Abstract
Musculoskeletal malignancies consist of a heterogenous group of mesenchymal tumors, often with high inter- and intratumoral heterogeneity. The early and accurate diagnosis of these malignancies can have a substantial impact on optimal treatment and quality of life for these patients. Several new applications and techniques have emerged in molecular imaging, including advances in multimodality imaging, the development of novel radiotracers, and advances in image analysis with radiomics and artificial intelligence. This review highlights the recent advances in molecular imaging modalities and the role of non-invasive imaging in evaluating tumor biology in the era of precision medicine.
Keywords: Artificial intelligence, heterogeneity, molecular imaging, musculoskeletal, precision medicine, radiomics
INTRODUCTION
The number of new cancer cases in 2011–2015 was 439.2/100,000 persons/year, with approximately 163.5 cancer-related deaths/100,000 persons/year.[1] Cancers arise from complex biochemical cellular processes secondary to alterations in normal DNA, often resulting in uncontrolled rapid cellular proliferation. Tumor biomarkers are essential in the diagnosis, risk-stratification, and treatment planning of tumors. With the continual growing emphasis on genomics, proteomics, and radiomics, as well as advances in molecular imaging, personalized precision medicine is becoming a tangible reality. This manuscript aims to provide an overview of molecular imaging for musculoskeletal (MSK) malignancies, highlighting the role it may play in the era of precision medicine.
TUMOR HETEROGENEITY, GENOMIC BIOMARKERS, AND MOLECULAR IMAGING
Cancers consist of a heterogeneous collection of cell with various mutations, leading to different biologic properties, including degrees of differentiation and growth rate.[2,3] This heterogeneity serves as a strong internal mechanism for tumor cells to escape various oncologic treatments. Cancer cell heterogeneity can be categorized as intertumoral and intratumoral. Intertumoral heterogeneity alludes to various biological properties among different lesions of an identical malignancy. Intertumoral heterogeneity arises from a combination of intrinsic and extrinsic mechanisms, including genetic and epigenetic mutations and influences of the tumor microenvironment, causing varying biology of the same tumor type between patients or even different lesions within the same patient.[4] Intratumoral heterogeneity refers to the microheterogeneity within a tumor, in part secondary to imperfect rapid DNA replication in rapidly growing cancers. This leads to a diverse population of cancer cell types within a single lesion, creating difficulties in interpreting limited tissue sampling of a malignancy, such as a biopsy, and determining appropriate therapeutic management.[5,6]
Genetic mutations in tumors can consist of oncogenes (such as c-myc, fos, Ha-ras, Ki-ras, sis, met, SAS MFH, and MDM2), tumor suppressor genes (such as p53, Rb, NF1, and APC), and tumor-specific translocations (such as CHOP-FUS [TLS], EWS-FLI1, EWS-ATF1, SYT-SSX, and PAX3-FKHR).[3,7,8] Traditional medical management of tumors typically involves obtaining a single sample of a tumor and determining the appropriate therapeutic option from that encapsulating diagnosis. Precision medicine aims to capture both the inter- and intratumoral heterogeneity within a patient to create a personalized treatment plan. Molecular imaging noninvasively images the complex biochemical and genetic processes of cancers. This imaging consists of various physiologic imaging techniques targeting components such as peptides, antibodies, proteins, affibodies, aptamers, and nanoparticles, predominantly in the field of nuclear medicine, as well as analysis of quantitative data from cross-sectional imaging, such as computed tomography (CT), magnetic resonance imaging (MRI), and ultrasound (US). Utilizing various imaging techniques, molecular imaging provides a realistic method to better quantify tumor heterogeneity throughout a patient.[9,10,11] Molecular imaging not only provides an insight into initial personalized cancer treatment decisions, but also allows for continual monitoring during treatment. This may lead to the detection of new cancer mutations during treatment, which could prompt changes in therapy before other signs of tumor progression.[12,13,14,15] With continuing improvements in molecular imaging techniques and devices, recognition of new genetic and molecular targets, and new methods of analyzing and quantifying data with artificial intelligence, there is an increasing role of molecular imaging in the diagnosis and treatment of MSK malignancies [Figure 1].
Figure 1.
