Abstract
Background:
Curcumin likely has wound-healing properties, but its poor pharmacokinetic attributes inhibit its potential. To overcome these limitations, a novel nanoformulation was previously developed, wherein curcumin was loaded into mesoporous silica particles.
Objectives:
The objective of the study is to assess the efficiency of this nanocurcumin formulation as a wound-healing agent in an animal model.
Materials and Methods:
Curcumin was loaded onto mesoporous silica particles. Eighteen healthy, test-naive male Wistar rats were randomly separated into two groups of 9: Group 1 (control) rats were treated topically with a standard drug (sulfadiazine) and Group 2 with 1% curcumin formulation. A circular excision wound was made, and topical application was performed twice a day. The excision diameters were measured on days 3, 6, 9, 12, 15, 18 and 21 of treatment. Three rats from each group were sacrificed on days 7, 14 and 21, and a cross-section from skin specimen in the excision injury was obtained for histological assessment of inflammation, angiogenesis, fibroblast proliferation, presence of collagen and reepithelization.
Results:
Wound contraction percentage in rats treated with curcumin nanoformulation was nonsignificantly higher than that in the control group (P > 0.05). In both groups, inflammatory reactions considerably reduced by day 21 of treatment, the angiogenesis process was almost complete by day 7, fibroblast proliferation noticeably rose by day 14, and a high degree of wound reepithelization was achieved by day 21, with no significant differences between the groups. Interestingly, by day 21, the level of collagen significantly increased in curcumin nanoformulation-treated rats compared with those treated with sulfadiazine.
Conclusions:
Curcumin nanoformulation likely enhanced wound repair by inhibiting the inflammatory response, stimulating angiogenesis, inducing fibroblast proliferation as well as enhancing reepithelization and synthesis of collagen. Therefore, the curcumin nanoformulation used in this study may have potential as a wound-healing ethnomedicine.
Keywords: Circular excision, curcumin, mesoporous silica particles, nanoformulation, wound healing
INTRODUCTION
Curcumin 1,7-bis (4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione is a chemical compound extracted from the roots of Curcuma longa. It is broadly used but is most common in South Asian countries as a functional component of food and as a traditional medicine for treating various diseases. Curcumin has attracted attention owing to its wide-ranging biological and pharmacological roles, such as anti-inflammatory,[1,2,3] antioxidant,[4,5] anti-infectious[1,6,7] and antitumor activities.[8,9,10] Despite significant research worldwide, there are several obstacles that restrict the use of oral curcumin as a pharmacologically active agent. The extremely low solubility of curcumin in water[10] and its rapid degradation and excretion from the human body are the main obstacles limiting its oral use.[11] In addition, curcumin has poor bioavailability, as it is rapidly metabolized and removed due to the harsh physiological conditions of the gastrointestinal tract, p-glycoprotein efflux mode of action and liver first-pass effect.[10,12] These poor pharmacokinetic attributes inhibit curcumin from reaching its action site, and consequently, diminish its efficiency against targeted tissues. Nonetheless, it has been suggested that nanoparticle delivery devices may be considered as possible colloidal carriers that help overcome these difficulties.[10]
Recently, several studies have investigated the wound healing efficiency of curcumin as such or loaded into a range of nanoparticle carriers used as delivery devices.[13,14,15,16,17,18,19,20] Notably, several studies have shown that curcumin nanoformulations such as chitosan/γ-poly-γ-glutamic acid (PGA)/pluronic/curcumin nanoparticles,[13] poly (caprolactone nanofibers) containing curcumin,[14] and poly (lactic-co-glycolic acid) (PLGA)-loaded curcumin nanoparticles have shown to accelerate wound healing.[15] Similarly, Krausz et al.[16] showed that curcumin nanoparticles are slowly released when used in their new sol–gel formulation, and its topical application had both antimicrobial and wound-healing properties in vivo. Li et al.[19] found that applying a dressing of nanocurcumin plus chitosan/oxidized alginate hydrogel resulted in almost complete wound closure in animals by day 14 postwounding. Encapsulation of curcumin into these nanoparticles can serve as a pharmacologically active agent; however, several difficulties and limitations with the abovementioned delivery devices, such as moderate- or low-loading capacity, hinder their use for delivering curcumin as a preventive and therapeutic agent.
