Abstract
Pyrazinamide remains the only drug in the tuberculosis pharmacopeia to drastically shorten first-line therapy from nine to six months. Due to its unparalleled ability to sterilize non-replicating bacilli and reduce relapse rates, PZA is expected to be irreplaceable in future therapies against tuberculosis. While the molecular target of PZA is unclear, recent pharmacokinetic studies using small animal models and patient samples have highlighted the importance of host metabolism and immune responses in PZA efficacy. Delineating which host factors are important for PZA action will be integral to the design of next-generation therapies to shorten current TB drug regimens as well as to overcome treatment limitations in some patients. In this review, we discuss evidence for influence of the host environment on PZA activity, targets for PZA mechanism of action, recent studies in PZA pharmacokinetics, PZA antagonism and synergy with other first-line anti-TB drugs, and implications for future research.
Keywords: Pyrazinamide, Tuberculosis, CoA biosynthesis, Granuloma, Pharmacokintetics, Cell-mediated immunity
1. Introduction
Mycobacterium tuberculosis, the causative agent of tuberculosis (TB), is responsible for an estimated 1.6 million deaths each year [1]. As the case fatality rate for untreated TB is approximately 70%, the discovery and integration of combinatorial drug therapy in clinical practice marked a major milestone in the fight against TB. While first-line treatment of drug-susceptible TB achieves cure rates of up to 95%, success rates involving second-line treatments for drug resistant TB are on the order of 50% [1], [2]. Thus, a comprehensive understanding of the factors that underlie treatment success and failure are paramount in advancing toward global eradication of TB.
Pyrazinamide (PZA) is an irreplaceable drug used for treatment of both drug-susceptible and multidrug-resistant TB infections. Discovery efforts that led to identification of PZA were inspired by Vital Chorine's research on nicotinamide (vitamin B3) as a treatment for leprosy [3]. Chorine and others [4] found that treatment of M. leprae infected rats and mice with high concentrations of nicotinamide prolonged their survival. Chorine translated these findings to TB infected guinea pigs and demonstrated that subcutaneous administration of nicotinamide similarly delayed disease progression.
Eager to contribute other antitubercular drugs to the growing TB pharmacopeia, Lederle Laboratories of American Cynamid [5] and Merck Laboratories [6] developed numerous pyrazine analogues of nicotinamide with the hope that they would show increased potency compared to the parent compound. Contrary to standard practices for drug development and validation, these pyrazine analogues were exclusively screened in TB infected mice without prior in vitro susceptibility testing. Both laboratories found that the most active pyrazine analogue against M. tuberculosis was PZA. Testing in animals proved to be fortuitous as PZA showed potent activity in vivo paradoxical to inactivity of the drug on pure cultures of M. tuberculosis at near neutral pH. Future experimentation by McDermott and Thompsett would show that the culture medium needed to be mildly acidic (pH of 5.8) for PZA to inhibit growth of M. tuberculosis [7]. Subsequent studies revealed enhanced PZA activity in the presence of alkaline pH [7], low temperature [8], nutrient limitation [9], and hypoxia [10]. Further testing of experimentally infected mice and guinea pigs by Malone [11], Dessau [12] and colleagues showed that PZA treatment resulted in decreased lung pathology and showed superior antitubercular activity compared to para-aminosalicylic acid and nicotinamide. Clinical evaluation of PZA treatment in forty-three TB infected patients at Summit Park Sanatorium commenced from 1949 until 1951 [13]. Yeager, Monroe, and Dessau noted improvement of symptoms and disposition in patients treated with PZA, even those with infections that were resistant to streptomycin. However, rapid resistance to PZA was observed in patients, particularly those with large cavitary lesions. Favorable outcomes from PZA treatment led to the rapid inclusion of PZA in second-line therapy for drug resistant TB.
PZA was considered in the combinatorial first-line therapy after reports of synergistic activity between PZA and isoniazid (INH) in experimentally infected mice [14] and TB patients [15]. The addition of PZA to first-line therapy dramatically reduced relapse rates and enabled treatment shortening from nine to six months [16]. PZA has remained a cornerstone of TB therapy over the last 50 years and is projected to be a mainstay in future TB regimens. It is fortunate that PZA was first evaluated in infected animals as its inactivity in standard culture medium would have likely excluded this drug for further testing. The unique efficacy of PZA in vivo has also provided researchers with evidence of the importance of the host environment in antimicrobial drug action.
While the majority of basic science research surrounding PZA has focused on molecular and biochemical aspects of its antitubercular activity, recent studies have highlighted the indispensable role of the host environment in the sterilizing action of PZA in vivo [17], [18], [19], [20], [21]. Defining which aspects of the host response enhance PZA activity will provide key insight for development of host-directed therapies as adjunctive treatments to current antitubercular drugs. In this review, we focus on proposed modes of action for PZA, recent pharmacokinetic studies using host cells, patient samples and small animal models, PZA antagonism and synergy by other first-line antitubercular drugs, and implications for future TB drug research.
2. Proposed molecular targets for PZA
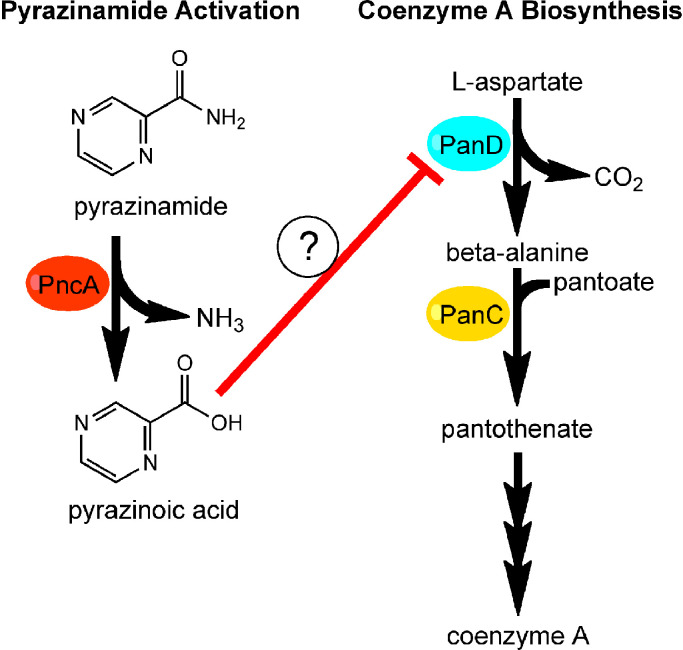
PZA is a pro-drug that is hydrolyzed to its active form, pyrazinoic acid (POA), by the M. tuberculosis intracellular pyrazinamidase/nicotinamidase (PncA, Fig. 1) [22]. This enzyme, encoded by pncA, is involved in the salvage pathway for the production of nicotinamide adenine dinucleotide (NAD+), a critical cofactor required for hundreds of biological reactions [23]. Since M. tuberculosis can also synthesize NAD+ by the de novo pathway, the NAD+ salvage pathway is non-essential for fitness in vivo [24], [25]. Thus, loss-of-function mutations in pncA represent the primary mechanism of PZA resistance in clinical isolates. Diverse pncA mutations, including single and multi-nucleotide polymorphisms and indels, have been reported in PZA resistant clinical isolates along the entire 561 base pair gene length [26], [27]. Recently, Yadon et al. [28] created a comprehensive library of PncA polymorphisms through saturating mutagenesis and identified over 300 substitutions, some of which were previously reported in clinical isolates, that conferred PZA resistance in vitro and in vivo. Interestingly, more PZA resistant substitutions were observed in the in vivo infection model, suggesting that there are unique selective pressures mediated by the host that are not captured by in vitro experimentation [28]. In addition, many non-synonymous substitutions that did not elicit PZA resistance were also observed. Biochemical and bioinformatic analysis indicated that most PZA resistance-conferring substitutions led to impaired PncA structure and stability or disrupted active site catalytic triad and iron coordinating residues [28]. While mutation of pncA is the primary molecular mechanism of PZA resistance, as much as 30% of PZA resistant clinical isolates encode wild type pncA and retain full PncA activity [29], [30]. PZA resistant isolates with functional PncA indicate that there are other as yet undescribed resistance mechanisms.
