Abstract
No studies have described physical therapy treatment for children with congenital Zika virus syndrome. In this case report, the authors aimed to improve postural control, mobility, and social skills in a 17- to 18-month-old child with congenital Zika virus syndrome through a period of 6-week home-based, intensive physical therapy intervention. Outcome measures were the Posture and Postural Ability Scale, Pediatric Evaluation of Disability Inventory, and Caregiver Priorities and Child Health Index of Life With Disabilities. From pre- to postintervention, the child’s Posture and Postural Ability Scale scores increased for level of postural ability in the prone position and postural alignment in all 4 positions (prone, supine, sitting, and standing). The authors saw an overall improvement in mobility and social skills from preintervention to follow-up 3 weeks after intervention. In conclusion, postural control, mobility, and social skills improved for a child with congenital Zika virus syndrome after physical therapy intervention, but future studies are required to confirm these findings.
Keywords: children, developmental disability, disability, epilepsy, pediatric, rehabilitation, treatment
Congenital Zika virus syndrome is a constellation of neurodevelopmental signs and symptoms that appear prenatally or during infancy and are caused by Zika virus disease.1 Microcephaly is the most obvious sign of congenital Zika virus syndrome and is strongly associated with cerebral palsy, intellectual disabilities, and epilepsy.2 Other manifestations include craniofacial deformity, irritability, and brain stem dysfunction including feeding difficulties, ocular abnormalities, and also findings on neuroimaging such as calcifications, cortical disorders, and ventriculomegaly.3 These signs and symptoms are associated with a general developmental impairment, and the severity seems to correlate with the timing of infection in the mother, being most severe in the first trimester.1,4
Studies have shown an extreme delay in motor development in children with congenital Zika virus syndrome.2,5-7 One study including 47 children with congenital Zika virus syndrome found that most children mastered some communication and gross motor skills at 6 to 8 months but none had age-appropriate skills at 16 months of age.8 Another study of 39 children found that their motor skills at 6, 12, and 18 months were equal to that of normal children at 2 to 3 months, 3 to 4 months, and 4 to 5 months, respectively.7 A high proportion met the criteria for the more severe levels of cerebral palsy,7 also supported by a study of 19- to 24-month-old children.6 Many children with congenital Zika virus syndrome will not be able to roll over, sit, or in some cases hold their heads up.2 A significant percentage will not be able to walk and those who learn can have challenges with gait, agility, and/or tremors during walking.2
Children with congenital Zika virus syndrome are at high risk for cognitive impairment,9 most likely to be in the profound to severe range.2 This impairs their ability for motor learning. Children with intellectual impairment need a greater number of repetitions to learn a task and they have slower response time and a more limited repertoire of motor responses.10 As a result, they learn a lesser number of things. Moreover, they have greater difficulty generalizing skills and maintaining skills that are not practiced regularly.10
For a child to acquire mobility skills, such as sitting, standing, and walking, it is critical that some basic postural prerequisites are acquired, such as functional head and trunk control in various positions. Postural control is also the basis of social skills such as looking around, maintaining gaze, and holding conversations.11,12 Unfortunately, there are no studies describing development of postural control or physical therapy interventions for children with congenital Zika virus syndrome. In children with cerebral palsy, trunk control is impaired and increasingly so with more severe levels of cerebral palsy.13 Gross motor task training is one of the interventions that has shown the best effect for improving postural control in children with cerebral palsy.12 Several studies support interventions that are intensive and that are maintained for at least 5 to 6 weeks.14-17 Home-based interventions have shown to be effective.18
The aim of this article is to describe the development of postural control, mobility, and social skills in a child with congenital Zika virus syndrome before and after a period of physical therapy intervention. This will contribute to an understanding of the motor consequences of congenital Zika virus syndrome and what type of physical therapy intervention can be offered to these children.
Case Description
This child was born in Southeast Asia. The medical report states that when 12 weeks’ pregnant, the mother was sick and developed red spots on her face. Ultrasound examination at week 22 confirmed microcephaly. At birth, gestational age was 40 weeks, weight was 2800 g, length was 49 cm, and head circumference was 28.5 cm. According to the World Health Organization child growth standards, these measures correspond to the 15th percentile in weight, the 50th percentile in length, and <3rd percentile in head circumference.19 At birth, the child’s medical condition was reported as alert, no fever, or dyspnea, oxygen saturation was measured to be 99%, and there was no skin rash. She had a systolic heart murmur, but lung function, infant reflexes, and optic nerves were considered normal.
When the child came to Norway at 4 months of age, further examinations were conducted. The examination period lasted for several months both because the family moved after first arriving to Norway and also due to the complexity of testing and analyses. Magnetic resonance imaging showed microcephaly, lissencephaly, and corpus callosum dysgenesis. Examination of vision and hearing showed normal findings. Heart examination confirmed a congenital ventricular septal defect. Further examination included blood samples of mother and child that were analyzed by a virologist. The mother tested positive for Zika virus immunoglobulin G but also for dengue virus immunoglobulin G. However, cross-reactivity to flavivirus other than Zika virus can occur some time after illness onset.20 Further blood testing of the child was conducted resulting in a positive result for Zika virus immunoglobulin G and negative for dengue virus immunoglobulin G. The level of immunoglobulin G values in the child’s blood was decreasing, and this was interpreted by the virologist that the Zika virus antibodies found in child’s blood came from the mother. Testing of the child’s cerebrospinal fluid was negative for Zika virus immunoglobulin G enzyme-linked immunosorbent assay and positive for Zika virus immunoglobulin G indirect immunofluorescence. Zika virus immunoglobulin M and polymerase chain reaction analyzed from urine were negative. The negative tests can be expected given the time elapsed from infection to testing.20,21 The conclusion that the microcephaly was caused by prenatal Zika virus infection was made by the pediatrician based on the mother’s infection in week 12, fetal microcephaly detected at ultrasound examination in week 22, abnormalities of the brain detected at magnetic resonance imaging, and blood sample analysis made by the virologist.