The production and potential application of mesoporous silica as a delivery system have attracted substantial consideration over the past 20 years since the finding of the first generation of silica with pore diameters of 2–50 nm (M41S).[21] These mesoporous silica nanoparticles have several unique attributes that make them outstanding candidates for a wide range of pharmacological and biomedical applications.[21,22] However, no delivery system using mesoporous silica nanoparticles had been designed for curcumin. Accordingly, we had developed a novel curcumin delivery device, in which curcumin was successfully loaded into mesoporous silica particles (pore diameters of 2–50 nm), and the resultant delivery system was characterized.[23] In this delivery device, the loading capacity was considerably increased (98%) because these nanoparticles possessed high surface area and large uniform pores that permitted effective loading.[23] Although curcumin alone is unstable and its hydrophobic nature affects its bioavailability, mesoporous silica nanoparticles loaded with curcumin were stable because these particles resist changes in pH, degradation and mechanical stress. As a subsequent work, the aim of the current study is to assess the potential efficiency of this nanocurcumin formulation as a wound-healing agent in an animal model. Animal models are useful tool in wound healing studies because they are relatively inexpensive, ethical, easily controlled or manipulated, flexible and have entirely replicating biological conditions (e.g., immune response, disease and healing).
MATERIALS AND METHODS
Ethical consideration
This study was approved by the Medical Ethical Committee at Pharmacology and Toxicology Department of Taif University on January 15, 2017 (Reference no.: 2017/TU/Pharmacy/05) and complied with the United Kingdom Animals (Scientific Procedures) Act, 1986, and the EU Directive 2010/63/EU for animal studies.
Materials
Oleic acid (cis-9-octadecenoic acid), curcumin, and silica gel 60 were purchased from Sigma-Aldrich (Steinheim, Germany). Carbopol-934 was purchased from HiMedia Laboratories (Mumbai, India). Sulfadiazine (Flamazine) was obtained from Riyadh Pharmaceutics, Riyadh, Kingdom of Saudi Arabia.
Preparation of curcumin nanoformulation
Mesoporous silica particles were prepared as described earlier.[23] Curcumin loading into mesoporous silica particles was performed as detailed previously.[23] Briefly, curcumin solution was prepared by dissolving 100 mg of curcumin into 10 g of oleic acid and 10 g of ethanol. This curcumin solution was sonicated for 30 min using an ultrasonic water bath (Elmasonic, E-15H, Elma, Singen, Germany). Mesoporous silica particle (500 mg) powder and curcumin solution (100 mg) were thoroughly mixed using pestle and mortar. The mixture was heated in an oven at 50°C for 2 h to get rid of ethanol.
Characterization of the resultant curcumin mesoporous silica particles using particle size and zeta-potential measurements and scanning electron microscope was performed as detailed previously.[23] The prepared curcumin formulation was dissolved in distilled water (10 ml). Centrifugation at 5300 ×g (Labofuge™ 200, Thermo Scientific, Hanau, Germany) was performed to separate free curcumin, and then quantified spectrophotometrically (UV-visible spectroscopy, UV-1800, Shimadzu, Kyoto, Japan) at 425 nm. Loading efficacy was calculated to be 98% using the following formula:
Loading efficacy = (Wtotal− Wfree/Wtotal) × 100, where W is the curcumin weight in mg.
Characterization of mesoporous silica particles
Zeta potential measurements for particle size
Dynamic light scattering (Nano ZS, Malvern, Worcestershire, UK) was used for measuring the particle size of each sample. The average of at least three measurements was recorded, and the zeta potential was measured or determined on the basis of electrophoretic mobility beneath the electrical field as an average value of the three measurements.
Differential scanning calorimetry
Differential scanning calorimetry measurement was performed using a calibrated STA449 F3 Jupiter (Netzsch, Selb, Germany). A scan rate of 10°C/min was used, with a temperature range of 25°C–350°C. About 10–15 mg of the sample was examined, while an empty pan was used as a reference. The following samples were prepared for thermal analysis: curcumin as such, unloaded mesoporous silica particles and curcumin-loaded into mesoporous silica particles.
Fourier transform infrared spectroscopy
About 5 mg of each sample was triturated with 100 mg potassium bromide powder. Each tested sample was scanned by a Fourier-transform Infrared spectrophotometer (IRPrestige-21, Shimadzu, Kyoto, Japan) from 400 to 4000/cm. The following samples were analyzed: curcumin, mesoporous silica particles, oleic acid, curcumin/oleic acid/mesoporous silica particle physical mixture and curcumin loaded onto mesoporous silica particles.
Scanning electron microscope
Each tested sample was mounted on a metal stub and sputtered with a thin coating of gold under vacuum using a sputter coater (Cressington 108 auto, Cressington Scientific Instruments Ltd, Watford, UK) and studied by means of analytical scanning electron microscope (JSM-6390 LA, JEOL, Tokyo, Japan).
