Abstract
Pleuroparenchymal fibroelastosis (PPFE) is an unusual pulmonary disease with unique clinical, radiological, and pathological characteristics. Designated a rare idiopathic interstitial pneumonia in 2013, its name refers to a combination of fibrosis involving the visceral pleura and fibroelastotic changes predominating in the subpleural lung parenchyma. Although a number of disease associations have been described, no single cause of PPFE has been unequivocally identified. A diagnosis of PPFE is most commonly achieved by identifying characteristic abnormalities on computed tomographic scans. The earliest changes are consistently located in the upper lobes close to the lung apices, the same locations where subsequent disease progression is also most conspicuous. When sufficiently severe, the disease leads to progressive volume loss of the upper lobes, which, in combination with decreased body mass, produces platythorax. Once regarded as a slowly progressing entity, it is now acknowledged that some patients with PPFE follow an inexorably progressive course that culminates in irreversible respiratory failure and early death. In the absence of effective medical drug treatment, lung transplant remains the only therapeutic option for this disorder. This review focuses on improving early disease recognition and evaluating its pathophysiological impact and discusses working approaches for its management.
Keywords: pleuroparenchymal fibroelastosis, interstitial lung disease, intraalveolar fibrosis, elastosis
Historical Aspects of Pleuroparenchymal Fibroelastosis
By the time of its formal recognition as a rare idiopathic interstitial pneumonia (IIP) in 2013, the term “idiopathic pleuroparenchymal fibroelastosis” (iPPFE) had been in use for nearly a decade, and the clinical entity that it described had been acknowledged for at least 20 years (1–3). The first five cases formally labeled as “pleuroparenchymal fibroelastosis” (PPFE) shared a pattern of chronic interstitial and pleural fibrosis that did not fit within other categories of IIP (2). Instead, they were descriptively similar to historical cases of pulmonary upper lobe fibrosis (PULF) and contemporaneous cases of idiopathic upper lobe fibrosis, the vast majority of which had been reported from Japan (1, 4–8). Amitani and colleagues’ report of PULF included historical examination of lung tissue from biopsies and autopsy, but interpretation of radiological imaging was limited to plain chest radiography (1). However, PULF and PPFE do appear to be the same disease (4, 6, 9). Differences in described clinical characteristics and longitudinal disease behavior between the Asian and Western cases may partly relate to disparities in case ascertainment, although variations in disease-associated factors (e.g., pathogenetic mechanisms) cannot be excluded. Such differences have not been easy to delineate, partly because the term “PULF” is no longer in common use but also because published studies of PPFE, owing to its rarity, have been limited to relatively small case numbers.
Incidence and Prevalence
The true incidence and prevalence of PPFE are not known, owing to uncertainties in its detection, misdiagnosis, and the absence of agreed criteria for its identification. In general, the frequency of finding PPFE in individuals referred for investigation of an interstitial lung disease (ILD) is less than among patients who are awaiting lung transplant.
In one study, 5.9% of 205 biopsied cases from a total pool of 1,622 patients undergoing an ILD workup were ultimately diagnosed with PPFE (9). iPPFE also formed 7.7% of consecutive cases of IIP referred to a single tertiary center over a 10-year period (10). In comparison, one-fourth of 118 patients with fibrotic ILD who were listed for lung transplant over a 5-year period had radiological changes consistent with PPFE in addition to their principal ILD (11).
Information on the prevalence of PPFE developing after transplant is sparse. Data from a single institutional registry spanning a 9-year period showed that 7.5% and 0.28% of 53 lung and 700 bone marrow transplant recipients, respectively, developed PPFE (12). An earlier study had reported a 2% rate of post–lung transplant upper lobe fibrosis that met a definition of chronic lung allograft dysfunction, which shares key histological similarities with PPFE (13).
Pathogenesis of PPFE
It has been suggested that acute or subacute lung injury, including diffuse alveolar damage, causing exuberant interstitial inflammation is central to the pathological cascade that culminates in PPFE (14). However, the exact nature of the injurious stimuli involved in triggering this process remains unknown. The presence of diffuse alveolar damage has also been reported in the setting of post-transplant PPFE (15). Failure of parenchymal lung injury to adequately resolve risks promoting aberrant tissue repair, which can leave behind dense and permanent obliterative fibroelastosis and atelectasis that ultimately contribute to the development of PPFE (16). PPFE and alveolar fibroelastosis developing in post-transplant restrictive allograft syndrome (RAS) have common pathological and gene profile characteristics (15, 17, 18). The reasons why such injuries should produce chronic well-demarcated and predominantly subpleural elastin-rich fibrotic abnormalities remain unexplained.
Disease Associations of Nonidiopathic PPFE
A number of potential initiating factors for PPFE have been reported, the commonest of which are bone marrow and hematopoietic stem cell transplant as well as lung transplant (13, 17, 19, 20). A history of chemotherapy treatment, autoimmune or connective tissue disease, acute lung injury particularly with infective complications, chronic hypersensitivity pneumonitis (HP), and occupational exposure to asbestos and aluminum have also been associated with PPFE (21–26) (Table 1).
Table 1.
Initiating, underlying, and other disease-associated factors of pleuroparenchymal fibroelastosis
| Type of PPFE | References |
|---|---|
| Idiopathic PPFE | |
| Nonidiopathic PPFE | |
| As a form of restrictive allograft syndrome complicating lung, bone marrow, and hematopoietic stem cell transplant (also known as “restrictive chronic allograft dysfunction”) | 12, 13, 17, 20 |
| Fibrotic interstitial lung disease (e.g., usual interstitial pneumonia, hypersensitivity pneumonitis) | 23, 27, 31, 32 |
| Chronic or recurrent bronchopulmonary infection (e.g., Aspergillus, nontuberculous mycobacteria) | 1, 26, 27, 29, 30 |
| Autoimmune or connective tissue disease (e.g., scleroderma, rheumatoid arthritis, inflammatory bowel disease) | 22, 27 |
| Familial history of pulmonary fibrosis | 1, 2, 34, 35, 36 |
| Short telomere lengths resulting from mutations of genes encoding the telomerase complex | 35, 36 |
| Anticancer/cytotoxic chemotherapy (e.g., cyclophosphamide and carmustine) and radiation therapy | 21, 45 |
| Occupational dust inhalation (e.g., asbestos and aluminum) | 24, 25 |
Definition of abbreviation: PPFE = pleuroparenchymal fibroelastosis.