The child was referred to physical therapy in the municipality, and she has been treated from the age of 5 months. The child’s overall development was severely delayed. At first, all activity was restricted by severe spasticity and she had no intentional movements. She was uncomfortable, cried a lot, and feeding and sleeping were difficult. The initial physical therapy sessions were home based and focused on easing spasms, finding good positions for rest and activity and supporting the parents. She also received health-care services from other health-care professionals at the hospital and in the municipality.
At 8 months of age, she was diagnosed with epilepsy. Electroencephalogram was analyzed by neurophysiologists. The initial electroencephalogram performed at 4 months of age was difficult to analyze, as the child was uneasy and cried a lot during examination. A sleep-deprived electroencephalogram at 6 months showed multifocal epileptiform activity. This was confirmed by a 24-hour assessment at 7 months. This assessment also revealed periods of 10 to 15 seconds of sharp-wave activity. After starting on Keppra and Buccolam (when indicated) and a more nutrient-dense diet, she became more comfortable and showed increased endurance. The home-based physical therapy sessions were then carried out twice a week with the aim to increase postural control of the trunk. Activities included play and transfers in different positions, such as rolling, prone lying on the floor, and using a crawling device. She learned to move independently using this device by pushing herself forward using her feet. The next step was to introduce activities in the sitting position. Her favorite activity was to sit on a therapy ball while the physical therapist sang and moved the child forward, backward, and sideways. The occupational therapist also introduced her to manual activities while sitting in her chair. There were periods of several days where the child had seizures, constipation, sleeping difficulties, and/or infections. During these periods, the physical therapy sessions were often canceled or incomplete.
At 1 year of age, her postural control of the trunk had improved, and the aim was therefore advanced to include postural control of the head. As before, the physical therapy sessions were carried out twice a week, with periods of cancelations related to the child’s condition. The focus was on activities in sitting, standing (using a standing aid), and transfers from sitting to standing. The physical therapy also cooperated with the occupational therapist in practicing the skill of holding a toy (placed in her hand) and leading it toward her mouth. Some of the sessions were carried out together with a physical therapist from the local hospital.
At the age of 14 to 16 months, there was a period of intensive training performed by the occupational therapist, focusing on manual activities. The child then learned to hold toys and lead them to her mouth. The physical therapy sessions were then carried out only twice a month focusing on maintaining skills in sitting and standing. During this period, the child also started the medication Apodorm in addition to Keppra and Buccolam, due to increased epileptic activity.
The long-term goal was that the child should be able to walk independently with a suitable walking aid. The intervention described in this article was carried out when the child was 17- to 18 months old. The child was scheduled to start in kindergarten at 21 months, and the parents’ primary concern was her participation in physical and social settings. This the authors chose to address.
Intervention
The primary goal of the intervention was to improve postural control in various positions. Secondary goals were to improve mobility and social skills to enable participation in activities in kindergarten. The intervention was carried out for 6 weeks with home-based physical therapy sessions twice a week and activities performed by the parents 5 days a week. Both the physical therapy and parent sessions consisted of 3 activities lasting for 15 to 20 minutes in total (Table 1). The frequency and duration were chosen according to the child’s capacity and based on what has been shown to be effective in previous studies in children with cerebral palsy.16,17
Table 1.
Activities Included in the Physical Therapy and Parent Sessions for a Child With Congenital Zika Virus Syndrome.
| PT Activity 1 | PT Activity 2 | PT Activity 3/Parent Activity 1 | Parent Activity 2 | Parent Activity 3 | |
|---|---|---|---|---|---|
| Movement | Sideways and circular movements with child lying prone on therapy ball | Transfer from sitting on PT’s lap to standing in front of sofa looking in a mirror | Forward, backward, and sideway movements with child sitting on lap facing PT/parent | Standing supported against therapy ball. Transfer to prone on ball | Standing supported in standing frame |
| Purpose | Hold head up (focus on PT) and keep body stable (with support) | Hold the head through the transfer, stabile while looking into mirror | Hold the head in different positions while the body is moving | Hold the head stable to the transfer and hold head up in prone position | Hold head stable during activities motivating for the child |
| Predictable cue | Song describing different movement on verse and refrain | Movement was initiated by counting “1-2-3-up” | Song to initiate movements and make them predictable | Movement was initiated by counting “1-2-3-up” | Introducing the standing frame |
Abbreviation: PT, physical therapy.
The activities conducted by the physical therapist included supporting the child lying prone on a therapy ball performing sideways and circular movements, transferring from sitting to standing, and moving forward, backward, and sideways sitting on the physical therapy’s lap (Table 1). Parent activities included transferring from standing to prone on a therapy ball, moving forward, backward, and sideways on a therapy ball, and standing in a standing aid. The activities were a combination of familiar and new activities. The familiar activities included the third physical therapy activity and the first parent activity (Table 1). In addition, the child was familiarized with the standing aid.
All activities were gross motor activities challenging postural control. As activities that are meaningful to the child have been found to enhance neuroplasticity,14 the selection was based on what the child found amusing and the activities were incorporated into play. It was important to give the child the opportunity to initiate the movements, thereby increasing her participation. The most demanding activity was put first and the one she liked best at the end of each session to increase her motivation for the next session.