Wound healing
Experimental animals
Eighteen healthy, test-naive male Wistar rats, aged 8–10 weeks, and weighting 200–250 g, were purchased from the Animal Facility of King Abdulaziz University, Jeddah, Kingdom of Saudi Arabia. The rats were allowed to adapt for 14 days following transportation from Jeddah to Taif. The rats were housed in standard conditions of temperature 25°C ± 2°C, 12-h light/dark cycle and a relative humidity of 60% ± 5%. The animals were housed in specific pathogen-free animal facility. The rats were randomly separated into two equal groups. Simple randomization was applied as follows: animals were given a specific number from 1 to 18 on small pieces of paper and then drawn out of a bag for each cage or treatment. Group 1 was control and treated topically with sulfadiazine (Flamazine, Riyadh Pharmaceutics, KSA), which is the standard drug for wound healing. Group 2 was the experimental group and was treated with 1% curcumin nanoformulation in a carbopol 934 gel.
Experimental procedure
The rats were sedated by injection of 0.8 ml (ip) chloral hydrate at a dose of 400 mg/kg. Then, the dorsal area of each rat was shaved and a round excision was performed. The diameter of the circular excision was approximately 25 mm. The circle area was calculated using the standard formula and ranged from 400 to 450 mm2. The respective topical applications were made twice daily for all rats: the first application was made at 9:00 am and the second at 3:00 pm. The diameter of each circular excision was measured with a digital caliper on days 3, 6, 9, 12, 15, 18 and 21 postwounding.
On day 7 postwounding, three rats were randomly selected from each group and sacrificed with an overdose of anesthesia, and then, a cross-section from the skin specimen in the excision injury was taken and fixed in 10% formalin for 24 h at room temperature for histological study. On day 14 after injury, another three rats were randomly selected from each group and sacrificed, and on day 21, the remaining three rats in each group were sacrificed; skin specimens were obtained for histology studies from sacrificed rats of both days as described for day 7. No adverse event was noted in any rat from either group during the course of this study.
Wound contraction percent was calculated using the following formula: Percent wound contraction = (initial wound size − wound size on specific day)/initial wound size) × 100.
Histological study
Fixed specimens in 10% formalin at ambient temperature for 24 h were embedded in paraffin, and thin sections (of 5 μm) were made using a microtome (CUT4062; SLEE Medical Gmbh, Mainz, Germany). These sections were then processed with a standard procedure,[24] and stained with hematoxylin and eosin (H and E). Although Masson's Trichrome staining is better for differentiating cells from neighboring connective cell and tissue compared with H and E, it was unavailable for the authors during this study.
The stained sections were mounted using DPX (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Stained sections were tested under a light microscope (magnification, ×10–40) to assess for histological alterations.
Outcome measured
In the histological study, five main histological changes were assessed, namely, inflammation, formation of new blood vessels (angiogenesis), fibroblast proliferation, presence of collagen and reepithelization. The assessor was blinded regarding the grouping for the histological analysis. The prepared slides (treatments) were placed into three case slide sets (total slides: 81), and given a random number, and then assessed in a single-blinded manner by an expert in histopathology. The results of the control and experimental groups were compared to assess for any differences.
Data analysis
Analysis of variance (one-way) was used to compare the three groups. Because there was a substantial difference, Tukey post hoc analysis was used to make pair-wise comparisons between the three groups (day 7, 14 and 21 postwounding) to determine exact differences. Data analysis was conducted using SPSS 22 (IBM Corp., Armonk, NY, USA). The significance of the differences was identified at a confidence interval of 95%, and P < 0.05 was considered statistically significance. Data were expressed as the mean ± standard deviation. The number of replicates was 9.
RESULTS
Wound contraction
Figure 1 and Table 1 show that from days 3 to 18 postwounding, the wound contraction percent in rats treated with the curcumin nanoformulation (Group 2) was higher compared with that in rats treated with sulfadiazine (Group 1); however, this difference was not statistically significant (P > 0.05). On the last day of measurement (day 21), both treatment groups stimulated complete closure of wounds.
Figure 1.
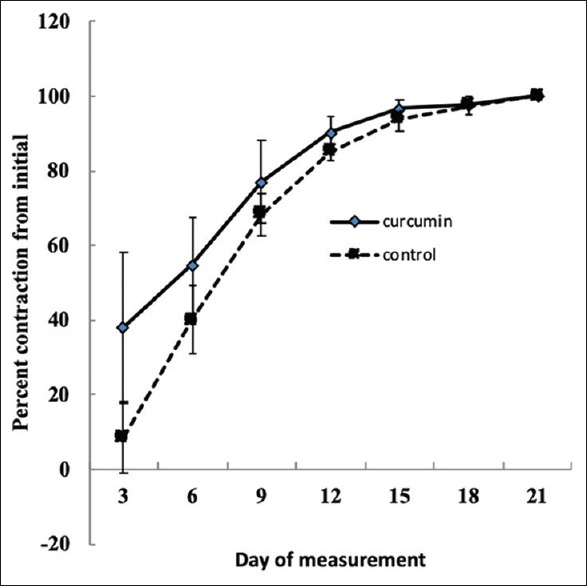
Impact of topical application of curcumin nanoformulation on percent wound contraction in Wistar rats
Table 1.
