Abstract
Rationale: The 2016 guidelines for hospital-acquired pneumonia (HAP) suggest applying a universal antibiogram resistance threshold in addition to patient criteria to determine empiric coverage. The impact of these recommendations is unknown.
Objectives: 1) Describe national antibiotic use and microbiology patterns for HAP among patients with noninfectious admissions, 2) measure the predictive performance of the antibiogram threshold and risk factors, and 3) estimate the change in practice with guideline implementation.
Methods: We conducted a retrospective analysis of all hospitalizations without initial infection but with secondary pneumonia diagnoses at Veterans Affairs Medical Centers between October 1, 2012, and September 30, 2015. For each hospitalization we extracted: presence of methicillin-resistant Staphylococcus aureus (MRSA) and resistant gram-negative rods (R-GNR) in cultures, anti-MRSA and antipseudomonal antimicrobial administration, and facility-level prevalence of MRSA and R-GNR. We calculated the percentage of hospitalizations with resistant organisms, broad-spectrum antibiotics, and the predictive performance of patient characteristics and prevalence thresholds for MRSA.
Results: Among 3,562 cases, 5.17% were positive for MRSA and 2.30% for R-GNR. The recommended MRSA prevalence threshold was 100.00% sensitive (95% confidence interval [CI], 98.02–100.00%) and 0.03% specific (95% CI, 0.00–0.16%) for MRSA-positive culture, leading to overtreatment of 94.81% (95% CI, 94.02–95.50%) of patients. Pressor order (odds ratio [OR], 3.89; 95% CI, 1.17–12.91) and intravenous antibiotics within the past 90 days (OR, 1.98; 95% CI, 1.03–3.81) were associated with MRSA. Mechanical ventilation was associated with R-GNR (OR, 4.37; 95% CI, 1.52–12.57).
Conclusions: The guideline-recommended antibiogram threshold and characteristics did not improve prediction of MRSA or R-GNR and would have led to an increase in MRSA treatment.
Keywords: hospital-acquired pneumonia, antibiograms, resistance
Hospital-acquired pneumonia (HAP) is a common nosocomial infection with high mortality and an association with resistant bacteria (1–6). Antimicrobial therapy, including empiric therapy with activity against resistant organisms, should be tailored to individual patients, but the magnitude of risk for resistant infections is largely unknown (6–13). Recent guidelines from the American Thoracic Society (ATS) and Infectious Disease Society of America (IDSA) for the diagnosis and management of HAP provide recommendations for empiric antimicrobial selection on the basis of perceived risk factors for resistant infections. They strongly recommend that each hospital generate antibiograms and provide thresholds for the proportion of resistance that would indicate need for coverage of methicillin-resistant Staphylococcus aureus (MRSA). Specifically, for HAP, coverage for MRSA is recommended if prevalence of resistance is >20% or unknown (4, 14). MRSA coverage and two antipseudomonals are also recommended if the patient has received prior intravenous antibiotics in the preceding 90 days, is in shock, or requires ventilatory support. These guidelines were intended to “minimize patient harm and exposure to unnecessary antibiotics and reduce the development of antibiotic resistance” (4). However, the implications of these guidelines for antibiotic use are unknown, as is the prevalence of resistance nationally.
Using a large national database we sought to 1) describe existing national practice and microbiology patterns before the guidelines, including the frequency of initial treatment for multidrug-resistant organisms and culture methods for HAP; 2) measure the predictive performance of the specified risk factors and antibiogram resistance threshold to identify patients with resistant organisms; and 3) estimate the change in practice with guideline implementation.