Fig. 1.
Working mode for pyrazinamide action. Pyrazinamide is activated by PncA to pyrazinoic acid (left). Pyrazinoic acid impairs coenzyme A metabolism possibly through interaction with PanD. Intermediates of coenzyme A synthesis antagonize pyrazinamide and pyrazinoic acid activity.
Over the last twenty years, several mechanisms of action for PZA have been proposed [31], [32], [33], [34], [35], [36]. Some models, such as POA-mediated acidification of the M. tuberculosis cytoplasm, inhibition of fatty acid synthase I (FAS-I), and inhibition of ribosome rescue have been challenged by multiple research groups [31], [37], [38], [39]. Recently, numerous studies have shown that a component of the coenzyme A (CoA) biosynthetic pathway may be a molecular target of POA (Fig. 1) [40], [41], [42], [43], [44], [45]. Analysis of spontaneous PZA and POA resistant isolates with functional PncA revealed missense mutations in panD that encodes l-aspartate-α-decarboxylase, a rate-limiting step in the CoA biosynthetic pathway [42], [44]. In the CoA pathway, l-aspartate undergoes decarboxylation by PanD to form β-alanine (Fig. 1) [46]. PanC then catalyzes ligation β-alanine with pantoate to form pantothenate that is further modified to ultimately yield CoA (Fig. 1), an essential cofactor for numerous metabolic pathways [47]. While M. tuberculosis PanD shows sequence and structural similarity to other members of the PanD family, it has a unique 13 amino acid C-terminal extension [48]. The C-terminal extension appears to be a hot spot for POA resistance mutations [45]. In addition to isolation of panD mutant strains in vitro, Gopal et al. recovered POA resistant panD mutant strains (82% of POA resistant strains) from the lungs of mice that were infected with M. tuberculosis and treated with POA [41]. Mice infected with a panD missense mutant strain had bacterial lung burdens comparable to those infected with wild-type M. tuberculosis [41], indicating that panD mutant strains maintain fitness in vivo.
In support of PanD as a target of POA, Gopal et al. showed binding of POA to PanD (KD = 6.1 μM ± 0.88 μM), which was abrogated with resistant variants [40]. Treatment of wild-type M. bovis BCG with POA was also found to decrease CoA abundance after 24–48 h of exposure [42], and a mutant strain disrupted in the acyl-CoA ligase FadD2 showed heightened susceptibility to POA [49]. CoA depletion appears to be specific for POA as the structural analogues, nicotinic acid and benzoic acid, failed to significantly change CoA levels [40]. Multiple studies demonstrate that POA-mediated inhibition of M. tuberculosis growth can be antagonized by precursors of the CoA biosynthetic pathway (Fig. 1), such as β-alanine, pantothenate and pantetheine [42], [43], [44]. These data are consistent with a model in which POA is able to bind PanD and ultimately inhibit production of CoA. However, it is important to note that the pantothenate auxotrophic strain M. tuberculosis mc27000 (ΔpanCD) remains susceptible to PZA when cultivated with sub-antagonistic concentrations of panthetheine, indicating that there must be other factors in the CoA biosynthetic pathway involved in PZA-mediated growth inhibition [43]. While data supporting PanD as a target of POA are intriguing, panD missense mutations identified in the laboratory have yet to reported in PZA resistant clinical isolates [50]. Additional in vitro, in vivo and clinical studies are essential to better understand the association between CoA metabolism and the sterilizing activity of PZA in TB therapy.
3. PZA and the host environment
While the majority of efforts toward dissecting PZA action have focused on its direct interaction with M. tuberculosis, recent studies have also explored host related aspects of PZA activity [51], [52], [53], [54]. The current understanding of TB indicates that the disease presents as a spectrum of responses to infection rather than a simple binary distribution of pathology in the host [55]. Recent studies have shown multiple lesions types within a single host that vary in cell composition, microenvironment, metabolic activity, and bacterial burden [56]. Differences in microenvironments govern the support and suppression of M. tuberculosis subpopulations [57] as well as drug penetration, activity, and availability [17]. Ultimately, a few granulomas may dictate treatment responses and disease outcome. Understanding drug activity in different cell and lesion types is essential to optimize drug combinations, dosing, and timing.
Drug distribution and efficacy studies involving PZA have been conducted in macrophages, small animal models, patient sera and lung tissues collected from surgical resections. PZA is most efficacious during the first two months of therapy and it is generally assumed to target non- or slowly-replicating bacilli residing in acidic compartments such as the macrophage phagosome [58], [59]. Studies with cultured macrophages have shown that PZA accumulates intracellularly within 3 h of exposure independent of the cellular metabolic state [60], suggesting that the drug can enter cells through passive diffusion. Despite this observation, PZA and POA antitubercular activity in macrophages range from no inhibition [61] to bacteriostatic [62] to sterilizing [63]. These disparate results stem from use of different experimental designs, including source and activation state of macrophages, drug concentration, and time of drug exposure. It is also important to note that culture conditions, such as the inclusion of fetal bovine serum [64] and carbon dioxide [65], can be antagonistic for the antitubercular activity of PZA and POA.
Several therapeutic drug monitoring studies have been conducted to assess the concentration of circulating PZA and POA in plasma and the impact of disease status on drug absorption and clearance. Clinical data indicate that the susceptibility breakpoint at which PZA therapy fails is 100 µg/mL [66], while PZA peak plasma concentrations are 20–60 µg/mL at 2 h post-dose in most patients [67]. Plasma clearance of PZA ranges from 1.7 L/h to 3.42 L/h, whereas the rate for POA is approximately 1.9 L/h [21], [68]. There also appears to be slow and fast absorbers of PZA [68]. PZA pharmacokinetic studies in children with pulmonary TB demonstrate a slower rate of absorption and clearance compared to adults when dosage is based on body weight (mg/kg) [69]. Amendments to the WHO guidelines for drug dosage in children recommended increased concentrations of antitubercular drugs and the PZA range was changed to 30–40 mg/kg [70]. The few studies that have conducted therapeutic drug monitoring in children using the revised WHO guidelines report that target plasma PZA concentrations were typically achieved [66], [67], [68].
Absorption and clearance of PZA in the context of TB-HIV co-infection is less clear. Some studies report that co-infection with HIV reduces PZA plasma concentrations [69], [71], [72]; while others state that no significant differences are observed between TB only and co-infected patients [73], [74]. Factors that may influence disparate drug concentrations include nutritional status [75], access to antiretroviral therapy, and state of immunosuppression. Few studies have examined disease outcome with respect to low PZA and POA concentrations in the plasma. Three studies within the last eight years have shown an association between low serum concentrations of PZA and poor disease outcome [76], [77], [78]. For example, Chideya et al. [76] reported that patients with inadequate PZA levels in serum (adjusted for HIV status and CD4+ T cell counts) were at three times greater risk for poor outcome compared to patients with normal concentrations of PZA. However, data collected by Park et al. [79] indicated that low serum concentrations of isoniazid rather than PZA had a slight association with TB recurrence and drug resistance.
