Abstract
Primary refractory acute myeloid leukemia (AML), or primary induction failure, represents a continued challenge in clinical management. This review presents an overview of primary refractory disease and a discussion of risk factors for induction failure, including current evidence regarding the impact of karyotype and molecular mutation status on responsiveness to chemotherapy. We review the evidence for various treatment options for refractory AML including salvage chemotherapy regimens, allogeneic hematopoietic stem cell transplantation, targeted agents, and non-intensive therapies such as hypomethylating agents. A therapeutic approach to this patient population is presented, and several new and emerging therapies are reviewed.
Keywords: acute myeloid leukemia, primary refractory, induction failure, salvage chemotherapy
INTRODUCTION
Approximately 10–40% of adults with acute myeloid leukemia (AML) will have persistent leukemia following intensive induction chemotherapy[1–4]. Despite significant progress that has been made in AML therapy, the treatment of primary refractory disease (PRD) remains challenging due to relatively low response rates to salvage chemotherapy and poor overall survival (OS) rates. Allogeneic hematopoietic stem cell transplantation (HSCT) represents the best hope for long-term cure in this group of patients, although many patients with PRD are not candidates for transplantation due to factors such as poor performance status or advanced age. This review discusses risk factors for PRD and provides an update on current treatment options and promising emerging therapies.
DEFINITION
For the purposes of this review, we will define PRD--also known as primary induction failure--as the lack of a complete remission after two courses of standard-dose cytarabine-based induction chemotherapy (e.g. cytarabine and anthracycline “7+3”)[5,6] or at least one course of high-dose cytarabine-based induction[7]. For this definition, complete remission (CR) is defined as fewer than 5% blasts on morphologic examination of the bone marrow along with recovery of the absolute neutrophil count (ANC) to greater than 1.0 × 109/L and recovery of the platelet count to at least 100 × 109/L. Patients with all criteria for CR except either residual neutropenia or thrombocytopenia (CR with incomplete blood count recovery, CRi)[5,8] do have inferior outcomes compared to those who achieve a full CR[9], but patients with CRi are not included in the primary refractory group.
It should be noted that the definition of a complete remission is evolving as the prognostic significance of measurable residual disease (MRD) by multiparameter flow cytometry (MFC) or molecular-based assays becomes more clear[10,11]. The 2017 European LeukemiaNet (ELN) recommendations include CR without MRD (CRMRD-) as a separate response category[5]. Patients who achieve a CRMRD- have improved outcomes compared to those who achieve a CR with persistent MRD, including those who undergo allogeneic HSCT[12–18]. A lack of standardization among laboratories and variation in sensitivity depending on the type of assay used has slowed widespread adoption of MRD monitoring in AML [10,19]. Therefore, for the remainder of this review article we will continue to use the traditional definition of PRD as persistent leukemia by light microscopy.
RISK FACTORS
Risk factors for primary refractory AML include a complex or monosomal karyotype, advanced age, an increased time to blast clearance in the bone marrow or peripheral blood, a high white blood cell (WBC) count at diagnosis, secondary AML, and the presence of certain molecular mutations such as TP53[20–23].
Cytogenetics
Karyotype is the most important factor in estimating prognosis. The revised MRC cytogenetic classification system stratifies karyotypes into favorable, intermediate and adverse risk, which are associated with 10-year OS rates of 69%, 33%, and 12%, respectively[24,25]. Karyotype is also an important predictor of whether a patient will achieve a CR with induction chemotherapy[20,21,26]. A large prospective analysis of 1213 adults with de novo AML who were treated with “7+3” induction chemotherapy on 5 Cancer and Leukemia Group B (CALGB) studies found that only 30% of subjects with ≥ 5 unrelated cytogenetic abnormalities and 47% of subjects with 3–4 cytogenetic abnormalities achieved a CR, as compared to 68% of subjects with a normal karyotype (p<0.001 and p=0.002, respectively)[20,21].