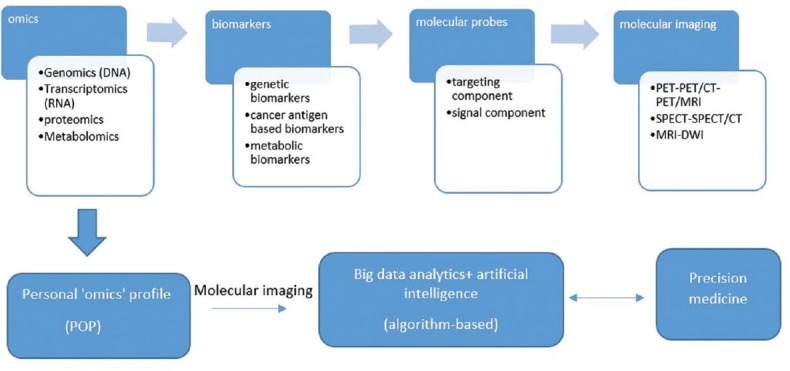
From omics to molecular imaging and precision medicine
PHYSIOLOGIC IMAGING
Bone scintigraphy
Nuclear medicine bone scintigraphy, most commonly with the use of 99mTc-methylene diphosphonate (MDP), is a functional measurement of bone metabolism. It can play a significant role in the evaluation of osseous metastases and cancer staging, and help distinguish metabolically-inactive treated bone metastases from active disease. The specificity, sensitivity, and accuracy for bone scintigraphy for the detection of osseous metastases are 80.9%–96%, 67%–95.2%, and 60%–80.3%, respectively.[16] Bone scintigraphy can be performed with either a singlestatic phase to identify regions of bone with high osteoblast activity, or as a dynamic threephase study, with additional perfusion and blood pool phases to help distinguish inflammatory conditions and changes in blood supply. With a high sensitivity, bone scans are useful in identifying new metastatic lesions. However, the study is limited due to radiotracer uptake up by a variety of other disease processes, including metabolic bone diseases, infections, traumatic injury, and inflammatory conditions.[17,18,19,20]
Single-photon emission tomography
Single-photon emission computed tomography (SPECT) scans are spatial three-dimensional acquisitions of radionuclides. With multiplanar reconstruction, SPECT allows for better contrast resolution and improvement lesion localization. In addition, SPECT can be fused with CT to allow for concurrent anatomical and functional imaging, resulting in improved specificity, sensitivity, and spatial resolution for MSK malignancies.[21,22,23] In particular, SPECT-CT has been shown to reduce equivocal interpretations compared to SPECT or planar scintigraphy in MSK malignancies.[21,24,25,26,27,28]
Positron emission tomography
The development and advances in positron emission tomography (PET) have revolutionized functional imaging. With the use 18F-fluorodeoxyglucose (18F-FDG) to evaluate tumor metabolism, and various other radiopharmaceuticals targeting specific molecular targets, PET has now plays a big role in the accurate staging and monitoring of MSK malignancies, and can also serve as a predictor for treatment outcomes [Figures 2–4].[29,30,31,32]
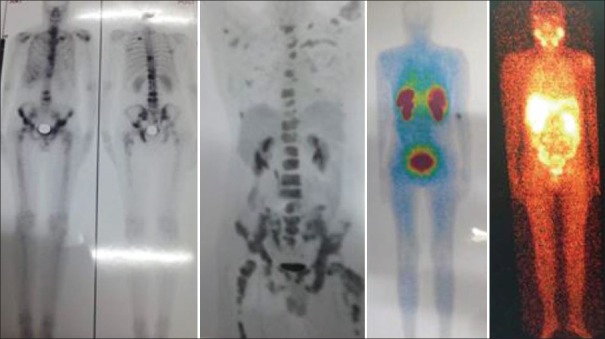
Figure 2.
