Abstract
When a protein is covalently and irreversibly bound to DNA (i.e., a DNA–protein cross-link [DPC]), it may obstruct any DNA-based transaction, such as transcription and replication. DPC formation is very common in cells, as it can arise from endogenous factors, such as aldehyde produced during cell metabolism, or exogenous sources like ionizing radiation, ultraviolet light, and chemotherapeutic agents. DPCs are composed of DNA, protein, and their cross-linked bonds, each of which can be targeted by different repair pathways. Many studies have demonstrated that nucleotide excision repair and homologous recombination can act on DNA molecules and execute nuclease-dependent DPC repair. Enzymes that have evolved to deal specifically with DPC, such as tyrosyl-DNA phosphodiesterases 1 and 2, can directly reverse cross-linked bonds and release DPC from DNA. The newly identified proteolysis pathway, which employs the proteases Wss1 and SprT-like domain at the N-terminus (SPRTN), can directly hydrolyze the proteins in DPCs, thus offering a new venue for DPC repair in cells. A deep understanding of the mechanisms of each pathway and the interplay among them may provide new guidance for targeting DPC repair as a therapeutic strategy for cancer. Here, we summarize the progress in DPC repair field and describe how cells may employ these different repair pathways for efficient repair of DPCs.
Keywords: DNA–protein cross-link, SPRTN, NER, HR, TDP1/TDP2
Background
DNA in eukaryotic cells is coated with proteins and forms a highly compact and dynamic chromatin structure. Interactions between DNA and proteins are important for numerous cellular processes, such as cell division, transcription, and replication. These interactions are mostly transient and dynamic, guaranteeing that these remarkable complex reactions occur in a time- and space-regulated manner. However, proteins can be accidently covalently linked with DNA molecules, which can block not only interactions between other proteins and DNA but also DNA transactions that must slide-through DNA molecules. We call this covalent, irreversible binding of protein to DNA a DNA–protein cross-link (DPC), which is considered a type of DNA damage.
The first report of DPCs in living cells was in 1962, when researchers found that the extractability of bacterial DNA from these cells after ultraviolet irradiation decreased in a dose-dependent manner [1]. It was realized later that DPCs can be induced by a lot exogenous and endogenous agents, such as ionizing radiation, ultraviolet light, metals and metalloids, aldehyde, and chemotherapeutic drugs [2–5]. These agents induce DPCs via distinct chemical mechanisms, resulting in various types of DPCs [2]. These covalently DNA-bound proteins pose a physical challenge to all types of DNA transactions and are therefore harmful to cells. Thus, knowing how DPCs form in different situations, the consequences of DPCs, how cells deal with DPCs, and how we can use the underlying knowledge for cancer therapy is important.
Depending on the properties of DPCs, which are diverse, cells employ different repair pathways to deal with them. Investigators have shown that nucleotide excision repair (NER) and homologous recombination (HR) target the damaged DNA and remove DPCs with different size limits for proteins [6–11]. Direct reversal of specific DPCs by hydrolysis, chelation, and targeted enzymes like tyrosyl-DNA phosphodiesterase 1 (TDP1) and TDP2 were also reported [12]. However, repair mechanisms that target covalently bound proteins were not clear until the discovery of the proteases Wss1 in yeast and SprT-like domain at the N-terminus (SPRTN) in humans [13–18]. Wss1 and SPRTN, which is also known as C1orf124, SPARTAN, or DVC1 (DNA damage-targeting VCP p97 adaptor C1orf124), can directly degrade proteins that are covalently bound to DNA and allow other repair factors to access the damage sites. Studies have also implicated involvement of proteasomes in the degradation of the covalently bound proteins [19, 20], but the detailed mechanism of how it functions remains unclear. Herein we summarize the progress in the DPC repair field and describe how cells may employ these different repair pathways for efficient repair of DPCs.
Types of DPCs
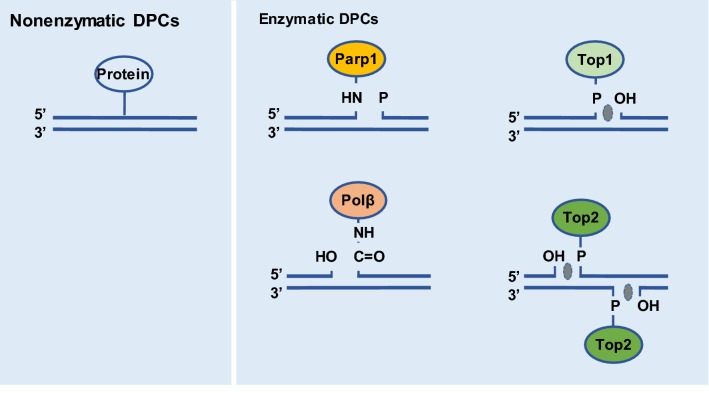
Unlike other types of DNA lesions, DPCs can be produced by any nuclear proteins that are located in the vicinity of DNA and therefore could be cross-linked with DNA [21, 22]. Based on the properties of cross-linked proteins, DPCs can be classified as enzymatic or nonenzymatic (Fig. 1) [23, 24].
Fig. 1.
DPCs can be categorized as nonenzymatic or enzymatic based on the properties of the cross-linked proteins. Any proteins located in the vicinity of DNA can result in nonspecific DPCs triggered by various agents, including reactive compounds like aldehydes, metal ions, and several types of radiation. These are defined as nonenzymatic DPCs. Also, many DNA-related enzymatic reactions produce intermediates in which transient covalent linking between DNA and the enzyme occurs. Enzymes, such as DNA TOPs, DNA polymerases, and DNA methyltransferases, can be trapped and therefore form stable DPCs under certain circumstances. These are defined as enzymatic DPCs
Enzymatic DPCs
Many DNA-related enzymatic reactions produce intermediates in which transient covalent linking of DNA with an enzyme occurs. Typically, the enzymes involved in such reactions are DNA topoisomerases (TOPs), DNA polymerases, DNA methyltransferases, DNA glycosylases, or apurinic or apyrimidinic lyases (Fig. 1) [25–27]. Generally, these intermediates are not stable, and the covalent linking can be reversed very quickly. However, under certain circumstances, such transient intermediates can be trapped, thereby forming stable DPCs. The most well-known enzymatic DPCs are the covalent links between DNA and TOPs. Specifically, TOP1 relieves the torsional stress of DNA supercoiling by cleaving on a single strand of DNA. The 3′ end of the resulting single-strand break is covalently bound to TOP1, whereas the 5′-OH end is free and can rotate around the intact DNA strand to release the torsional stress. Afterward, TOP1 catalyzes annealing of the single-strand break and is then released from DNA. However, TOP1-dependent annealing of single-strand breaks can be easily inhibited because successful ligation of the breaks can only be achieved if the two DNA ends or strands are properly aligned. This means that any distortion of the DNA structure that disturbs the alignment of DNA strands will lead to permanent trapping of TOP1 and therefore formation of a stable DPC at the site of the single-strand break. Typically, such distortion of DNA strands can be caused by nearby DNA lesions like abasic sites. Alternatively, small molecules like camptothecin and its derivatives used in chemotherapy may prevent ligation of these strands [28]. Similarly, TOP2 can be trapped in DNA and contribute to the formation of DPCs [29]. Because TOP2 induces double-strand breaks (DSBs), the TOP2-associated DPCs are generally located at the terminal ends of DSBs. Therefore, enzymatic DPCs are normally accompanied by DNA lesions, such as single-strand DNA breaks for TOP1 and DSBs for TOP2.
