Abstract
Background
Neuroblastoma is a paediatric tumour originated from sympathoadrenal precursors and characterized by its heterogeneity and poor outcome in advanced stages. Intra-tumoral cellular heterogeneity has emerged as an important feature in neuroblastoma, with a potential major impact on tumour aggressiveness and response to therapy. CD44 is an adhesion protein involved in tumour progression, metastasis and stemness in different cancers; however, there has been controversies about the significance of CD44 expression in neuroblastoma and its relationship with tumour progression.
Methods
We have performed transcriptomic analysis on patient tumour samples studying the outcome of patients with high CD44 expression. Adhesion, invasion and proliferation assays were performed in sorted CD44high neuroblastoma cells. Tumoursphere cultures have been used to enrich in undifferentiated stem-like cells and to asses self-renewal and differentiation potential. We have finally performed in vivo tumorigenic assays on cell line-derived or Patient-derived xenografts.
Findings
We show that high CD44 expression is associated with low survival in high-grade human neuroblastoma, independently of MYCN amplification. CD44 is expressed in a cell population with neural crest stem-like features, and with the capacity to generate multipotent, undifferentiated tumourspheres in culture. These cells are more invasive and proliferative in vitro. CD44 positive cells obtained from tumours are more tumorigenic and metastatic, giving rise to aggressive neuroblastic tumours at high frequency upon transplantation.
Interpretation
We describe an unexpected intra-tumoural heterogeneity within cellular entities expressing CD44 in neuroblastoma, and propose that CD44 has a role in neural crest stem-like undifferentiated cells, which can contribute to tumorigenesis and malignancy in this type of cancer.
Funding
Research supported by grants from the “Asociación Española contra el Cáncer” (AECC), the Spanish Ministry of Science and Innovation SAF program (SAF2016-80412-P), and the European Research Council (ERC Starting Grant to RP).
Keywords: CD44, Neuroblastoma, Differentiation, Neural crest stem cells, Biomarker, Intra-tumour heterogeneity, Cancer
Research in context
Evidence before this study
Neuroblastoma originates during neural crest development and is characterized by a great heterogeneity. At the cellular level, these paediatric tumours contain phenotypically divergent cells which have been classified transcriptionally into an adrenergic/neuronal cell population and an undifferentiated, neural crest-like mesenchymal cell population. These later cells are thought to be more aggressive and resistant to therapy. CD44 is an adhesion transmembrane glycoprotein that mediates cell responses to the cellular microenvironment, regulating cell growth, differentiation and motility. Despite its relationship with tumour progression and aggressiveness in other tumours, its role in neuroblastoma has been controversial.
Added value of the study
We show that high CD44 expression on stage 4 NB patient tumours can be indicative of low survival. We demonstrate that CD44 is highly expressed in undifferentiated, multipotent neural crest-like NB cells that are highly tumorigenic and metastatic in vivo. High CD44 expression delineates the aggressive undifferentiated/neural-crest-like cell population in neuroblastoma.
Implications of all the available evidence
We help to clarify the controversies around CD44 expression in NB at the cellular level postulating that CD44 could have a role not only in terminally differentiated glial cells but also in neural crest-like undifferentiated cells that can contribute to tumorigenesis. We offer new possibilities to isolate and characterize these cells, explore their contribution to neuroblastoma relapses and aggressiveness and promote their targeting.
1. Introduction
Neuroblastoma (NB) is a paediatric tumour that originates from sympathoadrenal precursors during neural crest development [1,2]. It is characterized by a great heterogeneity, ranging from spontaneously regressing tumours to metastatic aggressive forms that are incurable to date. Despite recent improvements in patient risk stratification and genetic profiling, neuroblastoma is still the most lethal extracranial solid tumour in children.
Currently available prognostic markers for NB (amplified MYCN, loss of heterozygosity in chromosome 1p or DNA ploidy, among others) fail to predict the outcome of all patients efficiently. Amplification of the MYCN oncogene is the best prognostic factor to date and is associated with poor outcome. However, this amplification is only found in approximately 22% of neuroblastoma tumours [3,4]. Current approaches frequently fail to correctly classify the rest of patients with unfavourable course, indicating the need for new markers or the re-evaluation of existing ones [5,6]. Moreover, there is still an incomplete understanding of the biology of this malignancy at the cellular level, making difficult to find therapeutically relevant molecular targets.
Neuroblastomas seem to recapitulate neural crest development, with the formation of multiple cellular lineages after spontaneous or induced differentiation from neural crest progenitors. The degree of differentiation and stromal content has allowed the histologic stratification of patients into risk-groups, with most aggressive tumours being the most undifferentiated ones [7,8]. At the cellular level, these paediatric tumours typically contain phenotypically divergent cells, with at least a neuroblastic/adrenergic population together with a non-neuronal/mesenchymal component, which are both supposed to share a common origin [9]. More recently, transcriptional profiling on neuroblastoma tumours and cell lines have confirmed this heterogeneity, showing the existence of at least two main cell identities: an undifferentiated, neural crest-like mesenchymal cell and an adrenergic committed cell [10,11].
CD44 is an adhesion transmembrane glycoprotein that mediates cell responses to the cellular microenvironment, regulating cell growth, differentiation and motility. This protein works as the main receptor for the extracellular matrix ligand hyaluronic acid (HA) as well as other extracellular matrix components. CD44 also acts as co-receptor for other signalling proteins like MET or integrins [12]. This adhesion protein has been described to have a role in tumour progression and has been linked to metastatic spreading in various tumours such as colon or breast carcinomas [13], [14], [15]. By contrast, CD44 expression presents an inverse correlation with tumour progression in other tumours like prostate carcinoma or neuroblastoma [16,17]. TCGA (The Cancer Genome Atlas) expression data shows a higher expression in human tumours compared with healthy tissues in colon adenocarcinoma, glioblastoma, renal cancer, amyloid leukaemia, ovarian and stomach cancer, among others. Meanwhile there is lower expression in thymomas, uterine and endometrial carcinoma tumours. The reasons for this cancer type-dependent discrepancy are not clear. This panorama is further complicated due to the existence of CD44 splice variants with different functions in tumorigenesis, but whose relevance in NB is not yet clear [18], [19], [20], [21].
CD44 has also been associated with the differentiation status of neural precursor cells, being highly expressed in differentiated cells of the glial lineage. NB cell differentiation to Schwann-like cells is normally associated to improved prognosis and increased CD44 expression [18,22,23]. In fact, CD44 expression was identified preferentially in a group of neuroblastoma patients with favourable outcome and maturing phenotype [24]. These observations, and the fact that MYCN directly represses CD44 expression [17], explain why, in neuroblastoma, the lack of overall CD44 expression is a statistically significant factor for poor prognosis [18,25]. However, patients with CD44+ stage 4 tumours still behave quite poorly, and the presence of CD44 expression does not allow a definitive low-grade stratification [26]. Furthermore, there is a minor CD44 positive cell population still present in stage 4 undifferentiated neuroblastomas, whose role, if any, remains to be established. Hence, there is a clear need for the clarification of CD44 function at the cellular level in neuroblastoma tumours with CD44 expression and poor outcome.
Given these preliminary evidences, we decided to shed light on the function of CD44 in NB cells, especially in MYCN non-amplified, high grade tumours. We describe here that CD44 is highly expressed in undifferentiated, multipotent neural crest-like NB cells that are highly tumorigenic and metastatic in vivo. Moreover, we show that high CD44 expression on stage 4 NB patient tumours is indicative of low survival. We postulate that CD44 could have a role not only in terminally differentiated glial cells but also in neural crest stem-like undifferentiated cells that can contribute to tumorigenesis, clarifying the controversies around CD44 expression in NB at the cellular level.
2. Materials and methods
2.1. Gene expression analysis
CD44 gene expression analysis was performed with R2: Genomics Analysis and Visualization Platform (http://r2.amc.nl; Academic Medical Centre, Amsterdam). Human neuroblastoma datasets used were GEO IDs GSE45547 (649 patient samples) and GSE90805 (34 samples, including neuroblastoma cell lines with mesenchymal state (7), adrenergic state (22) or human neural crest cell lines (5) [11]. INSS stratification system is used on these collections dividing tumours into stages 1, 2, 3 and 4, with 4 being the most aggressive. INRG stratification system is used on the home patient samples collection, dividing tumours into stages MS, L1, L2 and M, with M being the most aggressive. CD44hi expression cut-off in survival curves is stablished according to CD44 expression distribution, established on the samples with a CD44 expression value above the 10th percentile of the distribution, and with a minimal group size of 8 samples.
