
| Any grade | 30.2 (26.6–34.2) | 77.5 (74.2–80.6) | |
| Grade 3–4 | 12.6 (10.1–15.6) | 40.3 (36.6–44.1) | |
| Cumulative incidence of VOD on day +28 post-transplant,% (95% CI) | 5.2 (3.6–7.6) | NA | |
| Cumulative incidence of chronic GVHD on year +1 post-transplant,% (95% CI) | 38.4 (34.2–43.0) | NA | |
Abbreviations: ALL, acute lymphoblastic leukaemia; AlloSCT, allogeneic stem cell transplantation; AML, acute myeloid leukaemia; ATG, antithymocyte globulin; CI, confidence interval; GVHD, graft-versus-host-disease; IL-18, interleukin-18; IQR, interquartile range; MDS, myelodysplastic syndrome; MAC: myeloablative conditioning; MM, multiple myeloma; MMUD, mismatched unrelated donor; MPN, myeloproliferative neoplasm; MUD, matched unrelated donor; NA, not available; RD, related donor; RIC: reduced intensity conditioning; VOD, veno-occlusive disease.
According to Gratwohl et al. [17].
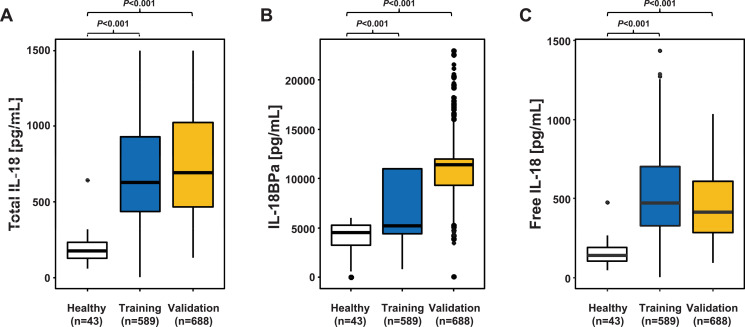
As compared to healthy control subjects, pre-conditioning serum levels of both total IL-18 and free IL-18 were significantly (approximately threefold) higher in both cohorts, but also IL-18BPa serum levels were significantly increased in patients (Fig. 1).
Fig. 1.
Comparison of median serum levels of total IL-18, IL-18BPa and free IL-18 between healthy individuals and allografted patients of both cohorts.
Median serum levels of total IL-18 (A), IL-18BPa (B), and free IL-18 (C) in healthy individuals and in allografted patients of the training and the validation cohort assessed prior to transplant. As compared to healthy individuals (median IL-18 141 pg/mL, IQR 102-189), median pre-transplant levels of free IL-18 were significantly and approximately 3-fold higher in both patients of the training cohort (470 pg/mL, IQR 326-703) and in patients of the validation cohort (415 pg/mL, IQR 284-607). Box plots are depicted. P values were derived by Mann-Whitney U test.
Abbreviations: IL-18, interleukin-18; IL-18BPa, IL-18-binding protein A.
Patient characteristics reflecting the individual's health and nutritional status prior to alloSCT, the immunosuppressive regimen post-transplant and pre-transplant levels of ST2 were only available for the training cohort and are summarised in supplemental Table S1. Median serum levels of pre-transplant free IL-18 were higher in patients with increased (>5 mg/L) pre-transplant CRP levels and in patients with lower (≤80%) KPS. Pre-transplant free IL-18 levels trended to be higher with advanced age and higher HCT-CI (for further details see Supplement, section on Results and Figure S1).
3.2. Pre-transplant serum levels of free IL-18 and outcome measures following alloSCT in the training cohort
In univariable analysis, higher levels of pre-transplant free IL-18 (per 1-log2 increase, i.e. doubling of serum pre-transplant free IL-18 concentration) were associated with a shorter OS. This was due to a significantly higher hazard of NRM and not relapse (Table 2). Pre-transplant free IL-18 showed no correlation with the incidence of acute GVHD, VOD or chronic GVHD. Increasing pre-conditioning free IL-18 serum levels tended to be associated with poorer OS and NRM after onset of acute GVHD but this did not reach statistical significance (supplemental Table S2).
Table 2.
Univariable analysis of outcome associations of pre-transplant free interleukin-18 serum levels in the training and validation cohorts.
| Training cohorta | Validation cohortb | |||
|---|---|---|---|---|
| n = 589 | n = 688 | |||
| Free IL-18 per 1-log2 increasec | Free IL-18 per 1-log2 increasec | |||
| HR 95%CI | P | HR 95%CI | P | |
| Endpoint | ||||
| OS | 1.26 (1.08–1.48) | 0.004 | 1.29 (1.12–1.48) | <0.001 |
| CHR 95%CI | P | CHR 95%CI | P | |
| NRM | 1.37 (1.06–1.76) | 0.02 | 1.33 (1.11–1.59) | 0.002 |
| Relapse | 1.11 (0.93–1.32) | 0.24 | 1.16 (0.96–1.40) | 0.12 |
| Acute GVHD any grade | 1.03 (0.88–1.19) | 0.75 | 0.92 (0.82–1.03) | 0.14 |
| Acute GVHD grade 3–4 | 1.13 (0.89–1.44) | 0.33 | 1.07 (0.92–1.26) | 0.37 |
| VOD | 0.78 (0.60–1.01) | 0.07 | – | |
| Chronic GVHD | 0.93 (0.82–1.07) | 0.31 | – | |
Abbreviations: CHR, cause-specific hazard ratio; CI, confidence interval; IL-18, interleukin-18; HR, hazard ratio; OS, overall survival; NRM, non-relapse mortality; VOD, veno-occlusive disease.