A 44-year-old man with carcinoma of unknown primary. The bone 99mTc-methylene diphosphonate scintigraphy demonstrated several skeletal lesions throughout the body, 99mTc-prostate-specific membrane antigen scintigraphy and 18F-fluorodeoxyglucose positron emission tomography images showed avid lesions only in the pelvis, and 99mTc-octreotide scintigraphy demonstrated no activity, highlighting the intertumoral heterogeneity
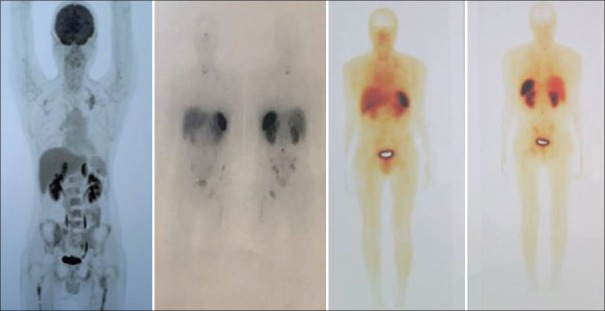
Figure 4.
An 8-year-old boy with Stage IV neuroblastoma. 18F-fluorodeoxyglucose positron emission tomography-computed tomography images demonstrated faint-18F-fluorodeoxyglucose-avid lesions throughout the skeleton (standardized uptake value <2), while 68Ga-DOTATATE positron emission tomography-computed tomography showed numerous 68Ga-DOTATATE-avid lesions in the same region (standardized uptake value >10)
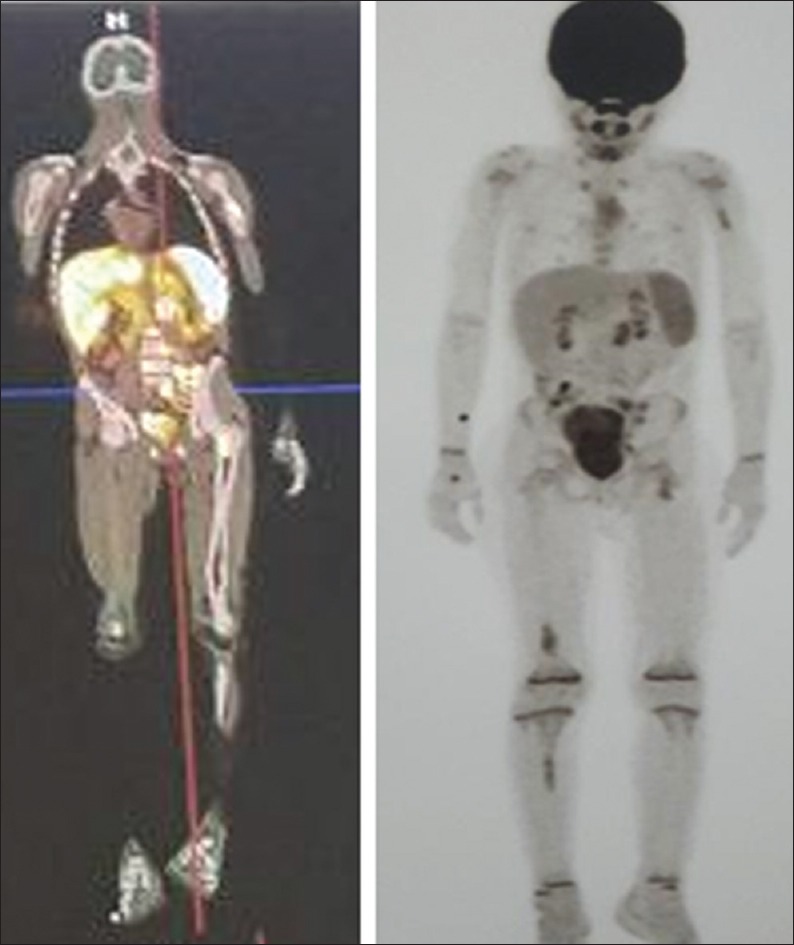
Figure 3.