Nonenzymatic DPCs
Besides particular enzymes surrounding DNA strands, other proteins located in the vicinity of DNA can result in nonspecific DPCs under certain circumstance (Fig. 1). Cross-linking of proteins with DNA to form these nonenzymatic DPCs can be triggered by various agents, including reactive compounds like aldehydes, metal ions, and several types of radiation [3, 30–33]. Regarding aldehydes, formaldehyde (FA) is generated from histone demethylation [30], and acetaldehyde is a metabolic product of ethanol oxidation [34]. FA produces DPCs by forming methylene bridges between DNA bases and nucleophilic amino acid residues [30, 35, 36]. The mechanisms underlying ionizing radiation-induced DPC formation are unclear, but researchers have suggested that this kind of DPC formation has important clinical potential [37–39]. As far as we know, ionizing radiation leads to radiolysis of water molecules, which results in high levels of free radicals and reactive oxygen species in a locally restricted environment. These highly reactive species trigger multiple types of DNA lesions, including DPCs. Nonenzymatic DPCs normally involve proteins attached to undisrupted DNA strands and are therefore very different from enzymatic DPCs, especially TOP-associated DPCs.
Mechanisms of DPC repair
As stated above, DPCs are composed of DNA, protein, and cross-linked bonds of them [40] and can arise via different mechanisms, which results in diversity of any of the three DPC components. Cells likely cannot detect DPCs using highly specific sensors. Several repair pathways are reported to be involved in the repair of DPCs [12, 23, 24, 33, 40, 41]. Below we summarize these repair pathways, placing them in three categories based on the DPC components they target (Fig. 2).
Fig. 2.
DPCs are composed of DNA, protein, and their cross-linked bonds, which can be targeted by different repair pathways. NER and HR are nuclease-dependent pathways that can directly cleave DNA molecules. The chemical bond between TOP1/TOP2 and DNA can be directly hydrolyzed by TDP1 and TDP2/ZNF451. Also, proteasomes, SPRTN/Wss1, and ACRC/GCNA-1 are related to proteolysis-dependent removal of covalently bound proteins
Nuclease-dependent repair mechanisms targeting DNA molecules: NER, HR, and others
The first insight into the involvement of NER and HR in DPC repair came in early genetic studies of Escherichia coli. By characterizing the survival and mutagenic effects of DPC-inducing agents like FA and 5-aza-2′-deoxycytidine, researchers found that uvrA and recA mutants, which are defective in NER and HR, respectively, were sensitive to FA-based treatment [42, 43]. However, the recA but not the uvrA mutants were sensitive to treatment with 5-aza-2′-deoxycytidine [44, 45]. Later, several lines of biochemical and genetic evidence further demonstrated that the NER and HR pathways cooperate closely but commit differentially to DPC repair [9, 10]. NER repairs DPCs with cross-linked proteins smaller than 12–14 kDa, whereas HR mainly repairs oversized DPCs. The limitation of NER in repairing oversized proteins is determined by the loading efficiency of UvrB, which influences the incision efficiency of DNA by UvrABC complex during NER [10]. Similarly, genetic studies with yeast demonstrated the involvement of the NER and HR pathways in the repair of FA-induced DPCs, with NER having a dominant role in repair following treatment with acute high doses of FA and HR aiding repair following treatment with chronic low doses of FA [6]. NER also seems to eliminate particular types of DPCs in mammalian cells [11, 46]. However, because the size of the cross-linked protein in NER based DPC removal is limited to 8–10 kDa, employment of NER alone in repairing DPCs in vivo is limited [47]; preprocessing of the cross-linked protein by a proteasome or protease may be required.
The involvement of HR in DPC repair seems to be conserved in mammalian cells [48, 49]. Mammalian cells treated with FA accumulate DSBs and RAD51 foci and also have increased rates of sister chromatin exchange events, all of which indicate an activated HR pathway [50]. Unlike with the direct digestion of DNA around DPCs by NER, evidence of the function of HR regarding intact DPCs is lacking. The involvement of HR in repair of intact DPCs likely depends on the formation of DSBs near DPCs. One example for this is the MRE11, RAD50, and NBS1 (MRN) complex [51], which is an important nuclease complex in the initiation of resection of the HR pathway. Use of the MRN complex in resolving DNA ends correlates with its evolutionally conserved role in DPC repair [7, 52–54]. In particular, repair of antitumor agent-induced TOP-DNA cross-links in T4 bacteriophages was dependent on the MR complex (i.e., gp46/47) [52, 53]. Also, the E. coli SbcCD (MR) complex was able to nucleolytically process protein-bound DNA ends [54]. Similarly, in yeast, Mre11-deficient strains were highly sensitive to treatment with TOP inhibitors [55]. In addition, DSBs with proteins covalently bound to the 5′ termini ends generated by Spo11 during meiotic recombination were endonucleolytically cleaved by the Mre11/Rad50/Xrs2 (homologs of MRN) complex, resulting in the release of Spo11 attached to an oligonucleotide [7, 56–58]. As a note, the yeast meiotic specific protein Spo11 shares sequence homology with archaeal topoisomerase VI and reacts just like topoisomerase to generate Spo11-DNA intermediate. Similarly, biochemical analysis of Xenopus egg extracts demonstrated the cooperation of the MRN complex, CtIP, and BRCA1 in removal of Top2-DNA covalent adducts and subsequent resection of DSB ends [59]. Consistent with these observations, the MRN complex also facilitates removal of TOP2-DNA covalent adducts from mammalian cells [60, 61]. However, deletion of MRE11 in mammalian cells by small interfering RNA did not increase the total number of DPCs formed in vivo under unperturbed conditions [16], demonstrating that multiple pathways may be involved in the processing and repair of these DPCs.
The nuclease-dependent DPC repair mechanisms targeting DNA molecules are restricted by the accessibility of nucleases to substrates. Large proteins (> 8–10 kDa) can block loading of the NER repair machinery and reduce the incision efficiency of NER nucleases. Preprocessing pathways that can reduce the protein size or relax the structure of bound proteins may be needed before the NER pathway can access and repair these DPCs. Additionally, DPCs without any DNA ends cannot be recognized by an MRN-directed HR pathway. Prenucleolytic cleavage of DNA by other pathways, such as NER, may produce a substrate that can be subsequently repaired by the HR pathway. Therefore, evaluating the participation of NER and/or HR in DPC repair is critical, as their involvement in this repair may vary according to the type of DPC.
Hydrolysis of the chemical bond between proteins and DNA by TDP1 and TDP2/ZNF451
As mentioned above, the chemical bonds between proteins and DNA in DPCs are quite diverse, which makes involvement of a specific enzyme in reversing each type of covalent bonds impossible. However, some types of enzymatic DPCs occur frequently, and cells have evolved specific enzymes to induce direct hydrolysis of these chemical bonds. For example, TDP1 and TDP2 are two enzymes that can specifically reverse covalent bonds of DNA with TOP1 and TOP2, respectively [12].