While activation of PZA to POA is typically attributed to hydrolysis by M. tuberculosis, recent evidence indicates that TB-independent host-mediated activation may be relevant for drug action [21]. Following oral administration of PZA to uninfected mice, guinea pigs, rabbits and primates, a substantial level of POA could be detected in plasma [21]. Further, POA was found to penetrate and accumulate within the lung and pulmonary lesions of rabbits infected with M. bovis lacking PncA activity [21]. While PZA shows no activity against most other PncA deficient mycobacteria [53], additional studies are required to discern whether host mediated activation of PZA can be exploited to enhance exposure of tubercle bacilli to POA.
Small animal models have been informative in pharmacokinetic and pharmacodynamic studies involving antituberculosis drugs [80], [81]. Murine models of TB infection have been extensively used in assessing TB drugs due to the availability of reagents, tractability of host genetics, cost-effectiveness, and ability to achieve statistical power [82], [83], [84]. The majority of classical murine models display one disease state in the TB spectrum [85], [86]. BALB/C and C57/BL6 mice represent immunocompetent hosts that experience a prolonged chronic infection [87], [88]. BALB/C and C57/BL6 mice develop aerobic and diffuse lesions that fail to progress to an advanced state, which is atypical in human TB infection [89], [90]. The inability of BALB/C and C57/BL6 mice to capture the full gamut of granuloma heterogeneity has led researchers to explore other small animal models for TB drug studies. One such model, C3HeB/FeJ mice (also referred to as the Kramnik model), forms necrotic, hypoxic, liquefying granulomas followed by occasional cavitation in response to M. tuberculosis challenge due to a mutation in the Ipr1 (intracellular pathogen resistance) isoform of the Ifi75 (interferon-inducible-75) gene [91]. Drug efficacy studies pioneered by Anne Lenaerts and colleagues using the Kramnik mouse model showed a refractory response to PZA monotherapy at 7–8 weeks post M. tuberculosis infection [52]. Extension of the post-infection time course by six weeks prior to PZA monotherapy resulted in the separation of two distinct response groups within Kramnik mice; one group showed bactericidal effects similar to BALB/C control mice and the other group displayed little to no reduction in bacterial burden [92]. The lungs from Kramnik mice from the bactericidal group contained a relatively homogenous population of lesions comparable to BALB/C mice, while the other group developed a majority of necrotic, caseous lesions [92] despite having similar PZA concentration profiles in plasma and lesions [20]. PZA and POA were found to accumulate to within cellular and necrotic lesions by 0.5–1 h post-dose [17]. However, a higher concentration of PZA was distributed throughout lesions compared to POA. PZA penetration into lesions was also observed in TB infected rabbits [19]. Granulomas isolated from patients undergoing lung resection for drug refractory TB also showed PZA and POA distribution throughout the cellular cuff and caseum [93]. Combined, PZA and POA spatial distribution data in animals and human subjects suggest that PZA is able to penetrate into critical lesion compartments where recalcitrant populations of bacilli are thought to reside.
Given the importance of the host environment in PZA action, assessment of PZA within host compartments is imperative. The pH of liquefied caseum in lesions from the PZA unresponsive Kramnik mice was near neutral (pH 7.4), which provides an intriguing explanation for PZA inactivity [17], [92]. The pH of caseum in lesions from TB infected guinea pigs was also a circumneutral (pH 7.2) environment and is thought to be the basis for inadequate PZA treatment response in this model [20]. Data involving use of the in vitro hollow-fiber model of TB suggested PZA sterilizing activity may be primarily directed against extracellular bacteria found within acidic fluid lining the alveolar epithelium [94]. While acidic pH is not a strict requirement for PZA activity, acidic conditions may be important to drive PZA susceptibility in acellular environments that present limited host-induced stresses. Further supports for this concept comes from the observation that PZA promotes dramatic reduction of M. tuberculosis burden in immune competent mice, such as BALB/C and C57BL/6, which have cellular restricted bacilli [87], [88]. M. tuberculosis restricted to cellular compartments of the host experience multiple immune mediated stresses irrespective of low pH. These conditions are likely responsible for enhanced PZA activity in vivo. In fact, multiplexed in situ imaging of cytokine and immune effector transcripts in TB infected Kramnik mice showed that cellular granulomas displayed networks, like CD68-iNOS (inducible nitric oxide synthase) and IFNG (gamma interferon), associated with a robust response against M. tuberculosis [95]. This is in stark contrast to enriched transcript signatures found in necrotic granulomas, such as IL10 and FOXP3, which are associated with immunosuppression [95]. Additional studies using the Kramnik model should consider mapping immune transcripts in multiple granulomas that are responsive and refractory to PZA treatment.
In addition to Kramnik mice, TB infected athymic nude mice showed similar PZA inactivity after 7–8 weeks of treatment [53]. While the basis for this drug inactivity has not been elucidated, it is consistent with the need for macrophage activation and sufficient phagosomal acidification for PZA to be efficacious against intracellular M. tuberculosis [62]. It has also been demonstrated that PZA can dampen expression of pro-inflammatory cytokines and chemokines [54]. Some studies have suggested that PZA potentiation by the host may be related to drug upregulation of the autophagy pathway [96]. Multiple studies have reported the occurrence of dysregulated autophagy during diseases that cause altered immune states [97], [98], [99], [100] While many outstanding questions remain, these studies indicate that the appropriate cell-mediated responses are critical for optimal PZA efficacy, and both local and systemic immune modulation are highly impactful for PZA efficacy.
It was recently hypothesized that some antituberculosis drugs fail to diffuse through lesions due to caseum macromolecule binding. There was no discernable amount of PZA binding to caseum in contrast to another TB drug, bedaquiline [101]. Caseum binding provides a rationale as to why PZA can distribute throughout lesions as opposed to drugs like bedaquiline [17], which are retained in the cellular regions surrounding the caseum. Collectively, these exciting data suggest that lung pathology heterogeneity and lesion microenvironment have a dramatic influence on PZA efficacy and are important considerations for future TB drug discovery and design [102].
4. Other considerations
Another intriguing aspect of PZA is its paradoxical relationship to another first-line TB pro-drug, isoniazid (INH). Although PZA and INH are structurally similar and appear to target different metabolic populations of M. tuberculosis, INH has been shown to antagonize PZA action in vivo in a dose dependent manner [103], [104], [105]. It is well established that INH is activated via the M. tuberculosis catalase-peroxidase KatG to form an isonicotinoyl radical [106], [107], [108], [109], [110]. This radical reacts with NAD+ to produce an INH—NAD adduct that is a potent inhibitor of the enoyl-ACP reductase (InhA) involved in mycolic acid biosynthesis [111], [112]. It is possible that INH accelerates metabolism and clearance of PZA. Alternatively, INH may more directly interfere with PZA action at the site of the bacilli. Pharmacokinetic studies measuring the concentrations and distribution of PZA and POA within lesions isolated from mice co-treated with PZA and INH may shed light on the mechanism behind this antagonism.
Interestingly, the addition of INH to combination therapy with rifampicin (RIF) and PZA has an ameliorating affect in reducing hepatotoxicity in uncomplicated active TB cases [113]. This observation is in stark contrast to the severe hepatotoxicity and occasional fatalities noted in individuals treated with a combination of RIF and PZA for latent TB infection [113], [114], [115], [116]. Hepatotoxicity is thought to occur due to the accumulation of toxic, reactive drug metabolites [117] or drug-metabolite adducts within the liver [118], [119], [120], [121]. Drug-metabolites may impair critical cellular functions [122], [123]. For example, POA is hydroxylated by the host xanthine oxidase to form 5-hydroxypyrazinoic acid (5-OH-PA) [21], [124], which was shown to be cytotoxic in Hep-G2 cells [125] and has been correlated with hepatotoxicity in rats [117], [125] and TB patients [126]. Co-administration with the amidase inhibitor, bis-p-nitrophenyl phosphate, reduced or eliminated PZA induced hepatotoxicity in M. tuberculosis infected rats [125]. This observation suggests that limiting host-mediated conversion of PZA to POA and subsequent formation of 5-OH-PA may suppress deleterious side-effects of PZA. Future studies examining INH, RIF, and PZA mono- and combination treatment in TB infection animal models should consider measuring the accumulation of PZA drug metabolites in the liver to explore important drug-drug interactions.
