Abstract
Background
Newer treatments for HIV and hepatitis C virus (HCV) have decreased mortality in HIV/HCV patients. Nonalcoholic fatty liver disease (NAFLD) has increased globally; therefore, the prevalence and mortality of NAFLD among HIV (+) patients was assessed.
Methods
Using Medicare denominator, inpatient, and outpatient files (random 5% sample per year), serial cross-sectional analysis (2006 to 2016) was performed. Joinpoint trend analysis evaluated prevalence and mortality with average annual percent change (AAPC). HIV (+) patients and liver diseases (LDs) were identified using International Classification of Diseases 9/10 codes. NAFLD was presumed using diagnosis codes or codes for metabolic dysfunction and obesity in absence of other LDs. Liver-related HIV (+) indicated HIV (+) patients with LDs.
Results
Among 28 675 887 Medicare beneficiaries, 47 062 were HIV (+) (mean [SD] age, 51.4 [11.3] years); 11 920 had liver diseases (6923 HCV, 2019 hepatitis B virus [HBV], 2472 presumed NAFLD, 278 alcoholic liver disease [ALD], and 1653 other LDs); 2882 HIV (+) patients died; 1260 had LDs. The prevalence and mortality for non-liver-related HIV (+) decreased (AAPC, –1.1% and –9.1%). Liver-related HIV (+) increased (AAPC, 1.7%; P = .007); mortality leveled off. Prevalence and mortality worsened for presumed NAFLD (AAPC, 9.7% and 10.0%) and improved for HBV and HCV (HBV: AAPC, –3.5% and –8.8%; HCV: AAPC, –0.7% and –4.9%). After adjustments, HCV (odds ratio [OR], 2.00; 95% confidence interval [CI], 1.24–172), HBV (OR, 2.40; 95% CI, 2.09–2.77), ALD (OR, 5.70; 95% CI, 4.34–7.48), and presumed NAFLD (OR, 1.46; 95% CI, 1.24–1.72) increased 1-year mortality.
Conclusions
Among HIV (+) subjects, viral hepatitis remains the leading LD for increased 1-year mortality, but the prevalence and mortality with presumed NAFLD are increasing.
Keywords: liver disease, mortality, HCV, HBV, trends
In the United States, as of 2016, ~1 008 929 people were living with HIV [1]. As the HIV virus affects killer T cells, leaving the infected individuals unable to mount an effective response to infections, today’s antiretroviral regimens for HIV are geared toward increasing the number of killer cells by stopping HIV transcription within the cells (viral suppression). In addition to the incidence of HIV declining in most groups, the use of highly effective antiretroviral regimens has combined to successfully increase patients’ life expectancy from 12 years after diagnosis in the 1980s up to 47 years following a diagnosis in 2017 [1–4].
It is important to remember that HIV shares common risks with other blood-borne infections, specifically hepatitis B virus (HBV) and hepatitis C virus (HCV) [5, 6]. As treatment of HIV has changed over the years, this highly lethal disease has become a chronic and manageable disease. As such, HCV-related liver disease then became a major cause of death among those who were co-infected with HCV [7]. However, this too is changing due to the advent of highly effective and well tolerated direct-acting antiviral agents (DAAs) for HCV, which have provided a curative option for all HCV-infected patients. In fact, DAAs provide a >95% cure rate regardless of HIV co-infection [8, 9]. Therefore, as HCV is being aggressively treated among HIV-infected patients, it is expected that the risk of liver disease from HCV in HIV-infected patients will fall [7].
In the United States, in addition to viral hepatitis, nonalcoholic fatty liver disease (NAFLD) is another very common liver disease [10]. NAFLD is associated with metabolic risk factors such as obesity and type 2 diabetes [11–13]. In this context, as survival increases among HIV-infected patients, they are now at risk for obesity and diabetes, similar to the rest of the population [14–17]. In addition, although most of the new antiretroviral agents don’t cause metabolic disturbances, a few may still cause lipodystrophy, which can worsen the metabolic profile and predispose patients to NAFLD [15]. In fact, the newer combined antiretroviral therapies (cART) may be inducing an increase in weight gain beyond the “return to health,” exposing patients with HIV to the potential development of diabetes mellitus and cardiovascular disease [18–22]. Recent studies have indicated that treatment with nucleoside reverse transcriptase inhibitors (NRTIs) and protease inhibitors (PIs) increases the chance of developing metabolic syndrome by around 2.4 times [23]. Studies remain ongoing to determine the exact mechanisms by which cART may cause obesity, especially in the face of the obesity epidemic and fewer patients now appearing underweight for cART therapy, as was the hallmark of advanced HIV disease [24–27]. Nonetheless, these factors combined may increase the incidence and prevalence of NAFLD in this population independent of lipodystrophy.
The prevalence of NAFLD has been studied in subgroups, but the prevalence and outcomes data in HIV-infected patients have not been fully reported [28–34]. Therefore, the purposes of this study were to assess the prevalence and mortality outcomes and resource utilization of Medicare beneficiaries with HIV infection who also had NAFLD or other types of liver disease.
METHODS
Data Source and Study Population
This was a serial cross-sectional analysis of Medicare beneficiaries between 2006 and 2016 using Medicare denominator, inpatient, and outpatient files (a random 5% sample for each of our study years). Eligible patients were fee-for-service Medicare enrollees with a medical record (inpatient or outpatient) with an International Classification of Diseases (ICD) 9/10 code for HIV (042-044 in ICD 9 or B20-B24 in ICD 10 code) at any position. Supplementary Table 1 displays the ICD 9/10 codes used to identify the liver diseases (such as hepatitis B virus [070.2, 070.3, 070.42, 070.52, V02.61, B16, B17.0, B18.0, B18.1, B19.1, Z2251], hepatitis C virus [070.7, 070.41, 070.44, 070.51, 070.54, V02.62, B17.1, B18.2, B19.2, Z2252], alcoholic liver disease [571.0–571.3 and K70], NAFLD, etc.).
At this point, we observed that NAFLD was underdiagnosed and undercoded in the Medicare data sets. Indeed, we found that the prevalence of NAFLD through ICD codes (K76.0 and K76.89) among Medicare beneficiaries in 2016 was 0.19. Therefore, we extended NAFLD cases with an ICD code for NAFLD and nonalcoholic steatohepatitis (NASH), an ICD code for “cryptogenic liver disease or cirrhosis,” and those with metabolic abnormality [35], defined as all 3 ICD codes for diabetes, hypertension, and hyperlipidemia or 2 ICD codes for diabetes and obesity. To reduce the possibility of misclassifying NAFLD, we excluded NAFLD cases with any other liver diseases (viral hepatitis, ALD, iron overload, autoimmune, Wilson disease, hemochromatosis, and alpha-1-antitrypsin deficiency) or alcohol abuse. This extended NAFLD definition was validated using the NHANES database with clinical and laboratory data. We found that 92.5% of Medicare-aged subjects from NHANES who fulfilled our last NAFLD definition had evidence of NAFLD, as determined by an improved Fatty Liver Index for the US population (US FLI), a surrogate for the clinical diagnosis of NAFLD using race, age, gamma glutamyltransferase, waist circumference, insulin, and glucose, in which a cutoff score of 30 was found to be the most accurate, with an area under the curve of 0.80 (95% confidence interval [CI], 0.77–0.83). (Supplementary Table 2) [36].