Assessments
Assessments were carried out at 3 weeks before the start of the intervention (baseline) when the child was 16 months + 1 week of age, at the start of the intervention (preintervention) at 17 months of age, at the end of the intervention (postintervention) at 18 months + 2 weeks of age, and at 3 weeks after the end of the intervention (follow-up) at 19 months + 1 week of age. Postural control was evaluated by using the Posture and Postural Ability Scale,22 which measures the level of postural ability (“quantity” of posture) and alignment (quality of posture) in the frontal and sagittal plane in 4 different positions: supine, prone, sitting, and standing. Postural ability refers to the stabilizing of body segments relative to each other and the supporting surface. The level of postural ability is assessed by 1 item, with scores ranging from 1 to 7, where higher scores indicate better postural ability. The difference between levels 1 to 2 and 3 to 7 is the ability to maintain the position with or without support.22 Postural alignment refers to the alignment of body structures in relation to each other and the surroundings. Alignment in the sagittal and frontal plane is assessed by 6 items each: head, trunk, pelvis, legs, arms, and weight distribution. Scores for each item range from 0 (asymmetry) to 1 (symmetry) giving a total alignment score ranging from 0 to 6 in each plane, where higher scores indicate better alignment.22
The Posture and Postural Ability Scale was designed to assess posture in individuals of all ages with lower levels of gross motor function who were not able to sit or walk independently. Psychometric properties have been evaluated in children with cerebral palsy and found satisfactory with regard to validity and reliability.22 However, the change in score required to indicate true change has not yet been established. The Posture and Postural Ability Scale was chosen because it was a measure familiar to the first author and it is the only clinical assessment tool designed to assess quality and “quantity” of posture separately.22
Mobility and social skills were evaluated by using the Pediatric Evaluation of Disability Inventory23 and the Caregiver Priorities and Child Health Index of Life With Disabilities.24 The Pediatric Evaluation of Disability Inventory is a parental interview which evaluates the function of disabled children aged 6 months to 7.5 years in 3 domains: self-care, mobility, and social function.23 It measures capability through identification of functional skills (197 items) and performance in terms of caregiver assistance (20 items) and modifications or adaptive equipment used (20 items). Each item is scored from 0 (incapable) to 1 (capable). The Pediatric Evaluation of Disability Inventory yields raw scores, normative standard scores, and scaled scores, the latter recommended as an outcome measure. The scaled scores range from 0 to 100 and provide an indication of performance of the child along a continuum of relatively easy (lower score) to relatively difficult (higher score) items in a domain. In this study, the authors chose to use the functional skills domains of mobility (59 items) and social function (65 items) in addition to caregiver assistance related to these domains (7 items for mobility, 5 items for social function), as this was relevant to the aims of the physical therapy intervention. According to the test developers,23 a change in more than 2 standard errors is thought to reflect a change in the child’s functional performance beyond random factors. However, some have suggested that a change as large as 11 points is needed in order to be clinically meaningful.25 For the functional skills domains of mobility and social function, the standard error varies according to the score obtained. Accordingly, 2 standard errors range from 7.4 to 23.4 points for mobility and from 9.0 to 22.1 points for social function.23
The authors used the Norwegian version of Pediatric Evaluation of Disability Inventory, which has shown high reliability.26 Due to a discrepancy in normative standard scores between Norwegian and United States children, the scaled scores are recommended for Norwegian children.27
The Caregiver Priorities and Child Health Index of Life With Disabilities measures caregiver’s perspectives on health status, comfort, well-being, functional abilities, and ease of caregiving of children with severe developmental disabilities.24 It was developed to measure the effectiveness of interventions intended to improve or preserve these outcomes.24 The Caregiver Priorities and Child Health Index of Life With Disabilities consists of 37 items divided into 6 different sections: personal care/activities of daily living; positioning; transferring and mobility; communication and social interaction; comfort and emotions; and health and overall quality of life. Each item describes both the caregiver’s assessment of difficulties in performing the task and the level of assistance needed. In addition, the caregiver can give details in free text within each item. Raw scores range from 0 to 6 or 0 to 9 in each section and standard scores range from 0 to 100, where higher scores indicate better function.24 In the present study, only the 2 domains of positioning; transferring and mobility; and communication and social interaction mostly related to the intervention goal were addressed.
The Caregiver Priorities and Child Health Index of Life With Disabilities is found to be a reliable and valid measure.28,29 As there is no validated clinically significant change for the Caregiver Priorities and Child Health Index of Life With Disabilities, a difference larger than the mean absolute difference in scores between 2 measurements in a test–retest reliability study24 was used as an indication of true change. The mean absolute difference was 8.97 points for positioning and transferring and mobility and 10.58 points for communication and social interaction.24
The tests and questionnaires were also scored by an experienced physical therapist blinded to the order of the assessments and the intervention and following the scoring procedures in the respective test manuals. The results were then compared to the original scoring by the first author. There was no discrepancy for the Posture and Postural Ability Scale, the Pediatric Evaluation of Disability Inventory, or the Caregiver Priorities and Child Health Index of Life With Disabilities domains of positioning, transferring and mobility, and communication and social interaction.
Results
During the intervention period, the physical therapy sessions were conducted as planned in 10 of 12 sessions. One session was canceled and one was incomplete. The child had a period of days of not feeling well in which the sessions were postponed within the same week and the parent activities were canceled. The parent activities were performed daily, but the standing aid was only used during the first week of intervention as it was defect. A walking aid was introduced in the third week, and it replaced the standing aid for the rest of the intervention.
In the Caregiver Priorities and Child Health Index of Life With Disabilities questionnaire, the parents reported problems with teething, constipation, seizures, parent’s being afraid of seizures, and the child being afraid of lying down. At preintervention, no problems were reported. At postintervention, problems with teething, the child being afraid of lying down, and a stuffy nose was reported, and at follow-up, a constipation problem was reported.
