Abstract
As our population grows older, age-related pathologies are becoming more prevalent. Deterioration of skeletal muscle and the immune system manifests as sarcopenia and immune senescence respectively. The disease burden of these pathologies emphasizes the need for a better understanding of the underlying mechanisms. Skeletal muscle has emerged as a potent regulator of immune system function. As such, skeletal muscle might be the central integrator between sarcopenia and immune senescence in an aging biological system. Therapeutic approaches targeting skeletal muscle might be able to restore both muscle and immune system function. In this review, we therefore outline the current - however still fragmentary - knowledge about the potential communication pathways of muscle and immune system, how they are affected by aging of skeletal muscle and discuss possible treatment strategies. The review intends to be hypothesis-generating and should thereby stimulate further research in this important scientific field.
Keywords: Skeletal muscle; Sarcopenia; Immune senescence; Myokines; IL-6, IL-7; IL-15
- AMPK
5′-AMP-activated protein kinase;
- BDNF
brain-derived neurotrophic factor;
- CCL-21
CC-chemokine ligand 21;
- EAM
experimental autoimmune myositis;
- MAPK
mitogen-activated protein kinase;
- NFAT
nuclear factor of activated T-cells;
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells;
- NK
natural killer;
- NKG2D
natural killer group 2D;
- LIF
leukaemia inhibitory factor;
- STAT
signal transducer and activator of transcription;
- SASP
senescence-associated secretory phenotype;
- TNF-α
tumour necrosis factor α;
- PD-L1
programmed cell death 1 ligand
1. Introduction
Biological aging is defined by the loss of physiological integrity. Almost every organ in the human body is affected by the detrimental effects of aging. Skeletal muscle is no exception. Muscle mass and function decline with age [1]. This age-dependent loss of muscle quality and quantity defines the sarcopenic phenotype according to the European Working Group on Sarcopenia in Older People [2]. Since 2018, sarcopenia is considered a muscle disease and muscle strength is superior to muscle mass in predicting adverse outcomes. Thus, muscle strength is considered the primary parameter defining sarcopenia [2].
The importance of a clinical definition identifying sarcopenic patients is highlighted by an increasingly aging population. Currently, around 10% of elderly patients are considered sarcopenic. This number is expected to rise dramatically. In Europe, a 72% increase in the number of sarcopenic patients until 2045 is expected, severely impacting the quality of life [3].. However, an exact understanding of the underlying mechanisms leading to sarcopenia and its clinical consequences is still lacking. Immunological dysregulation and chronic inflammation have been discussed in the multifaceted pathogenesis of sarcopenia. The interaction between the immune system and the muscle compartment has been thought to be unilateral. Lately however, skeletal muscle has been shown to regulate immunological processes and the inflammatory response [4]. With respect to immune function, although lacking an undisputed definition, the term immune senescence is commonly used to summarize the age-dependent deterioration of the immune system. Key features of immune senescence are thymic atrophy, accumulation of senescent T-cells, impaired function of innate immune cells such NK-cells, macrophages and neutrophils, and defective maintenance and functional response of lymphocytes [5,6] Age-dependent alterations of the immunological function of skeletal muscle have also been observed [7,8] Therefore, sarcopenia and immune senescence might be linked/interact via the skeletal muscle. In this review, we will discuss a potential central role of skeletal muscle in regulating its own and immune system function during aging.
2. The disease burden of sarcopenia: a risk factor for infections
Recently, several adverse outcomes of sarcopenia have been identified. These include but are not limited to an increased risk of falls leading to fractures, disability and functional impairment, dysphagia, lower quality of life, and all-cause mortality [2].
Sarcopenia predicts the risk for infection after surgery [9]. Additionally, after three weeks of hospitalization patients diagnosed with sarcopenia showed a two-fold increased risk of developing nosocomial infections [10]. The impact of sarcopenia on the risk of infection in community-dwelling patient is less clear as the lack of epidemiological studies precludes a conclusive statement. However, sarcopenia predicts both the risk for community-acquired pneumonia in the elderly [11] as well as 90-day mortality in patients suffering from aspiration pneumonia [12].
Although the studies outlined above do not establish causality, they suggest a link between impaired muscle function and an impaired immune response to pathogens. Given the high incidence for sarcopenia and the increased risk for infections in elderly patients, the implications are profound as sarcopenia might constitute both a clinical predictor for patients at risk as well as a potential therapeutic target to ameliorate infection-associated morbidity in the elderly.
3. Skeletal muscle as a potential central regulator of immune system function
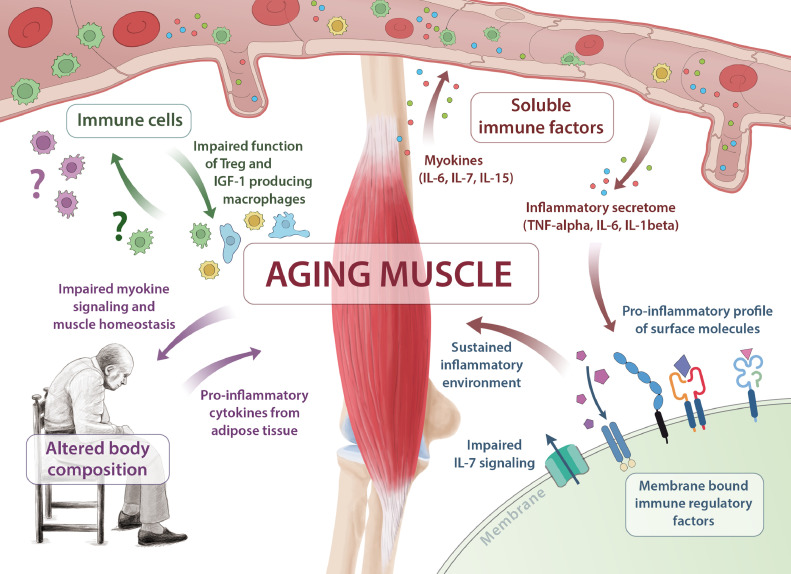
In the last two decades, the perception of skeletal muscle as a pure locomotors unit has shifted. Muscle is increasingly recognized as an organ with immune regulatory properties. As such, skeletal muscle cells modulate immune function by signalling through different soluble factors, cell surface molecules or cell-to-cell interactions [4]. Although our knowledge of the muscle-immune system interplay has advanced considerably, the impact of age is relatively unknown. Sarcopenia may severely disturb this interaction, providing a potential explanation for the observed clinical outcomes of sarcopenic patients. In the following chapters we will discuss the possible mechanisms responsible for the impact of aging skeletal muscle on immune system function and vice versa (Fig. 1).
Fig. 1.
Aging of skeletal muscle is central in the pathogenesis of immune senescence and sarcopenia. Multiple pathways are affected, including insufficient myokine signalling (IL-6, IL-7, IL-15), shifting of membrane bound immune regulatory factors towards a pro-inflammatory profile, impaired immune cell function and altered body composition.