Researchers first identified TDP1 in yeast based on its activity in hydrolyzing phosphotyrosyl bonds at the 3′ ends of DNA [62, 63]. Also, studies demonstrated that TDP1 repairs covalent TOP1-DPCs in vivo [63, 64]. TDP1 is conserved in eukaryotic cells, and deficiency of TDP1 confers sensitivity to TOP1 inhibitors in cells and in organisms ranging from yeast to humans [64–71]. TDP1 not only can hydrolyze 3′-tyrosine but also is active against a wide range of other 3′ DNA end-blocking adducts, such as those produced by oxidative DNA damage [12]. TDP1 functions as a monomer and processes its substrates via formation of transient covalent intermediates [72, 73]. After hydrolysis by TDP1, the DNA has a 3′-phosphate end, which must be further processed by polynucleotide kinase phosphatase to generate a 3′-hydroxyl end that can be extended by polymerases. Mutations in the TDP1 catalytic domain result in accumulation of TDP1-DNA intermediates and lead to the rare autosomal recessive neurodegenerative disease spinocerebellar ataxia with axonal neuropathy [69, 74].
Researchers discovered the function of TDP2 in repairing DPCs in a genetic screen designed to identify suppressors of camptothecin sensitivity in tdp1- and rad1-deficient yeast cells with expression of human cDNAs [75]. TDP2 exhibited prominent activity toward 5′-tyrosyl DNA ends [75, 76], and cells deficient in TDP2 were hypersensitive to treatment with TOP2 inhibitors [75–78]. Although investigators have broadly identified homologs of TDP2 in eukaryotic cells, yeast homologs have yet to be discovered. Unlike for TDP1, two divalent metals are required for TDP2′s catalytic activity, and TDP2 does not form covalent-linked intermediates [75, 79, 80]. TDP2 generates 5′-phosphate DNA ends, which can be directly processed by ligases. Homozygous mutations of the TDP2 gene were associated with spinocerebellar ataxia autosomal recessive 23, a disease characterized by intellectual disability, seizures, and ataxia [77].
Similar to the nuclease-dependent DPC repair pathways, TDP1 and TDP2 are restricted by the accessibility to substrates, which are easily buried by covalently bound proteins. Both TDP1 and TDP2 were unable to remove full-length TOP1 or TOP2 and needed prehydrolysis of these proteins by a proteasome [77, 81–84]. However, a recent study demonstrated that the small ubiquitin-related modifier (SUMO) ligase ZATT (ZNF451) can mediate direct resolution of the TOP2-DNA covalent complex (TOP2-cc) by TDP2 [85]. Researchers showed that ZNF451 can directly bind to and SUMOylate TOP2-cc, which enhances the hydrolase activity of TDP2 and promotes its efficient recruitment to damage sites [85]. Further studies are needed to identify any other mechanisms of promoting the direct hydrolytic activity of TDP1 and TDP2 toward TOP1-cc and TOP2-cc, respectively.
Proteolysis-dependent repair mechanisms targeting cross-linked proteins: proteasomes, SPRTN/Wss1, and acidic repeat-containing protein/germ cell nuclear antigen-1
Proteolysis of covalently bound proteins during DPC repair has been observed for quite some time [19, 81, 84, 86, 87] and originally attributed to the function of proteasomes. The 26S proteasome is the principle proteolytic machine for regulated protein degradation in eukaryotic cells [88, 89]. Normally, proteins are marked by polyubiquitin chains before they are recognized and degraded by proteasomes [88, 89]. Indeed, researchers observed ubiquitination of TOP1 after treating cells with TOP1 inhibitors [81, 87, 90]. Also, blockage of proteasome activity by inhibitors like MG132 and lactacystin hindered the proteolysis of TOP1-cc [81, 87, 90]. Furthermore, degradation of TOP1 was blocked when the E1 ubiquitin-activating enzyme was inactivated in ts85 cell lines [81, 87, 90]. Investigators also observed proteasome-dependent degradation for of TOP2-cc [84] and FA-induced DPCs [19]. However, deficiency of cytosolic ATP-dependent proteases in bacteria, which are the counterparts of eukaryotic proteasomes, did not affect cell survival following treatment with FA or 5-aza-2′-deoxycytidine [10]. A study using Xenopus egg extract demonstrated that inhibition of proteasome activity had no obvious effect on DPC repair in vitro, but that adding ubiquitin-vinyl sulfone, a deubiquitylation enzyme inhibitor, blocked the degradation of proteins in DPCs [91]. Moreover, adding free ubiquitin back to the reaction restored the destruction of proteins in DPCs [91]. Therefore, the authors concluded that the presence of free ubiquitin but not the activity of deubiquitylation enzymes or proteasomes is required for the repair of DPCs. These contradictory conclusions may be due to the use of proteasome inhibitors for the experiments, which not only inhibit proteasome activity but also deplete the free ubiquitin pool that may affect other ubiquitin-dependent functions. More recently, a study using an in vitro DPC repair system identified the accumulation of proteasome proteins on replicating DPC plasmids and found that proteasome-mediated degradation of polyubiquitinated DPCs requires the action of the E3 ligase TRAIP [92]. Further studies are needed to define the exact roles of proteasomes in DPC repair in vivo.
In recent years, investigators identified a more specific proteolytic pathway with the finding of Wss1 in yeast cells and SPRTN in mammalian cells. Wss1, a weak suppressor of smt3-331, is a metalloprotease that was first linked with the SUMO pathway in yeast [93, 94]. The discovery of Wss1 functions in DPC repair came in a synthetic interaction screening of a tdp1-knockout yeast strain [13]. Researchers found that co-deletion of wss1 and tdp1 led to extremely slow growth of yeast cells and hypersensitivity to camptothecin treatment, which could be relieved by deletion of Top1 [13]. Further in vitro biochemical studies showed that Wss1 can cleave the DNA-binding protein Top1, histone H1, high mobility group protein 1, and itself in a DNA-dependent manner. Cells lacking wss1 were hypersensitive to FA-based treatment. Additionally, interaction studies demonstrated that Wss1 works with Cdc48 in processing genotoxic SUMO conjugates [13, 95]. Recent report also indicated the involvement of Wss1 in DNA replication stress response [96]. They found that deletion of wss1 in yeast sensitized cells to hydroxyurea-based treatment and that further deletion of another protease, ddi1, made the cells even more sensitive to this treatment, suggesting a strong genetic interaction between wss1 and ddi1 [96, 97]. However, whether the proteolytic activity of Wss1 is required for its involvement in replication stress response has yet to be addressed.
In a bioinformatic analysis based on sequence similarity and domain organization, researchers speculated that SPRTN is a functional homolog of Wss1 [24]. Both SPRTN and Wss1 contain a protease domain with a conserved HEXXH active site and harbor the motif responsible for interaction of the protein with the segregase Cdc48 (p97 in higher eukaryotes). Moreover, both Wss1 and SPRTN contain modification-directed binding domains, a SUMO-interacting motif, or the ubiquitin interaction domain UBZ, respectively. SPRTN also harbors a proliferating cell nuclear antigen (PCNA)-interacting motif (PIP box), which directs its binding to PCNA. Indeed, more recent studies revealed a similar function of SPRTN in proteolysis of proteins on DPCs [14–18].
However, before discovery of its function in DPC repair, SPRTN was first characterized as a PCNA interacting protein involved in translesion synthesis [98–104]. SPRTN can be recruited to DNA damage sites via a PIP box and UBZ domain [98–104]. Conflicting results showed the dependence of damage-induced SPRTN localization on RAD18 and PCNA ubiquitin [100–102, 104] and the independence of this localization on them [98, 99]. Knockdown of SPRTN sensitized cells to treatment with ultraviolet radiation and increased mutagenesis during replication of ultraviolet radiation-damaged DNA [98–104]. SPRTN also interacts with VCP/p97 via the SHP domain [98–104]. Whether SPRTN promotes the recruitment of Polη to damage sites (TLS polymerase) [101, 102] or its release from damage sites [98, 99] is under debate.