A history of pulmonary infections is frequently encountered in individuals with PPFE (26, 27). Historical studies of PULF and more recent reports using the diagnostic label of PPFE have highlighted the presence of Aspergillus, although the frequency of clinically significant aspergillosis has not been determined (1, 7, 28, 29). Reddy and colleagues reported recurrent pulmonary infection, attributed to different potential pathogens, in over half of their cases of PPFE (27). Elevated titers of IgG antibody against Aspergillus and isolation of nontuberculous mycobacteria have also been reported during the disease course of patients with PPFE (30). Thus, although the role of infection in the pathogenesis of PPFE remains unclear, progression of mild to more extensive disease, including the formation of upper lobe fibrocystic changes, is often accompanied by evidence of severe or repeated bronchopulmonary infection.
The commonest pattern of fibrotic ILD to coexist with PPFE is usual interstitial pneumonia (UIP), reported in one-fourth to one-half of cases (27, 31, 32). Coexistent UIP or even nonspecific interstitial pneumonia (NSIP) occurs most frequently in the lower lobes, away from the main areas of PPFE, but in common with the latter, each pattern will typically progress over time (6, 32). PPFE has also been reported in patients diagnosed with chronic HP (23, 27). The development of PPFE has not been linked to cigarette smoking or specific immunodeficiency states (6, 11, 33).
Familial and Genetic Associations of PPFE
A history of familial pulmonary fibrosis is often elicited from individuals with PPFE or bilateral upper lobe pleuroparenchymal fibrosis (1, 2, 34, 35). The presence of a familial link among these patients has been reported in up to 57% of cases (2, 7, 36).
Genetic mutations may be detected in patients with PPFE even when a family history of lung disease is absent. Profiling of genes that are involved in maintaining telomere integrity and telomerase function, including TERT (telomerase reverse transcriptase) and TERC (telomerase RNA component), has revealed a link between clinically significant pathological variants and abnormally shortened telomeres in PPFE (35). Furthermore, the presence of such mutations has been strongly associated with a progressive disease phenotype similar to that observed in UIP. TERT and TERC mutations have also been reported in half of a cohort of patients with PPFE, most of whom were female and had a low body mass index (BMI) (36).
Clinical Features of PPFE
PPFE has been reported in children and the elderly, but most patients present between 40 and 70 years of age. A review of 78 cases from different series published up to 2013 revealed a bimodal age distribution ranging from 13 to 85, with a mean age of 49 years (33). The majority were labeled as PULF rather than PPFE, and a sizable number developed PPFE after transplant rather than as an idiopathic entity. Younger patients were overall more likely to be female, particularly in the nontransplant setting. Female preponderance was also observed in two recent reports, including a genetic study of telomere gene mutations (10, 35). By contrast, other contemporaneous reports have not identified a clear sex difference (11, 24, 37). Male preponderance of PPFE has rarely been reported (12).
The duration of symptoms before presentation varies from 6 to 24 months. The commonest of these are progressive breathlessness and cough; nonspecific chest discomfort and pleuritic pain are reported, but persisting pain is unusual in the absence of pneumothorax. Progressive weight loss is frequently reported during the disease course and may raise the possibility of an intercurrent infection or occult malignancy. Apart from one report, most of the information on low BMI in PPFE has come from Japanese studies (14, 32, 36, 38, 39).
Auscultatory findings may be normal because inspiratory crackles or squawks are detected only when the PPFE has extended outside the upper zones or when there is coexistent UIP, NSIP, or HP. Clubbing of the fingers is uncommon.
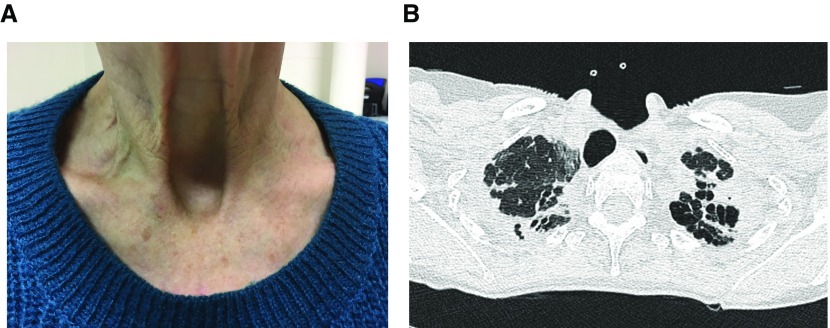
A significant proportion of patients with PPFE develop platythorax as a result of marked upper lobe volume contraction in conjunction with reduced chest wall bulk associated with weight loss. This causes the anteroposterior thoracic depth to decrease and produces flattening of the frontal chest aspect (40, 41). In some individuals, the suprasternal notch deepens considerably and becomes highly noticeable clinically and radiologically (Figure 1).
Figure 1.
(A) Deepened suprasternal notch in a patient with pleuroparenchymal fibroelastosis. (B) Computed tomography of the same patient at the level of the lung apices demonstrating a prominent suprasternal notch, anteroposterior flattening of the thorax, and retraction of the trachea so that its posterior margin “overlaps” with the anterior border of the adjacent vertebra.
Establishing a Diagnosis
The main diagnostic differentials for PPFE include conditions associated with upper lobe disease, including HP, sarcoidosis, IIP with extension of disease to the upper zones (including UIP), atypical including nontuberculous mycobacterial infection, post–lung injury remodeling, pneumoconiosis, malignancy, and apical pleural cap. Criteria for diagnosing PPFE incorporating computed tomography (CT) and histopathology were first proposed in 2012 by Reddy and colleagues, who subcategorized cases as “definite,” “consistent with” PPFE, or “inconsistent with” PPFE on the basis of earlier histological descriptions from small case series (2, 9, 31) (Table 2). Additional albeit more condensed pathological criteria have subsequently been suggested (42). In practice, a diagnosis of PPFE should ideally be reached after multidisciplinary consideration of clinical; radiological; and, when available, pathological information. Surgical biopsy is often avoided because of a recognized risk of complications such as iatrogenic pneumothorax, pneumomediastinum, or the development of a bronchopleural fistula that could cause a protracted “air leak” (9, 43).
Table 2.
Proposed diagnostic criteria for pleuroparenchymal fibroelastosis
| Category | Histopathology | High-Resolution Computed Tomography |
|---|---|---|
| Definite PPFE | Upper zone pleural fibrosis with subjacent intraalveolar fibrosis accompanied by alveolar septal elastosis | Pleural thickening with associated subpleural fibrosis concentrated in the upper lobes with less marked or no lower lobe involvement |
| Consistent with PPFE | Intraalveolar fibrosis present but 1) not accompanied by significant pleural fibrosis, 2) not predominantly subpleural, or 3) not present in an upper lobe biopsy | Upper lobe pleural thickening with associated subpleural fibrosis but 1) distribution not concentrated in the upper lobes or 2) with features of coexistent disease elsewhere |
| Inconsistent with PPFE | Absence of features in “definite PPFE” and “consistent with PPFE” categories | Absence of features in “definite PPFE” and “consistent with PPFE” categories |
Definition of abbreviation: PPFE = pleuroparenchymal fibroelastosis.