3.1. Soluble factors
Muscle is increasingly recognized as an endocrine organ producing and releasing cytokines and other peptides, which exert autocrine, paracrine and endocrine activity on numerous tissues. Consequently, these soluble factors are commonly termed myokines. Proteomic profiling has been applied to the secretome of skeletal muscle and identified more than 300 potential myokines [13]. Myokines such as IL-6, IL-7, IL-15 or LIF have been shown to modulate the immune system [13]. Remarkably, serum concentrations of myokines such as IL-7 and IL-15 are inversely correlated with age, suggesting a link between skeletal muscle and age-dependent loss of immune system function [7,8,14]
IL-15: a recipe for sustained immune function?
IL-15 belongs to the same family of cytokines as IL-2, communicating down-stream effects via the Janus kinases 1 and 3, STAT3 and STAT5 pathways. IL-15 is expressed in various cells and tissues, including muscle and there is a growing evidence implicating IL-15 as a myokine [15]. IL-15 mRNA expression and IL-15 immunoreactivity in muscle were significantly elevated in obese rats undergoing treadmill training in comparison with sedentary rats [16]. Corroborating the effects seen in rodents, biopsies obtained from humans completing a strenuous resistance exercise protocol displayed 2fold higher IL-15 mRNA levels after 24 h of recovery[17] and increased IL-15 plasma levels were observed in untrained, male subjects after exercise [18]. Of note, IL-15 release in response to exercise seems to be pulsatile and is not sustained over a longer period of time [18]. On a molecular level, the 5′-AMP-activated protein kinase (AMPK) acts as a sensor of intracellular energy levels in skeletal muscle [19]. Consequently, AMPK is crucial for exercise induced IL-15 production and release since transgenic mice with a functionally inactive AMPK showed reduced levels of both IL-15 mRNA and plasma concentration [15]. Interestingly, AMPK activity declines with aging, thus providing a potential molecular mechanism underlying impaired IL-15 signalling in aged muscle [15,20] The implications of exercise-induced regulation of systemic IL-15 levels are profound: IL-15 is crucial in maintaining immune function while also stimulating myogenesis and reducing adipose tissue distribution. Acting independently or in synergy with IGF-1, IL-15 is able to induce myosin heavy chain synthesis. Additionally, IL-15 affects other cell types that compose skeletal muscle such as resident fibro-adipogenic progenitors (FAP). IL-15 stimulates the proliferation of FAPs while also inhibiting differentiation of FAPs into adipocytes, thereby promoting muscle regeneration [21]. IL-15 also exerts a catabolic effect on adipose tissue, effectively reducing adiposity therefore regulating body composition [22]. Given the emerging role of adipose tissue in the pathogenesis of sarcopenia, impaired IL-15 signalling may contribute to the coalescence of sarcopenia and obesity.
Moreover, IL-15 is crucial in the development and maintenance of immune cells. The proliferation, activation and distribution of NK-cells are regulated by IL-15. Similarly to NK-cells, IL-15 modulates CD8 T-cell homeostasis and promotes survival of naïve T-cells as well as proliferation of B-cells [23]. NK-cells and CD8 T-cells are necessary for the effective clearance of viral pathogens and the destruction of tumour cells. Consequently, the immune response in IL-15 knockout mice infected with the vaccinia virus was impaired [24]. IL-15 is also implicated in the innate immune response by enhancing neutrophil migration and phagocytosis [25].
IL-15 signalling is therefore essential in maintaining body composition and immune function. However, a decline in serum levels of IL-15 and soluble IL-15Rα was observed in murine aging models and expression of intramuscular IL-15 and IL-15Rα decreases in aged rats [8,14] Substantiating this data, an age-dependent decline in IL-15 serum levels in humans has been reported [7]. Although the impact of IL-15 in an aging system is not yet fully understood, IL-15 signalling is implicated in sustaining NK-cells, neutrophils and lymphocyte function in old age.
On a knife´s edge: The dual role of IL-6
IL-6 exerts a complex biological profile. Broadly, IL-6 can exert both pro- and anti-inflammatory effects and even promote muscle anabolism or catabolism depending on the target structure, the predominant cytokine environment and the mode of release.
-
(1)
pro-inflammatory and catabolic effects
As part of the inflammatory secretome, IL-6 is secreted in response to infection or tissue damage by a plethora of cells such as neutrophils, T- and B-cells, macrophages and endothelial cells [26]. Pro-inflammatory effects of IL-6 signalling include enhanced T-cell recruitment and expansion, stimulation of antibody production from B-cells, abrogation of de novo regulatory T-cell (Treg) differentiation and enhanced lymphocyte trafficking due to upregulation of cell adhesion molecules such as ICAM-1 and CCL-21 [26]. Besides activation of immune system function in response to pathogens, IL-6 signalling is part of age-related chronic low-grade inflammation and implicated in the pathogenesis of sarcopenia [27], [28], [29]. Under physiological conditions the activity of IL-6 is limited to the duration of the injury, while chronic low-grade inflammation is associated with prolonged exposure to IL-6 signalling.
IL-6 was shown to facilitate muscle atrophy by blunting muscle anabolism and energy homeostasis and may also directly mediate muscle catabolism [30]. Transgenic mice chronically overexpressing IL-6 displayed a marked loss of muscle mass coinciding with increased cathepsin activity. Treating these mice with an IL-6 receptor antibody mitigated the detrimental effect of IL-6 on muscle [31]. Interestingly, IL-6 is also produced by FAPs and was shown to promote muscle atrophy and fibrosis mediated by aberrant STAT3-IL-6 signalling. Therefore, this mechanism may contribute to the sarcopenic phenotype seen in various neuromuscular diseases or in the elderly [32].
However, IL-6 knockout mice displayed no significant difference in muscle catabolism as compared to wild type mice in experimentally induced sepsis [33]. These findings indicate that the sole action of IL-6 is not sufficient to induce muscle wasting; instead, the catabolic effect of IL-6 is dependent on the synergistic interaction with other factors mediating the inflammatory response. Thus, IL-6 mediated muscle catabolism depends on (a) the chronic exposure to IL-6 and (b) the concomitant activity of other pro-inflammatory cytokines such as TNF-α.
-
(1)
anti-inflammatory and anabolic effects
Interestingly, IL-6 can also act as a major myokine. The concentration of circulating IL-6 increases drastically following exercise and depends on intensity of muscle exercise implicating that IL-6 is released from skeletal muscle in order to restore muscle homeostasis after exercise [34]. Indeed, IL-6 signalling is pivotal for skeletal muscle regeneration and hypertrophy by regulating satellite cell function and enhancing glucose metabolism [35].
