Abstract
The discovery of X-rays and radioactivity in 1895/1896 triggered a flood of studies and applications of radiation in medicine that continues to this day. They started with imaging fractures/organs and progressed to treating diseases by exposing areas to radiation from external and internal sources. By definition, low-dose treatments stimulate damage control (or adaptive protection) systems that remedy diseases. Publications are identified on low-dose ionizing radiation (LDIR) therapies for different cancers, infections, inflammations, and autoimmune and neurodegenerative diseases. The high rate of endogenous DNA damage, due to leakage of oxygen from aerobic metabolism, and the damage control systems that deal with this are discussed. Their stimulation and inhibition by radiation are described. The radium dial painter studies revealed the radium ingestion threshold for malignancy and the dose threshold for bone sarcoma. The radiation scare that misled the medical profession and the public is a barrier to LDIR therapies. Many studies on nasal radium irradiation demonstrated that children are not unduly radiation sensitive. Omissions in the medical textbooks misinform physicians about the effects of LDIR therapy, which blocks clinical trials to determine optimal doses, efficacy, and thresholds for onset of harm. Information from many recent case reports on LDIR therapies, including successes with radon therapy, is provided.
Keywords: radiation hormesis, radon therapy, cancer remediation, inflammation, autoimmune disease, low-dose ionizing radiation
Introduction
After the discovery of X-rays in 1895 and nuclear radiations in 1896, medical practitioners began applying these penetrating radiations to image internal injuries and assess many diseases. They soon found out that such exposures were often followed by important changes in the patient’s medical condition. A large X-ray dose (Gy or joules/kg) produced a painful burn, but a small dose often resulted in beneficial health effects that were attributed to stimulation of biological protection mechanisms. Thousands of articles about radiation-induced health effects of X-rays began to appear in medical journals and other publications. There are old articles and books, in English, French, German, and Polish, about the many applications of radium in medicine.1 Brucer’s remarkable chronology of nuclear medicine covers the period from 1600 to 1989.2 Rowland’s review of US studies on radium in humans mentions the production and ingestion of radium as an elixir and, inadvertently, while painting radium phosphor on instrument dials. His report focuses on health effects of radium on the dial painters.3 More recently, Sanders’ radiation hormesis books compile and discuss the enormous amount of medical evidence of many low-dose radiation therapies.4,5 This article describes the author’s presentation on low-dose medical therapies at the 2019 Polish Radiation Research Society Symposium.6
What is a low dose of ionizing radiation (LDIR)? It is essential to define this term here because its meaning is different from what is considered to be a low dose in nuclear safety analysis or in radiation protection, where the linear no-threshold (LNT) model, adopted in the 1960s, is used to calculate the risk of radiation-induced cancer.7
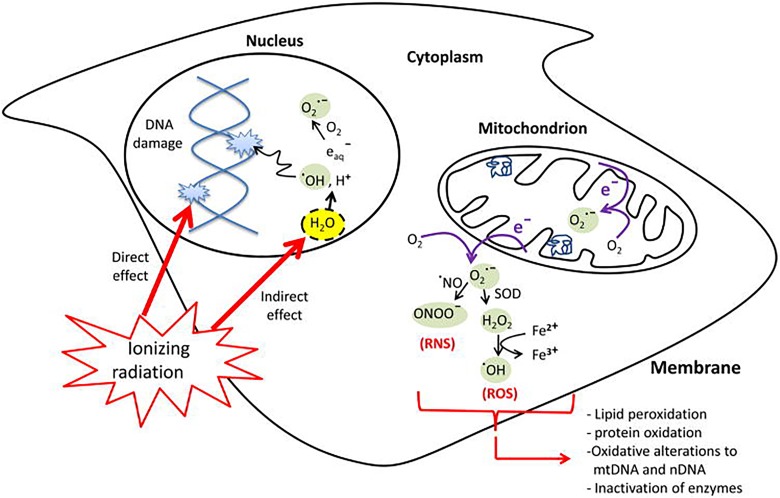
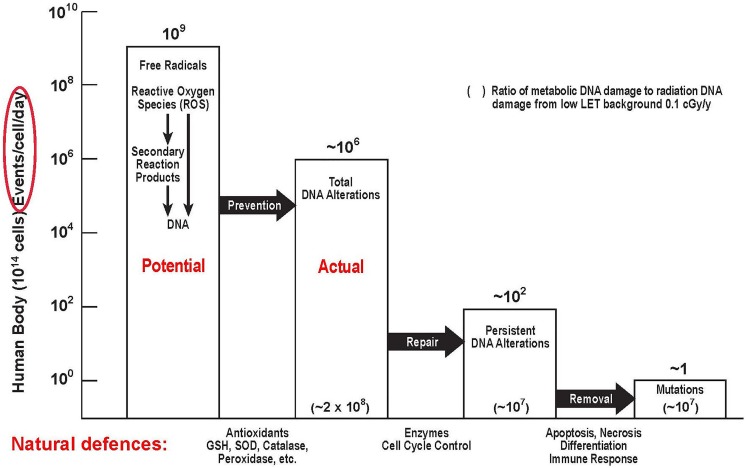
As discussed in the section “Low Dose of Ionizing Radiation Stimulation of Protection Systems in Aerobic Organisms,” every air-breathing organism has very powerful damage control or protective systems, which prevent, repair, and scavenge the naturally occurring DNA damage that happens at a very high rate due to natural leakage of oxygen from aerobic metabolism. These protection systems are essential for an organism to survive. Figure 1 shows ionizing radiation (IR) hitting a DNA molecule and also ionizing water, producing a range of reactive oxygen species (ROS). In addition to damaging DNA (and the other biomolecules) locally, “hits” and ROS send signals that affect the protection systems in the irradiated areas and also in the nonirradiated areas of the body (bystander effects).8 These systemic effects are very complicated.
Figure 1.
Leakage of reactive oxygen species (ROS) from oxygen metabolism and ionizing radiation (IR) effect on DNA and biomolecules.8
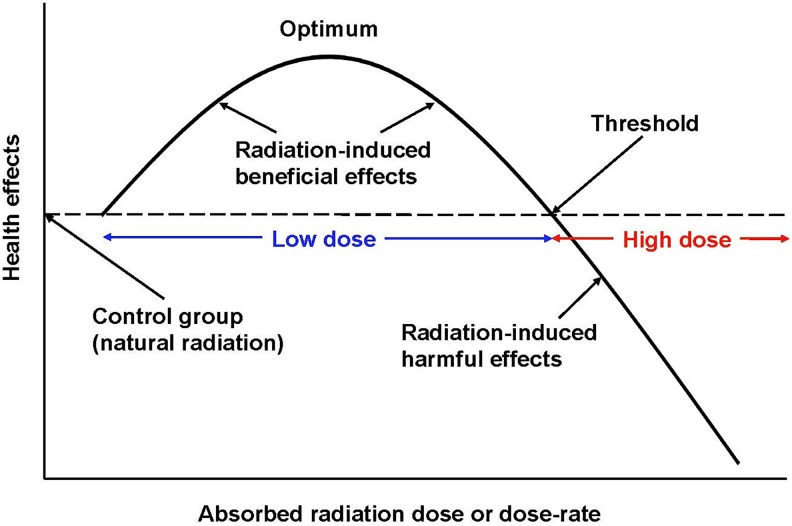
A low dose of IR is a dose that creates a burst of hits and ROS that is adequate to stimulate the protective systems and produce observable health benefits. A high dose is defined as a dose that is large enough to inhibit or damage the protective systems sufficiently to cause observable harm, immediate or latent. Figure 2 illustrates the meaning of low-dose and high-dose radiation.
Figure 2.
Biphasic dose–response model.9 Definition of a low dose and a high dose of radiation.
Harmful Effects and Radiation Protection Limits
Roentgen’s discovery of X-rays in 1895 started a flood of studies on their application for the treatment of many medical conditions. In the very early days, the practitioners were unaware of the harmful effects of a high dose. There were no instruments to measure X-ray dose. Calibration was based on the amount of skin reddening (erythema) produced when the operator’s hand was placed in the X-ray beam. The erythema dose after a 20-minute exposure to a standard beam was about 600 rad or 6 Gy. Numerous unexpected injuries to patients, physicians, and scientists led to studies for setting exposure limits.10
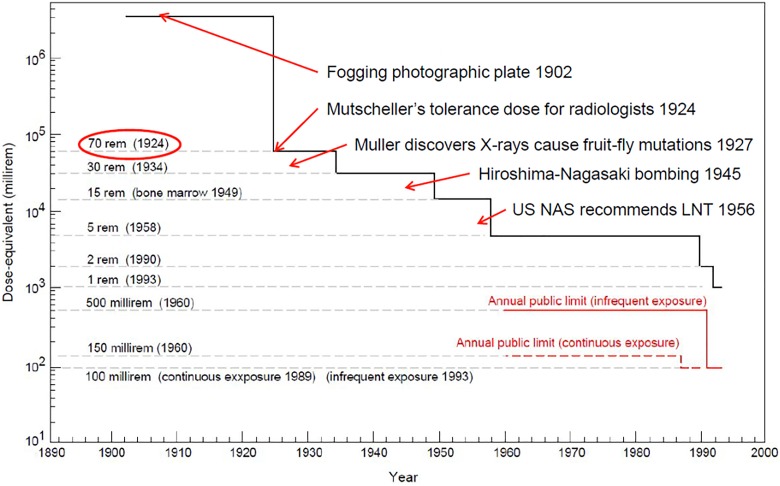
In September 1924 at a meeting of the American Roentgen Ray Society, Arthur Mutscheller was the first to recommend a “tolerance” dose rate for radiation workers, a dose rate that in his judgment could be tolerated indefinitely. This limit is 0.2 roentgen (r) per day, about 70 rad (700 mGy) per year, and it was accepted by the ICRP in 1934.7 Subsequently, the annual dose limit decreased stepwise, driven by changing attitudes toward acceptable risk (Figure 3).9
Figure 3.
