Abstract
Injection into the suprachoroidal space (SCS) allows drug delivery targeted to sclera, choroid and retina. Here, we studied SCS injection formulated with collagenase to expand drug delivery coverage and increase posterior drug targeting within SCS by breaking down collagen fibrils that link sclera and choroid in the SCS. When 1 μm latex microparticles were injected with a collagenase formulation using microneedles into the SCS of rabbit eyes ex vivo and incubated at 37°C for 4 h, microparticle delivery coverage increased from 20% to 45% and enhanced posterior drug targeting. Collagenase concentration was optimized to 0.5 mg/mL to maximize expanded posterior delivery and minimize tissue damage. Effects of collagenase injection within SCS increased and then plateaued 4 h after injection. Simultaneous injection of collagenase and microparticles had a greater effect on expanded delivery in the SCS compared to sequential injection. Collagenase injection into the SCS of rabbit eyes in vivo was also effective to expand delivery and was generally well tolerated, showing transiently lowered IOP, but no apparent lasting adverse effects on ocular tissues such as sclera, choroid, and retina, as determined by analyzing histology, sclera tensile strength and fundus imaging. We conclude that addition of collagenase during SCS injection can expand drug delivery coverage and increase posterior drug targeting.
Keywords: ocular drug delivery, microneedles, suprachoroidal space, collagenase
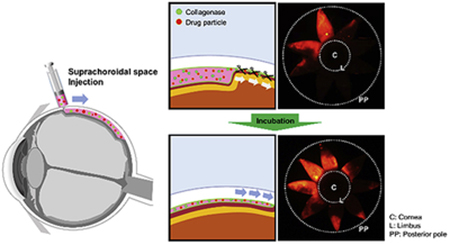
Graphical Abstract
1. Introduction
Ocular diseases such as age-related macular degeneration (AMD), macular edema, and uveitis are leading causes of blindness in the industrialized world (Lee et al., 2015; Yang et al., 2016). Current ophthalmic drug delivery (i.e. topical eye drops, intra- and peri-ocular injection and systemic drug delivery) is constrained by the anatomical and physiological barriers of the eye, which prevent many drugs from effectively or selectively reaching their sites of pharmacological action (Holz et al., 2014; Rowe-Rendleman et al., 2014).
To achieve better targeting to posterior segment tissues, drugs have been injected into the suprachoroidal space (SCS), which is a potential space between the sclera and choroid that provides a novel drug delivery route targeting the chorioretina (Jung et al., 2019; Moisseiev et al., 2016; Patel et al., 2011). SCS injection can improve the bioavailability of drugs targeting their site of action with significant dose sparing (Abarca et al., 2013; Chen et al., 2015). In addition, SCS injection allows drugs to flow circumferentially from an anterior injection site near the limbus posteriorly to the macula and optic nerve. Initial studies targeted the SCS using catheters or other devices requiring surgical procedures, but SCS injection has more recently and extensively been performed using a microneedle due to its simplicity and safety (Chiang et al., 2018; Wang and Eliott, 2017).
Microneedles used for SCS delivery are similar to hypodermic needles but measure only ~1 mm in length. This design facilitates perpendicular penetration across the conjunctiva and sclera to the sclera-choroid interface, which targets injection into the SCS in a minimally invasive way (Habot-Wilner et al., 2019; Patel et al., 2012; Patel et al., 2011; Patel and Prausnitz, 2016; Rai et al., 2015). Since scleral thickness is different among patients, the proper length of a microneedle is sometimes determined based on ultrasound measurement of scleral thickness (Goldstein, 2014). SCS injection using a microneedle has been shown to be safe and well tolerated in several clinical trials, including Phase III studies (ClinicalTrials.gov, 2017b, 2018; Goldstein et al., 2016; Lampen et al., 2018).
Typical SCS injection volume (in rabbits) is 50 μL, which some studies have shown to be insufficient to inject material uniformly throughout the SCS, but instead localizes material preferentially near the site of injection (Chiang et al., 2016b; Gu et al., 2015). Nonetheless, SCS delivery has been successfully used to reach target sites in the posterior retina, such as treatment of macular edema after injection of drug near the limbus (ClinicalTrials.gov, 2017a, b, c). It is, however, possible that increased delivery to the posterior SCS would give still better results.
In some cases, localization at the site of injection can be useful for drug delivery, for example, to the ciliary body, which is located next to the anterior SCS, to treat glaucoma (Chiang et al., 2016a). However, it is sometimes desirable to deliver drug to choroid and/or retina throughout the eye, such as when treating uveitis or proliferative diabetic retinopathy, which generally occurs over a large areas of the posterior segment (Goldstein et al., 2016; Jung et al., 2019); (Bressler et al., 2011; Chappelow et al., 2012a) . Other times it is desirable to target drug delivery toward the posterior pole to access the macula or optic nerve, such as when treating macular degeneration, which is localized to the macular region (Schwartz et al., 2014; Weiss et al., 2015).
Although the delivery coverage can be expanded both laterally and posteriorly by increasing injection volume (Chiang et al., 2017a), very large injection volumes can generate highly elevated intraocular pressure and other possible complications to the eye, as well as increase the pain of the injection (Goldstein, 2014; Gu et al., 2015). Therefore, it is important to develop methods other than injecting large volumes to achieve expanded drug delivery coverage (Jung et al., 2019).
There have been previous studies addressing the issue of controlled and expanded drug delivery in the SCS. For example, iontophoresis was used to transfer charged compounds through the SCS toward the posterior pole of the eye (Jung et al., 2018a). In another approach, biodegradable hydrogel was injected into the SCS to push drug particles toward the back of the eye as the hydrogel swelled (Jung et al., 2018b). A high-density nanoparticle emulsion was also developed to direct drug delivery to the back (or front) of the eye in the SCS by using the force of gravity (Kim et al., 2014). Expanded drug delivery coverage was also achieved by injecting non-Newtonian fluids into the SCS, which resulted in particle spreading throughout the SCS (Kim et al., 2015). These previous studies have required novel formulations, special injection procedures or additional instrumentation.
In this study, we developed a new method of SCS injection using collagenase to expand drug delivery coverage and increase posterior drug targeting. Because the interface between sclera and choroid contains loose collagen fibrils traversing the SCS (Yiu et al., 2014), we hypothesized that degradation of the collagen fibrils should make it easier to open up the SCS and thereby expand drug delivery coverage. Outside of the SCS, a similar strategy based on this hypothesis is commonly used. For example, hyaluronidase is often added to an anesthetic formulation to facilitate dispersion and absorption of the anesthetic administered by retrobulbar and peribulbar injection prior to eye surgery (Lv et al., 2015; Silverstein et al., 2012). To investigate our hypothesis, we injected collagenase formulations into the SCS, and then determined the drug delivery area and distribution in the SCS in the rabbit eye ex vivo and in vivo. Because sclera and choroid contain many more and much denser collagen fibrils compared to the SCS and collagenase injection occurs in the SCS, we further hypothesized that SCS collagen fibrils could be significantly degraded while minimizing effects on sclera and choroid. Using this approach, expanded delivery in the SCS was achieved not only physically by the injection itself but also biochemically by collagenase activity to degrade collagenous barriers in the SCS. In this way, we propose that collagenase can be used as a simple, effective, and economical enhancer of SCS delivery without complex experimental steps or instrumentation.
2. Experimental Section
2.1. Formulation preparation
A control solution (Particle) was prepared for SCS injection containing 0.2 wt% negatively charged, carboxylate-modified, red-fluorescent latex microparticles with 1 μm diameter (FluoSpheres, λex=580 nm/λem=605 nm, Life Technologies, Carlsbad, CA) in Hank’s balanced salt solution (HBSS, Mediatech, Manassas, VA). In some cases, collagenase (Sigma-Aldrich, St. Louis, MO) was added to the solution at a concentration of 0.05, 0.1, 0.5, or 1.0 mg/ml (Particle Colla). To stabilize collagenase activity, CaCl2 (Sigma-Aldrich) was added as a cofactor at a concentration of 3 mM (Particle Colla CaCl2). The prepared formulations were analyzed by dynamic light scattering (Zetasizer Nano ZS, Malvern Panalytical, Westborough, MA) to identify possible particle aggregation.