5. Outstanding questions and conclusion
Since its introduction into clinical use, PZA has become part of the bedrock of TB drug regimens. Early screening of pyrazine analogues in small animal models revealed that the host environment is an integral factor PZA efficacy. Recent studies show this drugs works optimally in conjunction with an antitubercular cell-mediated immune response and the granuloma microenvironment may help to drive PZA activity. Evidence provided by Anne Lenaerts and colleagues [17] show that PZA fails to sterilize M. tuberculosis in caseous granulomas with neutral pH. However, it is unclear if pH is the sole host stress that is required for drug activity or if other host responses may similarly potentiate PZA. It is possible that low pH may be required for PZA efficacy in the caseum but other host factors, including oxidative/nitrosative stress and nutrient limitation, may be sufficient at the periphery of the granuloma. In order to appreciate the diversity of host stresses with respect to spatial and temporal considerations, future studies should employ a combination of multiplexed in situ imaging of immune mRNAs and proteomics to map the landscapes of various granulomas after PZA treatment. Furthermore, studies should seek to characterize PZA metabolites in the caseum throughout the spectrum of TB pathology. Recently, an elegant study reported by Eric Rubin and colleagues [127] characterized the host proteome of multiple lesion types. This study showed that the centers of the granulomas contained antimicrobial peptides, reactive oxygen species, and pro-inflammatory signals, while the surrounding tissues had anti-inflammatory associated markers. Important host factors mediating PZA activity in vivo may also be identified through the use of mutant mouse strains with defined knockdowns of antimicrobial peptides and factors for cell-mediated immunity. Delineating which factors drive PZA action in vivo may reveal novel therapeutic approaches to further decrease the length of the TB drug regimen as well as illuminate the basis for PZA failure in some patients.
6. Search strategy and selection criteria
Data for this review were identified by searches of PubMed, MEDLINE, and references from relevant articles using the search terms “pyrazinamide”, “tuberculosis”, “host immune system”, “mode of action”, “pharmacokinetics”, and “mouse models.” Articles between 1945–2019 were included.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
Acknowledgements
Research relevant for this review was supported by NIH grant R01 AI123146 to A.D.B. E.A.L was supported by a Postdoctoral Fellowship from the Ford Foundation of the National Academies of Science, Engineering, and Mathematics, U.S.A and the NIH diversity supplement award R01AI123146-03S1.
References
- 1.World Health Organization. Global tuberculosis report. 2017.
- 2.Sotgiu G., Centis R., D'Ambrosio L., Migliori G.B. Tuberculosis treatment and drug regimens. Cold Spring Harb Perspect Med. 2015;5(5) doi: 10.1101/cshperspect.a017822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chorine V. Action de l'amide nicotinique sur les bacilles du genre mycobacterium. Comptes Rendus de l'Académie des Sciences - Series III. 1945;220:150–151. [Google Scholar]
- 4.McKenzie D., Malone L., Kushner S., Oleson J.J., SubbaRow Y. The effect of nicotinic acid amide on experimental tuberculosis of white mice. J Lab Clin Med. 1948;33(10):1249–1253. [PubMed] [Google Scholar]
- 5.Kushner S., Dalalian H., Sanjurjo J.L. Experimental chemotherapy of tuberculosis. II. the synthesis of pyrazinamides and related compounds1. J Amer Chem Soc. 1952;74(14):3617–3621. [Google Scholar]
- 6.Solotorovsky M., Gregory F.J., Ironson E.J., Bugie E.J., O'Neill R.C., Pfister R., 3rd. Pyrazinoic acid amide; an agent active against experimental murine tuberculosis. Proc Soc Exp Biol Med. 1952;79(4):563–565. doi: 10.3181/00379727-79-19447. [DOI] [PubMed] [Google Scholar]
- 7.McDermott W., Tompsett R. Activation of pyrazinamide and nicotinamide in acidic environments in vitro. Am Rev Tuberc. 1954;70(4):748–754. doi: 10.1164/art.1954.70.4.748. [DOI] [PubMed] [Google Scholar]
- 8.den Hertog A.L., Menting S., Pfeltz R., Warns M., Siddiqi S.H., Anthony R.M. Pyrazinamide is active against mycobacterium tuberculosis cultures at neutral pH and low temperature. Antimicrob Agents Chemother. 2016;60(8):4956–4960. doi: 10.1128/AAC.00654-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Q., Chen Z.F., Li Y.Y. Nutrient-starved incubation conditions enhance pyrazinamide activity against mycobacterium tuberculosis. Chemotherapy. 2007;53(5):338–343. doi: 10.1159/000107723. [DOI] [PubMed] [Google Scholar]
- 10.Wade M.M., Zhang Y. Anaerobic incubation conditions enhance pyrazinamide activity against mycobacterium tuberculosis. J Med Microbiol. 2004;53(Pt 8):769–773. doi: 10.1099/jmm.0.45639-0. [DOI] [PubMed] [Google Scholar]
- 11.Malone L., Schurr A., Lindh H., McKenzie D., Kiser J.S., Williams J.H. The effect of pyrazinamide (aldinamide) on experimental tuberculosis in mice. Am Rev Tuberc. 1952;65(5):511–518. [PubMed] [Google Scholar]
- 12.Dessau F.I., Yeager R.L., Burger F.J., Williams J.H. Pyrazinamide (aldinamide) in experimental tuberculosis of the guinea pig. Am Rev Tuberc. 1952;65(5):519–522. [PubMed] [Google Scholar]
- 13.Yeager R.L., Munroe W.G., Dessau F.I. Pyrazinamide (aldinamide) in the treatment of pulmonary tuberculosis. Am Rev Tuberc. 1952;65(5):523–546. [PubMed] [Google Scholar]
- 14.McDermott W., Ormond L., Muschenheim C., Deuschle K., McCune R., Tompsett R. Pyrazinamide-isoniazid in tuberculosis. Am Rev Tuberc. 1954;69(3):319–333. doi: 10.1164/art.1954.69.3.319. [DOI] [PubMed] [Google Scholar]
- 15.Muschenheim C., McDermott W., McCune R., Deuschle K., Ormond L., Tompsett R. Pyrazinamide-isoniazid in tuberculosis. II. results in 58 patients with pulmonary lesions one year after the start of therapy. Am Rev Tuberc. 1954;70(4):743–747. doi: 10.1164/art.1954.70.4.743. [DOI] [PubMed] [Google Scholar]
- 16.Geiter L.J., O'Brien R.J., Combs D.L., Snider D.E., Jr. United states public health service tuberculosis therapy trial 21: preliminary results of an evaluation of a combination tablet of isoniazid, rifampin and pyrazinamide. Tubercle. 1987;68(2 Suppl):41–46. doi: 10.1016/0041-3879(87)90021-3. [DOI] [PubMed] [Google Scholar]