Reprinted by permission from Reference 27.
Laboratory Tests
There are no diagnostic laboratory tests for PPFE per se. Variably elevated levels of circulating KL-6 (Kerbs von Lungren 6 antigen) and SP-D (surfactant protein D) in affected individuals have been reported, but their clinical significance is unclear (6, 14, 38). Serum autoantibodies, including rheumatoid factor and myeloperoxidase–antineutrophil cytoplasmic antibody, have also been reported (2, 25, 38, 44). In patients with a separate underlying connective tissue disease, serological abnormalities may simply reflect the dysregulated immunity inherent to their connective tissue disease. Similarly, detection of elevated IgG antibodies against fungi (principally Aspergillus) may reflect colonization or opportunistic infection rather than indicate a direct pathogenic role. Elevated levels of urinary desmosine have been reported in a preliminary study of individuals with biopsy-proven PPFE compared with patients with idiopathic pulmonary fibrosis, patients with chronic obstructive pulmonary disease, and healthy control subjects, suggesting its potential utility as a noninvasive diagnostic marker in suspected but unbiopsied cases of PPFE (43). It remains to be seen if any of these biological markers will ultimately be validated for clinical application.
Radiological Imaging of PPFE
Descriptions of a plain radiographic profile that bore more than a passing resemblance to PPFE emerged in the 1970s (45, 46). Later, in 2004, Frankel and colleagues, in proposing the term “PPFE,” described its characteristic manifestations of bilateral upper zone pleural thickening and lobar volume loss (2). Their observation that radiographic changes could be progressive was confirmed in subsequent studies (26, 42, 47). Patients with recurrent pneumothoraces and an unusual form of parenchymal fibrosis complicating allogeneic bone marrow transplant were also identified before the formal adoption of the term “PPFE,” ahead of subsequent recognition of a pathological link to the disease (17, 48). As a differential diagnosis, apical “pleural caps” occur more frequently in older individuals, rarely progress, and are typically restricted to the uppermost 5 mm of each hemithorax (49) (Figure 2D). The pleural thickening in PPFE, in contrast, is variable in magnitude, occasionally asymmetric, and may extend for some distance caudally (2).
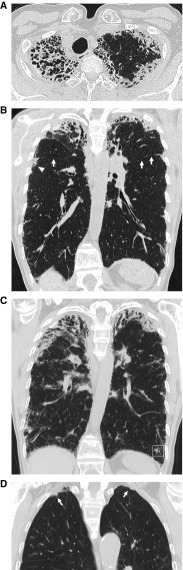
Figure 2.
(A) Computed tomography (CT) through the lung apices demonstrating classical features of pleuroparenchymal fibroelastosis (PPFE), including pleural thickening, subpleural consolidation with coarse reticulation, and striking traction bronchiolectasis/bronchiectasis. (B) Coronal reconstruction of the same study as in A showing bilateral upper zone PPFE that extends caudally to the level of the fourth rib in the right hemithorax (arrowhead). Marked volume loss can be judged by elevation of the interlobar fissures (arrows). (C) Unequivocal progression of PPFE evident on a follow-up CT scan 2 years later. (D) Coronal CT image of bilateral apical pleural caps, more prominent on the right (arrow).
The principal and ancillary CT findings of PPFE have been highlighted in multiple studies (17, 27, 38, 47, 48, 50). For diagnostic purposes, cases should be examined using both standard axial and coronal reconstruction images (Figure 2). Reddy and coworkers proposed CT criteria for “definite” PPFE, including upper lobe pleural thickening with subpleural fibrosis and limited, if any, lower lobe involvement (27). Tractional distortion of the airways within areas of PPFE is common, reflecting the dense surrounding fibrosis of the disease (38, 47, 48). The presence of “free-standing” bronchiectasis and mosaic attenuation of the lung parenchyma has also been reported (12, 15, 17, 51). Overt lung fibrosis of varying patterns can coexist with PPFE, most frequently UIP, NSIP, or HP (9, 23, 27, 31, 32). PPFE manifesting as a hypermetabolic lung nodule in conjunction with more usual appearances of biapical fibroelastosis has also been reported (52). Whether this represents another rare variant of PPFE remains unclear.
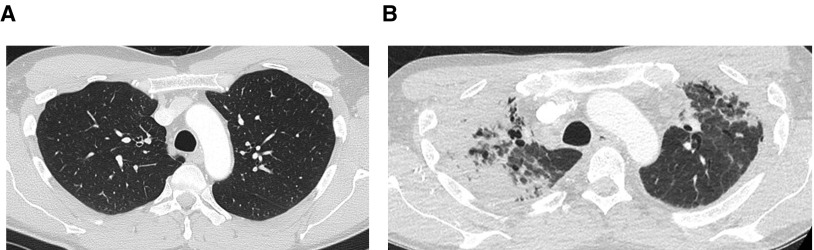
Anteroposterior flattening of the chest, or platythorax, occurs commonly in PPFE (50) and has been correlated with decreased BMI, suggesting its potential role as a surrogate marker of weight loss in PPFE (29, 38, 50). Two other observations associated with platythorax but hitherto not reported bear mentioning: 1) “overlapping” of the posterior tracheal border and spine (a consequence of reduced anteroposterior thoracic depth) and 2) the appearance of a deep suprasternal notch resulting from reduced upper thoracic volume and progressive weight loss (Figure 1). Cases of PPFE for which there is documentation of radiologically normal premorbid lungs are valuable for studying the possible etiological circumstances of the disease, particularly if a history of interval ill health is also available (Figure 3).
Figure 3.
(A) Normal premorbid computed tomography (CT) through the upper lobes 12 months before the patient underwent a Nuss procedure to correct a pectus excavatum deformity. (B) CT through the same region scanned 3 years after surgery that was complicated by recurrent postoperative pulmonary infection showing dense asymmetric pleuroparenchymal fibroelastosis. Dramatic reduction of the anteroposterior thoracic distance is accompanied by anatomic distortion, including a change in the relationship between the posterior wall of the trachea and the vertebral body.