2.2. Ex vivo injection procedure
Prior to SCS injection, frozen rabbit eyes (Pel-Freez Biological, Rogers, AR) were thawed at room temperature for 30 min, after which most fat and conjunctiva were removed to expose a clear injection spot and facilitate analysis of the specimen. To adjust intraocular pressure (IOP) to a physiological range (10-15 mmHg, as determined by a tonometer (iCare Tonovet, Helsinki, Finland)), HBSS was injected through the inferior sclera into the vitreous humor using a hypodermic needle. To maintain IOP throughout the experiment, we confirmed that there was no leakage of HBSS from the eyes after injection.
A 30-gauge hollow microneedle measuring 750 μm in length (Clearside Biomedical, Alpharetta, GA) was inserted perpendicularly to the sclera surface 3 mm posterior from the limbus to make a 50 μL injection of control (Particle) or collagenase formulation (Particle Colla CaCl2). To investigate the effect of collagenase or CaCl2 along on SCS delivery, we also injected a formulation containing only collagenase (Particle Colla) or CaCl2 (Particle CaCl2). The injection was performed within 5 s, while the microneedle was left in place for an additional 90 s to avoid formulation backflow. The injected rabbit eyes were then incubated in HBSS at 37 °C for 4 h, which is a characteristic time over which collagenase is expected to react (according to the manufacture’s collagenase protocol). The incubated eyes ex vivo were still intact, even after 4 hour incubation (Jung et al., 2018b).
To investigate the temporal and spatial nature of the interactions between microparticles, collagenase, and the SCS, we separately injected microparticles and collagenase into the SCS. First, collagenase formulation without microparticles (Colla CaCl2) was injected into the SCS and incubated at 37 ° C for 4 h. Then, microparticle solution without collagenase (Particle) was injected at the same injection site.
2.3. In vivo injection procedure
All in vivo experiments were carried out with approval from the Georgia Institute of Technology Institutional Animal Care and Use Committee. Practices complied with the ARVO statement of the Use of Animals in Ophthalmic and Vision Research. New Zealand White rabbits (Charles River Breeding Laboratories, Wilmington, MA) were anesthetized by subcutaneous injection of ketamine (17.5 mg/kg) and xylazine (8.5 mg/kg). Isoflurane gas was administered to continue anesthesia during the SCS injection. Topical anesthesia, 0.5% proparacaine (Akorn, Lake Forest, IL) was administered to the eye 3 min before the SCS injection.
Rabbit eyes were proptosed by pushing the eyelid back and fixing it with a latex band to facilitate the SCS injection. A 50 μL injection of control (Particle) or collagenase formulation (Particle Colla CaCl2) was administered into the SCS 3 mm posterior to the limbus in the supranasal quadrant of both eyes using a 30-gauge hollow microneedle measuring 750 μm in length (Clearside Biomedical). The injection was carried out within 5 s, after which the microneedle was left in place for an additional 90 s to avoid formulation backflow.
After every SCS injection, anti-inflammatory ointment (neomycin and polymyxin B sulfate and Bacitracin zinc ophthalmic ointment USP, Baush+Lomb, Rochester, NY) was applied to prevent inflammation at the injection site. In addition, buprenorphine HCl (0.3 mg/mL; Par Pharmaceuticals, Spring Valley, NY) was injected intramuscularly at a dose of 0.03 mg/kg for pain control.
In some cases, bright-field fundus images of the eye after SCS injection were obtained with a RetCam II (Clarity Medical Systems, Pleasanton, CA). The images were measured over time (before injection, 1 hour and 7 days after the injection) with images taken over the whole retinal surface that was visible. After SCS injection, the rabbits were checked daily for 10 days by the Georgia Tech animal facility veterinary staff and anything noted by clinical exam was reported. The rabbits were euthanized under anesthesia with an injection of 150 mg/kg pentobarbital through the central ear artery. The eyes were enucleated immediately after death and utilized for analysis of particle distribution and histology.
Three rabbits were used each for in vivo particle distribution analysis and histology analysis. Three additional rabbits were used for fundus imaging and IOP measurement. At least three eyes were used for each experimental analysis.
2.4. Analysis of particle delivery in the SCS
After SCS injection ex vivo or in vivo, rabbit eyes were immersed in isopropyl alcohol and immediately chilled by liquid nitrogen to conserve the particle distribution in the SCS. The fully frozen eyes were dissected from the optic nerve to the limbus so that eight identical radial eye cuts could be made. Each of the eight tissue pieces was then splayed and peeled away outward, exposing the chorioretinal surface inside the eye. The vitreous humor was removed.
Bright-field and fluorescence images of the ocular tissues were taken using a digital camera (Cannon 60d, Melville, NY). A green LED light (Bluewind Multicolor RGB, HitLight, Baton Rouge, LA) was used to excite the fluorescent microparticles, and fluorescence images were obtained by a red camera filter (610 ± 10 nm; Edmunds Optics, Barrington, NJ). ImageJ software was used to analyze fluorescent microparticle distribution in the SCS. To quantify particle distribution, each of the eight petals was cut into four segments every 3 mm from the limbus (0–3, 3–6, 6–9, and >9 mm). Then, the SCS segments were immersed in radioimmunoprecipitation assay buffer (Abcam, Cambridge, MA) and disrupted by a sonicator (Sonics, Newtown, CT). After incubation for 1 day at 4 °C, the supernatant in each solution was transferred to a 96-well plate. The fluorescence signals of the extracted microparticles were measured by a plate reader (Synergy Microplate Reader, Winooski, VT). The average particle distance (APD) was obtained as the sum of the center distances from the limbus of each SCS segment (e.g., the 0-3 mm SCS segment had a center distance of 1.5 mm) multiplied by the percent of microparticles in that SCS segment obtained from the particle distribution data.
2.5. Tensile test for identification of scleral tissue damage
To identify possible scleral tissue damage by collagenase, we carried out tensile tests of sclera tissues and compared their elastic moduli. After SCS injection of a formulation with or without collagenase into rabbit eyes ex vivo, the eyes were incubated at 37 °C for 4 h. Then, the sclera was cut into pieces measuring 5 × 20 mm (width × length). Both of the short edges of the cut sclera were fixed by jaws, which were mounted on a digital force gauge (Mark-10 Series 5, Copiague, NY). Initial distance between the jaws, i.e., the sclera length, was set at 10 mm. Samples were then stretched at a constant displacement rate of 5 mm/min. Tensile stress was calculated from the axial force measured by the digital force gauge and an initial cross-sectional area of the sclera. Strain was defined as the ratio of the change in length and initial length (i.e., 10 mm). We generated a stress-strain curve based on the measured data of tensile stress and strain. Finally, elastic modulus (MPa) was measured at the point of 5% strain (Hatami-Marbini and Rahimi, 2015; Liu and Wang, 2017).
2.6. Histology
Histological analysis was conducted to assess the safety of the collagenase formulation in the SCS. The enucleated eyes were immediately incubated in modified Davison’s fixative (Polyscience, Warrington, PA) for 2 days, then incubated in 10% formalin solution for ≥12 h to fix the entire ocular tissue. Using a microtome, the eyes were sectioned to ~10 μm thickness and stained with hematoxylin-eosin (H&E). Stained samples were imaged to produce cross-sectional views of the tissue around the injection site using a microscope (DP71; Olympus, Tokyo, Japan)
2.7. IOP measurement
After SCS injection, we measured IOP of the rabbit eye in vivo daily for 9 days using a rebound tonometer (TonoVet, iCare, Espoo, Finland). Because ketamine anesthetic can affect IOP in the rabbit, we did not measure IOP on the injection day. To obtain baseline IOP values, we measured IOP daily for 1 week before injection. Each IOP value was averaged from 5 measurements.