2.2. Cell culture
SK-N-SH and SK-N-DZ are spontaneously immortalized, human NB cell lines that have not been subjected to selection in culture and present a mixed population reminiscent of neuroblastoma cell heterogeneity, with neuronal-like cells and more spread glial-like or undifferentiated cells [27,28]. They were obtained from ECACC (Salisbury, UK) and grown in DMEM supplemented with 10% FCS. GI-M-EN and GI-CAN are also heterogeneous cell neuroblastoma cell lines and were obtained from the laboratory of Dr. M. Ponzoni (Genova, Italy). They were grown on DMEM and RPMI respectively, supplemented with 10% FCS. CHLA-15 and CHLA-20 cell lines were obtained from the Children's Oncology Group (COG) Cell Line and Xenograft Repository (Texas Tech University Health Sciences Center) and grown on IMDM media + 10% FBS. NB39T and NB48T PDX primary cell lines were obtain from PDXs after mechanical dissociation and culture on IMDM+15%FBS. All media were supplemented with 2 mM glutamine, 100 μg/ml streptomycin and 100 U/ml penicillin. Cells were also cultured, when indicated, in Neural Crest complete media (NCM) composed of d-MEM:F-12 + 15% FBS, 1% N2 supplement, 2% B27 supplement, 1% penicillin/streptomycin (all from Gibco, Paisley, UK), 10 ng/ml human FGF, 20 ng/ml human IGF-1, and 20 ng/ml human EGF (R&D Systems, Minneapolis, MN, USA). Serum deprived NCM (NCMlow) was similar but without FBS.
2.3. Immunofluorescence and IHC
For immunofluorescence, cells or frozen sections were fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton X-100 in PBS, blocked with 1% BSA in PBS and incubated with the corresponding antibodies. Antibodies used were: S100β (α-mouse Sigma or α-goat R&D; 1:500), CD44 (BD, San Jose, CA, USA; 1:500), DDC (Cell signalling Technology, Hitchin, UK; 1:500), GFAP (DAKO, Carpinteria, CA, USA; 1:500), Nestin (α-mouse R&D or α-rabbit Millipore; 1:1000), αSMA (Sigma; 1:10,000), CD114 (PE-conjugated α-mouse BDPharmigen; 1:500) and Tuj1 (Sigma; 1:2000). Alexa546-fluor phalloidin was used for F-actin visualization and DAPI used for nuclear staining (Life Technologies, Carlsbad, CA, USA). Secondary antibodies used were Alexa568-goat-α-mouse-IgG, Alexa568-goat-α-rabbit-IgG, Alexa488-donkey-α-rabbit-IgG, Alexa633-sheep-α-goat-IgG and Alexa488-donkey-α-mouse-IgG (Life technologies; 1:500). Images were acquired on an epifluorescence (Olympus BX-61, Tokyo, Japan) or confocal (LSM 710, Zeiss, Oberkochen, Germany) microscope.
For immunohistochemistry staining, paraffin embedded sections (5μm) were obtained from tissues, hydrated, treated for antigen retrieval with citrate buffer and incubated with the corresponding antibodies. Vectastain ABC kit and DAB peroxidase substrate kit (Vector Laboratories, Burlingame, CA, USA) were used following recommended procedures. Anti-nuclein antibody (Cell Signaling; 1:1000) was used to specifically mark human cells.
2.4. Fluorescence-activated cell sorting (FACS)
Flow cytometry analyses and sorting were performed in a cytometer BD FACS Aria (BD, Franklin Lakes, NJ, USA). Cells were detached with versene, resuspended in FACS medium (L15 medium + BSA 2 mg/ml, 1% Hepes and 1% penicillin/streptomycin) and incubated with the corresponding primary antibody for 40 min at 4ºC, followed by secondary fluorescently labelled antibody for 20 min when necessary. Antibodies used were CD44 (BD, San Jose, CA, USA; 1:500), CD114 (PE-conjugated α-mouse BDPharmigen; 1:500), c-kit (PE-conjugated, clone A3C6E2, Miltenyi Biotec; 1:500), CD133 (AC133 pure human Miltenyi Biotec; 1:300) and Alexa488-donkey-α-mouse-IgG (Life Technologies; 1:500). Cells highly positive and negative for CD44 staining were sorted and used for subsequent experiments. Gating strategy considered CD44 expression distribution levels in each cell line, defining the population with high CD44 expression compared to negative cells.
2.5. QPCR
RNA was extracted from tumourspheres or cell cultures using RNeasy micro kit (Qiagen, Chadstone Centre, VIC, Australia). cDNA was synthesized with the QuantiTec Reverse transcription kit (Qiagen) and Real-time quantitative PCR was performed in an ABI Prism 7500 Sequence Detection System using the thermocycler conditions recommended (Applied Biosystems, Grand Island, NY, USA). Detection was performed with SYBR Green PCR Master Mix and analysis with the ΔCt method. Oligonucleotides used can be seen on Supplementary Table S1.
2.6. Tumoursphere formation assay
For tumoursphere formation, cells were cultured in ultra-low binding 6-well plates (Corning, NY, USA) at 5000 cells per well (2.5 cells/μl) in Neural Crest media (NCM) or serum deprived NCM (NCMlow), as indicated. Tumourspheres were allowed to form for 7 days before imaging and collection for OCT inclusion, seeding on top of adherent substrate or mechanical dispersion after 0.02% trypsin treatment. For single-cell tumoursphere formation assays, single cells (1 cell per well) were sorted onto single wells from ultralow binding 96-well plates (Corning) with 100 μl neural crest complete medium, and grown 10–15 days before quantification and imaging of tumourspheres. Except where indicated, imaged and quantified tumourspheres were from second passages. TS formation efficiency is an indication of the percentage of TS forming cells in the original cell culture and is defined as the percentage of originally seeded cells in clonogenic dilution conditions that successfully form TS. Tumourspheres in suspension were concentrated in the field of view before imaging.
2.7. Adhesion, spreading, migration and proliferation assays
Sorted cell cultures were detached with Versene and seeded on culture plates coated with 100 μg/ml Matrigel (BD Biosciences) or uncoated. For adhesion assay, plates were washed with PBS, and cells were fixed and stained with Crystal violet solution 20 min after seeding. Adhesion is expressed as the number of adhered cells per condition after washing, relative to the ones found to adhere from the CD44hi population on TC plastic, which was set to 100% for normalization. For cell spreading, cells were allowed to spread for 1 h before fixing with paraformaldehyde without washing. Cells were then stained for F-actin, and area quantified with Cell Profiler software [29]. More than 100 cells from at least three different experiments were analysed.
For migration studies in 3D, cells were mixed with Matrigel (5 μg/ml) and placed on Chemotaxis 3D microslides (Ibidi, Martinsried, Germany). Bright field images were acquired at 37 °C every 5 min for 24 h on a Nikon Eclipse microscope (Tokyo, Japan). Migration was analysed using the ImageJ Manual tracking and Chemotaxis tool plugins.
For clonal proliferation assay, time-lapse images from single cells after sorting were acquired (5 min/frame) and the number of cell divisions in the colony formed quantified.
Where indicated, cells were transfected with 100 nM of RNAi against CD44 (Dharmacon ON-TARGET plus SMART pool #L-009999-00) using Lipofectamine 2000 48 h before performing the assay.
2.8. Differentiation assay
Differentiation of neuroblastoma cells was induced following a modification of the method described [30]. Cells or tumourspheres seeded on cell culture dishes were incubated in neural low serum media (NM media: D-MEM:F-12, 1% N2 supplement, 2% B27 supplement, 1% penicillin/streptomycin) to promote neuronal differentiation, NM media + 5% FBS + 10 μM Forskolin (FK, R&D) to induce glial differentiation, NM media + 5 ng/ml TGFβ3 (R&D) to induce differentiation into mesenchymal lineage or 10 μM all-trans retinoic acid (ATRA, Sigma), used in the clinic for differentiation therapy. Media was replaced every 3 days for 10 days before fixing and staining the cells with appropriate markers. CD44hi cells after cell sorting were also treated with NM media for differentiation for 6 days before ARN extraction.