Impacts of topical application of curcumin nanoformulation on inflammation, angiogenesis, fibroblasts proliferation, collagen content and reepithelization in circular excision wound model in Wistar rats
| Histological parameters | Positive control | Curcumin nanoformulation |
|---|---|---|
| Inflammation | ||
| Day 7 | 3.667 ± 0.500A | 3.889 ± 0.333A |
| Day 14 | 2.333 ± 0.500B | 3.444 ± 0.726a,B |
| Day 21 | 1.444 ± 0.726C | 1.222 ± 0.441B |
| Angiogenesis | ||
| Day 7 | 3.556 ± 0.726A | 4.000 ± 0.000A |
| Day 14 | 2.778 ± 0.667b,B | 3.889 ± 0.333a,A |
| Day 21 | 1.556 ± 1.130B | 1.556 ± 0.726B |
| Fibroblast | ||
| Day 7 | 3.111 ± 0.333B | 2.889 ± 0.333B |
| Day 14 | 4.000 ± 0.000A | 4.000 ± 0.000A |
| Day 21 | 1.889 ± 0.782C | 1.556 ± 0.527C |
| Collagen | ||
| Day 7 | 1.778 ± 0.833B | 1.889 ± 0.333C |
| Day 14 | 3.000 ± 0.707A | 2.556 ± 0.527B |
| Day 21 | 2.667 ± 0.500A | 3.667 ± 0.500a,A |
| Reepithelization | ||
| Day 7 | 0.000 ± 0.000C | 0.000 ± 0.000C |
| Day 14 | 0.444 ± 0.527B | 0.000 ± 0.000a,B |
| Day 21 | 2.000 ± 0.00A | 2.000 ± 0.000A |
Key for inflammation, angiogenesis, fibroblast and collagen: 0 – Absent; 1 – Occasionally present; 2 – Scattered in the field; 3 – Abundant; 4 – Confluent. For reepithelization: 0 – Absent; 1 – Presence of one layer; 2 – Presence of more than one layer. Lowercase superscript alphabets within the same row indicate statistical significance (P < 0.05); different uppercase superscript alphabets in the same column indicate statistical significance
Histological examination
Histological assessment showed that on day 7 after wound injury, the inflammatory response in both group rats was similar [Table 1] [Figure 2Aa and Ba], and there was no significant difference between the groups. The examination also revealed an increasing area of inflammation in the early postwounding stages. On day 14 postinjury, Group 2 rats showed marginal or no change in inflammatory reaction, whereas those in Group 1 showed a considerable change compared with the group's day 7 findings [Table 1] [Figure 2Ab and Bb]. On day 21 postwounding, the inflammatory reaction was considerably lowered in both groups compared with their respective day 7 findings; the difference between both groups was statistically insignificant.
Figure 2.
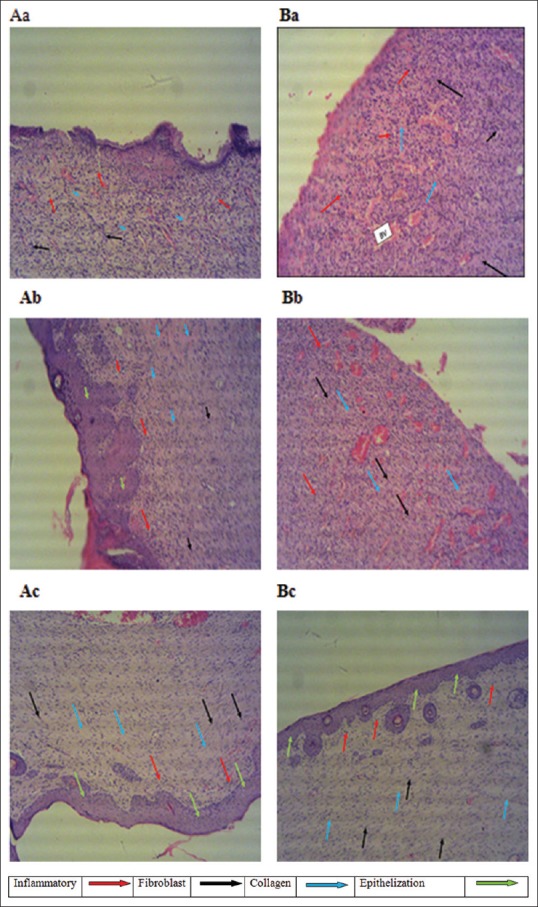
Histological observation of H and E-stained sections of rats received 1% of sulfadiazine and curcumin nanoformulation. Sections of rats treated with sulfadiazine on (Aa) day 7, (Ab) day 14 and (Ac) day 21 of treatment. Sections of rats treated with curcumin nanoformulation on (Ba) day 7, (Bb) day 14 and (Bc) day 21 of treatment
Angiogenesis
The angiogenesis process was almost complete by day 7 postwounding in both groups, and there was negligible difference between the groups [Table 1]. In curcumin formulation-treated animals, angiogenesis declined with time [Table 1]. On day 21 postinjury, insignificant difference in the level of formation of new blood vessels was noted between both groups, suggesting that this curcumin nanoformulation may have a similar impact on wound angiogenesis as sulfadiazine.
