Abstract
Background
MiR-182-5p, a cancer-related microRNA (miRNA), modulates tumorigenesis and patient outcomes in various human malignances. This study interroted the clinicopathological significance and molecular mechanisms of miR-182-5p in non-small cell lung cancer (NSCLC).
Methods
The clinical significance of miR-182-5p in NSCLC subtypes was determined based on an analysis of 124 samples (lung adenocarcinomas [LUADs], n = 101; lung squamous cell carcinomas [LUSCs], n = 23) obtained from NSCLC patients and paired noncancer tissues and an analysis of data obtained from public miRNA-seq database, miRNA-chip database, and the scientific literature. The NSCLC samples (n = 124) were analyzed using the real-time quantitative polymerase chain reaction (RT-qPCR). Potential targets of miR-182-5p were identified using lists generated by miRWalk v.2.0, a comprehensive atlas of predicted and validated targets of miRNA-target interactions. Molecular events of miR-182-5p in NSCLC were unveiled based on a functional analysis of candidate targets. The association of miR-182-5p with one of the candidate target genes, homeobox A9 (HOXA9), was validated using in-house RT-qPCR and dual-luciferase reporter assays.
Results
The results of the in-house RT-qPCR assays analysis of data obtained from public miRNA-seq databases, miRNA-chip databases, and the scientific literature all supported upregulation of the expression level of miR-182-5p level in NSCLC. Moreover, the in-house RT-qPCR data supported the influence of upregulated miR-182-5p on malignant progression of NSCLC. In total, 774 prospective targets of miR-182-5p were identified. These targets were mainly clustered in pathways associated with biological processes, such as axonogenesis, axonal development, and Ras protein signal transduction, as well as pathways involved in axonal guidance, melanogenesis, and longevity regulation, in multiple species. Correlation analysis of the in-house RT-qPCR data and dual-luciferase reporter assays confirmed that HOXA9 was a direct target of miR-182-5p in NSCLC.
Conclusions
The miR-182-5p expression level was upregulated in NSCLC tissues. MiR-182-5p may exert oncogenic influence on NSCLC through regulating target genes such as HOXA9.
Keywords: miR-182-5p, Non-small cell lung cancer, RT-qPCR, miRNA-seq, miRNA-chips, HOXA9
Background
According to data from the National Comprehensive Cancer Network, lung cancer (LC) is responsible for the majority of cancer-associated deaths worldwide [1]. There are two types of LC: non-small cell lung cancer (NSCLC) and small cell lung cancer [2]. Of these, NSLC is the most common and accounts for the majority cases of LC [1–8].
Although improvements in screening (i.e., diagnostic imaging and laboratory tests) and drug therapy have contributed greatly to NSCLC outcomes, the clinical outcome of NSCLC remains poor due to a lack of effective biomarkers for NSCLC [4]. At the time of diagnosis, most of patients have advanced stage disease because of atypical symptoms in the early stage of the disease [2]. Thus, NSCLC survival is poor, with 5-year survival lower than 20% [5]. Therefore, the development of novel screening and therapeutic strategies are of crucial importance for NSCLC patients.
MicroRNAs (miRNAs) are small, noncoding RNAs that regulate gene expression by binding specifically to the complimentary sequence of target mRNAs in the 3′ untranslated region (3′-UTR), thereby silencing the translation process and accelerating the degradation of target mRNAs [5, 8–16]. A number of previous studies demonstrated that miRNAs played essential roles in various cancers, including LC, via their effects on various biological events, such as differentiation, apoptosis, and proliferation, at the post-transcriptional level [9, 17–24]. Studies also reported that the miRNA miR-182-5p participated in the occurrence and progression of various human cancers [25–30]. In previous work, we demonstrated a tumor-promoting effect of upregulated miR-182-5p in lung squamous cell carcinomas (LUSCs) [31].
The aim of the present study was to examine the clinicopathological value and molecular mechanisms of miR-182-5p in non-small cell lung cancer (NSCLC). With this aim in mind, we examined miR-182-5p overexpression patterns in lung adenocarcinomas (LUADs) and NSCLC.
We expect that the current study will facilitate understanding of the role of miR-182-5p in the pathogenesis of NSCLC and its potential value as a marker of NSCLC subtypes.
Methods
Tissue collection from NSCLC patients
NSCLC tissue samples and paired noncancer tissue samples were obtained from 124 NSCLS patients (LUADs, n = 101; LUSCs, n = 23) undergoing surgery at the First Affiliated Hospital of Guangxi Medical University between January 2012 and February 2014. All the NSCLC cases were diagnosed by two independent pathologists with no involvement in the study. There were 74 males and 50 females in this study.
The study was approved by the ethics committee of the First Affiliated Hospital of Guangxi Medical University, and written informed consent was obtained from all the patients.
All 124 NSCLC tissues were formalin fixed and paraffin embedded for subsequent experiments.
In-house real-time quantitative polymerase chain reaction (RT-qPCR)
Isolation and normalization of RNA, and the RT-qPCR were carried out as previously described [31–38]. RNU6B was selected as an endogenous control and reference gene of miR-182-5p [34]. The sequences of miR-182-5p and RNU6B were as follows: miR-182-5p: UUUGGCAAUGGUAGAACUCACACU (Cat. no. 4427975–002334); RNU6B: CGCAAGGAUGACACGCAAAUUCGUGAAGCGUUCCAUAUUUUU (Cat. no. 4427975–000490). The relative miR-182-5p expression level was computed using the method of 2-Δcq. The statistical analysis for the RT-qPCR was as described previously [39].
MiR-182-5p expression in NSCLC using miRNA-seq data
Level 3 IlluminaHiSeq miRNA-seq data on miR-182-5p expression in NSCLC were obtained from recomputed and normalized the cancer genome atlas (TCGA) data in UCSC Xena (https://xena.ucsc.edu/). Alterations in the expression of miR-182-5p in LUADs, non-LUADs, NSCLC, and non-NSCLC, in addition to the distribution of miR-182-5p in groups of different clinical variables, were calculated to determine the clinical significance of miR-182-5p. The statistical analysis of the miRNA-seq data has been described in detail elsewhere [39].
Analysis of miR-182-5p expression in NSCLC based on data in public miRNA-chip databases
The gene expression omnibus (GEO) database was searched for data deposited up to 12 April 2019. The search terms used were as follows: (cancer OR carcinoma OR adenocarcinoma OR tumour OR tumor OR malignanc* OR neoplas*) AND (lung OR pulmonary OR respiratory OR respiration OR aspiration OR bronchi OR bronchioles OR alveoli OR pneumocytes OR “air way”). All miRNA-chips meeting the listed inclusion criteria were considered eligible for inclusion in the study: (1) the experimental subjects were humans, and (2) miR-182-5p expression was reported for both NSCLC and healthy lung tissues. Only studies where the NSCLC sample size exceeded three and included paired healthy tissues were included. The data extraction and statistical analysis of miR-182-5p expression have been described in detail elsewhere [40].
Search of the scientific literature for data on miR-182-5p expression in NSCLC
We searched the literature for information of miR-182-5p differential expression in NSCLC and noncancer lung tissues. The following databases were searched using the keywords: (cancer OR carcinoma OR adenocarcinoma OR tumour OR tumor OR malignanc* OR neoplas*) AND (Lung OR pulmonary OR respiratory OR respiration OR aspiration OR bronchi OR bronchioles OR alveoli OR pneumocytes OR “air way”) AND (miR-182 OR miRNA-182 OR microRNA-182 OR miR182 OR miRNA182 OR microRNA182 OR “miR 182” OR “miRNA 182” OR “microRNA 182”OR miR-182-5p OR miRNA-182-5p OR microRNA-182-5p): PubMed, Wiley Online Library, EBSCO, Cochrane Central Register of Controlled Trials, Web of Science, Google Scholar, Ovid, EMBASE, and LILACS. Studies that reported expression data for miR-182-5p in NSCLC subtypes and paired noncancer samples were included in a meta-analysis. The meta-analysis included all the in-house RT-qPCR, miRNA-seq, miRNA-chip, and literature data. Pooling of the standard mean difference (SMD) and creation of summary receiver operating characteristic (SROC) curves from all the included studies was done to determine the differential expression miR-182-5p and its potential utility in distinguishing NSCLC and noncancer cases. Details on the data processing and statistical analysis in the meta-analysis have been described in previous studies [40, 41].
Prediction of target genes
An online program, MiRWalk v.2.0, which incorporates 12 prediction platforms (miRanda, Microt4, miRWalk, miRDB, miRbridge, miRMap, Pictar2, miRNAMap, PITA, RNAhybrid, RNA22, and Targetscan), was used for predicting the targets of miR-182-5p. Predicted genes that appeared in at least eight of the 12 prediction platforms were regarded as candidate targets of miR-182-5p.
Functional annotation of candidate target genes of miR-182-5p and construction of a protein–protein interaction (PPI) network
Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses were conducted using the ClusterProfiler package in R software v.3.5.2 to explore the enrichment of candidate target genes in biological process, cellular component, and molecular function pathways. Items with a p < 0.05 were considered statistically significant. The top 15 significant biological process, cellular component, and molecular function terms, as well as the top 10 significant KEGG pathway terms, were visualized as a bubble plot and chord plot using the GOplot package of R software v.3.5.2. A PPI network was subsequently built using the Search Tool for the Retrieval of Interacting Genes to illustrate the interactions between target genes.
Validation of miR-182-5p targeting of HOXA9
In a previous study, we detected the expression level of HOXA9 in the same cohort of NSCLC patients using the RT-qPCR [42]. The primers for HOXA9 and the internal control: glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were as follows: 5′-GCTGAGAATGAGAGCGGC-3′ (HOXA9 forward); 5′-CAGTTCCAGGGTCTGGTGTT-3′ (HOXA9 reverse); 5-′TGCACCACCAACTGCTTA-3′ (GAPDH forward); and 5′-GGATGCAGGGATGATGTTC-3′ (GAPDH reverse). The student’s paired t test in SPSS v.22.0 was performed to compare the expression levels of HOXA9 and miR-182-5p. The correlation between HOXA9 and miR-182-5p expression was assessed using Pearson’s correlation test in GraphpadPrism v.7.0.
Data on predictive binding sites between HOXA9 and miR-182-5p were obtained from TargetScanHuman v.7.2. A dual luciferase reporter assay was performed to validate the direct target binding between HOXA9 and miR-182-5p. The 3’UTR of HOXA9 (wild type or mutation type) comprising putative miR-182-5p binding sites was cloned into a psiCHECK-2 luciferase reporter vector (Promega, USA) to generate psiCHECK-HOXA9 3′-UTRs or psiCHECK-HOXA9-mut 3′UTRs. HEK-293 T cells were co-transfected with an miR-182-5p mimic, a negative mimic control, and a reporter vector of the psiCHECK-HOXA9 3′UTR or psiCHECK-HOXA9-mut 3′UTR. After incubation for 27 h, the luciferase activity was measured using dual luciferase assay (Promega, USA) according to the manufacturer’s protocol. Luciferase activity was inferred based on the ratios of Renilla and firefly luciferase activity. Each experiment was repeated three times.