The ability of IL-6 to induce skeletal muscle anabolism as well might be explained by a different biological profile. Exercise leads to a Ca2+ release from the sarcoplasmic reticulum [36]. In response to Ca2+, calcineurin, an activator of the nuclear factor of activated T-cells (NFAT), mediates IL-6 production in myocytes. Of note, elevating intracellular Ca2+levels in turn inhibit TNF-α expression [37]. Consequently, in response to exercise only IL-6 is secreted, while TNF-α levels remain largely unchanged. Only strenuous exercise, such as running a marathon, provoked a minor TNF-α response [38]. Further independent pathways such as p38 MAPK are induced by exercise mediated glycogen-usage and lead to increased IL-6 mRNA levels [39]. Therefore, exercise-induced IL-6 release displays a markedly different biological profile than IL-6 exposure seen in chronic, low-grade inflammation. Indeed, subjects, who were injected with a low dose of Escherichia coli endotoxin after three hours of cycling, did not mount a TNF-α response, while a resting control group injected with the same amount of toxin displayed a TNF-α increase up to 3fold as compared to baseline levels [40]. The intricate link between IL-6 and TNF-α is of importance, because TNF-α is not only implicated in the pathogenesis of sarcopenia but also able to reduce CD28 expression on T-cells resulting in the accumulation of CD28 negative T-cells, a hallmark of immune senescence [41,42]
Under certain circumstances IL-6 might also have anti-inflammatory effects. Human IL-6 induced secretion of IL-1 receptor antagonist (IL-1ra) and IL-10 in healthy volunteers [43]. IL-1ra inhibits the inflammatory effect mediated by IL-1, IL-1α and IL-1β and recombinant IL-1ra is currently tested in the treatment of autoimmune diseases [44]. IL-10 limits Th 1 inflammatory response, inhibits NF-κB signalling and TNF-α release [45]. Of note, IL-10 is also implied in B-cell differentiation, which is impaired in aging individuals as part of immune senescence [45].
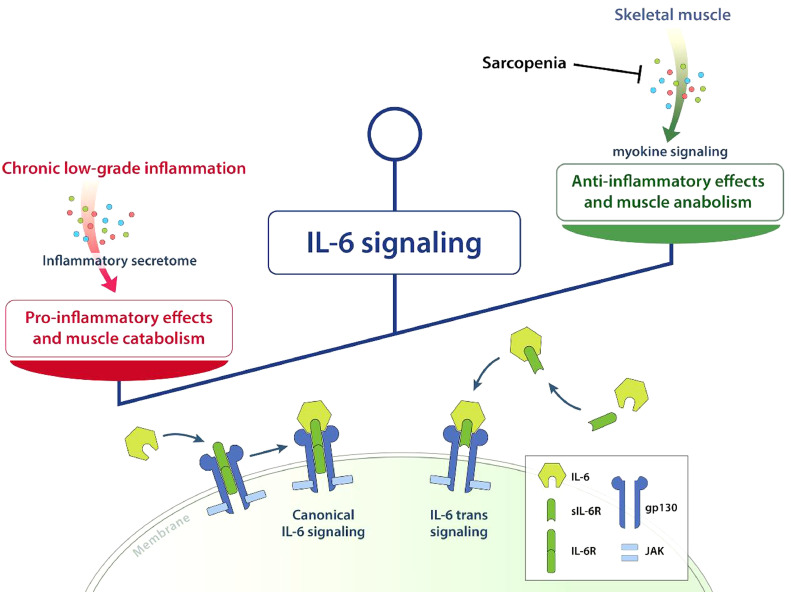
In conclusion, IL-6 is indeed a “double-edged sword” [34]. Chronic exposure and the concomitant action of pro-inflammatory cytokines such as TNF-α cause IL-6 to exhibit pro-inflammatory properties, ultimately leading to muscle catabolism and disruption of immune homeostasis. However, in the absence of pro-inflammatory cytokines, pulsatile IL-6 release in response to exercise is crucial for myogenesis and the regulation of the inflammatory response. In the context of sarcopenia, we propose that IL-6 signalling may shift towards the pro-inflammatory profile as skeletal muscle function is gradually lost in an aging system (Fig. 2).
Fig. 2.
Aging tips the scales of IL-6 signalling. Chronic exposure to IL-6 and the concomitant release of pro-inflammatory cytokines promote pro-inflammatory effects and muscle catabolism due to IL-6 signalling. The pulsatile release of IL-6 in response to exercise is impaired in sarcopenia resulting in reduced anti-inflammatory effects and impaired muscle anabolism mediated by IL-6. The biological effect of IL-6 is mediated both by canonical and by trans-signalling.
IL-7
IL-7 signalling plays an important role in the development and maintenance of immature lymphocytes while also supporting thymic function [7]. IL-7 is primarily produced by the stromal and epithelial cells of the thymus [46]. The expression of IL-7 declines with age in mice [47]. Accordingly, an age dependent decrease of IL-7 serum levels has been reported in humans [7]. Recently, IL-7 has been classified as a myokine due to its expression and secretion from skeletal muscle cells [48]. While the impact of age on skeletal muscle as a source of IL-7 remains to be investigated, exercise can restore the decline of IL-7 levels observed in old age, highlighting skeletal muscle as a potential source of IL-7 [7]. However, down-stream signalling of the IL-7 receptor mediated by Jak1 and −3 activation resulting in phosphorylation of STAT-molecules is impaired with age [49]. In line with this data, IL7 receptor knock-out mice display defects of early lymphopoiesis characteristic for immune senescence [50]. Thus, defective IL-7 signalling is implicated as a link between skeletal muscle and the aging phenotype of immune senescence [7].
3.2. Membrane bound factors and the senescence-associated secretory phenotype
Membrane bound factors constitute a pivotal aspect of the immune-muscle system interface. As such, skeletal muscle expresses a number of crucial immune modulatory molecules such as ICAM-1, ICOSL, CD40, B7-H1, NKG2DL or PD-L1 [4]. The direct effect of aging on the expression of these molecules remains to be elucidated. However, senescent cells, namely endothelial and epithelial cells, that accumulate during aging often express a senescence-associated secretory phenotype (SASP) with overexpression of IL-1β, IL-6, IL-8, TNF-α and IFN-γ among others [51]; Senescence of cells resident in skeletal muscle itself might induce changes in aged muscle cells and fibres by the senescence-associated secretory phenotype, since muscle injury induces senescent cell accumulation in young mice[52] and xenotransplantation of senescent cells into mouse skeletal muscle induced markers of senescence in muscle fibres [53]. Thus, senescence of tissue resident cells might exert a paracrine effect through the SASP and modulate aged muscle cells, possibly providing a further link between aging of skeletal muscle and immune senescence. Correspondingly, cytokines associated with the SASP were shown to induce the expression of pro-inflammatory surface molecules such as ICAM-1 or B7-H1 (CD80) in human muscle cells [54], [55], [56]. Therefore, the SASP associated with cells resident in skeletal muscle might also modulate the expression of further surface molecules in skeletal muscle cells and promote an inflammatory environment. However, further research investigating this link is clearly needed. Of note, elimination of senescent cells in old mice through pharmacological or genetic interventions was able to highly improve function of aged muscle, thus delineating a potential treatment modality for the management of sarcopenia and its associated ailments [57].