Number of events: OS, n = 268; NRM, n = 110; relapse, n = 206.
Number of events: OS, n = 375; NRM, n = 223; relapse, n = 203.
Doubling of serum pre-transplant free IL-18 concentration.
The association of higher levels of pre-transplant free IL-18 with worse OS was confirmed in the multivariable models adjusting for patient age, disease stage, diagnosis, donor type, conditioning intensity, antithymocyte globulin (ATG) treatment, and sex of donor and recipient. Again, this was based on an increased hazard of NRM rather than relapse. Other factors with a significantly adverse impact on OS were advanced disease stage, transplant from a mismatched unrelated donor, and myeloablative conditioning (Table 3). When pre-transplant free IL-18 was analysed in multivariable models including age, CRP, BMI, KPS and HCT-CI as confounding variables, the association of higher levels of pre-transplant free IL-18 with worse OS due to higher NRM remained significant, with similar effect sizes (supplemental Table S3).
Table 3.
Multivariable analysis of predictors of overall survival (OS), non-relapse mortality (NRM) and relapse after allogeneic stem cell transplantation in the training and the validation cohorts.
| Training cohorta | Validation cohortb | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
n = 589 |
n = 688 |
|||||||||||
| OS |
NRM* |
Relapse* |
OS |
NRM* |
Relapse* |
|||||||
| HR 95% CI | P | HR 95% CI | P | HR 95% CI | P | HR 95% CI | P | HR 95% CI | P | HR 95% CI | P | |
| Covariate | ||||||||||||
| Free IL-18 per 1-log2 increasec | 1.25 (1.06–1.47) | 0.008 | 1.39 (1.07–1.81) | 0.01 | 1.11 (0.93–1.32) | 0.27 | 1.22 (1.06–1.41) | 0.006 | 1.24 (1.03–1.50) | 0.02 | 1.15 (0.95–1.40) | 0.14 |
| Age per 1-year increase | 1.01 (1.00–1.02) | 0.22 | 1.03 (1.01–1.05) | 0.003 | 1.00 (0.99–1.01) | 0.76 | 1.01 (1.00–1.02) | 0.04 | 1.01 (1.00–1.03) | 0.02 | 1.00 (0.99–1.02) | 0.57 |
| Disease staged | ||||||||||||
| Early/intermediate | Ref | <0.001 | Ref | 0.53 | Ref | <0.001 | Ref | <0.001 | Ref | <0.001 | Ref | 0.05 |
| Late | 1.60 (1.24–2.05) | 1.14 (0.76–1.70) | 1.87 (1.41–2.48) | 1.72 (1.37–2.16) | 1.71 (1.28–2.29) | 1.38 (1.01–1.91) | ||||||
| Donor | ||||||||||||
| RD/MUD | Ref | 0.03 | Ref | 0.01 | Ref | 0.42 | Ref | <0.001 | Ref | <0.001 | Ref | 0.76 |
| MMUD | 1.38 (1.03–1.83) | 1.77 (1.14–2.75) | 1.15 (0.82–1.61) | 1.57 (1.26–1.97) | 1.75 (1.31–2.33) | 1.05 (0.78–1.46) | ||||||
| Donor sex | ||||||||||||
| Female | Ref | 0.80 | Ref | 0.77 | Ref | 0.70 | Ref | 0.35 | Ref | 0.95 | Ref | 0.78 |
| Male | 0.97 (0.74–1.25) | 0.94 (0.63–1.41) | 1.06 (0.79–1.44) | 0.90 (0.72–1.13) | 0.99 (0.74–1.33) | 1.04 (0.77–1.42) | ||||||
| Patient sex | ||||||||||||
| Female | Ref | 0.74 | Ref | 0.80 | Ref | 0.12 | Ref | 0.46 | Ref | 0.59 | Ref | 0.47 |
| Male | 0.96 (0.75–1.23) | 1.05 (0.71–1.56) | 0.80 (0.60–1.06) | 0.92 (0.74–1.14) | 0.93 (0.70–1.22) | 0.90 (0.67–1.20) | ||||||
| Diagnosise | ||||||||||||
| Myeloid | Ref | 0.27 | Ref | 0.13 | Ref | 0.07 | Ref | <0.001 | Ref | 0.001 | Ref | 0.11 |
| Lymphoid | 1.17 (0.89–1.53) | 1.38 (0.91–2.11) | 1.33 (0.98–1.81) | 1.52 (1.21–1.93) | 1.65 (1.22–2.23) | 1.30 (0.95–1.78) | ||||||
| ATG treatment | ||||||||||||
| No | Ref | 0.21 | Ref | 0.005 | Ref | 0.39 | Ref | 0.06 | Ref | 0.02 | Ref | 0.55 |
| Yes | 0.83 (0.63–1.11) | 0.53 (0.34–0.83) | 0.87 (0.63–1.20) | 0.81 (0.65–1.01) | 0.72 (0.54–0.95) | 0.91 (0.68–1.22) | ||||||
| Conditioningf | ||||||||||||
| MAC | Ref | 0.04 | Ref | 0.003 | Ref | 0.70 | Ref | 0.03 | Ref | 0.02 | Ref | 0.59 |
| RIC | 0.70 (0.51–0.98) | 0.46 (0.27–0.77) | 0.93 (0.63–1.37) | 1.41 (1.04–1.92) | 1.72 (1.11–2.64) | 0.90 (0.62–1.32) | ||||||
Abbreviations: ATG, antithymocyte globulin; IL-18, interleukin-18; MAC: myeloablative conditioning; MMUD, mismatched unrelated donor; MUD, matched unrelated donor; RD, related donor; RIC: reduced intensity conditioning.