Results
Evaluation of the clinicopathological significance of miR-182-5p in NSCLC
Analysis of RT-qPCR data
The analysis of the RT-qPCR data demonstrated that miR-182-5p was significantly upregulated in LUAD tissues as compared with that in paired non-LUAD lung tissues (P < 0.001, Table 1, Additional file 1: Fig. S1). In general, miR-182-5p expression level was markedly higher in the majority of NSCLC tissues than in paired noncancer tissues (P < 0.001, Table 2, Additional file 2: Fig. S2). Overexpression of miR-182-5p in LUAD and NSCLC was strongly associated with clinical parameters including tumor size, TNM stage, and lymph node metastasis (P < 0.05, Tables 1 and 2). As shown by the ROC curve in Additional files 3 and 4: Fig. S3 and S4, miR-182-5p performed moderately well in differentiating LUAD from noncancer lung tissues and better in differentiating NSCLC tissues from noncancer lung tissues (area under the curve [AUC] = 0.68 and 0.82, respectively). Kaplan–Meier survival curves revealed insignificant difference in the survival outcomes of LUAD and NSCLC patients with low or high miR-182-5p expression (data not shown).
Table 1.
MiR-182-5p expression in LUAD data from RT-qPCR
| Clinicopathological parameters | n | Relevant expression of miR-182-5p (2−ΔCq) | |||
|---|---|---|---|---|---|
| Mean ± SD | t/F-value | p-value | |||
| Tissue | LUAD | 101 | 30.371 ± 5.475 | 14.035 | < 0.001* |
| Noncancerous | 101 | 22.908 ± 3.728 | |||
| Gender | Male | 56 | 29.923 ± 5.567 | 1.150 | 0.253 |
| Female | 45 | 28.609 ± 5.879 | |||
| Age (years) | < 60 | 41 | 30.642 ± 5.238 | 1.964 | 0.053 |
| ≥60 | 60 | 28.446 ± 5.899 | |||
| Smoke | No | 26 | 31.159 ± 5.435 | 0.602 | 0.550 |
| Yes | 18 | 30.138 ± 5.671 | |||
| Tumor size | ≤3 cm | 53 | 25.041 ± 4.651 | −13.541 | < 0.001* |
| > 3 cm | 48 | 34.082 ± 1.343 | |||
| Vascular invasion | No | 70 | 28.986 ± 5.839 | −0.927 | 0.356 |
| Yes | 31 | 30.130 ± 5.441 | |||
| TNM | I-II | 44 | 26.730 ± 5.442 | −4.377 | < 0.001* |
| III-IV | 57 | 31.350 ± 5.114 | |||
| Lymph node metastasis | No | 45 | 26.478 ± 5.243 | −5.023 | < 0.001* |
| Yes | 56 | 31.635 ± 5.036 | |||
| Pathological grading | I | 17 | 27.642 ± 5.863 | 0.915b | 0.404 |
| II | 61 | 29.753 ± 5.713 | |||
| III | 23 | 29.489 ± 5.641 | |||
LUAD: lung adenocarcinoma; SD: standard deviation
* The results were statistically significant (P < 0.05)
Table 2.
MiR-182-5p expression in NSCLC data from RT-qPCR
| Clinicopathological parameters | n | Relevant expression of miR-182-5p (2−ΔCq) | |||
|---|---|---|---|---|---|
| Mean ± SD | t/F-value | p-value | |||
| Tissue | NSCLC | 124 | 29.615 ± 5.616 | 13.979 | < 0.001* |
| Noncancerous | 124 | 22.859 ± 3.669 | |||
| Gender | Male | 74 | 30.144 ± 5.497 | 1.278 | 0.204 |
| Female | 50 | 28.833 ± 5.755 | |||
| Age (years) | < 60 | 56 | 30.913 ± 5.052 | 2.413 | 0.017* |
| ≥60 | 68 | 28.547 ± 5.864 | |||
| Smoke | No | 38 | 31.184 ± 5.185 | 0.720 | 0.474 |
| Yes | 29 | 30.237 ± 5.530 | |||
| Histological type | Adenocarcinoma | 101 | 29.337 ± 5.717 | −1.245 | 0.221 |
| Squamous carcinoma | 23 | 30.836 ± 5.086 | |||
| Tumor size | ≤3 cm | 60 | 24.995 ± 4.570 | −14.353 | < 0.001* |
| > 3 cm | 64 | 33.947 ± 1.620 | |||
| Vascular invasion | No | 90 | 29.456 ± 5.720 | −0.512 | 0.609 |
| Yes | 34 | 30.037 ± 5.391 | |||
| TNM | I-II | 54 | 27.131 ± 5.424 | −4.632 | < 0.001* |
| III-IV | 70 | 31.532 ± 5.007 | |||
| Lymph node metastasis | No | 56 | 26.621 ± 5.128 | −6.095 | < 0.001* |
| Yes | 68 | 32.081 ± 4.759 | |||
| Pathological grading | I | 17 | 27.642 ± 5.863 | 1.246a | 0.291 |
| II | 77 | 29.853 ± 5.721 | |||
| III | 30 | 30.124 ± 5.131 | |||
NSCLC: non-small cell lung cancer; SD: standard deviation
a One-way analysis of variance (ANOVA) was performed
* The results were statistically significant (P < 0.05)
Analysis of miRNA-seq data
In total, miRNA-seq data were obtained for 784 NSCLC tissues (LUAD, n = 448; LUSC, n = 336) and 89 noncancer tissues. The clinicopathological significance of miR-182-5p in LUAD and NSCLC is summarized in Table 3 and Table 4, respectively. In both LUAD and NSCLC tissues, miR-182-5p expression was markedly higher as compared with that in noncancer tissues (P < 0.001, Additional files 1 and 2: Fig. S1 and S2, Tables 3 and 4). As shown by the ROC curves in Additional files 3 and 4: Fig. S3 and S4, miR-182-5p expression appeared to distinguish LUAD and NSCLC from noncancer tissues (AUC = 0.98 and AUC = 0.96, respectively). The Kaplan–Meier curves revealed no significant relationship between miR-182-5p expression and survival of NSCLC patients and LUAD patients (data not shown). Thus, the prognostic role of miR-182-5p in NSCLC remains unclear and needs to be studied in future work.
Table 3.
MiR-182-5p expression in LUAD from miRNA-seq data
| Characteristics | n | Relevant expression of miR-182-5p (log2x) | |||
|---|---|---|---|---|---|
| Mean ± SD | t/F-value | P-value | |||
| Tissue | LUAD | 448 | 14.273 ± 0.947 | 17.368 | < 0.001* |
| Noncancerous | 45 | 11.644 ± 1.160 | |||
| Gender | Male | 209 | 14.259 ± 0.902 | −0.306 | 0.760 |
| Female | 239 | 14.286 ± 0.987 | |||
| Age(years) | ≤50 | 33 | 14.295 ± 1.205 | 0.175 | 0.862 |
| > 50 | 396 | 14.257 ± 0.932 | |||
| T | T1 + T2 | 388 | 14.294 ± 0.954 | 0.679 | 0.497 |
| T3 + T4 | 57 | 14.203 ± 0.871 | |||
| Nodes | No | 293 | 14.252 ± 0.966 | −1.025 | 0.306 |
| Yes | 146 | 14.349 ± 0.892 | |||
| Metastasis | No | 285 | 14.326 ± 0.898 | −0.366 | 0.715 |
| Yes | 19 | 14.404 ± 1.027 | |||
| Pathologic stage | I-II | 351 | 14.249 ± 0.958 | −0.810 | 0.419 |
| III-IV | 92 | 14.340 ± 0.918 | |||
| Anatomic neoplasm subdivision | L-Lower | 70 | 14.229 ± 0.825 | 0.512a | 0.727 |
| L-Upper | 108 | 14.206 ± 0.998 | |||
| R-Lower | 85 | 14.214 ± 0.940 | |||
| R-Middle | 18 | 14.281 ± 0.789 | |||
| R-Uppr | 155 | 14.351 ± 0.986 | |||
| Tumor location | Peripheral | 113 | 14.309 ± 0.940 | −0.743 | 0.459 |
| Central | 54 | 14.429 ± 1.051 | |||
LUAD: lung adenocarcinoma; SD: standard deviation
a One-way analysis of variance (ANOVA) was performed
* The results were statistically significant (P < 0.05)
Table 4.