Stepwise decrease in annual occupational dose limit from 70 rem (1924) to 5 rem (1958).9
Health concerns began soon after Muller discovered in 1927 that X-rays produced mutations in fruit flies. Being a geneticist, he was interested in gene mutations, which “form the chief basis of organic evolution.” And being a eugenicist, he lamented that “the study of these mutations, and, through them, of the genes themselves, has heretofore been very seriously hampered by the extreme infrequency of their occurrence under ordinary conditions, and by the general unsuccessfulness of attempts to modify decidedly, and in a sure and detectable way, this sluggish natural mutation rate. Modification of the innate nature of organisms, for more directly utilitarian purposes, has of course been subject to these same restrictions, and the practical breeder has hence been compelled to remain content with the mere making of recombinations of the material already at hand, providentially supplemented, on rare and isolated occasions, by an unexpected mutational windfall. To these circumstances are due the widespread desire on the part of biologists to gain some measure of control over the hereditary changes within the genes.”11(p. 84)
For exposure times of 12, 24, 36, and 48 minutes, Muller found that the mutation rate in fruit flies was about proportional to dose. In 1930, he determined that natural background radiation is inadequate to explain the frequency of natural mutations.12 His usual X-ray treatment caused a mutation rate that was about 150 times the mutation rate of untreated germ cells. This did not correspond to his calculated dose rate that was 200 000 times the natural background dose rate. He did not identify what other cause produced the observed natural mutation rate.12 Recently, Calabrese showed that the dose rate Muller used, 81.4 r per minute, is actually about 95 million times the natural background dose rate, rather than 200 000-fold.13 So Muller’s Proportional Rule is based on exposures that were very high indeed. Linear extension of the dose received by his flies to the dose received by unexposed flies spans a range of about 8 orders of magnitude! In recommending the LNT model in his 1946 Nobel Prize speech, Muller ignored Caspari’s evidence of a dose threshold at about 50 rad or 0.5 Gy when he declared that there was “no escape from the conclusion that there is no threshold.”14,15
After atomic bombs were used to end World War II, strong political activity arose against the ongoing testing and development of nuclear weapons. Muller’s speech was communicated widely and exploited to create extreme fear of radiation-induced mutations from radioactive bomb fallout as a means to stop testing. This has been well described by Jaworowski.16 In 1956, the US National Academy of Sciences (NAS) recommended an abrupt change from use of a threshold model to determine onset of harm, to the LNT dose–response model to assess risk of radiation-induced mutations.17 Over the past 10 years, Calabrese has been carefully studying the history of how radiation protection changed from using a safe dose limit and a safe dose-rate limit to calculating risks of harmful mutations and cancer that increase linearly with cumulative dose. This change ignored natural biological protection and its renewal processes. Calabrese discovered that the NAS genetics panel, which included Muller, was ideologically motivated. He produced evidence that it falsified and fabricated the research record. It was funded, managed, and directed by the Rockefeller Foundation, created by the petroleum energy industry. The NAS recommendation had far-reaching influence; it misled the world community. All regulators of radiation adopted the unscientific LNT model for cancer risk assessment. This affected cancer risk communication, nuclear energy, public health, and medical practices in the United States and worldwide.18 By 1958, the annual occupational dose limit had decreased stepwise from about 70 to 5 rem per year (Figure 3).10
Early Medical Therapies
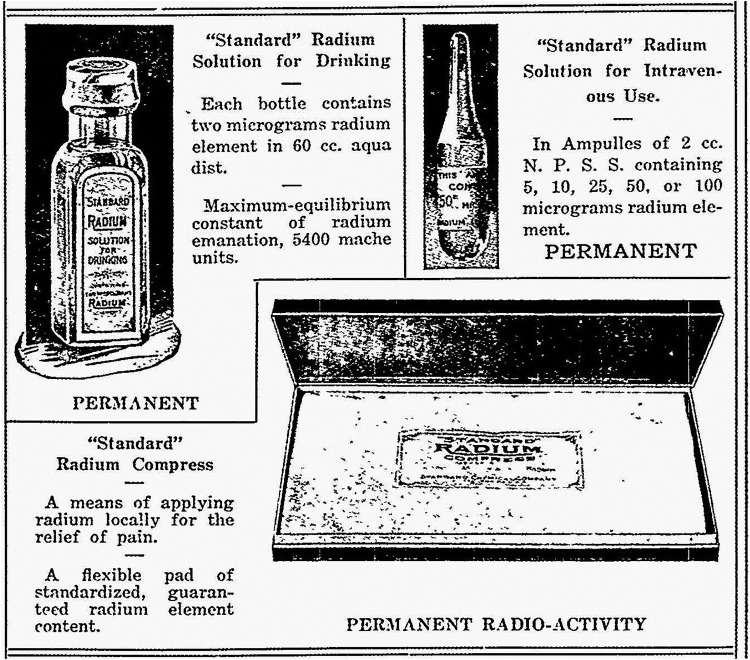
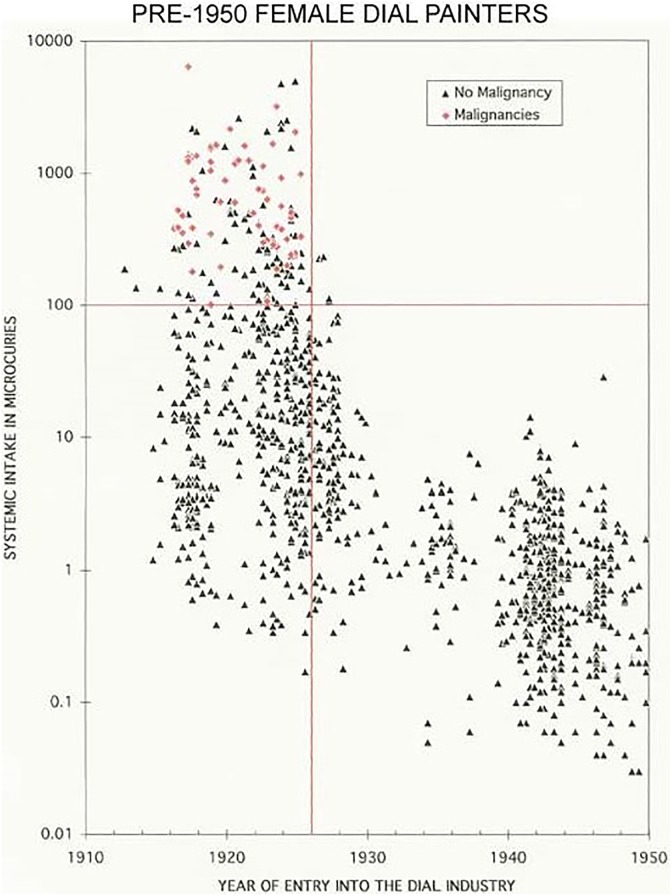
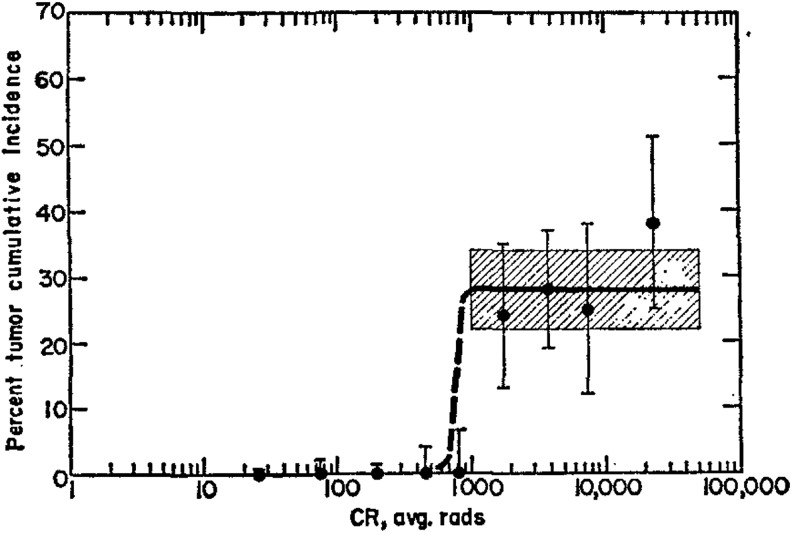
In 1910, the Standard Chemical Company began to extract radium for commercial applications, including treatments of many diseases through internal or external exposures.3 Physicians treated hundreds of patients, orally and intravenously, with 10 μg amounts of radium every week, with total doses ranging between 100 and 300 μg. The best known form available to the public in the 1920s was a solution, sold over the counter or by mail (Figure 4). Each 60 cm3 bottle was advertised to contain 2 μg (or 2 μCi) of radium. During the period 1925 to 1930, about 400 000 bottles were sold at $1 per bottle. Radium, like calcium, accumulates in bones. These sales ended in 1932 after the death of a prominent businessman from radium poisoning.3 He had consumed about 5000 μCi. Studies on the dial painters established that a systemic intake of 100 μCi of radium (Figure 5) is the threshold for malignancies; 10 Gy is the cumulative dose threshold for bone sarcoma (Figure 6).19,20
Figure 4.
Standard radium solution for drinking, 2 μg or 2 μCi.3
Figure 5.
Study of 1468 female radium dial painters indicated a 100 μCi systemic intake threshold for malignancies. There were 56 malignancies among the 1468 dial painters in this study.19
Figure 6.
Cumulative incidence of bone sarcoma versus cumulative rad (CR) from radium ingested by dial painters indicates a 10 Gy threshold. Note the plateau at about 30%, from 10 to 500 Gy.20
Early on, radiation exposures were used against cancers to reduce tumor size or slow their progression and eliminate cancer metastases. Cancer cells are perceived as foreign pathogens, which are targeted by the patient’s immune system. Physicians discovered that a low dose of radiation stimulates immunity. They also observed that radiation stimulates a patient’s protection systems leading to faster wound healing. X-ray treatments cured a wide variety of infections and inflammations, including sinus, inner ear, pertussis, adenoids, pneumonia, gas gangrene, carbuncles, and boils. Radiation was also very effective in relieving many kinds of inflammatory conditions, such as asthma, arthritis, and immune system disorders. Calabrese et al performed historical reviews of many of these diseases that were treated by radiation stimulation (hormesis) of the patients’ natural defences.21-28 There were no reports of increased cancer incidence among these patients.
An assessment of the radiotherapy treatments of more than 37 000 patients with inflammatory diseases and conditions (Table 1) concluded that the optimal dose is between 30 and 100 r. This assessment also reviewed recent findings, which indicated that a low dose of radiation, like certain chemical agents, can mediate its anti-inflammatory effects by inducing a response involving the polarization of macrophages toward an anti-inflammatory M2-like phenotype.29
Table 1.
Diseases/Conditions Treated With LDIR Therapy.a
| Number of Patients | Successful Treatment, % | Studies, N | References29 | |
|---|---|---|---|---|
| Arthritis | >5000 | ∼85 | Cumulative experience | Kahlmeter and Kuhns and Morrison |
| Bronchial asthma | ∼4000 | 75-80 | 57 | Calabrese et al |
| Carbuncles | 187 | 60-90 | 5 | Calabrese |
| Cervical adenitis | 893 | 75-90 | 11 | Calabrese and Dhawan |
| Deafness | 15 000 | >95%; performed prior to age 15 | Cumulative experience | Crowe and Baylor |
| Furuncles | 420 | 75-95 | 5 | Calabrese |
| Gas gangrene | 365 | Mortality rate decreased from | 13 | Calabrese and Dhawan |
| 40% to 10% | ||||
| Otitis media/mastoides | 564 | ∼90 | 16 | Calabrese and Dhawan |
| Pertussis | ∼2400 | ∼80 | 22 | Calabrese et al |
| Pneumonia | 863 | 80-85 | 18 | Calabrese and Dhawan |
| Sinus infection | 4492 | 75-90 | 16 | Calabrese and Dhawan |
| Tendonitis/bursitis | 3333 | 70-90 | 31 | Calabrese and Dhawan |
| 37 517 |
a The High Success Rates.