- 17.Irwin S.M., Prideaux B., Lyon E.R. Bedaquiline and pyrazinamide treatment responses are affected by pulmonary lesion heterogeneity in mycobacterium tuberculosis infected C3HeB/FeJ mice. ACS Infect Dis. 2016;2(4):251–267. doi: 10.1021/acsinfecdis.5b00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blanc L., Sarathy J.P., Alvarez Cabrera N. Impact of immunopathology on the antituberculous activity of pyrazinamide. J Exp Med. 2018;215(8):1975–1986. doi: 10.1084/jem.20180518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kjellsson M.C., Via L.E., Goh A. Pharmacokinetic evaluation of the penetration of antituberculosis agents in rabbit pulmonary lesions. Antimicrob Agents Chemother. 2012;56(1):446–457. doi: 10.1128/AAC.05208-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanoix J.P., Ioerger T., Ormond A. Selective inactivity of pyrazinamide against tuberculosis in C3HeB/FeJ mice is best explained by neutral pH of caseum. Antimicrob Agents Chemother. 2016;60(2):735–743. doi: 10.1128/AAC.01370-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Via L.E., Savic R., Weiner D.M. Host-Mediated bioactivation of pyrazinamide: implications for efficacy, resistance, and therapeutic alternatives. ACS Infect Dis. 2015;1(5):203–214. doi: 10.1021/id500028m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butler W.R., Kilburn J.O. Susceptibility of mycobacterium tuberculosis to pyrazinamide and its relationship to pyrazinamidase activity. Antimicrob Agents Chemother. 1983;24(4):600–611. doi: 10.1128/aac.24.4.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scorpio A., Zhang Y. Mutations in pncA, a gene encoding pyrazinamidase/nicotinamidase, cause resistance to the antituberculous drug pyrazinamide in tubercle bacillus. Nat Med. 1996;2(6):662–667. doi: 10.1038/nm0696-662. [DOI] [PubMed] [Google Scholar]
- 24.Boshoff H.I., Xu X., Tahlan K. Biosynthesis and recycling of nicotinamide cofactors in mycobacterium tuberculosis. an essential role for NAD in nonreplicating bacilli. J Biol Chem. 2008;283(28):19329–19341. doi: 10.1074/jbc.M800694200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vilchèze C., Weinrick B., Wong K.-.W., Chen B., Jacobs W.R., Jr. NAD(+) auxotrophy is bacteriocidal for the tubercle bacilli. Mol Microbiol. 2010;76:365–377. doi: 10.1111/j.1365-2958.2010.07099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allana S., Shashkina E., Mathema B. pncA gene mutations associated with pyrazinamide resistance in drug-resistant tuberculosis, south africa and georgia. Emerg Infect Dis. 2017;23(3):491–495. doi: 10.3201/eid2303.161034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoon J.H., Nam J.S., Kim K.J., Ro Y.T. Characterization of pncA mutations in pyrazinamide-resistant mycobacterium tuberculosis isolates from Korea and analysis of the correlation between the mutations and pyrazinamidase activity. World J Microbiol Biotechnol. 2014;30(11):2821–2828. doi: 10.1007/s11274-014-1706-0. [DOI] [PubMed] [Google Scholar]
- 28.Yadon A.N., Maharaj K., Adamson J.H. A comprehensive characterization of PncA polymorphisms that confer resistance to pyrazinamide. Nat Commun. 2017;8(1):588. doi: 10.1038/s41467-017-00721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stoffels K., Mathys V., Fauville-Dufaux M., Wintjens R., Bifani P. Systematic analysis of pyrazinamide-resistant spontaneous mutants and clinical isolates of mycobacterium tuberculosis. Antimicrob Agents Chemother. 2012;56(10):5186–5193. doi: 10.1128/AAC.05385-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhuju S., Fonseca Lde S., Marsico A.G. Mycobacterium tuberculosis isolates from Rio de Janeiro reveal unusually low correlation between pyrazinamide resistance and mutations in the pncA gene. Infect Genet Evol. 2013;19:1–6. doi: 10.1016/j.meegid.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Boshoff H.I., Mizrahi V., Barry C.E., 3rd. Effects of pyrazinamide on fatty acid synthesis by whole mycobacterial cells and purified fatty acid synthase I. J Bacteriol. 2002;184(8):2167–2172. doi: 10.1128/JB.184.8.2167-2172.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zimhony O., Vilcheze C., Arai M., Welch J.T., Jacobs W.R., Jr. Pyrazinoic acid and its n-propyl ester inhibit fatty acid synthase type I in replicating tubercle bacilli. Antimicrob Agents Chemother. 2007;51(2):752–754. doi: 10.1128/AAC.01369-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y., Scorpio A., Nikaido H., Sun Z. Role of acid pH and deficient efflux of pyrazinoic acid in unique susceptibility of mycobacterium tuberculosis to pyrazinamide. J Bacteriol. 1999;181(7):2044–2049. doi: 10.1128/jb.181.7.2044-2049.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y., Wade M.M., Scorpio A., Zhang H., Sun Z. Mode of action of pyrazinamide: disruption of mycobacterium tuberculosis membrane transport and energetics by pyrazinoic acid. J Antimicrob Chemother. 2003;52(5):790–795. doi: 10.1093/jac/dkg446. [DOI] [PubMed] [Google Scholar]
- 35.Zimhony O., Cox J.S., Welch J.T., Vilcheze C., Jacobs W.R., Jr. Pyrazinamide inhibits the eukaryotic-like fatty acid synthetase I (FASI) of mycobacterium tuberculosis. Nat Med. 2000;6(9):1043–1047. doi: 10.1038/79558. [DOI] [PubMed] [Google Scholar]
- 36.Shi W., Zhang X., Jiang X. Pyrazinamide inhibits trans-translation in mycobacterium tuberculosis. Science. 2011;333(6049):1630–1632. doi: 10.1126/science.1208813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baughn A.D., Deng J., Vilcheze C. Mutually exclusive genotypes for pyrazinamide and 5-chloropyrazinamide resistance reveal a potential resistance-proofing strategy. Antimicrob Agents Chemother. 2010;54(12):5323–5328. doi: 10.1128/AAC.00529-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peterson N.D., Rosen B.C., Dillon N.A., Baughn A.D. Uncoupling environmental pH and intrabacterial acidification from pyrazinamide susceptibility in mycobacterium tuberculosis. Antimicrob Agents Chemother. 2015;59(12):7320–7326. doi: 10.1128/AAC.00967-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dillon N.A., Peterson N.D., Feaga H.A., Keiler K.C., Baughn A.D. Anti-tubercular activity of pyrazinamide is independent of trans-Translation and RpsA. Sci Rep. 2017;7(1):6135. doi: 10.1038/s41598-017-06415-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gopal P., Nartey W., Ragunathan P. Pyrazinoic acid inhibits mycobacterial coenzyme a biosynthesis by binding to aspartate decarboxylase PanD. ACS Infect Dis. 2017;3(11):807–819. doi: 10.1021/acsinfecdis.7b00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gopal P., Tasneen R., Yee M. In vivo-selected pyrazinoic acid-resistant M. tuberculosis strains harbor missense mutations in the aspartate decarboxylase PanD and the unfoldase ClpC1. ACS Infect Dis. 2017;3:492–501. doi: 10.1021/acsinfecdis.7b00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gopal P., Yee M., Sarathy J. Pyrazinamide resistance is caused by two distinct mechanisms: prevention of coenzyme a depletion and loss of virulence factor synthesis. ACS Infect Dis. 2016;2(9):616–626. doi: 10.1021/acsinfecdis.6b00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dillon N.A., Peterson N.D., Rosen B.C., Baughn A.D. Pantothenate and pantetheine antagonize the antitubercular activity of pyrazinamide. Antimicrob Agents Chemother. 2014;58(12):7258–7263. doi: 10.1128/AAC.04028-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi W., Chen J., Feng J. Aspartate decarboxylase (PanD) as a new target of pyrazinamide in mycobacterium tuberculosis. Emerg Microbes Infect. 2014;3(8):e58. doi: 10.1038/emi.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang S., Chen J., Shi W., Liu W., Zhang W., Zhang Y. Mutations in panD encoding aspartate decarboxylase are associated with pyrazinamide resistance in mycobacterium tuberculosis. Emerg Microbes Infect. 2013;2(6):e34. doi: 10.1038/emi.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Webb M.E., Smith A.G., Abell C. Biosynthesis of pantothenate. Nat Prod Rep. 2004;21(6):695–721. doi: 10.1039/b316419p. [DOI] [PubMed] [Google Scholar]