MiR-182-5p expression in NSCLC from miRNA-seq data
| Clinicopathological feature | n | Relevant expression of miR-182-5p (log2x) | |||
|---|---|---|---|---|---|
| Mean ± SD | t/F-value | P-value | |||
| Tissue | Lung cancer | 784 | 14.332 ± 1.042 | 21.214 | < 0.001* |
| Noncancerous | 89 | 11.962 ± 0.994 | |||
| Histological type | Adenocarcinoma | 448 | 14.273 ± 0.947 | −1.785 | 0.075 |
| Squamous carcinoma | 336 | 14.411 ± 1.154 | |||
| Gender | Male | 460 | 14.367 ± 1.042 | 1.137 | 0.256 |
| Female | 324 | 14.282 ± 1.042 | |||
| Age(years) | ≤60 | 213 | 14.396 ± 1.040 | 1.169 | 0.243 |
| > 60 | 546 | 14.297 ± 1.055 | |||
| T | T1 + T2 | 654 | 14.358 ± 1.038 | 1.242 | 0.214 |
| T3 + T4 | 127 | 14.232 ± 1.051 | |||
| Nodes | No | 509 | 14.315 ± 1.050 | −1.182 | 0.238 |
| Yes | 260 | 14.408 ± 1.006 | |||
| Metastasis | No | 540 | 14.376 ± 1.041 | 0.328 | 0.743 |
| Yes | 21 | 14.302 ± 1.029 | |||
| Pathologic stage | I-II | 631 | 14.320 ± 1.063 | −0.481 | 0.631 |
| III-IV | 145 | 14.366 ± 0.962 | |||
| Anatomic organ subdivision | L-Lower | 112 | 14.285 ± 1.029 | 0.662a | 0.618 |
| L-Upper | 198 | 14.240 ± 0.995 | |||
| R-Lower | 160 | 14.390 ± 1.073 | |||
| R-Middle | 29 | 14.416 ± 1.008 | |||
| R-Uppr | 249 | 14.359 ± 1.026 | |||
| Tumor location | Peripheral | 187 | 14.342 ± 1.013 | −0.210 | 0.834 |
| Central | 162 | 14.366 ± 1.130 | |||
NSCLC: non-small cell lung cancer; SD: standard deviation
a One-way analysis of variance (ANOVA) was performed
* The results were statistically significant (P < 0.05)
Analysis of miRNA-chip data
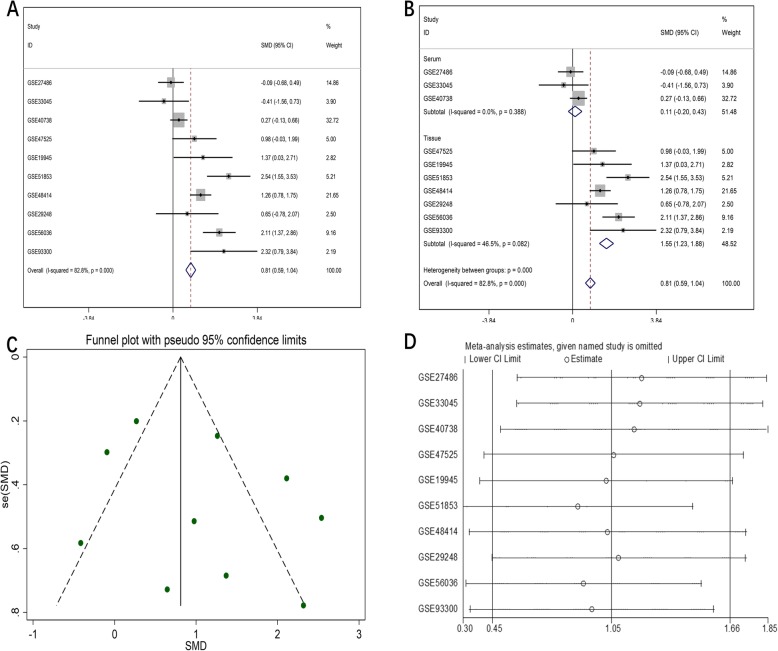
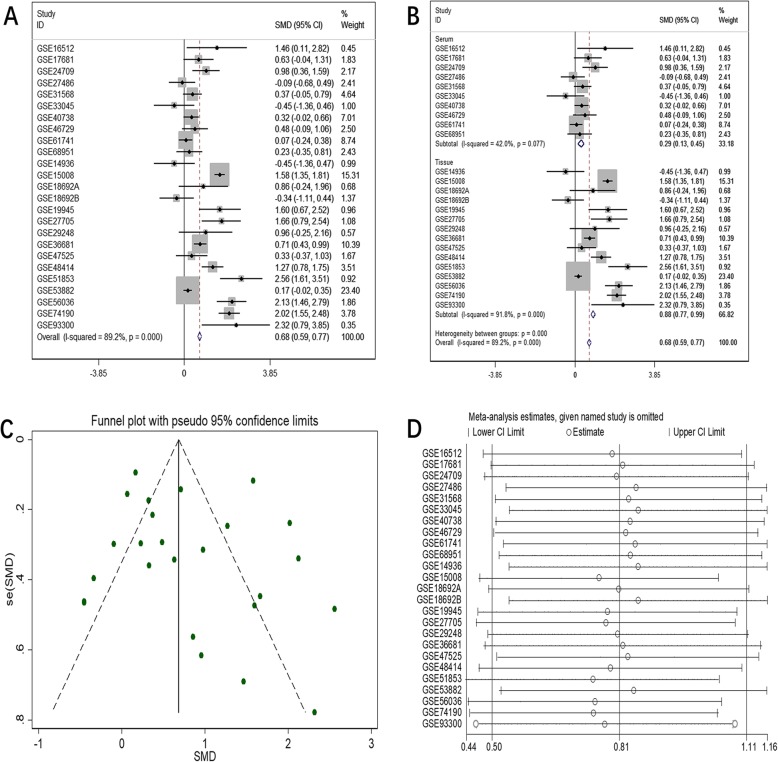
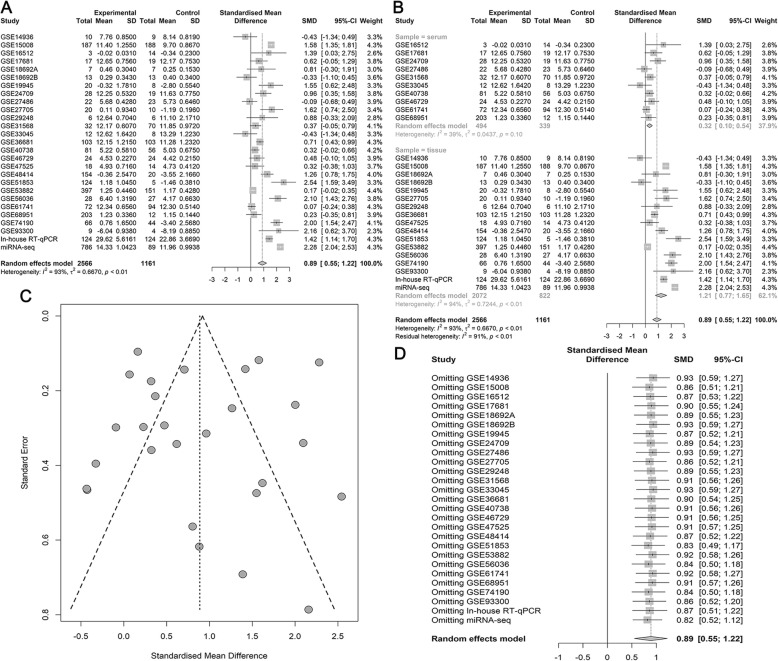
The initial search of the GEO database revealed 3204 studies. Of these, 248 studies were excluded after screening the titles and abstracts. The final analysis included data of 25 eligible miRNA-chips on 1656 NSCLC samples (LUAD, n = 350) and 948 noncancer samples. Several of the datasets that contained information on miR-182-5p expression in LUSC (GSE29248, GSE47525, GSE19945, GSE51853, and GSE74190) have been mined in previous work [31]. The characteristics of all the included miRNA-chip data are listed in Table 5. The differential expression of miR-182-5p and the discriminatory ability of miR-182-5p in distinguishing LUAD and NSCLC tissues from noncancer tissues are displayed in Additional file 1-6: Fig. S1–6. The forest plots in Figs. 1 and 2 support a noticeable increase in the miR-182-5p level in LUAD and NSCLC as compared with the level in noncancer lung samples (SMD = 0.81, 95% confidence interval [CI] = 0.59–1.04; SMD = 0.68, 95% CI = 0.59–0.77).
Table 5.
Characteristics of included GEO miRNA-chips
| First author | Experiment type | Sample type | Platform | Cancer (N) | Cancer (M) | Cancer (SD) | Noncancer (N) | Noncancer (M) | Noncancer (SD) | TP | FP | FN | TN | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GSE14936 | Seike M | Non-coding RNA profiling by array | tissue | GPL8879 | 10 | 7.763 | 0.850 | 9 | 8.137 | 0.819 | 8 | 7 | 2 | 2 |
| GSE15008 | Tan X | Non-coding RNA profiling by array | tissue | GPL2009 | 187 | 11.401 | 1.255 | 188 | 9.697 | 0.867 | 44 | 1 | 143 | 187 |
| GSE16512 | Lodes MJ | Non-coding RNA profiling by array | serum | GPL8686 | 3 | −0.024 | 0.031 | 14 | −0.338 | 0.23 | 1 | 1 | 2 | 13 |
| GSE17681 | Keller A | miRNA Profiling | serum | GPL9040 | 17 | 12.652 | 0.756 | 19 | 12.174 | 0.753 | 7 | 1 | 10 | 18 |
| GSE18692A | Puisségur MP | Non-coding RNA profiling by array | tissue | GPL4717 | 7 | 0.458 | 0.304 | 7 | 0.251 | 0.153 | 5 | 1 | 2 | 6 |
| GSE18692B | Puisségur MP | Non-coding RNA profiling by array | tissue | GPL4718 | 13 | 0.288 | 0.343 | 13 | 0.403 | 0.34 | 10 | 11 | 3 | 2 |
| GSE19945 | Ohba T | Non-coding RNA profiling by array | tissue | GPL9948 | 20 | −0.325 | 1.781 | 8 | −2.799 | 0.554 | 15 | 0 | 5 | 8 |
| GSE24709 | Andreas Keller | Non-coding RNA profiling by array | serum | GPL9040 | 28 | 12.253 | 0.532 | 19 | 11.626 | 0.775 | 17 | 0 | 11 | 19 |
| GSE27486 | Santosh Kumar Patnaik | Non-coding RNA profiling by array | serum | GPL11432 | 22 | 5.680 | 0.428 | 23 | 5.731 | 0.646 | 11 | 10 | 12 | 13 |
| GSE27705 | Chris Fenton | Non-coding RNA profiling by array | tissue | GPL11432 | 20 | 0.106 | 0.934 | 10 | −1.187 | 0.196 | 19 | 1 | 1 | 9 |
| GSE29248 | lina ma | Non-coding RNA profiling by array | tissue | GPL8179 | 6 | 12.643 | 0.704 | 6 | 11.099 | 2.171 | 3 | 0 | 3 | 6 |
| GSE31568 | Andreas Keller | Non-coding RNA profiling by array | serum | GPL9040 | 32 | 12.173 | 0.607 | 70 | 11.847 | 0.972 | 12 | 5 | 20 | 65 |
| GSE33045 | Sonia Molina-Pinelo | Expression profiling by RT-PCR | serum | GPL13987 | 12 | 12.618 | 1.642 | 8 | 13.291 | 1.223 | 12 | 7 | 0 | 1 |
| GSE36681 | Jin Sung Jang | Non-coding RNA profiling by array | tissue | GPL8179 | 103 | 12.151 | 1.215 | 103 | 11.282 | 1.232 | 16 | 1 | 87 | 102 |
| GSE40738 | Santosh Kumar Patnaik | Non-coding RNA profiling by array | serum | GPL16016 | 81 | 5.225 | 0.581 | 56 | 5.025 | 0.675 | 8 | 1 | 73 | 55 |
| GSE46729 | Zongli Xu | Non-coding RNA profiling by array | serum | GPL8786 | 24 | 4.530 | 0.227 | 24 | 4.423 | 0.215 | 5 | 1 | 19 | 23 |
| GSE47525 | Maikel Wouters | Non-coding RNA profiling by array | tissue | GPL17222 | 18 | 4.927 | 0.716 | 14 | 4.727 | 0.412 | 7 | 1 | 11 | 13 |
| GSE48414 | Maria Moksnes Bjaanæs | Non-coding RNA profiling by array | tissue | GPL16770 | 154 | −0.364 | 2.547 | 20 | −3.548 | 2.166 | 120 | 0 | 34 | 20 |
| GSE51853 | Takashi Takahashi | Non-coding RNA profiling by array | tissue | GPL7341 | 124 | 1.177 | 1.045 | 5 | −1.459 | 0.381 | 120 | 0 | 4 | 5 |
| GSE53882 | Heng-Ying Pu | Expression profiling by array | tissue | GPL18130 | 397 | 1.245 | 0.446 | 151 | 1.172 | 0.428 | 11 | 1 | 386 | 150 |
| GSE56036 | Satoshi Kondo | Non-coding RNA profiling by array | tissue | GPL15446 | 28 | 6.397 | 1.319 | 27 | 4.166 | 0.663 | 25 | 1 | 3 | 26 |
| GSE61741 | Andreas Keller | Non-coding RNA profiling by array | serum | GPL9040 | 72 | 12.342 | 0.656 | 94 | 12.302 | 0.514 | 26 | 25 | 46 | 69 |
| GSE68951 | Christina Backes | Non-coding RNA profiling by array | serum | GPL16770 | 203 | 1.225 | 0.336 | 12 | 1.149 | 0.144 | 92 | 1 | 111 | 11 |
| GSE74190 | Lu Shaohua | Non-coding RNA profiling by array | tissue | GPL19622 | 66 | 0.761 | 1.650 | 44 | −3.404 | 2.568 | 36 | 1 | 30 | 43 |
| GSE93300 | qu lili | Non-coding RNA profiling by array | tissue | GPL21576 | 9 | −6.044 | 0.938 | 4 | −8.187 | 0.885 | 8 | 0 | 1 | 4 |
Note: N: number; M: median; SD: standard deviation; TP: true positivity; FP: false positivity; FN: false negativity; TN: true negativity
Fig. 1.