Cause-specific hazards from a competing risks analysis for relapse and NRM.
Number of events: OS, n = 268; NRM, n = 110; relapse, n = 206.
Number of events: OS, n = 375; NRM, n = 223; relapse, n = 203.
Doubling of serum pre-transplant free IL-18 concentration.
According to Gratwohl et al. [17].
Myeloid: acute myeloid leukaemia, myelodysplastic and myeloproliferative syndromes; lymphoid: acute lymphoblastic leukaemia, chronic lymphocytic leukaemia, lymphoma and multiple myeloma.
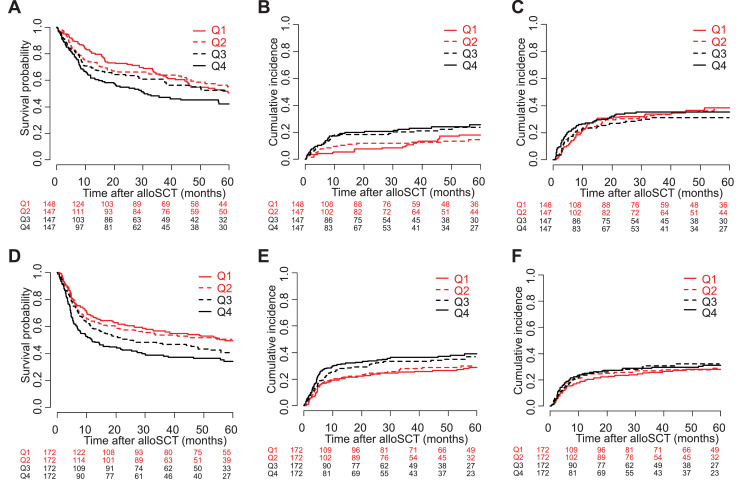
In order to illustrate the continuous associations of pre-transplant free IL-18 with all endpoints, patients were grouped according to four increasing intervals of pre-transplant free IL-18 levels determined by the quartiles of the free IL-18 distribution. The corresponding plots showing OS, NRM and relapse in the training cohort are given in Fig. 2A–C.
Fig. 2.
Outcome after allogeneic stem cell transplantation stratified according to pre-transplant free IL-18 quartiles. (A) In the training cohort, higher pre-transplant serum levels of free IL-18 were associated with lower probability of overall survival (OS). (B) Higher pre-transplant free IL-18 correlated with an increased cumulative incidence of non-relapse mortality (NRM) in the training cohort. (C) In contrast, relapse incidences were similar between the quartiles of patients stratified on the basis of pre-transplant free IL-18 in the training set. (D, E, F) In the validation cohort, a similar association of pre-transplant free IL-18 with OS, due to a higher incidence of NRM rather than relapse, was observed.
Note: Quartiles were chosen for reasons of visualization of the associations of outcome measures with continuous pre-transplant free IL-18 (assessed for every twofold change in uni- and multivariable analyses [cf. Table 2 and 3]).
Abbreviations: IL-18, interleukin-18; Q1-4, quartiles of the pre-transplant free IL-18 distribution.
3.3. Pre-transplant serum levels of free IL-18 and post-transplant outcome in the validation cohort
The results obtained in the training set could be largely replicated in the validation cohort. In univariable analysis, similar to the training cohort, higher pre-transplant free IL-18 (as a continuous variable) showed a significant association with NRM and OS but not with relapse (Table 1). Although showing no association with the incidence of acute GVHD, pre-transplant free IL-18 levels were also significantly correlated with non-relapse and overall mortality after onset of acute GVHD in the validation cohort (supplemental Table S2). The associations of higher pre-transplant free IL-18 levels with worse OS and NRM were also confirmed in the corresponding multivariable models of the validation cohort (Table 3; Fig. 2D–F).
3.4. Predictive value of pre-transplant free IL-18 and validation of the findings
The predictive performance of pre-transplant free IL-18 is described in the Supplement (supplemental Figures S2 and S3).
As regards the validation, for both OS (HR 0.98 95%CI 0.85–1.13) and NRM (HR 0.89 95%CI 0.74–1.08) the effect of pre-transplant free IL-18 estimated additionally to the offset (i.e. the effect in the model based on patients from the training cohort) was not significantly different from 1. This means the effect on non-relapse and overall mortality in the validation cohort was not significantly different from the effect in the training set and thus can be considered validated. Furthermore, the prediction ability is about the same, if the effect of pre-transplant free IL-18 was transferred from the multivariable model based on the training set and included as an offset (supplemental Figure S4).
3.5. Relationship between pre-transplant serum levels of total and free IL-18, IL-18BPa and CXCL9/MIG
IL-18 is known to induce IFNγ, and IFNγ conversely increases the gene expression and synthesis of both IL-18BP and CXCL9/MIG (illustrated in supplemental Figure S5A). Hence, we analysed the relationship between pre-transplant cytokine serum levels in both cohorts. In both training and validation cohort, IL-18 (total and free) and IL-18BPa were significantly positively correlated to CXCL9/MIG, although the correlations were only weak to moderate (supplemental Figure S5B).