2.9. PDXs and xenograft experiments
SK-N-SH neuroblastoma cells were resuspended in cold 5 mg/ml Matrigel in DMEM media at a concentration of 5 × 106 cells/ml. 6–8 weeks old C.B-17 SCID mice (Harlan Laboratories) were injected subcutaneously with 200 μl of cell suspension in the shaved right flank. Tumours formed were collected after 6–9 weeks, minced in small pieces, dissociated in dissociation solution (HBSS buffer + 0.05% Collagenase, 0.005% Elastase, 0.025% Trypsin and 0.04% DNAase) and incubated for 5 min in ACK buffer (155 mM NH4Cl). Cells were resuspended in FACS media stained and sorted as described into CD44- and CD44hi populations. 1–5 × 104 of the sorted cells were injected in new SCID mice after recovery, following the same procedure. Once secondary tumours appeared, they were measured and processed for paraffin sectioning. 9 mice total were injected per condition. Micrometastasis analysis was performed by Q-PCR detection of human cells in mouse tissue, using a variation of the method used in [31]. Quantification and comparison of the grade of metastasis was performed by a relativization of the detected human genomic DNA to the amount of mouse tissue. Nuclein staining in paraffin sections of lungs and liver tissues were used to visualize micro metastatic clones.
PDXs were stablished from freshly-obtained human stage 4, neuroblastoma tumour samples. Briefly, a 1 × 1 mm fragment of original tumour was placed on a subcutaneous pocket performed on SCID mice. Engrafted tumours were maintained in mice until they reach a volume enough to collect new fragments and transfer them to new recipient mice. Four different PDXs were used for the experiments. Relevant clinical information and characteristics are presented on Supplementary Table S2. Cells were dissociated from early passages of these PDXs and treated as described before for TS formation, cell sorting or cell line derivation.
3. Statistical analysis
Statistical analysis was performed on R2 genomics platform or Graphpad Prism software.
One-way (2-way on grouped differentiation data) analysis of variance ANOVA or un-paired t-test were used for statistical analysis. Results were assumed significant when p < 0.05. In gene expression analysis plots, box and whiskers graphs show median, 10–90 percentiles and outliers as dots. Bonferroni test was used for the statistical analysis on the Kaplan survival curves. In all other box and whiskers plots, boxes show median and 25th and 75th percentiles and whiskers show 95% percentile. Bar graphs show average +/-SEM in all cases. Number of measurements or samples (n) is indicated in each case. Results from cell populations after cell sorting are expressed as mean % +/− SD.
4. Ethics statement
All animal experiments were conducted according to procedures approved by the Ethics Committee from the Universidad de Sevilla and complying with animal use guidelines. Patient samples for the generation of the PDXs where obtained through the Andalusian tissue Biobank after informed consent of the patients following all stablished regulations. Clinical information on patient cohorts for expression analysis is openly available for research use and has been anonymised.
5. Results
5.1. High CD44 expression correlates with poor survival in neuroblastoma tumours
CD44 mRNA expression has been associated with glial differentiation and good prognosis in neuroblastoma tumours [24]. Although CD44 mRNA expression is significantly higher in low grade neuroblastoma tumours than in high stage ones, a subset of unfavourable stage 3 and 4 neuroblastomas also presents high CD44 expression levels. We analysed the expression of CD44 using array datasets from a cohort of human neuroblastoma tumours (Fig. 1). Effectively, CD44 expression is significantly lower in stage 4 compared to stage 1 or 2 tumours (Fig. 1a). The expression of CD44 in high-risk neuroblastomas is inversely correlated with MYCN amplification status (Fig. 1b), which is in agreement with the reported role of MYCN as an inhibitor of CD44 transcription [17]. However, CD44 is frequently highly expressed on high-risk tumours without MYCN amplification. We could detect high CD44-expressing samples in tumours from all neuroblastoma INSS stages, benign and aggressive, notably including also some stage 4 tumours (Fig. 1c). Interestingly, patients with the highest CD44 expression among stage 4 tumours (top 10% expressing samples from the series) presented significantly worse outcome when compared to the rest, showing a reduced survival (Fig 1d). This was true independently of MYCN status, meaning that high CD44 expression could discriminate the most aggressive patients, with worse outcome, with or without MYCN amplification.
Fig. 1.
CD44 expression in human neuroblastoma tumours. a, CD44 expression in NB tumours according to INSS stage (stages 1 to 4). (ANOVA; **: p < 0.005). b, CD44 expression in high-risk neuroblastoma tumours according to their MYCN amplification status. (t-test; ***: p < 0.001). In brackets, number of tumour samples. c, Expression of CD44 in human neuroblastoma tumour samples sorted by INSS stage (stages 1 to 4) and showing their MYCN status (red= MYCN amplified). d, Survival curves showing overall survival probability for stage 4 tumours with high CD44 vs rest when considering all tumour samples (i), MYCN-amplified tumour samples only (ii) or MYCN non-amplified tumour samples only (iii). Patient samples per group are shown in brackets. p values: Bonferroni. e, CD44 staining on tissue samples from NB tumours with unfavourable histology. % of CD44 cells in tissue sample is indicated. Clinical information from these tumours can be seen on Supplementary Table S1. Bar: 200 μm. Bar on inset: 25 μm. Arrowhead: CD44 expressing cells. Asterisk: examples of unspecific red blood cells staining.
We were also able to detect high CD44 protein expression in our in-house collection of human neuroblastoma patient samples, with significant presence in some aggressive, undifferentiated NB tumours, both MYCN non-amplified and amplified (Fig. 1e and Supplementary Table S3). CD44+ cells in these tumour sections account for 3.8%(+/−1.1) of cells. Taken together, these observations reveal that there is a subset of aggressive neuroblastoma tumours, mostly but not exclusively MYCN non-amplified, with high CD44 expression and worse survival, and suggest that CD44 might be expressed on cells other than benign differentiated glial derivatives in neuroblastoma.
5.2. CD44 is expressed in undifferentiated, tumoursphere-forming neuroblastoma cells
To study CD44 expression and its role in NB cell heterogeneity and differentiation, we initially used the phenotypically heterogeneous, MYCN non-amplified, SK-N-SH neuroblastoma cell line. We and others have used serum-rich neural crest media (NCM) for the culture of undifferentiated neural crest-derived progenitor cells from adult tissues and neuroblastoma. These cells grow as clonal spheres on low adherence conditions and can be later induced to differentiate [30,32,33]. We set up the culture of SK-N-SH cells in NCM to enrich for undifferentiated cells, or in serum deprived NCM (NCMlow) for comparison (Fig. 2 and Supplementary Fig. S2). NCM culture of neuroblastoma cells selects for undifferentiated tumoursphere-forming cells with higher self-renewal and proliferative capacity, expressing high levels of the undifferentiated progenitor marker Nestin and low levels of neuronal markers like Tuj1 or DDC, which are present mainly at the periphery of the tumoursphere (TS). The culture of this spheres on NCMlow media provokes a marked neuronal differentiation, best observed in cell morphology upon adherence (Fig. 2a and Supplementary Movie S1). This was not exclusive for SK-N-SH cells and could be observed also in other neuroblastoma cell lines, like GI-M-EN, GI-CAN and the MYCN amplified cell line SK-N-DZ (Supplementary Fig. 2 and data not shown).
Fig. 2.
Neural crest media selects for CD44+ cells. a, Tumourspheres placed on adherent substrate after growing on the indicated media. The undifferentiation marker Nestin and the neuronal differentiation marker DDC are shown. Bar: 50 μm. b, CD44 and DDC staining in SK-N-SH cells growing on NCM or NCMlow. Representative images and quantification are shown. (t-test; *: p < 0.05). Bar: 25 μm. c, CD44 and DDC labelling of tumourspheres from SK-N-SH cells grown on NCM or NCMlow media. Tumourspheres were either sectioned after OCT inclusion (i) or seeded on adherent substrate (ii) before staining. Representative images and quantification are shown. (t-test; *: p < 0.05). Bars: 50 μm (i) and 100 μm (ii). d, Representative dot-plots showing CD44 and CD114 expression on the cell lines indicated. % of cells in every quadrant is shown. e, Representative confocal image of a CD44+ cell-derived TS from NB39T sample, showing CD44 and CD114 staining. Yellow arrows point to areas of overlapping expression. Bar: 100 μm.