Histopathological Examination
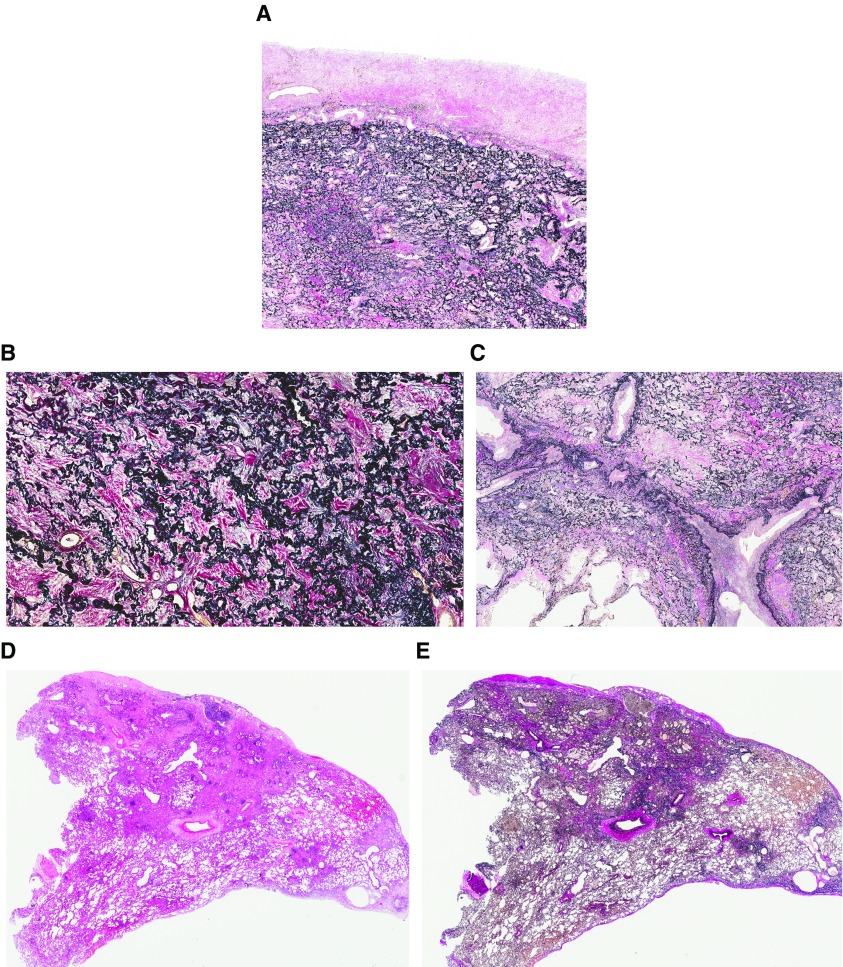
A histopathological diagnosis of PPFE requires demonstration of intraalveolar fibrosis and elastosis (IAFE), ideally with visceral pleural fibrosis (1–3, 27, 30). The latter may be absent in biopsies because of its patchy distribution (29). IAFE comprises dense collagenous fibrosis filling alveolar spaces, with the residual alveolar walls highlighted by elastin deposition (Figure 4A). These features dominate in the upper lobes and are more readily seen on elastin van Gieson stain, with the alveolar parenchyma sometimes appearing “petrified” by the fibrosis (Figure 4B). Foci of loose fibroblastic proliferation may be present at the interface between established fibrosis and normal lung parenchyma (30). Inflammation is typically mild and nonspecific, but intimal fibrosis may be evident, appearing prominently within the pulmonary vasculature, particularly the pulmonary veins (Figure 4C). At low power, IAFE commonly appears just deep to the visceral pleura, although it may extend into the deeper parenchyma, typically around interlobular septa and bronchovascular bundles (Figures 4D and 4E).
Figure 4.
(A) Elastic van Gieson staining showing a combination of visceral pleural fibrosis and intraalveolar fibrosis with elastosis (IAFE). IAFE comprising dense collagenous fibrosis fills the alveolar spaces, whereas the residual alveolar walls are highlighted by elastin deposition. (B) At higher power, the alveolar parenchyma can appear “petrified” by the fibrosis, its architecture highlighted by abundant elastosis. (C) Intimal fibrosis within the pulmonary vasculature, particularly the pulmonary veins, is a common finding in pleuroparenchymal fibroelastosis. (D and E) Although IAFE predominates in the subpleural lung parenchyma, it may extend into the deeper lung, typically around interlobular septa and bronchovascular bundles, as shown to the right of both panels. Elastic van Gieson staining is shown in A, B, C, and E; hematoxylin and eosin staining is shown in D.
Foci of granulomatous inflammation may be present in approximately 15% of cases (27, 30, 53, 54), although it is unclear whether they represent a coexistent pathology such as HP or infection. Its presence but not that of other histological findings has been associated with better prognosis, possibly by highlighting potential opportunities for targeting an underlying infection (30). No features of IAFE have been found to distinguish between iPPFE and secondary PPFE (17, 55, 56), although PPFE forming part of the post-transplant RAS typically includes features of obliterative bronchiolitis (57).
IAFE with pleural fibrosis in the upper lobes may coexist with a separate pattern of UIP in the lower lobes (13, 27, 58). Coexistent HP-like features and myeloperoxidase–antineutrophil cytoplasmic antibody positivity have also been reported (30, 44). The importance of obtaining biopsies from at least two sites cannot be overstated, both to achieve a confident diagnosis of PPFE and to ensure that a second histological pattern, especially when the radiological abnormalities extend beyond the upper lobes. Nonsurgical sampling techniques, including bronchoscopic transbronchial cryobiopsies, conventional transbronchial biopsies, and transthoracic core needle biopsies, have been used with varying degrees of success (44, 47, 59, 60). In these situations, a conclusive diagnosis of PPFE will be even more reliant on expert multidisciplinary review.
Other patterns of lung injury that may show IAFE-dominant pathology include apical “cap,” radiation-induced lung injury, pulmonary paraquat toxicity, and chronic postinjury remodeling due to failure of acute respiratory distress syndrome to resolve. IAFE is readily differentiated from organizing pneumonia, desquamative interstitial pneumonia, lymphoid interstitial pneumonia, and NSIP. Like UIP, IAFE is predominantly subpleural, has sparse inflammation, and may feature fibroblastic proliferation at the interface between normal and abnormal lung. However, it does not incorporate honeycomb change, and its hallmark dense alveolar elastotic framework contrasts against generally more fragmented and less conspicuous elastin deposition in UIP (61). Immunohistochemical studies have recently shown that, unlike in UIP, myofibroblasts in PPFE stain positively for podoplanin (D2-40) (62). This finding is in accord with a separate observation that lymphatic proliferation is increased in PPFE but not in UIP (63).
Pulmonary Function Profile
The progressive diminution of lung volume in PPFE produces a characteristic restrictive ventilatory defect denoted by decreased forced vital capacity (FVC), decreased total lung capacity (TLC), and increased ratio of forced expiratory volume in 1 second to FVC. Reduced TLC may be accompanied by mild or moderately increased residual volume (31). The reasons for this remain unclear but may include inhomogeneous lung emptying or increased end-expiratory air trapping. A subtle mixed ventilatory pattern may also be encountered (41). In patients with progressive PPFE, the FVC may follow a rapidly declining trajectory that is comparable to or even greater in magnitude than that of UIP (10, 35). A mean annual FVC loss of 300 ml characterized the accelerated progression of disease in patients with PPFE who had specific telomere gene abnormalities (35).