3.6. Non-relapse causes of death
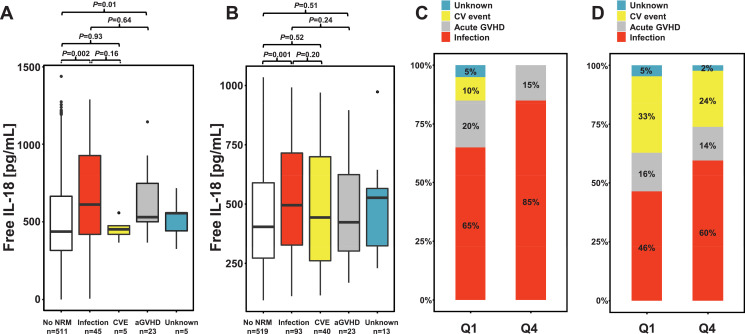
To further explore the association of pre-transplant free IL-18 with NRM a detailed analysis of non-relapse causes of death in both cohorts was performed focusing on the first post-transplant year. In the training cohort, a total of 78 non-relapse deaths occurred within the first post-transplant year. These were caused by severe infections (including sepsis) in 45 (62%), acute GVHD (i.e. lethal complications of acute GVHD and/or its treatment) in 23 (32%), and cardiovascular (CV) events (including haemorrhage) in 5 (7%) patients. In 5 patients the non-relapse cause of death could not be ascertained. In the validation cohort, a total of 169 non-relapse deaths occurred. Again, the most common non-relapse cause of death was infection/sepsis in 93 (60%) followed by CV events and lethal acute GVHD in 40 (26%) and 23 (15%) patients, respectively.
Of note, as compared to patients not succumbing to NRM, in both cohorts, serum levels of pre-transplant free IL-18 were higher in patients who died of severe infectious complications (Fig. 3A and B). When causes of NRM were compared between the first and last quartile of the pre-transplant IL-18 distribution, in both cohorts, the proportion of NRM events due to severe infections was higher in patients of the upper quartile of the pre-transplant IL-18 distribution (Fig. 3C and D).
Fig. 3.
Comparison of pre-transplant serum levels of free IL-18 according to different non-relapse causes of death of patients of the training and the validation cohort in the first post-transplant year.
Non-relapse causes of death were grouped into four categories: severe infection/sepsis, cardiovascular events (CVE, including haemorrhage), death due to acute GVHD (i.e. lethal complications of acute GVHD and/or its treatment), and unknown causes. Median pre-transplant serum levels of free IL-18 were compared to patients not succumbing to NRM and among each category.
(A, B) In both the training and the validation cohort, median pre-transplant serum levels of free IL-18 (611 and 495 pg/mL, respectively) were most pronounced in patients who succumbed to severe infectious complications. Box plots are depicted. Number of patients/events for each group is indicated. P values were derived by Mann-Whitney U test.
(C, D) When NRM causes were compared between the first (Q1) and fourth quartile (Q4) of the pre-transplant IL-18 distribution, in both cohorts, the proportion of NRM events due to severe infections was higher in patients of the upper quartile of the pre-transplant IL-18 distribution.
Abbreviations: CV, cardiovascular; GVHD, graft-versus-host disease; IL-18, interleukin-18; Q1 and Q4, first and fourth quartile of the pre-transplant free IL-18 distribution, respectively.
3.7. Pre-transplant free IL-18 and neutrophil engraftment
Data on absolute neutrophil counts (ANC) on d + 28 was available for 360 and 681 patients of the training and the validation set, respectively. In both, training and validation cohort pre-transplant free IL-18 serum levels were not associated with d + 28 ANC (Spearman's rho: 0.11, P = 0.84 and −0.06, P = 0.10, respectively).
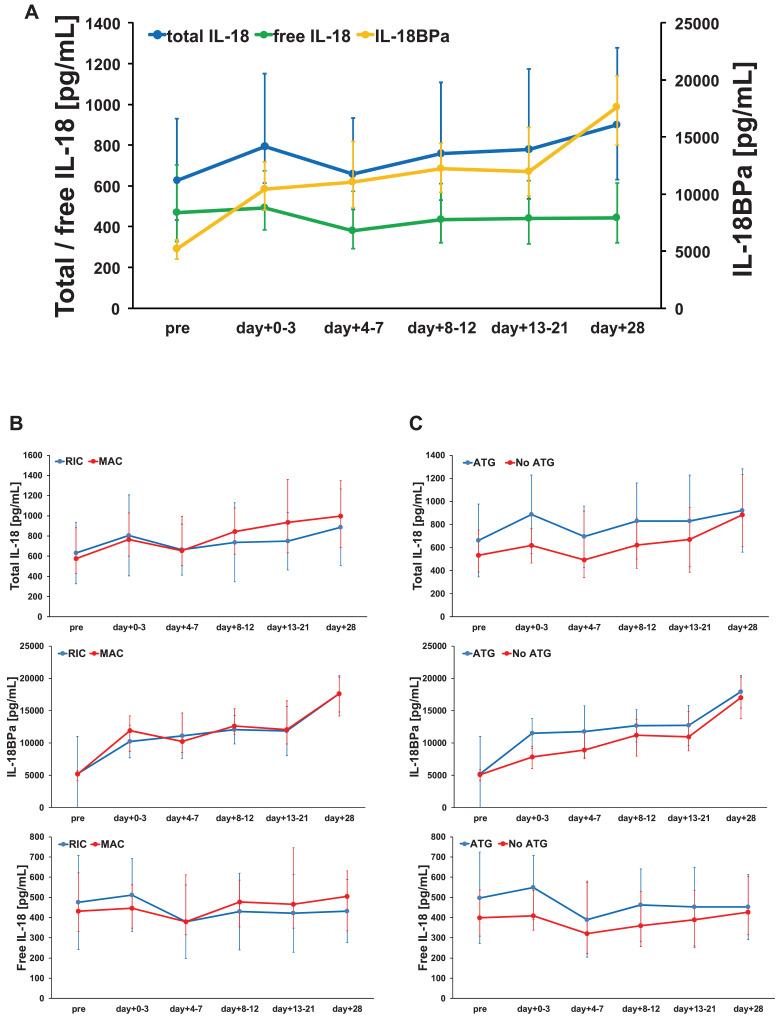
3.8. Time course of post-transplant free IL-18 serum levels in the training cohort
Consecutive pre- and post-transplant serum levels were available for 395 patients of the training cohort. When looking at the post-transplant time course, a parallel increase in both total IL-18 and IL-18BPa was observed. Consequently, the median serum levels of free IL-18 remained stable during the first 28 days after alloSCT (Fig. 4A). Conditioning intensity or ATG use did not substantially influence the time course of total IL-18, IL-18BPa and free IL-18 (Fig. 4B and C).