Meta-analysis of miRNA-chip data for LUAD. a. Forest plot for overall SMD; b. Subgroup analysis; c. Funnel plot of publication bias; d. Sensitivity analysis
Fig. 2.
Meta-analysis of miRNA-chip data for NSCLC. a. Forest plot for overall SMD; b. Subgroup analysis; c. Funnel plot of publication bias; d. Sensitivity analysis
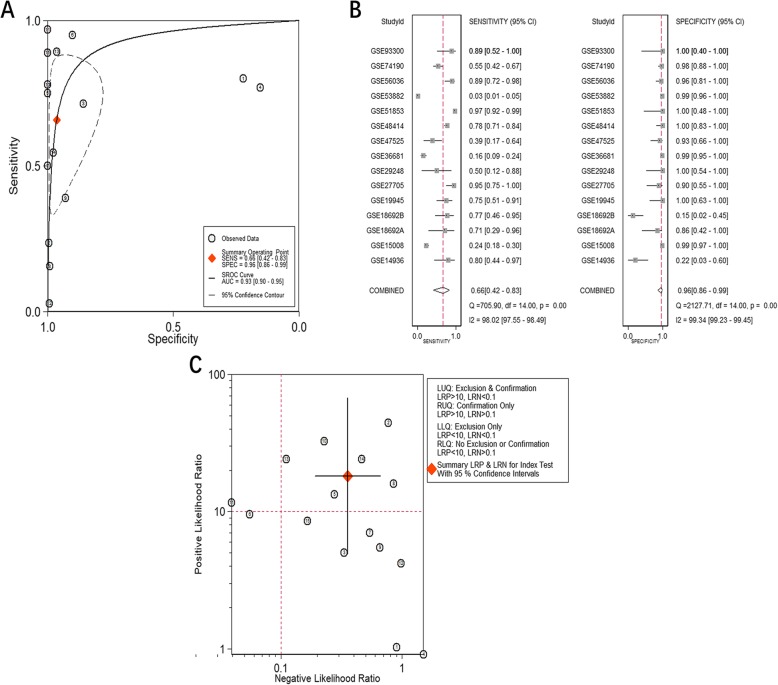
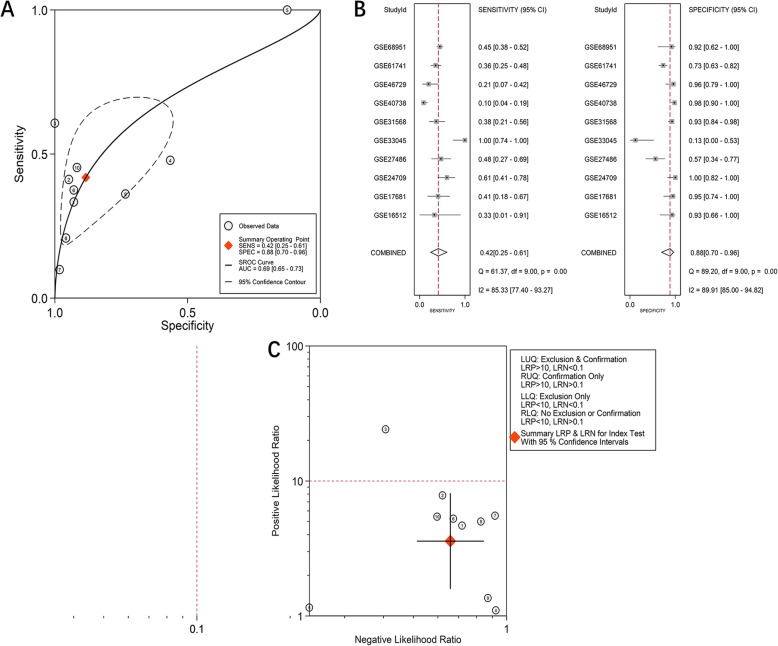
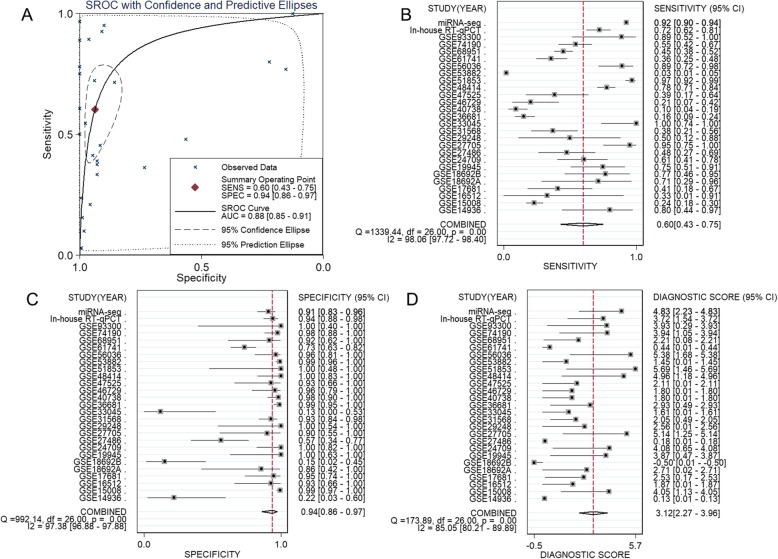
Due to obvious heterogeneity among individual studies (I2 = 82.8%, P < 0.001; I2 = 89.2%, P < 0.001), random-effect models were applied to merge the estimates. A subgroup analysis based on the source of the samples was employed to determine the origin of the heterogeneity. The pooled SMD of miR-182-5p expression in LUAD serum and tissue samples was 0.11 (− 0.20–0.43) and 1.55 (1.23–1.88), respectively (Fig. 1). For NSCLC, the pooled SMD of miR-182-5p expression in NSCLC serum and tissue samples was 0.29 (0.13–0.45) and 0.88 (0.77–0.99), respectively, suggesting that upregulation of miR-182-5p expression was more obvious in the tissue samples than in the serum samples (Fig. 2). A subsequent sensitivity analysis and test for publication bias reported no eccentric study and no publication bias (Figs. 1 and 2). SROC curves accompanied by forest plots of the sensitivity, specificity, positive likelihood ratio, and negative likelihood ratio for NSCLC suggested that miR-182-5p expression in tissue better discriminated cancerous versus noncancerous tissue than miR-182-5p expression in serum (AUC = 0.93 and AUC = 0.69, respectively; Figs. 3 and 4).
Fig. 3.
The distinguishing ability of miR-182-5p in NSCLC tissues based on data from miRNA-chips. a. SROC curves; b. Forest plot for sensitivity and specificity; c. Summary of positive likelihood ratio and negative likelihood ratio
Fig. 4.
The distinguishing value of miR-182-5p in NSCLC serum based on data from miRNA-chips. a. SROC curves; b. Forest plot for sensitivity and specificity; c. Summary of positive likelihood ratio and negative likelihood ratio
Results of the meta-analysis incorporating in-house RT-qPCR data, miRNA-seq data and miRNA-chip data
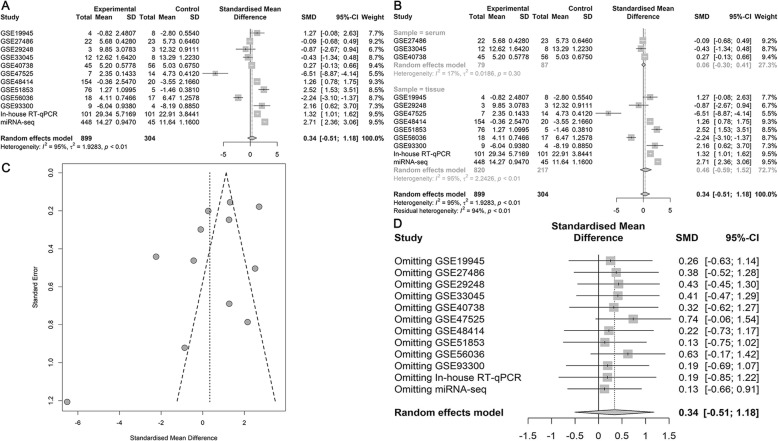
Based on the inclusion and exclusion criteria for the literature search, no studies were eligible for inclusion in the meta-analysis. Thus, the meta-analysis included 2564 NSCLC samples (LUAD, n = 899) and 1161 noncancer samples obtained from the in-house RT-qPCR, miRNA-seq, and miRNA-chip data analyses. The results of this comprehensive meta-analysis were consistent with those of the GEO meta-analysis, which confirmed upregulation of miR-182-5p in NSCLC tissues (SMD = 0.89 (0.55–1.22) Figs. 6). Upregulation of miR-182-5p expression was more apparent in the tissue samples than in the serum samples, and miR-182-5p expression in the tissue samples had stronger discriminating power in terms of cancer versus noncancer than miR-182-5p expression did in serum samples (Figs. 3, 4, 5, 6 and 7).
Fig. 6.
The comprehensive meta-analysis for miR-182-5p expression in NSCLC. a. Forest plot for overall SMD; b. Subgroup analysis; c. Funnel plot of publication bias; d. Sensitivity analysis
Fig. 5.
The comprehensive meta-analysis for miR-182-5p expression in LUAD. a. Forest plot for overall SMD; b. Subgroup analysis; c. Funnel plot of publication bias; d. Sensitivity analysis
Fig. 7.