- 47.Leonardi R., Zhang Y.M., Rock C.O., Jackowski S. Coenzyme A: back in action. Prog Lipid Res. 2005;44(2–3):125–153. doi: 10.1016/j.plipres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 48.Chopra S., Pai H., Ranganathan A. Expression, purification, and biochemical characterization of mycobacterium tuberculosis aspartate decarboxylase, PanD. Protein Expr Purif. 2002;25(3):533–540. doi: 10.1016/s1046-5928(02)00039-6. [DOI] [PubMed] [Google Scholar]
- 49.Rosen B.C., Dillon N.A., Peterson N.D., Minato Y., Baughn A.D. Long-Chain fatty acyl coenzyme a ligase FadD2 mediates intrinsic pyrazinamide resistance in mycobacterium tuberculosis. Antimicrob Agents Chemother. 2017;61(2):e02130–e02216. doi: 10.1128/AAC.02130-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maslov D.A., Zaichikova M.V., Chernousova L.N. Resistance to pyrazinamide in Russian mycobacterium tuberculosis isolates: pncA sequencing versus bactec MGIT 960. Tuberculosis (Edinb) 2015;95(5):608–612. doi: 10.1016/j.tube.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 51.Mendez S., Traslavina R., Hinchman M. The antituberculosis drug pyrazinamide affects the course of cutaneous leishmaniasis in vivo and increases activation of macrophages and dendritic cells. Antimicrob Agents Chemother. 2009;53(12):5114–5121. doi: 10.1128/AAC.01146-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Driver E.R., Ryan G.J., Hoff D.R. Evaluation of a mouse model of necrotic granuloma formation using C3HeB/FeJ mice for testing of drugs against mycobacterium tuberculosis. Antimicrob Agents Chemother. 2012;56(6):3181–3195. doi: 10.1128/AAC.00217-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Almeida D.V., Tyagi S., Li S. Revisiting anti-tuberculosis activity of pyrazinamide in mice. Mycobact Dis. 2014;4:145. doi: 10.4172/2161-1068.1000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manca C., Koo M.S., Peixoto B., Fallows D., Kaplan G., Subbian S. Host targeted activity of pyrazinamide in mycobacterium tuberculosis infection. PLoS ONE. 2013;8(8):e74082. doi: 10.1371/journal.pone.0074082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Subbian S., Tsenova L., Kim M.J. Lesion-Specific immune response in granulomas of patients with pulmonary tuberculosis: a pilot study. PLoS ONE. 2015;10(7) doi: 10.1371/journal.pone.0132249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ly L.H., Russell M.I., McMurray D.N. Microdissection of the cytokine milieu of pulmonary granulomas from tuberculous guinea pigs. Cell Microbiol. 2007;9(5):1127–1136. doi: 10.1111/j.1462-5822.2006.00854.x. [DOI] [PubMed] [Google Scholar]
- 57.Liu Q., Via L.E., Luo T. Within patient microevolution of mycobacterium tuberculosis correlates with heterogeneous responses to treatment. Sci Rep. 2015;5:17507. doi: 10.1038/srep17507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mackaness G.B. The intracellular activation of pyrazinamide and nicotinamide. Am Rev Tuberc. 1956;74(5):718–728. doi: 10.1164/artpd.1956.74.5.718. [DOI] [PubMed] [Google Scholar]
- 59.Rohde K., Yates R.M., Purdy G.E., Russell D.G. Mycobacterium tuberculosis and the environment within the phagosome. Immunol Rev. 2007;219:37–54. doi: 10.1111/j.1600-065X.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- 60.Acocella G., Carlone N.A., Cuffini A.M., Cavallo G. The penetration of rifampicin, pyrazinamide, and pyrazinoic acid into mouse macrophages. Am Rev Respir Dis. 1985;132(6):1268–1273. doi: 10.1164/arrd.1985.132.6.1268. [DOI] [PubMed] [Google Scholar]
- 61.Rastogi N., Potar M.C., David H.L. Pyrazinamide is not effective against intracellularly growing mycobacterium tuberculosis. Antimicrob Agents Chemother. 1988;32(2):287. doi: 10.1128/aac.32.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salfinger M., Crowle A.J., Reller L.B. Pyrazinamide and pyrazinoic acid activity against tubercle bacilli in cultured human macrophages and in the BACTEC system. J Infect Dis. 1990;162(1):201–207. doi: 10.1093/infdis/162.1.201. [DOI] [PubMed] [Google Scholar]
- 63.Crowle A.J., Sbarbaro J.A., May M.H. Inhibition by pyrazinamide of tubercle bacilli within cultured human macrophages. Am Rev Respir Dis. 1986;134(5):1052–1055. doi: 10.1164/arrd.1986.134.5.1052. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Y., Permar S., Sun Z. Conditions that may affect the results of susceptibility testing of mycobacterium tuberculosis to pyrazinamide. J Med Microbiol. 2002;51(1):42–49. doi: 10.1099/0022-1317-51-1-42. [DOI] [PubMed] [Google Scholar]
- 65.Butler W.R., Kilburn J.O. Improved method for testing susceptibility of mycobacterium tuberculosis to pyrazinamide. J Clin Microbiol. 1982;16(6):1106–1109. doi: 10.1128/jcm.16.6.1106-1109.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gumbo T., Chigutsa E., Pasipanodya J. The pyrazinamide susceptibility breakpoint above which combination therapy fails. J Antimicrob Chemother. 2014;69(9):2420–2425. doi: 10.1093/jac/dku136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alsultan A., Peloquin C.A. Therapeutic drug monitoring in the treatment of tuberculosis: an update. Drugs. 2014;74(8):839–854. doi: 10.1007/s40265-014-0222-8. [DOI] [PubMed] [Google Scholar]
- 68.Wilkins J.J., Langdon G., McIlleron H., Pillai G.C., Smith P.J., Simonsson U.S. Variability in the population pharmacokinetics of pyrazinamide in South African tuberculosis patients. Eur J Clin Pharmacol. 2006;62(9):727–735. doi: 10.1007/s00228-006-0141-z. [DOI] [PubMed] [Google Scholar]
- 69.Graham S.M., Bell D.J., Nyirongo S., Hartkoorn R., Ward S.A., Molyneux E.M. Low levels of pyrazinamide and ethambutol in children with tuberculosis and impact of age, nutritional status, and human immunodeficiency virus infection. Antimicrob Agents Chemother. 2006;50(2):407–413. doi: 10.1128/AAC.50.2.407-413.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.World Health Organization. Rapid advice: treatment of tuberculosis in children. Geneva; 2010. [PubMed]
- 71.Antwi S., Yang H., Enimil A. Pharmacokinetics of the first-line antituberculosis drugs in ghanaian children with tuberculosis with or without HIV coinfection. Antimicrob Agents Chemother. 2017;61(2) doi: 10.1128/AAC.01701-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sahai J., Gallicano K., Swick L. Reduced plasma concentrations of antituberculosis drugs in patients with HIV infection. Ann Intern Med. 1997;127(4):289–293. doi: 10.7326/0003-4819-127-4-199708150-00006. [DOI] [PubMed] [Google Scholar]