2.8. Statistical analysis
Mean and standard error of the mean were caulaulated from the data obtained with at least three replicates. One-tailed t-test was utilized to determine the statistical significance in pairwise comparisons of changes in microparticle delivery coverage and average particle distance. One-way analysis of variance (ANOVA) was utilized to determine statistical significance of changes in the percent of microparticles delivered to the posterior SCS (i.e., >9 mm posterior to the limbus) depending on the collagenase concentration and incubation time. In all cases, a p value < 0.05 was considered statistically significant. A p value < 0.05, < 0.01, or < 0.005 is indicated with one (*), two (**) or three (***) asterisks in the graphs.
3. Results
3.1. Microparticle Delivery Enhanced by Collagenase Formulation
To determine if injection of collagenase could expand microparticle delivery coverage in the SCS, 50 μL of a formulation containing 1 μm fluorescent microparticles either with control (Particle) or collagenase formulation (Particle Colla CaCl2) was injected into the SCS of a rabbit eye ex vivo and incubated for up to 4 h, after which the microparticle delivery coverage and distribution in the SCS were analyzed (Fig. 1). CaCl2 was also included in the collagenase formulation as a cofactor to stabilize collagenase activity.
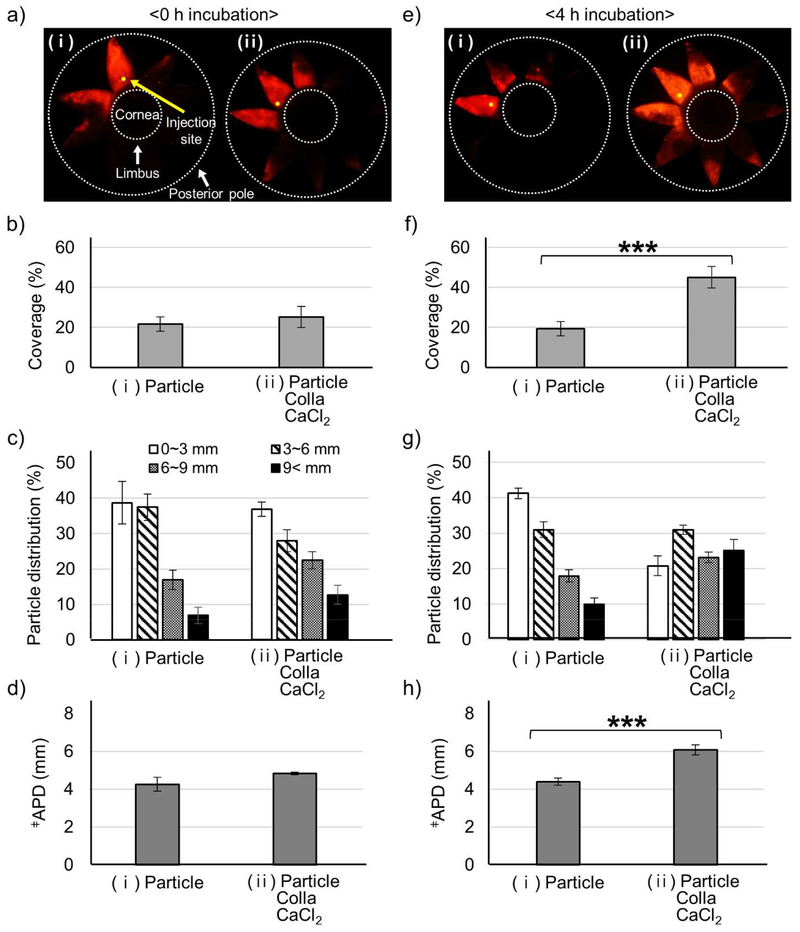
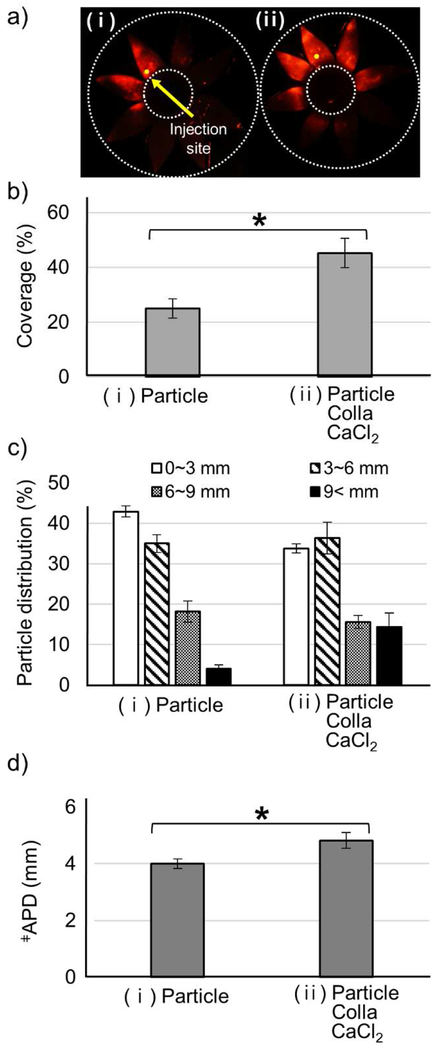
Figure 1.
Microparticle delivery in SCS enhanced by collagenase formulation. Microparticles (Particle) were delivered alone (i) or with collagenase (Colla) and CaCl2 (ii) to the SCS of rabbit eyes ex vivo. Imaging and analysis were performed immediately (a-d) or following incubation at 37 °C for 4 h (e-h) after injection. Representative fluorescence micrographs (a, e), particle delivery coverage (i.e., percent of SCS containing microparticles) (b, f), particle distribution in the SCS (expressed as distance posteriorly from the limbus) (c, g), and average particle delivery distance (APD, expressed as the average particle distance posteriorly from the limbus) (d, h) of red-fluorescent microparticles in the SCS. Fluorescence micrographs (a, e) show splayed eyes after dissection with 8 radial cuts from the posterior pole to the limbus to form “eye petals.” Yellow dots identify the SCS injection site. The cornea is located in the center of the petals, and the posterior pole is at the tips of the petals. Two white dashed lines indicate the location of the limbus and the posterior pole. APD (d, h) was calculated based on the particle distribution in (c, g). ǂAPD (mm) = (1.5 mm × % of particles in 0-3 mm region) + (4.5 mm × % of particles in 3-6 mm region) + (7.5 mm × % of particles in 6-9 mm region) + (10.5 mm × % of particles in >9 mm region). *** indicates significant difference (one tailed t-test, p<0.005). Graphs show mean ± SEM (n=3 in (a-d) and n=6 in (e-f)).
Immediately after SCS injection, there was no significant difference in the particle delivery coverage, between the control (Particle) and collagenase formulation (Particle Colla CaCl2), which was 20 – 25% of SCS surface area in both cases (Fig. 1b). There was also no significant difference in particle distribution (Fig. 1c), which can be described as an average particle distance from the limbus (APD, Fig. 1d), which was 4 – 5 mm in both cases.
When eyes were incubated in a 37 °C HBSS bath for 4 h after injection, the particle delivery coverage of the control solution was not significantly different before (Fig. 1b(i)) versus after (Fig. 1f(i)) the incubation (p>0.05). In contrast, the delivery coverage of microparticles injected in the collagenase formulation was approximately doubled to 45% (Fig. 1f(ii)) relative to the delivery coverage after incubation without collagenase (Fig. 1f(i)). Delivery coverage was also approximately doubled relative to delivery coverage before incubation, either with collagenase (Fig. 1b(ii)) or without collagenase (Fig. 1b(i)).
Similarly in the particle distribution analysis, no significant change was found in the control injection before incubation versus after incubation (Fig. 1c(i) and Fig. 1g(i)). In the collagenase formulation, the particle distribution was shifted posteriorly (Fig. 1g(ii)) and the APD increased (Fig. 1h(ii)) after incubation compared to before incubation (Fig. 1 c(ii) and 1d(ii), respectively) and compared to injection without collagenase (Fig. 1g(i) and 1h(i), respectively). The percent of microparticles delivered to the posterior pole (i.e., >9 mm posterior to the limbus) increased from 10% without collagenase (Fig. 1g(i)) to 25% with collagenase (Fig. 1g(ii)) after incubation.