Fig. 4.
Time course of post-transplant free IL-18 serum levels in the training cohort (n = 395). (A) Due to a parallel increase in both total IL-18 and IL-18BPa, serum levels of free IL-18 remained relatively stable during the first 28 days after alloSCT. (B) Conditioning intensity or (C) antithymocyte globulin (ATG) use did not substantially influence the time of course of total IL-18, IL-18BPa and free IL-18.
Median serum levels are given. Error bars represent the corresponding interquartile range relative to the median value.
When comparing the distribution of the individual patients according to the quartiles of pre-transplant free IL-18 and d + 28 free IL-18, the majority of the patients remained in their respective quartiles (i.e. the majority of patients who constituted the upper two quartiles pre-transplant remained in the upper two quartiles of the IL-18 distribution on d + 28 post-transplant, see alluvial diagram in supplemental Figure S6).
Next, the performance of pre-transplant free IL-18 and d + 28 free IL-18 as continuous variables in the context of post-transplant outcome were compared. ROC and prediction error analysis indicated similar prognostic associations of pre-transplant and d + 28 IL-18 with both post-transplant OS and NRM (supplemental Figure S7). Accordingly, as compared to pre-transplant free IL-18, the univariable associations with shorter OS and higher NRM were similar for d + 28 free IL-18 as illustrated in supplemental Figure S8.
Free IL-18 serum levels measured on d + 28 post-transplant were not significantly associated with incidence of acute GVHD (any grade) and chronic GVHD in the training cohort (HR per 1-log2 increase, 1.25 95%CI 0.96–1.62, P = 0.10 and HR 0.96 95%CI 0.75–1.23, P = 0.74, respectively).
3.9. SNP analyses of the IL-18 and the IL18BP gene in the training cohort
Genomic DNA for SNP analyses was available for 395 patients of the training cohort. A total of 5 polymorphisms for IL-18 and 4 polymorphisms for IL-18BP were selected. Hardy-Weinberg equilibrium was observed for all SNPs with the exception of the IL-18BP SNP (rs3793941). A heat map from Haploview is provided in supplemental Figure S9.
Both median pre-transplant and day+28 serum levels of total IL-18, IL-18BPa and free IL-18 were not correlated to specific SNPs in the IL-18 and IL-18BP gene (supplemental Table S4 and Table S5, respectively).
3.10. Optimised cut-off of pre-transplant free IL-18
Finally, since continuous effects are less instructive and often difficult to interpret, particularly with regard to potential interventions, an optimal cut-off determination with respect to post-transplant NRM was conducted in the training set yielding multiple cut-points (maxima) (supplemental Figure S10A, details on the statistical methodology are provided in the Supplement, section on Methods).
The maximum at 470 pg/mL (=28.876) which also represents the median of the pre-transplant free IL-18 distribution, was chosen to stratify patients of both cohorts in high (≥470 pg/mL) and low (<470 pg/mL) free IL-18 groups pointing to an increased non-relapse (HR ∼1.5–1.8) and overall mortality (HR ∼1.3–1.4) in patients allografted with high pre-transplant free IL-18 (supplemental Figure S10B-E).
4. Discussion
Cytokine dysregulation and sequential activation of T-cells and monocytes contribute to acute GVHD that substantially affects mortality after alloSCT [21,22]. Hence it is not surprising that involvement of the pro-inflammatory cytokine IL-18 in acute GVHD has been demonstrated as early as two decades ago [23]. However, most prior data on IL-18 in acute GVHD were derived from murine transplantation models. Since IL-18 activity strongly depends on the immunologic context, the exact role in the pathophysiology of acute GVHD proved highly complex [7]. In particular, although serum levels of IL-18 were increased in experimental models of acute GVHD, paradoxical effects of IL-18 on the severity and outcome of acute GVHD were described [7,24]. Similarly, a correlation of IL-18 and GVHD severity was found in some alloSCT studies [25,26] but not in others [27]. Furthermore, clinical studies on IL-18 in allografted patients were difficult to interpret given the small numbers of investigated individuals (<40) in most series and the focus on total IL-18 rather than free IL-18 [25], [26], [27], [28]. Accordingly, the significance of (free) IL-18 in terms of outcome after alloSCT remained to be elucidated.