Liver-related HIV (+) subjects were identified as HIV (+) patients having liver diseases including hepatitis B and C virus (HBV and HCV), alcoholic liver disease (ALD), NAFLD, autoimmune liver disease, Wilson disease, hemochromatosis, iron overload, alpha-1-antitrypsin deficiency, liver disorder, or unknown etiologies in the presence of hepatocellular carcinoma, cirrhosis, or hepatic failure. Non-liver-related HIV (+) subjects were defined as HIV (+) patients without liver disease. Figure 1 shows a flow diagram outlining the inclusion and exclusion criteria of the study cohort.
Figure 1.
Flow diagram of the study cohort. Abbreviations: ALD, alcoholic liver disease; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis.
Mortality status was available within 1 year of an encounter per year. All Medicare expenditures were adjusted using the medical component of Consumer Price Index, with 2016 as the index year (https://www.bls.gov/cpi/). Charlson Comorbidity Index (CCI) was derived using the enhanced ICD 9/10 codes [37].
Data Analysis
The present study was comprised of 2 components: (1) a trend analysis was performed for prevalence and mortality in Medicare patients positive for HIV with and without liver disease and (2) an association analysis was conducted to determine the impact of HIV and liver diseases on mortality and resource utilization. Temporal trend prevalence and death rates (per 100 000 Medicare population) were evaluated using the Joinpoint Regression Program (version 4.6.0.0; National Cancer Institute) with estimates of average annual percent change (AAPC). An increasing or decreasing trend was defined if the AAPC was significantly different from 0; otherwise, a stable or level trend was defined. The Cochran-Armitage test for linear trends in proportions was also performed.
After calendar year, age, gender, race/ethnicity, and beneficiary entitlement adjustments, multivariable regression analysis was performed on 1-year mortality (logistic model), charges (generalized linear regression model [GLM] with Gamma distribution), and length of stay (GLM with Poisson distribution) [38]. To show group homogeneity in our extended NAFLD definition, we performed sensitivity analyses for trends in prevalence and mortality among HIV (+) patients who were diagnosed with NAFLD through ICD codes, had cryptogenic liver disease and cirrhosis, or had a metabolic abnormality in the absence of any other cause of chronic liver disease or excessive alcohol consumption, as defined by ICD codes. All analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Demographics
Among 28 675 887 Medicare beneficiaries between 2006 and 2016, there were 47 062 HIV (+) patients (mean age [SD], 51.4 [11.3] years; non-Hispanic white 45.9%; non-Hispanic black 44.4%; Hispanic 6.6%; and male 74.2%). The majority of HIV (+) patients (84.9%) were receiving Medicare benefits under the category of disability or end-stage renal disease (ESRD). The highest number of HIV (+) patients observed was in the South (42.1%). Liver cancer was noted in 92 (0.2%), and cirrhosis was noted in 1319 (2.8%). The top 7 comorbidities were complications of hypertension (34.0%), followed by hyperlipidemia (27.0%), depression (15.8%), diabetes (15.4%), lung disease (14.6%), cardiovascular disease (25.6%), and renal disease (11.9%). Overall, 6.12% (n = 2882) of HIV (+) patients died within 1 year of their encounter (Table 1).
Table 1.
Characteristics of HIV Patients, Stratified By Liver Disease Status: Medicare Population (2006–2016)
| All HIV | Liver-Related HIV | Non-Liver-Related HIV | P | |
|---|---|---|---|---|
| Subjects’ Characteristics | 47 062 | 11 920 | 35 142 | |
| Age, mean ± SD, y | 51.38 ± 11.28 | 51.01 ± 11.62 | 52.46 ± 10.14 | <.0001 |
| Age, No. (%) | ||||
| <65 y | 40 428 (85.90) | 10 394 (87.20) | 30 034 (85.46) | <.0001 |
| 65–74 y | 5594 (11.89) | 1334 (11.19) | 4260 (12.12) | .0066 |
| ≥75 y | 1040 (2.21) | 192 (1.61) | 848 (2.41) | <.0001 |
| Male, No. (%) | 34 936 (74.23) | 9065 (76.05) | 25 871 (73.62) | <.0001 |
| Race, No. (%) | ||||
| Non-Hispanic white | 21 621 (45.94) | 5147 (43.18) | 16 474 (46.88) | <.0001 |
| Non-Hispanic black | 20 895 (44.40) | 5583 (46.84) | 15 312 (43.57) | <.0001 |
| American Asian | 388 (0.82) | 81 (0.68) | 307 (0.87) | .0429 |
| Hispanic | 3087 (6.56) | 842 (7.06) | 2245 (6.39) | .0101 |
| American Native | 278 (0.59) | 105 (0.88) | 173 (0.49) | <.0001 |
| Other race | 793 (1.69) | 162 (1.36) | 631 (1.80) | .0014 |
| Region, No. (%) | ||||
| Northeast | 11 887 (25.26) | 3387 (28.41) | 8500 (24.19) | <.0001 |
| South | 19 806 (42.08) | 5095 (42.74) | 14 711 (41.86) | .0920 |
| Midwest | 7044 (14.97) | 1504 (12.62) | 5540 (15.76) | <.0001 |
| West | 8158 (17.33) | 1907 (16.00) | 6251 (17.79) | <.0001 |
| Entitlement, No. (%) | ||||
| Age | 7094 (15.07) | 1658 (13.91) | 5436 (15.47) | <.0001 |
| Disability/ERSD | 39 968 (84.93) | 10 262 (86.09) | 29 706 (84.53) | <.0001 |
| Liver cancer, No. (%) | 92 (0.20) | 92 (0.77) | 0 (0.00) | <.0001 |
| Cirrhosis, No. (%) | 1319 (2.80) | 1319 (11.07) | 0 (0.00) | <.0001 |
| Comorbidities, No. (%) | ||||
| Hypertension | 15 992 (33.98) | 6258 (52.50) | 9734 (27.70) | <.0001 |
| Diabetes | 7225 (15.35) | 3987 (33.45) | 3238 (9.21) | <.0001 |
| Hyperlipidemia | 12 681 (26.95) | 4262 (35.76) | 8419 (23.96) | <.0001 |
| Obese | 2144 (4.56) | 1027 (8.62) | 1117 (3.18) | <.0001 |
| Cardiovascular disease | 6522 (13.86) | 2773 (23.26) | 3749 (10.67) | <.0001 |
| Depression | 7455 (15.84) | 2740 (22.99) | 4715 (13.42) | <.0001 |
| Dementia | 716 (1.52) | 260 (2.18) | 456 (1.30) | <.0001 |
| Lung disease | 6858 (14.57) | 2705 (22.69) | 4153 (11.82) | <.0001 |
| Renal disease | 5607 (11.91) | 2628 (22.05) | 2979 (8.48) | <.0001 |
| Neurological disorder | 3239 (6.88) | 1337 (11.22) | 1902 (5.41) | <.0001 |
| Nonliver cancer | 2101 (4.46) | 751 (6.30) | 1350 (3.84) | <.0001 |
| Alcohol abuse | 2193 (4.66) | 1092 (9.16) | 1101 (3.13) | <.0001 |
| Lipodystrophy | 687 (1.46) | 251 (2.11) | 436 (1.24) | <.0001 |
| CCI, mean ± SD | 7.00 ± 1.53 | 8.05 ± 1.84 | 6.64 ± 1.21 | <.0001 |
| 1-y all-cause mortality, No. (%) | 2882 (6.12) | 1260 (10.57) | 1622 (4.62) | <.0001 |
Abbreviations: CCI, Charlson comorbidity index; ESRD, end-stage renal disease.