As additional controls, we measured microparticle delivery in the SCS using solutions containing only CaCl2 (Particle CaCl2) and containing only collagenase (Particle Colla) (Fig. S1). The addition of CaCl2 had no significant effect on particle delivery coverage (Fig. S1b(i) vs. (ii)), particle distribution (Fig. S1c(i) vs. (ii)) or APD (Fig. S1d(i) vs. (ii)). The addition of collagenase (without CaCl2) significantly increased particle delivery coverage (Fig. S1b(i) vs. (iii)), particle distribution (Fig. S1c(i) vs. (iii)) and APD (Fig. S1d(i) vs. (iii)). However, collagenase without CaCl2 had less particle delivery coverage (Fig. S1b(iii) vs. (iv)) and less posterior particle distribution (Fig. S1c(iii) vs. (iv)), but APD was not significantly different (Fig. S1d(iii) vs. (iv)) compared to injection of collagenase with CaCl2.
3.2. Microparticle Aggregation by Collagenase and CaCl2 Formulation
To assess possible microparticle aggregation during incubation in the SCS, each formulation was incubated under the same conditions as the injected eye incubation (i.e., 37 ° C for 4 h, Fig. S2). As determined by dynamic light scattering, particle aggregation was not observed in the control, where particle size remained ~1.2 μm (Fig. S2a(i) and b(i)). Incubation with CaCl2 led to extensive aggregation that increased effective particle size to almost 2.5 μm (Fig. S2a(ii) and 2b(ii)). This probably occurred because the divalent cation, Ca2+, attracted and aggregated the negatively charged microparticles (Chen et al., 2006). Microparticle aggregation was not observed in the solution containing only collagenase (Fig. S2a(iii) and 2Sb(iii)) or the collagenase formulation containing CaCl2 (Fig. S2a(iv) and 2b(iv)), indicating that CaCl2 was associated with collagenase, thereby increasing its enzymatic activity and avoiding association with the microparticles leading to aggregation.
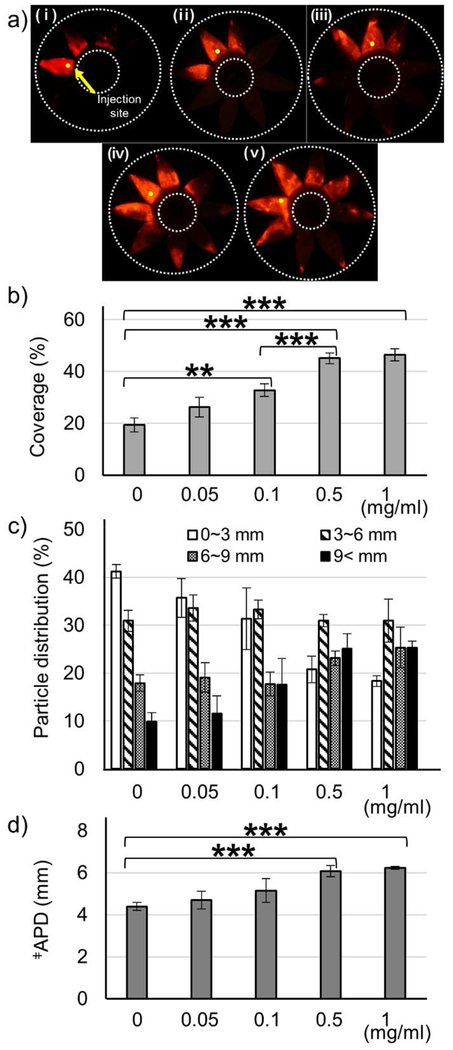
3.3. Effect of Collagenase Concentration on Microparticle Delivery
It is important to optimize collagenase concentration to maximize enhancement of drug delivery while minimizing possible tissue damage by collagenase. We found that as collagenase concentration increased, particle delivery coverage increased (Fig. 2a and b). At the highest collagenase concentration (1 mg/mL), delivery coverage was the largest (46%), but this was not siginificantly different from the delivery coverage at 0.5 mg/mL (45%) (Fig. 2b). In addition, delivery coverage using 0.5 mg/mL collagenase was significantly bigger than when using 0.1 mg/mL collagenase or without collagenase. Particle distribution analysis showed that increasing collagenase concentration also increased posterior SCS delivery (i.e., >9 mm posterior to the limbus) and APD, with maximum effects seen at 0.5 mg/mL (Fig. 2c and d). At this concentration, 25% of microparticles were delivered to the posterior SCS and the APD was 6.1 mm. This suggests that 0.5 mg/mL may be the highest collagenase concentration needed.
Figure 2.
Effect of collagenase concentration on microparticle delivery in the SCS. Collagenase formulation at different concentrations was injected into the SCS of rabbit eyes ex vivo and incubated at 37 °C for 4 h. Representative fluorescence micrographs (a), particle delivery coverage (b), particle distribution in the SCS (c), and APD (d) of red-fluorescent microparticles after injection into the SCS. (i) 0 (ii) 0.05 (iii) 0.1 (iv) 0.5 (v) 1 mg/mL collagenase with 3 mM CaCl2. ǂAverage particle distance. Graphs show mean ± SEM (n=6 in (i and iv) and n=3 in (ii, iii, and v)). *** indicates significant difference (one tailed t-test, p<0.005).
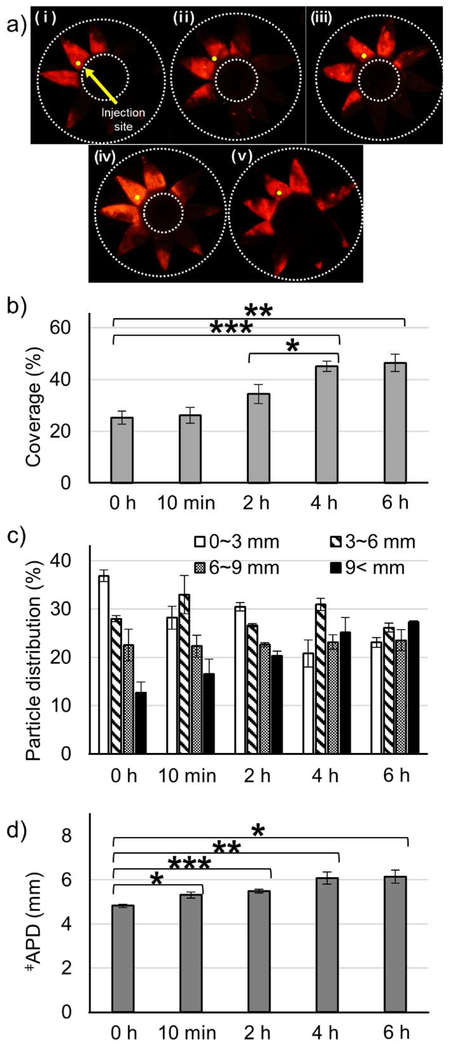
3.4. Effect of Collagenase Incubation Time on Microparticle Delivery
To determine how long the collagenase activity lasts in the SCS, the incubation time was varied from 0 to 6 hours and the effect on microparticle delivery was analyzed (Fig. 3). We found that as incubation time increased, particle delivery coverage increased (Fig. 3b). However, the delivery coverage after incubation for 4 or 6 hours were not significantly different from each other, but were significantly higher compared to incubation for 2 h or less. The particle distribution to the posterior SCS (Fig. 3c) and the APD (Fig. 3d) also increased with increasing incubation time, such that a 4 h incubation maximized posterior SCS delivery delivery and APD, and incubating for 6 h showed no further improvement. Altogether, this suggests that 4 h may be the longest incubation time needed. This may be the case because collagenase loses activity after ~4 h in the SCS, because all of the collagen in the SCS has been effectively degraded after 4 h or other factors.