A 29-year-old man with poorly differentiated neuroendocrine tumor (Ki-67 = 28%). 99mTc-octreotide scintigraphy and post-177Lu-DOTATATE therapy images showed intense uptake within the skeletal lesions, predicting a good response to 177Lu-DOTATATE therapy in patients with somatostatin-expressing neuroendocrine tumors. However, 18F-fluorodeoxyglucose positron emission tomography-computed tomography images demonstrated numerous 18F-fluorodeoxyglucose-avid lesions throughout the skeleton and marrow, representing a poor prognosis
Sarcomas are one of the less common malignancies, and despite current treatments, patients have poor outcomes and life expectancy.[33,34] 18F-FDG uptake in sarcomas has been shown to be reflective of tumor biology and has a valid predictor for tumor aggressiveness and patient outcomes.[30,35] In addition, PET has a growing role in the evaluation of intra- and intertumoral heterogeneity.[36] Piperkova et al. demonstrated advantages of 18F-FDG PET-CT for the initial staging, restaging, and evaluation of the treatment response for bone and soft-tissue sarcomas.[31] PET studies fused with cross-sectional imaging, PET-CT or PET-MRI, allow for more accurate disease localization, detection, and as a guide for biopsies.[37] Furthermore, 18F-FDG PET-CT has been shown to better differentiate soft-tissue and osseous malignancies from benign lesions compared to PET or CT alone.[38,39,40,41]
In addition to 18F-FDG, several novel PET radiotracers have shown promising results. 18F-Fluoroestradiol, which targets estrogen receptors (ER) has been shown to have a high sensitivity for the detection of ER-positive skeletal metastases and is useful for quantifying in vivo ER expression without the need for biopsy.[42] Similar results have been seen for identifying osseous metastases of thyroid malignancy with 124I.[43] 18F-Fluorothymidine (FLT), a radiotracer which measures tumor proliferation, has shown promise in imaging bone and soft-tissue sarcomas. 18F-FLT can help differentiate between high- and low-grade sarcomas and may be useful in evaluating changes in tumor biology over time and assessing intratumoral heterogeneity.[44] Furthermore, the use of dual tracer “cocktail scans” are actively being investigated. lagaru et al. have shown increased detection of osseous metastases with combined 18F-NaF and 18F-FDG PET-CT compared to the modalities individually.[45,46,47,48]
Radiomics and artificial intelligence
Radiomics utilizes quantifiable data from imaging modalities to provide insight into tumor biology and heterogeneity. In the era of “-omics” this data can be combined with genetic and other data to obtain a comprehensive understanding of a patient's tumor biology. In addition, radiomics can aid in the diagnosis of tumor cell type, potentially negating the need for tissue biopsy in some cases and providing a better understanding of intratumoral heterogeneity, which is an intrinsic limitation of limited tissue sampling.[49,50,51,52,53] Imaging features analyzed with radiomics have been shown to have prognostic implications for a diversity of tumors.[54,55,56,57] In patients with soft-tissue sarcomas of the extremities, Vallières et al. demonstrated an association between extracted texture features from 18F-FDG PET-CT and a propensity for developing lung metastases.[58] Radiomic MRI features have also been shown to help distinguish intermediate- and high-grade soft-tissue sarcomas.[59] Associations such as these aid in risk assessment at diagnosis and may help guide first-line therapy choices.
As this field continues to grow, and imaging databases become larger, new trends may arise from mining these large datasets. A current major limitation to the clinical applications of radiomics is the lack of effective autosegmentation techniques, with the majority of current studies performed with either manual or semi-automated segmentation. However, since machine learning techniques are becoming more sophisticated, the possibility of seamless autosegmentation in clinical practice is becoming more realistic.[51,52,53,54,55] Indeed, these algorithms and programs may soon be able to rapidly synthesize the imaging data with other clinical data points to provide even more diagnostic and prognostic information, allowing for more personalized treatment planning.[60,61,62,63,64]