Methods
Setting and Subject Selection
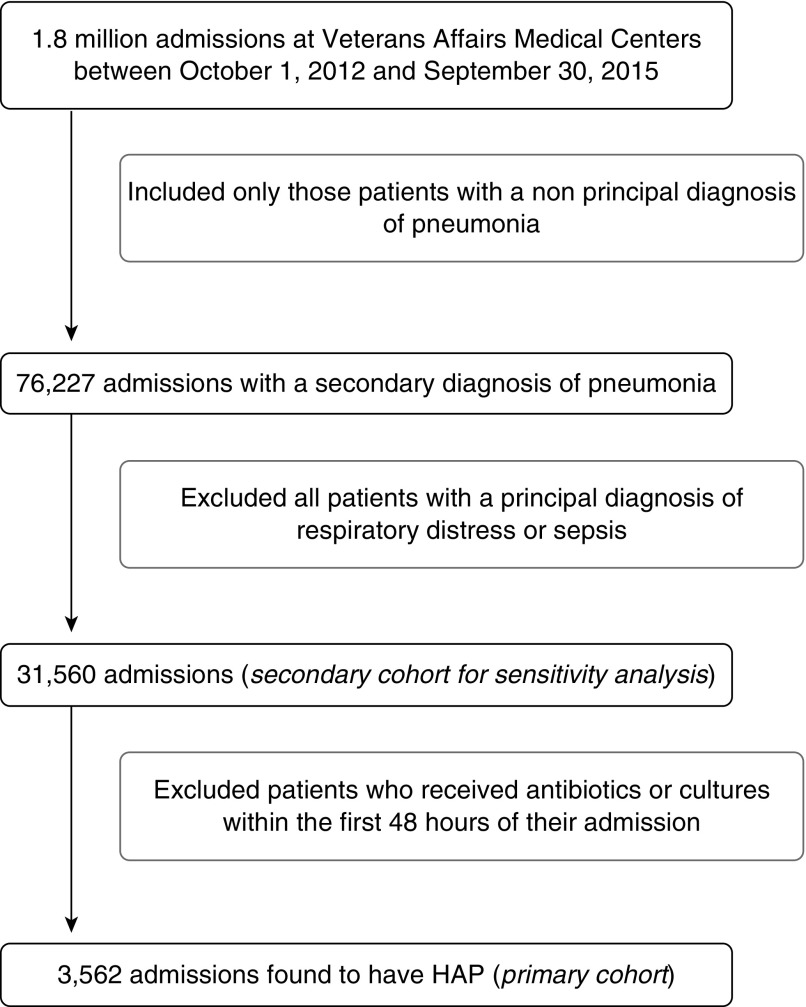
We conducted a retrospective analysis of HAP across all Veterans Affairs Medical Centers (VAMCs). We included all patients admitted between October 1, 2012 and September 30, 2015 admitted to acute care medical or surgical wards, an observation unit, or an intensive care unit (ICU), with a nonprincipal diagnosis of pneumonia by International Classification of Diseases, 9th Revision (ICD-9; 481–486). We ended our sample period in 2015 to avoid the effect of change between ICD-9-Clinical Modification (CM) and ICD-10-CM. We then limited our sample to noninfectious admissions by excluding those with a principal diagnosis of pneumonia, respiratory distress, or sepsis (507.0, 518.81, 518.84, 799.1, 785.52, 995.91, 995.92) and those who received antibiotics or cultures within the first 48 hours to improve our ability to specifically identify HAP (Figure 1). These were excluded because although HAP can occur after an infection present on admission, retaining these admissions could lead to frequent misclassification of community-acquired pneumonia as HAP. Timing of infection was defined as the date of earliest cultures or antibiotics. We validated the precision of our approach through chart review of 100 charts.
Figure 1.
Study population. HAP = hospital-acquired pneumonia.
In an effort to ensure the internal validity of our study, we initially limited our cohort of patients with HAP to those who did not receive antibiotics or cultures during the first 48 hours of admission. To explore the generalizability of our findings, we subsequently completed a sensitivity analysis among all patients with a secondary diagnosis of pneumonia, including those who received antibiotics or cultures in the initial 48 hours (Figure 1).
Data were accessed and analyzed using Veterans Informatics and Computing Infrastructure (15).
Patient-Level Measurements
After identifying our cohort of patients with HAP, we then evaluated the following patient characteristics: age, sex, admission service, ICU admission, orders for vasopressors, average length of stay, 30-day mortality, and presence of comorbid disease, including cerebrovascular disease, congestive heart failure, diabetes, renal disease, and neoplastic disease. Next, we measured the percentage of patients with HAP who received empiric antimicrobial agents with activity against MRSA and two-drug therapy for resistant gram-negative rods (R-GNR) as recommended in the 2016 ATS/IDSA guidelines for HAP (4). For MRSA, the suggested agents were vancomycin and linezolid. For R-GNR, the suggested agents were piperacillin-tazobactam, cefepime, ceftazidime, imipenem, meropenem, aztreonam, ciprofloxacin, levofloxacin, amikacin, gentamicin, tobramycin, colistin, and polymyxin B. Of note, Veterans Affairs pharmacy benefits management leaves all decisions regarding which antibiotics to carry to the local hospital or station. They state that these decisions should be based on local culture and sensitivity patterns. In addition, we focused on the number of antipseudomonals being used to evaluate concern for resistance by the provider in an effort to accommodate prescribing practices. We also measured the percentage of hospitalizations that had blood and respiratory cultures obtained at time of treatment. We measured the percentage of hospitalizations in which cultures detected MRSA and GNR resistant to piperacillin-tazobactam. Nasal MRSA polymerase chain reaction screening data were not included in the estimates of resistance prevalence.