Figure 3.
Effect of collagenase incubation time on microparticle delivery in the SCS. Collagenase formulation (0.5 mg/mL collagenase with 3 mM CaCl2) was injected into the SCS of rabbit eyes ex vivo and incubated at 37 °C for different lengths of time. Representative fluorescence micrographs (a), particle delivery coverage (b), particle distribution (c), and APD (d) of red-fluorescent microparticles after injection into the SCS followed by different incubation times: (i) 0 h (ii) 10 min (iii) 2 h (iv) 4 h (v) 6 h. ǂAverage particle distance. Graphs show mean ± SEM (n=3 in (i, ii, iii, and v) and n=6 in (iv)). *,**,*** indicate significant difference (one tailed t-test, p<0.05,0.01,0.005, respectively).
3.5. Effect of Simultaneous Versus Sequential Injection of Microparticles and Collagenase on Microparticle Delivery
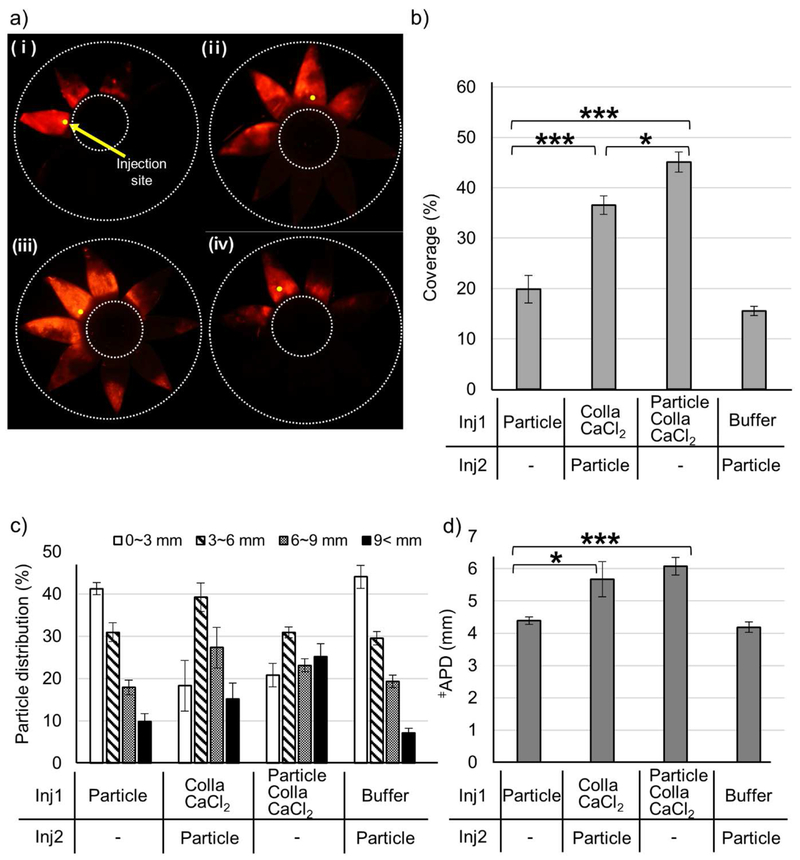
We wanted to further study the temporal and spatial nature of the interactions between microparticles, collagenase, and the SCS. In the experiments already discussed, microparticles and collagenase were injected simultaneously. If the kinetics of collagenase activity to open up the SCS are slow, then it might be beneficial to inject collagenase first and then later inject the microparticles. On the other hand, if collagenase and microparticles have a synergistic interaction, then sequential injection of collagenase and microparticles may be less effective at expanding delivery coverage.
To assess these hypotheses, we injected the collagenase formulation without microparticles (Colla CaCl2) into the SCS, incubated the eye at 37 ° C for 4 h, and then injected a microparticle solution without collagenase (Particle) at the same injection site (Fig. 4). Sequential injection of collagenase and microparticles was more effective than injection of microparticles alone (i.e., with no pre-injection of collagenase), as shown by increased particle delivery coverage (Fig. 4b), particle distribution to the posterior SCS (Fig. 4c) and APD (Fig. 4d). Sequential injection in this way could be useful in a scenario where collagenase interacts with the drug, and the two compounds need to be isolated from each other. However, simultaneous injection of collagenase and microparticles was even more effective than sequential injection, as shown by even larger increases in delivery coverage (Fig. 4b) and particle distribution to the posterior SCS (Fig. 4c), while APD was not significantly different (Fig. 4d). Preceding microparticle injection with a pre-injection of buffer (i.e., without collagenase) had no significant effect on microparticle delivery (Fig. 4). Altogether, these findings suggest that temporal and spatial colocalization of collagenase and microparticles is beneficial for enhanced microparticle delivery in the SCS.
Figure 4.
Effect of simultaneous versus sequential injection of microparticles and collagenase on microparticle delivery in the SCS. A first injection (inj1) was made into the SCS of rabbit eyes ex vivo: (i) control solution containing microparticles in HBSS (Particle), (ii) collagenase formulation without microparticles (Colla CaCl2) in HBSS, (iii) collagenase formulation with microparticles (Particle Colla CaCl2) in HBSS and (iv) HBSS. After incubation at 37 ° C for 4 h, a second injection (inj2) was made into the SCS at the same injection site: (i) no injection (ii) control microparticle solution (Particle), (iii) no injection and (iv) control microparticle solution (Particle). Representative fluorescence micrographs (a), particle delivery coverage (b), particle distribution (c), and APD (d) of red-fluorescent microparticles after the second injection in the SCS. ǂAverage particle distance. Graphs show mean ± SEM (n=6 in (i and iii), n=3 in (ii), and n=5 in (iv)). *,*** indicate significant difference (one tailed t-test, p<0.05,0.005, respectively).
3.6. In vivo Microparticle Delivery With Collagenase
To determine if our ex vivo results can be reproduced in vivo, we injected microparticles either with or without collagenase into the SCS of New Zealand White rabbit eyes in vivo. Four hours after the injection, rabbits were euthanized and eyes were collected to analyze microparticle delivery coverage and distribution (Fig. 5). As expected, particle delivery coverage (Fig. 5b), particle distribution to the posterior SCS (Fig. 5c) and APD (Fig. 5d) were all larger after microparticle injection with collagenase compared to without collagenase in vivo. Moreover, there we no significant differences between microparticle delivery ex vivo versus in vivo, as characterized by particle delivery coverage (Fig. 1f vs. 5b). However, particle distribution to the posterior SCS (Fig. 1g vs. 5c) and APD (Fig. 1h vs. 5d) were increased to a lesser extent in vivo than ex vivo.
Figure 5.
In vivo microparticle delivery with collagenase. Collagenase formulation (0.5 mg/mL collagenase with 3 mM CaCl2) was injected into the SCS of rabbit eyes in vivo. Representative fluorescence micrographs (a), particle delivery coverage (b), particle distribution (c), and APD (d) of red-fluorescent microparticles in the SCS determined 4 h after the injection of (i) control solution containing microparticles in HBSS (Particle) and (ii) collagenase formulation with microparticles (Particle Colla CaCl2). ǂAverage particle distance. Graphs show mean ± SEM (n=3). * indicates significant difference (one tailed t-test, p<0.05).