CD44 expression has been already described in adherent neuroblastoma cell lines in comparison to more neuronal and poorly adherent cell lines [18]. Our results indicated that expression of CD44 is almost exclusively restricted to NCM selected, non-neuronal, tumoursphere-forming cells (Fig. 2b). CD44 was also mainly present in TS growing on NCM, especially in the main body of the tumoursphere (Fig. 2c). Few CD44 positive cells could be observed in NCMlow tumourspheres, and they were always at the core of the sphere, and negative for DDC expression. CD44 expression was also restricted to NCM growing tumorspheres in other cell lines (Supplementary Fig. S2d). Sympathetic noradrenergic neuroblastic cell lines like CHLA-15 or CHLA20 did not present CD44 expression or generated undifferentiated TS in these conditions (Supplementary Fig. S3b). We also analysed by RT-PCR the expression of different CD44 splice variants on these cells but could not find a consistent pattern of expression among the different conditions and tumourspheres (data not shown).
Some markers for stemness in neuroblastoma cells have been proposed [34]. We decided to evaluate the expression of CD114 (CSF3R), CD133 (prominin1) and CD117 (c-kit) in the context of CD44 expression in neuroblastoma cell lines (Fig. 2d and Supplementary Fig. S3c). We detected co-expression of CD44 with c-kit and, more importantly, with CD114, in a minor population of cells in SK-N-SH cell line and in primary PDX derived cell lines. These overlappings account for a considerable percentage of CD44 positive cells in the case of CD114 (ranging from 36% in the case of SK-N-SH to 14% in the case of NB39T cells). We also detected co-expression of CD44 and CD114 in tumorspheres derived from CD44hi cells (Fig. 2e).
These observations indicate that self-renewing undifferentiated TS growing on NCM media are enriched in CD44+ cells, and that differentiated DDC positive cells are negative for CD44 expression.
5.3. CD44 marks an invasive and proliferative neuroblastoma cell population
CD44 has been suggested to mediate a metastatic profile in neuroblastoma [35] and this seems to be its main role in other solid tumours [12]. We wanted to elucidate the function of CD44 protein in NB cells and for that we separated CD44 high expressing (CD44hi) and CD44 negative (CD44-) cell populations from heterogeneous SK-N-SH neuroblastoma cells. In agreement with a previous report [36], an average of 39.7% (+/−16.2) of SK-N-SH cells were CD44 positive in normal growing conditions (Supplementary Fig. S3a). The expression of CD44 in these cells distinguished two heterogeneous but distinct cell populations, with morphology and characteristics that are maintained in culture for at least 14 days after sorting (Fig. 3a and Supplementary Fig. S4a).
Fig. 3.
CD44 marks a motile and proliferative NB cell population. a, Representative dot-plot of SK-N-SH cells labelled for CD44 showing FACS-sorted populations. Immunostaining show sorted cells 7 days after sorting. Bar: 50 μm. b, Adhesion of the indicated cell populations onto different substrates. Measures show number of cells adhered relative to the levels observed on CD44hi population over plastic condition (set at 100%). (ANOVA; *: p < 0.05, **: p < 0.01). c, Velocity and distance reached by cells from the indicated cell populations in 3D migration assays. (t-test; **: p < 0.01). d, Velocity and distance reached by cells from the indicated cell populations in 2D migration assays. (t-test; *: p < 0.01). e, Number of cell divisions per each colony, starting from a single cell with the indicated phenotypes. Line indicates median. (t-test; **: p < 0.01).
The CD44hi cell population presented increased cell adhesion and spreading, both onto tissue culture plastic and onto an HA-rich extracellular matrix like Matrigel (Fig. 3b and Supplementary Fig. S4b). Controlled cell adhesion to extracellular matrix is needed for cancer cell motility and invasion, and binding of SK-N-SH cells to HA-rich matrix has been previously shown to be CD44-dependent [37]. CD44hi neuroblastoma cells are enriched in F-Actin and, when embedded on a 3D extracellular matrix, form groups that extend actin-rich protrusions (Supplementary Fig. S4a and c). Both presence of actin polymerization and extension of protrusions are indicative of a motile and invasive phenotype. Migration of CD44hi cells on a 3D environment is significantly increased compared to CD44- cells (Fig. 3c). Of note, adhesion, morphology and migration phenotypes were only partially abrogated after CD44 downregulation by RNAi, indicating that CD44 marks an adhesive cell population but other adhesion molecules could be also involved in the phenotype (Fig. 3b and c and Supplementary Fig. S4d).
In addition, CD44hi cells seeded clonally, divide more than the negative population (Fig. 3e), indicating a higher proliferative capacity in vitro.
These results show that high CD44 expression, in MYCN non-amplified neuroblastoma cells, marks a population that interacts better with extracellular matrix substrates, is more motile and protrusive in 3D, and proliferates faster than CD44- cells.
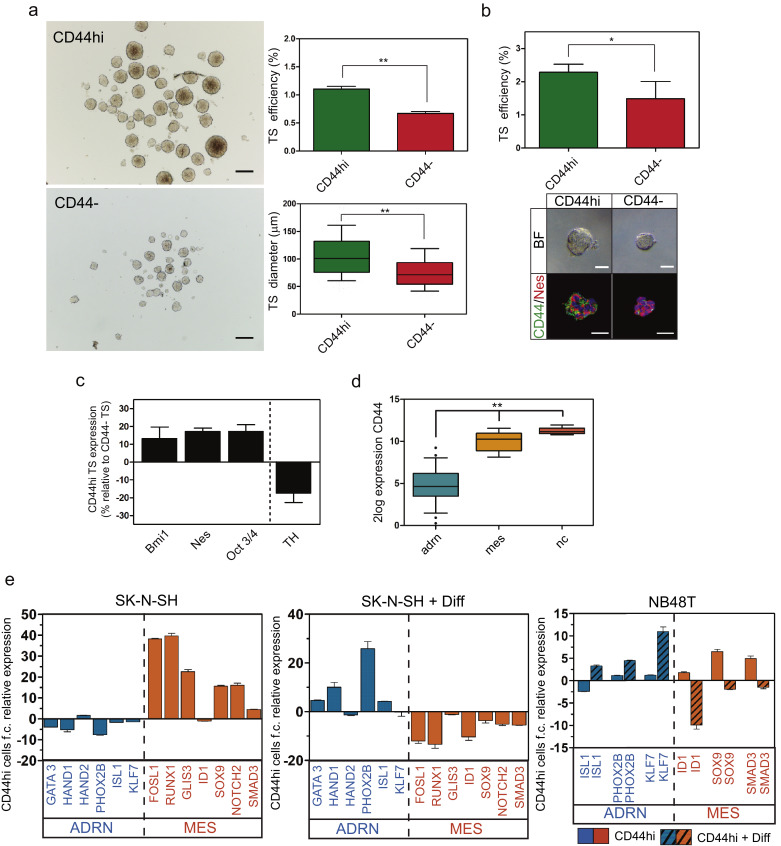
5.4. CD44hi tumoursphere-forming neuroblastoma cells behave as neural crest-like multipotent progenitor cells in culture
Neural crest progenitors give rise to clonal TS in low adherence conditions, from which differentiated cells, corresponding to different neural crest-derived lineages, can be obtained. In order to explore the nature of CD44hi neuroblastoma cells, we tested the tumoursphere forming ability of CD44hi and CD44- cells. The CD44hi cell population is enriched in tumoursphere-forming cells (accounting for 1% of the sorted CD44hi cells) with higher proliferative potential, reflected on an increased TS diameter (Fig. 4a). Single cells sorted on ultra-low binding 96 well plates gave the same results, demonstrating clonogenicity of the formed tumourspheres (Fig. 4b). These spheres were mainly composed by CD44+/Nestin+ cells (bottom panel on Fig. 4b). Same results could be observed with PDX-derived cells (Supplementary Fig. S5a)
Fig. 4.