Calabrese has recently urged the evaluation of low-dose radiotherapy as a potential adjunct treatment for necrotizing fasciitis, also known as flesh-eating disease.30 Other than prompt surgical removal of the infected tissue, there is no other remedy for this life-threatening disease.
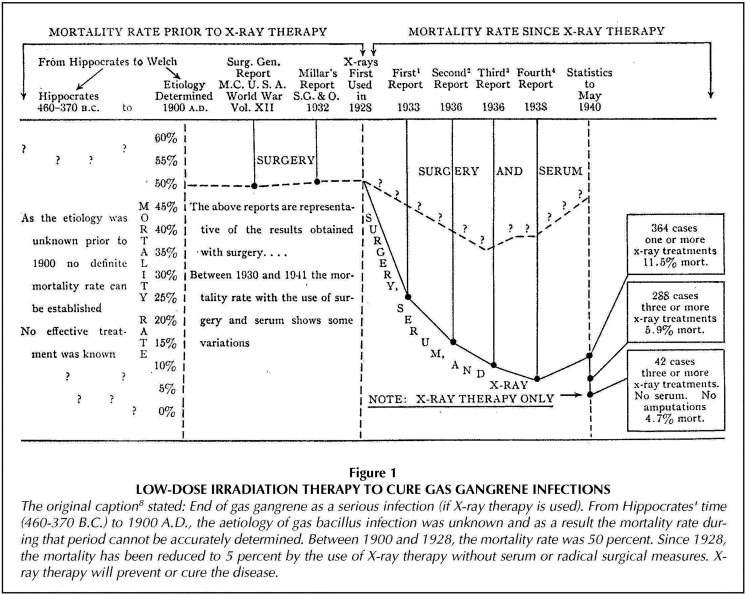
A study of gas gangrene cases prior to 1940 indicated that 3 or more X-ray treatments of 50 r, to prevent or cure the disease, could lower mortality from about 50% to only 5% (Figures 7 and 8).31
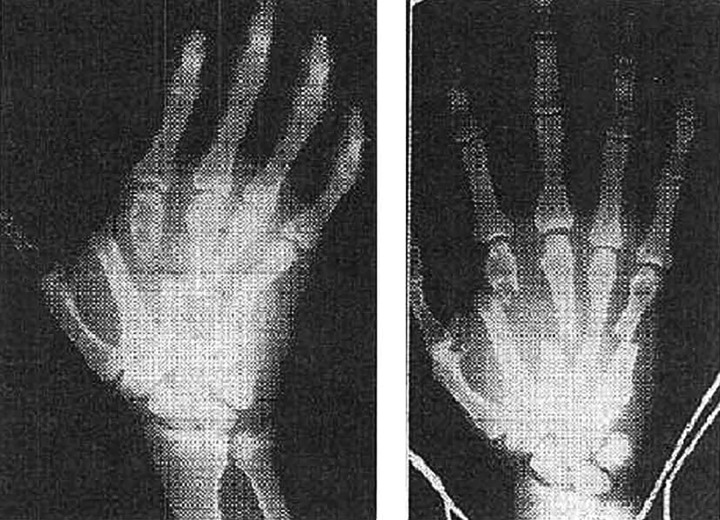
Figure 7.
Severe hand injury with some gas in tissue (left) and same hand a few days after prophylactic X-ray irradiation (right).31
Figure 8.
Reduction in gas gangrene mortality from 50% to 5% by using X-ray therapy.31
“Nasal radium irradiation” (NRI) was widely used from 1940 through 1970 to treat ear dysfunction in children (Figure 9) and military personnel. Use of NRI was stopped when concern arose about possible adverse effects, including cancer. The purpose of NRI was to shrink swollen tissue in the nasopharyngeal cavity—the opening between the nose and mouth. The treatment involved inserting 2 radium probes through the nostrils into the cavity for short periods of time (Figure 10). Some radiation exposure to the salivary, thyroid, and pituitary glands and to brain tissue also occurred during the process. Nasal radium irradiation was used in several European countries, Canada, and the United States. In the United States, it is estimated that between 0.5 million and 2.5 million children and at least 8000 military personnel were treated with NRI. Children are considered to be the most vulnerable to radiation-related cancers. At this time, worldwide studies have not confirmed a definite link between NRI exposure and any disease.”32-34
Figure 9.
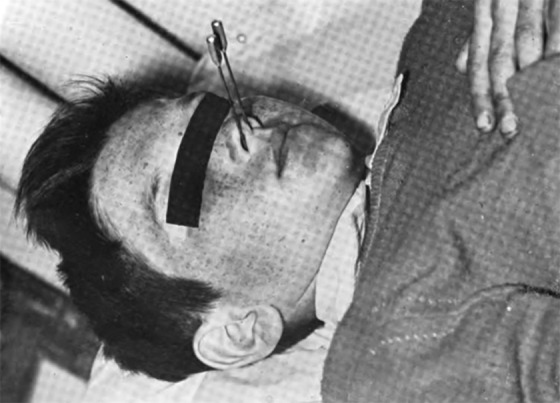
Child receiving nasal radium irradiation treatment.35
Figure 10.
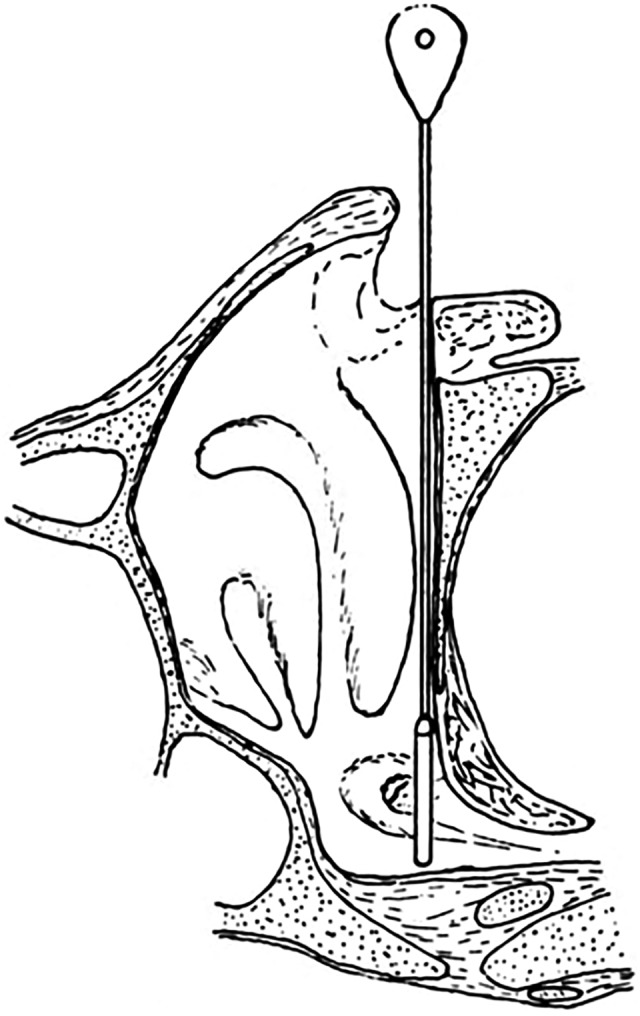
Position of the 2 radium probes.35
For a 12-minute treatment, the gamma dose on contact was about 20 Gy; the dose at 1-cm depth was 2 Gy. The beta dose from each applicator was 0.7 Gy. The standard treatment is 3 times with intervals of 2 weeks between applications. Beneficial results may be expected within 6 to 12 weeks.35 The evidence of so many treated safely by NRI and the lack of any evidence of harm justify the dismissal of any concerns that children are particularly sensitive to LDIR exposures.
Present-Day Physicians Avoid Treatments With Low Doses of Radiation
Treatments with LDIR became very controversial after the 1956 NAS recommendation was issued. Physicians began prescribing antibiotics and chemical treatments instead of treatments with low doses of radiation. For many decades, radiologists have been carefully taught the LNT ideology that any exposure to IR carries a risk of cancer.36 They are constantly urged to avoid any use of such radiations and to minimize the dose of diagnostic X-rays and computed tomography (CT) scans.37,38 The potential benefit of any procedure that uses IR is to be weighed against the risk of cancer, as calculated by the LNT model. It appears to be unacceptable for physicians to learn about or use LDIR therapy.
Medical textbooks fail to mention an important characteristic of the normal aerobic metabolism, namely that the mitochondria leak ROS, which cause endogenous damage to DNA and other biomolecules at a very high rate.39 Pollycove and Feinendegen have pointed out that very powerful adaptive protection systems have evolved, which act against this high rate of DNA and other biomolecular damage.40 Physicians are not taught the experience of the past 120 years that low doses of radiation stimulate the protection systems, including the immune system, which involve more than 150 genes. They do not learn about the biphasic dose–response model (Figure 2) and are unaware of dose thresholds for the onset of radiogenic cancer. Without an informed medical community, it is impossible for researchers to initiate clinical studies of LDIR therapies that would stimulate a patient’s protection systems. When conventional treatments fail to remedy a patient’s life-threatening disease and an LDIR therapy is provided as a last resort, a case report may be issued that describes the significant benefits observed.