The distinguishing power of miR-182-5p in NSCLC tissues based on data from all studies. a. SROC curves; b. Forest plot for sensitivity; c. Forest plot for specificity; d. Summary of diagnostic scores
Molecular mechanism of miR-182-5p in NSCLC
Functional annotation of candidate target genes in a PPI network
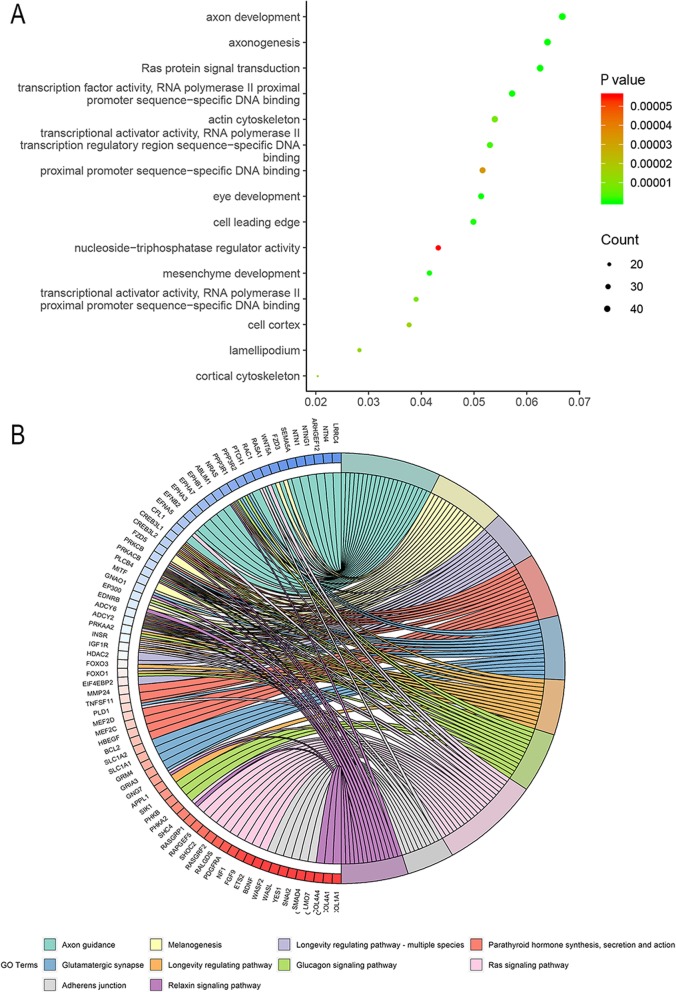
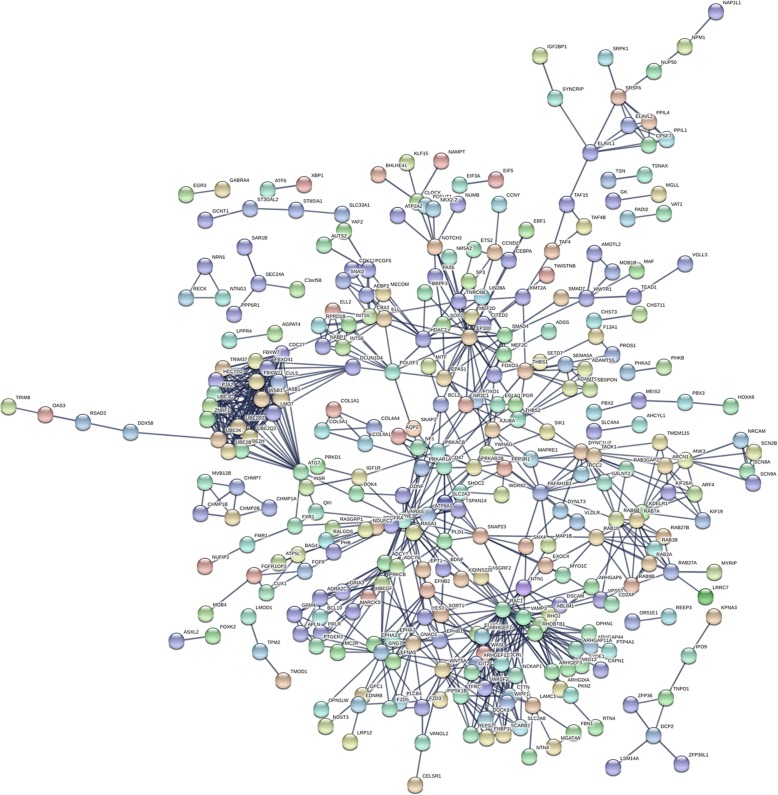
In total, 774 genes were identified as candidate target genes in eight of the 12 prediction platforms (Additional file 7). As shown in Fig. 8 and Table 6, these candidate target genes were significantly enriched in biological processes, such as axonogenesis, axonal development, and Ras protein signal transduction. According to the chord plot in Fig. 8, these target genes appeared to mainly participate in pathways involved in axonal guidance, melanogenesis, and longevity regulation in multiple species. The complicated interactions between the candidate target genes were illustrated in a PPI network (Fig. 9).
Fig. 8.
Functional enrichment analysis for candidate target genes of miR-182-5p. a. Bubble plot for gene ontology enrichment; b. Chord plot for Kyoto Encyclopedia of Genes and Genomes pathway analysis
Table 6.
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis of candidate target genes of miR-182-5p
| Category | Item | Count | P-value |
|---|---|---|---|
| GO-BP | axonogenesis | 46 | < 0.001 |
| GO-BP | axon development | 48 | < 0.001 |
| GO-BP | Ras protein signal transduction | 45 | < 0.001 |
| GO-BP | eye development | 37 | < 0.001 |
| GO-BP | mesenchyme development | 30 | < 0.001 |
| GO-CC | cell leading edge | 37 | < 0.001 |
| GO-CC | actin cytoskeleton | 40 | 0.002 |
| GO-CC | cortical cytoskeleton | 15 | 0.002 |
| GO-CC | lamellipodium | 21 | 0.002 |
| GO-CC | cell cortex | 28 | 0.002 |
| GO-MF | transcription factor activity, RNA polymerase II proximal promoter sequence-specific DNA binding | 41 | < 0.001 |
| GO-MF | transcriptional activator activity, RNA polymerase II transcription regulatory region sequence-specific DNA binding | 38 | < 0.001 |
| GO-MF | transcriptional activator activity, RNA polymerase II proximal promoter sequence-specific DNA binding | 28 | < 0.001 |
| GO-MF | proximal promoter sequence-specific DNA binding | 37 | < 0.001 |
| GO-MF | nucleoside-triphosphatase regulator activity | 31 | < 0.001 |
| KEGG | Axon guidance | 21 | < 0.001 |
| KEGG | Melanogenesis | 15 | < 0.001 |
| KEGG | Longevity regulating pathway - multiple species | 11 | < 0.001 |
| KEGG | Parathyroid hormone synthesis, secretion and action | 14 | < 0.001 |
| KEGG | Glutamatergic synapse | 14 | < 0.001 |
Note: BP: biological process; CC: cellular component; MF: molecular function
Fig. 9.
PPI network for candidate target genes of miR-182-5p. Nodes and strings in the network represented target genes and interactions between target genes
Validation of miR-182-5p targeting of HOXA9
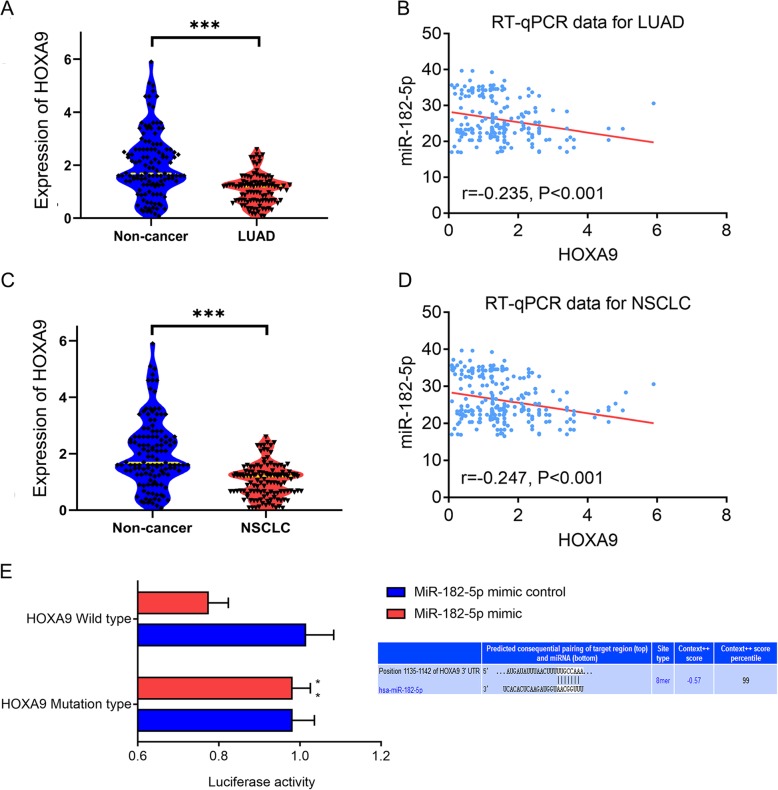
Among the candidate target genes, we selected HOXA9 and studied the relationship between it and miR-182-5p. As expected, HOXA9 was downregulated in 101 LUAD tissue samples and all 125 NSCLC tissue samples, as shown by the RT-qPCR data (P < 0.001, Figs. 10A and C). Importantly, miR-182-5p expression was negatively correlated with HOXA9 expression in 101 LUAD cases and 125 NSCLC cases (r = − 0.235, r = − 0.247, P < 0.001, Figs. 10B and D). The predictive binding sites for miR-182-5p in the 3′-UTR of HOXA9 mRNA were imported from TargetScanHuman v.7.2. (Fig. 10E). According to a luciferase reporter assay, HEK-293 T cells co-transfected with psiCHECK-2/HOXA9 3′-UTR and miR-182-5p mimics showed significantly reduced luciferase activity as compared with that in a control group (P < 0.01, Fig. 10E).
Fig. 10.
Validation of the targeting regulatory relationship between HOXA9 and miR-182-5p. a. Differential expression of HOXA9 in LUAD and noncancer tissues from RT-qPCR data; b. Correlation analysis based on in-house RT-qPCR data for miR-182-5p and HOXA9 expression in LUAD; c. Differential expression of HOXA9 in NSCLC and noncancer tissues from RT-qPCR data; d. Correlation analysis based on in-house RT-qPCR data for miR-182-5p and HOXA9 expression in NSCLC; e. Dual-luciferase reporter assay
Discussion
MiRNAs are important in the occurrence and development of LC [43–48]. Recent studies reported that dysregulation of the expression of multiple miRNAs, including miR-182-5p, was significantly correlated with tumorigenesis of LC [34]. Although several studies have demonstrated the oncogenic effect of miR-182-5p in NSCLC [49–52], interactions between miR-182-5p and target genes in NSCLC remained unclear. In particular, the molecular mechanism of miR-182-5p in NSCLC was unclear.
We previously demonstrated the oncogenic consequences of miR-182-5p in LUSCs through a combinatory analysis of data from RT-qPCR assays of 23 samples obtained from LUSC patients treated in our hospital, miRNA-seq data, and miRNA-chip data. We hypothesized that the expression pattern of miR-182-5p was similar in all the subtypes of NSCLC. In the present study, miR-182-5p was overexpressed in LUADs according to data from RT-qPCR assays of 124 samples obtained from NSCLC patients treated in our hospital, miRNA-seq data, and miRNA-chip data. Thus, we investigated the clinicopathological significance of miR-182-5p in NSCLC using in-house RT-qPCR data, miRNA-seq data, miRNA-chip data, and data in the scientific literature to explore the underlying molecular mechanism via a functional analysis of target genes.
The aforementioned data supported marked upregulation of miR-182-5p in NSCLC. Furthermore, the results of the RT-qPCR assays supported the influence of upregulated miR-182-5p on malignant clinical progression of NSCLC, which was consistent with the findings of previous studies [49–52]. It should be noted that there were some contradictions between the results of the RT-qPCR assays and those of the miRNA-seq data analysis. The discord might stem from different sources of patient cohorts and methods for calculating miR-182-5p expression. The expression of miR-182-5p in the NSCLC samples analyzed using the in-house RT-qPCR was calculated based on the 2-Δcq algorithm In contrast, miR-182-5p expression in the miRNA-seq data was log2 (total_ reads per million + 1) transformed in IlluminaHiSeq_miRNASeq platform. Nevertheless, miR-182-5p was upregulated in both the LUAD and NSCLC cohorts according to the miRNA-seq data, and miR-182-5p exhibited a trend toward elevated expression in samples from patients with malignant clinical progression of NSCLC, which was in agreement with the overall results.