Trend in Prevalence and Mortality for HIV (+) Patients in Medicare Beneficiaries
Between 2006 and 2017, the prevalence rate of HIV per 100 000 Medicare beneficiaries decreased 5.1%, with an AAPC of –0.5% (95% CI, –0.8% to –0.2%; P < .001). The significant decrease in HIV rates occurred in 2011–2016 with an annual percent change (APC) of –1.9%, after a continuous increase between 2006 and 2011 with an APC of 1.0%. The majority of cases occurred in males (271.1 cases per 100 000 compared with 76.9 cases per 100 000 for females). Interesting, although the HIV rate for males decreased continuously during the 2006–2016 (AAPC, –1.3%; 95% CI, –1.6% to –1.0%; P < .001), the rate for females actually increased until 2013 (APC, 2.7%; 95% CI, 1.4% to 4.0%; P = .002) and then leveled off between 2013 and 2016 (APC, –3.0%; 95% CI, –7.0% to 1.2%; P = .129). In fact, males and females experienced different trends for HIV (P < .001). The rates for HIV with cardiovascular disease, diabetes, hypertension, hyperlipidemia, and obesity (AAPC, 3.9%, 3.8%, 4.1%, 4.0%, and 15.7%, respectively; all P < .001) increased, whereas those with alcohol abuse and lipodystrophy plateaued (P = .112 and .728, respectively) (Table 3). Characteristics of HIV (+) patients from 2006 to 2016 are reported in Supplementary Table 3.
Table 3.
Prevalence and Mortality for those with HIV (per 100 000 Medicare Population): Medicare Population 2006–2016
| Prevalence of HIV (per 100 000): Medicare Population (2006–2016) | ||||
|---|---|---|---|---|
| Count (Rate per 100 000), No. (%) | Average APC | |||
| 2006–2016a | 2006 | 2016 | ||
| HIV | 47 062 (164.36) | 3702 (162.83) | 4614 (154.45) | –0.49 (–0.75 to –0.22)b |
| Sex | ||||
| Female | 12 126 (76.85) | 857 (67.68) | 1251 (77.13) | 0.95 (–0.29 to 2.21) |
| Male | 34 936 (271.06) | 2845 (282.46) | 3363 (246.32) | –1.34 (–1.63 to –1.04)b |
| HIV with | ||||
| Alcohol abuse | 2193 (7.68) | 209 (9.19) | 222 (7.43) | –1.34 (–3.02 to 0.38) |
| CVD | 6522 (22.50) | 427 (18.78) | 826 (27.65) | 3.88 (2.84 to 4.94)b |
| Diabetes | 7225 (24.93) | 467 (20.54) | 866 (28.99) | 3.77 (2.82 to 4.73)b |
| Hypertension | 15 992 (55.17) | 956 (42.05) | 1987 (66.51) | 4.12 (2.86 to 5.40)b |
| Hyperlipidemia | 12 681 (43.78) | 717 (31.54) | 1450 (48.54) | 3.98 (2.63 to 5.35)b |
| Obese | 2144 (7.20) | 69 (3.03) | 344 (11.52) | 15.16 (12.16 to 18.24)b |
| Lipodystrophy | 687 (2.42) | 51 (2.24) | 47 (1.57) | –1.31 (–8.40 to 6.32) |
| Mortality for HIV (per 100 000): Medicare Population (2006–2016) | ||||
| Count (Rate per 100 000), No. (%) | Average APC | |||
| 2006–2016a | 2006 | 2016 | ||
| Overall | 2882 (10.21) | 284 (12.49) | 237 (7.93) | –5.30 (–6.54 to –4.04)b |
| Sex | ||||
| Female | 662 (4.25) | 56 (4.42) | 55 (3.39) | –3.69 (–7.40 to 0.16) |
| Male | 2220 (17.49) | 228 (22.64) | 182 (13.33) | –5.98 (–7.05 to –4.89)b |
| HIV with: | ||||
| Alcohol abuse | 250 (0.88) | 31 (1.36) | 18 (0.6) | –4.75 (–8.99 to –0.31)b |
| CVD | 1161 (4.05) | 90 (3.96) | 126 (4.22) | –0.14 (–3.05 to 2.86) |
| Diabetes | 653 (2.27) | 44 (1.94) | 63 (2.11) | 1.39 (–1.96 to 4.85) |
| Hypertension | 1396 (4.86) | 101 (4.44) | 144 (4.82) | 0.40 (–1.78 to 2.62) |
| Hyperlipidemia | 533 (1.81) | 16 (0.7) | 77 (2.58) | 9.6 (5.24 to 14.15)b |
| Obese | 94 (0.32) | 1 (0.04) | 12 (0.4) | 21.75 (8.04 to 37.21)b |
Average annual percent change is a weighted average of the APCs (a maximum of 2 joinpoints was allowed).
Abbreviations: APC, annual percent change; CVD, cardiovascular disease.
aRates were averaged across 2006–2016.
bSignificantly different from 0 (P < .05).
The mortality rate for HIV (+) beneficiaries was 10.2 deaths per 100 000 Medicare population. The mortality rate significantly decreased from 12.5 cases in 2006 to 7.9 cases in 2016, with an AAPC of –5.3% (–6.54% to –4.04%; P < .001). The mortality rate for HIV (+) males decreased (AAPC, –6.0%; P < .001), whereas the rate for HIV (+) females leveled off (P = .058). The mortality rates for HIV (+) with hyperlipidemia and obesity (AAPC, 9.6% and 15.7%, respectively; all P < .001) increased, whereas the mortality rates for those with alcohol abuse decreased (AAPC, –4.7%; P = .039) (Table 3).
Trends in Prevalence and Mortality for HIV (+) Patients by Liver Disease in Medicare Beneficiaries
During the study period, there were 11 920 (25.32%) HIV (+) beneficiaries (mean [SD] age, 51.4 [11.3] years) with liver diseases (6923 HCV, 2019 HBV, 2472 NAFLD, 278 ALD, and 1653 other liver diseases such as autoimmune, cholestatic, etc.). Compared with non-liver-related HIV (+) subjects, liver-related HIV (+) subjects were younger, more commonly male, more commonly non-Hispanic black, and less commonly non-Hispanic white, and had higher CCI scores and higher 1-year all-cause mortality (all P < .001) (Table 1).
The overall age of liver-related HIV (+) subjects (SD) was 51.0 (11.6) years, with a range (SD) of 50.2 (9.9) for HBV to 56.1 (10.4) for NAFLD. The majority of liver-related HIV (+) patients were male (76.1%), with a range of 88.3% for HBV to 66.9% for NAFLD. The HIV (+) patients’ race/ethnicity varied by liver disease. Non-Hispanic white and Hispanic more commonly had ALD, whereas non-Hispanic black less commonly had ALD (Table 2).
Table 2.