3.3. Cellular factors: muscle-immune cell interactions
The regenerative potential of skeletal muscle is dependent on the interaction between skeletal muscle and immune cells. In response to injury, immune cells infiltrate skeletal muscle in order to restore muscle homeostasis by removing necrotic cells and secreting growth factors necessary for satellite cell proliferation and differentiation [58]. The physiological functions of immune cells are gradually lost with advanced age leading to impaired regenerative capacity of skeletal muscle [59].
A major subset of T-cells infiltrating skeletal muscle are CD4+FoxP3+ Tregs, which constitute around 50% of CD4+ T-cells infiltrating muscle, and control muscle inflammation upon tissue damage. Of note, Treg homeostasis is impaired in aged mice due to increased turn-over rates and reduced recruitment from the circulating T cell pool [60]. Treg function is dependent on IL-33 signalling. In the context of skeletal muscle, IL-33 is mainly expressed by cells resembling fibro/adipogenic progenitor cells in response to tissue injury. In older animals, IL-33 signalling is significantly blunted due to reduced IL-33 expression and reduced number of IL-33 secreting fibro/adipogenic progenitor-like cells [60]. Rescuing Treg function in old mice by IL-33 injection improved muscle regeneration as evidenced by histological analysis, which is attributed to an increased satellite cell expansion and a shift of the muscle transcriptome towards a muscle repair signature. In part, Treg and satellite cell interaction is mediated by amphiregulin, a growth factor directly acting on satellite cells [61]. Besides promoting tissue regeneration, Tregs are potent regulators of inflammation able to suppress muscle inflammation. Therefore, defects in Treg function may promote the catabolic effect of inflammation in aged muscle. [58] A rodent model of experimental autoimmune myositis substantiates this data, as EAM mice depleted of Tregs displayed increased disease severity, whereas injection of polyclonal Tregs into EAM mice ameliorated disease activity [62]. Consequently, integrity of Treg activity is pivotal for sustaining muscle health into old age, as Tregs regulate both inflammation and regeneration of skeletal muscle.
Besides Tregs, macrophages regulate tissue regeneration and inflammation in response to injury. Based on the corresponding stimuli, macrophages exhibit a continuum of phenotypes and are able to exhibit both pro- and anti-inflammatory properties [63]. As such, IGF-1 produced by anti-inflammatory macrophages is a major driver of tissue regeneration and limits tissue inflammation [64]. While systemic IGF-1 is mainly produced in the liver and serum levels decline with age, little is known about the impact of age on the population of IGF-1 producing anti-inflammatory macrophages resident in skeletal muscle [65]. Interestingly, impaired macrophage polarization has been reported in old mice [66]. Consequently, it can be hypothesized that impaired activity of anti-inflammatory macrophages in old age contributes both to atrophy and inflammatory responses of skeletal muscle. However, further research is clearly necessary to understand the complex interplay between skeletal muscle cells and immune cells.
In conclusion, skeletal muscle might be central in the pathogenesis of sarcopenia and immune senescence. Myokines, membrane bound factors and cell-to-cell interactions are potential communication pathways affected by ageing of skeletal muscle; a process that results in a plethora of adverse effects (Table 1).
Table 1.
Consequences of skeletal muscle ageing.
| Consequences of skeletal muscle ageing | ||
|---|---|---|
| Effect | Potential mechanisms | References |
| Impaired immunological protection | Impaired proliferation, activation and maintenance of NK cells and proliferation and survival of naïve T-cells an CD8 T-cells due to impaired IL-15 signalling | (7, 8, 23, 24) |
| Defective neutrophil migration and phagocytosis due to impaired IL-15 signalling | (7, 8, 25) | |
| Impaired IL-7 signalling might lead to defective maintenance and development of T- and B-lymphocytes and thymic atrophy | (7, 48, 49, 50) | |
| Establishment of a pro-inflammatory environment | Impaired IL-6 signalling as a myokine | |
| Increased TNF-α production | (40) | |
| Reduced IL-1ra and IL-10 levels | (43, 44, 45) | |
| Expression of pro-inflammatory surface molecules in response to inflammatory secretome | (5, 53–56) | |
| Impaired muscle regeneration | Impaired function muscle resident Tregs | (59–51) |
| Impaired function of IGF-1 producing macrophages | (62 - 65) | |
| Altered body composition | Reduced IL-15 signalling leads to increased adiposity | (22) |
| Enhanced skeletal muscle wasting | Impaired IL-15 induced myosin chain synthesis | (22) |
| Enhanced IL-6 signalling as part of the inflammatory secretome induces muscle catabolism | (30–34) | |
| Accumulation of senescent cells in skeletal muscle | Detrimental effect of the SASP on bystander-cells | (51, 52, 53) |
| Possible establishment of an inflammatory environment | (53, 54, 55) |
4. Sarcopenia and immune senescence: a bidirectional link
As humans age, the immune system undergoes drastic changes. As detailed before, the umbrella term immune senescence is used to encompass these changes. Moreover, ageing is associated with increased serum levels of pro-inflammatory molecules, in particular CRP, TNF-α, and IL-6 [27], [28], [29]. While the source and complex regulation of this low-grade inflammation are subject of current research and beyond the scope of this review, cross sectional studies suggest an impact of low-grade inflammation on muscle health, since IL-6 is a significant predictor for sarcopenia [27], [28], [29]. Additionally, elevated serum levels of both TNF-α and CRP were associated with sarcopenia [27]. However, it should be noted that these studies are cross-sectional. Longitudinal studies examining the association of sarcopenia with markers of low-grade inflammation are sparse. A five-year prospective cohort study correlating cytokine levels and muscle mass concluded that elevated serum levels of IL-6 and CRP were significantly associated with the loss of total appendicular skeletal muscle [29]. Interestingly, a meta-analysis from 2016 reported a significant association of CRP levels with sarcopenia, while serum IL-6 and TNF-α did not differ between sarcopenic patients and controls [27].
So far, we have outlined that skeletal muscle exhibits immune regulatory properties and that chronic, low-grade inflammation may induce muscle wasting. The concept of skeletal muscle as a regulator of immune function is relatively new and adds a new layer of complexity to the muscle-immune system link. Consequently, the muscle-immune system connection might be bidirectional: Chronic, low-grade inflammation induces muscle catabolism via pleiotropic mechanisms mediated by the inflammatory secretome [28]. Concurrently, homeostasis of skeletal muscle is, in part, responsible for healthy immune function. However, when dysregulated, insufficient myokine signalling, alteration of membrane bound factors towards a pro-inflammatory profile and impaired regenerative capacities of immune cells might result in disruption of immune system function.