CONCLUSION
Musculoskeletal malignancies have a wide array of intra- and inter-tumoral heterogeneity. With continued advances in molecular imaging, noninvasive methods of understanding tumor biology show promising results. This may aid in the diagnosis, prognosis, and treatment planning and monitoring of musculoskeletal malignancies.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Forouzanfar MH, Afshin A, Alexander LT, Anderson HR, Bhutta ZA, Biryukov S, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: A systematic analysis for the global burden of disease study 2015. Lancet. 2016;388:1659–724. doi: 10.1016/S0140-6736(16)31679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alic L, Niessen WJ, Veenland JF. Quantification of heterogeneity as a biomarker in tumor imaging: A systematic review. PLoS One. 2014;9:e110300. doi: 10.1371/journal.pone.0110300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thakur ML. Seminars in nuclear medicine. WB Saunders: 2009. Genomic biomarkers for molecular imaging: Predicting the future; pp. 236–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutherland KD, Visvader JE. Cellular mechanisms underlying intertumoral heterogeneity. Trends Cancer. 2015;1:15–23. doi: 10.1016/j.trecan.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Gatenby RA, Grove O, Gillies RJ. Quantitative imaging in cancer evolution and ecology. Radiology. 2013;269:8–15. doi: 10.1148/radiol.13122697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chowdhury R, Ganeshan B, Irshad S, Lawler K, Eisenblätter M, Milewicz H, et al. The use of molecular imaging combined with genomic techniques to understand the heterogeneity in cancer metastasis. Br J Radiol. 2014;87:20140065. doi: 10.1259/bjr.20140065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cormier JN, Pollock RE. Soft tissue sarcomas. CA Cancer J Clin. 2004;54:94–109. doi: 10.3322/canjclin.54.2.94. [DOI] [PubMed] [Google Scholar]
- 8.Uchida A, Seto M, Hashimoto N, Araki N. Molecular diagnosis and gene therapy in musculoskeletal tumors. J Orthop Sci. 2000;5:418–23. doi: 10.1007/pl00021460. [DOI] [PubMed] [Google Scholar]
- 9.Mankoff DA. A definition of molecular imaging. J Nucl Med. 2007;48:18N, 21N. [PubMed] [Google Scholar]
- 10.Ghasemi M, Nabipour I, Omrani A, Alipour Z, Assadi M. Precision medicine and molecular imaging: New targeted approaches toward cancer therapeutic and diagnosis. Am J Nucl Med Mol Imaging. 2016;6:310–27. [PMC free article] [PubMed] [Google Scholar]
- 11.Blasberg RG, Tjuvajev JG. Molecular-genetic imaging: Current and future perspectives. J Clin Invest. 2003;111:1620–9. doi: 10.1172/JCI18855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pysz MA, Gambhir SS, Willmann JK. Molecular imaging: Current status and emerging strategies. Clin Radiol. 2010;65:500–16. doi: 10.1016/j.crad.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James ML, Gambhir SS. A molecular imaging primer: Modalities, imaging agents, and applications. Physiol Rev. 2012;92:897–965. doi: 10.1152/physrev.00049.2010. [DOI] [PubMed] [Google Scholar]
- 14.Cai W, Olafsen T, Zhang X, Cao Q, Gambhir SS, Williams LE, et al. PET imaging of colorectal cancer in xenograft-bearing mice by use of an 18F-labeled T84.66 anti-carcinoembryonic antigen diabody. J Nucl Med. 2007;48:304–10. [PubMed] [Google Scholar]