Characteristics of HIV Patients With Liver Disease, Stratified By Etiology: Medicare Population (2006–2016)
| NAFLD | HCV | HBV | ALD | Other Liver Diseasesa | |
|---|---|---|---|---|---|
| Subjects, No. | 2472 | 6293 | 2019 | 278 | 1653 |
| Age, mean ± SD, y | 56.12 ± 10.38 | 52.04 ± 8.97 | 50.18 ± 9.85 | 53.37 ± 9.38 | 50.70 ± 12.32 |
| Age, No. (%) | |||||
| <65 y | 1909 (77.22) | 5708 (90.70) | 1840 (91.13) | 242 (87.05) | 1419 (85.84) |
| 65–74 y | 474 (19.17) | 546 (8.68) | 158 (7.83) | 30 (10.79) | 187 (11.31) |
| ≥75 y | 89 (3.60) | 39 (0.62) | 21 (1.04) | 6 (2.16) | 47 (2.84) |
| Male, No. (%) | 1654 (66.91) | 4883 (77.59) | 1783 (88.31) | 241 (86.69) | 1178 (71.26) |
| Race, No. (%) | |||||
| Non-Hispanic white | 1073 (43.41) | 2674 (42.49) | 920 (45.57) | 152 (54.68) | 679 (41.08) |
| Non-Hispanic black | 1109 (44.86) | 2974 (47.26) | 930 (46.06) | 86 (30.94) | 845 (51.12) |
| American Asian | 26 (1.05) | 38 (0.60) | 11 (0.54) | 3 (1.08) | 8 (0.48) |
| Hispanic | 190 (7.69) | 484 (7.69) | 116 (5.75) | 25 (8.99) | 89 (5.38) |
| American Native | 15 (0.61) | 61 (0.97) | 23 (1.14) | 11 (3.96) | 8 (0.48) |
| Other race | 59 (2.39) | 62 (0.99) | 19 (0.94) | 1 (0.36) | 24 (1.45) |
| Region, No. (%) | |||||
| Northeast | 644 (26.05) | 2093 (33.26) | 533 (26.40) | 62 (22.30) | 317 (19.18) |
| South | 1146 (46.36) | 2474 (39.31) | 815 (40.37) | 121 (43.53) | 845 (51.12) |
| Midwest | 341 (13.79) | 709 (11.27) | 280 (13.87) | 38 (13.67) | 241 (14.58) |
| West | 336 (13.59) | 1001 (15.91) | 388 (19.22) | 56 (20.14) | 245 (14.82) |
| Entitlement, No. (%) | |||||
| Age | 600 (24.27) | 662 (10.52) | 196 (9.71) | 37 (13.31) | 237 (14.34) |
| Disability/ERSD | 1872 (75.73) | 5631 (89.48) | 1823 (90.29) | 241 (86.69) | 1416 (85.66) |
| HCC, No. (%) | 4 (0.16) | 66 (1.05) | 25 (1.24) | 7 (2.52) | 11 (0.67) |
| Cirrhosis, No. (%) | 226 (9.14) | 772 (12.27) | 242 (11.99) | 210 (75.54) | 103 (6.23) |
| Etiology, No. (%) | |||||
| HCV | 0 (0.00) | 6293 (100.00) | 647 (32.05) | 127 (45.68) | 0 (0.00) |
| HBV | 0 (0.00) | 647 (10.28) | 2019 (100.00) | 49 (17.63) | 0 (0.00) |
| ALD | 0 (0.00) | 127 (2.02) | 49 (2.43) | 278 (100.00) | 0 (0.00) |
| NAFLD | 2472 (100.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Other liver diseasesa | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1653 (100.00) |
| Comorbidities, No. (%) | |||||
| Hypertension | 2131 (86.21) | 2712 (43.10) | 869 (43.04) | 133 (47.84) | 792 (47.91) |
| Diabetes | 2176 (88.03) | 1199 (19.05) | 350 (17.34) | 68 (24.46) | 367 (22.20) |
| Hyperlipidemia | 1976 (79.94) | 1370 (21.77) | 490 (24.27) | 65 (23.38) | 513 (31.03) |
| Obese | 574 (23.22) | 261 (4.15) | 81 (4.01) | 15 (5.40) | 128 (7.74) |
| Cardiovascular disease | 712 (28.80) | 1265 (20.10) | 449 (22.24) | 85 (30.58) | 512 (30.97) |
| Depression | 479 (19.38) | 1549 (24.61) | 476 (23.58) | 91 (32.73) | 401 (24.26) |
| Dementia | 51 (2.06) | 123 (1.95) | 46 (2.28) | 7 (2.52) | 57 (3.45) |
| Lung disease | 568 (22.98) | 1435 (22.80) | 451 (22.34) | 81 (29.14) | 411 (24.86) |
| Renal disease | 651 (26.33) | 1079 (17.15) | 415 (20.55) | 69 (24.82) | 628 (37.99) |
| Neurological disorder | 192 (7.77) | 681 (10.82) | 259 (12.83) | 44 (15.83) | 297 (17.97) |
| Nonliver cancer | 160 (6.47) | 326 (5.18) | 168 (8.32) | 17 (6.12) | 141 (8.53) |
| Alcohol abuse | 0 (0.00) | 719 (11.43) | 208 (10.30) | 278 (100.00) | 142 (8.59) |
| Lipodystrophy | 116 (4.69) | 93 (1.48) | 24 (1.19) | 1 (0.36) | 24 (1.45) |
| CCI, mean ± SD | 8.49 ± 1.80 | 7.84 ± 1.79 | 7.99 ± 1.92 | 9.45 ± 2.18 | 8.32 ± 1.95 |
| CCI, median (Q1–Q3) | 8 (7–9) | 7 (7–9) | 7 (7–9) | 9 (8–11) | 8 (7–9) |
| 1-y all-cause mortality, No. (%) | 173 (7.00) | 640 (10.17) | 274 (13.57) | 85 (30.58) | 268 (16.21) |
Abbreviations: CCI, Charlson comorbidity index; ESRD, end-stage renal disease; LD, liver disease.
aIncludes autoimmune, Wilson’s disease, hemochromatosis, iron overload, alpha-1-antitrypsin deficiency, Budd-Chiari syndrome, cholangitis, liver disorders, and unknown etiologies for hepatocellular carcinoma, cirrhosis, and liver failure.
Among liver-related HIV (+) patients, 0.8% of HIV (+) patients had liver cancer, but 2.5% of HIV (+) patients with ALD had liver cancer. In addition, 11.1% had cirrhosis, but 75.5% of ALD-related HIV (+) subjects had cirrhosis. Among 5 etiology groups (NAFLD, HCV, HBV, ALD, and other liver diseases), NAFLD-related HIV (+) subjects were more likely to have hypertension, diabetes, hyperlipidemia, and to be obese and had a higher mean CCI score (SD) of 8.5 (1.8) (Table 2). The characteristics of liver-related and non-liver-related HIV (+) patients from 2006 to 2016 are also reported in Supplementary Tables 4 and 5.
The prevalence rate for non-liver-related HIV (+) was 3 times higher than liver-related HIV (+) (123 vs 41.4 per 100 000). Between 2006 and 2016, the prevalence and mortality for non-liver-related HIV (+) decreased (AAPC, –1.1% and –9.1%; P values < .001). In contrast, liver-related HIV (+) prevalence increased (AAPC, 1.7%; P = .007) and mortality leveled off (P = .825) (Table 4). Joinpoint analysis showed that the prevalence rate for non-liver-related HIV (+) began to decrease annually (APC, –2.4%; P < .001) after a stable trend in the 2006–2010 period (APC, 0.8%; P = .187), whereas the prevalence for liver-related HIV (+) increased annually until 2012 (APC, 1.7%; P < .001) and then stabilized between 2012 and 2016 (APC, –0.5%; P = .676) (Figure 2).
Table 4.