We propose that biological aging may disturb the equilibrium of muscle-immune system homeostasis with skeletal muscle acting as a potential central link between sarcopenia and immune senescence. Healthy muscle function is gradually lost in an aging biological system due to physical inactivity, metabolic changes and the accumulation of chronic, low-grade inflammation. In turn, impaired muscle function curtails skeletal muscle cell signalling needed for immune regulation and maintenance, cumulating in a vicious cycle in which immune and muscle system dysfunction sustain each other [7,28]
Sarcopenic patients are at increased risk of infection, implicating a clinical correlate of impaired immune function [9,10] The impact of sarcopenia on immune function with respect to autoimmune disease and cancer is less clear. The incidence of cancer and autoimmune disorders increases as people age, while, on average, muscle mass declines [67]. Although suggestive, causality cannot be deduced. Longitudinal studies investigating the influence of sarcopenia on the incidence of cancer and autoimmune diseases are needed.
5. Outlook: treating the muscle-immune system
Demographic change is a major challenge of our time. As our population grows older, age associated pathologies become more prevalent. Two of these pathologies, sarcopenia and immune senescence, might be intrinsically linked by skeletal muscle as central integrator and demand a feasible and effective treatment.
Exercise, a broad term summarizing physical activity with the goal of preserving or improving physical fitness, has already proven effective. In regard to sarcopenia, exercise has emerged as the most important and consistent treatment option, improving skeletal muscle metabolism and function [68]. Importantly, physical activity has also been shown to support immune function in old age, specifically improving vaccine responses and reducing chronic inflammation [69]. Physically active elders display higher numbers of naïve T-cells and recent thymic emigrants, two major hallmarks of immune senescence, as compared to sedentary age-matched controls [7]. Additionally, exercise is associated with improved neutrophil function, NK-cell cytotoxicity and vaccine responses [70]. The observed effects might be attributed to the thymoprotective effect of increased IL-7 levels seen in active individuals [7]. Thus, exercise may protect immune and muscle function against the detrimental effects of aging. As the level of physical activity is inversely correlated with all-cause mortality, exercise is emerging as a major driver of longevity [71]. Although ambitious to implement, exercise may be critical in protecting an aging population against the clinical consequences of sarcopenia and immune senescence. In addition to exercise, nutritional strategies might be able to improve function of aged skeletal muscle. Specifically, an increase of muscle strength relative to body mass has been observed in patients undergoing caloric restriction [72]. While the intricacies of the underlying mechanisms warrant further research, at least in part, the impact of caloric restriction on muscle function might be attributed to a reduction of circulating pro-inflammatory cytokine levels [73] as well as a potential senostatic effect of caloric restriction [53], providing a further possible link between muscle and immune system function.
Another promising treatment modality has been delineated by studies investigating models of heterochronic parabiosis. Here, the circulation of a young and an old rodent are surgically joined, elegantly demonstrating that soluble factors derived from the young environment are able to induce signalling pathways in the aged system. Subsequently, aged rodents in a heterochronic pairing displayed enhanced muscle regeneration as compared to old rodents in a homochronic pairing [74]. To date, the exact identity of the factor(s) mediating this rejuvenating effect remains elusive. Attributing the effect of parabiosis to a single factor seems doubtful, instead, the interplay of various factors may constitute a rejuvenating secretome able to maintain immune system and skeletal muscle function into old age. As detailed before, possible candidates for this profile are IL-7 and IL-15 among others. Although promising, mindfulness in regard to cytokine therapies is warranted as dysregulation of cytokine homeostasis is implicated in autoimmune disease, e.g. IL-15 plays a role in the pathogenesis of inflammatory myopathies [75]. Further research into the rejuvenating potential of exercise-linked cytokines is needed in order to engineer treatment strategies targeting age-associated pathologies.
6. Conclusion
Skeletal muscle regulates immune system functions via myokine signalling and the expression of immune modulatory surface molecules. Immune cells in turn critically influence muscle mass and function. Therefore, skeletal muscle may act as a central integrator between sarcopenia and immune senescence. Given their individual and socioeconomic burden innovative therapeutic approaches are urgently needed and targeting skeletal muscle might be able to restore both skeletal muscle and immune system function in aging individuals.
Outstanding questions
To translate sarcopenia and immune senescence research into clinical practice the following questions should be addressed by future research:
Exercise has proven effective in combating age-associated pathologies. In part, this effect is mediated by myokines secreted by skeletal muscle. Which are the myokines responsible for this beneficial effect? Does the muscle secretome differ between young and old individuals? And if so, which are the soluble factors responsible for the rejuvenating effect seen in models of heterochronic parabiosis? Can these factors be employed to ameliorate age-associated pathologies?
In the context of soluble factors, IL-15 is a promising candidate for the treatment of both immune senescence and sarcopenia. Recently, short term administration of IL-15 for the treatment of cancer has been proven to be safe. What are the adverse effects of long-term IL-15 treatment? Similarly, the thymoprotective effect of IL-7 was able to ameliorate features of immune senescence in aged athletes. Can IL-7 an IL-15 restore immune system and skeletal muscle function in old age? Can these myokines “mimic” the effect of exercise?
Immune cells, in particular infiltrating Tregs, are crucial for maintaining homeostasis of skeletal muscle. Therefore, it is crucial to identify the mechanisms responsible for the age-associated deterioration of Tregs. Can substitution of factors secreted by Tregs improve skeletal muscle pathology? Are cytokines such as IL-2 able to restore Treg function in aged skeletal muscle? What is the role of other immune cell subsets and their age-related alterations?
Finally, a holistic treatment strategy targeting age-associated pathologies should combine exercise, nutritional strategies and pharmacological interventions. However, given the complex and interlinked nature of sarcopenia and immune senescence, further research identifying the underlying mechanisms is needed. Which pathways are responsible for the coalescence of these pathologies? And which pathways enable certain patients to age “successfully”? How can we utilize these pathways?
Search strategy and selection criteria
Data for this Review were identified by searches of MEDLINE, PubMed, Google scholar and references from relevant articles using the search terms “sarcopenia”, “immune senescence”, “skeletal muscle” and “myokine”. Only articles published in English between 1996 and 2019 were included.
Author contributions
CN and TR conceptualized and wrote the original draft of the manuscript; SGM, RD and JM reviewed and edited the manuscript. All authors have read and approved the final version of the manuscript.