Recent Medical Therapies
Low-Dose X-Ray Therapy of Cancer
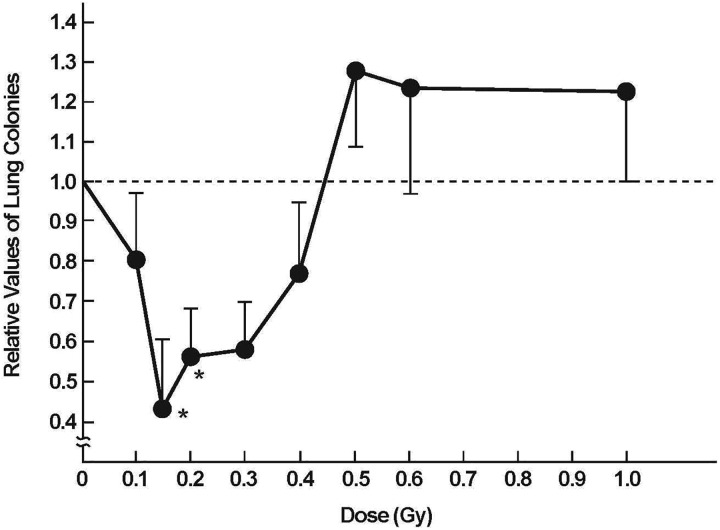
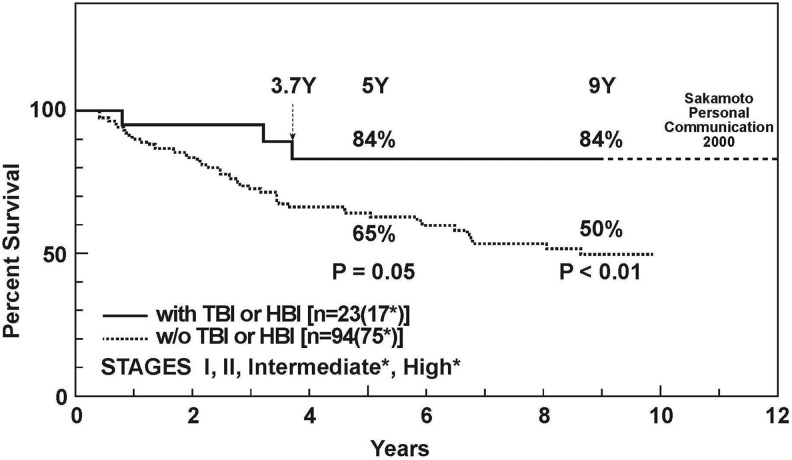
Sakamoto carried out a series of fundamental studies on mice in the early 1990s to understand the effects of X-rays on the immune system. He discovered that a low dose of total- or half-body radiation (TBI/HBI) stimulates immunity. He also found that tumor cell killing is enhanced by a high dose of radiation, locally to the tumor, given 12 hours after delivering 0.1 Gy of TBI. Furthermore, distant metastases can be suppressed by TBI treatments of 0.1 or 0.15 Gy (Figure 11). With this very important evidence, he and his team members were able to carry out a successful clinical trial on about 200 patients with non-Hodgkin’s lymphoma, demonstrating that low-dose TBI or HBI therapy provides a significantly better cure rate than conventional therapies (Figures 12 and 13).41 Unfortunately, government funding to carry out more clinical studies was not provided.
Figure 11.
Low dose of ionizing radiation depresses lung metastases in mice when total body radiation is given 12 days after tumor cell transplantation into groin. Lung colonies were counted 20 days after TBI occurred.41
Figure 12.
Survival of patients with non-Hodgkin lymphoma treated by the combined treatment of low-dose total body radiation (TBI) and high-dose local irradiation (solid line) versus patients treated by high-dose local irradiation alone (dotted line).41
Figure 13.
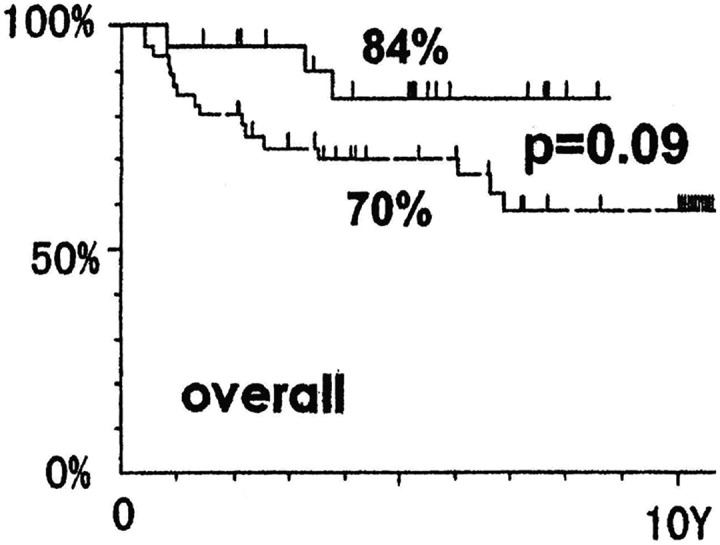
Survival of patients with non-Hodgkin lymphoma, stage I and II, treated by the combined therapy of low-dose total body radiation (TBI) and high-dose local irradiation (solid line) versus patients treated by the combined therapy of high-dose local irradiation followed by chemotherapy (dashed line).41
One of the members in Sakamoto’s team developed advanced ovarian cancer. She was given a course of TBI therapy (1.5 Gy) that totally removed the malignancy.41 Another had advanced colon cancer and was treated by this therapy successfully. In 1999, Dr Sakamoto mentioned to the author that he himself had received this therapy plus a repeat course, 6 months later, as a “booster” to prevent recurrence.
The author is very familiar with the case of a patient with Waldenstrom macroglobulinemia who received 2 courses of low-dose TBI X-ray therapy in 1999. Significant improvement was observed, and a case report about this treatment was later published.42 A close colleague had Hurthle cell thyroid cancer, which metastasized to his lungs. The progression of the lung tumors was arrested in 2009 by low-dose TBI. The author’s wife had grade 3 uterine cancer at stage 1. After surgery in 2011, she received low-dose HBI as a prophylaxis against cancer recurrence. The concentration of the NK cells in her blood was significantly elevated. The oncologist did not write case reports about these 2 patients and did not treat any other patients with LDIR therapy.
Pollycove provided an excellent description of the radiobiological basis of low-dose irradiation in the prevention and therapy of cancer.43 He furnished much evidence, pointing out that LDIR, including acute doses up to 0.3 Gy, stimulates each component of the homeostatic antimutagenic control system of antioxidant prevention, enzymatic repair, and immunologic and apoptotic removal of DNA alterations. On the other hand, high-dose IR suppresses each of these protective components. He urged that clinical trials be carried out on LDIR immunotherapy of breast, prostate, colorectal, and ovarian cancers and lymphomas to optimize this therapy.43 The current protocol for treating humans, 10 whole-body, 0.15-Gy dose fractions, is still based on the mouse data that Sakamoto measured more than 25 years ago.41
A pilot study was proposed recently to treat pancreatic cancer with LDIR therapy.44 However, the oncologists were unwilling to apply for funding and did not submit an application to their research ethics board.
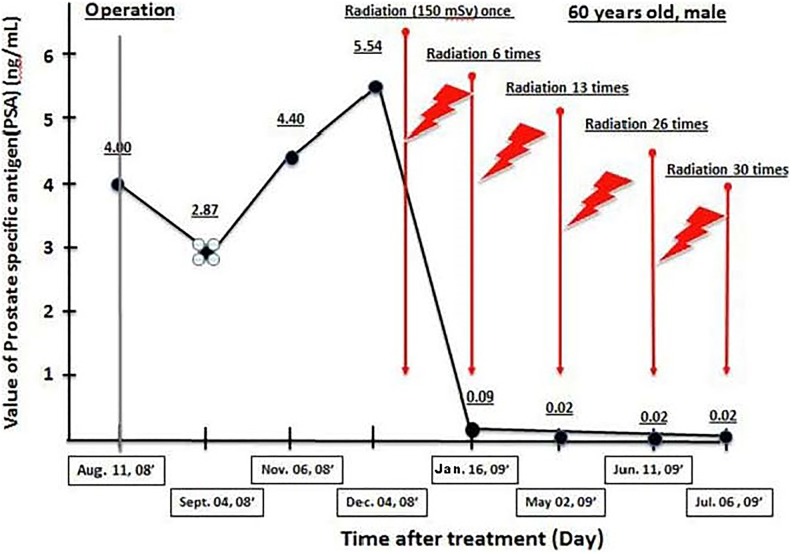
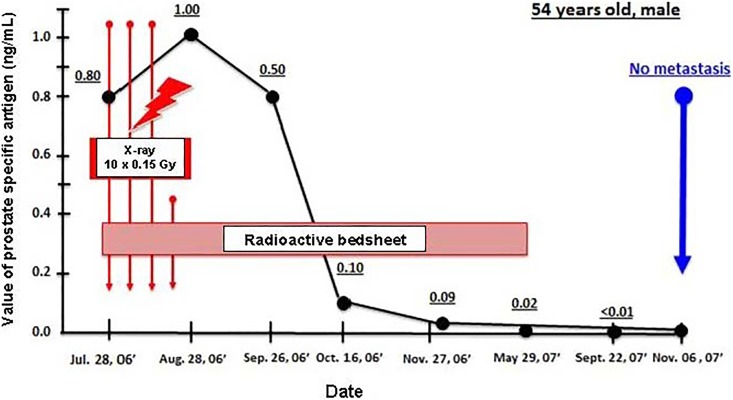
An article by Kojima et al described case reports on the successful treatment of 3 patients in 2017. Sakamoto’s TBI method was used on 2 patients with metastasized prostate cancer. The third patient had colitis and received radon therapy.45 (The 3 had received standard treatments for their diseases with unsatisfactory outcomes.) The first patient received a 0.15 Gy dose of X-rays (125 kV, 3 mA) once a week, 30 times in total. His prostate-specific antigen (PSA) value started decreasing immediately from 5.54 and reached 0.085 by the sixth treatment (Figure 14). The second patient had end-stage prostate cancer with bone metastases. His PSA had been 860 and, following chemotherapy at several hospitals, had been reduced to 5.8 and then 1.0. After it rose to 4.8, he came for hormesis treatments and received ten 0.15 Gy treatments over 3 weeks. He also used a radon bedsheet for 6 hours each night for about 10 months. His PSA value decreased to 0.008 (Figure 15); the metastases were not apparent in the 99mTc-HMDP bone scans.45 The third patient had intractable ulcerative colitis since age 15. He received LDIR therapy in the hormesis (radon) room for 40 minutes, twice a week, and drank 200 mL of water containing 330 Bq/L of radon with every meal. He used a radon bedsheet each night. Bleeding and swelling completely disappeared after 8 months of treatment. A year after starting the treatment, he was in good health.45
Figure 14.
Prostate-specific antigen (PSA) change shows the improvement in the condition of patient with prostate cancer after 30 X-ray treatments of 0.15 Gy each.45
Figure 15.