Prevalence and Mortality for HIV, by Liver Diseases (per 100 000 Medicare Population (2006–2016)
| Prevalence for HIV, by Liver Diseases (per 100 000 Medicare Population (2006–2016) | ||||
|---|---|---|---|---|
| Count (Rate per 100 000), No. (%) | Average APC | |||
| 2006–2016a | 2006 | 2016 | ||
| Non-liver-related HIV | 35 142 (122.99) | 2868 (126.15) | 3338 (111.74) | –1.13 (–1.63 to –0.63)c |
| Liver-related HIV | 11 920 (41.37) | 834 (36.68) | 1276 (42.71) | 1.68 (0.45 to 2.92)c |
| NAFLD | 2472 (8.42) | 92 (4.05) | 353 (11.82) | 9.72 (6.69 to 12.84)c |
| HCV | 6293 (21.99) | 519 (22.83) | 595 (19.92) | –0.67 (–1.44 to 0.10) |
| HBV | 2019 (7.11) | 187 (8.23) | 182 (6.09) | –3.46 (–4.81 to –2.08)c |
| ALD | 278 (0.96) | 21 (0.92) | 32 (1.07) | 1.93 (–1.50 to 5.48) |
| Other liver diseasesb | 1653 (5.71) | 104 (4.57) | 172 (5.76) | 2.86 (–0.94 to 6.80) |
| Mortality for HIV, by Liver Diseases (per 100 000 Medicare population (2006–2016) | ||||
| Count (Rate per 100 000) | Average APC | |||
| 2006–2016a | 2006 | 2016 | ||
| Non-liver-related HIV | 1622 (5.81) | 185 (8.14) | 109 (3.65) | –9.14 (–11.02 to –7.22)c |
| Liver-related HIV | 1260 (4.39) | 99 (4.35) | 128 (4.28) | –0.29 (–3.18 to 2.68) |
| NAFLD | 173 (0.58) | 1 (0.04) | 22 (3.65) | 10.01 (2.05 to 18.6)c |
| HCV | 640 (2.26) | 74 (3.25) | 64 (2.14) | –4.85 (–9.00 to –0.51)c |
| HBV | 274 (0.98) | 33 (1.45) | 20 (0.67) | –8.78 (–11.20 to –6.29)c |
| ALD | 85 (0.3) | 10 (0.44) | 5 (0.17) | –6.37 (–15.32 to 3.52) |
| Other liver diseasesb | 268 (0.91) | 9 (0.4) | 27 (0.9) | 7.2 (–0.51 to 15.51) |
Average annual percent change is a weighted average of the APCs (a maximum of 2 joinpoints was allowed).
Abbreviations: ALD, alcoholic liver disease; APC, annual percent change; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease.
aRates were averaged across 2006–2016.
bInclude autoimmune, Wilson disease, hemochromatosis, iron overload, alpha-1-antitrypsin deficiency, Budd-Chiari syndrome, cholangitis, liver disorders, and unknown etiologies for HCC, cirrhosis, and liver failure.
cSignificantly different from 0 (P < .05).
Figure 2.
Prevalence and mortality rates for HIV+ Medicare patients with/without liver disease from 2006 to 2016. Abbreviations: AAPC, average annual percent change; APC, annual percent change.
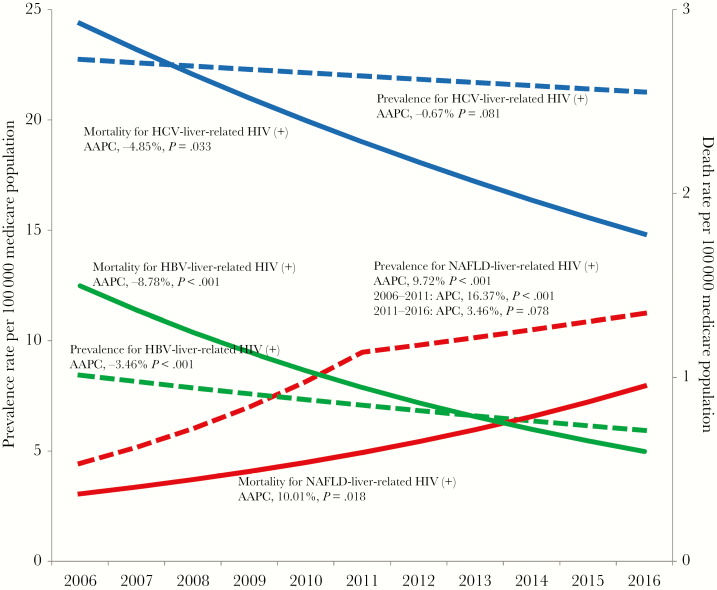
In 2016, HCV was the leading cause of liver-related HIV (+) (46.6%), followed by NAFLD (27.7%), HBV (14.3%), other LDs (13.5%), and ALD (2.5%) (Table 4). Among liver diseases, NAFLD was the driver of the increase in the prevalence rate for liver-related HIV (+) with an AAPC increase of 9.7% (P < .001), whereas HBV had an AAPC decrease of –3.4% (P < .001) and HCV, ALD, and other liver diseases were noted to have stable trends (Figure 3 and Table 4).
Figure 3.
Prevalence and mortality rates for HIV+ Medicare patients with nonalcoholic fatty liver disease, hepatitis C virus, and hepatitis B virus from 2006 to 2016. Abbreviations: AAPC, average annual percent change; APC, annual percent change; HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease.
Of 2882 HIV (+) patients who died, 1260 had liver diseases (640 HCV, 274 HBV, 173 NAFLD, 85 ALD, and 268 other liver diseases). During the study period, worsening trends in mortality for NAFLD-related diagnosis in HIV (+) subjects were observed (AAPC, 10.0%; P = .018), as compared with improving trends for HBV- and HCV-related HIV (+) (HBV: AAPC, –8.8%; P < .001; HCV: AAPC, –4.9%; P = .033) and stable trends for ALD and other-liver-related HIV (+) (P = .172 and .068).
As a sensitivity analysis, each classification used for our extended NAFLD definition experienced similar increasing trends in prevalence (pairwise comparison for test of parallelism: all P > .05), demonstrating the homogeneity of our extended NAFLD definition (Supplemental Table 6). This result suggests that we did not inadvertently increase the prevalence of NAFLD through the use of our NAFLD definition. In addition, similar increasing trends in mortality for each definition were also confirmed by biennial or triennial trend analyses. Of note, annual trend analysis was not possible because of a small event size (data not shown).
In the multivariate analysis after adjustments for calendar year, age, sex, race/ethnicity, region, and beneficiary entitlement, the presence of liver disease was associated with an increased risk of 1-year mortality compared with those with HIV but without liver disease: HCV (odds ratio [OR], 2.00; 95% CI, 1.24–172), HBV (OR, 2.40; 95% CI, 2.09–2.77), ALD (OR, 5.70; 95% CI, 4.34–7.48), and NAFLD (OR, 1.46; 95% CI, 1.24–1.72) (Table 5).
Table 5.
One-Year All-Cause Mortality, Total Charges, and Length of Stay Among HIV Patients, by Liver Diseases: Medicare Population (2006–2016)
| 1-y All-Cause Mortality | Length of Stay | Total Charges | ||
|---|---|---|---|---|
| Inpatient | Outpatient | |||
| OR (95% CI) | % Change (95% CI) | % Change (95% CI) | % Change (95% CI) | |
| NAFLD | 1.46 (1.24 to 1.72) | 24.58 (14.80 to 35.20) | 58.73 (50.50 to 67.41) | 12.31 (9.94 to 14.74) |
| HCV | 2.00 (1.81 to 2.21) | 39.61 (32.67 to 46.90) | 50.82 (45.57 to 56.26) | 29.65 (28.09 to 31.23) |
| HBV | 2.40 (2.09 to 2.77) | 45.26 (34.61 to 56.76) | 75.70 (64.98 to 87.13) | 48.99 (46.58 to 51.43) |
| ALD | 5.70 (4.34 to 7.48) | 15.26 (–0.50 to 33.53) | 60.75 (26.4 to 104.43) | 17.25 (13.32 to 21.31) |
All models were adjusted for calendar year, age, sex, race/ethnicity, region, and beneficiary entitlement.