Funding
This work was supported by Innovative Medizinische Forschung (IMF) Münster (I-RU211405 and I-RU211811 to TR), the Else-Kröner-Fresenius-Stiftung (2018_A03 to TR) and by the German Research Foundation (DFG, RU 2169/2-1 to TR). The funders had no role in the design, creation or writing of the manuscript.
Declaration of Competing Interest
Dr. Nelke has nothing to disclose. Dr. Dziewas has nothing to disclose. Dr. Minnerup has nothing to disclose. Dr. Meuth has nothing to disclose. Dr. Ruck reports grants from German Ministry of Education, Science, Research and Technology, grants from Else-Kröner-Fresenius Stiftung, grants from German Research Foundation during the conduct of the study.
Acknowledgements
We thank Heike Blum for excellent graphical illustration.
References
- 1.Dodds R.M., Syddall H.E., Cooper R., Benzeval M., Deary I.J., Dennison E.M. Grip strength across the life course: normative data from twelve British studies. PLoS ONE. 2014;9(12) doi: 10.1371/journal.pone.0113637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyere O., Cederholm T. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ethgen O., Beaudart C., Buckinx F., Bruyere O., Reginster J.Y. The future prevalence of sarcopenia in Europe: a claim for public health action. Calcif Tissue Int. 2017;100(3):229–234. doi: 10.1007/s00223-016-0220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afzali A.M., Muntefering T., Wiendl H., Meuth S.G., Ruck T. Skeletal muscle cells actively shape (auto)immune responses. Autoimmun Rev. 2018;17(5):518–529. doi: 10.1016/j.autrev.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Elias R., Hartshorn K., Rahma O., Lin N., Snyder-Cappione J.E. Seminars in oncology. Elsevier; 2018. Aging, immune senescence, and immunotherapy: a comprehensive review. [DOI] [PubMed] [Google Scholar]
- 6.Fulop T., Larbi A., Dupuis G., Le Page A., Frost E.H., Cohen A.A. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front Immunol. 2017;8:1960. doi: 10.3389/fimmu.2017.01960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duggal N.A., Pollock R.D., Lazarus N.R., Harridge S., Lord J.M. Major features of immunesenescence, including reduced thymic output, are ameliorated by high levels of physical activity in adulthood. Aging Cell. 2018;17(2) doi: 10.1111/acel.12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quinn L.S., Anderson B.G., Strait-Bodey L., Wolden-Hanson T. Serum and muscle interleukin-15 levels decrease in aging mice: correlation with declines in soluble interleukin-15 receptor alpha expression. Exp Gerontol. 2010;45(2):106–112. doi: 10.1016/j.exger.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakanishi R., Oki E., Sasaki S., Hirose K., Jogo T., Edahiro K. Sarcopenia is an independent predictor of complications after colorectal cancer surgery. Surg Today. 2018;48(2):151–157. doi: 10.1007/s00595-017-1564-0. [DOI] [PubMed] [Google Scholar]
- 10.Cosquéric G., Sebag A., Ducolombier C., Thomas C., Piette F., Weill-Engerer S. Sarcopenia is predictive of nosocomial infection in care of the elderly. Br J Nutr. 2007;96(5):895–901. doi: 10.1017/bjn20061943. [DOI] [PubMed] [Google Scholar]
- 11.Altuna-Venegas S., Aliaga-Vega R., Maguina J.L., Parodi J.F., Runzer-Colmenares F.M. Risk of community-acquired pneumonia in older adults with sarcopenia of a hospital from Callao, Peru 2010-2015. Arch Gerontol Geriatr. 2019;82:100–105. doi: 10.1016/j.archger.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maeda K., Akagi J. Muscle mass loss is a potential predictor of 90-day mortality in older adults with aspiration pneumonia. J Am Geriatr Soc. 2017;65(1):e18–e22. doi: 10.1111/jgs.14543. [DOI] [PubMed] [Google Scholar]
- 13.Giudice J., Taylor J.M. Muscle as a paracrine and endocrine organ. Curr Opin Pharmacol. 2017;34:49–55. doi: 10.1016/j.coph.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marzetti E., Carter C.S., Wohlgemuth S.E., Lees H.A., Giovannini S., Anderson B. Changes in IL-15 expression and death-receptor apoptotic signaling in rat gastrocnemius muscle with aging and life-long calorie restriction. Mech Ageing Dev. 2009;130(4):272–280. doi: 10.1016/j.mad.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crane J.D., MacNeil L.G., Lally J.S., Ford R.J., Bujak A.L., Brar I.K. Exercise-stimulated interleukin-15 is controlled by AMPK and regulates skin metabolism and aging. Aging Cell. 2015;14(4):625–634. doi: 10.1111/acel.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang H., Chang J., Chen W., Zhao L., Qu B., Tang C. Treadmill exercise promotes interleukin 15 expression in skeletal muscle and interleukin 15 receptor alpha expression in adipose tissue of high-fat diet rats. Endocrine. 2013;43(3):579–585. doi: 10.1007/s12020-012-9809-6. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen A.R., Mounier R., Plomgaard P., Mortensen O.H., Penkowa M., Speerschneider T. Expression of interleukin-15 in human skeletal muscle – effect of exercise and muscle fibre type composition. J Physiol. 2007;584(1):305–312. doi: 10.1113/jphysiol.2007.139618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamura Y., Watanabe K., Kantani T., Hayashi J., Ishida N., Kaneki M. Upregulation of circulating IL-15 by treadmill running in healthy individuals: is IL-15 an endocrine mediator of the beneficial effects of endurance exercise? Endocr J. 2011;58(3):211–215. doi: 10.1507/endocrj.k10e-400. [DOI] [PubMed] [Google Scholar]
- 19.Kjobsted R., Hingst J.R., Fentz J., Foretz M., Sanz M.N., Pehmoller C. AMPK in skeletal muscle function and metabolism. Faseb J. 2018;32(4):1741–1777. doi: 10.1096/fj.201700442R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salminen A., Kaarniranta K., Kauppinen A. Age-related changes in AMPK activation: role for AMPK phosphatases and inhibitory phosphorylation by upstream signaling pathways. Ageing Res Rev. 2016;28:15–26. doi: 10.1016/j.arr.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Kang X., Yang M.Y., Shi Y.X., Xie M.M., Zhu M., Zheng X.L. Interleukin-15 facilitates muscle regeneration through modulation of fibro/adipogenic progenitors. Cell Commun Signaling. 2018;16(1):42. doi: 10.1186/s12964-018-0251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalinkovich A., Livshits G. Sarcopenic obesity or obese sarcopenia: a cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev. 2017;35:200–221. doi: 10.1016/j.arr.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Conlon K.C., Lugli E., Welles H.C., Rosenberg S.A., Fojo A.T., Morris J.C. Redistribution, hyperproliferation, activation of natural killer cells and CD8 t cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J. Clin Oncol. 2015;33(1):74–82. doi: 10.1200/JCO.2014.57.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennedy M.K., Glaccum M., Brown S.N., Butz E.A., Viney J.L., Embers M. Reversible defects in natural killer and memory CD8 t cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191(5):771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Girard D., Paquet M.E., Paquin R., Beaulieu A.D. Differential effects of interleukin-15 (IL-15) and IL-2 on human neutrophils: modulation of phagocytosis, cytoskeleton rearrangement, gene expression, and apoptosis by IL-15. Blood. 1996;88(8):3176. [PubMed] [Google Scholar]