CD44hi tumoursphere-forming NB cells have an undifferentiated/neural crest-like identity. a, Representative images and quantification of forming efficiency and diameter of TS from CD44hi and CD44- sorted cells. (t-test; **: p < 0.01). Bar: 200 μm. b, Representative images and quantification of TS-forming efficiency on a single cell sorting assay. Data is from 2 independent experiments with a total of 960 sorted cells per condition. (t-test; *: p < 0.05). Bar: 50 μm. c, Gene expression of representative undifferentiation (Bmi1, Nes and OCT3/4) and differentiation (TH) marker genes on TS derived from CD44hi cells, relative to the expression of CD44 negative-derived TS. d, CD44 expression from data in GEO ID: GSE90805 on transcriptionally defined adrenergic neuronal-like (22 cell lines) or mesenchymal undifferentiated neuroblastoma cell lines (7 cell lines), and human neural crest cells (5 cell lines). (ANOVA; **: p < 0.01). e, Relative gene expression in CD44hi cells compared to CD44- or to CD44hi cells after differentiation, as indicated. Genes corresponding to ADR signature or MES signature are indicated. f.c.: fold change. Diff: differentiation.
Gene expression analysis showed that CD44hi-derived TS express higher levels of undifferentiation markers compared to CD44- cell-derived TS (Fig. 4c). Analysis of available expression datasets shows that CD44 expression is significantly higher on neural crest cells or mesenchymal-undifferentiated (mes) neuroblastoma cells compared to adrenergic (adr) neuronal-like ones (Fig. 4d). Moreover, CD44hi cells have an increase in mes/neural-crest-like gene expression signature, further reinforcing the connection between CD44 expression and the undifferentiated phenotype (Fig. 4e and Supplementary Fig. S5b). This expression pattern can be reversed after neuronal differentiation, with a drop in mes signature genes and an increase in adr signature genes (Fig. 4e).
While tumourspheres formed from CD44- neuroblastoma cells were mainly composed of DDC+/S100β low/Nestin low neuroblasts, TS coming from sorted CD44hi cells were composed mostly by CD44hi/Nestin high undifferentiated cells (Fig. 5a). Furthermore, some DDC+ and GFAP+ cells could be seen at the periphery of CD44hi cell-derived TS, indicating that CD44 positive cells could give rise to neural derivatives.
Fig. 5.
CD44hi TS-forming cells are multipotent. a, Immunostaining showing the indicated markers on secondary tumourspheres sections from CD44hi or CD44- sorted SK-N-SH cells. Arrowheads: DDC positive cells in TS formed from CD44hi cells. Asterisks: GFAP positive cells in TS formed from CD44hi cells. Bar: 50 μm. b, CD44-sorted SK-N-SH neuroblastoma cells after treatment with different media, as described in the methods section, and stained with the indicated markers. DAPI is used in all images for nuclear staining. Arrowheads point to αSMA positive cells in TGFβ3 treated cells. Graphs show quantification of positive cells for each marker and condition. (ANOVA; *: p < 0.05, ***: p < 0.001), ns: not significant. Bar: 50 μm.
To further show that CD44hi tumoursphere-forming cells were multipotent, we decided to direct their differentiation into all three lineages typically obtained from sympatho-adrenal neural crest precursors, following a modification of the protocol described elsewhere [30] (Fig. 5b and Supplementary Fig. S5c). Culturing in neural low-serum media (NM) promotes neuronal differentiation, the addition of Forskolin (FK) enriches the culture in glial cells, and the presence of TGFβ3 promotes differentiation of neural crest cells into the mesenchymal lineage. We have also used all-trans retinoic acid (ATRA), commonly used in the clinic as a differentiation agent for neuroblastoma treatment. Independently of the treatment applied, we observed mostly neuronal differentiation from CD44- cells, measured by the expression of DDC and Tuj1. This confirms our previous observations indicating that CD44- cells were mostly restricted to the neuronal lineage. On the other hand, tumourspheres from CD44hi precursor cells also gave rise to DDC+ neuronal cells, demonstrating that not all CD44+ cells are differentiated glial derivatives, and that CD44hi undifferentiated cells retain neuronal differentiation capacity. Moreover, these TS were also able to produce glial (S100β+) and mesenchymal (αSMA+) derivatives when induced to differentiate with the appropriate media, demonstrating their multipotent potential (Fig. 5b). The distinct capacity of CD44hi-derived TS to differentiate towards both mesenchymal and neuronal lineages was further demonstrated by the differentiation of TS coming from sorted single cells (Supplementary Fig. S5d and e).
In conclusion, these results show that a small proportion of CD44hi cells in neuroblastoma seem to be neural crest-derived multipotent progenitors able to give rise to diverse cell types.
5.5. CD44hi neuroblastoma tumour cells are more tumorigenic and metastatic
High-grade neuroblastomas are vascularized tumours, whose major component are proliferative neuroblasts. Rare, non neuroblastic cell populations are also present in these tumours, but their contribution to tumour aggressiveness is not clear. To explore the contribution of undifferentiated CD44 positive cells to tumour formation we sorted CD44hi and negative cells from primary xenografts performed on immune-compromised mice and injected them on secondary recipient mice (Supplementary Figs. S6a and b). SK-N-SH cells form neuroblastic tumours that recapitulate, histologically and morphologically, human neuroblastomas [35], and that present a low proportion of CD44 positive cells (7.1+/−6.8% of CD44+ cells). CD44 positive cells sorted from these tumours generated secondary tumours more efficiently, and more aggressively than CD44 negative cells (Fig. 6a and b). These tumours also metastasize more frequently and aggressively to the lungs (Fig. 6c). CD44hi cells-derived tumours have the same histologic characteristics that the ones formed by the unsorted population and were composed mainly of CD44 negative neuroblasts, with a 24.2% (+/−12.9) of CD44 positive cells. Interestingly, xenografts formed by CD44- tumour cells also presented a CD44+ population (14+/−1.4% of CD44+ cells within the tumours) suggesting some plasticity in vivo. These cells continue forming undifferentiated tumourspheres in culture with higher efficiency than CD44 negative cells sorted from the same tumours (Fig. 6d and Supplementary Fig. S6c).
Fig. 6.
CD44hi cells present increased tumorigenic potential. a, Representative xenograft obtained after SK-N-SH engraftment, stained for CD44. Arrowheads: CD44 positive cells. Bar: 100 μm. b, Volume of the appeared tumours and incidence obtained at 8 weeks after injection with the indicated cell populations. (t-test; *: p < 0.05). c, Incidence and extent of micrometastasis in lungs and liver of xenografted mice. Representative immunostainings showing micrometastatic colonies of human cells in lungs are shown. Arrowheads: NB cell colonies. Bar: 100 μm. d, Immunostaining of sectioned tumourspheres from CD44hi and CD44- sorted cells from tumours. Bar: 50 μm. e, PDX tumour volume and incidence obtained at 8 weeks after injection with the indicated cell populations. (t-test; *: p < 0.01). f, Representative images of paraffin sections from tumours obtained from CD44hi or CD44- PDX cells, stained with the indicated antibodies. Bar: 100 μm (200 μm for αSMA images). (t-test; *: p < 0.05). g, Representative confocal image from PDX tissue section stained for CD44, Nestin and S100β. White arrowhead points to a CD44+/Nes+/S100β- cell. Yellow arrowhead points to a CD44+/Nes-/S100β cell. Bar: 50 μm.
Finally, we sorted freshly dissociated cells from neuroblastoma patient derived xenografts (PDXs) growing in immune-compromised mice (Supplementary Fig. S6d). When re-injected in recipient mice, CD44hi cells generated highly vascularized undifferentiated neuroblastic tumours with higher efficiency than CD44 negative cells (Fig. 6e and f and Supplementary Fig. S6e). Examining the histology of these PDX tumours in detail, we could detect CD44+/Nes+ cells and CD44+/S100β+ elongated cells with schwannian morphology (Fig. 6g), with Nes and S100β staining being mutually exclusive, corroborating the existence of two distinct CD44+ cell populations and the presence of CD44+ undifferentiated cells. Of note, CD44 expression was restricted to the membrane in CD44+/Nes+ cells.
Altogether, our in vivo data suggest that CD44 marks, both in neuroblastoma cell line-derived xenografts and in human PDXs, a tumorigenic undifferentiated cell population, able to form neuroblastic aggressive tumours upon re-injection into secondary recipient mice.