Postural Control
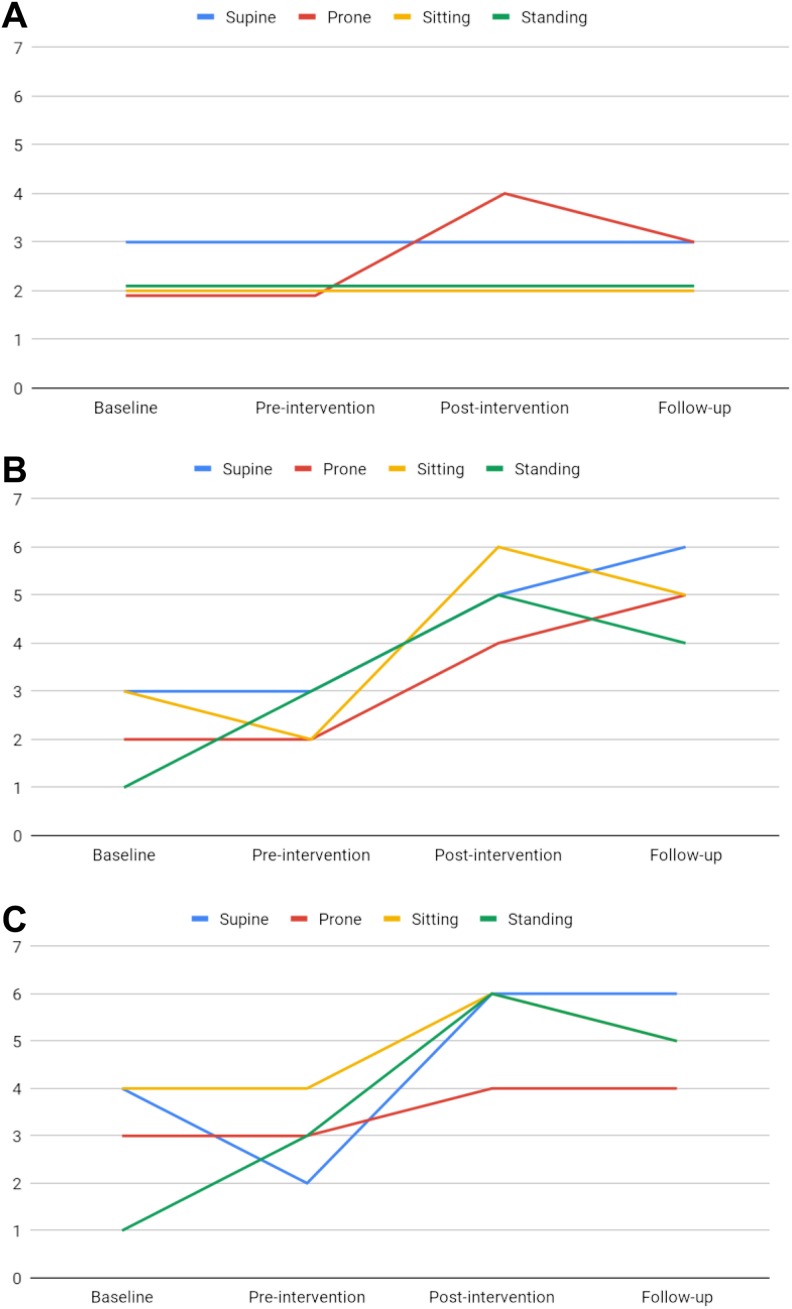
The Posture and Postural Ability Scale scores for level of postural ability remained unchanged at 3 points in the supine position and 2 points for sitting and standing from baseline to follow-up (Table 2; Figure 1A). In the prone position, scores increased from 2 points at baseline and preintervention to 4 and 3 points at postintervention and follow-up.
Table 2.
Scores for Level of Postural Ability and Postural Alignment in the Frontal and Sagittal Plane in Supine, Prone, Sitting, and Standing for a Child With Congenital Zika Virus Syndrome, Measured by the Posture and Postural Ability Scale at Baseline, Preintervention, Postintervention, and Follow-Up.
| Baseline | Preintervention | Postintervention | Follow-Up | |
|---|---|---|---|---|
| Supine | ||||
| Level | 3 | 3 | 3 | 3 |
| Frontal alignment | ||||
| Total | 3 | 3 | 5 | 6 |
| Head | 1 | 1 | 1 | 1 |
| Trunk | 1 | 0 | 1 | 1 |
| Sagittal alignment | ||||
| Total | 4 | 2 | 6 | 6 |
| Head | 1 | 1 | 1 | 1 |
| Trunk | 1 | 0 | 1 | 1 |
| Prone | ||||
| Level | 2 | 2 | 4 | 3 |
| Frontal alignment | ||||
| Total | 2 | 2 | 4 | 5 |
| Head | 0 | 0 | 0 | 0 |
| Trunk | 1 | 1 | 1 | 1 |
| Sagittal alignmenta | ||||
| Total | 3 | 3 | 4 | 4 |
| Trunk | 1 | 0 | 1 | 0 |
| Sitting | ||||
| Level | 2 | 2 | 2 | 2 |
| Frontal alignment | ||||
| Total | 3 | 2 | 6 | 6 |
| Head | 0 | 0 | 1 | 0 |
| Trunk | 1 | 0 | 1 | 1 |
| Sagittal alignment | ||||
| Total | 4 | 4 | 6 | 5 |
| Head | 0 | 0 | 1 | 0 |
| Trunk | 0 | 0 | 1 | 1 |
| Standing | ||||
| Level | 2 | 2 | 2 | 2 |
| Frontal alignment | ||||
| Total | 1 | 3 | 5 | 4 |
| Head | 0 | 0 | 1 | 0 |
| Trunk | 0 | 1 | 1 | 0 |
| Sagittal alignment | ||||
| Total | 1 | 3 | 6 | 5 |
| Head | 0 | 0 | 1 | 1 |
| Trunk | 0 | 0 | 1 | 0 |
a Head alignment in the sagittal plane is not measured in the prone position.