Prostate-specific antigen (PSA) change shows the improvement in the condition of patient with prostate cancer after 10 X-ray treatments of 0.1 Gy and radon bedsheet therapy each night, for 10 months.45
Radon Therapy
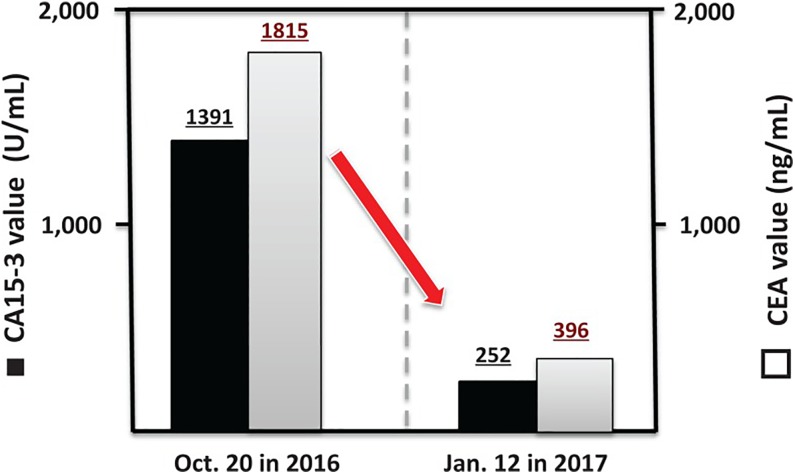
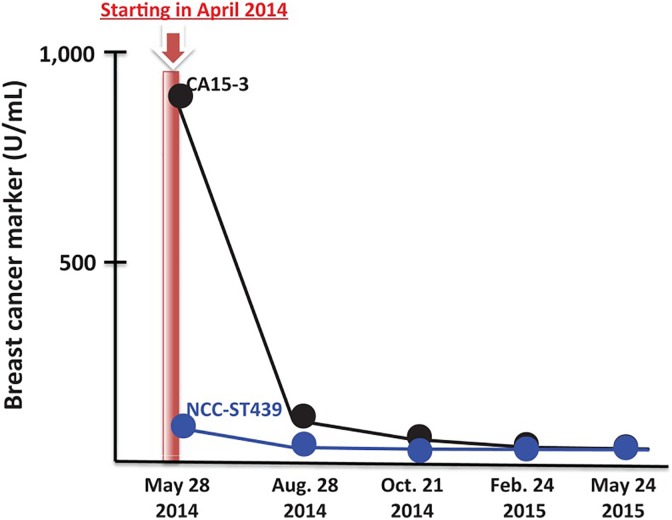
The second and third patients, described in the abovementioned case reports, received radon therapy that was very effective.45 A subsequent article discussed present and future prospects of cancer therapy using targeted and nontargeted α-emitters.46 It presented evidence of 2 patients with metastatic breast cancer who had refused conventional treatments. The first patient, with metastases to her brain, chest, and lumbar vertebrae, received radon therapy. Three days per week she inhaled 0.5 to 1.0 MBq/m3 for 40 minutes, twice, from a radon generator. All of her breast cancer symptoms were significantly alleviated after 8 months of treatment. Figure 16 shows the decrease in the markers CA15-3 and CEA. The second patient, with metastases to her bones, received LDIR for 40 minutes twice daily in a hormesis room. The radon concentration was 9800 Bq/m3, and the γ-radiation level was 11 μSv/h. After a year of these treatments, the cancer markers improved to normal values (Figure 17) and her cancer symptoms disappeared.46
Figure 16.
Cancer markers show improvement in condition of patient with breast cancer after inhaling radon, 0.5 to 1.0 MBq/m3, for 40 minutes, twice, 3 days per week.46
Figure 17.
Cancer markers show improvement in condition of patient with breast cancer after 40 minutes of hormesis room therapy, twice daily (radon = 9800 Bq/m3; γ-radiation level = 11 μSv/h).46
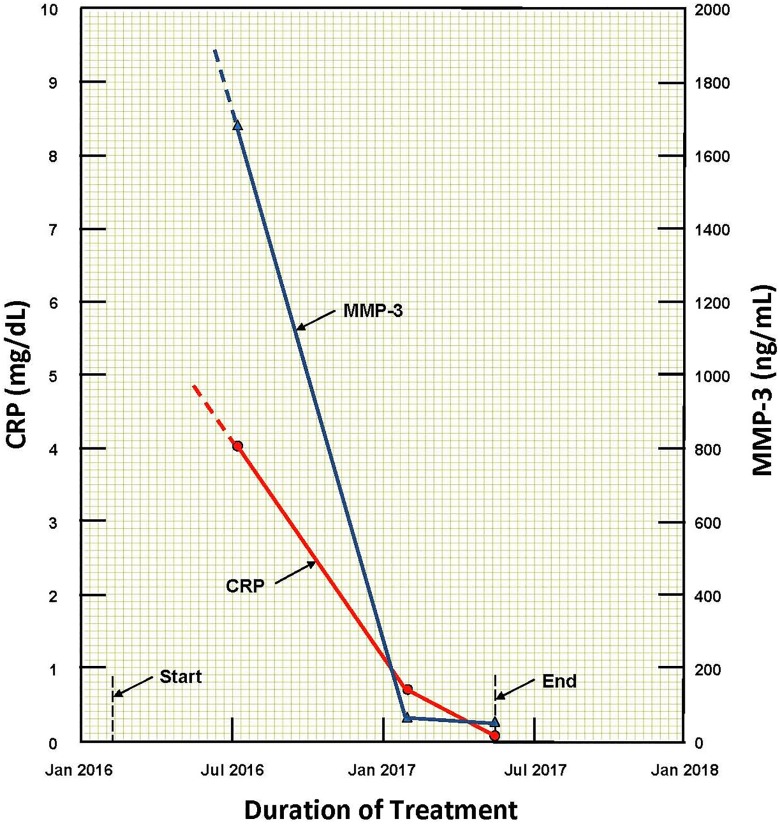
A patient recovered from advanced rheumatoid arthritis after 15 months of LDIR treatments. She received a daily 40-minute exposure in a hormesis therapy room followed by 10 consecutive radio-nebulizer treatments.47 The concentration of radon in the room was 0.2 MBq/m3; the average γ-radiation dose rate was 11 μGy/hour. The ultrasonic nebulizer vaporized 15 mL of radon water, which the patient inhaled in about 6 minutes. After 15 months of this therapy, the inflammation subsided and the pain throughout her body almost disappeared. She continues to take this therapy twice each month to prevent recurrence. Figure 18 shows the decrease in the values of the 2 markers as the patient gradually recovered from this autoimmune disease.47
Figure 18.
Changes in values of 2 markers show the improvement in the condition of a patient with advanced rheumatoid arthritis after receiving hormesis room and radon nebulizer therapies.47
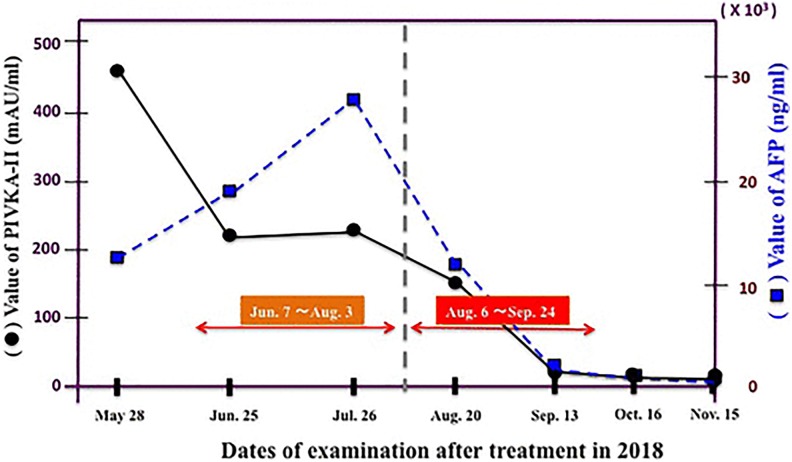
Treatment of 4 different types of cancer was reported by Kojima et al.48 The patient with stage IVB colorectal cancer and the patient with advanced uterine body cancer both received radon room therapy. The concentration was 0.2 MBq/m3, and the duration of each treatment was 40 minutes. The 3 markers for the patient with colon cancer decreased to their normal range. The patient with uterine cancer experienced significant reductions in the size of the tumors in her lungs. Radon therapy is ongoing for both patients. The patient with stage IV lung cancer and the patient with stage IVB hepatocellular cancer both received therapy from a radon generator. In addition to chemotherapy, the patient with lung cancer inhaled radon at a concentration of 2 MBq/m3 for 40 minutes, 3 times a week. The markers improved significantly, and his brain metastases disappeared; the treatments continue. The patient with liver cell cancer received conventional treatments; however, they did not provide adequate improvement. He started radon therapy with a concentration of 1 MBq/m3, but the markers did not improve. When the concentration was increased 6-fold to 6 MBq/m3, the markers decreased dramatically to the normal values (Figure 19). Treatments were stopped when the metastatic tumors were no longer detectable.48 There appears to be an optimal radon concentration for treating liver cell cancer and likely for other cancer types.
Figure 19.
Cancer markers show improvement in condition of patient with liver cell cancer after the radon concentration was increased 6-fold to 6 MBq/m3 on August 6, 2018.48
This success was followed by the treatment of 2 patients with autoimmune diseases.49 The first patient had severe pemphigus, a skin disease. After receiving conventional treatments for a month, she began hormesis room therapy for 40 minutes, followed by 1 hour of inhalation of radon, 1 MBq/m3, from a generator. After receiving these treatments, once or twice each week, for about 3 months, the disease largely subsided, as shown in Figures 20A and 20B (before and after these novel therapies). However, the treatments have been continued at the patient’s request. The treatment of the second patient, who has diabetes mellitus, confirmed the improvement potential of radon therapy for type I diabetes. He received 2 hormesis room treatments, once a week, for the first 2 months. Then he received 2 treatments, 4 times a week, for 3 months. His elevated marker decreased progressively to the upper limit of the normal range, 8 months after the start of the therapy.49
Figure 20.
Pemphigus autoimmune disease subsided after patient received hormesis room and radon generator therapies.49
Potential Therapy to Remedy Alzheimer Dementia and Parkinson Disease
A patient with advanced Alzheimer dementia (AD) was transferred to hospice in April 2015. Her husband asked the author whether LDIR could extend her life. The author recalled reviewing a paper in 2013 which presented evidence that LDIR could upregulate adaptive protection to control neurodegenerative diseases.50 The husband arranged for standard CT scans of the patient’s brain and observed the partial restoration of her cognition, memory, speech, movement, and appetite (Figure 21).51 Ongoing CT scan treatments were provided to sustain the improvements. The husband, who has Parkinson disease, arranged to receive a standard CT scan of his brain. He perceived a significant reduction of tremor. After repeated treatments, he determined that an interval of 4 to 6 weeks is optimal. He also experienced improved vision and hearing. This novel discovery was published in a 2016 case report and 2 update letters in 2017 and 2018.51-53 After 3 years of improved quality of life, the patient with AD deteriorated and died.
Figure 21.