6. Discussion
We show here that CD44 is expressed in a cell population with characteristics of undifferentiated neural crest-like progenitors, which are able to give rise to multipotent tumourspheres in culture and present a high tumorigenic potential. There has been some controversy over the significance of CD44 expression in neuroblastic tumours, with some studies describing that CD44 expression can be a significant factor for the prediction of survival in neuroblastomas, associated with good prognosis [25]. However, most studies classify tumour patient samples as CD44 positive when more than 10% of the cells are immune-reactive for anti-CD44 antibodies in immunohistochemistry, ignoring less frequent cell populations. Moreover, they do not normally address CD44 active membrane localization or the level of expression. A study performed on dissociated tumour cells showed CD44 expression upregulation in non-MYCN human NB metastatic tumours with unfavourable histology, compared to benign ones [38]. Our results also suggest that, among high-risk NB tumours, a high CD44 expression confers worse survival, and that this CD44 expression could be indicative of the presence of undifferentiated neural crest-like cells with malignant properties, different than the neuroblastic adrenergic population that normally forms the bulk of the tumour. Recent works have suggested a similar cellular heterogeneity on high-grade neuroblastoma tumours at the transcriptional level [10,11].
The presence of CD44 is frequently associated with differentiated tumours with higher glial and stromal content (schwannian subtype) and therefore with a better prognosis [18,23]. This is the case for most low stage, MYCN non-amplified tumours and explains previous observations on those tumours. However, there is still a subset of NB patients with no amplification of MYCN that progresses badly and is more difficult to classify. Our results show that a high CD44 expression could also be associated with tumours with worse outcome and presence of undifferentiated cells, with invasive and proliferative properties, that could contribute to tumour aggressiveness. The characterization of CD44 expressing cells in these tumours could be of help for the early detection and treatment of patients.
The expression of CD44 shows an inverse correlation with MYCN oncogene expression [18]. This introduces difficulties to the study of the role of CD44 in neuroblastoma tumours, as MYCN amplification is a strong predictor and driver of aggressiveness. However, event-free survival probability among patients with high-risk decreases in case of high CD44 expression, both in MYCN non-amplified or amplified tumours, illustrating how CD44 could be indicative of the presence of undifferentiated malignant cells in these tumours, and further highlighting the different meanings of CD44 expression in NB.
Our data confirm that CD44hi NB cells have increased adhesion and migration, likely contributing to their aggressiveness. CD44+ cells have been shown to express more adhesion molecules than CD44- cells in NB [36]. However, we only get partial suppression of these phenotypes after CD44 expression knockdown, indicating that there might be other adhesion molecules implicated. Nevertheless, cell sorting of NB cells based on CD44 expression is enough to identify this motile and adhesive cell population. A differential metastatic pattern in CD44+ cell-induced NB tumours, and the accumulation of CD44+ NB cells in hyaluronic-rich areas of the lungs after metastatic dissemination have been suggested [35,37]. Our xenograft experiments show an increase in metastasis, specifically to the lungs, in CD44hi-derived tumours. Matrix-CD44 interaction has been linked to increased tumour cell survival, increased epithelial-to-mesenchymal transition (EMT) and metastasis, cancer stem cell self-renewal and drug resistance [39]. Metastasis in neuroblastoma might be defined by an adhesion imbalance in the tumour microenvironment [40]. The direct contribution of the migratory and adhesive properties of CD44+ cells to NB metastasis and aggressiveness remains to be elucidated.
We have shown that CD44hi cells sorted from cell lines, mouse xenografts or PDXs, are able to form multipotent undifferentiated tumourspheres with increased proliferative and self-renewal capacity. The origin of neuroblastoma is thought to be an incompletely differentiated neural crest precursor during development [1,41]. The existence of cells with stem-like properties in NB tumours has been suggested and their possible contribution to tumour aggressiveness proposed [38,[42], [43], [44]]. Nevertheless, the discovery of a definitive marker allowing the isolation and characterization of this cell population in tumours has been elusive [43] and most probably a combination of cellular markers in specific cancer settings will be needed [45,46]. CD44 permits the enrichment for cancer stem cells (CSCs) in tumours of diverse origin [39,47], and promotes tumour progression by mediating CSC-driven metastasis [48]. Our results point to CD44 as a marker for tumour cells with shared characteristics with neural precursors. CD44 expression in neural precursor cells improves transendothelial migration and invasion [49] and it has been detected in normal neuronal precursors migrating into the adrenal gland [50]. In a transcriptome profile study with normal embryonic neuroblasts, CD44 appeared more expressed in these primordial cell clusters, putative origin of neuroblastomas, than in control adjacent cortical cells [51]. Our results suggest that CD44 might be a non-exclusive marker for undifferentiated neural crest stem-like cells in neuroblastoma, although its expression in other differentiated cell populations within tumours, as in glial S100β+ derivatives, preclude the use of CD44 in solitary for the identification of these cells in this type of cancer. The co-expression of CD44 with putative neuroblastoma stem cell markers, like CD114 or c-kit, described here, opens new possibilities to isolate undifferentiated subpopulations from tumours. Interestingly, we have detected CD44+/Nestin+/S100β- cells in PDX tumours in which CD44 pattern of expression in the membrane is compatible with an active adhesive function. These cells present a clearly different morphology than CD44+/Nestin-/S100β+ schwannian cells.
Cellular heterogeneity has emerged has a hallmark for NB tumours. Histology describes cells with different differentiation status and cells with morphology and expression patterns reminiscent of neural crest-derived lineages [7,8]. Recent transcriptional and expression profiles have defined the presence of at least a mesenchymal neural crest-like undifferentiated identity and an adrenergic differentiated identity, both proliferative and able to interconvert to some extent [10,11]. Although the contribution of the different cell populations to tumour progression needs to be defined, undifferentiated cells might be more malignant and resistant to chemotherapy. Our results confirm that CD44 is a marker for the undifferentiated neural crest-like cell population, delineating the mesenchymal/neural crest-like state from the adrenergic state in NB cells. It will help to define the contribution of these cells to NB aggressiveness. Our experiments also indicate that there is heterogeneity in the tumour forming ability within the CD44hi cell population growing in normal culture conditions. Nearly 40% of SK-N-SH cells are positive for CD44 expression in culture, although only about 7% of cells are CD44+ in SK-N-SH-derived xenografts. We found evidences of higher tumorigenicity on CD44hi cells sorted from tumour xenografts either derived from cell lines or from human tumour samples. Our results also show that, consistent with previous reports [36], there are tumorigenic CD44 negative tumourspheres-forming progenitors on our cell cultures. However, these cells seem to lack multipotency and seem to differentiate early in vitro to neuron-like cells.
In conclusion, we have characterized CD44 function in neuroblastoma tumours at the cellular level, taking into consideration NB cellular intratumoural heterogeneity and its contribution to tumour progression. We propose that, in NB tumours, a high CD44 expression marks a proliferative, multipotent undifferentiated cell population with tumorigenic potential, in addition to labelling other differentiated benign cell types, explaining the different association of CD44 expression with patient survival. Our data stablishes CD44 as a possible biomarker with prognostic value for a subset of patients. Moreover, it points to a significant contribution of undifferentiated CD44hi precursors to neuroblastoma aggressiveness and opens the way to the design of strategies for their identification and targeting in neuroblastoma patients.
CRediT authorship contribution statement
Francisco M. Vega: Conceptualization, Supervision, Writing - original draft, Methodology, Data curation, Formal analysis, Writing - review & editing. Ana Colmenero-Repiso: Data curation. María A. Gómez-Muñoz: Data curation, Resources, Writing - review & editing. Ismael Rodríguez-Prieto: Methodology, Data curation, Resources, Writing - review & editing. Diana Aguilar-Morante: Methodology, Resources, Writing - review & editing. Gema Ramírez: Methodology, Writing - review & editing. Catalina Márquez: Methodology, Writing - review & editing. Rosa Cabello: Resources, Writing - review & editing. Ricardo Pardal: Conceptualization, Supervision, Writing - original draft, Formal analysis, Writing - review & editing.