The present study, to our knowledge, is the first to systematically explore the clinical associations of pre-transplant IL-18 in the setting of alloSCT in independent large patient cohorts. Higher pre-transplant levels of free IL-18 were associated with worse survival due to increased NRM. In addition, although a significant association with acute GVHD incidence was not observed, mortality after onset of acute GVHD seemed to be correlated with pre-transplant free IL-18.
The median serum levels of total and free IL-18, and IL-18BPa observed in this study in healthy individuals are in accordance with the literature [29]. Data on free IL-18 serum levels are scarce for alloSCT patients. However, with respect to total IL-18 and IL18-BPa assessed prior to alloSCT, our results are in line with previous studies [27,30]. However, median levels of pre-transplant free IL-18 were nearly threefold higher in patients as compared to healthy controls. We can only speculate about the reasons for this difference. As pre-transplant free IL-18 levels were correlated to patient performance status and HCT-CI in the training set, IL-18 may possibly reflect a pro-inflammatory state due to a poorer overall health status in patients with haematologic malignancies as compared to healthy controls. In addition, since IL-18 may be released from dying endothelium [6], and in one of our previous reports, pre-transplant levels of free IL-18 were positively correlated with serum levels of ADMA [4], endothelial dysfunction or an impaired endothelial homeostasis in patients due to the previous treatment of the underlying malignancy may also be implicated. The observed positive inter-correlations between IL-18, IL18-BPa, and CXCL9/MIG in patients suggest the involvement of the IFNγ-pathway.
The incidence of severe acute GVHD was substantially higher in the validation cohort, which may probably be related to the post-transplant patient management (particularly the immunosuppressive regimen and the tapering of immunusuppressants) which differed between the centres. Furthermore, patients of the training and validation cohorts showed several differences with regard to patient and treatment characteristics (disease status prior to transplant, gender distribution, proportion of patients allografted for myeloid diseases, and ATG treatment). These differing characteristics were all included as confounders in the multivariable models and we were able to validate the association of higher pre-transplant free IL-18 levels with increased non-relapse and overall mortality.
Since the prognostic relevance of biomarkers assessed by single-point measurements often needs to be interpreted with caution, the post-transplant kinetics of serum IL-18 were carefully examined in the training cohort. Median serum levels of free IL-18 were relatively steady over the first 28 days after alloSCT and were apparently not affected by the intensity of the conditioning treatment or the use of ATG. The time-course of free IL-18 remained stable due to a parallel increase in both, total IL-18 and its natural inhibitor IL-18BP. Also, the composition of the patient distribution according to free IL-18 serum levels remained relatively stable between d + 28 and the time prior to transplant. Accordingly, in the training cohort, higher levels of free IL-18 on day+28 were similarly associated with non-relapse and overall mortality. Altogether, these findings suggest that elevated levels of free-IL18 possibly may reflect both, a pro-inflammatory state and an intrinsic characteristic of the recipients and their response to the treatment-related challenges of alloSCT. The lack of correlation with polymorphisms in the IL-18 and IL18-BP genes, together with the observed association of pre-transplant levels of free IL-18 with age, CRP, HCT-CI and patient performance status in the training cohort support the hypothesis that IL-18 reflects a pro-inflammatory condition. Importantly, free IL-18 remained the strongest predictor of NRM in multivariable analyses including all these variables as confounders. However, it should be noted that data on post-transplant free IL-18 levels, patient comorbidities and performance status, and gene polymorphisms were only available for the training cohort.
In-depth analysis of the non-relapse causes of death in both cohorts revealed pre-transplant serum levels of free IL-18 to be most pronounced in patients who later succumbed to infectious complications. However, this is merely a descriptive account. It should be clarified that our results do not imply a higher incidence or risk of post-transplant infectious complications per se in the context of elevated free IL-18.
IL-18 is a known component of the human systemic inflammatory response. It facilitates type 1 T-helper cell responses and natural killer cell activation which both protect during host defence against infection [31]. However, some of the pathologic consequences of infection are, in part, also mediated by IL-18, and outside the transplant setting, correlations with infection severity have been reported [8,31,32]. Notably, by promoting organ injury and lethal shock high serum IL-18 was demonstrated to be an early predictor of worse outcome in sepsis patients [9]. Therefore, the association of free IL-18 levels with NRM in the present study may be suggestive of a pro-inflammatory disposition of the recipient, by which infections in an IL-18-rich environment are more likely to result in fatal complications and excess mortality, particularly in the early post-transplant period.
The main limitation of our study is represented by its retrospective and observational design making the findings vulnerable to unknown confounders. It is obvious that further studies are needed to elucidate the mechanistic association of IL-18 with post-transplant outcome, ideally by performing prospective and interventional studies. With regard to the latter, two recently developed IL-18 inhibitor drugs have entered clinical trial phase and bear potential for inflammatory auto-immune diseases. GSK1070806 is a monoclonal antibody that neutralises human IL-18, and Tadekinig alfa is a recombinant human IL-18BP. Notably, Tadekinig alfa given three times per week for 12 weeks to patients with adult-onset Still's disease showed a favourable safety profile and was associated with early signs of efficacy [33]. Based on literature data and our results, the cut-off ≥470 pg/mL for pre-transplant free IL-18 (i.e. threefold higher than in healthy subjects) could be used to define high-risk patients targeted for intervention.