Notably, biallelic germline mutations in SPRTN have caused Ruijs–Aalfs syndrome, a human autosomal recessive disorder characterized by genomic instability and early-onset hepatocellular carcinoma [105]. Also, SPRTN insufficiency in mice recapitulated some of the characteristics of human patients with Ruijs–Aalfs syndrome, such as chromosomal instability, premature aging, and early-onset age-related phenotypes [17, 106]. In vivo studies revealed that SPRTN-deficient cells are hypersensitive to treatment with DPC-inducing agents, are defective in removing DPCs, and accumulate nonspecific and TOP-involved DPCs in vivo due to defective protease activity [14–18]. In vitro biochemical assays further proved that SPRTN is a protease that can degrade histones, TOP, and itself in a DNA-dependent manner [14–18]. Studies also suggested that SPRTN travels with the replication fork and removes DPCs depending on the presence of DNA replication [16, 91]. Furthermore, the protease activity of SPRTN is tightly regulated with a switch that depends on its DNA binding, ubiquitination, and autocleavage [14–18]. Both single- and double-stranded DNA can activate the protease activity of SPRTN, with single-stranded DNA being more effective [14–16, 107]. SPRTN can be monoubiquitinated, but only unmodified SPRTN binds to chromatin [15]. Therefore, investigators proposed that DPCs somehow cause SPRTN deubiquitination, which promotes the binding of SPRTN to DNA and its activation [15]. Researchers have also observed autocleavage of SPRTN, which they proposed to be a mechanism of its tight regulation and prevention of unnecessary degradation of proteins other than DPCs on chromatin [14–16, 107]. Whether some or all of these mechanisms are involved in the regulation of SPRTN function remains to be determined.
Structure analysis showed that the catalytic centers of Wss1 and SPRTN are highly solvent-exposed and lack a substrate-binding cleft, which can explain the lack of specificity of their activity [15, 107, 108]. A recent study reported that SPRTN can degrade nonubiquitylated DPCs [92]. Thus, how SPRTN acts with VCP/p97 segregase and/or proteasomes must be investigated further.
A more recent study proposed that acidic repeat-containing (ACRC) protein is an SPRTN-related protease [41]. It contains a conserved catalytic domain just as those in Wss1 and SPRTN and is in proximity to SPRTN based on phylogenetic analysis results [41]. In a comprehensive proteomic profiling study aimed at characterizing SUMOylation response to DPC induction in human cells, researchers showed that ACRC protein interacted with a polySUMO chain and could be recruited to FA-induced foci, which was dependent on SUMOylation [109]. In addition, in Caenorhabditis elegans, the ACRC protein ortholog germ cell nuclear antigen (GCNA)-1 promoted survival after DPC induction [109]. Determining whether ACRC protein and GCNA-1 function as proteases in proteolysis of DPCs in vivo and how they may interplay with Wss1 and SPRTN requires further experimentation.
Even after proteolysis by a proteasome or Wss1/SPRTN, DPCs are not fully removed from DNA strands [91]. Small peptides are left covalently bound to the DNA, which can be further processed by NER, HR, or TDP1/TDP2. In addition, bypass of peptide-DNA conjugates may rely on the translesion synthesis pathway [91].
Conclusions
The finding of specific proteases such as Wss1 and SPRTN in direct proteolysis covalently bound proteins inspires the current working hypothesis that a specific DPC repair pathway exists in vivo. Insightful mechanistic studies of Wss1 and SPRTN may help uncover their “co-workers” in DPC repair and provide a comprehensive understanding of this specific DNA repair pathway. Questions remain about how cells choose different repair pathways, including NER, HR, TDP1/TDP2, proteasomes, and Wss1/SPRTN, for DPC repair and how these pathways may interplay with each other. Given the critical roles of DPC repair in the physiological setting as well as following treatment with many antitumor modalities, DPC repair is likely a meaningful target for cancer treatment, especially in combination with inhibition of other repair and/or checkpoint pathways.
Acknowledgements
We thank all the members of the Chen laboratory for their help and constructive discussions. We also thank Norwood Donald R in Scientific Publications, Research Medical Library at The University of Texas MD Anderson Cancer Center for help in scientific editing of the manuscript.
Abbreviations
- ACRC
acidic repeat-containing
- DPC
DNA–protein cross-link
- DSB
double-strand break
- FA
formaldehyde
- GCNA
germ cell nuclear antigen
- HR
homologous recombination
- MRN
MRE11, RAD50 and NBS1
- NER
nucleotide excision repair
- SUMO
small ubiquitin-related modifier
- SPRTN
SprT-like domain at the N terminus
- TDP
tyrosyl-DNA phosphodiesterase
- TOP
topoisomerase
- TOP2-cc
TOP2-DNA covalent complex
Authors’ contributions
HZ, YX, and JC wrote the paper. All authors read and approved the final manuscript.
Funding
This work was supported in part by a Cancer Prevention & Research Institute of Texas award (RP160667) to J.C. J.C. also received support from the NIH/NCI under award number P01CA193124, for which J.C. serves as the leader of Project 4, and award numbers CA210929, CA216911, and CA216437. In addition, J.C. is the Pamela and Wayne Garrison Distinguished Chair in Cancer Research.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Smith KC. Dose dependent decrease in extractability of DNA from bacteria following irradiation with ultraviolet light or with visible light plus dye. Biochem Biophys Res Commun. 1962;8:157–163. doi: 10.1016/0006-291X(62)90255-3. [DOI] [PubMed] [Google Scholar]
- 2.Barker S, Weinfeld M, Murray D. DNA–protein crosslinks: their induction, repair, and biological consequences. Mutat Res. 2005;589(2):111–135. doi: 10.1016/j.mrrev.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Costa M, Zhitkovich A, Gargas M, Paustenbach D, Finley B, Kuykendall J, Billings R, Carlson TJ, Wetterhahn K, Xu J, et al. Interlaboratory validation of a new assay for DNA–protein crosslinks. Mutat Res. 1996;369(1–2):13–21. doi: 10.1016/S0165-1218(96)90043-9. [DOI] [PubMed] [Google Scholar]
- 4.Zwelling LA, Anderson T, Kohn KW. DNA–protein and DNA interstrand cross-linking by cis- and trans-platinum(II) diamminedichloride in L1210 mouse leukemia cells and relation to cytotoxicity. Cancer Res. 1979;39(2 Pt 1):365–369. [PubMed] [Google Scholar]