Antibiogram Development Feasibility and Application to HAP Cohort
We extracted all inpatient culture data from all VAMCs between 2011 and 2014 to determine the ability of hospitals to generate antibiograms by location (hospital-wide vs. ICU) and culture type (respiratory vs. all). We defined a hospital’s ability to generate an antibiogram as the presence of 30 or more cultures during the year before the pneumonia (16). For each facility, we measured the ability to develop the following antibiograms in 2014: hospital-wide S. aureus resistance among all cultures, hospital-wide S. aureus resistance among respiratory samples only, ICU-level S. aureus resistance among all cultures, ICU-level S. aureus resistance among respiratory samples, ICU-level GNR resistance among all cultures, and ICU-level GNR resistance among respiratory samples. Last, we developed yearly antibiograms for each facility between 2011 and 2014 to determine the hypothetical recommended use of broad-spectrum antibiotics for each patient with HAP on the basis of the ATS/IDSA-provided thresholds.
Statistical Analysis
To assess the ATS/IDSA guidelines’ ability to accurately identify patients at risk for resistant pathogens, we examined the alignment between the recommendation for broad-spectrum antimicrobials and the recovery of relevant pathogens. In our analysis, we evaluated both the providers’ behavior and the performance characteristics of the guidelines. We calculated provider sensitivity, defined as the proportion of admissions with positive cultures for MRSA or R-GNR that received an anti-MRSA drug or more than one antipseudomonal, and also the sensitivity of the guidelines, defined as the proportion of cultures positive for a resistant pathogen that would be recommended to receive broad-spectrum treatment. Similarly, we calculated provider specificity (the proportion of admissions without a resistant infection on cultures that did not receive empiric treatment for resistance) and guideline specificity (those lacking positive cultures who would not be recommended to receive treatment for resistance by the guidelines). We calculated the potential overtreatment and undertreatment of those with HAP if guideline recommendations were followed. Potential overtreatment was defined as the proportion of all patients who had negative cultures but were treated. Potential undertreatment was defined as the proportion of all patients who had positive cultures but were not treated. We then completed both a univariable and multivariable regression analysis to determine the predictive ability of the provided thresholds and patient characteristics believed to confer increased risk of resistance for HAP. Patient characteristics included intravenous antibiotics in the previous 90 days, mechanical ventilation in the 48 hours before admission, and pressor order (4–6, 17, 18).
In our sensitivity analysis among all patients with a secondary diagnosis of pneumonia who did receive antibiotics or cultures in the initial 48 hours, we measured the rate of MRSA and R-GNR detection and estimated the empiric use of broad-spectrum antibiotics if guideline recommendations were followed.
All statistical analyses were performed using STATA, Version 12.0 (StataCorp) and R (http://cran.r-project.org). This study was reviewed and approved by the institutional review board of the University of Utah office of research and development and the Salt Lake City VA Human Research Protection Program
Results
Patient Characteristics, Antimicrobial Coverage, and Culture and Detection
Of a total of 1.8 million hospitalizations at 113 facilities over 3 years, 76,227 (4.23%) hospitalizations had a secondary diagnosis of pneumonia. After exclusion of those admitted for respiratory distress or sepsis, and subsequently those who received antibiotics or cultures in the first 48 hours of admission, the remaining 3,562 (0.20%) met our criteria for HAP, leading to an incidence rate of 3.7 HAPs per 10,000 hospital days in patients without initial infection on admission. Manual chart review (conducted by A.D.B.) to validate the precision of our definition of patients with HAP found a positive predictive value of 92.00%. Among the 3,562 hospitalizations, median patient age was 69 years (mean, 71 yr; interquartile range [IQR], 64–79 yr), and median length of stay was 16 days (mean, 22 d; IQR, 10–26 d). A total of 2,676 (75.13%) admissions were to a medical service, and 885 (24.85%) were to a surgical service. See Table 1for associated comorbidities. A total of 764 (21.45%) patients spent at least 1 day in the ICU during their admission, 72 patients (2.02%) had an order for vasopressors, and 94 patients (2.64%) received mechanical ventilation in the 48 hours before the HAP episode. A total of 579 (16.25%) patients died within 30 days of their admission. Regarding cultures, 3,042 (85.40%) had blood cultures, 1,761 (49.44%) had respiratory cultures, and 1,447 (40.62%) had both. Of those patients mechanically ventilated, 78 (82.98%) had respiratory cultures obtained. MRSA was detected by culture in 184 patients (5.17%) and R-GNR were detected in 82 patients (2.30%), despite positive blood or respiratory cultures in 1,199 (33.66%) patients (Table 1). The most common pathogens identified were S. aureus, Pseudomonas aeruginosa, and Klebsiella (see Table E1 in the online supplement).