In summary, this retrospective cohort study points to an association between free IL-18 and mortality after alloSCT. The associations with non-relapse and overall mortality could be attributed to mainly fatal infectious complications. The results may provide a rationale for prospective studies evaluating IL-18 status and interference with the IL-18/IL-18BP axis in patients undergoing alloSCT.
CRediT authorship contribution statement
Aleksandar Radujkovic: Conceptualization, Data curation, Writing - original draft. Lambros Kordelas: Data curation, Writing - original draft. Hao Dai: Data curation, Writing - original draft. David Schult: Data curation, Writing - original draft. Joshua Majer-Lauterbach: Data curation, Writing - original draft. Dietrich Beelen: Data curation, Writing - original draft. Carsten Müller-Tidow: Data curation, Writing - original draft. Peter Dreger: Data curation, Writing - original draft. Thomas Luft: Conceptualization, Supervision, Data curation, Writing - original draft.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Acknowledgments
The authors wish to acknowledge the great work of the physicians and the nursing staff of both transplant units, and, of course, the patients for making the study possible. The authors also thank Michael Hess and Alexandra Hof for their expert technical assistance and the construction and maintenance of the serum biobank.
This work was supported by the Helmholtz-Alliance on Immunotherapy of Cancer, B.L.U.T. e.V. (Weingarten, Germany), the Wilhelm-Sander-Stiftung (grant no. 2008.068.1), and the China Scholarship Council. The funders had no role in study design, data collection, data analysis, interpretation, or writing of the report.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2019.10.024.
Appendix. Supplementary materials
References
- 1.Carreras E., Diaz-Ricart M. The role of the endothelium in the short-term complications of hematopoietic SCT. Bone Marrow Transplant. 2011;46:1495–1502. doi: 10.1038/bmt.2011.65. [DOI] [PubMed] [Google Scholar]
- 2.Tatekawa S., Kohno A., Ozeki K. A novel diagnostic and prognostic biomarker panel for endothelial cell damage-related complications in allogeneic transplantation. Biol Blood Marrow Transplant. 2016;22:1573–1581. doi: 10.1016/j.bbmt.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 3.Luft T., Dietrich S., Falk C. Steroid-refractory GVHD: T-cell attack within a vulnerable endothelial system. Blood. 2011;118:1685–1692. doi: 10.1182/blood-2011-02-334821. [DOI] [PubMed] [Google Scholar]
- 4.Radujkovic A., Dai H., Kordelas L. Asymmetric dimethylarginine serum levels are associated with early mortality after allogeneic stem cell transplantation. Haematologica. 2019;104:827–834. doi: 10.3324/haematol.2018.202267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplanski G. Interleukin-18: biological properties and role in disease pathogenesis. Immunol Rev. 2018;281:138–153. doi: 10.1111/imr.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinarello C.A., Novick D., Kim S., Kaplanski G. Interleukin-18 and IL-18 binding protein. Front Immunol. 2013;4:289. doi: 10.3389/fimmu.2013.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy P., Ferrara J.L. Role of interleukin-18 in acute graft-vs-host disease. J Lab Clin Med. 2003;141:365–371. doi: 10.1016/S0022-2143(03)00028-3. [DOI] [PubMed] [Google Scholar]
- 8.Grobmyer S.R., Lin E., Lowry S.F. Elevation of IL-18 in human sepsis. J Clin Immunol. 2000;20:212–215. doi: 10.1023/a:1006641630904. [DOI] [PubMed] [Google Scholar]
- 9.Emmanuilidis K., Weighardt H., Matevossian E. Differential regulation of systemic IL-18 and IL-12 release during postoperative sepsis: high serum IL-18 as an early predictive indicator of lethal outcome. Shock. 2002;18:301–305. doi: 10.1097/00024382-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Blankenberg S., Tiret L., Bickel C. Interleukin-18 is a strong predictor of cardiovascular death in stable and unstable angina. Circulation. 2002;106:24–30. doi: 10.1161/01.cir.0000020546.30940.92. [DOI] [PubMed] [Google Scholar]
- 11.Baldassarre D., Porta B., Camera M. Markers of inflammation, thrombosis and endothelial activation correlate with carotid IMT regression in stable coronary disease after atorvastatin treatment. Nutr Metab Cardiovasc Dis. 2009;19:481–490. doi: 10.1016/j.numecd.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Hung J., McQuillan B.M., Chapman C.M., Thompson P.L., Beilby J.P. Elevated interleukin-18 levels are associated with the metabolic syndrome independent of obesity and insulin resistance. Arterioscler Thromb Vasc Biol. 2005;25:1268–1273. doi: 10.1161/01.ATV.0000163843.70369.12. [DOI] [PubMed] [Google Scholar]
- 13.Esposito K., Pontillo A., Ciotola M. Weight loss reduces interleukin-18 levels in obese women. J Clin Endocrinol Metab. 2002;87:3864–3866. doi: 10.1210/jcem.87.8.8781. [DOI] [PubMed] [Google Scholar]