Author beside patient with Parkinson disease and patient with Alzheimer dementia. Computed tomography scans of brain-stimulated protection against oxidative stress, restoring a degree of cognition, memory, speech, movement, and appetite.51-53
The author urged neurologists in several cities to carry out studies to repeat this experience and was somewhat surprised at their reluctance to perform this harmless, but controversial method of treatment. Fortunately in May 2017, 2 hospitals in Toronto agreed to carry out a pilot study. Progress has been slow due to lack of funding and delays in recruiting participants like the patient described in the 2016 case report.51 The procedure has been to evaluate each patient as prescribed in the study protocol, before and after providing the same 3 initial CT scan as in 2015. A more objective and quantitative approach to assess efficacy would be the measurement and tracking of good markers for oxidative distress in AD, such as F2-isoprostanes in cerebrospinal fluid.54 It could allow the optimal dose and repeat interval to be determined. However, sampling and analyzing these markers is invasive and costly.
Low Dose of Ionizing Radiation Stimulation of Protection Systems in Aerobic Organisms
Antimutagenic DNA damage control is the central component of the homeostatic regulation that is essential for survival. These complex protective systems evolved to control the vast number of DNA alterations produced naturally by ROS, generated principally by leakage of free radicals from mitochondrial metabolism of oxygen (Figure 1).8,40 Aging, mortality, and cancer are associated with stem cell accumulation of permanent alterations of DNA, that is, the accumulation of mutations. In a young adult, the protective systems of prevention, repair, and the removal of DNA alterations reduces ∼1 million alterations per cell per day to ∼1 mutation per cell per day (Figure 22). DNA alterations from background radiation produce about 1 additional mutation per 10 million cells per day. As mutations accumulate and gradually degrade the protective systems, aging progresses at an increasing rate, mortality increases correspondingly, and cancer incidence increases exponentially with age. During the past 5 decades, genomic, cellular, animal, and human data have shown that LDIR (acute doses of up to 0.3 Gy) stimulates each component of the protective systems of antioxidant prevention, enzymatic repair, and immunologic and apoptotic removal of DNA alterations. On the other hand, high-dose IR suppresses each of these protective components. Both studies of cancer in animals and clinical trials of patients with cancer also show the beneficial effects of low-dose radiation.40,43
Figure 22.
DNA damage control biosystem, showing the effects of the 3 types of natural adaptive protection systems against reactive oxygen species (ROS)-induced damage in human cells.40
Ionizing radiation causes targeted and nontargeted molecular damage due to energy deposition along tracks of charged particles (Figure 1). Each burst of absorbed energy damages biomolecules. Since about three-fourths of tissue is water, the majority of initial damage breaks water apart, mostly to toxic ROS and H2O2. The energy deposited per unit of tissue mass is the dose, and the amount of dose per unit of time is the dose rate. A long interval between “hits” allows the cell to operate its protective mechanisms without interference from the next hit. Indeed, the amount of damage is smaller per unit of dose when the dose rate is low than when it is high.55
If not immediately repaired or removed, primary damage becomes persistent damage and/or causes secondary cellular responses. Genetic changes of different kinds occur. After a low dose, changes in intra- and intercellular cell signaling of the stress response type appear to dominate. Later in life, diseases such as cancer arise from cells that have acquired mutations and genomic instability and have become resistant to protective mechanisms in the body.55
Primary radiogenic damage triggers secondary responses at the molecular, cellular, and tissue levels. These responses may propagate and amplify damage but may also activate tissue-inherent protective mechanisms of damage prevention (scavenging of toxins), repair (including DNA repair), and damaged cell removal in various ways (such as by apoptosis and immune responses). These secondary responses operate in a nonlinear fashion with respect to dose. At low doses, damage prevention and handling in irradiated tissues are dominant. The biological responses to radiation are genetically determined. Thus, some people are more radiosensitive.55
Following a low dose, responses are induced both instantly and after delay. The immediate responses can result in both the propagation of damage and the induction of repair and reconstitution of functional homeostasis. Delayed responses are similar to stress responses as consequences of changed cellular signaling in and between cells. They appear in cells and tissues, within hours after a low-dose irradiation, and can express upregulation of various physiological protections in terms of damage prevention, repair, and removal. This is accompanied by changes in gene expression. The delayed responses express system adaptation, following sublethal system disturbances, and are referred to as adaptive responses or adaptive protections. More than 150 genes are active in this adaptive protection.55
The efficacy of adaptive protections rises to a maximum at about 0.1 Gy and increasingly vanishes as the radiation dose increases above about 0.2 Gy. The dose–response model is biphasic, as shown in Figure 2. Adaptive protections may persist for hours to months and even for a lifetime, stimulating damage prevention, repair, and removal in the irradiated organism, regardless of the causal history of the damage, be it radiogenic or nonradiogenic.
Some damage, such as cell transformation, may escape defenses and evolve into, for instance, cancer cells. However, the degree of protection by the immune system against cancer cells tends to be relatively high after low doses and in young people. There is no proportionality between the expression of these mechanisms and the radiation dose.55
Adaptive protections are readily and easily observed experimentally. This also follows from the fact that, at low doses, the ratio of probabilities of a cell signaling event, which is considered a radiogenic burst of signaling substrates (per average hit), to a DNA double-strand break event is about 100. This ratio speaks in favor of benefit far outweighing the risk of detriment at low doses because stress response signaling is viewed as the gatekeeper to survival, its stimulation thus being beneficial.55
Evidence of a Dose Threshold for Radiation-Induced Cancer
When LDIR therapy is discussed, concerns are expressed about it increasing the risk of cancer. Cancers are complex diseases that are not well understood, with many causes and confounding factors that affect incidence and morbidity. Based on the therapeutic evidence presented in this article, LDIR therapy is expected to decrease the risk of cancer because it stimulates the immune system to destroy cancer cells more effectively than it would without the LDIR stimulation.
A radiation dose can increase cancer risk by 2 mechanisms. First, a high dose will inhibit or damage the immune system, allowing more nonradiogenic cancer cells to evade immune attack. If an organism survives a short-term, high-dose challenge and recovers without succumbing to infectious pathogens, the immune system may regain much of its former efficacy, including the suppression of cancer. Notable examples are the atomic bomb survivors and Chernobyl workers who recovered from high, acute exposures and achieved remarkably long lifespans.
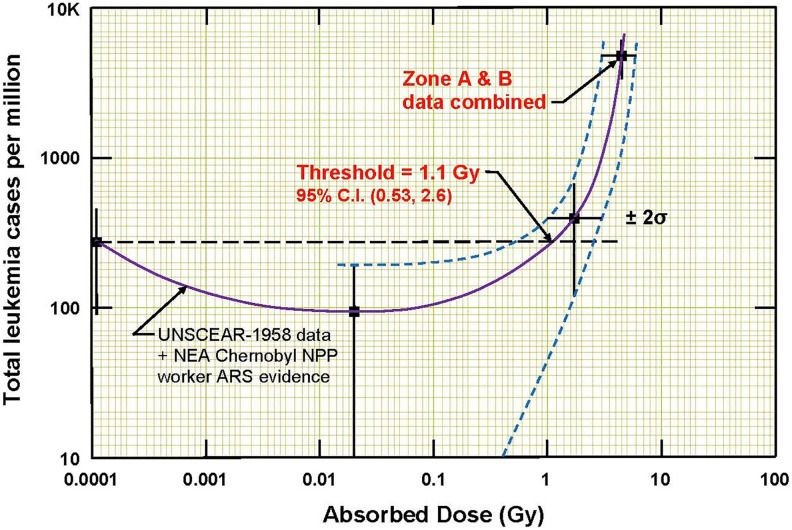
Second, a dose mutates stem cells, and some of them will progress over the course of years into cancer cells that can evade the immune system. Since blood-forming stem cells in the bone marrow of mammals are very radiation sensitive,56 radiation-induced blood cancer (leukemia) should be expected after a high exposure. Recently, data on the incidence of leukemia among 95 819 Hiroshima atomic bomb survivors were analyzed, and this analysis indicated that leukemia was induced only in those who received a dose in excess of about 1.1 Gy (Figure 23).57,58 This apparent threshold for an acute dose to induce cancer is much higher than the doses that are used in LDIR therapy. The incidence started to increase about 5 years after the exposures, and it returned to the natural incidence level about 10 years later, which is what might be expected for radiation-induced cancer. It is very important to note that the incidence of this radiogenic cancer was quite low, only about 0.5% of the survivors who were in the very high radiation areas.57,58
Figure 23.
Evidence of a threshold at 1.1 Gy for radiation-induced leukemia from an analysis of the data of 95 819 Hiroshima atomic bomb survivors.57,58
This evidence suggests that radiation induction of cancer, in cells that are less radiosensitive than blood-forming cells, would likely occur at a threshold higher than 1.1 Gy and that the incidence of such cancers would likely be lower than that for radiogenic leukemia.
Evidence of Long-Term Survival Following High-Dose Radiation Exposures
Follow-up on the health of 106 workers, who recovered from very severe radiation exposures received during the 1986 Chernobyl nuclear reactor accident, provides evidence that contradicts the hypothesis or assumption of acute radiation-induced delayed adverse health effects in humans. Table 2 shows the radiation exposures of the workers who were hospitalized for acute radiation syndrome. The dosimeters they wore were overexposed, so biological dosimetry was used to determine their radiation doses.59
Table 2.
Radiation Doses of Persons Hospitalized for Acute Radiation Syndrome.59
| Number of Patients | Estimated Dose, Gy | Deaths During the First Weeks |
|---|---|---|
| 21 | 6-16 | 20 |
| 21 | 4-6 | 7 |
| 55 | 2-4 | 1 |
| 140 | <2 | 0 |
| Total: 237 | 28 |
Of the 237 hospitalized, 134 were heavily irradiated, 28 died, and 106 persons remained alive. From among these 106 persons, 22 died during the next 19 years, which gives the mortality rate of 1.09% per year, much lower than the average mortality rate in 2000 in Russia (1.4%).60
From among the 106 persons who remained alive, 26 died during the following 30 years,61 which gives the mortality rate of 0.82% per year. The number of cancer deaths is 27% of the 26 deaths, which is about the same as the fraction of cancer deaths among all mortality causes for Central Europe.
Conclusions
This article is about the author’s presentation at the symposium of the Polish Radiation Research Society held in Kielce on September 17 to 18, 2019. It outlines notable medical therapies that used LDIR, more than 80 years ago, and describes recent therapies that the author has been associated with.