- 73.Rockwood N., Meintjes G., Chirehwa M. HIV-1 coinfection does not reduce exposure to rifampin, isoniazid, and pyrazinamide in South African tuberculosis outpatients. Antimicrob Agents Chemother. 2016;60(10):6050–6059. doi: 10.1128/AAC.00480-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van Oosterhout J.J., Dzinjalamala F.K., Dimba A. Pharmacokinetics of antituberculosis drugs in HIV-Positive and HIV-Negative adults in Malawi. Antimicrob Agents Chemother. 2015;59(10):6175–6180. doi: 10.1128/AAC.01193-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McIlleron H., Rustomjee R., Vahedi M. Reduced antituberculosis drug concentrations in HIV-infected patients who are men or have low weight: implications for international dosing guidelines. Antimicrob Agents Chemother. 2012;56(6):3232–3238. doi: 10.1128/AAC.05526-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chideya S., Winston C.A., Peloquin C.A. Isoniazid, rifampin, ethambutol, and pyrazinamide pharmacokinetics and treatment outcomes among a predominantly HIV-infected cohort of adults with tuberculosis from Botswana. Clin Infect Dis. 2009;48(12):1685–1694. doi: 10.1086/599040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ramachandran G., Kumar A.K., Kannan T. Low serum concentrations of rifampicin and pyrazinamide associated with poor treatment outcomes in children with tuberculosis related to HIV status. Pediatr Infect Dis J. 2016;35(5):530–534. doi: 10.1097/INF.0000000000001069. [DOI] [PubMed] [Google Scholar]
- 78.Pasipanodya J.G., McIlleron H., Burger A., Wash P.A., Smith P., Gumbo T. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J Infect Dis. 2013;208(9):1464–1473. doi: 10.1093/infdis/jit352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park J.S., Lee J.Y., Lee Y.J. Serum levels of antituberculosis drugs and their effect on tuberculosis treatment outcome. Antimicrob Agents Chemother. 2015;60(1):92–98. doi: 10.1128/AAC.00693-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yasinskaya Y., Sacks L. Models and approaches for anti-TB drug testing. Expert Rev Anti Infect Ther. 2011;9(7):823–831. doi: 10.1586/eri.11.64. [DOI] [PubMed] [Google Scholar]
- 81.Nuermberger E. Using animal models to develop new treatments for tuberculosis. Semin Respir Crit Care Med. 2008;29(5):542–551. doi: 10.1055/s-0028-1085705. [DOI] [PubMed] [Google Scholar]
- 82.Martinez-Hoyos M., Perez-Herran E., Gulten G. Antitubercular drugs for an old target: GSK693 as a promising InhA direct inhibitor. EBioMedicine. 2016;8:291–301. doi: 10.1016/j.ebiom.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blanco D., Perez-Herran E., Cacho M. Mycobacterium tuberculosis gyrase inhibitors as a new class of antitubercular drugs. Antimicrob Agents Chemother. 2015;59(4):1868–1875. doi: 10.1128/AAC.03913-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nikonenko B.V., Apt A.S. Drug testing in mouse models of tuberculosis and nontuberculous mycobacterial infections. Tuberculosis (Edinb) 2013;93(3):285–290. doi: 10.1016/j.tube.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 85.Cooper A.M. Mouse model of tuberculosis. Cold Spring Harb Perspect Med. 2014;5(2) doi: 10.1101/cshperspect.a018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kramnik I., Beamer G. Mouse models of human TB pathology: roles in the analysis of necrosis and the development of host-directed therapies. Semin Immunopathol. 2016;38(2):221–237. doi: 10.1007/s00281-015-0538-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Apt A., Kramnik I. Man and mouse TB: contradictions and solutions. Tuberculosis (Edinb) 2009;89(3):195–198. doi: 10.1016/j.tube.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McMurray D.N., Collins F.M., Dannenberg A.M., Jr., Smith D.W. Pathogenesis of experimental tuberculosis in animal models. Curr Top Microbiol Immunol. 1996;215:157–179. doi: 10.1007/978-3-642-80166-2_7. [DOI] [PubMed] [Google Scholar]
- 89.Tsai M.C., Chakravarty S., Zhu G. Characterization of the tuberculous granuloma in murine and human lungs: cellular composition and relative tissue oxygen tension. Cell Microbiol. 2006;8(2):218–232. doi: 10.1111/j.1462-5822.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 90.Aly S., Wagner K., Keller C. Oxygen status of lung granulomas in mycobacterium tuberculosis-infected mice. J Pathol. 2006;210(3):298–305. doi: 10.1002/path.2055. [DOI] [PubMed] [Google Scholar]
- 91.Pan H., Yan B.-.S., Rojas M. Ipr1 gene mediates innate immunity to tuberculosis. Nature. 2005;434(7034):767–772. doi: 10.1038/nature03419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lanoix J.P., Lenaerts A.J., Nuermberger E.L. Heterogeneous disease progression and treatment response in a C3HeB/FeJ mouse model of tuberculosis. Dis Model Mech. 2015;8(6):603–610. doi: 10.1242/dmm.019513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Prideaux B., Via L.E., Zimmerman M.D. The association between sterilizing activity and drug distribution into tuberculosis lesions. Nat Med. 2015;21(10):1223–1227. doi: 10.1038/nm.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gumbo T., Dona C.S., Meek C., Leff R. Pharmacokinetics-pharmacodynamics of pyrazinamide in a novel in vitro model of tuberculosis for sterilizing effect: a paradigm for faster assessment of new antituberculosis drugs. Antimicrob Agents Chemother. 2009;53(8):3197–3204. doi: 10.1128/AAC.01681-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Carow B., Hauling T., Qian X., Kramnik I., Nilsson M., Rottenberg M.E. Spatial and temporal localization of immune transcripts defines hallmarks and diversity in the tuberculosis granuloma. Nat Commun. 2019;10(1):1823. doi: 10.1038/s41467-019-09816-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim J.J., Lee H.M., Shin D.M. Host cell autophagy activated by antibiotics is required for their effective antimycobacterial drug action. Cell Host Microbe. 2012;11(5):457–468. doi: 10.1016/j.chom.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 97.Nardacci R., Ciccosanti F., Marsella C., Ippolito G., Piacentini M., Fimia G.M. Role of autophagy in HIV infection and pathogenesis. J Intern Med. 2017 doi: 10.1111/joim.12596. [DOI] [PubMed] [Google Scholar]
- 98.Li X., Li Y., Fang S. Downregulation of autophagy-related gene ATG5 and GABARAP expression by IFN-lambda1 contributes to its anti-HCV activity in human hepatoma cells. Antiviral Res. 2017;140:83–94. doi: 10.1016/j.antiviral.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 99.Massey D.C., Parkes M. Genome-wide association scanning highlights two autophagy genes, ATG16L1 and IRGM, as being significantly associated with Crohn's disease. Autophagy. 2007;3(6):649–651. doi: 10.4161/auto.5075. [DOI] [PubMed] [Google Scholar]