- 5.Pommier Y. DNA topoisomerase I inhibitors: chemistry, biology, and interfacial inhibition. Chem Rev. 2009;109(7):2894–2902. doi: 10.1021/cr900097c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Graaf B, Clore A, McCullough AK. Cellular pathways for DNA repair and damage tolerance of formaldehyde-induced DNA–protein crosslinks. DNA Repair (Amst) 2009;8(10):1207–1214. doi: 10.1016/j.dnarep.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neale MJ, Pan J, Keeney S. Endonucleolytic processing of covalent protein-linked DNA double-strand breaks. Nature. 2005;436(7053):1053–1057. doi: 10.1038/nature03872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salem AM, Nakano T, Takuwa M, Matoba N, Tsuboi T, Terato H, Yamamoto K, Yamada M, Nohmi T, Ide H. Genetic analysis of repair and damage tolerance mechanisms for DNA–protein cross-links in Escherichia coli. J Bacteriol. 2009;191(18):5657–5668. doi: 10.1128/JB.00417-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minko IG, Zou Y, Lloyd RS. Incision of DNA–protein crosslinks by UvrABC nuclease suggests a potential repair pathway involving nucleotide excision repair. Proc Natl Acad Sci U S A. 2002;99(4):1905–1909. doi: 10.1073/pnas.042700399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakano T, Morishita S, Katafuchi A, Matsubara M, Horikawa Y, Terato H, Salem AM, Izumi S, Pack SP, Makino K, et al. Nucleotide excision repair and homologous recombination systems commit differentially to the repair of DNA–protein crosslinks. Mol Cell. 2007;28(1):147–158. doi: 10.1016/j.molcel.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 11.Baker DJ, Wuenschell G, Xia L, Termini J, Bates SE, Riggs AD, O’Connor TR. Nucleotide excision repair eliminates unique DNA–protein cross-links from mammalian cells. J Biol Chem. 2007;282(31):22592–22604. doi: 10.1074/jbc.M702856200. [DOI] [PubMed] [Google Scholar]
- 12.Pommier Y, Huang SY, Gao R, Das BB, Murai J, Marchand C. Tyrosyl-DNA-phosphodiesterases (TDP1 and TDP2) DNA Repair (Amst) 2014;19:114–129. doi: 10.1016/j.dnarep.2014.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stingele J, Schwarz MS, Bloemeke N, Wolf PG, Jentsch S. A DNA-dependent protease involved in DNA–protein crosslink repair. Cell. 2014;158(2):327–338. doi: 10.1016/j.cell.2014.04.053. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Mosqueda J, Maddi K, Prgomet S, Kalayil S, Marinovic-Terzic I. Terzic J. Dikic I: SPRTN is a mammalian DNA-binding metalloprotease that resolves DNA–protein crosslinks. Elife; 2016. p. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stingele J, Bellelli R, Alte F, Hewitt G, Sarek G, Maslen SL, Tsutakawa SE, Borg A, Kjaer S, Tainer JA, et al. Mechanism and regulation of DNA–protein crosslink repair by the DNA-dependent metalloprotease SPRTN. Mol Cell. 2016;64(4):688–703. doi: 10.1016/j.molcel.2016.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaz B, Popovic M, Newman JA, Fielden J, Aitkenhead H, Halder S, Singh AN, Vendrell I, Fischer R, Torrecilla I, et al. Metalloprotease SPRTN/DVC1 orchestrates replication-coupled DNA–protein crosslink repair. Mol Cell. 2016;64(4):704–719. doi: 10.1016/j.molcel.2016.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maskey RS, Flatten KS, Sieben CJ, Peterson KL, Baker DJ, Nam HJ, Kim MS, Smyrk TC, Kojima Y, Machida Y, et al. Spartan deficiency causes accumulation of topoisomerase 1 cleavage complexes and tumorigenesis. Nucleic Acids Res. 2017;45(8):4564–4576. doi: 10.1093/nar/gkx107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morocz M, Zsigmond E, Toth R, Enyedi MZ, Pinter L, Haracska L. DNA-dependent protease activity of human Spartan facilitates replication of DNA–protein crosslink-containing DNA. Nucleic Acids Res. 2017;45(6):3172–3188. doi: 10.1093/nar/gkw1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quievryn G, Zhitkovich A. Loss of DNA–protein crosslinks from formaldehyde-exposed cells occurs through spontaneous hydrolysis and an active repair process linked to proteosome function. Carcinogenesis. 2000;21(8):1573–1580. doi: 10.1093/carcin/21.8.1573. [DOI] [PubMed] [Google Scholar]
- 20.Gao R, Schellenberg MJ, Huang SY, Abdelmalak M, Marchand C, Nitiss KC, Nitiss JL, Williams RS, Pommier Y. Proteolytic degradation of topoisomerase II (Top2) enables the processing of Top2.DNA and Top2.RNA covalent complexes by tyrosyl-DNA-phosphodiesterase 2 (TDP2) J Biol Chem. 2014;289(26):17960–17969. doi: 10.1074/jbc.M114.565374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakano T, Ouchi R, Kawazoe J, Pack SP, Makino K, Ide H. T7 RNA polymerases backed up by covalently trapped proteins catalyze highly error prone transcription. J Biol Chem. 2012;287(9):6562–6572. doi: 10.1074/jbc.M111.318410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakano T, Miyamoto-Matsubara M, Shoulkamy MI, Salem AM, Pack SP, Ishimi Y, Ide H. Translocation and stability of replicative DNA helicases upon encountering DNA–protein cross-links. J Biol Chem. 2013;288(7):4649–4658. doi: 10.1074/jbc.M112.419358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaz B, Popovic M, Ramadan K. DNA–Protein crosslink proteolysis repair. Trends Biochem Sci. 2017;42(6):483–495. doi: 10.1016/j.tibs.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Stingele J, Habermann B, Jentsch S. DNA–protein crosslink repair: proteases as DNA repair enzymes. Trends Biochem Sci. 2015;40(2):67–71. doi: 10.1016/j.tibs.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Maslov AY, Lee M, Gundry M, Gravina S, Strogonova N, Tazearslan C, Bendebury A, Suh Y, Vijg J. 5-aza-2′-deoxycytidine-induced genome rearrangements are mediated by DNMT1. Oncogene. 2012;31(50):5172–5179. doi: 10.1038/onc.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, Nava Rodrigues D, Robinson D, Omlin A, Tunariu N, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373(18):1697–1708. doi: 10.1056/NEJMoa1506859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murai J, Huang SY, Das BB, Renaud A, Zhang Y, Doroshow JH, Ji J, Takeda S, Pommier Y. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. 2012;72(21):5588–5599. doi: 10.1158/0008-5472.CAN-12-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pommier Y, Marchand C. Interfacial inhibitors: targeting macromolecular complexes. Nat Rev Drug Discov. 2011;11(1):25–36. doi: 10.1038/nrd3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nitiss JL. Targeting DNA topoisomerase II in cancer chemotherapy. Nat Rev Cancer. 2009;9(5):338–350. doi: 10.1038/nrc2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA, Shi Y. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119(7):941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Costa M, Zhitkovich A, Harris M, Paustenbach D, Gargas M. DNA–protein cross-links produced by various chemicals in cultured human lymphoma cells. J Toxicol Environ Health. 1997;50(5):433–449. doi: 10.1080/00984109708984000. [DOI] [PubMed] [Google Scholar]