Fibroblast proliferation
In both groups, histological analysis revealed noticeable fibroblast proliferation, mainly on day 14 postinjury [Table 1] [Figure 2Ba and Bb]. The level of fibroblast proliferation was the same in both groups on day 14, and there was no statistical difference found between the two groups in the day 7 findings. On day 21, the level of proliferation of fibroblast was reduced by ~60% in Group 2 rats and by ~52% in Group 1 rats; however, the difference between the two groups was not statistically significant [Table 1] [Figure 2Ca and Cb].
Collagen levels
On day 21 postinjury, the level of collagen in the curcumin formulation-treated rats was significantly higher than that in the sulfadiazine group (3.667 vs. 2.667) [Table 1]. This indicates that topically applied curcumin formulation may increase the levels of collagen.
Wound reepithelization
A high degree (2.00 ± 0.00) of wound reepithelization was observed in both groups on day 21 postwounding [Table 1] [Figure 2Ac and Bc], with no significant difference between the groups. In addition, analysis of the day 21 skin sections showed epithelization with well-defined and differentiated epithelial tissues.
DISCUSSION
Curcumin has gained significance because of its promising biological effects and low toxicity and is widely used as a pharmacologically active natural product, despite its poor pharmacokinetic properties that limit its efficiency.[1,2,3,10,11,12] To overcome these limitations, we had previously developed a delivery device with curcumin loaded into mesoporous silica particles, which significantly enhanced the solubility of curcumin. In the current study, we found that topical application of this nanoformulation on the wounds of male Wistar rats resulted in a nonsignificantly higher percent wound contraction than that with sulfadiazine, the standard drug, for all days of measurements.
The findings of the current study are in line with that of similar studies using different curcumin nanoformulations. Lin et al.[13] found that chitosan/γ-PGA/pluronic/curcumin nanoparticle delivery device had higher percent wound closure efficiency than chitosan dressing plus unloaded curcumin and chitosan dressing alone. Similarly, Merrell et al.[14] found that wound closure in animals treated with polycaprolactone nanofibers containing curcumin for 10 days had about 80% wound closure, while those treated with the delivery vehicle alone had about 60% wound closure.[14] In another study, Chereddy et al.[15] found that mice treated with PLGA-loaded curcumin nanoparticles for 10 days had almost complete wound closure, while those treated with unloaded curcumin and the delivery device alone had 75% wound closure. Nguyen et al.,[25] using a self-assembled nanoplex of curcumin and oligomer of chitosan, reported a superior efficiency of wound healing compared with unloaded curcumin with injury or wound closure of >90% on day 7 versus day 9 for the curcumin as such.[25] This superior wound healing could be attributed to the availability of high amount of curcumin at the sites of the wound.[25]
Ahmad et al.[26] prepared and characterized a new self-nanoemulsifying delivery devise for enhanced solubility and skin penetration for curcumin and showed that contraction of wounds in rats was enhanced until day 24 postwounding in the tested groups. In addition, newly developed curcumin delivery system and the positive control (fusidic acid) significantly enhanced the contraction of wound from day 12 to day 24 compared with control animals.[26] In another study by Ahmad et al.,[27] a new curcumin nanoemulsion was prepared through an ultrasonication technique. The wound healing impacts of this curcumin nanoemulsion were shown to be considerably similar to those by the positive control (fusidic acid).[27]
In terms of healing burn wounds, Krausz et al.[16] found that curcumin-loaded nanoparticles effectively healed burn wounds in addition to displaying antimicrobial properties. Durgaprasad et al.[17] applied curcumin preparation (20%) on burn wounds in an animal model and found that the percent of wound contraction was considerably higher in these animals compared with those treated with sulfadiazine, a finding similar to that observed in the current study. Similarly, El-Refaie et al.[18] found that rats treated with a novel curcumin-loaded self-assembled nanogel (gel-core hyaluosome) had considerable improvements at day 7, and no scar was noted on day 11, likely because of the combined antioxidant and anti-inflammatory effect of curcumin and the scar-reducing effect of hyaluosome. Li et al.[19] found that the application of a newly developed wound dressing comprising nanocurcumin and chitosan/oxidized alginate hydrogel resulted in almost complete wound closure in animals by day 14 postwounding, which was significantly better than in other treatment groups; our findings are in agreement with this finding.