- 100.Van Grol J., Subauste C., Andrade R.M., Fujinaga K., Nelson J., Subauste C.S. HIV-1 inhibits autophagy in bystander macrophage/monocytic cells through Src-Akt and STAT3. PLoS ONE. 2010;5(7):e11733. doi: 10.1371/journal.pone.0011733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sarathy J.P., Zuccotto F., Hsinpin H. Prediction of drug penetration in tuberculosis lesions. ACS Infect Dis. 2016;2(8):552–563. doi: 10.1021/acsinfecdis.6b00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dartois V., Barry C.E., 3rd. A medicinal chemists' guide to the unique difficulties of lead optimization for tuberculosis. Bioorg Med Chem Lett. 2013;23(17):4741–4750. doi: 10.1016/j.bmcl.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Singh P., Mishra A.K., Malonia S.K. The paradox of pyrazinamide: an update on the molecular mechanisms of pyrazinamide resistance in mycobacteria. J Commun Dis. 2006;38(3):288–298. [PubMed] [Google Scholar]
- 104.Almeida D., Nuermberger E., Tasneen R. Paradoxical effect of isoniazid on the activity of rifampin-pyrazinamide combination in a mouse model of tuberculosis. Antimicrob Agents Chemother. 2009;53(10):4178–4184. doi: 10.1128/AAC.00830-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Grosset J., Truffot-Pernot C., Lacroix C., Ji B. Antagonism between isoniazid and the combination pyrazinamide-rifampin against tuberculosis infection in mice. Antimicrob Agents Chemother. 1992;36(3):548–551. doi: 10.1128/aac.36.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee J.H., Ammerman N.C., Nolan S. Isoniazid resistance without a loss of fitness in mycobacterium tuberculosis. Nat Commun. 2012;3:753. doi: 10.1038/ncomms1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mahapatra S., Woolhiser L.K., Lenaerts A.J. A novel metabolite of antituberculosis therapy demonstrates host activation of isoniazid and formation of the isoniazid-NAD+ adduct. Antimicrob Agents Chemother. 2012;56(1):28–35. doi: 10.1128/AAC.05486-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wengenack N.L., Rusnak F. Evidence for isoniazid-dependent free radical generation catalyzed by mycobacterium tuberculosis KatG and the isoniazid-resistant mutant KatG(S315T) Biochemistry. 2001;40(30):8990–8996. doi: 10.1021/bi002614m. [DOI] [PubMed] [Google Scholar]
- 109.Johnsson K., Schultz P.G. Mechanistic studies of the oxidation of isoniazid by the catalase peroxidase from mycobacterium tuberculosis. J Amer Chem Soc. 1994;116(16):7425–7426. [Google Scholar]
- 110.Lei B., Wei C.J., Tu S.C. Action mechanism of antitubercular isoniazid. activation by mycobacterium tuberculosis KatG, isolation, and characterization of inha inhibitor. J Biol Chem. 2000;275(4):2520–2526. doi: 10.1074/jbc.275.4.2520. [DOI] [PubMed] [Google Scholar]
- 111.Rawat R., Whitty A., Tonge P.J. The isoniazid-NAD adduct is a slow, tight-binding inhibitor of InhA, the mycobacterium tuberculosis enoyl reductase: adduct affinity and drug resistance. Proc Natl Acad Sci U S A. 2003;100(24):13881–13886. doi: 10.1073/pnas.2235848100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vilcheze C., Weisbrod T.R., Chen B. Altered NADH/NAD+ ratio mediates coresistance to isoniazid and ethionamide in mycobacteria. Antimicrob Agents Chemother. 2005;49(2):708–720. doi: 10.1128/AAC.49.2.708-720.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.van Hest R., Baars H., Kik S. Hepatotoxicity of rifampin-pyrazinamide and isoniazid preventive therapy and tuberculosis treatment. Clin Infect Dis. 2004;39(4):488–496. doi: 10.1086/422645. [DOI] [PubMed] [Google Scholar]
- 114.McNeill L., Allen M., Estrada C., Cook P. Pyrazinamide and rifampin vs isoniazid for the treatment of latent tuberculosis: improved completion rates but more hepatotoxicity. Chest. 2003;123(1):102–106. doi: 10.1378/chest.123.1.102. [DOI] [PubMed] [Google Scholar]
- 115.Centers for Disease Control and Prevention Fatal and severe hepatitis associated with rifampin and pyrazinamide for the treatment of latent tuberculosis infection–New York and Georgia, 2000. Morb Mortal Wkly Rep. 2001;50(15):289–291. [PubMed] [Google Scholar]
- 116.Stout J.E., Engemann J.J., Cheng A.C., Fortenberry E.R., Hamilton C.D. Safety of 2 months of rifampin and pyrazinamide for treatment of latent tuberculosis. Am J Respir Crit Care Med. 2003;167(6):824–827. doi: 10.1164/rccm.200209-998OC. [DOI] [PubMed] [Google Scholar]
- 117.Whitehouse L.W., Lodge B.A., By A.W., Thomas B.H. Metabolic disposition of pyrazinamide in the rat: identification of a novel in vivo metabolite common to both rat and human. Biopharm Drug Dispos. 1987;8(4):307–318. doi: 10.1002/bdd.2510080402. [DOI] [PubMed] [Google Scholar]
- 118.Ramappa V., Aithal G.P. Hepatotoxicity related to anti-tuberculosis drugs: mechanisms and management. J Clin Exp Hepatol. 2013;3(1):37–49. doi: 10.1016/j.jceh.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mitchell J.R., Zimmerman H.J., Ishak K.G. Isoniazid liver injury: clinical spectrum, pathology, and probable pathogenesis. Ann Intern Med. 1976;84(2):181–192. doi: 10.7326/0003-4819-84-2-181. [DOI] [PubMed] [Google Scholar]
- 120.Knowles S.R., Uetrecht J., Shear N.H. Idiosyncratic drug reactions: the reactive metabolite syndromes. Lancet. 2000;356(9241):1587–1591. doi: 10.1016/S0140-6736(00)03137-8. [DOI] [PubMed] [Google Scholar]
- 121.Guo H.L., Hassan H.M., Zhang Y. Pyrazinamide induced rat cholestatic liver injury through inhibition of fxr regulatory effect on bile acid synthesis and transport. Toxicol Sci. 2016;152(2):417–428. doi: 10.1093/toxsci/kfw098. [DOI] [PubMed] [Google Scholar]
- 122.Tailor A., Faulkner L., Naisbitt D.J., Park B.K. The chemical, genetic and immunological basis of idiosyncratic drug-induced liver injury. Hum Exp Toxicol. 2015;34(12):1310–1317. doi: 10.1177/0960327115606529. [DOI] [PubMed] [Google Scholar]
- 123.Koen Y.M., Galeva N.A., Metushi I.G., Uetrecht J., Hanzlik R.P. Protein targets of isoniazid-reactive metabolites in mouse liver in vivo. Chem Res Toxicol. 2016;29(6):1064–1072. doi: 10.1021/acs.chemrestox.6b00098. [DOI] [PubMed] [Google Scholar]
- 124.Yamamoto T., Moriwaki Y., Takahashi S., Hada T., Higashino K. In vitro conversion of pyrazinamide into 5-hydroxypyrazinamide and that of pyrazinoic acid into 5-hydroxypyrazinoic acid by xanthine oxidase from human liver. Biochem Pharmacol. 1987;36(19):3317–3318. doi: 10.1016/0006-2952(87)90654-x. [DOI] [PubMed] [Google Scholar]
- 125.Shih T.Y., Pai C.Y., Yang P., Chang W.L., Wang N.C., Hu O.Y. A novel mechanism underlies the hepatotoxicity of pyrazinamide. Antimicrob Agents Chemother. 2013;57(4):1685–1690. doi: 10.1128/AAC.01866-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lacroix C., Hoang T.P., Nouveau J. Pharmacokinetics of pyrazinamide and its metabolites in healthy subjects. Eur J Clin Pharmacol. 1989;36(4):395–400. doi: 10.1007/BF00558302. [DOI] [PubMed] [Google Scholar]
- 127.Marakalala M.J., Raju R.M., Sharma K. Inflammatory signaling in human tuberculosis granulomas is spatially organized. Nat Med. 2016;22(5):531–538. doi: 10.1038/nm.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]