- 15.Liu K, Wang MW, Lin WY, Phung DL, Girgis MD, Wu AM, et al. Molecular imaging probe development using microfluidics. Curr Org Synth. 2011;8:473–87. doi: 10.2174/157017911796117205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Catalano OA, Nicolai E, Rosen BR, Luongo A, Catalano M, Iannace C, et al. Comparison of CE-FDG-PET/CT with CE-FDG-PET/MR in the evaluation of osseous metastases in breast cancer patients. Br J Cancer. 2015;112:1452–60. doi: 10.1038/bjc.2015.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sudoł-Szopińska I, Cwikła JB. Current imaging techniques in rheumatology: MRI, scintigraphy and PET. Pol J Radiol. 2013;78:48–56. doi: 10.12659/PJR.889138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palestro CJ. Radionuclide imaging of musculoskeletal infection: A review. J Nucl Med. 2016;57:1406–12. doi: 10.2967/jnumed.115.157297. [DOI] [PubMed] [Google Scholar]
- 19.Brown ML, O'Connor MK, Hung JC, Hayostek RJ. Technical aspects of bone scintigraphy. Radiol Clin North Am. 1993;31:721–30. [PubMed] [Google Scholar]
- 20.Palestro CJ. Seminars in Nuclear Medicine. WB Saunders: Elsevier; 1994. The current role of gallium imaging in infection. [DOI] [PubMed] [Google Scholar]
- 21.Saha S, Burke C, Desai A, Vijayanathan S, Gnanasegaran G. SPECT-CT: Applications in musculoskeletal radiology. Br J Radiol. 2013;86:20120519. doi: 10.1259/bjr.20120519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buck AK, Nekolla S, Ziegler S, Beer A, Krause BJ, Herrmann K, et al. SPECT/CT. J Nucl Med. 2008;49:1305–19. doi: 10.2967/jnumed.107.050195. [DOI] [PubMed] [Google Scholar]
- 23.Hasegawa BH, Wong KH, Iwata K, Barber WC, Hwang AB, Sakdinawat AE, et al. Dual-modality imaging of cancer with SPECT/CT. Technol Cancer Res Treat. 2002;1:449–58. doi: 10.1177/153303460200100605. [DOI] [PubMed] [Google Scholar]
- 24.Bybel B, Brunken RC, DiFilippo FP, Neumann DR, Wu G, Cerqueira MD, et al. SPECT/CT imaging: Clinical utility of an emerging technology. Radiographics. 2008;28:1097–113. doi: 10.1148/rg.284075203. [DOI] [PubMed] [Google Scholar]
- 25.Lu SJ, Ul Hassan F, Vijayanathan S, Gnanasegaran G. Radionuclide bone SPECT/CT in the evaluation of knee pain: Comparing two-phase bone scintigraphy, SPECT and SPECT/CT. Br J Radiol. 2018;91:20180168. doi: 10.1259/bjr.20180168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palestro CJ, Love C, Schneider R. The evolution of nuclear medicine and the musculoskeletal system. Radiol Clin North Am. 2009;47:505–32. doi: 10.1016/j.rcl.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Pachowicz M, Staśkiewicz G, Florek K, Chrapko BE. The usefulness of SPECT/CT in characterization of skeletal and soft tissue lesions – Report of two cases. Nucl Med Rev Cent East Eur. 2014;17:29–34. doi: 10.5603/NMR.2014.0007. [DOI] [PubMed] [Google Scholar]
- 28.Upadhyay B, Mo J, Beadsmoore C, Marshall T, Toms A, Buscombe J. Technetium-99m methylene diphosphonate single-photon emission computed tomography/computed tomography of the foot and ankle. World J Nucl Med. 2017;16:88–100. doi: 10.4103/1450-1147.203077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aboagye EO, Kraeber-Bodéré F. Highlights lecture EANM 2016: “Embracing molecular imaging and multi-modal imaging: A smart move for nuclear medicine towards personalized medicine”. Eur J Nucl Med Mol Imaging. 2017;44:1559–74. doi: 10.1007/s00259-017-3704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jadvar H, Velez E, Desai B, Ji L, Colletti PM, Quinn DI. Prediction of time to hormonal treatment failure in metastatic castrate-sensitive prostate cancer with (18)F-FDG PET/CT. J Nucl Med. 2019 doi: 10.2967/jnumed.118.223263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piperkova E, Mikhaeil M, Mousavi A, Libes R, Viejo-Rullan F, Lin H, et al. Impact of PET and CT in PET/CT studies for staging and evaluating treatment response in bone and soft tissue sarcomas. Clin Nucl Med. 2009;34:146–50. doi: 10.1097/RLU.0b013e3181966f9d. [DOI] [PubMed] [Google Scholar]
- 32.Feldman F, van Heertum R, Manos C. 18FDG PET scanning of benign and malignant musculoskeletal lesions. Skeletal Radiol. 2003;32:201–8. doi: 10.1007/s00256-003-0623-3. [DOI] [PubMed] [Google Scholar]
- 33.Hawkins DS, Rajendran JG, Conrad EU, 3rd, Bruckner JD, Eary JF. Evaluation of chemotherapy response in pediatric bone sarcomas by [F-18]-fluorodeoxy-D-glucose positron emission tomography. Cancer. 2002;94:3277–84. doi: 10.1002/cncr.10599. [DOI] [PubMed] [Google Scholar]