Abbreviations: ALD, alcoholic liver disease; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease; OR, odds ratio.
Health Care Utilization Among HIV (+) Patients With Liver Disease
Overall, 24.7% of HIV+ patients were hospitalized with a mean length of stay (LOS) of 14.2 ± 24.5 days (median LOS [IQR], 7 [3–17] days), and 7.3% of patients died while hospitalized. The mean inpatient charge was $116 234 ± $218 248, whereas the mean inpatient payment was $3646 ± $52 356. On average, overall, HIV+ patients experienced 4.0 ± 4.3 (median [IQR], 3 [1–5] visits) outpatient visits per year with a mean outpatient charge of $6450 ± $18 225 and a mean outpatient payment of $1423 ± $4038 (Supplementary Table 7).
Among those who were HIV+ with LDs, those with HBV had the longest LOS at 19.3 ± 33.0 days, with 10.1% of this group dying while hospitalized, compared with those HIV (+) but without LDs, whose mean LOS was 12.6 ± 25.3 days (median [IQR], 6 [3–14] days; 6.1% died while hospitalized). The mean inpatient charges were also highest for those with HBV at $165 621 ± $230 073 (median [IQR], $78 044 [$33 676–$205 663]) with a mean inpatient payment of $53 352 ± $63 925 (median [IQR], $26 984 [$16 063–$65 417]), compared with those who were HIV (+) without LDs, whose mean inpatient charge was $95 659 ± $160 403 (median [IQR], $44 993 [$21 461–$99 404]). (Supplementary Table 7).
Predictors for longer inpatient stays are noted in Table 5. For both groups, those with HBV experienced greater odds of having a longer length of stay and greater inpatient charges and outpatient charges.
DISCUSSION
Over the past few decades, treatment of HIV infection has changed this highly lethal disease to a manageable chronic disease. Nevertheless, as AIDS-related deaths among HIV (+) patients have declined, death from HCV-related liver disease has became an important cause of death [14]. This paradigm has also shifted with the advent of the highly effective DAAs for HCV treatment [39–41]. In contrast, the epidemic of NAFLD is affecting all sectors of the society and may impact the long-term outcome of HIV-infected patients. Therefore, we sought to assess the prevalence and outcomes of different liver diseases in HIV (+) Medicare beneficiaries in the United States [42].
Overall, our results indicated that the prevalence of HIV among Medicare beneficiaries has been decreasing over the past decade (2006–2016). However, this overall decrease showed some gender differences, with leveling off of the rates of HIV positivity for female subjects. These same findings were noted when reviewing the deaths among Medicare beneficiaries with HIV. In this context, the overall deaths per 100 000 significantly decreased over the study time frame an average of 5.30% per year, primarily in men, without significant decreases in the female subjects.
We also found that among this group of Medicare beneficiaries with HIV, 22% had some form of liver disease. Viral hepatitis was still the most prevalent disease, followed by NAFLD. Interestingly, although the prevalence of viral hepatitis decreased at an average rate of 0.9% per year, the prevalence of NAFLD significantly increased at an average of 7.2% per year, primarily between 2006 and 2011. This was not only significant for the prevalence rates of different liver diseases, but also death rates related to different types of liver disease. This may have important ramifications as HIV+ patients with NAFLD may have more rapid progression of their liver disease, so it becomes imperative to effectively work and counsel patients to provide the most appropriate treatment [42].
Although previous studies have suggested that the presence of viral hepatitis in HIV (+) patients hastens disease progression and possibly death [43–46], our study highlights the changing profile of liver disease from viral hepatitis to NAFLD in HIV (+) subjects. This is an urgent issue, as NAFLD is expected to become more common among those with HIV, who are now living longer and are thus more at risk for developing chronic diseases such as T2DM and NAFLD. In addition, HIV (+) patients on the newer HIV treatments have been reported to be at a higher risk for obesity and type 2 diabetes, both metabolic conditions associated with NAFLD [20–24, 47]. In fact, in our study, we found that among those with HIV, the prevalence of obesity increased an average of 15.16% per year from 2006–2016, whereas lipodystrophy, a condition associated with the older HIV treatments, has plateaued. The change in the prevalence of obesity carried over to mortality, where among those with HIV and obesity there was an increasing trend of mortality, an average of 21.75% per year. Nevertheless, it is important to recognize that the sample size is small and caution must be exercised when interpreting these results. Type 2 diabetes mellitus and cardiovascular disease followed the same stable trends over time. Nonetheless, we recommend further study to investigate the impact of the newer HIV therapies, the obesogenic environment, and genetic links on this rapid rise in obesity, especially as it is obesity, which is helping to drive the global increase in NAFLD [11, 12, 20–24, 47].
On the other hand, it is important to note that our results suggest a significant decrease in the prevalence and death rates from viral hepatitis in HIV (+) patients. This trend is probably a result of the new curative treatment regimens for HCV that have been shown to be very successful in patients coinfected with HIV while at the same improving patients’ health-related quality of life, work productivity, and levels of fatigue [39, 40, 42, 48]. The same holds true for those with HBV, as there are now excellent suppressive therapies for HBV infection. In fact, the nucleotide analogs such as tenofovir disoproxil fumarate (TDF) and tenofovir alafenamide (TAF) have activity against both HIV and HBV, and the recommended treatment regimen for patients with both HIV and HBV includes (TAF or TDF) plus emtricitabine (FTC) or lamivudine (3TC) as the nucleoside reverse transcriptase inhibitor [46].
In addition to mortality outcomes, we assessed resource utilization in this group of patients. Our analyses show that HIV (+) patients with liver diseases do have a significant health care resource utilization burden. As noted in this study, compared with those with HIV but without liver disease, HIV (+) patients with liver disease had longer length of hospital stays, higher inpatient mortality, higher hospital charges, more outpatient visits, and higher outpatient charges. The exact mechanism of the increased utilization was not investigated in this study; however, as noted in previous studies, the likely causes may not be just liver related but rather related to the number of comorbidities that these patients may now have as they live longer, and as seen in the higher CCI among those with HIV and liver disease vs those without. This is especially concerning for those with HIV and NAFLD as the latest economic forecasts for patients with NAFLD indicate a significant economic burden by the year 2030 due to the vast number of people who may be affected by NAFLD unless better diagnostic tools and treatments become available [49, 50].
There are several study limitations that we need to acknowledge. First, because the diagnoses of HIV positivity and liver diseases were based on electronic medical record and ICD codes, some misclassification may have occurred. In this context, there is a possibility of a systematic under- or overcoding of medical conditions known to be less or more common. Indeed, we found that hypertension, hyperlipidemia, obesity, alcohol abuse, and NAFLD were underestimated (ie, HIV patients with metabolic abnormalities were classified as not having these conditions). Such a misclassification would lead to an underestimation of such disease states and bias our results and potentially attenuate the effect of NAFLD on mortality and health utilization. This is an inherent limitation of Medicare data. Second, to mitigate the likelihood of misclassification of NAFLD, we extended the definition of NAFLD to include metabolic dysfunction and obesity in the absence of other cause of liver diseases or alcohol abuse. Our extended definition of NAFLD has been validated with the use of NHANES data and the USFLI, a reliable surrogate for the clinical diagnosis of NAFLD in the US population [36]. Unfortunately, we were not able to validate the definition with liver biopsies; however, this diagnostic method is impractical for population-based studies such as NHANES and Medicare. Therefore, we also conducted a sensitivity analysis on our extended NAFLD definition and determined the homogeneity of the NAFLD subgroup for temporal trends in prevalence and mortality. However, readers are cautioned to think of NAFLD as presumed NAFLD, as we discussed earlier.