Figure 1.
Level of postural ability (A), postural alignment in the frontal plane (B), and postural alignment in the sagittal plane (C) in supine, prone, sitting, and standing for a child with congenital Zika virus syndrome, measured by the Posture and Postural Ability Scale at baseline, preintervention, postintervention, and follow-up.
Overall, scores for postural alignment in the frontal and sagittal plane increased in all 4 positions (supine, prone, sitting, and standing) from baseline and preintervention to postintervention and follow-up (Table 2; Figure 1B and C). In the frontal plane, the increase was 2 to 3 points for the various positions from pre- to postintervention. In the sagittal plane, the increase ranged from 1 to 4 points for different positions, being largest in supine and standing and smallest in the prone position. In the supine and prone position in the frontal plane, the scores continued to increase by 1 point from postintervention to follow-up.
More specifically, scores for postural alignment increased for the head or trunk in all 4 positions from pre- to postintervention in both planes, except for the prone position in the sagittal plane (Table 2). The improvement was most pronounced in sitting and standing, as scores for both head and trunk alignment increased in the sagittal plane and for sitting also in the frontal plane. In total, the scores for head and trunk alignment in the 4 positions in both planes increased from 4 to 14 points from pre- to postintervention but decreased to 8 at follow-up.
Mobility and Social Skills
The Pediatric Evaluation of Disability Inventory score for functional skills increased by 9.1 points for mobility and 17.2 points for social function from pre- to postintervention (Table 3). For social function, the score continued to increase from postintervention to follow-up. Of the single items included in these 2 domains, the following showed an increase in score from pre- to postintervention: 4 items related to walking, using gestures with clear meaning, noticing the presence of other children, vocalizing and gesturing toward peers, and manipulating toys with intent (Table 4).
Table 3.
Scaled Scores for the Mobility and Social Function Domains of the Pediatric Evaluation of Disability Inventory (PEDI) and Standard Scores for the Positioning, Transfers and Mobility, and Communication and Social Interaction sections of the Caregiver Priorities and Child Health Index of Life with Disabilities (CPCHILD) at Baseline, Preintervention, Postintervention, and Follow-Up for a Child With Congenital Zika Virus Syndrome.
| PEDI | Baseline | Preintervention | Postintervention | Follow-Up | ||||
|---|---|---|---|---|---|---|---|---|
| Scaled Score | SE | Scaled Score | SE | Scaled Score | SE | Scaled Score | SE | |
| Mobility | 18.2 | 3.8 | 18.2 | 3.8 | 27.3 | 3.1 | 27.3 | 3.1 |
| Social function | 10.5 | 3.2 | 10.5 | 3.2 | 27.7 | 2.7 | 30.0 | 2.1 |
| CPCHILD | Standard Score | AMD | Standard Score | AMD | Standard Score | AMD | Standard Score | AMD |
| Positioning, transfers, and mobility | 52 | 8.97 | 64 | 8.97 | 46 | 8.97 | 71 | 8.97 |
| Communication and social interaction | 36 | 10.58 | 27 | 10.58 | 37 | 10.58 | 38 | 10.58 |
Table 4.
Single Items From the Pediatric Evaluation of Disability Inventory (PEDI) and the Caregiver Priorities and Child Health Index of Life With Disabilities (CPCHILD) Relevant to Posture With Change in Scores From Pre- to Postintervention for a Child With Congenital Zika Virus Syndrome.
| Items Related to Mobility | Pre | Post | Items Related to Social Skills | Pre | Post |
|---|---|---|---|---|---|
| PEDI | |||||
| Item 33: Changes physical location purposefully | 0 | 1 | Item 16: Using gestures with clear meaning | 0 | 1 |
| Item 38: Walks, but holds onto objects, caregiver, or devices for support | 0 | 1 | Item 31: Notices presence of other children; can vocalize and gesture toward peers | 0 | 1 |
| Item 40: Moves 10-50 ft (1-5 car lengths) | 0 | 1 | Item 36: Manipulates toys, objects, or body with intent | 0 | 1 |
| Item 45: Walks outdoor level surfaces (smooth sidewalks, driveways) | 0 | 1 | |||
| CPCHILD | |||||
| Item 12: Sitting in a wheelchair | 6 | 9 | Item 27: Difficulties understanding the caregiver | 1 | 2 |
| Item 15: Moving about outdoor | 5 | 6 | Item 28: Difficulties being understood by you | 1 | 3 |
| Item 10: Getting in and out of bed | 6 | 0 | |||
| Item 11: Transferring into/out of a wheelchair/chair | 6 | 0 | |||
| Item 16: Getting in and out of a motor vehicle | 6 | 0 |
Abbreviations: CPCHILD, Caregiver Priorities and Child Health Index of Life with Disabilities; PEDI, Pediatric Evaluation of Disability Inventory.
The score for Caregiver Assistance in these domains remained unchanged at 0 points from baseline to follow-up (data not shown). The Caregiver Priorities and Child Health Index of Life With Disabilities domain score for position, and transferring and mobility decreased by 18 points from pre- to postintervention. However, both the baseline and follow-up scores were higher than the postintervention score (Table 3). In contrast, the domain score for communication and social interaction increased by 10 points from pre- to postintervention and was unchanged at follow-up. However, the baseline score was higher than the preintervention score.
Of the single items included in these 2 domains, the following showed an increase in score from pre- to postintervention: sitting in a wheelchair, moving outdoors and items related to the child’s understanding, communication with others, and being able to play alone (Table 4). Transfers in and out of bed, chair, and car showed a decrease in the same period.