Gas transfer factor (synonymous with the diffusing capacity of the lung for carbon monoxide) is decreased in PPFE, usually with preserved or mildly reduced rate of alveolar carbon monoxide uptake. In cases in which the pleural component of PPFE is extensive, the rate of alveolar carbon monoxide uptake may be elevated as a result of extrapulmonary restriction.
Patients with established or progressed PPFE have a predilection for hypoxemic respiratory failure with a typically widened alveolar–arterial gradient due to a reduced arterial oxygen pressure. The arterial carbon monoxide pressure is usually normal or nearly normal but may be increased in late-stage disease, owing to hypoventilation or extrapulmonary restriction, but hypercarbic death in PPFE has not been reported frequently (41). Platythorax itself has been suggested as a cause of ventilation–perfusion mismatch, but this too has not been evaluated in detail (24).
Natural History and Clinical Outcome
Amitani and colleagues originally reported PULF as a condition that progressed gradually over 10–20 years (1). In a subsequent analysis of 85 patients, including historical cases from the 1960s and later cases specifically labeled as PPFE, a median survival of 11 years was highlighted (24). However, a divergence in longitudinal disease behavior is increasingly recognized in acknowledgment of a subgroup of patients who are prone to inexorably advancing disease. This so-called progressive disease phenotype has a typical median survival of less than 5 years and has been described in patients with iPPFE as well as in individuals with additional disease-associated factors.
The progressive PPFE phenotype was initially exemplified by five surgically biopsied patients with iPPFE who died within 2–3 years of diagnosis (37). Subsequent to that, two patterns of FVC decline in PPFE were described: one rapid, occurring over 2–6 years, and another that followed a more insidious course (37). In another study of iPPFE, one-third of 36 cases progressed from diagnosis to death within 12 months, resulting in a cohort median survival of 24 months (58). Rates of decline and survival that were similar to UIP were also observed in a genetic study of progressive ILD in which telomerase gene mutations were identified (35). Notably, patients with iPPFE with a Gender-Age-Physiology Index severity score of 2–3 were shown to have a poorer prognosis than those with UIP of similar disease severity (10, 64).
Although a number of studies have shown coexistent UIP to be a determinant of worse outcome in PPFE (28, 58), others have failed to demonstrate a negative prognostic impact specifically attributable to UIP (32). In a small subgroup of patients with UIP listed for lung transplant, the presence of coexistent PPFE based on CT similarly did not correlate with worse survival (11). However, PPFE has been shown to be an independent determinant of higher mortality in patients diagnosed with chronic HP (23). Current evidence therefore suggests that some patients may be more susceptible to progressive disease, including those with iPPFE, short telomere lengths, and concomitant UIP or HP.
Pneumothorax and pneumomediastinum are frequent complications of PPFE (1, 2, 27, 37). Among patients with ILD who are listed for lung transplant, a history of pneumothorax has been shown to be at least three times more common in those with PPFE (11). Pneumothorax that fails to resolve despite intervention is also prognostically important in PPFE (10, 28). However, the prevalence and clinical significance of pulmonary hypertension in this condition are not known.
Management
No treatment has been shown to be effective in PPFE. Low-dose prednisolone, used empirically, may have useful, although unproven, immunomodulatory effects (10). The use of larger doses of corticosteroids or the use of immunosuppressive agents such as azathioprine or methotrexate is usually avoided in view of the heightened risk of infection in these patients. Pirfenidone, an antifibrotic agent, has been used in isolated cases of iPPFE and PPFE occurring in RAS, with varying anecdotal results (10, 36, 65; see also PIRCLAD trial [Pirfenidone for Restrictive Chronic Lung Allograft Dysfunction; www.clinicaltrials.gov identifier NCT03359863]). It is also being evaluated in a phase 2 trial of restrictive chronic lung allograft dysfunction (66).
Patients with PPFE who are prone to frequent pulmonary infections may benefit from prophylactic antibiotics. Antifungal therapy is usually reserved for those with radiological or microbiological evidence of such infection. Oxygen assessment, nutritional input, psychological support, and pulmonary rehabilitation should ideally form part of the standard of care of PPFE, although the availability of each service will vary geographically. Pneumothorax or pneumomediastinum should be expediently managed in conjunction with interventional radiologists and surgeons. Lung transplant is an option, although extensive pleural thickening can pose technical challenges with explantation of the native lung. Successfully transplanted cases have been reported, including one involving a living donor and a patient who received bilateral lung transplant (67–69).
Conclusions
The number of reported cases of PPFE has increased considerably in the last 10 years. Commensurate with the role of CT as its commonest detection method, knowledge of the radiological characteristics of PPFE has significantly expanded. Similarly, there is increasing confidence among pathologists in distinguishing the histopathological phenotype of PPFE from other entities with similar features. By closely integrating clinical, radiological, and pathological information, a multidisciplinary approach to its management can minimize misdiagnosis, particularly when the presenting abnormalities are limited in extent. Recent studies have shown that a subgroup of patients has a very poor outcome due to rapid clinical deterioration. The additional realization that those with coexistent PPFE and a separate fibrotic lung disease such as UIP fare badly has similarly important implications for clinical care. The prognostic determinants of PPFE, like its etiological factors, remain ill defined and are the focus of ongoing studies. Above all, finding an effective treatment for PPFE remains a major clinical challenge.