- 14.Frayling T.M., Rafiq S., Murray A. An interleukin-18 polymorphism is associated with reduced serum concentrations and better physical functioning in older people. J Gerontol A Biol Sci Med Sci. 2007;62:73–78. doi: 10.1093/gerona/62.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He M., Cornelis M.C., Kraft P. Genome-wide association study identifies variants at the IL18-BCO2 locus associated with interleukin-18 levels. Arterioscler Thromb Vasc Biol. 2010;30:885–890. doi: 10.1161/ATVBAHA.109.199422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durpès M.C., Morin C., Paquin-Veillet J. PKC-β activation inhibits IL-18-binding protein causing endothelial dysfunction and diabetic atherosclerosis. Cardiovasc Res. 2015;106:303–313. doi: 10.1093/cvr/cvv107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gratwohl A., Stern M., Brand R. Risk score for outcome after allogeneic hematopoietic stem cell transplantation: a retrospective analysis. Cancer. 2009;115:4715–4726. doi: 10.1002/cncr.24531. [DOI] [PubMed] [Google Scholar]
- 18.Sorror M.L., Maris M.B., Storb R. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerds T.A., Schumacher M. Consistent estimation of the expected Brier score in general survival models with right-censored event times. Biom J. 2006;48:1029–1040. doi: 10.1002/bimj.200610301. [DOI] [PubMed] [Google Scholar]
- 20.Gerds T.A., Kattan M.W., Schumacher M., Yu C. Estimating a time-dependent concordance index for survival prediction models with covariate dependent censoring. Stat Med. 2013;32:2173–2184. doi: 10.1002/sim.5681. [DOI] [PubMed] [Google Scholar]
- 21.Zeiser R., Blazar B.R. Acute graft-versus-host disease - Biologic Process, prevention, and therapy. N Engl J Med. 2017;377:2167–2179. doi: 10.1056/NEJMra1609337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill G.R., Krenger W., Ferrara J.L. The role of cytokines in acute graft-versus-host disease. Cytokines Cell Mol Ther. 1997;3:257–266. [PubMed] [Google Scholar]
- 23.Hu H.Z., Li G.L., Lim Y.K., Chan S.H., Yap E.H. Kinetics of interferon-gamma secretion and its regulatory factors in the early phase of acute graft-versus-host disease. Immunology. 1999;98:379–385. doi: 10.1046/j.1365-2567.1999.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Min C.K., Maeda Y., Lowler K. Paradoxical effects of interleukin-18 on the severity of acute graft-versus-host disease mediated by CD4+ and CD8+ T-cell subsets after experimental allogeneic bone marrow transplantation. Blood. 2004;104:3393–3399. doi: 10.1182/blood-2004-02-0763. [DOI] [PubMed] [Google Scholar]
- 25.Shaiegan M., Iravani M., Babaee G.R., Ghavamzadeh A. Effect of IL-18 and sIL2R on aGVHD occurrence after hematopoietic stem cell transplantation in some iranian patients. Transpl Immunol. 2006;15:223–227. doi: 10.1016/j.trim.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Fujimori Y., Takatsuka H., Takemoto Y. Elevated interleukin (IL)-18 levels during acute graft-versus-host disease after allogeneic bone marrow transplantation. Br J Haematol. 2000;109:652–657. doi: 10.1046/j.1365-2141.2000.02095.x. [DOI] [PubMed] [Google Scholar]
- 27.Scholl S., Sayer H.G., Mügge L.O. Increase of interleukin-18 serum levels after engraftment correlates with acute graft-versus-host disease in allogeneic peripheral blood stem cell transplantation. J Cancer Res Clin Oncol. 2004;130:704–710. doi: 10.1007/s00432-004-0603-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura H., Komatsu K., Ayaki M. Serum levels of soluble IL-2 receptor, IL-12, IL-18, and IFN-gamma in patients with acute graft-versus-host disease after allogeneic bone marrow transplantation. J Allergy Clin Immunol. 2000;106:S45–S50. doi: 10.1067/mai.2000.106774. [DOI] [PubMed] [Google Scholar]
- 29.Puxeddu I., Italiani P., Giungato P. Free IL-18 and IL-33 cytokines in chronic spontaneous urticaria. Cytokine. 2013;61:741–743. doi: 10.1016/j.cyto.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Zecchina G., Novick D., Rubinstein M., Barak V., Dinarello C., Nagler A. Interleukin-18 binding protein in acute graft versus host disease and engraftment following allogeneic peripheral blood stem cell transplants. J Hematother Stem Cell Res. 2001;10:769–776. doi: 10.1089/152581601317210863. [DOI] [PubMed] [Google Scholar]
- 31.Dinarello C.A., Fantuzzi G. Interleukin-18 and host defense against infection. J Infect Dis. 2003;187:S370–S384. doi: 10.1086/374751. [DOI] [PubMed] [Google Scholar]
- 32.Feng M., Sun T., Zhao Y., Zhang H. Detection of serum interleukin-6/10/18 levels in sepsis and its clinical significance. J Clin Lab Anal. 2016;30:1037–1043. doi: 10.1002/jcla.21977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gabay C., Fautrel B., Rech J. Open-label, multicentre, dose-escalating phase II clinical trial on the safety and efficacy of tadekinig alfa (IL-18BP) in adult-onset still's disease. Ann Rheum Dis. 2018;77:840–847. doi: 10.1136/annrheumdis-2017-212608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bacigalupo A., Ballen K., Rizzo D. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bornhäuser M., Kienast J., Trenschel R. Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: a prospective, open-label randomised phase 3 trial. Lancet Oncol. 2012;13:1035–1044. doi: 10.1016/S1470-2045(12)70349-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.