Discussion
This is the first report to describe the development of postural control, mobility, and social skills in a 17- to 18-month-old child with congenital Zika virus syndrome before and after a period of intensive, home-based physical therapy. Measured by the Posture and Postural Ability Scale, the child showed an increase in level of postural ability in the prone position and postural alignment in all 4 positions from pre- to postintervention. The increase was largely related to head and trunk alignment. Measured by the Pediatric Evaluation of Disability Inventory and the Caregiver Priorities and Child Health Index of Life With Disabilities, the child showed an overall improvement in mobility from preintervention to follow-up; however, there was a transient drop in the Caregiver Priorities and Child Health Index of Life With Disabilities scores at postintervention. The child’s social skills improved by both measures from pre- to postintervention.
Although findings from case reports cannot be generalized, they can provide a valuable description, especially in relation to rare disorders such as the congenital Zika virus syndrome, where larger studies are difficult to perform.30 Moreover, the descriptions can provide useful information when planning future studies.30 Assessments 3 weeks before the start of intervention (baseline), at the start and at the end of intervention, and at 3 weeks after the end of intervention (follow-up) make it possible to evaluate whether results are due to the intervention or random factors and if there is a short-term lasting change. Furthermore, this study includes the use of several standardized measures of postural control, mobility, and social skills important for participation in kindergarten’s daily life. Although these measures have not been psychometrically evaluated in children with congenital Zika virus syndrome, they are found valid and reliable for use in children with cerebral palsy having severe disability. Those disabilities resemble those in the present case. As the same physical therapist carried out the examinations and the intervention, observer bias could have affected the results. However, the tests and questionnaires were independently scored by a second physical therapist, blinded as to the order of the assessments and the intervention. There was a high consensus between the 2 physical therapists, indicating that observer bias was less likely.
The intervention was based on current knowledge on what has been shown effective in children with cerebral palsy, such as including parents in a home-based and intensive period of training.12,14,18 Activities incorporated in play were chosen to make the intervention meaningful to the child and increase the child’s motivation to participate. Active participation has been shown to affect brain function and organization by refining synaptic connections,31,32 possibly enhancing the effect of intervention.14 Some of the activities in the intervention were well known to the child as they had been included in previous physical therapy sessions. This could have enabled the child to use more of her capacity in performing the activity, thereby increasing the quality and possibly the effect of therapy. The well-known activities could also have given the child a sense of achievement that could have increased her motivation. As the child had received periods of physical therapy from the age of 5 months, the authors believe that the previous interventions were essential in obtaining the level of postural control prior to this intensive period of intervention at 17 to 18 months of age. Nevertheless, assessing her postural control before and after the 3-week period of no intervention both prior to and after the intervention made it possible to assess the effect of the intervention described in this report.
The primary goal of the intervention was to improve postural control in various positions. Measured by the Posture and Postural Ability Scale, the child’s “quantity” of posture (level of postural ability) in the prone position improved from pre- to postintervention. Before the intervention, the child was not able to hold her head up by herself, whereas after the intervention, she could both hold and move her head in a given position. The scores remained unchanged in supine, sitting, and standing. In the supine position, the child was already able to hold the position without support, and a further increase in score would mean lifting head or feet off the floor, which cannot be considered relevant to the goals of the intervention. To increase scores in sitting and standing, the child should go from supported to unsupported sitting and standing, which was not considered realistic to achieve during the period of intervention. Thus, for these more demanding positions, the change in the quality of posture (postural alignment) can be more relevant to consider.
The increased ability to align body structures in relation to each other and surroundings in all 4 positions from pre- to postintervention is likely to be a result of the intervention, as there was no increase in scores from baseline to preintervention. The increase from 0 to 1 point in both head and trunk alignment in the sitting and standing position means that the child had developed from not being able to hold her head and trunk aligned to being able to. These new skills give the child increased possibilities to observe peers in kindergarten and thereby being able to interact and participate with others more effectively. Thus, these improvements in postural ability and alignment can be considered a prerequisite for the secondary goals of the intervention, which were to increase mobility and social skills in kindergarten.
Measured by the Pediatric Evaluation of Disability Inventory and the Caregiver Priorities and Child Health Index of Life With Disabilities, mobility increased from preintervention to follow-up. However, while the Pediatric Evaluation of Disability Inventory scores increased to postintervention, the Caregiver Priorities and Child Health Index of Life With Disabilities scores had an intermediate drop. The increased Pediatric Evaluation of Disability Inventory scores were larger than 2 standard error,23 indicative of a true change in functional performance, although the change was a little less than Iyer et al’s25 suggestion of 11 points for a clinically significant change. Nevertheless, Pediatric Evaluation of Disability Inventory mobility items indicated that new and significant skills were learned, such as independent walking with a suitable walking aid. In accordance with this, the parents rated walking outside, along with sitting in a chair, as less difficult on the Caregiver Priorities and Child Health Index of Life With Disabilities at postintervention. However, the decrease in Caregiver Priorities and Child Health Index of Life With Disabilities score at postintervention was larger than the absolute mean difference according to the manual,24 indicating that parents rated several skills as more difficult than at preintervention, such as transfers in and out of bed, chair, and car. The authors speculate that the assessment of these items can be related to an increased number of problems reported at postintervention, such as the child being afraid to lie down. At follow-up, the Pediatric Evaluation of Disability Inventory score was unchanged, but the Caregiver Priorities and Child Health Index of Life With Disabilities score increased by almost 3 times the absolute mean difference. At the same time, problems such as the child being afraid to lie down are not reported. This can support the speculation that the Caregiver Priorities and Child Health Index of Life With Disabilities scores at postintervention were influenced by the child’s overall comfort.