Li et al.[20] found that all days of measurements (day 3, 7, and 14 postwounding), the rate of wound healing among rats treated with curcumin chitosan film was considerably better than that of animals treated with the film alone, which is consistent with our findings. These findings indicate that curcumin nanoformulation may have improved wound repairing by inhibiting the inflammatory reaction, which results in injured skin tissues rapidly entering the later stages of wound repairing, especially tissue formation and reconstruction. Similarly, Lin et al.[13] found that reduced inflammation response was noted at the site of wound when tripolymeric composite injury dressing/curcumin was used. On the contrary, no inflammatory cells were noted upon topical application of the curcumin-loaded self-assembled nanogels (gel-core hyaluosome), highlighting the anti-inflammatory effect of curcumin.[18] In the current study, inflammatory reactions considerably reduced by day 21 of treatment in both the curcumin- and sulfadiazine-treated rats. The present study did not examine the mode of action by which the curcumin nanoformulation modulated inflammation; therefore, further studies are recommended to explore the mechanism of action of such formulation, particularly to examine the impact of such formulation on tumor necrosis factor-α and interleukin-1, the two cytokines with central roles in the inflammatory reaction regulation.[28]
Angiogenesis is an important process in wound healing, and blood flow to wounded tissues is crucial in the repair process. In our study, the process of angiogenesis was almost complete by day 7 of treatment in both groups. These findings are similar to that reported by Li et al.,[19] who found topical application of nanocurcumin for 7 days had a marked effect on capillary formation. Notably, the process of angiogenesis declined faster with time (60% change between day 14 and 21 postwounding) in curcumin-treated rats than those treated with sulfadiazine (44% change between day 14 and 21 postinjury). These results are in contrast with the findings of Krausz et al.,[16] who found that formation of new blood vessels, was significantly higher in injured tissues received curcumin-encapsulated nanoparticles compared with other treatments. Currently, there are no explanations for this finding, and thus further investigation is required to shed light on this finding.
Fibroblasts play a critical role in the process of wound healing, and its migration to wounded tissues is facilitated through several growth factors. This migration process is critical for granular tissue formation as well as for collagen production and deposition.[28] In the current study, enhanced wound-healing responses in the curcumin nanoformulation-treated rats could be attributed to the rise in fibroblast proliferation at day 14 of treatment, as fibroblasts are involved in the synthesis of collagen.
Collagen is the principal protein of the skin extracellular matrix, as it constitutes about 70%–80% of the skin structure and has a fundamental role in homeostasis. Collagen is responsible for the strength and integrity of the wound matrix. The definitive goal of wound repair is the formation of the scar tissue; consequently, adequate collagen production and deposition at the wound site are critical for wound healing.[28] In this study, by day 21 of treatment, the level of collagen significantly increased in collagen nanoformulation-treated rats compared with the sulfadiazine-treated rats. These results correspond with the findings of Lin et al.,[13] who found that collagen was regenerated in rat wounds receiving chitosan/polyglutamic/pluronic/curcumin nanoparticles. El-Refaie et al.[18] found that wound of rats treated with the curcumin-loaded self-assembled nanogels showed increased levels of collagen and that the fibers of collagen were densely packed in a parallel arrangement. Krausz et al.[16] found that after treatment with their sol–gel-based nanoformulation, the wounds exhibited well-arranged compact bundles of collagen. Moreover, Krausz et al.[16] used trichome stain and found significantly high collagen intensity; studying the extent of collagen deposition in repaired tissues could not be done in the present study, as the sections were not stained with Masson's trichrome stain. With regard to reepithelization, our study findings are in agreement with those of Chereddy et al.,[15] who found mice treated with PLGA-loaded curcumin nanoparticles for 10 days had complete epithelization. Similarly, Krausz et al.[16] demonstrated that curcumin-encapsulated nanoparticles displayed expedited epidermis and dermis maturation.
The collective evidence from our study and that in the literature suggests that curcumin nanoformulations have a positive effect on wound healing. An advantage of the current formulation was that the mesoporous silica particles had a high loading capacity (98%). Future studies can be conducted that compare the effectiveness of various curcumin nanoformulations as wound healing agents. In addition, the authors recommend that future studies should explore the mode of action of curcumin formulation, particularly to test the effects of such formulation on tumor necrosis factor-α and interleukin-1, the two cytokines with key roles in the inflammatory reaction regulation.