The SROC curves generated from all the datasets suggested that miR-182-5p could differentiate between LUAD or NSCLC and noncancer lung tissues. We believe that the large number of LC samples (NSCLC, N = 2564; non cancer, N = 1161) included in the present study support the findings.
To yield a deeper understanding of the molecular basis of the role of miR-182-5p in the carcinogenesis of NSCLC, we carried out functional annotations for candidate target genes and created a PPI network. The results indicated that miR-182-5p may exert an oncogenic influence on NSCLC via involvement in various biological processes, such as axonogenesis, axonal development, and Ras protein signal transduction, as well as in pathways including axonal guidance, melanogenesis, and longevity regulation in multiple species. The intricate regulatory network between the candidate target genes in the PPI network indicated that cooperation or antagonism between target genes may constitute an important link in the course of NSCLC.
Among the candidate target genes, HOXA9, a member of the HOX gene family, encodes a series of transcription factors with critical roles in cancer [53]. Previous research showed that HOXA9 had oncogenic functions in hematologic cancers and anticancer effects in breast cancer and NSCLC [54, 55]. In this study, to shed light on the regulatory relationship between miR-182-5p and HOXA9, we studied the expression level of HOXA9 in NSCLC and verified the relationships between miR-182-5p and HOXA9 through a correlation analysis and dual-luciferase reporter assay. The results showed that upregulation of miR-182-5p in LUAD or NSCLC was significantly correlated with downregulation of HOXA9 in LUAD or NSCLC. The direct regulatory association between miR-182-5p and HOXA9 was confirmed by the dual-luciferase reporter assay. Based on these findings, we conclude that miR-182-5p may affect the initiation and development of NSCLC by targeting HOXA9 to diminish the tumor-inhibitory effect of HOXA9 on NSCLC.
Several limitations of this study should be acknowledged. First, we did not validate the oncogenic effect of miR-182-5p on biological events of NSCLC through in vitro or in vivo experiments. Second, this study focused on the clinicopathological significance of miR-182-5p and the miR-182-5p-centered molecular mechanism in NSCLC. Alterations in the expression of various genes, such as EGFR, ALK, ROS1, KRAS, and BRAF, play essential roles in NSCLC, and these genes serve as targets of chemotherapy [56]. We did not explore the interactions between miR-182-5p and these genes in NSCLC. Third, the diagnostic value of miR-182-5p in serum was not verified in a large clinical NSCLC sample. Exosomal miRNAs have potential as diagnostic biomarkers for cancers because of their stability, nondegradability, and ease of detection [57]. The diagnostic value of exosomal miR-182-5p in NSCLC was not studied in current work.
Conclusions
In conclusion, the oncogenic role of miR-182-5p in NSCLC was confirmed by comprehensively analyzing data obtained from RT-qPCR assays, miRNA-seq and miRNA-chip database. Multiple target genes, including HOXA9, may play a role in the molecular mechanism of HOXA9 in NSCLC.
Supplementary information
Additional file 1: Figure S1. Differential expression of miR-182-5p in LUAD and noncancer lung tissues based on data from in-house RT-qPCR, miRNA-seq and miRNA-chips. The distribution of miR-182-5p in LUAD and noncancer lung tissues was illustrated in the color of blue and red, respectively. A: GSE19945; B: GSE27486; C: GSE29248; D: GSE33045; E: GSE40738; F: GSE47525; G: GSE48414; H: GSE56036; I: GSE93300; J: GSE51853; K: in-house RT-qPCR; L: miRNA-seq
Additional file 2: Figure S2. Differential expression of miR-182-5p in NSCLC and noncancer lung tissues based on data from 9 miRNA-chips, in-house RT-qPCR and miRNA-seq. The distribution of miR-182-5p in NSCLC and noncancer lung tissues was illustrated in the color of blue and red, respectively. A: GSE29248; B: GSE56036; C: GSE93300; D: GSE53882; E: GSE18692-GPL4717; F: GSE18692-GPL4718; G: GSE27705; H: GSE33045; I: GSE36881; J: in-house RT-qPCR; K: miRNA-seq
Additional file 3: Figure S3. ROC curves for distinguishing power of miR-182-5p in LUAD based on data from in-house RT-qPCR, miRNA-seq and miRNA-chips. AUC: area under curves. An AUC value ranging from 0.1–1 indicated the increasing distinguishing power of miR-182-5p in LUAD. A: GSE19945; B: GSE27486; C: GSE29248; D: GSE33045; E: GSE40738; F: GSE47525; G: GSE48414; H: 51853; I: GSE56036; J: GSE93300; K: miRNA-seq; L: in-house RT-qPCR
Additional file 4: Figure S4. ROC curves for distinguishing power of miR-182-5p in NSCLC based on data from 9 miRNA-chips, miRNA-seq and in-house RT-qPCR. AUC: area under curves. An AUC value ranging from 0.1–1 indicated the increasing distinguishing effect of miR-182-5p in NSCLC. A: GSE56036; B: GSE93300; C: GSE53882; D: GSE18692-GPL4717; E: GSE18692-GPL4718; F: GSE27705; G: GSE33045; H: GSE36881; I: GSE24709; J: miRNA-seq; K: in-house RT-qPCR.
Additional file 5: Figure S5. Differential expression of miR-182-5p in NSCLC and noncancer lung tissues based on data from 16 miRNA-chips. The distribution of miR-182-5p in NSCLC and noncancer lung tissues was illustrated in the color of blue and red, respectively. A: GSE16612; B: GSE17681; C: GSE27486; D: GSE31668; E: GSE40738; F: GSE46729; G: GSE61741; H: GSE19945; I: GSE68951; J: GSE14936; K: GSE15008; L: GSE74190; M: GSE47525; N: GSE48414; O: GSE51853; P: GSE24709.
Additional file 6: Figure S6. ROC curves for distinguishing power of miR-182-5p in NSCLC based on data from 16 miRNA-chips. AUC: area under curves. An AUC value ranging from 0.1–1 indicated the increasing distinguishing effect of miR-182-5p in NSCLC. A: GSE16512; B: GSE17681; C: GSE27486; D: GSE31568; E: GSE40738; F: GSE46729; G: GSE61741; H: GSE68951; I: GSE14936; J: GSE15008; K: GSE19945; L: GSE47525; M: GSE48414; N: GSE51853; O: GSE74190; P: GSE29248.
Additional file 7: Table S1. Predicted target genes of hsa-miR-182-5p from miRWalk database. Putative target genes of hsa-miR-182-5p were predicted by 12 algorithms within mRNA selected regions.
Acknowledgements
We expressed sincere thanks to the patients included in this study, the target gene prediction tools (miRWalk, Microt4, miRanda, mirbridge, miRDB, miRMap, miRNAMap, Pictar2, PITA, RNA22, RNAhybrid, Targetscan, Tarbase and mirTarbase), and the TCGA, GEO and STRING databases.
Abbreviations
- AUC
area under the curve
- CI
confidence interval
- GEO
Gene Expression Omnibus
- GO
Gene Ontology
- HOXA9
homeobox A9
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- miRNA
microRNA
- NSCLC
non-small cell lung cancer
- PPI
protein–protein interaction
- RT-qPCR
real-time quantitative polymerase chain reaction
- SMD
standard mean difference
- SROC
summary receiver operating characteristic
- SROC
summary receiver operating characteristic (SROC)
- TCGA
The Cancer Genome Atlas
Author’s contributions
All authors have contributed to this study for submission. The contributions are as following: LG analyzed all experiment results, prepared for tables and figures, and revised the manuscript. SBY contributed to data analysis and paper writing. JY contributed to the conception, modification of the study and instruction of experiment and revision work. JLK contributed to the conception, modification of the study and instruction of experiment and revision work. KS contributed to the conception and modification of the study, and writing of the paper draft. FCM contributed to the conception and modification of the study, and writing of the paper draft. LZH helped to perform the RT-qPCR and analyze the RT-qPCR data. JL contributed to analyze the target genes by GO, KEGG pathway and PPI network and corrected the paper. SYY analyzed the data from RT-qPCR, GEO and TCGA database and wrote the results. RQH performed RT-qPCR, collected and analyzed the data from TCGA and GEO database and corrected the part of the results. XHH contributed to the design of the study, guided the study method, and corrected the paper. GC contributed to the design of the study, supervised all experiments and corrected the paper. All authors read and approved the final manuscript.
Funding
The study was supported by funds from National Natural Science Foundation of China (NSFC81560469, NSFC81360327), Natural Science Foundation of Guangxi, China (2015GXNSFCA139009, 2017GXNSFAA198016), Guangxi Medical University Training Program for Distinguished Young Scholars (2017) and Innovation Project of Guangxi Graduate Education (201710598080), Guangxi Degree and Postgraduate Education Reform and Development Research Projects, China (JGY2019050), Medical Excellence Award Funded by the Creative Research Development Grant from the First Affiliated Hospital of Guangxi Medical University, Science and Technology Innovation Training Program for College Students in the First Clinical Medical College of Guangxi Medical University in 2018. 2019 Guangxi Medical University Education and Teaching Reform Project (2019XJGZ04) and Guangxi Zhuang Autonomous Region Health and Family Planning Commission Self-financed Scientific Research Project (Z20180979). The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The datasets generated and/or analysed during the current study are available in the TCGA (TCGA-LUAD and TCGA-LUSC) (https://portal.gdc.cancer.gov/), GEO (GSE14936, GSE15008, GSE16512, GSE17681, GSE18692 (GPL4717 and GPL4718), GSE19945, GSE24709, GSE27486, GSE27705, GSE29248, GSE31568, GSE33045, GSE36681, GSE40738, GSE46729, GSE47525, GSE48414, GSE51853, GSE53882, GSE56036, GSE61741, GSE68951, GSE74190 and GSE93300) (https://www.ncbi.nlm.nih.gov/gds/), miRWalk (has-miR-182-5p binding site predictions within 3′-UTR region) (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/) and TargetScanHuman (TargetScan_7.2_ENST00000396345.1_predicted_targeting_details) (http://www.targetscan.org/cgi-bin/targetscan/vert_72/view_gene.cgi?rs=ENST00000396345.1&taxid=9606&members=miR-182-5p&showcnc=0&shownc=0&subset=1). Raw data of predicted target genes of miR-182-5p from miRWalk database was included in Additional file 7.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University. Written informed consent was obtained from all of the patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Li Gao, Shi-bai Yan, Xiao-hua Hu and Gang Chen contributed equally to this work.
Contributor Information
Li Gao, Email: 2339352006@qq.com.
Shi-bai Yan, Email: 522937211@qq.com.
Jie Yang, Email: yanglz2005@126.com.
Jin-liang Kong, Email: kjl071@126.com.
Ke Shi, Email: 529057611@qq.com.
Fu-chao Ma, Email: mafuchao@live.cn.
Lin-zhen Huang, Email: 944354533@qq.com.
Jie Luo, Email: 1164524563@qq.com.
Shu-ya Yin, Email: 349230442@qq.com.
Rong-quan He, Email: herongquan@gxmu.edu.cn.
Xiao-hua Hu, Email: huxiaohua@gxmu.edu.cn.