Table 1.
Demographics and clinical characteristics of patients with hospital-acquired pneumonia (N = 3,562)
| Demographic | |
|---|---|
| Age, yr, median (IQR) | 69 (64–79) |
| Male sex | 3,487 (97.89) |
| Clinical | |
| LOS, d, median (IQR) | 22 (10–26) |
| Admitted to medical service | 2,676 (75.13) |
| Admitted to surgical service | 885 (24.85) |
| Renal disease | 430 (12.07) |
| Heart failure | 587 (16.48) |
| Cerebrovascular disease | 428 (12.02) |
| Diabetes mellitus | 977 (27.43) |
| Cancer | 662 (18.39) |
| IV antibiotics in 90 d | 639 (17.94) |
| ICU admission | 764 (21.45) |
| Pressor order | 72 (2.00) |
| Mechanical ventilation | 94 (2.64) |
| 30-d mortality | 579 (16.25) |
| Antibiotics | |
| MRSA coverage | 2,010 (56.43) |
| No antipseudomonal | 720 (20.21) |
| 1 antipseudomonal | 1,743 (48.93) |
| ≥2 antipseudomonals | 809 (22.71) |
| Cultures | |
| Blood | 3,042 (85.40) |
| Respiratory | 1,761 (49.44) |
| Blood and respiratory | 1,447 (40.62) |
| Positive blood or respiratory culture | 1,199 (33.66) |
| MRSA positive | 184 (5.17) |
| R-GNR positive | 82 (2.30) |
Definition of abbreviations: ICU = intensive care unit; IQR = interquartile range; LOS = length of stay; MRSA = methicillin-resistant Staphylococcus aureus; R-GNR = resistant gram-negative rods.
Data presented as n (%) unless otherwise noted.
Antibiogram Feasibility and Utility
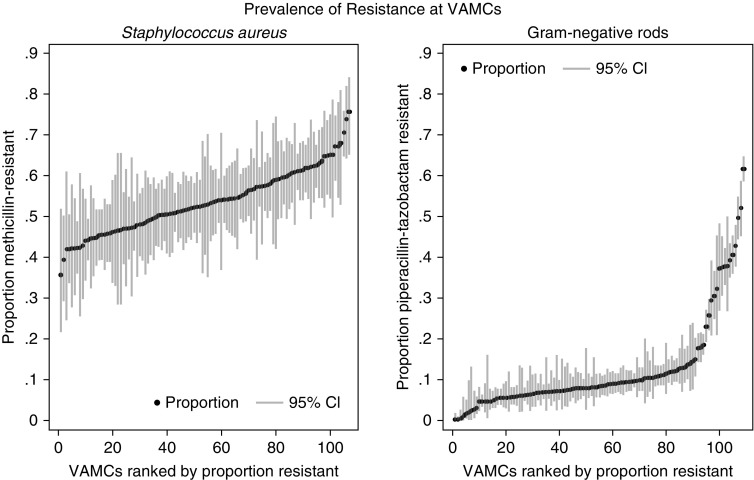
Among 113 VAMCs, we found 84 (74.34%) could generate annual, facility-wide S. aureus methicillin antibiograms from all hospital cultures according to Clinical and Laboratory Standards Institute guidelines (16). However, only 16 (14.16%) could generate antibiograms for MRSA from respiratory samples. For the ICUs, 17 (15.04%) could generate MRSA antibiograms and 68 (60.18%) could generate GNR antibiograms from all cultures, although when limited to respiratory cultures only 2 (1.77%) could generate antibiograms for MRSA and 14 (12.39%) for GNR resistance. When evaluating the prevalence of resistance between 2014 and 2017 among all centers, we found the median facility-level prevalence of MRSA was 49% (IQR, 44–54%) and the median R-GNR prevalence was 6.7% (IQR, 4.3–9.3%). Almost all VAMCs (112, 99.12%) had a prevalence of MRSA >20%. For resistant GNR, the prevalence among ICUs was much more varied, although the majority had a prevalence of resistance to piperacillin-tazobactam <20% (Figure 2).