Limitations
Histological study of the present study adopted a single-blinded fashion, which can result in certain bias, and thus further study needs to be performed using the double-blinded method. Further investigations should be carried out using three treatments, i.e., Group 1 (positive), Group 2 (curcumin formulation) and Group 3 (combination of positive control and curcumin formulation), to investigate the enhancement effects of such formulation. Despite Masson's trichrome being considered more suitable than H and E for differentiating cells from neighboring connective, this staining was unavailable throughout the course of the current study. Thus, it is recommended to conduct further histological study using Masson's trichrome staining. In vitro release of curcumin from mesoporous slica particles (with and without the presence of carbopol gel) should have been conducted in simulated wound fluid; however, it was not possible due to lack of funding. Therefore, the authors recommend that in future studies, results of the release study should be correlated with the in vivo studies with respect to various time points. Although software such as ImageJ that quantifies the histological parameters is very useful, unfortunately, it was unavailable in our laboratory.
CONCLUSION
This study found that the percent wound contraction stimulated in rats by application of curcumin-loaded mesoporous silica particles was nonsignificantly higher than that of sulfadiazine on all days of measurements. This novel nanoformulation likely enhanced wound healing through inhibition of the inflammatory reaction, enhancement of fibroblast proliferation, induction of collagen synthesis and promotion of reepithelization. Therefore, the curcumin nanoformulation used in this study may have potential as a wound-healing ethnomedicine.
Ethical considerations
This study was approved by the Medical Ethical Committee at Pharmacology and Toxicology Department of Taif University on January 15, 2017 (Reference no.: 2017/TU/Pharmacy/05), and complied with the United Kingdom Animals (Scientific Procedures) Act, 1986, and the EU Directive 2010/63/EU for animal studies.
Peer review
This article was peer reviewed by four independent and anonymous reviewers.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Nishikawa H, Tsutsumi J, Kitan S. Antiinflammatory and anti-oxidative effect of curcumin in connective tissue type mast cell. J Funct Foods. 2013;5:763–72. [Google Scholar]
- 2.Suzuki T, Kishimoto Y, Misawa M. Formalin- and carrageenan-induced inflammation attenuates place preferences produced by morphine, methamphetamine and cocaine. Life Sci. 1996;59:1667–74. doi: 10.1016/0024-3205(96)00498-5. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Jiang Y, Wang YW, Huang MT, Ho CT, Huang Q. Enhancing anti-inflammation activity of curcumin through O/W nanoemulsions. Food Chem. 2008;108:419–24. doi: 10.1016/j.foodchem.2007.10.086. [DOI] [PubMed] [Google Scholar]
- 4.Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, et al. Biological activities of curcumin and its analogues (Congeners) made by man and mother nature. Biochem Pharmacol. 2008;76:1590–611. doi: 10.1016/j.bcp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Cole GM, Lim GP, Yang F, Teter B, Begum A, Ma Q, et al. Prevention of Alzheimer's disease: Omega-3 fatty acid and phenolic anti-oxidant interventions. Neurobiol Aging. 2005;26(Suppl 1):133–6. doi: 10.1016/j.neurobiolaging.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Niu Y, Ke D, Yang Q, Wang X, Chen Z, An X, et al. Temperature-dependent stability and DPPH scavenging activity of liposomal curcumin at pH 7.0. Food Chem. 2012;135:1377–82. doi: 10.1016/j.foodchem.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 7.Tomren MA, Másson M, Loftsson T, Tønnesen HH. Studies on curcumin and curcuminoids XXXI. Symmetric and asymmetric curcuminoids: Stability, activity and complexation with cyclodextrin. Int J Pharm. 2007;338:27–34. doi: 10.1016/j.ijpharm.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Wu Q, Zhang Z, Yuan L, Liu X, Zhou L. Preparation of curcumin-loaded liposomes and evaluation of their skin permeation and pharmacodynamics. Molecules. 2012;17:5972–87. doi: 10.3390/molecules17055972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leow PC, Tian Q, Ong ZY, Yang Z, Ee PL. Antitumor activity of natural compounds, curcumin and PKF118-310, as wnt/β-catenin antagonists against human osteosarcoma cells. Invest New Drugs. 2010;28:766–82. doi: 10.1007/s10637-009-9311-z. [DOI] [PubMed] [Google Scholar]
- 10.Sun M, Su X, Ding B, He X, Liu X, Yu A, et al. Advances in nanotechnology-based delivery systems for curcumin. Nanomedicine (Lond) 2012;7:1085–100. doi: 10.2217/nnm.12.80. [DOI] [PubMed] [Google Scholar]