Gang Chen, Email: chengang@gxmu.edu.cn.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12920-019-0648-7.
References
- 1.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64(4):252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 2.Bai X, Meng L, Sun H, Li Z, Zhang X, Hua S. MicroRNA-196b inhibits cell growth and metastasis of Lung Cancer cells by targeting Runx2. Cell Physiol Biochem. 2017;43(2):757–767. doi: 10.1159/000481559. [DOI] [PubMed] [Google Scholar]
- 3.Yu N, Zhang Q, Liu Q, Yang J, Zhang S. A meta-analysis: microRNAs' prognostic function in patients with nonsmall cell lung cancer. Cancer Med. 2017;6(9):2098–2105. doi: 10.1002/cam4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pang L, Han S, Jiao Y, Jiang S, He X, Li P. Bu Fei decoction attenuates the tumor associated macrophage stimulated proliferation, migration, invasion and immunosuppression of non-small cell lung cancer, partially via IL-10 and PD-L1 regulation. Int J Oncol. 2017;51(1):25–38. doi: 10.3892/ijo.2017.4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Han J, Zhu H, Peng L, Chen Z. MiR181b5p mediates TGFbeta1-induced epithelial-to-mesenchymal transition in non-small cell lung cancer stem-like cells derived from lung adenocarcinoma A549 cells. Int J Oncol. 2017;51(1):158–168. doi: 10.3892/ijo.2017.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He RQ, Li XJ, Liang L, Xie Y, Luo DZ, Ma J, Peng ZG, Hu XH, Chen G. The suppressive role of miR-542-5p in NSCLC: the evidence from clinical data and in vivo validation using a chick chorioallantoic membrane model. BMC Cancer. 2017;17(1):655. doi: 10.1186/s12885-017-3646-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Q, Song Y, Zhang X, Chen GY, Zhong DS, Yu Z, Yu P, Zhang YP, Chen JH, Hu Y, et al. A multicenter survey of first-line treatment patterns and gene aberration test status of patients with unresectable stage IIIB/IV nonsquamous non-small cell lung cancer in China (CTONG 1506) BMC Cancer. 2017;17(1):462. doi: 10.1186/s12885-017-3451-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobayashi H, Tomari Y. RISC assembly: coordination between small RNAs and Argonaute proteins. Biochim Biophys Acta. 2016;1859(1):71–81. doi: 10.1016/j.bbagrm.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Goto A, Dobashi Y, Tsubochi H, Maeda D, Ooi A. MicroRNAs associated with increased AKT gene number in human lung carcinoma. Hum Pathol. 2016;56:1–10. doi: 10.1016/j.humpath.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Gu S, Lai Y, Chen H, Liu Y, Zhang Z. MiR-155 mediates arsenic trioxide resistance by activating Nrf2 and suppressing apoptosis in lung cancer cells. Sci Rep. 2017;7(1):12155. doi: 10.1038/s41598-017-06061-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yue PY, Ha WY, Lau CC, Cheung FM, Lee AW, Ng WT, Ngan RK, Yau CC, Kwong DL, Lung HL, et al. MicroRNA profiling study reveals miR-150 in association with metastasis in nasopharyngeal carcinoma. Sci Rep. 2017;7(1):12012. doi: 10.1038/s41598-017-10695-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gan CZ, Li G, Luo QS, Li HM. MiR-339-5p downregulation contributes to Taxol resistance in small-cell lung cancer by targeting alpha1,2-fucosyltransferase 1. IUBMB Life. 2017;69(11):841–849. doi: 10.1002/iub.1679. [DOI] [PubMed] [Google Scholar]
- 13.Lu C, Shan Z, Hong J, Yang L. MicroRNA-92a promotes epithelial-mesenchymal transition through activation of PTEN/PI3K/AKT signaling pathway in non-small cell lung cancer metastasis. Int J Oncol. 2017;51(1):235–244. doi: 10.3892/ijo.2017.3999. [DOI] [PubMed] [Google Scholar]
- 14.Zhao C, Lu F, Chen H, Zhao F, Zhu Z, Zhao X, Chen H. Clinical significance of circulating miRNA detection in lung cancer. Med Oncol. 2016;33(5):41. doi: 10.1007/s12032-016-0757-5. [DOI] [PubMed] [Google Scholar]
- 15.Wu Y, Song Y, Xiong Y, Wang X, Xu K, Han B, Bai Y, Li L, Zhang Y, Zhou L. MicroRNA-21 (Mir-21) promotes cell growth and invasion by repressing tumor suppressor PTEN in colorectal Cancer. Cell Physiol Biochem. 2017;43(3):945–958. doi: 10.1159/000481648. [DOI] [PubMed] [Google Scholar]
- 16.Yang X, Bai F, Xu Y, Chen Y, Chen L. Intensified Beclin-1 mediated by low expression of Mir-30a-5p promotes Chemoresistance in human small cell Lung Cancer. Cell Physiol Biochem. 2017;43(3):1126–1139. doi: 10.1159/000481754. [DOI] [PubMed] [Google Scholar]
- 17.Wong P, Hui A, Su J, Yue S, Haibe-Kains B, Gokgoz N, Xu W, Bruce J, Williams J, Catton C, et al. Prognostic microRNAs modulate the RHO adhesion pathway: a potential therapeutic target in undifferentiated pleomorphic sarcomas. Oncotarget. 2015;6(36):39127–39139. doi: 10.18632/oncotarget.3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallach S, Jantus-Lewintre E, Calabuig-Farinas S, Montaner D, Alonso S, Sirera R, Blasco A, Uso M, Guijarro R, Martorell M, et al. MicroRNA profiling associated with non-small cell lung cancer: next generation sequencing detection, experimental validation, and prognostic value. Oncotarget. 2017;8(34):56143–56157. doi: 10.18632/oncotarget.18603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halvorsen AR, Bjaanaes M, LeBlanc M, Holm AM, Bolstad N, Rubio L, Penalver JC, Cervera J, Mojarrieta JC, Lopez-Guerrero JA, et al. A unique set of 6 circulating microRNAs for early detection of non-small cell lung cancer. Oncotarget. 2016;7(24):37250–37259. doi: 10.18632/oncotarget.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan F, Mao H, Bu F, Tong X, Li J, Zhang S, Liu X, Wang L, Wu L, Chen R, et al. Sp1-mediated transcriptional activation of miR-205 promotes radioresistance in esophageal squamous cell carcinoma. Oncotarget. 2017;8(4):5735–5752. doi: 10.18632/oncotarget.13902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang LJ, Zhang KL, Zhang N, Ma XW, Yan SW, Cao DH, Shi SJ. Serum miR-26a as a diagnostic and prognostic biomarker in cholangiocarcinoma. Oncotarget. 2015;6(21):18631–18640. doi: 10.18632/oncotarget.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang CH, Wang Y, Sims M, Cai C, He P, Yue J, Cheng J, Boop FA, Pfeffer SR, Pfeffer LM. MiRNA203 suppresses the expression of protumorigenic STAT1 in glioblastoma to inhibit tumorigenesis. Oncotarget. 2016;7(51):84017–84029. doi: 10.18632/oncotarget.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang B, Zhao R, He Y, Fu X, Fu L, Zhu Z, Fu L, Dong JT. MicroRNA 100 sensitizes luminal a breast cancer cells to paclitaxel treatment in part by targeting mTOR. Oncotarget. 2016;7(5):5702–5714. doi: 10.18632/oncotarget.6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang M, Zhang L, Cui M, Ye W, Zhang P, Zhou S, Wang J. MiR-302b inhibits cancer-related inflammation by targeting ERBB4, IRF2 and CXCR4 in esophageal cancer. Oncotarget. 2017;8(30):49053–49063. doi: 10.18632/oncotarget.17041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li C, Yin Y, Liu X, Xi X, Xue W, Qu Y. Non-small cell lung cancer associated microRNA expression signature: integrated bioinformatics analysis, validation and clinical significance. Oncotarget. 2017;8(15):24564–24578. doi: 10.18632/oncotarget.15596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang W, Tian Y, Dong S, Cha Y, Li J, Guo X, Yuan X. The long non-coding RNA SNHG3 functions as a competing endogenous RNA to promote malignant development of colorectal cancer. Oncol Rep. 2017;38(3):1402–1410. doi: 10.3892/or.2017.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang K, Wang YW, Wang YY, Song Y, Zhu J, Si PC, Ma R. Identification of microRNA biomarkers in the blood of breast cancer patients based on microRNA profiling. Gene. 2017;619:10–20. doi: 10.1016/j.gene.2017.03.038. [DOI] [PubMed] [Google Scholar]
- 28.Sharifi M, Moridnia A. Apoptosis-inducing and antiproliferative effect by inhibition of miR-182-5p through the regulation of CASP9 expression in human breast cancer. Cancer Gene Ther. 2017;24(2):75–82. doi: 10.1038/cgt.2016.79. [DOI] [PubMed] [Google Scholar]
- 29.Wei Y, He R, Wu Y, Gan B, Wu P, Qiu X, Lan A, Chen G, Wang Q, Lin X, et al. Comprehensive investigation of aberrant microRNA profiling in bladder cancer tissues. Tumour Biol. 2016;37(9):12555–12569. doi: 10.1007/s13277-016-5121-z. [DOI] [PubMed] [Google Scholar]
- 30.Li CY, Liang GY, Yao WZ, Sui J, Shen X, Zhang YQ, Peng H, Hong WW, Ye YC, Zhang ZY, et al. Identification and functional characterization of microRNAs reveal a potential role in gastric cancer progression. Clin Transl Oncol. 2017;19(2):162–172. doi: 10.1007/s12094-016-1516-y. [DOI] [PubMed] [Google Scholar]
- 31.Luo J, Shi K, Yin SY, Tang RX, Chen WJ, Huang LZ, Gan TQ, Cai ZW, Chen G. Clinical value of miR-182-5p in lung squamous cell carcinoma: a study combining data from TCGA, GEO, and RT-qPCR validation. World J Surg Oncol. 2018;16(1):76. doi: 10.1186/s12957-018-1378-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen G, Kronenberger P, Teugels E, De Greve J. Influence of RT-qPCR primer position on EGFR interference efficacy in lung cancer cells. Biol Proced Online. 2010;13:1. doi: 10.1186/1480-9222-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen G, Kronenberger P, Umelo IA, Teugels E, De Greve J. Quantification of epidermal growth factor receptor T790M mutant transcripts in lung cancer cells by real-time reverse transcriptase-quantitative polymerase chain reaction. Anal Biochem. 2010;398(2):266–268. doi: 10.1016/j.ab.2009.11.034. [DOI] [PubMed] [Google Scholar]
- 34.Chen G, Umelo IA, Lv S, Teugels E, Fostier K, Kronenberger P, Dewaele A, Sadones J, Geers C, De Greve J. MiR-146a inhibits cell growth, cell migration and induces apoptosis in non-small cell lung cancer cells. PLoS One. 2013;8(3):e60317. doi: 10.1371/journal.pone.0060317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee YS, Kim H, Kim HW, Lee JC, Paik KH, Kang J, Kim J, Yoon YS, Han HS, Sohn I, et al. High expression of MicroRNA-196a indicates poor prognosis in resected pancreatic neuroendocrine tumor. Medicine. 2015;94(50):e2224. doi: 10.1097/MD.0000000000002224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang R, Zhong T, Dang Y, Zhang X, Li P, Chen G. Association between downexpression of MiR-203 and poor prognosis in non-small cell lung cancer patients. Clin Transl Oncol. 2016;18(4):360–368. doi: 10.1007/s12094-015-1377-9. [DOI] [PubMed] [Google Scholar]
- 37.Chen WJ, Gan TQ, Qin H, Huang SN, Yang LH, Fang YY, Li ZY, Pan LJ, Chen G. Implication of downregulation and prospective pathway signaling of microRNA-375 in lung squamous cell carcinoma. Pathol Res Pract. 2017;213(4):364–372. doi: 10.1016/j.prp.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 38.He R, Yang L, Lin X, Chen X, Lin X, Wei F, Liang X, Luo Y, Wu Y, Gan T, et al. MiR-30a-5p suppresses cell growth and enhances apoptosis of hepatocellular carcinoma cells via targeting AEG-1. Int J Clin Exp Pathol. 2015;8(12):15632–15641. [PMC free article] [PubMed] [Google Scholar]
- 39.He RQ, Gao L, Ma J, Li ZY, Hu XH, Chen G. Oncogenic role of miR1835p in lung adenocarcinoma: a comprehensive study of qPCR, in vitro experiments and bioinformatic analysis. Oncol Rep. 2018;40(1):83–100. doi: 10.3892/or.2018.6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao L, He RQ, Wu HY, Zhang TT, Liang HW, Ye ZH, Li ZY, Xie TT, Shi Q, Ma J, et al. Expression signature and role of miR-30d-5p in non-small cell Lung Cancer: a comprehensive study based on in Silico analysis of public databases and in vitro experiments. Cell Physiol Biochem. 2018;50(5):1964–1987. doi: 10.1159/000494875. [DOI] [PubMed] [Google Scholar]
- 41.Gao L, Li SH, Tian YX, Zhu QQ, Chen G, Pang YY, Hu XH. Role of downregulated miR-133a-3p expression in bladder cancer: a bioinformatics study. Oncol Targets Ther. 2017;10:3667–3683. doi: 10.2147/OTT.S137433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang XL, He RQ, He QC, Dang YW, Chen G. Expression of HOXA9 mRNA in non-small cell lung cancer and their clinical significance. Chin J Diagn Pathol. 2015; http://kns.cnki.net/kcms/detail/detail.aspx?FileName=ZDBL201509003&DbName=CJFQ2015.