3.7. Effect of Collagenase on Ocular Tissue Integrity
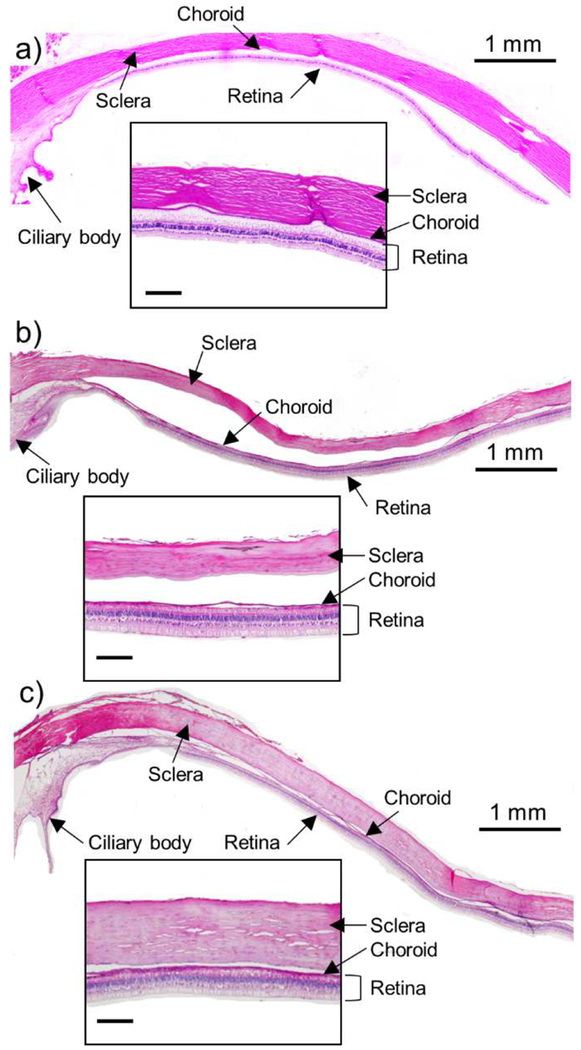
To assess possible tissue damage due to injection of collagenase into the SCS, we used histology to examine tissue sections from rabbit eyes enucleated and fixed 4 h after injecting collagenase formulation. Compared to untreated eye tissues (Fig. 6a and S3a), the sclera, choroid, and retina appeared normal and unchanged in eyes injected with control solution containing no collagenase (Fig. 6b and S3b), as well as in eyes injected with collagenase formulation (Fig. 6c and S3c). Gross clinical exam of the rabbits by a veterinary ophthalmologist revealed no adverse effects in any eyes, other than evidence of mild inflammation in the eyes treated with collagenase. These results suggest that the collagenase injection in the SCS did not produce safety concerns at the conditions examined, but more detailed safety analysis is needed to reach a firm conclusion about safety.
Figure 6.
Representative histological images of rabbit eye tissue collected 4 h after SCS injection in vivo. (a) Untreated eye, (b) eye injected with control solution containing microparticles in HBSS and (c) eye injected with collagenase containing microparticles in HBSS. Tissue was chemically fixed and stained with hematoxylin-eosin (H&E). Scale bars of the insets are 200 μm.
Because scleral structure is controlled to a large extent by collagen fibrils, we measured changes in elastic modulus of sclera from the rabbit eye ex vivo before and after SCS injection of collagenase as a measure of possible weakening of the sclera due to collagen degradation. There was no significant difference in the elastic modulus before (4.75 ± 0.89 MPa) and after (4.38 ± 0.58 MPa) collagenase injection, which suggests that the collagenase did not damage to the structural integrity of the sclera at the conditions studied (Fig. S4).
3.8. Fundus Imaging After Microparticle Delivery With Collagenase
Bright-field fundus imaging of the rabbit eye in vivo was performed to assess possible damage to intraocular tissues by the collagenase SCS injection (Figure S5). The fundus field of view was 130° and a series of nine images of the intraocular tissue were taken, including the injection site and optic nerve, to capture the all of the visible fundus. To assess immediate possible effects, fundus images were taken before SCS injection (Figure S5a) and 1 h after the injection (Figure S5b). Before SCS injection, the blood vessels and spaces between the blood vessels were clearly visible. One hour after the injection, the fundus image had an overall darker reddish appearance, which may be attributed to the red microparticles infused in the SCS as part of the collagenase formulation. Otherwise, the fundus appeared normal with no evidence of ocular tissue bleeding or damage caused by the SCS injection.
One week after the injection, fundus images of the rabbit eye were taken to identify possible damage to ocular tissue at this later time point (Figure S5c). Again, there was no evidence of bleeding or damage of ocular tissue in the fundus images. The red color attributed to the red microparticles was also gone and the choroid and its surrounding spaces were clearly visible like in the images before the injection.
3.9. IOP Measurement After Microparticle Delivery With Collagenase
As an additional measure of safety, IOP was measured in rabbit eyes after SCS injection with collagenase (Figure S6). After measurement of IOP baseline for 1 week, either a control (Particle) or collagenase formulation (Particle Colla CaCl2) was injected into the SCS of rabbit eyes in vivo. IOP was then measured daily from day 1 to day 9 after injection. IOP of control eyes was reduced by ~2 mmHg and IOP of collagenase-injected eyes was lowered by ~2.5 mmHg relative to baseline from 1 day to 5 days after injection. These IOP reductions were significant relative to baseline (one-way ANOVA, p < 0.0001). On the other hand, IOP reductions caused by control versus collagenase-injected eyes were not significantly different from each other (one-way ANOVA, p < 0.0003). After that, IOP began to recover, and on days 7 through 9, IOP values measured in control eyes, collagenase-injected eyes and pre-injection eyes (i.e., baseline) were statistically indistinguishable (two-way ANOVA, p > 0.63), indicating that the IOP returned to normal.
4. Discussion
This study demonstrates that injection of collagenase can expand drug delivery coverage and increase posterior drug targeting in the SCS, where 1 μm fluorescent microparticles were used as a model drug. In addition, co-injection with CaCl2, a cofactor of collagenase, further enhanced the delivery effect. These findings support the hypothesis that the observed delivery enhancement was due to enzymatic activity of collagenase that is hypothesized to degrade collagen structures in the SCS that impede fluid and/or microparticle movement. While CaCl2 caused aggregation of microparticles, the addition of collagenase prevented this aggregation, which is consistent with an interaction between collagenase and CaCl2 that inhibited interaction with microparticles causing aggregation.
Increasing collagen concentration increased delivery enhancement in the SCS, but the effect appeared to plateau at high concentration (i.e., ≥0.5 mg/mL). To minimize possible side effects of collagenase on ocular tissues, such as collagen-rich sclera, we concluded that 0.5 mg/mL was the optimal collagenase concentration under the conditions of this study.
Increasing collagenase incubation time also increased delivery enhancement in the SCS. This effect appeared to reach a plateau at long times (i.e., ≥4 h). While there are no active clearance mechanisms in the rabbit eye ex vivo, the effect of collagenase incubation time is especially important in vivo, where liquid injected into the SCS is cleared on a time scale on the order of 10 min in the ex vivo eye and within about 1 h in the in vivo eye (Chiang et al., 2017b; Jung et al., 2018a). Considering clearance of collagenase, it is known that macromolecules like bevacizumab and dextran remain in the SCS for hours (Chiang et al., 2017b; Patel et al., 2012). This means that the incubation time to achieve maximum effect from collagenase is well matched to the expected residence time of collagenase in the SCS (i.e., both are on the time scale of hours).
We used microparticles as a model drug in this study. While molecules are cleared from the SCS within hours, there is no apparent clearance mechanism for microparticles, which have been seen to remain in the SCS in vivo for months (Kim et al., 2015). Therefore, drug delivery to the SCS might best be achieved using a slow-release formulation that can maintain drug levels in the SCS without the need for frequent injections. For this reason, we used microparticles as our model drug to simulate a slow-release formulation of microparticles that slowly release encapsulated drug. While the microparticles used in this study were not biodegradable, drug delivery applications would probably use biodegradable microparticles so that they can break down and eventually be cleared from the SCS.
Delivery enhancement was better when collagenase was injected with the microparticles rather than beforehand, which suggests an interaction between the collagenase and microparticles. There could be non-specific binding of collagenase to the microparticle surface that stabilized the enzyme and increased the duration and/or intensity of its activity. There might also be a cooperative effect in expanding the SCS, where collagenase breaks down collagen-based barriers and the microparticles facilitate flow through the SCS to open it up. It might also be that co-injection increases co-localization of collagenase and microparicles, which maximizes the effect of collagenase to facilitate expanded delivery of the microparticles. Finally, from a practical point of view, co-injection of collagenase with microparticles is advantageous because it simplifies the administration protocol.