Among the subgroup of patients with adverse cytogenetics, inv(3)(q21q26), t(3;3)(q21;q26), and other abnormalities of 3q (excluding t(3;5)(q21~25;q31~35), which are relatively rare and occur in approximately 2% of adults with AML, are well-known risk factors for PRD[20,21,24,27–30]. A monosomal karyotype (MK), defined as having at least 2 autosomal monosomies or a single autosomal monosomy in the presence of at least 1 other structural chromosomal abnormality, is also associated with a very poor prognosis and an increased rate of PRD[22,31–33]. Breems et al. found that among 1975 patients with AML treated on various Dutch-Belgian Haemato-Oncology Cooperative Group (HOVON)/Swiss Group for Clinical Cancer Research trials, 52% of subjects with a MK failed to achieve a CR compared to 18% of patients overall, and subjects with a MK had a 4-year OS of only 4%[22]. Of note, HOVON conducted a prospective randomized trial in which patients with newly diagnosed AML were randomized to either standard-dose cytarabine (200mg/m2/day by continuous infusion on days 1–7) or high-dose cytarabine (HiDAC) (1000mg/m2 every 12 hours on days 1–5), both in combination with idarubicin[34]. Although there was no difference in CR rate or OS overall at a median follow-up of 5 years, a sub-group analysis found that subjects with a monosomal karyotype had improved 5-year event-free survival (13% vs. 0%) and OS (16% vs. 0%) with HiDAC, suggesting a HiDAC-based induction regimen be considered in patients with a known monosomal karyotype[34]. There has also been some enthusiasm for considering the inclusion of cladribine in the induction regimen of patients with adverse-risk karyotypes, though whether this benefits monosomal karyotype is uncertain[35–39].
Age
An older age is associated with an increased likelihood of PRD, which is primarily related to biological differences in AML which tend to occur with increasing age, including an increased incidence of complex and monosomal karyotypes[31,40]. Patients with an older age also have an increased likelihood of having a poor performance status (PS) or multiple other medical comorbidities. In addition to increasing the risk of treatment-related mortality, an Eastern Cooperative Oncology Group (ECOG) PS ≥ 2 has been independently associated with failure to achieve CR with induction therapy even in the absence of early death[23].
Time to blast clearance
The time to blast clearance in the bone marrow and peripheral blood after starting induction chemotherapy has also been recognized as a predictor of PRD[41–45]. Several studies have found that a higher blast percentage on a nadir bone marrow biopsy performed on day 14 to 16 of the first cycle of induction therapy is associated with an increased incidence of PRD[41–43].
Molecular mutation status
Among the recurrent somatic mutations commonly found in AML[27,46], some have been associated with a more favorable prognosis and others with a less favorable outcome[47]. With respect to achieving a CR with induction chemotherapy, both gene fusions and recurrent mutations have been predictive of induction success rates. For example, patients with core binding factor fusions have extremely high rates of CR, as do those with cytogenetically normal AML who have either a mutation in NPM1 or double mutations in CEBPA[27]. In addition, patients with these genotypes also have a relatively high 4-year OS rate (around 60%) in the absence of FLT3-ITD mutation[48]. On the other hand, TP53 mutations are associated with complex karyotype and a significantly worse outcome. This reflects both a low rate of initial remission as well as a high relapse rate, regardless of post-remission therapy delivered [49,50]. An analysis of adults with AML who were treated on various studies of the German-Austrian AML study group found that among 234 patients with complex karyotypes, those with mutations in TP53 were less likely than those who were TP53-wild type to achieve a CR with induction chemotherapy (28% vs. 50%, p=0.01) and had a dramatically worse 3-year OS (3% vs. 28%, p<0.0001)[49]. Interestingly, a non-randomized study of frontline decitabine therapy in TP53-mutated AML showed more favorable response rates and short-term outcomes in this group, suggesting hypomethylating agents might represent preferred agents for these patients[51]. The prognostic significance of other somatic mutations with respect to initial induction chemotherapy response is less clear[47,52–54].
TREATMENT
The treatment of patients who are refractory to standard induction chemotherapy remains extremely challenging and outcomes are overall poor. Therapeutic options include more intensively dosed or timed salvage chemotherapy, direct allogeneic HSCT, targeted agents, a hypomethylating agent (HMA), other non-intensive therapies such as low-dose cytarabine (LDAC), and enrollment to clinical trials.