Declaration of Competing Interest
Dr. Vega reports grants from Andalucía Talent Hub grant from “Agencia Andaluza del conocimiento, Junta de Andalucía” co-funded by the EU 7th Framework Program, Marie Skłodowska-Curie actions (COFUND-291780) and the Junta de Andalucía, during the conduct of the study; Dr. Pardal reports grants from “Asociación Española contra el Cáncer” (AECC), grants from Spanish Ministry of Science and Innovation SAF program (SAF2016-80412-P) co-funded by FEDER funds, grants from European Research Council (ERC Starting Grant to RP), during the conduct of the study; Dr. Gomez-Muñoz reports grants from “Asociación Niños Enfermos de Neuroblastoma (NEN)”., during the conduct of the study; Ms. Colmenero-Repiso, Mr. Rodríguez-Prieto, Dr. Aguilar-Morante, Dr. Ramirez, Dr. Cabello and Dr. Márquez have nothing to disclose.
Acknowledgments
Acknowledgements
We are grateful to Dr Eloy Rivas and the tissue Biobank at Virgen del Rocio University Hospital for their help with the human tumour samples and tissue microarray (TMA) histology. We thank María José Castro for her help with flow cytometry and Valentina Annese and Veronica Sobrino for their helpful discussion. We thank Dr. M. Ponzoni and Dr. D. Di Paolo for providing NB cell lines.
Funding
This research is supported by grants from the “Asociación Española contra el Cáncer” (AECC), the Spanish Ministry of Science and Innovation SAF program (SAF2016-80412-P) co-funded by FEDER funds, and the European Research Council (ERC Starting Grant to RP). FMV was partially supported by an Andalucía Talent Hub grant from “Agencia Andaluza del conocimiento, Junta de Andalucía” co-funded by the EU 7th Framework Program, Marie Skłodowska-Curie actions (COFUND – 291780) and the Junta de Andalucía. MAG is supported by a fellowship from the “Asociación Niños Enfermos de Neuroblastoma (NEN)”. The funders had no role in study design, data collection, data analysis, interpretation or writing of the report. The corresponding authors confirm that they have full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2019.10.041.
Contributor Information
Francisco M. Vega, Email: fmvega@us.es.
Ricardo Pardal, Email: rpardal@us.es.
Appendix. Supplementary materials
References
- 1.Tsubota S., Kadomatsu K. Origin and initiation mechanisms of neuroblastoma. Cell Tissue Res. 2018;372:211–221. doi: 10.1007/s00441-018-2796-z. [DOI] [PubMed] [Google Scholar]
- 2.Matthay K.K., Maris J.M., Schleiermacher G., Nakagawara A., Mackall C.L., Diller L. Neuroblastoma. Nat Rev Dis Prim. 2016;2:16078. doi: 10.1038/nrdp.2016.78. [DOI] [PubMed] [Google Scholar]
- 3.Neuroblastoma B.GM. biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–216. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 4.Pinto N.R., Applebaum M.A., Volchenboum S.L., Matthay K.K., London W.B., Ambros P.F. Advances in risk classification and treatment strategies for neuroblastoma. J Clin Oncol. 2015;33:3008–3017. doi: 10.1200/JCO.2014.59.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Louis C.U., Neuroblastoma S.J.M. molecular pathogenesis and therapy. Annu Rev Med. 2015;66:49–63. doi: 10.1146/annurev-med-011514-023121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oberthuer A., Warnat P., Kahlert Y., Westermann F., Spitz R., Brors B. Classification of neuroblastoma patients by published gene-expression markers reveals a low sensitivity for unfavorable courses of MYCN non-amplified disease. Cancer Lett. 2007;250:250–267. doi: 10.1016/j.canlet.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Mohlin S.A., Wigerup C., Påhlman S. Neuroblastoma aggressiveness in relation to sympathetic neuronal differentiation stage. Semin Cancer Biol. 2011;21:276–282. doi: 10.1016/j.semcancer.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Shimada H., Ambros I.M., Dehner L.P., Hata J., Joshi V.V., Roald B. The international neuroblastoma pathology classification (the Shimada system) Cancer. 1999;86:364–372. [PubMed] [Google Scholar]
- 9.Mora J., Cheung NK V., Juan G., Illei P., Cheung I., Akram M. Neuroblastic and Schwannian stromal cells of neuroblastoma are derived from a tumoral progenitor cell. Cancer Res. 2001;61:6892–6898. [PubMed] [Google Scholar]
- 10.Boeva V., Louis-Brennetot C., Peltier A., Durand S., Pierre-Eugène C., Raynal V. Heterogeneity of neuroblastoma cell identity defined by transcriptional circuitries. Nat Genet. 2017;49:1408–1413. doi: 10.1038/ng.3921. [DOI] [PubMed] [Google Scholar]
- 11.van Groningen T., Koster J., Valentijn L.J., Zwijnenburg D.A., Akogul N., Hasselt N.E. Neuroblastoma is composed of two super-enhancer-associated differentiation states. Nat Genet. 2017;49:1261–1266. doi: 10.1038/ng.3899. [DOI] [PubMed] [Google Scholar]
- 12.Orian-Rousseau V. CD44, a therapeutic target for metastasising tumours. Eur J Cancer. 2010;46:1271–1277. doi: 10.1016/j.ejca.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 13.Dhar D., Antonucci L., Nakagawa H., Kim J.Y., Glitzner E., Caruso S. Liver cancer initiation requires p53 inhibition by CD44-Enhanced growth factor signaling. Cancer Cell. 2018;33:1061–1077. doi: 10.1016/j.ccell.2018.05.003. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marangoni E., Lecomte N., Durand L., de Pinieux G., Decaudin D., Chomienne C. CD44 targeting reduces tumour growth and prevents post-chemotherapy relapse of human breast cancers xenografts. Br J Cancer. 2009;100:918–922. doi: 10.1038/sj.bjc.6604953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orian-Rousseau V. CD44 acts as a signaling platform controlling tumor progression and metastasis. Front Immunol. 2015;6:154. doi: 10.3389/fimmu.2015.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Marzo A.M., Bradshaw C., Sauvageot J., Epstein J.I., Miller G.J. CD44 and CD44v6 downregulation in clinical prostatic carcinoma: relation to Gleason grade and cytoarchitecture. Prostate. 1998;34:162–168. doi: 10.1002/(sici)1097-0045(19980215)34:3<162::aid-pros2>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 17.Shtivelman E., Bishop J.M. Expression of CD44 is repressed in neuroblastoma cells. Mol Cell Biol. 1991;11:5446–5453. doi: 10.1128/mcb.11.11.5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gross N., Beretta C., Peruisseau G., Jackson D., Simmons D., Beck D. CD44H expression by human neuroblastoma cells: relation to MYCN amplification and lineage differentiation. Cancer Res. 1994;54:4238–4242. [PubMed] [Google Scholar]
- 19.Komminoth P., Seelentag W.K., Saremaslani P., Heitz P.U., Roth J. CD44 isoform expression in the diffuse neuroendocrine system. II. Benign and malignant tumors. Histochem Cell Biol. 1996;106:551–562. doi: 10.1007/BF02473270. [DOI] [PubMed] [Google Scholar]
- 20.Mulder J.W., Kruyt P.M., Sewnath M., Oosting J., Seldenrijk C.A., Weidema W.F. Colorectal cancer prognosis and expression of exon-v6-containing CD44 proteins. Lancet. 1994;344:1470–1472. doi: 10.1016/s0140-6736(94)90290-9. [DOI] [PubMed] [Google Scholar]
- 21.Zeilstra J., Joosten S.P., van Andel H., Tolg C., Berns A., Snoek M. Stem cell CD44v isoforms promote intestinal cancer formation in Apc(min) mice downstream of Wnt signaling. Oncogene. 2014;33:665–670. doi: 10.1038/onc.2012.611. [DOI] [PubMed] [Google Scholar]
- 22.Munchar M.J.J., Sharifah N.A., Jamal R., Looi L.M. CD44s expression correlated with the international neuroblastoma pathology classification (Shimada system) for neuroblastic tumours. Pathology. 2003;35:125–129. [PubMed] [Google Scholar]