The improved ability to walk and sit facilitates social interaction as the child would have the same starting position as the peers in kindergarten. This is both important for improving the child’s ability to observe and also the possibility to be considered a playmate by the other children. Independent walking would also give the child the opportunity to move where she would like when she wants to. This will enhance her possibilities to interact with peers and participate in play. In fact, her social skills improved from pre- to postintervention. Both the social function domain of Pediatric Evaluation of Disability Inventory and the communication and social interaction domain of the Caregiver Priorities and Child Health Index of Life With Disabilities showed an increase in scores indicative of a true and clinically significant change. Several items requiring postural control improved, such as being able to use gestures with clear meaning, noticing the presence of other children, and manipulating toys with intent (Pediatric Evaluation of Disability Inventory) and items related to understanding and communicating (Caregiver Priorities and Child Health Index of Life With Disabilities). This supports the assumption that improvements in postural control are relevant to the child’s ability to interact with peers.11,14
Whether the changes measured from pre- to postintervention have lasting effects is difficult to answer from this study. For postural control, most Posture and Postural Ability Scale scores increased or remained unchanged from postintervention to follow-up. However, a small decline in postural ability in prone, as well as alignment in sitting and standing, makes it unclear whether the improvements in postural control are lasting. For mobility and social skills, the Pediatric Evaluation of Disability Inventory and Caregiver Priorities and Child Health Index of Life With Disabilities scores increased from postintervention to follow-up, indicating that the acquirement of these skills can potentially be lasting. Studies with longer follow-up and more assessment points are needed to fully consider the long-lasting effect of intervention.
In conclusion, this case study of a 17- to 18-month-old child with congenital Zika virus syndrome showed improvements in postural control after a 6-week intensive, home-based physiotherapy intervention. A large amount of increase was related to head and trunk alignment. The child showed an overall improvement in mobility and social skills from preintervention to follow-up, improving the child’s opportunities to participate in interaction with peers in kindergarten. Further studies are required to support the findings in this case report. As in children with cerebral palsy, intensive home-based interventions targeting gross motor skills can be a suitable physical therapy approach for children with congenital Zika virus syndrome.
Acknowledgments
The authors warmly thank the child and her parents for consenting to publication of the child’s test results and physiotherapy intervention. The authors are thankful to pediatrician Torunn Thune for her contribution and guidance regarding the medical information and physiotherapist Marie Tjelle Renli for assistance in scoring of test results. The authors are grateful to Anne E. Hansen, head of Unit for Physiotherapy Services in Trondheim Municipality, Norway, for giving the first author the opportunity to take time off from clinical work to write this article.
Footnotes
Author Contributions: KRA was responsible for acquisition of results. Both the authors contributed to conception and design, analysis and interpretation, drafted the manuscript, critically revised the manuscript, gave final approval, and agreed to be accountable for all aspects of the work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Kristine Rabben Amundsen  https://orcid.org/0000-0001-5116-6703
https://orcid.org/0000-0001-5116-6703
Ethical Approval: The parents gave written informed consent that the child’s test results could be published anonymously. The parents have read the final version of the article prior to submission. The PT intervention was a planned part of the child’s treatment documented in the child’s PT journal. Thus, ethical approval from the regional committee for medical and health research ethics was not needed. The issues regarding secure handling of information were considered and approved by the Department of Information Security in Trondheim Municipality.
References
- 1. World Health Organization. WHO Toolkit for the Care and Support of People Affected by Complications Associated with Zika Virus. Geneva, Switzerland: World Health Organization; 2017. [Google Scholar]
- 2. Wheeler AC. Development of infants with congenital Zika syndrome: what do we know and what can we expect? Pediatrics. 2018;141(suppl 2):S154–S160. doi:10.1542/peds.2017-2038D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Costello A, Dua T, Duran P, et al. Defining the syndrome associated with congenital Zika virus infection. Bull World Health Organ. 2016;94(6):406–506A. doi:10.2471/BLT.16.176990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chimelli L, Avvad-Portari E. Congenital Zika virus infection: a neuropathological review. Childs Nerv Syst. 2018;34(1):95–99. doi:10.1007/s00381-017-3651-3. [DOI] [PubMed] [Google Scholar]
- 5. Pessoa A, van der Linden V, Yeargin-Allsopp M, et al. Motor abnormalities and epilepsy in infants and children with evidence of congenital Zika virus infection. Pediatrics. 2018;141(suppl 2):S167–S179. doi:10.1542/peds.2017-2038F. [DOI] [PubMed] [Google Scholar]
- 6. Satterfield-Nash A, Kotzky K, Allen J, et al. Health and development at age 19-24 months of 19 children who were born with microcephaly and laboratory evidence of congenital Zika virus infection during the 2015 Zika virus outbreak - Brazil, 2017. MMWR Morb Mortal Wkly Rep. 2017;66(49):1347–1351. doi:10.15585/mmwr.mm6649a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marques FJP, Teixeira MCS, Barra RR, et al. Children born with congenital zika syndrome display atypical gross motor development and a higher risk for cerebral palsy. J Child Neurol. 2019;34(2):81–85. doi:10.1177/0883073818811234. [DOI] [PubMed] [Google Scholar]