Supplementary Material
Footnotes
CME will be available for this article at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Amitani R, Niimi A, Kuse F. Idiopathic pulmonary upper lobe fibrosis (IPUF) Kokyu. 1992;11:693–639. [Google Scholar]
- 2.Frankel SK, Cool CD, Lynch DA, Brown KK. Idiopathic pleuroparenchymal fibroelastosis: description of a novel clinicopathologic entity. Chest. 2004;126:2007–2013. doi: 10.1378/chest.126.6.2007. [DOI] [PubMed] [Google Scholar]
- 3.Travis WD, Costabel U, Hansell DM, King TE, Jr, Lynch DA, Nicholson AG, et al. ATS/ERS Committee on Idiopathic Interstitial Pneumonias. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iesato K, Ogasawara T, Masuda A, Okabe H, Tomita K, Nakamura H, et al. Idiopathic pulmonary upper lobe fibrosis; clinical and pathological features. Rinsho Hoshasen. 2005;50:13–25. [Google Scholar]
- 5.Watanabe K, Nagata N, Kitasato Y, Wakamatsu K, Nabeshima K, Harada T, et al. Rapid decrease in forced vital capacity in patients with idiopathic pulmonary upper lobe fibrosis. Respir Investig. 2012;50:88–97. doi: 10.1016/j.resinv.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Shiota S, Shimizu K, Suzuki M, Nakaya Y, Sakamoto K, Iwase A, et al. Seven cases of marked pulmonary fibrosis in the upper lobe [in Japanese] Nihon Kokyuki Gakkai Zasshi. 1999;37:87–96. [PubMed] [Google Scholar]
- 7.Kawabata Y, Matsuoka R. Pathology of idiopathic pulmonary upper lobe fibrosis. Nihon Kyobu Rinsho. 2003;62:S161–S202. [Google Scholar]
- 8.Nakatani T, Arai T, Kitaichi M, Akira M, Tachibana K, Sugimoto C, et al. Pleuroparenchymal fibroelastosis from a consecutive database: a rare disease entity? Eur Respir J. 2015;45:1183–1186. doi: 10.1183/09031936.00214714. [DOI] [PubMed] [Google Scholar]
- 9.Becker CD, Gil J, Padilla ML. Idiopathic pleuroparenchymal fibroelastosis: an unrecognized or misdiagnosed entity? Mod Pathol. 2008;21:784–787. doi: 10.1038/modpathol.2008.56. [DOI] [PubMed] [Google Scholar]
- 10.Shioya M, Otsuka M, Yamada G, Umeda Y, Ikeda K, Nishikiori H, et al. Poorer prognosis of idiopathic pleuroparenchymal fibroelastosis compared with idiopathic pulmonary fibrosis in advanced stage. Can Respir J. 2018;2018:6043053. doi: 10.1155/2018/6043053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanizawa K, Handa T, Kubo T, Chen-Yoshikawa TF, Aoyama A, Motoyama H, et al. Clinical significance of radiological pleuroparenchymal fibroelastosis pattern in interstitial lung disease patients registered for lung transplantation: a retrospective cohort study. Respir Res. 2018;19:162–172. doi: 10.1186/s12931-018-0860-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mariani F, Gatti B, Rocca A, Bonifazi F, Cavazza A, Fanti S, et al. Pleuroparenchymal fibroelastosis: the prevalence of secondary forms in hematopoietic stem cell and lung transplantation recipients. Diagn Interv Radiol. 2016;22:400–406. doi: 10.5152/dir.2016.15516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pakhale SS, Hadjiliadis D, Howell DN, Palmer SM, Gutierrez C, Waddell TK, et al. Upper lobe fibrosis: a novel manifestation of chronic allograft dysfunction in lung transplantation. J Heart Lung Transplant. 2005;24:1260–1268. doi: 10.1016/j.healun.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 14.Hirota T, Yoshida Y, Kitasato Y, Yoshimi M, Koga T, Tsuruta N, et al. Histological evolution of pleuroparenchymal fibroelastosis. Histopathology. 2015;66:545–554. doi: 10.1111/his.12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ofek E, Sato M, Saito T, Wagnetz U, Roberts HC, Chaparro C, et al. Restrictive allograft syndrome post lung transplantation is characterized by pleuroparenchymal fibroelastosis. Mod Pathol. 2013;26:350–356. doi: 10.1038/modpathol.2012.171. [DOI] [PubMed] [Google Scholar]
- 16.Tabaj GC, Fernandez CF, Sabbagh E, Leslie KO. Histopathology of the idiopathic interstitial pneumonias (IIP): a review. Respirology. 2015;20:873–883. doi: 10.1111/resp.12551. [DOI] [PubMed] [Google Scholar]
- 17.von der Thüsen JH, Hansell DM, Tominaga M, Veys PA, Ashworth MT, Owens CM, et al. Pleuroparenchymal fibroelastosis in patients with pulmonary disease secondary to bone marrow transplantation. Mod Pathol. 2011;24:1633–1639. doi: 10.1038/modpathol.2011.114. [DOI] [PubMed] [Google Scholar]
- 18.Jonigk D, Rath B, Borchert P, Braubach P, Maegel L, Izykowski N, et al. Comparative analysis of morphological and molecular motifs in bronchiolitis obliterans and alveolar fibroelastosis after lung and stem cell transplantation. J Pathol Clin Res. 2016;3:17–28. doi: 10.1002/cjp2.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parish JM, Muhm JR, Leslie KO. Upper lobe pulmonary fibrosis associated with high-dose chemotherapy containing BCNU for bone marrow transplantation. Mayo Clin Proc. 2003;78:630–634. doi: 10.4065/78.5.630. [DOI] [PubMed] [Google Scholar]
- 20.Konen E, Weisbrod GL, Pakhale S, Chung T, Paul NS, Hutcheon MA. Fibrosis of the upper lobes: a newly identified late-onset complication after lung transplantation? AJR Am J Roentgenol. 2003;181:1539–1543. doi: 10.2214/ajr.181.6.1811539. [DOI] [PubMed] [Google Scholar]
- 21.Beynat-Mouterde C, Beltramo G, Lezmi G, Pernet D, Camus C, Fanton A, et al. Pleuroparenchymal fibroelastosis as a late complication of chemotherapy agents. Eur Respir J. 2014;44:523–527. doi: 10.1183/09031936.00214713. [DOI] [PubMed] [Google Scholar]
- 22.Enomoto Y, Nakamura Y, Colby TV, Johkoh T, Sumikawa H, Nishimoto K, et al. Radiologic pleuroparenchymal fibroelastosis-like lesion in connective tissue disease-related interstitial lung disease. PLoS One. 2017;12:e0180283. doi: 10.1371/journal.pone.0180283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacob J, Odink A, Brun AL, Macaluso C, de Lauretis A, Kokosi M, et al. Functional associations of pleuroparenchymal fibroelastosis and emphysema with hypersensitivity pneumonitis. Respir Med. 2018;138:95–101. doi: 10.1016/j.rmed.2018.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe K. Pleuroparenchymal fibroelastosis: its clinical characteristics. Curr Respir Med Rev. 2013;9:299–237. doi: 10.2174/1573398X0904140129125307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu L, Rassaei N, Caruso C. Pleuroparenchymal fibroelastosis with long history of asbestos and silicon exposure. Int J Surg Pathol. 2018;26:190–193. doi: 10.1177/1066896917739399. [DOI] [PubMed] [Google Scholar]