- 23.Hasegawa G., Minami N., Kushida A., Inuyama H., Koga M., Wakabayashi K. Human neuroblastoma GOTO cells express CD44 and localize it into lipid rafts upon differentiation into Schwannian cells. Cell Biol Int. 2005;29:193–202. doi: 10.1016/j.cellbi.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Hiyama E., Hiyama K., Yamaoka H., Sueda T., Reynolds C.P., Yokoyama T. Expression profiling of favorable and unfavorable neuroblastomas. Pediatr Surg Int. 2004;20:33–38. doi: 10.1007/s00383-003-1077-3. [DOI] [PubMed] [Google Scholar]
- 25.Combaret V., Gross N., Lasset C., Frappaz D., Beretta-Brognara C., Philip T. Clinical relevance of CD44 cell surface expression and MYCN gene amplification in neuroblastoma. Eur J Cancer. 1997;33:2101–2105. doi: 10.1016/s0959-8049(97)00236-0. [DOI] [PubMed] [Google Scholar]
- 26.Kramer K., Cheung NK V., Gerald W.L., LaQuaglia M., Kushner B.H., LeClerc J.M. Correlation of MYCN amplification, Trk-A and CD44 expression with clinical stage in 250 patients with neuroblastoma. Eur J Cancer. 1997;33:2098–2100. doi: 10.1016/s0959-8049(97)00211-6. [DOI] [PubMed] [Google Scholar]
- 27.Ciccarone V., Spengler B a, Meyers M.B., Biedler J.L. Ross R a. Phenotypic diversification in human neuroblastoma cells: expression of distinct neural crest lineages. Cancer Res. 1989;49:219–225. [PubMed] [Google Scholar]
- 28.Ross R.A., Biedler J.L., Spengler B.A. A role for distinct cell types in determining malignancy in human neuroblastoma cell lines and tumors. Cancer Lett. 2003;197:35–39. doi: 10.1016/s0304-3835(03)00079-x. [DOI] [PubMed] [Google Scholar]
- 29.Carpenter A.E., Jones T.R., Lamprecht M.R., Clarke C., Kang I.H., Friman O. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7:R100. doi: 10.1186/gb-2006-7-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curchoe C.L., Maurer J., McKeown S.J., Cattarossi G., Cimadamore F., Nilbratt M. Early acquisition of neural crest competence during hESCs neuralization. PLoS One. 2010;5:e13890. doi: 10.1371/journal.pone.0013890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becker M., Nitsche A., Neumann C., Aumann J., Junghahn I., Fichtner I. Sensitive PCR method for the detection and real-time quantification of human cells in xenotransplantation systems. Br J Cancer. 2002;87:1328–1335. doi: 10.1038/sj.bjc.6600573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linares-Clemente P., Aguilar-Morante D., Rodríguez-Prieto I., Ramírez G., de Torres C., Santamaría V. Neural crest derived progenitor cells contribute to tumor stroma and aggressiveness in stage 4/M neuroblastoma. Oncotarget. 2017;8 doi: 10.18632/oncotarget.21128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Platero-Luengo A., González-Granero S., Durán R., Díaz-Castro B., Piruat J.I., García-Verdugo J.M. An O2-Sensitive Glomus cell-stem cell synapse induces carotid body growth in chronic hypoxia. Cell. 2014;156:291–303. doi: 10.1016/j.cell.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 34.Tomolonis J.A., Agarwal S., Shohet J.M. Neuroblastoma pathogenesis: deregulation of embryonic neural crest development. Cell Tissue Res. 2018;372:245–262. doi: 10.1007/s00441-017-2747-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valentiner U., Valentiner F.U., Schumacher U. Expression of CD44 is associated with a metastatic pattern of human neuroblastoma cells in a SCID mouse xenograft model. Tumour Biol. 2008;29:152–160. doi: 10.1159/000143401. [DOI] [PubMed] [Google Scholar]
- 36.Siapati E.K., Rouka E., Kyriakou D., Vassilopoulos G. Neuroblastoma cells negative for CD44 possess tumor-initiating properties. Cell Oncol. 2011;34:189–197. doi: 10.1007/s13402-011-0022-z. [DOI] [PubMed] [Google Scholar]
- 37.Schwankhaus N., Gathmann C., Wicklein D., Riecken K., Schumacher U., Valentiner U. Cell adhesion molecules in metastatic neuroblastoma models. Clin Exp Metastasis. 2014:1–14. doi: 10.1007/s10585-014-9643-8. [DOI] [PubMed] [Google Scholar]
- 38.Jensen T., Vadasz S., Phoenix K., Claffey K., Parikh N., Finck C. Descriptive analysis of tumor cells with stem like phenotypes in metastatic and benign adrenal tumors. J Pediatr Surg. 2015;50:1493–1501. doi: 10.1016/j.jpedsurg.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 39.Chanmee T., Ontong P., Kimata K., Itano N. Key roles of hyaluronan and its CD44 receptor in the stemness and survival of cancer stem cells. Front Oncol. 2015;5:180. doi: 10.3389/fonc.2015.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delloye-Bourgeois C., Bertin L., Thoinet K., Jarrosson L., Kindbeiter K., Buffet T. Microenvironment-Driven shift of cohesion/detachment balance within tumors induces a switch toward metastasis in neuroblastoma. Cancer Cell. 2017;32:427–443. doi: 10.1016/j.ccell.2017.09.006. e8. [DOI] [PubMed] [Google Scholar]
- 41.Marshall G.M., Carter D.R., Cheung B.B., Liu T., Mateos M.K., Meyerowitz J.G. The prenatal origins of cancer. Nat Rev Cancer. 2014;14:277–289. doi: 10.1038/nrc3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garner E., Beierle E. Cancer stem cells and their interaction with the tumor microenvironment in neuroblastoma. Cancers. 2015;8:5. doi: 10.3390/cancers8010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsu D.M., Agarwal S., Benham A., Coarfa C., Trahan D.N., Chen Z. G-CSF receptor positive neuroblastoma subpopulations are enriched in chemotherapy-resistant or relapsed tumors and are highly tumorigenic. Cancer Res. 2013;73:4134–4146. doi: 10.1158/0008-5472.CAN-12-4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ross R.A. Human neuroblastoma stem cells. Semin Cancer Biol. 2007;17:241–247. doi: 10.1016/j.semcancer.2006.04.006. S1044-579X(06)00038-1 [pii]10.1016/j.semcancer.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 45.Coulon A., Flahaut M., Mühlethaler-Mottet A., Meier R., Liberman J., Balmas-Bourloud K. Functional sphere profiling reveals the complexity of neuroblastoma tumor-initiating cell model. Neoplasia. 2011;13:991–1004. doi: 10.1593/neo.11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pandian V., Ramraj S., Khan F.H., Azim T., Aravindan N. Metastatic neuroblastoma cancer stem cells exhibit flexible plasticity and adaptive stemness signaling. Stem Cell Res Ther. 2015;6:2. doi: 10.1186/s13287-015-0002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zöller M. CD44, can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer. 2011;11:254–267. doi: 10.1038/nrc3023. [DOI] [PubMed] [Google Scholar]
- 48.Kinugasa Y., Matsui T., Takakura N. CD44 expressed on cancer-associated fibroblasts is a functional molecule supporting the stemness and drug resistance of malignant cancer cells in the tumor microenvironment. Stem Cells. 2014;32:145–156. doi: 10.1002/stem.1556. [DOI] [PubMed] [Google Scholar]
- 49.Deboux C., Ladraa S., Cazaubon S., Ghribi-Mallah S., Weiss N., Chaverot N. Overexpression of CD44 in neural precursor cells improves trans-endothelial migration and facilitates their invasion of perivascular tissues in vivo. PLoS One. 2013;8:e57430. doi: 10.1371/journal.pone.0057430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Combaret V., Coll J.L., Favrot M.C. Expression of integrin and CD44 adhesion molecules on neuroblastoma: the relation to tumor aggressiveness and embryonic neural-crest differentiation. Invasion Metastasis. 1994;14:156–163. [PubMed] [Google Scholar]
- 51.De Preter K., Vandesompele J., Heimann P., Yigit N., Beckman S., Schramm A. Human fetal neuroblast and neuroblastoma transcriptome analysis confirms neuroblast origin and highlights neuroblastoma candidate genes. Genome Biol. 2006;7:R84. doi: 10.1186/gb-2006-7-9-r84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.