- 8. Wheeler AC, Ventura CV, Ridenour T, et al. Skills attained by infants with congenital Zika syndrome: pilot data from Brazil. PLoS One. 2018;13(7):e0201495 doi:10.1371/journal.pone.0201495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Franca TLB, Medeiros WR, Souza NL, et al. Growth and development of children with microcephaly associated with congenital Zika virus syndrome in Brazil. Int J Environ Res Public Health. 2018;15(9). doi:10.3390/ijerph15091990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McEwen IR, Meiser MJ, Hansen LH. Children with motor and intellectual disabilities In: Campbell SK, Palisano RJ, Orlin MN, eds. Physical Therapy for Children. 4th ed St Louis, MO: Elsevier; 2012:539–576. [Google Scholar]
- 11. Adolph KE, Franchak JM. The development of motor behavior. Wiley Interdiscip Rev Cogn Sci. 2017;8(1-2) doi:10.1002/wcs.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dewar R, Love S, Johnston LM. Exercise interventions improve postural control in children with cerebral palsy: a systematic review. Dev Med Child Neurol. 2015;57(6):504–520. doi:10.1111/dmcn.12660. [DOI] [PubMed] [Google Scholar]
- 13. Heyrman L, Desloovere K, Molenaers G, et al. Clinical characteristics of impaired trunk control in children with spastic cerebral palsy. Res Develop Disabil. 2013;34(1):327–334. doi:10.1016/j.ridd.2012.08.015 [DOI] [PubMed] [Google Scholar]
- 14. Gordon AM, Magill RA. Motor learning: application of principles to pediatric rehabilitation In: Campell SK, Palisano RJ, Orlin MN, eds. Physical Therapy for Children. 4th ed St Louis, MO: Elsevier; 2012:151–174. [Google Scholar]
- 15. Rahman MA, Zaman MM, Rahman MM, et al. Effects of intensive versus non-intensive physical therapy on children with cerebral palsy. Mymensingh Med J. 2016;25(3):421–424. [PubMed] [Google Scholar]
- 16. Katz-Leurer M, Rotem H, Keren O, Meyer S. The effects of a ‘home-based’ task-oriented exercise programme on motor and balance performance in children with spastic cerebral palsy and severe traumatic brain injury. Clin Rehabilit. 2009;23(8):714–724. doi:10.1177/0269215509335293. [DOI] [PubMed] [Google Scholar]
- 17. Størvold GV, Jahnsen R. Intensive motor skills training program combining group and individual sessions for children with cerebral palsy. Pediatr Phys Ther. 2010;22(2):150–159. doi:10.1097/PEP.0b013e3181dbe379. [DOI] [PubMed] [Google Scholar]
- 18. Novak I, McIntyre S, Morgan C, et al. A systematic review of interventions for children with cerebral palsy: state of the evidence. Dev Med Child Neurol. 2013;55(10):885–910. doi:10.1111/dmcn.12246. [DOI] [PubMed] [Google Scholar]
- 19. World Health Organization. The WHO Child Growth Standards. https://www.who.int/childgrowth/standards/en/.
- 20. Griffin I, Martin SW, Fischer M, et al. Zika virus IgM detection and neutralizing antibody profiles 12-19 months after illness onset. Emerg Infect Dis. 2019;25(2):299–303. doi:10.3201/eid2502.181286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adebanjo T, Godfred-Cato S, Viens L, et al. Update: interim guidance for the diagnosis, evaluation, and management of infants with possible congenital Zika virus Infection—United States, October 2017. MMWR Morb Mortal Wkly Rep. 2017;66(41):1089–1099. doi:10.15585/mmwr.mm6641a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rodby-Bousquet E, Persson-Bunke M, Czuba T. Psychometric evaluation of the posture and postural ability scale for children with cerebral palsy. Clin Rehabilit. 2016;30(7):697–704. doi:10.1177/0269215515593612. [DOI] [PubMed] [Google Scholar]
- 23. Haley SM, Coster WJ, Ludlow LH, Haltiwanger JT, Andrellos PA. Pediatric Evaluation of Disability Inventory: Development, Standardization and Administration Manual. Boston, MA: Trustees of Boston University; 1992. [Google Scholar]
- 24. Narayanan UG, Weir S, Fehlings DL. CPCHILD Caregiver Priorities and Child Health Index of Life with Disabilities (CPCHILD (c)) Questionnaire. An instrument to assess the health status, comfort, wellbeing and ease of caregiving of children with severe cerebral palsy. In: Manual and Interpretation Guide. Canada; 2007. www.sickkids.ca. Accessed October 12, 2018. [Google Scholar]
- 25. Iyer LV, Haley SM, Watkins MP, Dumas HM. Establishing minimal clinically important differences for scores on the pediatric evaluation of disability inventory for inpatient rehabilitation. Phys Ther. 2003;83(10):888–898. [PubMed] [Google Scholar]
- 26. Berg M, Jahnsen R, Froslie KF, Hussain A. Reliability of the Pediatric Evaluation of Disability Inventory (PEDI). Phys Occupat Therap Pediat. 2004;24(3):61–77. [DOI] [PubMed] [Google Scholar]
- 27. Jahnsen R, Berg M, Dolva AS, Høyem R. Pediatric Evaluation of Disability Inventory (PEDI) [in Norwegian]. Oslo, Norway: Norsk Psykologforening; 2000. [Google Scholar]
- 28. Carlon S, Shields N, Yong K, Gilmore R, Sakzewski L, Boyd R. A systematic review of the psychometric properties of quality of life measures for school aged children with cerebral palsy. BMC Pediatr. 2010;10:81 doi:10.1186/1471-2431-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Narayanan UG, Fehlings D, Weir S, Knights S, Kiran S, Campbell K. Initial development and validation of the Caregiver Priorities and Child Health Index of Life With Disabilities (CPCHILD). Dev Med Child Neurol. 2006;48(10):804–812. doi:10.1017/S0012162206001745. [DOI] [PubMed] [Google Scholar]
- 30. Nissen T, Wynn R. The clinical case report: a review of its merits and limitations. BMC Res Notes. 2014;7:264 doi:10.1186/1756-0500-7-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Johnston MV. Clinical disorders of brain plasticity. Brain Develop. 2004;26(2):73–80. doi:10.1016/S0387-7604(03)00102-5. [DOI] [PubMed] [Google Scholar]
- 32. Herskind A, Greisen G, Nielsen JB. Early identification and intervention in cerebral palsy. Dev Med Child Neurol. 2015;57(1):29–36. doi:10.1111/dmcn.12531. [DOI] [PubMed] [Google Scholar]