- 26.Piciucchi S, Tomassetti S, Casoni G, Sverzellati N, Carloni A, Dubini A, et al. High resolution CT and histological findings in idiopathic pleuroparenchymal fibroelastosis: features and differential diagnosis. Respir Res. 2011;12:111–115. doi: 10.1186/1465-9921-12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddy TL, Tominaga M, Hansell DM, von der Thusen J, Rassl D, Parfrey H, et al. Pleuroparenchymal fibroelastosis: a spectrum of histopathological and imaging phenotypes. Eur Respir J. 2012;40:377–385. doi: 10.1183/09031936.00165111. [DOI] [PubMed] [Google Scholar]
- 28.George PM, Devaraj A, Nicholson AG, Chua F. An emerging interstitial lung disease. Lancet Respir Med. 2016;4:762. doi: 10.1016/S2213-2600(16)30242-9. [DOI] [PubMed] [Google Scholar]
- 29.Kurosaki F, Bando M, Nakayama M, Mato N, Nakaya T, Yamasawa H, et al. Clinical features of pulmonary aspergillosis associated with interstitial pneumonia. Intern Med. 2014;53:1299–1306. doi: 10.2169/internalmedicine.53.1578. [DOI] [PubMed] [Google Scholar]
- 30.Khiroya R, Macaluso C, Montero MA, Wells AU, Chua F, Kokosi M, et al. Pleuroparenchymal fibroelastosis: a review of histopathologic features and the relationship between histologic parameters and survival. Am J Surg Pathol. 2017;41:1683–1689. doi: 10.1097/PAS.0000000000000928. [DOI] [PubMed] [Google Scholar]
- 31.Oda T, Ogura T, Kitamura H, Hagiwara E, Baba T, Enomoto Y, et al. Distinct characteristics of pleuroparenchymal fibroelastosis with usual interstitial pneumonia compared with idiopathic pulmonary fibrosis. Chest. 2014;146:1248–1255. doi: 10.1378/chest.13-2866. [DOI] [PubMed] [Google Scholar]
- 32.Enomoto Y, Nakamura Y, Satake Y, Sumikawa H, Johkoh T, Colby TV, et al. Clinical diagnosis of idiopathic pleuroparenchymal fibroelastosis: a retrospective multicenter study. Respir Med. 2017;133:1–5. doi: 10.1016/j.rmed.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 33.von der Thüsen JH. Pleuroparenchymal fibroelastosis: its pathological characteristics. Curr Respir Med Rev. 2013;9:238–247. doi: 10.2174/1573398X113096660025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Azoulay E, Paugam B, Heymann MF, Kambouchner M, Haloun A, Valeyre D, et al. Familial extensive idiopathic bilateral pleural fibrosis. Eur Respir J. 1999;14:971–973. doi: 10.1034/j.1399-3003.1999.14d41.x. [DOI] [PubMed] [Google Scholar]
- 35.Newton CA, Batra K, Torrealba J, Kozlitina J, Glazer CS, Aravena C, et al. Telomere-related lung fibrosis is diagnostically heterogeneous but uniformly progressive. Eur Respir J. 2016;48:1710–1720. doi: 10.1183/13993003.00308-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nunes H, Jeny F, Bouvry D, Picard C, Bernaudin JF, Ménard C, et al. Pleuroparenchymal fibroelastosis associated with telomerase reverse transcriptase mutations. Eur Respir J. 2017;49:1602022. doi: 10.1183/13993003.02022-2016. [DOI] [PubMed] [Google Scholar]
- 37.Yoshida Y, Nagata N, Tsuruta N, Kitasato Y, Wakamatsu K, Yoshimi M, et al. Heterogeneous clinical features in patients with pulmonary fibrosis showing histology of pleuroparenchymal fibroelastosis. Respir Investig. 2016;54:162–169. doi: 10.1016/j.resinv.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Kusagaya H, Nakamura Y, Kono M, Kaida Y, Kuroishi S, Enomoto N, et al. Idiopathic pleuroparenchymal fibroelastosis: consideration of a clinicopathological entity in a series of Japanese patients. BMC Pulm Med. 2012;12:72. doi: 10.1186/1471-2466-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki Y, Yoshimura K, Enomoto Y, Yasui H, Hozumi H, Karayama M, et al. Distinct profile and prognostic impact of body composition changes in idiopathic pulmonary fibrosis and idiopathic pleuroparenchymal fibroelastosis. Sci Rep. 2018;8:14074. doi: 10.1038/s41598-018-32478-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harada T, Yoshida Y, Kitasato Y, Tsuruta N, Wakamatsu K, Hirota T, et al. The thoracic cage becomes flattened in the progression of pleuroparenchymal fibroelastosis. Eur Respir Rev. 2014;23:263–266. doi: 10.1183/09059180.00006713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watanabe S, Waseda Y, Takato H, Matsunuma R, Johkoh T, Egashira R, et al. Pleuroparenchymal fibroelastosis: distinct pulmonary physiological features in nine patients. Respir Investig. 2015;53:149–155. doi: 10.1016/j.resinv.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Rosenbaum JN, Butt YM, Johnson KA, Meyer K, Batra K, Kanne JP, et al. Pleuroparenchymal fibroelastosis: a pattern of chronic lung injury. Hum Pathol. 2015;46:137–146. doi: 10.1016/j.humpath.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 43.Oyama Y, Enomoto N, Suzuki Y, Kono M, Fujisawa T, Inui N, et al. Evaluation of urinary desmosines as a noninvasive diagnostic biomarker in patients with idiopathic pleuroparenchymal fibroelastosis (PPFE) Respir Med. 2017;123:63–70. doi: 10.1016/j.rmed.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 44.Yamakawa H, Oda T, Baba T, Ogura T. Pleuroparenchymal fibroelastosis with positive MPO-ANCA diagnosed with a CT-guided percutaneous needle biopsy. BMJ Case Rep. 2018;2018 doi: 10.1136/bcr-2017-223287. bcr-2017-223287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davies D. Ankylosing spondylitis and lung fibrosis. Q J Med. 1972;41:395–417. [PubMed] [Google Scholar]
- 46.Kentala E, Repo UK, Lehtipuu AL, Vuornos T. HLA-antigens and pulmonary upper lobe fibrocystic changes with and without ankylosing spondylitis: a report of seven cases. Scand J Respir Dis. 1978;59:8–12. [PubMed] [Google Scholar]