- 43.Gao X, Wu Y, Yu W, Li H. Identification of a seven-miRNA signature as prognostic biomarker for lung squamous cell carcinoma. Oncotarget. 2016;7(49):81670–81679. doi: 10.18632/oncotarget.13164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma Y, Liang AJ, Fan YP, Huang YR, Zhao XM, Sun Y, Chen XF. Dysregulation and functional roles of miR-183-96-182 cluster in cancer cell proliferation, invasion and metastasis. Oncotarget. 2016;7(27):42805–42825. doi: 10.18632/oncotarget.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie K, Wang C, Qin N, Yang J, Zhu M, Dai J, Jin G, Shen H, Ma H, Hu Z. Genetic variants in regulatory regions of microRNAs are associated with lung cancer risk. Oncotarget. 2016;7(30):47966–47974. doi: 10.18632/oncotarget.10299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei Q, Lei R, Hu G. Roles of miR-182 in sensory organ development and cancer. Thorac Cancer. 2015;6(1):2–9. doi: 10.1111/1759-7714.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang F, Zhong S, Zhang H, Zhang W, Zhang H, Wu X, Chen B. Prognostic value of MicroRNA-182 in cancers: a meta-analysis. Dis Markers. 2015;2015:482146. doi: 10.1155/2015/482146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dejima H, Iinuma H, Kanaoka R, Matsutani N, Kawamura M. Exosomal microRNA in plasma as a non-invasive biomarker for the recurrence of non-small cell lung cancer. Oncol Lett. 2017;13(3):1256–1263. doi: 10.3892/ol.2017.5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y, Zhang H, Gong H, Yuan Y, Li Y, Wang C, Li W, Zhang Z, Liu M. Liu H, et al. J Exp Clin Cancer Res. 2018;37(1):141. doi: 10.1186/s13046-018-0824-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang H, Liu YH, Wang LL, Wang J, Zhao ZH, Qu JF, Wang SF. MiR-182 promotes cell proliferation by suppressing FBXW7 and FBXW11 in non-small cell lung cancer. Am J Transl Res. 2018;10(4):1131–1142. [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu W, Zhou K, Zha Y, Chen D, He J, Ma H, Liu X, Le H, Zhang Y. Diagnostic value of serum miR-182, miR-183, miR-210, and miR-126 levels in patients with early-stage non-small cell Lung Cancer. PLoS One. 2016;11(4):e0153046. doi: 10.1371/journal.pone.0153046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ning FL, Wang F, Li ML, Yu ZS, Hao YZ, Chen SS. MicroRNA-182 modulates chemosensitivity of human non-small cell lung cancer to cisplatin by targeting PDCD4. Diagn Pathol. 2014;9:143. doi: 10.1186/1746-1596-9-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhatlekar S, Fields JZ, Boman BM. HOX genes and their role in the development of human cancers. J Mol Med (Berl) 2014;92(8):811–823. doi: 10.1007/s00109-014-1181-y. [DOI] [PubMed] [Google Scholar]
- 54.Gilbert PM, Mouw JK, Unger MA, Lakins JN, Gbegnon MK, Clemmer VB, Benezra M, Licht JD, Boudreau NJ, Tsai KK, et al. HOXA9 regulates BRCA1 expression to modulate human breast tumor phenotype. J Clin Invest. 2010;120(5):1535–1550. doi: 10.1172/JCI39534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Son JW, Jeong KJ, Jean WS, Park SY, Jheon S, Cho HM, Park CG, Lee HY, Kang J. Genome-wide combination profiling of DNA copy number and methylation for deciphering biomarkers in non-small cell lung cancer patients. Cancer Lett. 2011;311(1):29–37. doi: 10.1016/j.canlet.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 56.Zhuang X, Zhao C, Li J, Su C, Chen X, Ren S, Li X, Zhou C. Clinical features and therapeutic options in non-small cell lung cancer patients with concomitant mutations of EGFR, ALK, ROS1. KRAS BRAF Cancer Med. 2019. 10.1002/cam4.2183. [DOI] [PMC free article] [PubMed]
- 57.Taylor DD, Gercel TC. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110(1):13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Differential expression of miR-182-5p in LUAD and noncancer lung tissues based on data from in-house RT-qPCR, miRNA-seq and miRNA-chips. The distribution of miR-182-5p in LUAD and noncancer lung tissues was illustrated in the color of blue and red, respectively. A: GSE19945; B: GSE27486; C: GSE29248; D: GSE33045; E: GSE40738; F: GSE47525; G: GSE48414; H: GSE56036; I: GSE93300; J: GSE51853; K: in-house RT-qPCR; L: miRNA-seq
Additional file 2: Figure S2. Differential expression of miR-182-5p in NSCLC and noncancer lung tissues based on data from 9 miRNA-chips, in-house RT-qPCR and miRNA-seq. The distribution of miR-182-5p in NSCLC and noncancer lung tissues was illustrated in the color of blue and red, respectively. A: GSE29248; B: GSE56036; C: GSE93300; D: GSE53882; E: GSE18692-GPL4717; F: GSE18692-GPL4718; G: GSE27705; H: GSE33045; I: GSE36881; J: in-house RT-qPCR; K: miRNA-seq
Additional file 3: Figure S3. ROC curves for distinguishing power of miR-182-5p in LUAD based on data from in-house RT-qPCR, miRNA-seq and miRNA-chips. AUC: area under curves. An AUC value ranging from 0.1–1 indicated the increasing distinguishing power of miR-182-5p in LUAD. A: GSE19945; B: GSE27486; C: GSE29248; D: GSE33045; E: GSE40738; F: GSE47525; G: GSE48414; H: 51853; I: GSE56036; J: GSE93300; K: miRNA-seq; L: in-house RT-qPCR
Additional file 4: Figure S4. ROC curves for distinguishing power of miR-182-5p in NSCLC based on data from 9 miRNA-chips, miRNA-seq and in-house RT-qPCR. AUC: area under curves. An AUC value ranging from 0.1–1 indicated the increasing distinguishing effect of miR-182-5p in NSCLC. A: GSE56036; B: GSE93300; C: GSE53882; D: GSE18692-GPL4717; E: GSE18692-GPL4718; F: GSE27705; G: GSE33045; H: GSE36881; I: GSE24709; J: miRNA-seq; K: in-house RT-qPCR.
Additional file 5: Figure S5. Differential expression of miR-182-5p in NSCLC and noncancer lung tissues based on data from 16 miRNA-chips. The distribution of miR-182-5p in NSCLC and noncancer lung tissues was illustrated in the color of blue and red, respectively. A: GSE16612; B: GSE17681; C: GSE27486; D: GSE31668; E: GSE40738; F: GSE46729; G: GSE61741; H: GSE19945; I: GSE68951; J: GSE14936; K: GSE15008; L: GSE74190; M: GSE47525; N: GSE48414; O: GSE51853; P: GSE24709.
Additional file 6: Figure S6. ROC curves for distinguishing power of miR-182-5p in NSCLC based on data from 16 miRNA-chips. AUC: area under curves. An AUC value ranging from 0.1–1 indicated the increasing distinguishing effect of miR-182-5p in NSCLC. A: GSE16512; B: GSE17681; C: GSE27486; D: GSE31568; E: GSE40738; F: GSE46729; G: GSE61741; H: GSE68951; I: GSE14936; J: GSE15008; K: GSE19945; L: GSE47525; M: GSE48414; N: GSE51853; O: GSE74190; P: GSE29248.
Additional file 7: Table S1. Predicted target genes of hsa-miR-182-5p from miRWalk database. Putative target genes of hsa-miR-182-5p were predicted by 12 algorithms within mRNA selected regions.
Data Availability Statement
The datasets generated and/or analysed during the current study are available in the TCGA (TCGA-LUAD and TCGA-LUSC) (https://portal.gdc.cancer.gov/), GEO (GSE14936, GSE15008, GSE16512, GSE17681, GSE18692 (GPL4717 and GPL4718), GSE19945, GSE24709, GSE27486, GSE27705, GSE29248, GSE31568, GSE33045, GSE36681, GSE40738, GSE46729, GSE47525, GSE48414, GSE51853, GSE53882, GSE56036, GSE61741, GSE68951, GSE74190 and GSE93300) (https://www.ncbi.nlm.nih.gov/gds/), miRWalk (has-miR-182-5p binding site predictions within 3′-UTR region) (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/) and TargetScanHuman (TargetScan_7.2_ENST00000396345.1_predicted_targeting_details) (http://www.targetscan.org/cgi-bin/targetscan/vert_72/view_gene.cgi?rs=ENST00000396345.1&taxid=9606&members=miR-182-5p&showcnc=0&shownc=0&subset=1). Raw data of predicted target genes of miR-182-5p from miRWalk database was included in Additional file 7.