Figure 2.
Prevalence of resistance in antibiograms at 113 Veterans Affairs Medical Centers (VAMCs) from all hospital cultures. CI = confidence interval.
Predictive Performance of ATS/IDSA Thresholds and Patient Characteristics for Resistant Infection
Among patients with HAP, 2,010 (56.43%) were empirically treated for MRSA and 809 (22.71%) were empirically treated for R-GNR with two or more antipseudomonals. The sensitivity and specificity of the clinician’s empiric MRSA treatment was 69% (95% confidence interval [CI], 61.47–75.94%) and 39% (95% CI, 37.32–40.78%), respectively. For R-GNR, the sensitivity and specificity of clinician’s empiric treatment was 38% (95% CI, 26.36–49.70%) and 76% (95% CI, 74.04–77.04%), respectively. Using the ATS/IDSA-specified threshold, we found that 3,561 (99.97%) of patients with HAP were admitted to facilities with >20% prevalence of resistance, so that all but one patient would be recommended to have coverage for MRSA by the guidelines, although only 184 patients (5.17%) grew MRSA on relevant cultures. The sensitivity of this treatment threshold was 100% (95% CI, 98.02–100.00%) and the specificity was 0.03% (95% CI, 0.00–0.16%). This indicates that 94.81% (95% CI, 94.02–95.50%) of patients would be potentially overtreated using the 20% threshold for empiric coverage, and no patients would be undertreated for MRSA. When incorporating the ATS/IDSA patient characteristics, including intravenous antibiotics within the past 90 days, shock, and ventilator requirement at time of HAP, all patients but one would be recommended to receive empiric MRSA coverage, and 778 (21.84%) patients would be recommended to have R-GNR coverage with two antipseudomonal agents. By univariable and multivariable analysis, we did not find a MRSA prevalence of ≥20% or mechanical ventilation to be significantly associated with an increased rate of MRSA detection (threshold P < 0.05). We did find orders for vasopressors (odds ratio [OR], 3.89; 95% CI, 1.17–12.91; P = 0.03) and intravenous antibiotics in the last 90 days (OR, 1.98; 95% CI, 1.03–3.81; P = 0.04) to be associated with an increased risk of MRSA detection. For R-GNR, we did not find an association between increased risk of resistant infection and intravenous antibiotics in the last 90 days or vasopressor order. However, we did find mechanical ventilation to be associated with increased risk of culture-proven R-GNR infection (OR, 4.37; 95% CI, 1.52–12.57; P = 0.01) (Table 2).
Table 2.
Multivariable model for resistant infection in hospital-acquired pneumonia
| Odds Ratio (95% CI) | P Value | |
|---|---|---|
| Variables for MRSA resistance | ||
| 20% threshold | Undefined* | * |
| IV antibiotics in last 90 d | 1.98 (1.03–3.81) | 0.04 |
| Pressor order | 3.89 (1.17–12.91) | 0.03 |
| Mechanical ventilation | 1.82 (0.43–7.67) | 0.41 |
| AUC = 0.55 | ||
| Variables for GNR resistance | ||
| IV antibiotics in last 90 d | 1.00 (0.44–2.28) | 0.99 |
| Pressor order | 1.33 (0.18–9.86) | 0.78 |
| Mechanical ventilation | 4.37 (1.52–12.57) | 0.01 |
| AUC = 0.55 |
Definition of abbreviations: AUC = area under the receiver operating characteristic curve; CI = confidence interval; MRSA = methicillin-resistant Staphylococcus aureus; GNR = gram-negative rods.
The odds ratio is undefined, as no patients were in facilities where the prevalence of resistance was ≤20%; therefore, the denominator was 0.