- 26.Schaper F., Interleukin-6 Rose-John S. Biology, signaling and strategies of blockade. Cytokine Growth Factor Rev. 2015;26(5):475–487. doi: 10.1016/j.cytogfr.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Bano G., Trevisan C., Carraro S., Solmi M., Luchini C., Stubbs B. Inflammation and sarcopenia: a systematic review and meta-analysis. Maturitas. 2017;96:10–15. doi: 10.1016/j.maturitas.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Dalle S., Rossmeislova L., Koppo K. The role of inflammation in age-related sarcopenia. Front Physiol. 2017;8:1045. doi: 10.3389/fphys.2017.01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alemán H., Esparza J., Ramirez F.A., Astiazaran H., Payette H. Longitudinal evidence on the association between interleukin-6 and C-reactive protein with the loss of total appendicular skeletal muscle in free-living older men and women. Age Ageing. 2011;40(4):469–475. doi: 10.1093/ageing/afr040. [DOI] [PubMed] [Google Scholar]
- 30.Haddad F., Zaldivar F., Cooper D.M., Adams G.R. IL-6-induced skeletal muscle atrophy. J Appl Physiol. 2005;98(3):911–917. doi: 10.1152/japplphysiol.01026.2004. 1985. [DOI] [PubMed] [Google Scholar]
- 31.Tsujinaka T., Fujita J., Ebisui C., Yano M., Kominami E., Suzuki K. Interleukin 6 receptor antibody inhibits muscle atrophy and modulates proteolytic systems in interleukin 6 transgenic mice. J Clin Invest. 1996;97(1):244–249. doi: 10.1172/JCI118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madaro L., Passafaro M., Sala D., Etxaniz U., Lugarini F., Proietti D. Denervation-activated STAT3-IL-6 signalling in fibro-adipogenic progenitors promotes myofibres atrophy and fibrosis. Nat Cell Biol. 2018;20(8):917–927. doi: 10.1038/s41556-018-0151-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams A., Wang J.J., Wang L., Sun X., Fischer J.E., Hasselgren P.O. Sepsis in mice stimulates muscle proteolysis in the absence of IL-6. Am J Physiol. 1998;275(6):R1983–R1991. doi: 10.1152/ajpregu.1998.275.6.R1983. [DOI] [PubMed] [Google Scholar]
- 34.Muñoz-Cánoves P., Scheele C., Pedersen B.K., Serrano A.L. Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? FEBS J. 2013;280(17):4131–4148. doi: 10.1111/febs.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serrano A.L., Baeza-Raja B., Perdiguero E., Jardi M., Munoz-Canoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab. 2008;7(1):33–44. doi: 10.1016/j.cmet.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Benatti F.B., Pedersen B.K. Exercise as an anti-inflammatory therapy for rheumatic diseases-myokine regulation. Nat Rev Rheumatol. 2015;11(2):86–97. doi: 10.1038/nrrheum.2014.193. [DOI] [PubMed] [Google Scholar]
- 37.Keller C., Hellsten Y., Steensberg A., Klarlund Pedersen B. Differential regulation of IL-6 and TNF-α via calcineurin in human skeletal muscle cells. Cytokine. 2006;36(3):141–147. doi: 10.1016/j.cyto.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 38.Cullen T., Thomas A.W., Webb R., Hughes M.G. Interleukin-6 and associated cytokine responses to an acute bout of high-intensity interval exercise: the effect of exercise intensity and volume. Appl Physiol Nutr Metab. 2016;41(8):803–808. doi: 10.1139/apnm-2015-0640. [DOI] [PubMed] [Google Scholar]
- 39.Kumari N., Dwarakanath B.S., Das A., Bhatt A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016;37(9):11553–11572. doi: 10.1007/s13277-016-5098-7. [DOI] [PubMed] [Google Scholar]
- 40.Starkie R., Ostrowski S.R., Jauffred S., Febbraio M., Pedersen B.K. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. Faseb J. 2003;17(8):884–886. doi: 10.1096/fj.02-0670fje. [DOI] [PubMed] [Google Scholar]
- 41.Weng N-P, AN Akbar, Goronzy J. CD28− t cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30(7):306–312. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y., Welc S.S., Wehling-Henricks M., Tidball J.G. Myeloid cell-derived tumor necrosis factor-alpha promotes sarcopenia and regulates muscle cell fusion with aging muscle fibers. Aging Cell. 2018;17(6):e12828. doi: 10.1111/acel.12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steensberg A., Fischer C.P., Keller C., Moller K., Pedersen B.K. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab. 2003;285(2):E433–E437. doi: 10.1152/ajpendo.00074.2003. [DOI] [PubMed] [Google Scholar]
- 44.Schett G., Dayer J.M., Manger B. Interleukin-1 function and role in rheumatic disease. Nat Rev Rheumatol. 2016;12(1):14–24. doi: 10.1038/nrrheum.2016.166. [DOI] [PubMed] [Google Scholar]
- 45.Saxena A., Khosraviani S., Noel S., Mohan D., Donner T., Hamad A.R. Interleukin-10 paradox: a potent immunoregulatory cytokine that has been difficult to harness for immunotherapy. Cytokine. 2015;74(1):27–34. doi: 10.1016/j.cyto.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mazzucchelli R., Durum S.K. Interleukin-7 receptor expression: intelligent design. Nat. Rev. Immunol. 2007;7:144. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- 47.Andrew D., Aspinall R. Age-associated thymic atrophy is linked to a decline in IL-7 production. Exp Gerontol. 2002;37(2–3):455–463. doi: 10.1016/s0531-5565(01)00213-3. [DOI] [PubMed] [Google Scholar]
- 48.Haugen F., Norheim F., Lian H., Wensaas A.J., Dueland S., Berg O. IL-7 is expressed and secreted by human skeletal muscle cells. Am J Physiol-Cell Physiol. 2010;298(4):C807–C816. doi: 10.1152/ajpcell.00094.2009. [DOI] [PubMed] [Google Scholar]