Injection with collagenase enhanced SCS delivery in the rabbit eye in vivo as well as ex vivo. The delivery coverage in vivo (45%) was identical to the result ex vivo (45%). However, APD in vivo (4.8 mm) was smaller than the result ex vivo (6.1 mm). Similarly, the particle distribution shifted posteriorly with the addition of collagenase in vivo and ex vivo, but the posterior delivery effect in vivo was less efficacious compared to ex vivo. This difference may be explained by SCS that is more strongly held in place (i.e., by collagen fibers) in vivo, due to possible degradation of tissue ex vivo.
Histological analysis and clinical exam did not reveal safety concerns in this study (Figure 6 and S3). In addition, there was no significant weakening of the sclera (e.g., due to collagen break down) after SCS injection of collagenase (Figure S4). Analysis of fundus images and IOP measurement revealed initial ocular effects by the collagenase SCS injection that went away within one week. The change in fundus color seen 1 h after SCS injection may be attributed to the red microparticles injected as part of the collagenase formulation; this color disappeared within one week (Figure S5).
IOP was also reduced by SCS collagenase injection, but returned to normal within one week (Figure S6). IOP reduction can be caused by inflammation, which may have been caused by mechanical and enzymatic breaking of collagen fibrils between the choroid and the sclera or possibly damage to other ocular tissue structures. However, IOP recovered within a week without the use of anti-inflammatory agents (other than immediately after injection). Addition of further anti-inflammatory therapy could help restore the IOP more quickly. Another possible explanation for reduced IOP is an increase in uveoscleral outflow due to expansion of the SCS cause by injection into the SCS, which has been shown previously to lower IOP (Chiang et al., 2016a; Kaufman and Barany, 1976). Altogether, these findings suggest possible transient effects by SCS collagenase injection, but identified no lasting effects on the eye. Additional studies are needed to further evaluate safety.
Drug delivery to the SCS has been studied extensively for treatment of inflammation in the posterior segment using the steroid, triamcinolone acetonide (Chen et al., 2015; Gilger et al., 2013). Macular edema has been of significant interest, and increased drug targeting to the posterior SCS (i.e., where the macula is located) may improve the therapy. More recent studies are exploring additional applications, such as gene therapy, which in some cases would benefit from drug delivery targeted posteriorly and in other cases would benefit from pan-retinal delivery (Chappelow et al., 2012b; Touchard et al., 2012). Given the objectives and trends in the field, expanding drug delivery coverage and increase posterior drug targeting in the SCS using collagenase may be beneficial.
5. Conclusion
Treatment of many ocular diseases (e.g., AMD, macular edema, and uveitis) may be improved by drug delivery that is more targeted to sites of pharmacological action in the chorioretina, especially near the posterior pole (e.g., macula). Drug delivery to the SCS can improve drug targeting to the choroid and neighboring retina, but can be limited by insufficient delivery throughout the SCS or targeting to specific locations, such as the macula. In this study, we developed a simple method to improve drug delivery in the SCS by injecting collagenase to expand drug delivery coverage and increase posterior drug targeting.
This study found that increasing collagenase concentration (up to 0.5 mg/mL), increasing collagen incubation time in the SCS (up to 4 h) and inclusion of CaCl2 in the formulation (to act as an enzyme cofactor) all enhanced the spread and posterior delivery of microparticles used in this study as a model drug. In addition, particle spreading in the SCS was improved when collagenase was co-injected with the microparticles. Findings in the rabbit eye ex vivo were generally replicated in vivo, where collagenase similarly facilitated microparticle delivery coverage, although posterior targeting was not as effective. Injection of collagenase into the SCS showed transient effects on IOP, but otherwise appeared to be safe, based on histological analysis of ocular tissues, measurement of mechanical strength of the sclera and examination of fundus images, although a more complete analysis of safety is needed.
Overall, this study demonstrated that collagenase injection into the SCS can expand drug delivery coverage and increase posterior targeting in the SCS using a straightforward modification to the formulation used for SCS injection. We propose that SCS delivery using collagenase deserves further study as a means to improve treatment of ocular diseases of the posterior segment using drug delivery to the SCS.
Supplementary Material
Highlights.
Drug delivery in the suprachoroidal space (SCS) can target chorioretinal tissue.
The sclera and choroid, which bound SCS, are connected by loose collagen fibrils.
Collagenase delivery to SCS expanded drug delivery coverage by digesting collagen.
Collagenase delivery to SCS increased drug targeting to the posterior SCS.
Collagenase formulation was well tolerated by the rabbit eye in vivo.
Acknowledgements
Jae Hwan Jung and Sanghyun Park contributed equally to this work. The authors thank the veterinary staff at Georgia Tech for help with animal studies, Brandon Gerberich for assisting with mechanical testing and Donna Bondy for administrative support. This work was supported by grants from the National Institutes of Health (R01EY022097and R01EY025286).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supporting Information
Supporting Information is available.
Conflict of Interest Disclosure
MRP is an inventor of patents licensed to companies developing microneedle-based products, is a paid advisor to companies developing microneedle-based products and is a founder/shareholder of companies developing microneedle-based products (Clearside Biomedical). This potential conflict of interest has been disclosed and is managed by Georgia Tech and Emory University.
References
- Abarca EM, Salmon JH, Gilger BC, 2013. Effect of choroidal perfusion on ocular tissue distribution after intravitreal or suprachoroidal injection in an arterially perfused ex vivo pig eye model. J Ocul Pharmacol Th 29, 715–722. [DOI] [PubMed] [Google Scholar]
- Bressler NM, Beck RW, Ferris FL 3rd, 2011. Panretinal photocoagulation for proliferative diabetic retinopathy. N Engl J Med 365, 1520–1526. [DOI] [PubMed] [Google Scholar]
- Chappelow AV, Tan K, Waheed NK, Kaiser PK, 2012a. Panretinal photocoagulation for proliferative diabetic retinopathy: pattern scan laser versus argon laser. Am J Ophthalmol 153, 137–142 e132. [DOI] [PubMed] [Google Scholar]
- Chappelow AV, Tan K, Waheed NK, Kaiser PK, 2012b. Panretinal photocoagulation for proliferative diabetic retinopathy: pattern scan laser versus argon laser. American journal of ophthalmology 153, 137–142. e132. [DOI] [PubMed] [Google Scholar]
- Chen KL, Mylon SE, Elimelech M, 2006. Aggregation kinetics of alginate-coated hematite nanoparticles in monovalent and divalent electrolytes. Environ Sci Technol 40, 1516–1523. [DOI] [PubMed] [Google Scholar]
- Chen M, Li X, Liu J, Han Y, Cheng L, 2015. Safety and pharmacodynamics of suprachoroidal injection of triamcinolone acetonide as a controlled ocular drug release model. J Control Release 203, 109–117. [DOI] [PubMed] [Google Scholar]
- Chiang B, Jung JH, Prausnitz MR, 2018. The suprachoroidal space as a route of administration to the posterior segment of the eye. Adv Drug Deliver Rev 126, 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang B, Kim YC, Doty AC, Grossniklaus HE, Schwendeman SP, Prausnitz MR, 2016a. Sustained reduction of intraocular pressure by supraciliary delivery of brimonidine-loaded poly(lactic acid) microspheres for the treatment of glaucoma. J Control Release 228, 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang B, Venugopal N, Edelhauser HF, Prausnitz MR, 2016b. Distribution of particles, small molecules and polymeric formulation excipients in the suprachoroidal space after microneedle injection. Exp Eye Res 153, 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang B, Venugopal N, Grossniklaus HE, Jung JH, Edelhauser HF, Prausnitz MR, 2017a. Thickness and Closure Kinetics of the Suprachoroidal Space Following Microneedle Injection of Liquid Formulations. Invest Ophthalmol Vis Sci 58, 555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang B, Wang K, Ethier CR, Prausnitz MR, 2017b. Clearance Kinetics and Clearance Routes of Molecules From the Suprachoroidal Space After Microneedle Injection. Invest Ophthalmol Vis Sci 58, 545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ClinicalTrials.gov, 2017a. Suprachoroidal Injection of CLS-TA Alone or With Aflibercept in Subjects With Diabetic Macular Edema (HULK), p. .