Sensitivity Analysis
Among all patients with a secondary pneumonia without a principal diagnosis of respiratory distress or sepsis (31,560 patients), we found 27,024 (85.63%) were admitted to a medical service, 4,500 (14.26%) were admitted to a surgical service, and 36 patients (0.11%) were admitted to nonmedical and nonsurgical services. A total of 606 patients (1.92%) grew MRSA on relevant cultures, and 272 patients (0.86%) grew R-GNR. The most common pathogens identified were S. aureus, P. aeruginosa, and Klebsiella spp (Table E2). Using the ATS/IDSA-specified threshold for anti-MRSA therapy, we found that 31,516 patients (99.86%) would be recommended to receive anti-MRSA treatment. When including those who had received antibiotics in the past 90 days, this increased the number indicated to have anti-MRSA therapy to 31,520 (99.87%). In addition, when evaluating the number of patients recommended to receive dual antipseudomonal therapy from our larger cohort, we found that 23,724 (75.17%) received antibiotics on admission, which would lead to the recommendation for dual antipseudomonals at the time of HAP. Furthermore, when incorporating patient characteristics including shock and ventilator requirement at time of HAP, an additional 139 patients would be recommend to receive dual antipseudomonal therapy, indicating that a total of 23,863 (75.61%) would be recommended to have dual antipseudomonal coverage.
Discussion
Our study is the first to examine the potential consequences of the ATS/IDSA guidelines using resistance prevalence thresholds to help guide antimicrobial decisions and their performance as tools to identify resistant infections in a large multicenter study. We found the number of patients treated with antibiotics against MDR pathogens far exceeded the number of cultures found to display antibiotic resistance. In addition, when applying the guideline-recommended prevalence of resistance thresholds at which broad-spectrum coverage should be initiated, we found this would substantially increase the use of anti-MRSA antimicrobials compared with clinical practice without improved predictive performance had the guidelines been in place historically. The reason for this is likely multifactorial. It may in part be due to the inherent difficulty in a universal threshold when there is overall low prevalence of resistant infection. In addition, this finding may emphasize that the risk of infection with a resistant pathogen is more dependent on the particular pathogen in concert with a susceptible host. Factors influencing host susceptibility may include underlying lung disease, history of resistant infection, history of antimicrobial use, and ventilator-associated injury.
The rate of detection of resistant organisms in our population is similar to rates found in the VA system in HCAP, albeit lower than that described by Chung and colleagues in their cohort from Asia, although the most common pathogens were similar (19, 20). Our rates of resistance were more consistent with those found by Kollef and colleagues, potentially due to more similar populations, severity of illness, and sampling practices (21). Our findings are in concert with Ekren and colleagues, who also found very high sensitivity for the given resistant risk factors and very poor specificity leading to significant potential overtreatment (22). In addition, the risk factors we found to be significantly associated with resistant infection were consistent with the findings of Martin-Loeches and colleagues (i.e., including intravenous antibiotics in the past 90 days, shock, and mechanical ventilation) (23).
Our study has several limitations. We used administrative data that relied on principal diagnostic codes to identify cases of pneumonia and therefore may not have captured all HAPs because of inconsistent diagnostic coding practices or missed diagnosis. In addition, our detection rates of resistant infection were limited by the culturing practices of the providers. Although overall there was a high rate of blood cultures, respiratory cultures were obtained in only about half of admissions. A larger percentage of patients mechanically ventilated had respiratory cultures obtained, although overall the number mechanically ventilated in the 48 hours before their infection was small (2.64%). We defined gram-negative resistance as resistance to piperacillin-tazobactam for purposes of feasibility, because it is a common treatment for HAP and a primary agent recommend by the ATS/IDSA for HAP. In addition, it is the most common antipseudomonal that was administered for CAP across the VA after fluoroquinolones (8). However, because hospitals may have different antimicrobial practices, this may limit the generalizability of our results. Our primary analysis was limited to patients who were admitted to the hospital without receipt of antibiotics or cultures on admission, to ensure that we captured new diagnoses of pneumonia (rather than a late diagnosis of community-acquired pneumonia) and no additional infectious diseases that could complicate the antibiotic choices. As this approach substantially reduced our sample size and potentially skewed our population to a healthier subset of patients, this limits the generalizability of our findings. However, we also completed a sensitivity analysis of all patients with secondary diagnosis of pneumonia, including those who received antibiotics and cultures within the first 48 hours. This cohort demonstrated lower rates of detected resistance but suggested similar consequences of guidelines on treatment: all members would be recommended to receive anti-MRSA therapy and 75% would be recommended to receive dual antipseudomonals, given the guideline recommendations. Furthermore, given that the facility-level prevalence of MRSA in S. aureus antibiograms was >20% in almost all facilities over the time period evaluated, nearly universal anti-MRSA coverage would be recommended for HAP regardless of admission type. Therefore, despite the restricted nature of our primary HAP cohort, our study suggests that antibiogram-guided therapy at the recommended thresholds would substantially increase unnecessary broad-spectrum antibiotic use for HAP in more generalized patient populations. Last, our study included secondary diagnoses of pneumonia, not only culture-confirmed cases. Because of this, we likely included patients who lacked true infection. Unfortunately, this is the clinical reality of pneumonia: there is no gold standard for the diagnosis of pneumonia, and cultures are rarely revealing. Our chart review did, however, demonstrate a relatively high positive predictive value in our cohort of interest.