Regardless of these efforts, there may remain potential biases where ALD may have been misclassified as NAFLD due to the known under-reporting of ALD and excessive alcohol use in Medicare data [51, 52]. As such, caution must be used when interpreting our resource utilization findings, as they may not be solely attributed to NAFLD. Third, our follow-up was only a year after the medical encounter and the specific cause of death was not available in the Medicare data, which limits any robust interpretation of the mortality study results. Fourth, it is important to note that we were unable to distinguish the presence of nonalcoholic steatohepatitis (NASH), the more progressive form of NAFLD, or the stage of fibrosis, which is the main driver of liver-related mortality in patients with NAFLD [53]. We do recognize that the use of our extended definition of NAFLD to include metabolic dysfunction and obesity may have led to a bias of overestimating the impact of NAFLD on mortality, although we do not believe this to be the case, as was demonstrated in our sensitivity analysis. But, as we have reported previously, among those with presumed NAFLD, we do acknowledge that the risk of mortality increases as the number of metabolic components present increases, a result that was also demonstrated in this study [54].
Finally, we recognize that our findings may not be not generalizable to the general population as our patient population received their medical care through the governmental system, Medicare, where the majority of patients’ beneficiary status was based on their disability rather than age, such that their characteristics may be quite different than those patients whose health care expenses are covered through commercial or private insurance sectors. One must also keep in mind that some of the statistical results we have reported may be statistically significant but not relevant to clinical practice; for example, the ages at which we found a statistical difference between the groups were 51 vs 52. However, what is important to note is that the trend for aging was significant in that the HIV (+) population, which has become significantly older; this may hold clinical implications.
In summary, our results show that the prevalence of HIV among the Medicare population and the prevalence of liver disease among HIV (+) Medicare beneficiaries have decreased from 2011 to 2016. This decline in liver disease in HIV (+) subjects is most likely due to the introduction of curative treatment regimens for HCV and excellent viral suppressive drugs for HBV. However, despite the overall decrease, there was a significant increase in the prevalence of presumed NAFLD among those with HIV, a trend that is consistent worldwide for NAFLD alone. In fact, NAFLD may increase even more within the HIV (+) population compared with the general population due to newer cART therapy and its relationship with increased obesity. These results have substantial importance as the overall 1-year mortality decreased for all groups of HIV (+) patients with liver disease except for those with presumed NAFLD. In addition to prevalence and mortality, liver disease in HIV (+) Medicare beneficiaries was responsible for substantial high health care resource utilization. Given the increasing clinical and economic impact of NAFLD in HIV (+) patients, clinicians must be more vigilant in identifying and managing NAFLD in this patient population. Additionally, these data should inform policy-makers, payers, providers, and the pharmaceutical industry to better understand NAFLD among HIV (+) subjects and develop strategies to deal with NAFLD in a fashion similar to other important liver diseases such as HBV and HCV among patients with HIV.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Author contributions. James M. Paik: study design, data management, statistical analysis, statistical interpretation, manuscript writing, and critical review. Linda Henry: statistical interpretation, manuscript development and writing, and critical review. Pegah Golabi: study concept and design, critical review of manuscript. Saleh A. Alqahtani: critical review of manuscript. Gregory Trimble: critical review of manuscript. Zobair M. Younossi: study concept and design, statistical interpretation, manuscript writing, and critical review. Guarantor of the article: Zobair M. Younossi, MD, MPH.
Financial support. None.
Potential conflicts of interest. Z.M.Y. has received research funds or served as consultant to Gilead Sciences, Intercept, NovoNordisk, BMS, Abbvie, BMS, Terns, and Viking. S.A.A. consults for and has received grants from AbbVie, Bristol-Meyers Squibb, Gilead, Janssen, and Merck. All other authors have no disclosures.
References
- 1.Centers for Disease Control and Prevention. HIV at a glance. 2018. Available at: https://www.cdc.gov/hiv/statistics/overview/ataglance.html. Accessed 1 October 2019. [Google Scholar]
- 2. Antiretroviral Therapy Cohort Collaboration. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV 2017; 4:e349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas—2013. HIV Surveillance Supplemental Report 2015; 20(No. 2). 2015. Available at: http://www.cdc.gov/hiv/library/reports/surveillance. Accessed 1 October 2019. [Google Scholar]
- 4.Centers for Disease Control and Prevention. HIV prevention in the United States. New opportunities. New expectations. Available at: https://www.cdc.gov/hiv/pdf/policies/cdc-hiv-prevention-bluebook.pdf. Accessed 10 January 2019. [Google Scholar]
- 5. Kang W, Tong H-I, Sun Y, et al. Hepatitis C virus infection in patients with HIV-1: epidemiology, natural history and management. Expert Rev Gastroenterol Hepatol 2014; 8:247–66. [DOI] [PubMed] [Google Scholar]
- 6. Mandorfer M, Schwabl P, Steiner S, et al. Advances in the management of HIV/HCV coinfection. Hepatol Int 2016; 10:424–35. [DOI] [PubMed] [Google Scholar]
- 7. Salmon-Ceron D, Nahon P, Layese R, et al. HIV/HCV co-infected cirrhotic patients are no longer at higher risk for HCC or end-stage liver disease as compared to HCV mono-infected patients. Hepatology. 2019; 70:939–954. [DOI] [PubMed] [Google Scholar]
- 8. Sikavi C, Najarian L, Saab S. Similar sustained virologic response in real-world and clinical trial studies of hepatitis C/human immunodeficiency virus coinfection. Dig Dis Sci 2018; 63:2829–39. [DOI] [PubMed] [Google Scholar]
- 9. Milazzo L, Lai A, Calvi E, et al. Direct-acting antivirals in hepatitis C virus (HCV)-infected and HCV/HIV-coinfected patients: real-life safety and efficacy. HIV Med 2017; 18:284–91. [DOI] [PubMed] [Google Scholar]
- 10. Paik JM, Henry L, De Avila L, et al. Mortality related to nonalcoholic fatty liver disease is increasing in the United States. Hepatol Commun 2019; 3:1459–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Younossi Z, Tacke F, Arrese M, et al. Global perspectives on non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Hepatology. 2019; 69:2672–2682. [DOI] [PubMed] [Google Scholar]
- 12. Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018; 15:11–20. [DOI] [PubMed] [Google Scholar]
- 13. Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016; 64:73–84. [DOI] [PubMed] [Google Scholar]
- 14. Price JC, Thio CL. Liver disease in the HIV-infected individual. Clin Gastroenterol Hepatol 2010; 8:1002–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lebovics E, Thung SN, Schaffner F, Radensky PW. The liver in the acquired immunodeficiency syndrome: a clinical and histologic study. Hepatology 1985; 5:293–8. [DOI] [PubMed] [Google Scholar]