Many important treatments were discovered in the early 1900s while using X-rays to diagnose diseases. They were very successful for cancer, arthritis, tendonitis, asthma, carbuncles and boils, sinusitis, inner ear infections, deafness, inflamed adenoids, pneumonia, pertussis, and gas gangrene. X-ray therapy reduced gas gangrene mortality from 50% to 5%. A review of studies on 37 517 patients who received X-ray treatments found the optimal dose to be in the range from 0.3 to 1.0 Gy.
Patients were prescribed radium in the 1920s as a treatment, and many consumed 2 μCi amounts of radium as an elixir. Studies on the dial painters established the systemic intake of 100 μCi of radium to be the threshold for the onset of malignancies. A study of tumor cumulative incidence versus cumulative dose demonstrated 10 Gy to be the threshold for the onset of bone sarcoma.
Nasal radium irradiation was used from 1940 through 1970 to treat ear dysfunction. It was stopped when concerns arose about adverse effects, including cancer. In the United States, between 0.5 million and 2.5 million children were treated. Children are considered the most vulnerable to radiation-related cancers, but worldwide studies have not confirmed a definite link between NRI exposure and any disease. The evidence of so many treated safely and the lack of any evidence of harm justifies dismissing any concerns that children are sensitive to harm from LDIR therapy.
Sakamoto carried out fundamental studies on mice in the 1990s and gained an understanding of the effects of X-rays on the immune system. He discovered that low-dose TBI stimulates immunity and that a total body dose of about 0.15 Gy is optimal (for mice). He found tumor killing to be enhanced by the combined therapy of high-dose irradiation, locally to the tumor, given 12 hours after 0.1 Gy of TBI. He showed that TBI of 0.1 or 0.15 Gy suppresses metastases and then applied what he learned in a successful clinical study on 200 patients with non-Hodgkin lymphoma.
A key paper by Pollycove provides the radiobiological basis of using LDIR in the prevention and therapy of cancer. Papers by Feinendegen point out that stimulation of the adaptive protection systems, following a nontargeted low dose of radiation, occurs due to the signaling triggered by the burst of hits and the ROS produced.
Four recent papers by Kojima et al have detailed evidence of 12 patients who were successfully treated with nontargeted X-rays and/or radon. Two had metastasized prostate cancer; their PSA markers decreased to 0.085 and 0.008 after the therapy. Two had metastasized breast cancer that receded into remission. Four had advanced cancer: colorectal, uterine, lung, and liver cell. Their disease symptoms diminished significantly during the LDIR treatments. Four had advanced autoimmune diseases—colitis, rheumatoid arthritis, pemphigus, and type I diabetes. All were very satisfied by the improvements achieved following the LDIR therapies that they received. The radon concentrations employed in the 40-minute inhalation treatments were in the range from 0.2 to 6 MBq/m3.
Evidence was presented on 2 patients with neurodegenerative diseases who received CT scans of the brain. The patient with Alzheimer disease recovered some cognition, memory, speech, movement, and appetite. The patient with Parkinson disease benefitted from a decrease in the symptoms; he stopped taking medication. Improvement in his vision and hearing was also recorded.
Early radiologists had radiation burns and elevated cancer incidence until 1924 when a safe tolerance dose rate, 0.2 r per day, was proposed. The ICRP adopted this dose rate limit in 1934. However, the annual occupational dose limit decreased stepwise, from 70 rem in 1924 to 5 rem in 1958, because of changing attitudes toward acceptable risk. The ongoing adherence to LNT ideology requires that exposures be kept as low as reasonably achievable.
Muller’s X-ray treatment of fruit flies increased their mutation rate by a factor of 150 over that of the controls. He used a dose rate that was 8 orders of magnitude, ∼100 million times, greater than the average dose rate of natural background radiation. So, his “proportional rule” idea was based on very high treatment doses. He ignored evidence in 1946 of a dose threshold at about 0.5 Gy.
The US NAS started the unscientific radiation scare in 1956. The LDIR therapies were replaced by antibiotic and biochemical treatments. Physicians have been carefully taught the LNT ideology about cancer risks. They are constantly urged to avoid unnecessary radiation exposures and to minimize the dose. The benefit of any procedure must be weighed against the risk of cancer, as calculated by the LNT model.
Medical textbooks do not mention an important characteristic of the normal aerobic metabolism, namely that the mitochondria leak ROS, which cause endogenous damage to the DNA and other biomolecules at a very high rate. Also not discussed are the very powerful adaptive protection systems against this natural internal hazard. Physicians are not taught the experience of the past 120 years—that LDIR stimulates the protection systems, including the immune system, which involve more than 150 genes. The biphasic dose–response model is not taught nor is the existence of a dose threshold for the onset of radiogenic cancer. Without an informed medical community, how is it possible for researchers to initiate clinical studies on LDIR therapies that would stimulate a patient’s protection systems?
An LDIR is defined to be a dose that creates a burst of hits and ROS large enough to stimulate the protective systems and produce observable health benefits, but not greater than the threshold for the onset of observable harmful effects. The evidence of a dose threshold at about 1.1 Gy for radiation-induced leukemia, with an incidence of only 0.5%, was discussed, as well as the implications for other types of cancer. The doses used in successful LDIR therapies were below this threshold, suggesting that the likelihood is very low that they would increase the risk of cancer.
The actual data from the ongoing studies on the health of 106 workers, who recovered from very severe acute radiation exposures received during the 1986 Chernobyl accident, are evidence against the assumption that exposure to radiation induces latent adverse health effects.
Future of LDIR Therapies
The mechanism whereby a dose of IR induces signals that activate many adaptive protection system responses throughout an organism is very complex and poorly understood. Nevertheless, there are more than 120 years of human evidence—lifesaving medical remedies and welcome relief from chronic suffering that were provided by LDIR treatments.
As mentioned earlier, strong antinuclear political activity led the US NAS to recommend in 1956 an abrupt change from the threshold dose–response model to the LNT model to assess risk of radiogenic mutations/cancer. A robust international system of radiation protection is in place today that is based on the practice of as low as reasonably achievable, despite the long known dose thresholds for detrimental effects and Taylor’s 1980 statement: “Today we know about all we need to know for adequate protection from ionizing radiation. Therefore, I find myself charged to ask: why is there a radiation problem and where does it lie?”62
Fundamentally, there is an international consensus opinion on the attribution of radiation effects and the inference of radiation risk. In spite of the cancer crisis, factual evidence of the medical remedies by LDIR therapies is being ignored or shunned because it is not mainstream medicine and because it contradicts establishment opinion. The long-held beliefs and attitudes of physicians may begin to change in light of the case reports, discussed in this article, about recovery from different types of advanced cancer and autoimmune diseases following radon therapy. There is no remedy for many of the neurodegenerative diseases;63 however, a potential therapy for dementia using LDIR is mentioned in this article. Early results of a phase 1 clinical trial of this therapy are encouraging. The incidence of dementia is increasing dramatically as the population ages, and provision of long-term care is becoming very costly.64 If no other remedy is found, acceptance of this LDIR therapy by the medical community may become compelling.
Acknowledgments
Professor Ludwik Dobrzyński initiated the XVIII Polish Radiation Research Society Symposium on Applications of low radiation doses in medical diagnosis and therapy. The author is grateful for his invitation to participate and his encouragement to continue identifying and understanding the beneficial health effects of low doses of ionizing radiation. The author recognizes the very important contributions of Professor Ludwig Feinendegen to his understanding of the adaptive protection systems against radiogenic and nonradiogenic damage and the effects of IR on their performance. This article reflects the very significant contributions of Professor Edward Calabrese to the science of hormesis, the history of radiation hormesis, and its many medical applications. His comprehensive research into the history of the political activities that led to worldwide fear of radiation and his revelation of the scientific scandal will result in the eventual acceptance of very important old therapies and new low-dose therapies for cancer and neurodegenerative diseases. Finally, the author is grateful for the opportunity to participate in the research and development work of Professor Shuji Kojima on novel therapies against cancer and autoimmune diseases.
Authors’ Note: Now residing in Vaughan, Ontario, Canada, the author received his BASc&Eng diploma in engineering physics from the University Toronto in 1964 and his MSc and DSc diplomas in nuclear sciences from the Israel Institute of Technology in 1968 and 1971. In 1974, he was employed by Atomic Energy of Canada Limited to design and support the construction and operation of 25 nuclear energy reactors. In 2000, he began providing licensing-related services as a consultant to several nuclear plant owners. Since 1995, he has been collaborating with renowned medical scientists and radiobiologists to determine and understand the health effects of ionizing radiation.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jerry M. Cuttler  https://orcid.org/0000-0001-9532-9818
https://orcid.org/0000-0001-9532-9818
References
- 1. Historical Medical Library of the College of Physicians of Philadelphia. Radium. https://histmed.collegeofphysicians.org/for-students/radium/. Accessed December 11, 2019.
- 2. Brucer M. A Chronology of Nuclear Medicine. St Louis, MO: Heritage Publications; 1990. [Google Scholar]
- 3. Rowland RE. Radium in humans: a review of U.S. studies. Washington, DC; 1994. ANL/ER-3, UC-408. DOE. [Google Scholar]
- 4. Sanders CL. Radiation Hormesis and the Linear-No-Threshold Assumption. Berlin, Germany: Springer; 2010. [Google Scholar]
- 5. Sanders CL. Radiobiology and Radiation Hormesis: New Evidence and its Implications for Medicine and Society. Cham, Switzerland: Springer; 2017. [Google Scholar]
- 6. Applications of low radiation doses in medical diagnosis and therapy Paper presented at: Polish Radiation Research Society Symposium; September 17-18, 2019; Kielce, Poland. [Google Scholar]
- 7. Clarke R, Valentin J. A history of the international commission on radiological protection. Health Phys. 2005;88(5):407–422. [PubMed] [Google Scholar]
- 8. Azzam EI, Jay-Gerin J-P, Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012;327(1-2):48–60. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3980444/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Calabrese EJ. Biphasic dose responses in biology, toxicology and medicine: accounting for their generalizability and quantitative features. Environ Pollut. 2013;182:452–460. [DOI] [PubMed] [Google Scholar]
- 10. Inkret WC, Meinhold CB, Taschner JC. Radiation and risk—a hard look at the data. Los Alamos Sci. 1995;23:116–123. https://fas.org/sgp/othergov/doe/lanl/pubs/00326631.pdf. Accessed December 11, 2019. [Google Scholar]
- 11. Muller HJ. Artificial transmutation of the gene. Science. 1927;66:84–87. [DOI] [PubMed] [Google Scholar]
- 12. Muller HJ, Mott-Smith LM. Evidence that natural radioactivity is inadequate to explain the frequency of “natural” mutations. Proc Nat Acad Sci. 1930;16:277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Calabrese EJ. Muller’s Nobel Prize data: getting the dose wrong and its significance. Environ Res. 2019;176: 108528 doi:10.1016/j.envres.2019.108528. [DOI] [PubMed] [Google Scholar]
- 14. Caspari E, Stern C. The influence of chronic irradiation with gamma rays at low dosages on the mutation rate in drosophila melanogaster. Genetics. 1948;33:75–95. http://www.genetics.org/content/33/1/75.full.pdf+html?sid=cb861a39-fb63-48c4-bcbe-2433bb5c8d6a. Accessed December 11, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Calabrese EJ. Muller’s Nobel lecture on dose-response for ionizing radiation: ideology or science? Arch Toxicol. 2011;85(12):1495–1498. [DOI] [PubMed] [Google Scholar]
- 16. Jaworowski Z. Radiation hormesis—a remedy for fear. Human Exp Toxicol. 2010;29(4):263–270. [DOI] [PubMed] [Google Scholar]
- 17. National Academy of Sciences (NAS)/National Research Council (NRC). The biological effects of atomic radiation (BEAR): a report to the public. NAS/NRC, Washington. 1956. Published as, Genetic effects of atomic radiation. Science. 1956;124:1157–1164.17788880 [Google Scholar]
- 18. Calabrese EJ. LNTgate: The ideological history of cancer risk assessment. Tox Res App. 2017;1:1–3. doi:10.1177/2397847317694998. [Google Scholar]
- 19. Rowland RE. Radium dial painters: what happened to them? Circa 2000. http://www.rerowland.com/Dial_Painters.pdf. Accessed December 11, 2019.