Recommendations for the optimal empiric treatment of HAP must balance the need for effective patient treatment while minimizing unwarranted broad-spectrum antibiotic use. To achieve this, previous guidelines have emphasized limiting broad-spectrum use to those with either increased risk for resistant infection because of patient characteristics or the patient’s environmental exposure to resistant pathogens. At present, the evidence behind using a single antibiogram-based threshold to guide broad-spectrum antibiotic use is limited, making universal recommendations difficult. Focusing on refining our understanding of host susceptibility and host–pathogen interactions (and thus on patient-specific rather than population-level risk of infection by resistant pathogens), while working to establish improved rapid diagnostic testing and resistance prediction, are the most promising pathways for adequate empiric treatment. In the meantime, clinicians and stewardship programs will be wise to heed the guideline’s admonition to use local data to anticipate potential impact and inform local adaptation of guideline implementation. Future research evaluating the comparative benefits and harms of initial broad-spectrum treatment will be instrumental to evaluating the risk associated with different empiric strategies.
Supplementary Material
Footnotes
Supported by a training grant from the National Institutes of Health (A.D.B.) and Veterans Affairs Health Services Research and Development Service career development awards IK2HX001165 (M.J.) and IK2HX001908 (B.E.J.). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Author Contributions: A.D.B. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; B.E.J. contributed to data collection/management, study design, and manuscript preparation; R.P. contributed to study design and manuscript preparation; M.B.G. contributed to manuscript preparation; M.S. contributed to manuscript preparation; and M.J. contributed to data collection/management, study design, and manuscript preparation.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, et al. Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudeck MA, Weiner LM, Allen-Bridson K, Malpiedi PJ, Peterson KD, Pollock DA, et al. National Healthcare Safety Network (NHSN) report, data summary for 2012, device-associated module. Am J Infect Control. 2013;41:1148–1166. doi: 10.1016/j.ajic.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sopena N,Sabrià M; Neunos 2000 Study Group. Multicenter study of hospital-acquired pneumonia in non-ICU patients Chest 2005127213–219. [DOI] [PubMed] [Google Scholar]
- 4.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leroy O, d’Escrivan T, Devos P, Dubreuil L, Kipnis E, Georges H. Hospital-acquired pneumonia in critically ill patients: factors associated with episodes due to imipenem-resistant organisms. Infection. 2005;33:129–135. doi: 10.1007/s15010-005-4021-8. [DOI] [PubMed] [Google Scholar]
- 6.Torres A, Niederman MS, Chastre J, Ewig S, Fernandez-Vandellos P, Hanberger H, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT) Eur Respir J. 2017;50:1700582. doi: 10.1183/13993003.00582-2017. [DOI] [PubMed] [Google Scholar]
- 7.Chalmers JD, Rother C, Salih W, Ewig S. Healthcare-associated pneumonia does not accurately identify potentially resistant pathogens: a systematic review and meta-analysis. Clin Infect Dis. 2014;58:330–339. doi: 10.1093/cid/cit734. [DOI] [PubMed] [Google Scholar]