- 47.Esteves C, Costa FR, Redondo MT, Moura CS, Guimarães S, Morais A, et al. Pleuroparenchymal fibroelastosis: role of high-resolution computed tomography (HRCT) and CT-guided transthoracic core lung biopsy. Insights Imaging. 2016;7:155–162. doi: 10.1007/s13244-015-0448-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sverzellati N, Zompatori M, Poletti V, Geddes DM, Hansell DM. Small chronic pneumothoraces and pulmonary parenchymal abnormalities after bone marrow transplantation. J Thorac Imaging. 2007;22:230–234. doi: 10.1097/RTI.0b013e31802bddca. [DOI] [PubMed] [Google Scholar]
- 49.McLoud TC, Isler RJ, Novelline RA, Putman CE, Simeone J, Stark P. The apical cap. AJR Am J Roentgenol. 1981;137:299–306. doi: 10.2214/ajr.137.2.299. [DOI] [PubMed] [Google Scholar]
- 50.Ishii H, Watanabe K, Kushima H, Baba T, Watanabe S, Yamada Y, et al. Tokyo Diffuse Lung Disease Study Group. Pleuroparenchymal fibroelastosis diagnosed by multidisciplinary discussions in Japan. Respir Med. 2018;141:190–197. doi: 10.1016/j.rmed.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 51.Takeuchi Y, Miyagawa-Hayashino A, Chen F, Kubo T, Handa T, Date H, et al. Pleuroparenchymal fibroelastosis and non-specific interstitial pneumonia: frequent pulmonary sequelae of haematopoietic stem cell transplantation. Histopathology. 2015;66:536–544. doi: 10.1111/his.12553. [DOI] [PubMed] [Google Scholar]
- 52.Machuca JS, Niazi M, Diaz-Fuentes G. Pleuroparenchymal fibroelastosis presenting as a hypermetabolic lung nodule. J Bronchology Interv Pulmonol. 2011;18:65–68. doi: 10.1097/LBR.0b013e318207b396. [DOI] [PubMed] [Google Scholar]
- 53.Bargagli E, Rottoli P, Torricelli E, Allegrini C, Dubini A, Bennett D, et al. Airway-centered pleuroparenchymal fibroelastosis associated with non-necrotizing granulomas: a rare new entity. Pathobiology. 2018;85:276–279. doi: 10.1159/000492431. [DOI] [PubMed] [Google Scholar]
- 54.Cheng SKH, Chuah KL. Pleuroparenchymal fibroelastosis of the lung: a review. Arch Pathol Lab Med. 2016;140:849–853. doi: 10.5858/arpa.2015-0166-RS. [DOI] [PubMed] [Google Scholar]
- 55.Morán Álvarez P, Bachiller-Corral J, Gorospe Sarasúa L, de la Puente Bujidos C. Pleuroparenchymal fibroelastosis: a new entity of interstitial pneumonia related to connective tissue diseases. Reumatol Clin. doi: 10.1016/j.reuma.2018.09.003. [online ahead of print] 31 October 2018; DOI: 10.1016/j.reuma.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 56.Kinoshita Y, Watanabe K, Ishii H, Kushima H, Hamasaki M, Fujita M, et al. Pleuroparenchymal fibroelastosis as a histological background of autoimmune diseases. Virchows Arch. 2019;474:97–104. doi: 10.1007/s00428-018-2473-3. [DOI] [PubMed] [Google Scholar]
- 57.Montero MA, Osadolor T, Khiroya R, Salcedo MT, Robertus JL, Rice A, et al. Restrictive allograft syndrome and idiopathic pleuroparenchymal fibroelastosis: do they really have the same histology? Histopathology. 2017;70:1107–1113. doi: 10.1111/his.13171. [DOI] [PubMed] [Google Scholar]
- 58.Kato M, Sasaki S, Kurokawa K, Nakamura T, Yamada T, Sasano H, et al. Usual interstitial pneumonia pattern in the lower lobes as a prognostic factor in idiopathic pleuroparenchymal fibroelastosis. Respiration. 2019;97:319–328. doi: 10.1159/000494061. [DOI] [PubMed] [Google Scholar]
- 59.Kronborg-White S, Ravaglia C, Dubini A, Piciucchi S, Tomassetti S, Bendstrup E, et al. Cryobiopsies are diagnostic in pleuroparenchymal and airway-centered fibroelastosis. Respir Res. 2018;19:135. doi: 10.1186/s12931-018-0839-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kushima H, Hidaka K, Ishii H, Nakao A, On R, Kinoshita Y, et al. Two cases of pleuroparenchymal fibroelastosis diagnosed with transbronchial lung biopsy. Respir Med Case Rep. 2016;19:71–73. doi: 10.1016/j.rmcr.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kinoshita Y, Watanabe K, Ishii H, Kushima H, Fujita M, Nabeshima K. Proliferation of elastic fibres in idiopathic pulmonary fibrosis: a whole-slide image analysis and comparison with pleuroparenchymal fibroelastosis. Histopathology. 2017;71:934–942. doi: 10.1111/his.13312. [DOI] [PubMed] [Google Scholar]
- 62.Enomoto Y, Matsushima S, Meguro S, Kawasaki H, Kosugi I, Fujisawa T, et al. Podoplanin-positive myofibroblasts: a pathological hallmark of pleuroparenchymal fibroelastosis. Histopathology. 2018;72:1209–1215. doi: 10.1111/his.13494. [DOI] [PubMed] [Google Scholar]
- 63.Kinoshita Y, Watanabe K, Ishii H, Kushima H, Fujita M, Nabeshima K. Significant increases in the density and number of lymphatic vessels in pleuroparenchymal fibroelastosis. Histopathology. 2018;73:417–427. doi: 10.1111/his.13634. [DOI] [PubMed] [Google Scholar]
- 64.Ley B, Ryerson CJ, Vittinghoff E, Ryu JH, Tomassetti S, Lee JS, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156:684–691. doi: 10.7326/0003-4819-156-10-201205150-00004. [DOI] [PubMed] [Google Scholar]
- 65.Vos R, Verleden SE, Ruttens D, Vandermeulen E, Yserbyt J, Dupont LJ, et al. Pirfenidone: a potential new therapy for restrictive allograft syndrome? Am J Transplant. 2013;13:3035–3040. doi: 10.1111/ajt.12474. [DOI] [PubMed] [Google Scholar]
- 66.Sato S, Hanibuchi M, Takahashi M, Fukuda Y, Morizumi S, Toyoda Y, et al. A patient with idiopathic pleuroparenchymal fibroelastosis showing a sustained pulmonary function due to treatment with pirfenidone. Intern Med. 2016;55:497–501. doi: 10.2169/internalmedicine.55.5047. [DOI] [PubMed] [Google Scholar]
- 67.Chen F, Matsubara K, Miyagawa-Hayashino A, Tada K, Handa T, Yamada T, et al. Lung transplantation for pleuroparenchymal fibroelastosis after chemotherapy. Ann Thorac Surg. 2014;98:e115–e117. doi: 10.1016/j.athoracsur.2014.07.045. [DOI] [PubMed] [Google Scholar]
- 68.Righi I, Morlacchi L, Rossetti V, Mendogni P, Palleschi A, Tosi D, et al. Lung transplantation as successful treatment of end-stage idiopathic pleuroparenchymal fibroelastosis: a case report. Transplant Proc. 2019;51:235–238. doi: 10.1016/j.transproceed.2018.04.071. [DOI] [PubMed] [Google Scholar]
- 69.Hata A, Nakajima T, Yoshida S, Kinoshita T, Terada J, Tatsumi K, et al. Living donor lung transplantation for pleuroparenchymal fibroelastosis. Ann Thorac Surg. 2016;101:1970–1972. doi: 10.1016/j.athoracsur.2015.07.056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.