- 20. Evans RD. Radium in man. Health Phys. 1974;27:497–510. [Google Scholar]
- 21. Calabrese EJ. X-ray treatment of carbuncles and furuncles (boils): a historical assessment. Hum Exper Toxicol. 2013;32(8):817–827. [DOI] [PubMed] [Google Scholar]
- 22. Calabrese EJ, Dhawan G. The role of x-rays in the treatment of gas gangrene: a historical assessment. Dose-Response. 2012;10:626–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Calabrese EJ, Dhawan G. How radiotherapy was historically used to treat pneumonia: could it be useful today? Yale J Biol Med. 2013;86:555–570. [PMC free article] [PubMed] [Google Scholar]
- 24. Calabrese EJ, Dhawan G. The historical use of radiotherapy in the treatment of sinus infections. Dose-Response. 2013;11:469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Calabrese EJ, Dhawan G. Historical use of x-rays: treatment of inner ear infections and prevention of deafness. Hum Exper Toxicol. 2014;33(5):542–553. [DOI] [PubMed] [Google Scholar]
- 26. Calabrese EJ, Dhawan G, Kapoor R. Use of X-rays to treat shoulder tendonitis/bursitis: a historical assessment. Arch Toxicol. 2014;88:1503–1517. [DOI] [PubMed] [Google Scholar]
- 27. Calabrese EJ, Dhawan G, Kapoor R. The use of X-rays in the treatment of bronchial asthma: a historical assessment. Rad Res. 2015;184:180–192. [DOI] [PubMed] [Google Scholar]
- 28. Calabrese EJ, Dhawan G, Kapoor R. Radiotherapy for pertussis: an historical assessment. Dose-Response. 2017;2017:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Calabrese EJ, Dhawan G, Kapoor R, Kozumbo WJ. Radiotherapy treatment of human inflammatory diseases and conditions: optimal dose. Hum Exp Toxicol. 2019;38(8):888–898. [DOI] [PubMed] [Google Scholar]
- 30. Dhawan G, Kapoor R, Dhamija A, Singh R, Monga B, Calabrese EJ. Necrotizing fasciitis: low-dose radiotherapy as a potential adjunct treatment. Dose-Response. 2019;17(3):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kelly JF, Dowell DA. Twelve-year review of X-ray therapy of gas gangrene. Radiology. 1941;37:421–439. [Google Scholar]
- 32. National Cancer Institute. Nasopharyngeal radium irradiation (NRI) and cancers: fact sheet. 2003. https://stacks.stanford.edu/file/druid:st370yg4366/Fs3_87.pdf. Accessed December 11, 2019.
- 33. Centers for Disease Control and Prevention. Proceedings of 1995 workshop on the public health response to nasopharyngeal radium irradiation. J Otolaryngology Head Neck Surg. 1996;115:388–446. https://www.sciencedirect.com/journal/otolaryngology-head-and-neck-surgery/vol/115/issue/5. Accessed December 11, 2019. [Google Scholar]
- 34. Centers for Disease Control and Prevention. Centers for Disease Control and Prevention Brochure. Nasopharyngeal radium irradiation (NRI) 2014. http://www.cdc.gov/nceh/radiation/brochure/profile_nri.htm Accessed December 11, 2019. [Google Scholar]
- 35. Proctor DF. The Tonsils and Adenoids in Childhood. Springfield, IL: Thomas; 1960. Chap 2 https://babel.hathitrust.org/cgi/pt?id=mdp.39015000794811&view=1up&seq=7. Accessed December 11, 2019. [Google Scholar]
- 36. Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. New York, NY: Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 37. American Board of Internal Medicine. Choosing Wisely. https://www.abimfoundation.org/what-we-do/choosing-wisely. Accessed December 11, 2019.
- 38. Image Gently Alliance. Alliance for radiation safety in pediatric imaging. Image Gently 2007. https://www.imagegently.org/About-Us/The-Alliance. Accessed December 11, 2019.
- 39. Billen D. Spontaneous DNA damage and its significance for the “negligible dose” controversy in radiation protection. Rad Res. 1990;124:242–245. [PubMed] [Google Scholar]
- 40. Pollycove M, Feinendegen LE. Radiation-induced versus endogenous DNA damage: possible effects of inducible protective responses in mitigating endogenous damage. Hum Exp Toxicol. 2003;22:290–306. [DOI] [PubMed] [Google Scholar]
- 41. Sakamoto K, Myojin M, Hosoi Y, et al. Fundamental and clinical studies on cancer control with total or half body irradiation. J Jpn Soc Ther Radiol. 1997;9:161–175. [Google Scholar]
- 42. Welsh JS. Waldenstrom’s macroglobulinemia treated with fractionated low-dose total body irradiation. Case Rep Clin Pract Rev. 2004;5:425–431. http://www.crcpr-online.com/pub/case/vol_5/5638.pdf [Google Scholar]
- 43. Pollycove M. Radiobiological basis of low-dose irradiation in prevention and therapy of cancer. Dose-Response. 2007;5:26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cuttler JM, Garzon P, Mitchel REJ, Feinendegen LE, Sakamoto K, Welsh JS. Adjuvant therapy for resected exocrine pancreatic cancer by half-body low-dose irradiation. J Cancer Clin Trials. 2016;1:105 doi:10.4172/jcct.1000105. [Google Scholar]
- 45. Kojima S, Tsukimoto M, Shimura N, Koga H, Murata A, Takara T. Treatment of cancer and inflammation with low-dose ionizing radiation: three case reports. Dose-Response. 2017;15(1):1–7. doi:10.1177/1559325817697531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kojima S, Cuttler JM, Shimura N, Koga H, Murata A, Kawashima A. Present and future prospects of radiation therapy using α-emitting nuclides. Dose-Response. 2018;16(1):1–8. doi:10.1177/1559325817747387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kojima S, Thukimoto M, Cuttler JM, et al. Recovery from rheumatoid arthritis following 15 months of therapy with low doses of ionizing radiation: a case report. Dose-Response. 2018;16(3):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kojima S, Cuttler JM, Inoguchi K, et al. Radon therapy is very promising as a primary or an adjuvant treatment for different types of cancers: 4 case reports. Dose-Response. 2019;17(2):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kojima S, Cuttler JM, Shimura N, Koga H, Murata A, Kawashima A. Radon therapy for autoimmune diseases pemphigus and diabetes: 2 case reports. Dose-Response. 2019;17(2):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Doss M. Low dose radiation adaptive protection to control neurodegenerative diseases. Dose-Response. 2014;12(2):277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cuttler JM, Moore ER, Hosfeld VD, Nadolski DL. Treatment of Alzheimer disease with CT scans: a case report. Dose-Response. 2016;14(2):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cuttler JM, Moore ER, Hosfeld VD, Nadolski DL. Update on a patient with Alzheimer disease treated with CT scans. Dose-Response. 2017;15(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cuttler JM, Moore ER, Hosfeld VD, Nadolski DL. Second update on a patient with Alzheimer disease treated with CT scans. Dose-Response. 2018;16(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sies H, Berndt C, Jones DP. Oxidative stress. Annu Rev Biochem. 2017;86:715–748. [DOI] [PubMed] [Google Scholar]
- 55. Feinendegen LE, Cuttler JM. Biological effects from low doses and dose rates of ionizing radiation: science in the service of protecting humans: a synopsis. Health Phys. 2018;114(6):623–626. [DOI] [PubMed] [Google Scholar]
- 56. Fliedner TM, Graessle DH, Meineke V, Feinendegen LE. Hemopoietic response to low dose-rates of ionizing radiation shows stem cell tolerance and adaptation. Dose Response. 2012;10(4):644–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cuttler JM. Evidence of a dose threshold for radiation-induced leukemia. Dose-Response. 2018;16(4):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cuttler JM. Evidence of dose threshold for radiation-induced leukemia: absorbed dose and uncertainty. Dose-Response. 2019;17(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. OECD Nuclear Energy Agency. Chernobyl ten years on: radiological and health impact. 1995. https://www.oecd-nea.org/rp/chernobyl/chernobyl-1995.pdf. Accessed December 11, 2019.
- 60. Jaworowski Z. Comments by representative of Poland in UNSCEAR on The Chernobyl Forum Report. January 5, 2006 President’s Special Session on Low-Level Radiation and Its Implications for Fukushima Recovery. Chicago, IL: American Nuclear Society; 2012:131–141. [Google Scholar]
- 61. Barabanova AW. Prediction and actual data 30 years after the accident. Presentation at: International Conference on Health Effects of Chernobyl; Obninsk, Russia; May 17-19, 2016. [Google Scholar]
- 62. Taylor LS. Some nonscientific influences on radiation protection standards and practice. The 1980 Sievert Lecture. 5th International Congress of the International Radiation Protection Association. Jerusalem. Health Phys. 1980;39(12):851–874. http://www.irpa.net/irpa5/cdrom/vol.1/j1_64.pdf. [PubMed] [Google Scholar]
- 63. Aisen PS. Editorial: Failure after failure. What next in AD drug development? J Prev Alz Dis. 2019;6(3):150. [DOI] [PubMed] [Google Scholar]
- 64. Arvanitakis Z, Shah RC, Bennett DA. Diagnosis and management of dementia: Review. JAMA. 2019;322(16):1589–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]