- 8.Jones BE, Jones MM, Huttner B, Stoddard G, Brown KA, Stevens VW, et al. Trends in antibiotic use and nosocomial pathogens in hospitalized veterans with pneumonia at 128 medical centers, 2006-2010. Clin Infect Dis. 2015;61:1403–1410. doi: 10.1093/cid/civ629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vallés J, Martin-Loeches I, Torres A, Diaz E, Seijas I, López MJ, et al. Epidemiology, antibiotic therapy and clinical outcomes of healthcare-associated pneumonia in critically ill patients: a Spanish cohort study. Intensive Care Med. 2014;40:572–581. doi: 10.1007/s00134-014-3239-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giantsou E, Liratzopoulos N, Efraimidou E, Panopoulou M, Alepopoulou E, Kartali-Ktenidou S, et al. Both early-onset and late-onset ventilator-associated pneumonia are caused mainly by potentially multiresistant bacteria. Intensive Care Med. 2005;31:1488–1494. doi: 10.1007/s00134-005-2697-y. [DOI] [PubMed] [Google Scholar]
- 11.Berton DC, Kalil AC, Teixeira PJ. Quantitative versus qualitative cultures of respiratory secretions for clinical outcomes in patients with ventilator-associated pneumonia. Cochrane Database Syst Rev. 2014;(10):CD006482. doi: 10.1002/14651858.CD006482.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aliberti S, Reyes LF, Faverio P, Sotgiu G, Dore S, Rodriguez AH, et al. GLIMP investigators. Global Initiative for Methicillin-Resistant Staphylococcus aureus Pneumonia (GLIMP): an international, observational cohort study. Lancet Infect Dis. 2016;16:1364–1376. doi: 10.1016/S1473-3099(16)30267-5. [DOI] [PubMed] [Google Scholar]
- 13.Jones RN. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis. 2010;51:S81–S87. doi: 10.1086/653053. [DOI] [PubMed] [Google Scholar]
- 14.American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 15.U.S. Department of Veterans Affairs. VA Informatics and Computing Infrastructure (VINCI). [Updated/reviewed 2018 Oct 1; accessed 2017 Oct 1]. Available from: https://www.hsrd.research.va.gov/for_researchers/vinci/
- 16.Clinical and Laboratory Standards Institute (CLSI). Analysis and Presentation of Cumulative Antimicrobial Susceptibility Test Data. 2019. DOI: 10.1007/978-3-662-48986-4_300416.
- 17.Gross AE, Van Schooneveld TC, Olsen KM, Rupp ME, Bui TH, Forsung E, et al. Epidemiology and predictors of multidrug-resistant community-acquired and health care-associated pneumonia. Antimicrob Agents Chemother. 2014;58:5262–5268. doi: 10.1128/AAC.02582-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torres A, Niederman MS, Chastre J, Ewig S, Fernandez-Vandellos P, Hanberger H, et al. Summary of the international clinical guidelines for the management of hospital-acquired and ventilator-acquired pneumonia. ERJ Open Res. 2018;4:00028–2018. doi: 10.1183/23120541.00028-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung DR, Song JH, Kim SH, Thamlikitkul V, Huang SG, Wang H, et al. Asian Network for Surveillance of Resistant Pathogens Study Group. High prevalence of multidrug-resistant nonfermenters in hospital-acquired pneumonia in Asia. Am J Respir Crit Care Med. 2011;184:1409–1417. doi: 10.1164/rccm.201102-0349OC. [DOI] [PubMed] [Google Scholar]
- 20.Madaras-Kelly K, Jones M, Remington R, Caplinger CM, Huttner B, Jones B, et al. Antimicrobial de-escalation of treatment for healthcare-associated pneumonia within the Veterans Healthcare Administration. J Antimicrob Chemother. 2016;71:539–546. doi: 10.1093/jac/dkv338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest. 2005;128:3854–3862. doi: 10.1378/chest.128.6.3854. [DOI] [PubMed] [Google Scholar]
- 22.Ekren PK, Ranzani OT, Ceccato A, Li Bassi G, Muñoz Conejero E, Ferrer M, et al. Evaluation of the 2016 Infectious Diseases Society of America/American Thoracic Society guideline criteria for risk of multidrug-resistant pathogens in patients with hospital-acquired and ventilator-associated pneumonia in the ICU. Am J Respir Crit Care Med. 2018;197:826–830. doi: 10.1164/rccm.201708-1717LE. [DOI] [PubMed] [Google Scholar]
- 23.Martin-Loeches I, Deja M, Koulenti D, Dimopoulos G, Marsh B, Torres A, et al. EU-VAP Study Investigators. Potentially resistant microorganisms in intubated patients with hospital-acquired pneumonia: the interaction of ecology, shock and risk factors. Intensive Care Med. 2013;39:672–681. doi: 10.1007/s00134-012-2808-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.