- 32.Xie MZ, Shoulkamy MI, Salem AM, Oba S, Goda M, Nakano T, Ide H. Aldehydes with high and low toxicities inactivate cells by damaging distinct cellular targets. Mutat Res. 2016;786:41–51. doi: 10.1016/j.mrfmmm.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Ide H, Nakano T, Salem AMH, Shoulkamy MI. DNA–protein cross-links: formidable challenges to maintaining genome integrity. DNA Repair. 2018;71:190–197. doi: 10.1016/j.dnarep.2018.08.024. [DOI] [PubMed] [Google Scholar]
- 34.Brooks PJ, Zakhari S. Acetaldehyde and the genome: beyond nuclear DNA adducts and carcinogenesis. Environ Mol Mutagen. 2014;55(2):77–91. doi: 10.1002/em.21824. [DOI] [PubMed] [Google Scholar]
- 35.Szende B, Tyihak E. Effect of formaldehyde on cell proliferation and death. Cell Biol Int. 2010;34(12):1273–1282. doi: 10.1042/CBI20100532. [DOI] [PubMed] [Google Scholar]
- 36.Lu K, Ye W, Zhou L, Collins LB, Chen X, Gold A, Ball LM, Swenberg JA. Structural characterization of formaldehyde-induced cross-links between amino acids and deoxynucleosides and their oligomers. J Am Chem Soc. 2010;132(10):3388–3399. doi: 10.1021/ja908282f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakano T, Xu X, Salem AMH, Shoulkamy MI, Ide H. Radiation-induced DNA–protein cross-links: mechanisms and biological significance. Free Radic Biol Med. 2017;107:136–145. doi: 10.1016/j.freeradbiomed.2016.11.041. [DOI] [PubMed] [Google Scholar]
- 38.Zhang H, Koch CJ, Wallen CA, Wheeler KT. Radiation-induced DNA damage in tumors and normal tissues III. Oxygen dependence of the formation of strand breaks and DNA–protein crosslinks. Radiat Res. 1995;142(2):163–168. doi: 10.2307/3579024. [DOI] [PubMed] [Google Scholar]
- 39.Fornace AJ, Jr, Little JB. DNA crosslinking induced by x-rays and chemical agents. Biochim Biophys Acta. 1977;477(4):343–355. doi: 10.1016/0005-2787(77)90253-2. [DOI] [PubMed] [Google Scholar]
- 40.Stingele J, Bellelli R, Boulton SJ. Mechanisms of DNA–protein crosslink repair. Nat Rev Mol Cell Biol. 2017;18(9):563–573. doi: 10.1038/nrm.2017.56. [DOI] [PubMed] [Google Scholar]
- 41.Fielden J, Ruggiano A, Popovic M, Ramadan K. DNA protein crosslink proteolysis repair: from yeast to premature ageing and cancer in humans. DNA Repair. 2018;71:198–204. doi: 10.1016/j.dnarep.2018.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishioka H. Lethal and mutagenic action of formaldehyde in Hcr + and Hcr − strains of Escherichia coli. Mutat Res. 1973;17(2):261–265. doi: 10.1016/0027-5107(73)90175-9. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi K, Morita T, Kawazoe Y. Mutagenic characteristics of formaldehyde on bacterial systems. Mutat Res. 1985;156(3):153–161. doi: 10.1016/0165-1218(85)90058-8. [DOI] [PubMed] [Google Scholar]
- 44.Bhagwat AS, Roberts RJ. Genetic analysis of the 5-azacytidine sensitivity of Escherichia coli K-12. J Bacteriol. 1987;169(4):1537–1546. doi: 10.1128/JB.169.4.1537-1546.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lal D, Som S, Friedman S. Survival and mutagenic effects of 5-azacytidine in Escherichia coli. Mutat Res. 1988;193(3):229–236. doi: 10.1016/0167-8817(88)90033-8. [DOI] [PubMed] [Google Scholar]
- 46.Fornace AJ., Jr Detection of DNA single-strand breaks produced during the repair of damage by DNA–protein cross-linking agents. Cancer Res. 1982;42(1):145–149. [PubMed] [Google Scholar]
- 47.Nakano T, Katafuchi A, Matsubara M, Terato H, Tsuboi T, Masuda T, Tatsumoto T, Pack SP, Makino K, Croteau DL, et al. Homologous recombination but not nucleotide excision repair plays a pivotal role in tolerance of DNA–protein cross-links in mammalian cells. J Biol Chem. 2009;284(40):27065–27076. doi: 10.1074/jbc.M109.019174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ridpath JR, Nakamura A, Tano K, Luke AM, Sonoda E, Arakawa H, Buerstedde JM, Gillespie DA, Sale JE, Yamazoe M, et al. Cells deficient in the FANC/BRCA pathway are hypersensitive to plasma levels of formaldehyde. Cancer Res. 2007;67(23):11117–11122. doi: 10.1158/0008-5472.CAN-07-3028. [DOI] [PubMed] [Google Scholar]
- 49.Orta ML, Calderon-Montano JM, Dominguez I, Pastor N, Burgos-Moron E, Lopez-Lazaro M, Cortes F, Mateos S, Helleday T. 5-Aza-2′-deoxycytidine causes replication lesions that require Fanconi anemia-dependent homologous recombination for repair. Nucleic Acids Res. 2013;41(11):5827–5836. doi: 10.1093/nar/gkt270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shaham J, Bomstein Y, Melzer A, Ribak J. DNA–protein Crosslinks and sister chromatid exchanges as biomarkers of exposure to formaldehyde. Int J Occup Environ Health. 1997;3(2):95–104. doi: 10.1179/107735297800407695. [DOI] [PubMed] [Google Scholar]
- 51.Stracker TH, Petrini JH. The MRE11 complex: starting from the ends. Nat Rev Mol Cell Biol. 2011;12(2):90–103. doi: 10.1038/nrm3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woodworth DL, Kreuzer KN. Bacteriophage T4 mutants hypersensitive to an antitumor agent that induces topoisomerase-DNA cleavage complexes. Genetics. 1996;143(3):1081–1090. doi: 10.1093/genetics/143.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stohr BA, Kreuzer KN. Repair of topoisomerase-mediated DNA damage in bacteriophage T4. Genetics. 2001;158(1):19–28. doi: 10.1093/genetics/158.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Connelly JC, de Leau ES, Leach DR. Nucleolytic processing of a protein-bound DNA end by the E. coli SbcCD (MR) complex. DNA Repair. 2003;2(7):795–807. doi: 10.1016/S1568-7864(03)00063-6. [DOI] [PubMed] [Google Scholar]
- 55.Malik M, Nitiss JL. DNA repair functions that control sensitivity to topoisomerase-targeting drugs. Eukaryot Cell. 2004;3(1):82–90. doi: 10.1128/EC.3.1.82-90.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keeney S, Giroux CN, Kleckner N. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell. 1997;88(3):375–384. doi: 10.1016/S0092-8674(00)81876-0. [DOI] [PubMed] [Google Scholar]
- 57.Bergerat A, de Massy B, Gadelle D, Varoutas PC, Nicolas A, Forterre P. An atypical topoisomerase II from Archaea with implications for meiotic recombination. Nature. 1997;386(6623):414–417. doi: 10.1038/386414a0. [DOI] [PubMed] [Google Scholar]
