Abstract
Patients with radioiodine-refractory differentiated thyroid cancer (RrDTC) have only a limited or no benefit from radioiodine therapy. For these patients, tyrosine kinase inhibitors have demonstrated encouraging results in advanced RrDTC. Nevertheless, these therapies are related with substantial side effects and a high cost. Somatostatin receptor-based therapies have shown promising findings in advanced RrDTC. Recently, prostate-specific membrane antigen (PSMA) expression was seen in advanced RrDTC in imaging studies. This study presents a case of RrDTC who was treated with 177Lu-DOTATATE and 177Lu-PSMA, presumably as a first report in this regard.
Keywords: 177Lu, DOTATATE, prostate-specific membrane antigen, radioiodine-refractory differentiated thyroid cancer
INTRODUCTION
Patients with radioiodine-refractory differentiated thyroid cancer (RrDTC) have only a limited or no benefit from radioiodine therapy so that they need other treatment strategies such as surgery and/or external irradiation to provide a systemic approach to prevent metastatic tumor spread. Tyrosine kinase inhibitors have demonstrated encouraging results in advanced RrDTC but is associated with substantial side effects and a high cost. Somatostatin receptor (SSTR)-based therapies have shown promising findings in advanced RrDTC.[1,2] Recently, prostate-specific membrane antigen (PSMA) expression was seen in advanced RrDTC in imaging studies.[3]
This study presents a case of RrDTC who was treated with 177Lu-DOTATATE and 177Lu-PSMA, and is presumably the first report in this regard.
CASE REPORT
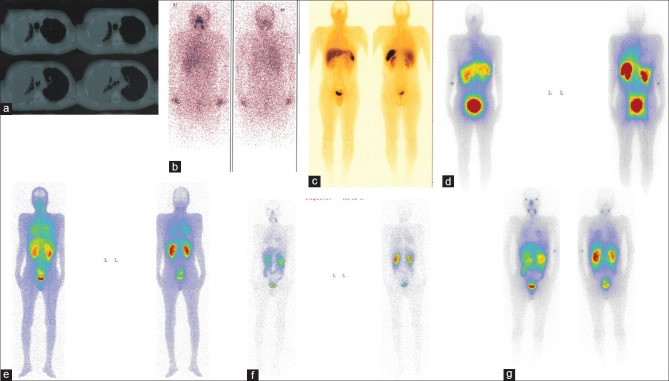
The patient was a 42-year-old man who had a progressive disease with neck and lung metastases and was treated with 25.9 GBq (700 mCi) 131I and also sorafenib for 6 months. The patient had progressive dyspnea due to massive pleural effusions and also repeated surgical intervention in thorax [Figure 1a]. The last radioiodine therapy with 7.4 GBq was given after a long period sorafenib treatment, which was associated with the patient experiencing severe side effects. The posttreatment whole-body imaging showed a faint uptake in the involved neck and lung regions [Figure 1b]. Few months later he underwent octreotide scintigraphy which depicted faint uptake in the neck and lung [Figure 1c], and he immediately received 7.4 GBq 177Lu-DOATATE, and also posttherapy whole-body imaging which demonstrated some uptake in the neck and lung [Figure 1d]. The patient showed continued disease progression and severe dyspnea which required repeated hospitalization. Based on the promising findings of 68Ga-PSMA,[3,4] Our multidisciplinary team proposed PSMA scanning which showed more uptake than octreotide scan in the involved regions [Figure 1e and f]. He then received 7.4GBq 177Lu-PSMA, and once more the posttherapy whole-body imaging revealed more uptake than posttherapy whole-body imaging following 177Lu-DOTATE [Figure 1g]. The patient died 2 weeks posttherapy unexpectedly due to cardiac arrest. It should be mentioned that the number of hospitalization after PRRT were fewer than before. Also, an interesting clinical point was that the respiratory function improved in the early days after therapy, so that although he could not walk alone before PRRT, he was able to walk alone for hours in the early days after PRRT.
Figure 1.
The different imaging modalities in a 42-year-old man with a radioiodine-refractory differentiated thyroid cancer. Computed tomography scan of thorax (a); post 131I ablation whole-body scan (b); 99mTc octreotide scintigraphy (c); post 177Lu-DOTATATE whole-body imaging (d); 2 and 24 h prostate-specific membrane antigen scintigraphy (e and f) and post 177Lu-prostate-specific membrane antigen whole-body imaging (g)
DISCUSSION
In this study, we present both 177Lu-DOTATATE and 177Lu-PSMA therapies in a patient with RrDTC.
Recently, Roll et al. presented findings of five RrDTC patients with limited further therapeutic options and documented expression of SSRT using 68Ga-DOTATATE-PET/CT received 2–4 cycles of PRRT with 177Lu-DOTATATE. They demonstrated that only one out of five patients depicted a partial response, whereas three patients had a progressive disease. One patient had discordant findings between stable imaging results albeit rising Tg levels.[2]
Overall, recent studies demonstrate heterogeneous results ranging from progressive disease under PRRT to favorable results with disease control in up to 60% of patients.[1,5,6] To the best of our knowledge, the literature is scarce regarding 177Lu-PSMA therapy in the patients with RrDTC, so further studies are needed to evaluate this subgroup of DTC.
Such cases illustrate the potential complexity, and interdisciplinary strategies that may end in managing dilemmas. It appears that there is a need for a multidisciplinary pragmatic approach for a change in the management of patients with RrDTC.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Salavati A, Puranik A, Kulkarni HR, Budiawan H, Baum RP. Peptide receptor radionuclide therapy (PRRT) of medullary and nonmedullary thyroid cancer using radiolabeled somatostatin analogues. Semin Nucl Med. 2016;46:215–24. doi: 10.1053/j.semnuclmed.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Roll W, Riemann B, Schäfers M, Stegger L, Vrachimis A. 177Lu-DOTATATE therapy in radioiodine-refractory differentiated thyroid cancer: A Single center experience. Clin Nucl Med. 2018;43:e346–51. doi: 10.1097/RLU.0000000000002219. [DOI] [PubMed] [Google Scholar]
- 3.Taywade SK, Damle NA, Bal C. PSMA expression in papillary thyroid carcinoma: Opening a new horizon in management of thyroid cancer? Clin Nucl Med. 2016;41:e263–5. doi: 10.1097/RLU.0000000000001148. [DOI] [PubMed] [Google Scholar]
- 4.Sager S, Vatankulu B, Uslu L, Sönmezoglu K. Incidental detection of follicular thyroid carcinoma in 68Ga-PSMA PET/CT imaging. J Nucl Med Technol. 2016;44:199–200. doi: 10.2967/jnmt.115.171660. [DOI] [PubMed] [Google Scholar]
- 5.Budiawan H, Salavati A, Kulkarni HR, Baum RP. Peptide receptor radionuclide therapy of treatment-refractory metastatic thyroid cancer using(90) Yttrium and (177)Lutetium labeled somatostatin analogs: Toxicity, response and survival analysis. Am J Nucl Med Mol Imaging. 2013;4:39–52. [PMC free article] [PubMed] [Google Scholar]
- 6.Versari A, Sollini M, Frasoldati A, Fraternali A, Filice A, Froio A, et al. Differentiated thyroid cancer: A new perspective with radiolabeled somatostatin analogues for imaging and treatment of patients. Thyroid. 2014;24:715–26. doi: 10.1089/thy.2013.0225. [DOI] [PubMed] [Google Scholar]