- 34.Hawkins DS, Conrad EU, 3rd, Butrynski JE, Schuetze SM, Eary JF. [F-18]-fluorodeoxy-D-glucose-positron emission tomography response is associated with outcome for extremity osteosarcoma in children and young adults. Cancer. 2009;115:3519–25. doi: 10.1002/cncr.24421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Treglia G, Salsano M, Stefanelli A, Mattoli MV, Giordano A, Bonomo L, et al. Diagnostic accuracy of18 F-FDG-PET and PET/CT in patients with ewing sarcoma family tumours: A systematic review and a meta-analysis. Skeletal Radiol. 2012;41:249–56. doi: 10.1007/s00256-011-1298-9. [DOI] [PubMed] [Google Scholar]
- 36.Basu S, Kwee TC, Gatenby R, Saboury B, Torigian DA, Alavi A, et al. Evolving role of molecular imaging with PET in detecting and characterizing heterogeneity of cancer tissue at the primary and metastatic sites, a plausible explanation for failed attempts to cure malignant disorders. Eur J Nucl Med Mol Imaging. 2011;38:987–91. doi: 10.1007/s00259-011-1787-z. [DOI] [PubMed] [Google Scholar]
- 37.O'Sullivan PJ, Rohren EM, Madewell JE. Positron emission tomography-CT imaging in guiding musculoskeletal biopsy. Radiol Clin North Am. 2008;46:475–86, v. doi: 10.1016/j.rcl.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Thomas L, Balmus C, Ahmadzadehfar H, Essler M, Strunk H, Bundschuh RA, et al. Assessment of bone metastases in patients with prostate cancer-A comparison between 99mTc-bone-scintigraphy and [68Ga]Ga-PSMA PET/CT. Pharmaceuticals (Basel) 2017;10:pii: E68. doi: 10.3390/ph10030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hongtao L, Hui Z, Bingshun W, Xiaojin W, Zhiyu W, Shuier Z, et al. 18F-FDG positron emission tomography for the assessment of histological response to neoadjuvant chemotherapy in osteosarcomas: A meta-analysis. Surg Oncol. 2012;21:e165–70. doi: 10.1016/j.suronc.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Benz MR, Evilevitch V, Allen-Auerbach MS, Eilber FC, Phelps ME, Czernin J, et al. Treatment monitoring by 18F-FDG PET/CT in patients with sarcomas: Interobserver variability of quantitative parameters in treatment-induced changes in histopathologically responding and nonresponding tumors. J Nucl Med. 2008;49:1038–46. doi: 10.2967/jnumed.107.050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bischoff M, Bischoff G, Buck A, von Baer A, Pauls S, Scheffold F, et al. Integrated FDG-PET-CT: Its role in the assessment of bone and soft tissue tumors. Arch Orthop Trauma Surg. 2010;130:819–27. doi: 10.1007/s00402-009-0937-2. [DOI] [PubMed] [Google Scholar]
- 42.Mahajan A, Azad GK, Cook GJ. PET imaging of skeletal metastases and its role in personalizing further management. PET Clin. 2016;11:305–18. doi: 10.1016/j.cpet.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 43.van Kruchten M, Glaudemans AW, de Vries EF, Beets-Tan RG, Schröder CP, Dierckx RA, et al. PET imaging of estrogen receptors as a diagnostic tool for breast cancer patients presenting with a clinical dilemma. J Nucl Med. 2012;53:182–90. doi: 10.2967/jnumed.111.092734. [DOI] [PubMed] [Google Scholar]
- 44.Kandathil A, Subramaniam RM. PET/Computed tomography and precision medicine: Musculoskeletal sarcoma. PET Clin. 2017;12:475–88. doi: 10.1016/j.cpet.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Iagaru A, Mittra E, Yaghoubi SS, Dick DW, Quon A, Goris ML, et al. Novel strategy for a cocktail 18F-fluoride and 18F-FDG PET/CT scan for evaluation of malignancy: Results of the pilot-phase study. J Nucl Med. 2009;50:501–5. doi: 10.2967/jnumed.108.058339. [DOI] [PubMed] [Google Scholar]
- 46.Samarin A, Burger C, Wollenweber SD, Crook DW, Burger IA, Schmid DT, et al. PET/MR imaging of bone lesions – Implications for PET quantification from imperfect attenuation correction. Eur J Nucl Med Mol Imaging. 2012;39:1154–60. doi: 10.1007/s00259-012-2113-0. [DOI] [PubMed] [Google Scholar]