- 11.Sharma RA, Gescher AJ, Steward WP. Curcumin: The story so far. Eur J Cancer. 2005;41:1955–68. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Boelsma E, Tanojo H, Boddé HE, Ponec M. Assessment of the potential irritancy of oleic acid on human skin: Evaluation in vitro and in vivo. Toxicol In Vitro. 1996;10:729–42. doi: 10.1016/s0887-2333(96)00053-7. [DOI] [PubMed] [Google Scholar]
- 13.Lin YH, Lin JH, Hong YS. Development of chitosan/poly-γ-glutamic acid/pluronic/curcumin nanoparticles in chitosan dressings for wound regeneration. J Biomed Mater Res B Appl Biomater. 2017;105:81–90. doi: 10.1002/jbm.b.33394. [DOI] [PubMed] [Google Scholar]
- 14.Merrell JG, McLaughlin SW, Tie L, Laurencin CT, Chen AF, Nair LS. Curcumin-loaded poly (epsilon-caprolactone) nanofibres: Diabetic wound dressing with anti-oxidant and anti-inflammatory properties. Clin Exp Pharmacol Physiol. 2009;36:1149–56. doi: 10.1111/j.1440-1681.2009.05216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chereddy KK, Coco R, Memvanga PB, Ucakar B, des Rieux A, Vandermeulen G, et al. Combined effect of PLGA and curcumin on wound healing activity. J Control Release. 2013;171:208–15. doi: 10.1016/j.jconrel.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Krausz AE, Adler BL, Cabral V, Navati M, Doerner J, Charafeddine RA, et al. Curcumin-encapsulated nanoparticles as innovative antimicrobial and wound healing agent. Nanomedicine. 2015;11:195–206. doi: 10.1016/j.nano.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durgaprasad S, Reetesh R, Hareesh K, Rajput R. Effect of a topical curcumin preparation (BIOCURCUMAX) on burn wound healing in rats. J Pharm Biomed Sci. 2011;8:1–3. [Google Scholar]
- 18.El-Refaie WM, Elnaggar YS, El-Massik MA, Abdallah OY. Novel curcumin-loaded gel-core hyaluosomes with promising burn-wound healing potential: Development, in vitro appraisal and in vivo studies. Int J Pharm. 2015;486:88–98. doi: 10.1016/j.ijpharm.2015.03.052. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Nan K, Li L, Zhang Z, Chen H. In vivo evaluation of curcumin nanoformulation loaded methoxy poly (ethylene glycol)-graft-chitosan composite film for wound healing application. Carbohydr Polym. 2012;88:84–90. [Google Scholar]
- 20.Li X, Chen S, Zhang B, Li M, Diao K, Zhang Z, et al. In situ injectable nano-composite hydrogel composed of curcumin, N, O-carboxymethyl chitosan and oxidized alginate for wound healing application. Int J Pharm. 2012;437:110–9. doi: 10.1016/j.ijpharm.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Hu YC, Zhao Q, Wu C, Zhao P, Jiang H, Jiang T, et al. 3D cubic mesoporous silica microsphere as a carrier for poorly soluble drug carvedilol. Microporous Mesoporous Mater. 2012;147:94–101. [Google Scholar]
- 22.Popova MD, Szegedi Á, Kolev IN, Mihály J, Tzankov BS, Momekov GT, et al. Carboxylic modified spherical mesoporous silicas as drug delivery carriers. Int J Pharm. 2012;436:778–85. doi: 10.1016/j.ijpharm.2012.07.061. [DOI] [PubMed] [Google Scholar]
- 23.Hamam F, Al-Remawi MF. Novel delivery system of curcumin through transdermal route using sub-micronized particles composed of mesoporous silica and oleic acid. J Fun Foods. 2014;8C:87–99. [Google Scholar]
- 24.Ruszymah BH, Chowdhury SR, Manan NA, Fong OS, Adenan MI, Saim AB. Aqueous extract of Centella asiatica promotes corneal epithelium wound healing in vitro. J Ethnopharmacol. 2012;140:333–8. doi: 10.1016/j.jep.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen MH, Lee SE, Tran TT, Bui CB, Nguyen TH, Vu NB, et al. Asimple strategy to enhance the in vivo wound-healing activity of curcumin in the form of self-assembled nanoparticle complex of curcumin and oligochitosan. Mater Sci Eng C Mater Biol Appl. 2019;98:54–64. doi: 10.1016/j.msec.2018.12.091. [DOI] [PubMed] [Google Scholar]
- 26.Ahmad N, Ahmad R, Al-Qudaihi A, Alaseel SE, Fita IZ, Khalid MS, et al. Anovel self-nanoemulsifying drug delivery system for curcumin used in the treatment of wound healing and inflammation 3. Biotech. 2019;9:360. doi: 10.1007/s13205-019-1885-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Ahmad N, Ahmad R, Al-Qudaihi1 A, Alaseel SE, Fita IZ, Khalid MS, et al. Preparation of a novel curcumin nanoemuslion by ultrasonication and its comparative in wound healing and the treatment of inflammation. RSC Adv. 2019;9:20192–206. doi: 10.1039/c9ra03102b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akbik D, Ghadiri M, Chrzanowski W, Rohanizadeh R. Curcumin as a wound healing agent. Life Sci. 2014;116:1–7. doi: 10.1016/j.lfs.2014.08.016. [DOI] [PubMed] [Google Scholar]