- 58.Rothenberg M, Kohli J, Ludin K. Ctp1 and the MRN-complex are required for endonucleolytic Rec12 removal with release of a single class of oligonucleotides in fission yeast. PLoS Genet. 2009;5(11):e1000722. doi: 10.1371/journal.pgen.1000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aparicio T, Baer R, Gottesman M, Gautier J. MRN, CtIP, and BRCA1 mediate repair of topoisomerase II-DNA adducts. J Cell Biol. 2016;212(4):399–408. doi: 10.1083/jcb.201504005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee KC, Padget K, Curtis H, Cowell IG, Moiani D, Sondka Z, Morris NJ, Jackson GH, Cockell SJ, Tainer JA, et al. MRE11 facilitates the removal of human topoisomerase II complexes from genomic DNA. Biol Open. 2012;1(9):863–873. doi: 10.1242/bio.20121834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoa NN, Shimizu T, Zhou ZW, Wang ZQ, Deshpande RA, Paull TT, Akter S, Tsuda M, Furuta R, Tsutsui K, et al. Mre11 Is essential for the removal of lethal topoisomerase 2 covalent cleavage complexes. Mol Cell. 2016;64(5):1010. doi: 10.1016/j.molcel.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 62.Yang SW, Burgin AB, Huizenga BN, Robertson CA, Yao KC, Nash HA. A eukaryotic enzyme that can disjoin dead-end covalent complexes between DNA and type I topoisomerases. Proc Natl Acad Sci U S A. 1996;93(21):11534–11539. doi: 10.1073/pnas.93.21.11534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pouliot JJ, Yao KC, Robertson CA, Nash HA. Yeast gene for a Tyr-DNA phosphodiesterase that repairs topoisomerase I complexes. Science. 1999;286(5439):552–555. doi: 10.1126/science.286.5439.552. [DOI] [PubMed] [Google Scholar]
- 64.Pouliot JJ, Robertson CA, Nash HA. Pathways for repair of topoisomerase I covalent complexes in Saccharomyces cerevisiae. Genes Cells. 2001;6(8):677–687. doi: 10.1046/j.1365-2443.2001.00452.x. [DOI] [PubMed] [Google Scholar]
- 65.Banerjee B, Roy A, Sen N, Majumder HK. A tyrosyl DNA phosphodiesterase 1 from kinetoplastid parasite Leishmania donovani (LdTdp1) capable of removing topo I-DNA covalent complexes. Mol Microbiol. 2010;78(1):119–137. doi: 10.1111/j.1365-2958.2010.07318.x. [DOI] [PubMed] [Google Scholar]
- 66.Maede Y, Shimizu H, Fukushima T, Kogame T, Nakamura T, Miki T, Takeda S, Pommier Y, Murai J. Differential and common DNA repair pathways for topoisomerase I- and II-targeted drugs in a genetic DT40 repair cell screen panel. Mol Cancer Ther. 2014;13(1):214–220. doi: 10.1158/1535-7163.MCT-13-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Katyal S, el-Khamisy SF, Russell HR, Li Y, Ju L, Caldecott KW, McKinnon PJ. TDP1 facilitates chromosomal single-strand break repair in neurons and is neuroprotective in vivo. EMBO J. 2007;26(22):4720–4731. doi: 10.1038/sj.emboj.7601869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.El-Khamisy SF, Saifi GM, Weinfeld M, Johansson F, Helleday T, Lupski JR, Caldecott KW. Defective DNA single-strand break repair in spinocerebellar ataxia with axonal neuropathy-1. Nature. 2005;434(7029):108–113. doi: 10.1038/nature03314. [DOI] [PubMed] [Google Scholar]
- 69.Interthal H, Chen HJ, Kehl-Fie TE, Zotzmann J, Leppard JB, Champoux JJ. SCAN1 mutant Tdp1 accumulates the enzyme–DNA intermediate and causes camptothecin hypersensitivity. EMBO J. 2005;24(12):2224–2233. doi: 10.1038/sj.emboj.7600694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miao ZH, Agama K, Sordet O, Povirk L, Kohn KW, Pommier Y. Hereditary ataxia SCAN1 cells are defective for the repair of transcription-dependent topoisomerase I cleavage complexes. DNA Repair. 2006;5(12):1489–1494. doi: 10.1016/j.dnarep.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 71.Gao R, Das BB, Chatterjee R, Abaan OD, Agama K, Matuo R, Vinson C, Meltzer PS, Pommier Y. Epigenetic and genetic inactivation of tyrosyl-DNA-phosphodiesterase 1 (TDP1) in human lung cancer cells from the NCI-60 panel. DNA Repair. 2014;13:1–9. doi: 10.1016/j.dnarep.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Interthal H, Pouliot JJ, Champoux JJ. The tyrosyl-DNA phosphodiesterase Tdp1 is a member of the phospholipase D superfamily. Proc Natl Acad Sci U S A. 2001;98(21):12009–12014. doi: 10.1073/pnas.211429198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Davies DR, Interthal H, Champoux JJ, Hol WG. The crystal structure of human tyrosyl-DNA phosphodiesterase, Tdp1. Structure. 2002;10(2):237–248. doi: 10.1016/S0969-2126(02)00707-4. [DOI] [PubMed] [Google Scholar]
- 74.Takashima H, Boerkoel CF, John J, Saifi GM, Salih MA, Armstrong D, Mao Y, Quiocho FA, Roa BB, Nakagawa M, et al. Mutation of TDP1, encoding a topoisomerase I-dependent DNA damage repair enzyme, in spinocerebellar ataxia with axonal neuropathy. Nat Genet. 2002;32(2):267–272. doi: 10.1038/ng987. [DOI] [PubMed] [Google Scholar]
- 75.Cortes Ledesma F, El Khamisy SF, Zuma MC, Osborn K, Caldecott KW. A human 5′-tyrosyl DNA phosphodiesterase that repairs topoisomerase-mediated DNA damage. Nature. 2009;461(7264):674–678. doi: 10.1038/nature08444. [DOI] [PubMed] [Google Scholar]
- 76.Zeng Z, Cortes-Ledesma F, El Khamisy SF, Caldecott KW. TDP2/TTRAP is the major 5′-tyrosyl DNA phosphodiesterase activity in vertebrate cells and is critical for cellular resistance to topoisomerase II-induced DNA damage. J Biol Chem. 2011;286(1):403–409. doi: 10.1074/jbc.M110.181016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gomez-Herreros F, Schuurs-Hoeijmakers JH, McCormack M, Greally MT, Rulten S, Romero-Granados R, Counihan TJ, Chaila E, Conroy J, Ennis S, et al. TDP2 protects transcription from abortive topoisomerase activity and is required for normal neural function. Nat Genet. 2014;46(5):516–521. doi: 10.1038/ng.2929. [DOI] [PubMed] [Google Scholar]
- 78.Gomez-Herreros F, Romero-Granados R, Zeng Z, Alvarez-Quilon A, Quintero C, Ju L, Umans L, Vermeire L, Huylebroeck D, Caldecott KW, et al. TDP2-dependent non-homologous end-joining protects against topoisomerase II-induced DNA breaks and genome instability in cells and in vivo. PLoS Genet. 2013;9(3):e1003226. doi: 10.1371/journal.pgen.1003226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gao R, Huang SY, Marchand C, Pommier Y. Biochemical characterization of human tyrosyl-DNA phosphodiesterase 2 (TDP2/TTRAP): a Mg(2 +)/Mn(2 +)-dependent phosphodiesterase specific for the repair of topoisomerase cleavage complexes. J Biol Chem. 2012;287(36):30842–30852. doi: 10.1074/jbc.M112.393983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Adhikari S, Karmahapatra SK, Karve TM, Bandyopadhyay S, Woodrick J, Manthena PV, Glasgow E, Byers S, Saha T, Uren A. Characterization of magnesium requirement of human 5′-tyrosyl DNA phosphodiesterase mediated reaction. BMC Res Notes. 2012;5:134. doi: 10.1186/1756-0500-5-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lin CP, Ban Y, Lyu YL, Desai SD, Liu LF. A ubiquitin-proteasome pathway for the repair of topoisomerase I-DNA covalent complexes. J Biol Chem. 2008;283(30):21074–21083. doi: 10.1074/jbc.M803493200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Interthal H, Champoux JJ. Effects of DNA and protein size on substrate cleavage by human tyrosyl-DNA phosphodiesterase 1. Biochem J. 2011;436(3):559–566. doi: 10.1042/BJ20101841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Debethune L, Kohlhagen G, Grandas A, Pommier Y. Processing of nucleopeptides mimicking the topoisomerase I-DNA covalent complex by tyrosyl-DNA phosphodiesterase. Nucleic Acids Res. 2002;30(5):1198–1204. doi: 10.1093/nar/30.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mao Y, Desai SD, Ting CY, Hwang JL, Liu LF. 26 S proteasome-mediated degradation of topoisomerase II cleavable complexes. J Biol Chem. 2001;276(44):40652–40658. doi: 10.1074/jbc.M104009200. [DOI] [PubMed] [Google Scholar]