- 47.Eiber M, Takei T, Souvatzoglou M, Mayerhoefer ME, Fürst S, Gaertner FC, et al. Performance of whole-body integrated 18F-FDG PET/MR in comparison to PET/CT for evaluation of malignant bone lesions. J Nucl Med. 2014;55:191–7. doi: 10.2967/jnumed.113.123646. [DOI] [PubMed] [Google Scholar]
- 48.Kogan F, Fan AP, Gold GE. Potential of PET-MRI for imaging of non-oncologic musculoskeletal disease. Quant Imaging Med Surg. 2016;6:756–71. doi: 10.21037/qims.2016.12.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lambin P, van Stiphout RG, Starmans MH, Rios-Velazquez E, Nalbantov G, Aerts HJ, et al. Predicting outcomes in radiation oncology – Multifactorial decision support systems. Nat Rev Clin Oncol. 2013;10:27–40. doi: 10.1038/nrclinonc.2012.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Orndal C, Rydholm A, Willén H, Mitelman F, Mandahl N. Cytogenetic intratumor heterogeneity in soft tissue tumors. Cancer Genet Cytogenet. 1994;78:127–37. doi: 10.1016/0165-4608(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 51.Sala E, Mema E, Himoto Y, Veeraraghavan H, Brenton JD, Snyder A, et al. Unravelling tumour heterogeneity using next-generation imaging: Radiomics, radiogenomics, and habitat imaging. Clin Radiol. 2017;72:3–10. doi: 10.1016/j.crad.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RG, Granton P, et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012;48:441–6. doi: 10.1016/j.ejca.2011.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Avanzo M, Stancanello J, El Naqa I. Beyond imaging: The promise of radiomics. Phys Med. 2017;38:122–39. doi: 10.1016/j.ejmp.2017.05.071. [DOI] [PubMed] [Google Scholar]
- 54.Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images are more than pictures, they are data. Radiology. 2016;278:563–77. doi: 10.1148/radiol.2015151169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Griethuysen JJ, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V, et al. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017;77:e104–7. doi: 10.1158/0008-5472.CAN-17-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choi ER, Lee HY, Jeong JY, Choi YL, Kim J, Bae J, et al. Quantitative image variables reflect the intratumoral pathologic heterogeneity of lung adenocarcinoma. Oncotarget. 2016;7:67302–13. doi: 10.18632/oncotarget.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5:4006. doi: 10.1038/ncomms5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vallières M, Freeman CR, Skamene SR, El Naqa I. A radiomics model from joint FDG-PET and MRI texture features for the prediction of lung metastases in soft-tissue sarcomas of the extremities. Phys Med Biol. 2015;60:5471–96. doi: 10.1088/0031-9155/60/14/5471. [DOI] [PubMed] [Google Scholar]
- 59.Corino VD, Montin E, Messina A, Casali PG, Gronchi A, Marchianò A, et al. Radiomic analysis of soft tissues sarcomas can distinguish intermediate from high-grade lesions. J Magn Reson Imaging. 2018;47:829–40. doi: 10.1002/jmri.25791. [DOI] [PubMed] [Google Scholar]
- 60.Beam AL, Kohane IS. Translating artificial intelligence into clinical care. JAMA. 2016;316:2368–9. doi: 10.1001/jama.2016.17217. [DOI] [PubMed] [Google Scholar]
- 61.Darcy AM, Louie AK, Roberts LW. Machine learning and the profession of medicine. JAMA. 2016;315:551–2. doi: 10.1001/jama.2015.18421. [DOI] [PubMed] [Google Scholar]
- 62.Jiang F, Jiang Y, Zhi H, Dong Y, Li H, Ma S, et al. Artificial intelligence in healthcare: Past, present and future. Stroke Vasc Neurol. 2017;2:230–43. doi: 10.1136/svn-2017-000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murff HJ, FitzHenry F, Matheny ME, Gentry N, Kotter KL, Crimin K, et al. Automated identification of postoperative complications within an electronic medical record using natural language processing. JAMA. 2011;306:848–55. doi: 10.1001/jama.2011.1204. [DOI] [PubMed] [Google Scholar]
- 64.Crown WH. Potential application of machine learning in health outcomes research and some statistical cautions. Value Health. 2015;18:137–40. doi: 10.1016/j.jval.2014.12.005. [DOI] [PubMed] [Google Scholar]