- 49.Bazdar D.A., Kalinowska M., Sieg S.F. Interleukin-7 receptor signaling is deficient in CD4+ t cells from HIV-infected persons and is inversely associated with aging. J Infect Dis. 2009;199(7):1019–1028. doi: 10.1086/597210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aspinall R. T cell development, ageing and interleukin-7. Mech Ageing Dev. 2006;127(6):572–578. doi: 10.1016/j.mad.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 51.Watanabe S., Kawamoto S., Ohtani N., Hara E. Impact of senescence-associated secretory phenotype and its potential as a therapeutic target for senescence-associated diseases. Cancer Sci. 2017;108(4):563–569. doi: 10.1111/cas.13184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chiche A., Le Roux I., von Joest M., Sakai H., Aguin S.B., Cazin C. Injury-Induced senescence enables in vivo reprogramming in skeletal muscle. Cell Stem Cell. 2017;20(3):407–414. doi: 10.1016/j.stem.2016.11.020. e4. [DOI] [PubMed] [Google Scholar]
- 53.da Silva P.F.L., Ogrodnik M., Kucheryavenko O., Glibert J., Miwa S., Cameron K. The bystander effect contributes to the accumulation of senescent cells in vivo. Aging Cell. 2019;18(1) doi: 10.1111/acel.12848. e12848-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marino M., Scuderi F., Mazzarelli P., Mannella F., Provenzano C., Bartoccioni E. Constitutive and cytokine-induced expression of MHC and intercellular adhesion molecule-1 (ICAM-1) on human myoblasts. J Neuroimmunol. 2001;116(1):94–101. doi: 10.1016/s0165-5728(01)00287-9. [DOI] [PubMed] [Google Scholar]
- 55.Wiendl H., Mitsdoerffer M., Schneider D., Chen L., Lochmuller H., Melms A. Human muscle cells express a B7-related molecule, B7-H1, with strong negative immune regulatory potential: a novel mechanism of counterbalancing the immune attack in idiopathic inflammatory myopathies. FASEB J. 2003;17(13):1892–1894. doi: 10.1096/fj.03-0039fje. [DOI] [PubMed] [Google Scholar]
- 56.Nagaraju K., Raben N., Merritt G., Loeffler L., Kirk K., Plotz P. A variety of cytokines and immunologically relevant surface molecules are expressed by normal human skeletal muscle cells under proinflammatory stimuli. Clin Exp Immunol. 1998;113(3):407–414. doi: 10.1046/j.1365-2249.1998.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu M., Pirtskhalava T., Farr J.N., Weigand B.M., Palmer A.K., Weivoda M.M. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24(8):1246–1256. doi: 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saini J., McPhee J.S., Al-Dabbagh S., Stewart C.E., Al-Shanti N. Regenerative function of immune system: modulation of muscle stem cells. Ageing Res Rev. 2016;27:67–76. doi: 10.1016/j.arr.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 59.Domingues-Faria C., Vasson M.P., Goncalves-Mendes N., Boirie Y., Walrand S. Skeletal muscle regeneration and impact of aging and nutrition. Ageing Res Rev. 2016;26:22–36. doi: 10.1016/j.arr.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 60.Kuswanto W., Burzyn D., Panduro M., Wang K.K., Jang Y.C., Wagers A.J. Poor repair of skeletal muscle in aging mice reflects a defect in local, interleukin-33-dependent accumulation of regulatory t cells. Immunity. 2016;44(2):355–367. doi: 10.1016/j.immuni.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burzyn D., Kuswanto W., Kolodin D., Shadrach Jennifer L., Cerletti M., Jang Y. A special population of regulatory t cells potentiates muscle repair. Cell. 2013;155(6):1282–1295. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Allenbach Y., Solly S., Gregoire S., Dubourg O., Salomon B., Butler-Browne G. Role of regulatory t cells in a new mouse model of experimental autoimmune myositis. Am J Pathol. 2009;174(3):989–998. doi: 10.2353/ajpath.2009.080422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murray P.J. Macrophage polarization. Annu Rev Physiol. 2017;79:541–566. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 64.Tonkin J., Temmerman L., Sampson R.D., Gallego-Colon E., Barberi L., Bilbao D. Monocyte/Macrophage-derived IGF-1 orchestrates murine skeletal muscle regeneration and modulates autocrine polarization. Molecular Therapy. 2015;23(7):1189–1200. doi: 10.1038/mt.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Junnila R.K., List E.O., Berryman D.E., Murrey J.W., Kopchick J.J. The GH/IGF-1 axis in ageing and longevity. Nat. Rev Endocrinol. 2013;9(6):366–376. doi: 10.1038/nrendo.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reidy P.T., McKenzie A.I., Mahmassani Z.S., Petrocelli J.J., Nelson D.B., Lindsay C.C. Aging impairs mouse skeletal muscle macrophage polarization and muscle-specific abundance during recovery from disuse. Am J. Physiol-Endocrinol Metab. 2019;317(1):E85–E98. doi: 10.1152/ajpendo.00422.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 68.Marzetti E., Calvani R., Tosato M., Cesari M., Di Bari M., Cherubini A. Physical activity and exercise as countermeasures to physical frailty and sarcopenia. Aging Clin Exp Res. 2017;29(1):35–42. doi: 10.1007/s40520-016-0705-4. [DOI] [PubMed] [Google Scholar]
- 69.Simpson R.J., Kunz H., Agha N., Graff R. Exercise and the regulation of immune functions. Prog Mol Biol Transl Sci. 2015;135:355–380. doi: 10.1016/bs.pmbts.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 70.Campbell J.P., Turner J.E. Debunking the myth of exercise-induced immune suppression: redefining the impact of exercise on immunological health across the lifespan. Front Immunol. 2018;9(648) doi: 10.3389/fimmu.2018.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Viña J., Rodriguez-Mañas L., Salvador-Pascual A., Tarazona-Santabalbina F.J., Gomez-Cabrera M.C. Exercise: the lifelong supplement for healthy ageing and slowing down the onset of frailty. J Physiol. 2016;594(8):1989–1999. doi: 10.1113/JP270536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Racette S.B., Rochon J., Uhrich M.L., Villareal D.T., Das S.K., Fontana L. Effects of two years of calorie restriction on aerobic capacity and muscle strength. Med Sci Sports Exerc. 2017;49(11):2240–2249. doi: 10.1249/MSS.0000000000001353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meydani S.N., Das S.K., Pieper C.F., Lewis M.R., Klein S., Dixit V.D. Long-term moderate calorie restriction inhibits inflammation without impairing cell-mediated immunity: a randomized controlled trial in non-obese humans. Aging. 2016;8(7):1416–1431. doi: 10.18632/aging.100994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Conboy I.M., Conboy M.J., Wagers A.J., Girma E.R., Weissman I.L., Rando T.A. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433(7027):760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 75.Ruck T., Bittner S., Afzali A.M., Göbel K., Glumm S., Kraft P. The NKG2D-IL-15 signaling pathway contributes to T-cell mediated pathology in inflammatory myopathies. Oncotarget. 2015;6(41):43230–44343. doi: 10.18632/oncotarget.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]