- 16. El-Sadr WM, Mullin CM, Carr A, et al. Effects of HIV disease on lipid, glucose and insulin levels: results from a large antiretroviral-naive cohort. HIV Med 2005; 6:114–21. [DOI] [PubMed] [Google Scholar]
- 17. van Welzen BJ, Mudrikova T, El Idrissi A, et al. A review of non-alcoholic fatty liver disease in HIV-infected patients: the next big thing? Infect Dis Ther. 2019; 8:33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Achhra AC, Mocroft A, Reiss P, et al. ; D:A:D Study Group Short-term weight gain after antiretroviral therapy initiation and subsequent risk of cardiovascular disease and diabetes: the D:A:D study. HIV Med 2016; 17:255–68. [DOI] [PubMed] [Google Scholar]
- 19. Herrin M, Tate JP, Akgün KM, et al. Weight gain and incident diabetes among HIV-infected veterans initiating antiretroviral therapy compared with uninfected individuals. J Acquir Immune Defic Syndr 2016; 73:228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McMahon CN, Petoumenos K, Hesse K, et al. High rates of incident diabetes and prediabetes are evident in men with treated HIV followed for 11 years. AIDS 2018; 32:451–9. [DOI] [PubMed] [Google Scholar]
- 21. Nansseu JR, Bigna JJ, Kaze AD, Noubiap JJ. Incidence and risk factors for prediabetes and diabetes mellitus among HIV-infected adults on antiretroviral therapy: a systematic review and meta-analysis. Epidemiology 2018; 29:431–41. [DOI] [PubMed] [Google Scholar]
- 22. Kumar S, Samaras K. The impact of weight gain during HIV treatment on risk of pre-diabetes, diabetes mellitus, cardiovascular disease, and mortality. Front Endocrinol (Lausanne) 2018; 9:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duro M, Manso MC, Barreira S, et al. Metabolic syndrome in human immunodeficiency virus-infected patients. Int J STD AIDS 2018; 29:1089–97. [DOI] [PubMed] [Google Scholar]
- 24. Freitas P, Carvalho D, Santos AC, et al. Adipokines, hormones related to body composition, and insulin resistance in HIV fat redistribution syndrome. BMC Infect Dis 2014; 14:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kosmiski LA, Bacchetti P, Kotler DP, et al. Relationship of fat distribution with adipokines in human immunodeficiency virus infection. J Clin Endocrinol Metab 2008; 93:216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morimoto HK, Simão AN, de Almeida ER, et al. Role of metabolic syndrome and antiretroviral therapy in adiponectin levels and oxidative stress in HIV-1 infected patients. Nutrition 2014; 30:1324–30. [DOI] [PubMed] [Google Scholar]
- 27. Moure R, Domingo P, Gallego-Escuredo JM, et al. Impact of elvitegravir on human adipocytes: alterations in differentiation, gene expression and release of adipokines and cytokines. Antiviral Res 2016; 132:59–65. [DOI] [PubMed] [Google Scholar]
- 28. Arshad T, Golabi P, Paik J, et al. Prevalence of nonalcoholic fatty liver disease in the female population. Hepatol Commun 2019; 3:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016; 64:73–84. [DOI] [PubMed] [Google Scholar]
- 30. Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019; 71:793–801. [DOI] [PubMed] [Google Scholar]
- 31. Kim D, Kim W, Adejumo AC, et al. Race/ethnicity-based temporal changes in prevalence of NAFLD-related advanced fibrosis in the United States, 2005-2016. Hepatol Int 2019; 13:205–13. [DOI] [PubMed] [Google Scholar]
- 32. Golabi P, Paik J, Hwang JP, et al. Prevalence and outcomes of non-alcoholic fatty liver disease (NAFLD) among Asian American adults in the United States. Liver Int 2019; 39:748–57. [DOI] [PubMed] [Google Scholar]
- 33. Li J, Zou B, Yeo YH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2019; 4:389–98. [DOI] [PubMed] [Google Scholar]
- 34. Le MH, Devaki P, Ha NB, et al. Prevalence of non-alcoholic fatty liver disease and risk factors for advanced fibrosis and mortality in the United States. PLoS One 2017; 12:e0173499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chronic Conditions Data Warehouse. CCW technical guidance: getting started with CMS Medicare administrative research files December 2017. Version 2.5. Available at: User/Downloads/ccw-technical-guidance-getting-started-with-cms-medicare-administrative-research-files%20(1).pdf Accessed 17 September 2019. [Google Scholar]
- 36. Ruhl CE, Everhart JE. Fatty liver indices in the multiethnic United States National Health and Nutrition Examination Survey. Aliment Pharmacol Ther 2015; 41:65–76. [DOI] [PubMed] [Google Scholar]
- 37. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43:1130–9. [DOI] [PubMed] [Google Scholar]
- 38.Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute. Joinpoint regression program, version 4.6.0.0 - April 2018. 2018. Available at: https://surveillance.cancer.gov/joinpoint/Joinpoint_Help_4.6.0.0.pdf. Accessed 12 December 2019. [Google Scholar]
- 39. Younossi ZM, Stepanova M, Sulkowski M, et al. Sofosbuvir and ribavirin for treatment of chronic hepatitis C in patients coinfected with hepatitis C virus and HIV: the impact on patient-reported outcomes. J Infect Dis 2015; 212:367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sulkowski MS, Naggie S, Lalezari J, et al. Sofosbuvir and ribavirin for hepatitis C in patients with HIV coinfection. JAMA 2014; 312:353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Molina J-M, Orkin C, Iser DM, et al. Sofosbuvir plus ribavirin for treatment of hepatitis C virus in patients co-infected with HIV (PHOTON-2): a multicentre, open-label, non-randomised, phase 3 study. Lancet 2015; 385:1098–106. [DOI] [PubMed] [Google Scholar]
- 42. Verna EC. Non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in patients with HIV. Lancet Gastroenterol Hepatol 2017; 2:211–23. [DOI] [PubMed] [Google Scholar]
- 43. Kaspar MB, Sterling RK. Mechanisms of liver disease in patients infected with HIV. BMJ Open Gastroenterol 2017; 4:e000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Spradling PR, Richardson JT, Buchacz K, et al. Prevalence of chronic hepatitis B virus infection among patients in the HIV Outpatient Study, 1996–2007. J Viral Hepat 2010; 17:879–86. [DOI] [PubMed] [Google Scholar]
- 45. Thio CL, Seaberg EC, Skolasky R Jr, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 2002; 360:1921–6. [DOI] [PubMed] [Google Scholar]
- 46.Veterans Administration. Liver disease, cirrhosis and HIV. Available at: https://www.hiv.va.gov/provider/manual-primary-care/liver-disease.asp. Accessed 1 October 2019. [Google Scholar]
- 47. Younossi ZM. Non-alcoholic fatty liver disease - a global public health perspective. J Hepatol 2019; 70:531–44. [DOI] [PubMed] [Google Scholar]
- 48.Molina JM, Orkin C, Iser DM, et al. PHOTON-2 study team. Sofosbuvir plus ribavirin for treatment of hepatitis C virus in patients co-infected with HIV (PHOTON-2): a multicentre, open-label, non-randomised, phase 3 study. Lancet 2015; 385:1098–106. . [DOI] [PubMed]
- 49. Estes C, Razavi H, Loomba R, et al. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018; 67:123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 2016; 64:1577–86. [DOI] [PubMed] [Google Scholar]
- 51. Quan H, Li B, Saunders LD, et al. ; IMECCHI Investigators Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res 2008; 43:1424–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Altamirano J, Miquel R, Katoonizadeh A, et al. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterol 2014; 146(5):1231–9.e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology 2017; 65:1557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Golabi P, Otgonsuren M, de Avila L, et al. Components of metabolic syndrome increase the risk of mortality in nonalcoholic fatty liver disease (NAFLD). Medicine (Baltimore) 2018; 97:e0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.