- ClinicalTrials.gov, 2017b. Suprachoroidal Injection of CLS-TA in Subjects With Macular Edema Associated With Non-infectious Uveitis (PEACHTREE).
- ClinicalTrials.gov, 2017c. Triamcinolone Acetonide With IVT Aflibercept in Subjects With Macular Edema Following RVO (SAPPHIRE), p. .
- ClinicalTrials.gov, 2018. Suprachoroidal Injection of CLS-TA in Subjects Non-infectious Uveitis (AZALEA).
- Gilger BC, Abarca EM, Salmon JH, Patel S, 2013. Treatment of acute posterior uveitis in a porcine model by injection of triamcinolone acetonide into the suprachoroidal space using microneedles. Invest Ophth Vis Sci 54, 2483–2492. [DOI] [PubMed] [Google Scholar]
- Goldstein DA, 2014. Achieving drug delivery via the suprachoroidal space. Retina Today. July/Aug, 82–87. [Google Scholar]
- Goldstein DA, Do D, Noronha G, Kissner JM, Srivastava SK, Nguyen QD, 2016. Suprachoroidal corticosteroid administration: a novel route for local treatment of noninfectious uveitis. Translational vision science & technology 5, 14–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu B, Liu J, Li X, Ma Q, Shen M, Cheng L, 2015. Real-Time Monitoring of Suprachoroidal Space (SCS) Following SCS Injection Using Ultra-High Resolution Optical Coherence Tomography in Guinea Pig EyesReal-Time Monitoring of Suprachoroidal Space. Invest Ophth Vis Sci 56, 3623–3634. [DOI] [PubMed] [Google Scholar]
- Habot-Wilner Z, Noronha G, Wykoff CC, 2019. Suprachoroidally injected pharmacological agents for the treatment of chorio-retinal diseases: a targeted approach. Acta Ophthalmologica. [DOI] [PubMed] [Google Scholar]
- Hatami-Marbini H, Rahimi A, 2015. Stiffening effects of riboflavin/UVA corneal collagen cross-linkingis hydration dependent. Journal of biomechanics 48, 1052–1057. [DOI] [PubMed] [Google Scholar]
- Holz FG, Schmitz-Valckenberg S, Fleckenstein M, 2014. Recent developments in the treatment of age-related macular degeneration. J Clin Invest 124, 1430–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JH, Chae JJ, Prausnitz MR, 2019. Targeting drug delivery within the suprachoroidal space. Drug Discov Today. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JH, Chiang B, Grossniklaus HE, Prausnitz MR, 2018a. Ocular drug delivery targeted by iontophoresis in the suprachoroidal space using a microneedle. J Control Release 277, 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JH, Desit P, Prausnitz MR, 2018b. Targeted Drug Delivery in the Suprachoroidal Space by Swollen Hydrogel Pushing. Invest Ophthalmol Vis Sci 59, 2069–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman PL, Barany EH, 1976. Loss of Acute Pilocarpine Effect on Outflow Facility Following Surgical Disinsertion and Retro-Displacement of Ciliary Muscle from Scleral Spur in Cynomolgus Monkey. Invest Ophth Visual 15, 793–807. [PubMed] [Google Scholar]
- Kim YC, Edelhauser HF, Prausnitz MR, 2014. Particle-Stabilized Emulsion Droplets for Gravity-Mediated Targeting in the Posterior Segment of the Eye. Advanced Healthcare Materials 3, 1272–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Oh KH, Edelhauser HF, Prausnitz MR, 2015. Formulation to target delivery to the ciliary body and choroid via the suprachoroidal space of the eye using microneedles. Eur J Pharm Biopharm 95, 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampen SIR, Khurana RN, Noronha G, Brown DM, Wykoff CC, 2018. Suprachoroidal Space Alterations Following Delivery of Triamcinolone Acetonide: Post-Hoc Analysis of the Phase 1/2 HULK Study of Patients With Diabetic Macular Edema. Ophthalmic Surg Lasers Imaging Retina 49, 692–697. [DOI] [PubMed] [Google Scholar]
- Lee R, Wong TY, Sabanayagam C, 2015. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye and Vision 2, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T-X, Wang Z, 2017. Biomechanics of sclera crosslinked using genipin in rabbit. International journal of ophthalmology 10, 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv SH, Rong SF, Cai BG, Guan SM, Li QQ, 2015. Property and current clinical applications of mammal hyaluronidase. Eur Rev Med Pharmacol Sci 19, 3968–3976. [PubMed] [Google Scholar]
- Moisseiev E, Loewenstein A, Yiu G, 2016. The suprachoroidal space: from potential space to a space with potential. Clin Ophthalmol 10, 173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SR, Berezovsky DE, McCarey BE, Zarnitsyn V, Edelhauser HF, Prausnitz MR, 2012. Targeted Administration into the Suprachoroidal Space Using a Microneedle for Drug Delivery to the Posterior Segment of the Eye. Invest Ophth Vis Sci 53, 4433–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SR, Lin AS, Edelhauser HF, Prausnitz MR, 2011. Suprachoroidal drug delivery to the back of the eye using hollow microneedles. Pharmaceutical research 28, 166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SR, Prausnitz MR, 2016. Targeted Drug Delivery Within the Eye Through the Suprachoroidal Space. J Ocul Pharmacol Th 32, 640–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai UDJP, Young SA, Thrimawithana TR, Abdelkader H, Alani AWG, Pierscionek B, Alany RG, 2015. The suprachoroidal pathway: a new drug delivery route to the back of the eye. Drug Discovery Today 20, 491–495. [DOI] [PubMed] [Google Scholar]
- Rowe-Rendleman CL, Durazo SA, Kompella UB, Rittenhouse KD, Di Polo A, Weiner AL, Grossniklaus HE, Naash MI, Lewin AS, Horsager A, Edelhauser HF, 2014. Drug and Gene Delivery to the Back of the Eye: From Bench to Bedside. Invest Ophth Vis Sci 55, 2714–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz SG, Scott IU, Flynn HW Jr., Stewart MW, 2014. Drug delivery techniques for treating age-related macular degeneration. Expert Opin Drug Deliv 11, 61–68. [DOI] [PubMed] [Google Scholar]
- Silverstein SM, Greenbaum S, Stern R, 2012. Hyaluronidase in Ophthalmology. Journal of Applied Research 12. [Google Scholar]
- Touchard E, Berdugo M, Bigey P, El Sanharawi M, Savoldelli M, Naud M-C, Jeanny J-C, Behar-Cohen F, 2012. Suprachoroidal electrotransfer: a nonviral gene delivery method to transfect the choroid and the retina without detaching the retina. Molecular Therapy 20, 1559–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JC, Eliott D, 2017. Accessing the Suprachoroidal Space for Therapeutic Delivery. Int Ophthalmol Clin 57, 179–192. [DOI] [PubMed] [Google Scholar]
- Weiss JN, Levy S, Benes SC, 2015. Stem Cell Ophthalmology Treatment Study (SCOTS) for retinal and optic nerve diseases: a case report of improvement in relapsing auto-immune optic neuropathy. Neural Regen Res 10, 1507–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Zhao J, Sun X, 2016. Resistance to anti-VEGF therapy in neovascular age-related macular degeneration: a comprehensive review. Drug Des Devel Ther 10, 1857–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu G, Pecen P, Sarin N, Chiu SJ, Farsiu S, Mruthyunjaya P, Toth CA, 2014. Characterization of the Choroid-Scleral Junction and Suprachoroidal Layer in Healthy Individuals on Enhanced-Depth Imaging Optical Coherence TomographyChoroid-Scleral Junction and Suprachoroidal LayerChoroid-Scleral Junction and Suprachoroidal Layer. JAMA Ophthalmology 132, 174–181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.