- 85.Schellenberg MJ, Lieberman JA, Herrero-Ruiz A, Butler LR, Williams JG, Munoz-Cabello AM, Mueller GA, London RE, Cortes-Ledesma F, Williams RS. ZATT (ZNF451)-mediated resolution of topoisomerase 2 DNA–protein cross-links. Science. 2017;357(6358):1412–1416. doi: 10.1126/science.aam6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82(2):373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 87.Desai SD, Liu LF, Vazquez-Abad D, D’Arpa P. Ubiquitin-dependent destruction of topoisomerase I is stimulated by the antitumor drug camptothecin. J Biol Chem. 1997;272(39):24159–24164. doi: 10.1074/jbc.272.39.24159. [DOI] [PubMed] [Google Scholar]
- 88.Tanaka K. The proteasome: overview of structure and functions. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85(1):12–36. doi: 10.2183/pjab.85.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bard JAM, Goodall EA, Greene ER, Jonsson E, Dong KC, Martin A. Structure and function of the 26S proteasome. Annu Rev Biochem. 2018;87:697–724. doi: 10.1146/annurev-biochem-062917-011931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Desai SD, Li TK, Rodriguez-Bauman A, Rubin EH, Liu LF. Ubiquitin/26S proteasome-mediated degradation of topoisomerase I as a resistance mechanism to camptothecin in tumor cells. Cancer Res. 2001;61(15):5926–5932. [PubMed] [Google Scholar]
- 91.Duxin JP, Dewar JM, Yardimci H, Walter JC. Repair of a DNA–protein crosslink by replication-coupled proteolysis. Cell. 2014;159(2):346–357. doi: 10.1016/j.cell.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Larsen NB, Gao AO, Sparks JL, Gallina I, Wu RA, Mann M, Raschle M, Walter JC, Duxin JP. Replication-Coupled DNA–Protein Crosslink Repair by SPRTN and the Proteasome in Xenopus Egg Extracts. Mol Cell. 2019;73(3):574–588.e577. doi: 10.1016/j.molcel.2018.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Biggins S, Bhalla N, Chang A, Smith DL, Murray AW. Genes involved in sister chromatid separation and segregation in the budding yeast Saccharomyces cerevisiae. Genetics. 2001;159(2):453–470. doi: 10.1093/genetics/159.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mullen JR, Chen CF, Brill SJ. Wss1 is a SUMO-dependent isopeptidase that interacts genetically with the Slx5-Slx8 SUMO-targeted ubiquitin ligase. Mol Cell Biol. 2010;30(15):3737–3748. doi: 10.1128/MCB.01649-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Balakirev MY, Mullally JE, Favier A, Assard N, Sulpice E, Lindsey DF, Rulina AV, Gidrol X, Wilkinson KD. Wss1 metalloprotease partners with Cdc48/Doa1 in processing genotoxic SUMO conjugates. Elife. 2015;4:e06763. doi: 10.7554/eLife.06763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Svoboda M, Konvalinka J, Trempe JF, Grantz Saskova K. The yeast proteases Ddi1 and Wss1 are both involved in the DNA replication stress response. DNA Repair. 2019;80:45–51. doi: 10.1016/j.dnarep.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 97.Costanzo M, VanderSluis B, Koch EN, Baryshnikova A, Pons C, Tan G, Wang W, Usaj M, Hanchard J, Lee SD, et al. A global genetic interaction network maps a wiring diagram of cellular function. Science. 2016 doi: 10.1126/science.aaf1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mosbech A, Gibbs-Seymour I, Kagias K, Thorslund T, Beli P, Povlsen L, Nielsen SV, Smedegaard S, Sedgwick G, Lukas C, et al. DVC1 (C1orf124) is a DNA damage-targeting p97 adaptor that promotes ubiquitin-dependent responses to replication blocks. Nat Struct Mol Biol. 2012;19(11):1084–1092. doi: 10.1038/nsmb.2395. [DOI] [PubMed] [Google Scholar]
- 99.Davis EJ, Lachaud C, Appleton P, Macartney TJ, Nathke I, Rouse J. DVC1 (C1orf124) recruits the p97 protein segregase to sites of DNA damage. Nat Struct Mol Biol. 2012;19(11):1093–1100. doi: 10.1038/nsmb.2394. [DOI] [PubMed] [Google Scholar]
- 100.Ghosal G, Leung JW, Nair BC, Fong KW, Chen J. Proliferating cell nuclear antigen (PCNA)-binding protein C1orf124 is a regulator of translesion synthesis. J Biol Chem. 2012;287(41):34225–34233. doi: 10.1074/jbc.M112.400135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Centore RC, Yazinski SA, Tse A, Zou L. Spartan/C1orf124, a reader of PCNA ubiquitylation and a regulator of UV-induced DNA damage response. Mol Cell. 2012;46(5):625–635. doi: 10.1016/j.molcel.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Juhasz S, Balogh D, Hajdu I, Burkovics P, Villamil MA, Zhuang Z, Haracska L. Characterization of human Spartan/C1orf124, an ubiquitin-PCNA interacting regulator of DNA damage tolerance. Nucleic Acids Res. 2012;40(21):10795–10808. doi: 10.1093/nar/gks850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim MS, Machida Y, Vashisht AA, Wohlschlegel JA, Pang YP, Machida YJ. Regulation of error-prone translesion synthesis by Spartan/C1orf124. Nucleic Acids Res. 2013;41(3):1661–1668. doi: 10.1093/nar/gks1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Machida Y, Kim MS, Machida YJ. Spartan/C1orf124 is important to prevent UV-induced mutagenesis. Cell Cycle. 2012;11(18):3395–3402. doi: 10.4161/cc.21694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lessel D, Vaz B, Halder S, Lockhart PJ, Marinovic-Terzic I, Lopez-Mosqueda J, Philipp M, Sim JC, Smith KR, Oehler J, et al. Mutations in SPRTN cause early onset hepatocellular carcinoma, genomic instability and progeroid features. Nat Genet. 2014;46(11):1239–1244. doi: 10.1038/ng.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Maskey RS, Kim MS, Baker DJ, Childs B, Malureanu LA, Jeganathan KB, Machida Y, van Deursen JM, Machida YJ. Spartan deficiency causes genomic instability and progeroid phenotypes. Nat Commun. 2014;5:5744. doi: 10.1038/ncomms6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li F, Raczynska JE, Chen Z, Yu H. Structural Insight into DNA-dependent activation of human metalloprotease spartan. Cell Rep. 2019;26(12):3336–3346.e3334. doi: 10.1016/j.celrep.2019.02.082. [DOI] [PubMed] [Google Scholar]
- 108.Yang X, Li Y, Gao Z, Li Z, Xu J, Wang W, Dong Y. Structural analysis of Wss1 protein from saccharomyces cerevisiae. Sci Rep. 2017;7(1):8270. doi: 10.1038/s41598-017-08834-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Borgermann N, Ackermann L, Schwertman P, Hendriks IA, Thijssen K, Liu JC, Lans H, Nielsen ML, Mailand N. SUMOylation promotes protective responses to DNA–protein crosslinks. EMBO J. 2019 doi: 10.15252/embj.2019101496. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.