Salvage chemotherapy
Many salvage chemotherapy regimens have been studied in primary refractory AML, including HiDAC, HAM, MEC, FLAG, FLAG-Ida, and CLAG-M (Table 2), among others. It is somewhat challenging to compare the outcomes of studies of salvage regimens due to both significant heterogeneity in the patients included in the studies and in the definitions by which PRD was defined. In general, CR rates with intensive salvage chemotherapy are in the range of 20 to 35%, although this varies based on the patient population studied as older patients and patients with high-risk cytogenetics are less likely to achieve a CR[55–58]. A large study of 1025 patients with PRD who were treated on German-Austrian AML Study Group trials and underwent salvage chemotherapy with various regimens including HAM (high-dose cytarabine and mitoxantrone), A-HAE (high dose-cytarabine, etoposide, and ATRA), and GO-A-HAM (gemtuzomab ozogamicin, ATRA, and HAM), found an overall CR/CRi rate of 36%[58]. There is not a standard of care regarding which salvage chemotherapy regimen should be chosen first line for a patient with PRD, but we favor a HiDAC-based regimen if the patient has not previously received high-dose cytarabine during the initial induction attempts [5,59].
Table 2.
Selected salvage chemotherapy regimens
| Abbrev. | Regimen | Total no. patients | Overall CR rate | No. pts. with PRD | CR rate for pts with PRD | OS | Early mortality rate/TRM | Ref. |
|---|---|---|---|---|---|---|---|---|
| HiDAC | Ara-C 3g/m2 q12h d1–6 | 81 | 26/81 (32%) | 27 | Not reported | Med OS 8 mo | 10/81 (12%) | [55] |
| HAM/ S-HAM |
Ara-C 3g/m2 q12h d1–3 MIT 12mg/m2 d2–3 Ara-C 3g/m2 q12h d1,2,8,9 MIT 10mg/m2 d3,4,10,11 |
150 | 42/150 (28%) | 150 | 42/150 (28%) | Not reported | 3/150 (2.0%) | [58] |
| GO-A-HAM | GO 2mg/m2 (5mg max) d1 ATRA 45mg/m2 po d4–6 and 15mg/m2 po d7–28 +HAM as above |
140 | 70/140 (50%) | 140 | 70/140 (50%) | Not reported | 2 (1.4%) | [58] |
| MEC | MIT 8mg/m2 d1–5 Etoposide 100mg/m2 d1–5 Ara-C 1g/m2 d1–5 |
63 | 16/63 (25%) | 17 | 3/17 (18%) | Med OS 5.4mo Med DFS 9.3mo |
7/63 (11%) | [56] |
| GCLAC | G-CSF 5mcg/kg d0-recovery Clofarabine 25mg/m2 d1–5 Ara-C 2g/m2 d1–5 |
50 | 21/46 (46%) | 18 | 12/18 (67%) | Med OS 8.8mo | 0/50 (0%) | [57,127] |
| FA/FLAG | Fludarabine 30mg/m2 d1–5 Ara-C 2g/m2 d1–5 ±G-CSF 400mcg/m2 d0-recovery |
FA: 81 FLAG: 20 |
FA: 22/81 (27%) FLAG: 4/20 (20%) |
FA: 20 FLAG: 3 |
FA: 2/20 (10%) FLAG: 0/3 (0%) |
FA: Med OS 3.4mo FLAG: Med OS 3.8mo |
FA: 18/81 (22%) FLAG: 4/20 (20%) |
[57] |
| FLAG-Ida | Fludarabine 25mg/m2 d1–5 Ara-C 2g/m2 d1–5 G-CSF d6-recovery |
34 | 15/34 (53.6%) | 11 | 7/11 (62.5%) | Med OS 22wks | 6/34 (17.6%) | [128] |
| CLAG-M | Cladribine 5mg/m2 d1–5 Ara-C 2g/m2 d1–5 G-CSF 300mcg d0–5 MIT 10mg/m2 d1–3 |
118 | 66/118 (58%) | 75 | 16/75 (21%) | 4y OS 14% 4y DFS 30% |
8/118 (7%) | [36] |
Abbreviations: CR, complete remission; OS, overall survival; PRD, primary refractory disease; TRM, treatment-related mortality; Ara-C, cytarabine; MIT, mitoxantrone; GO, gemtuzumab ozogamicin; ATRA, all-trans retinoic acid; G-CSF, granulocyte-colony stimulating factor
Unfortunately, the results of recent prospective randomized clinical trials that attempted to improve on these outcomes have been largely negative. The VALOR study, which was a phase III double-blind study of high-dose cytarabine alone or in combination with the quinolone derivative vosaroxin in patients with relapsed and refractory AML, found no improvement in OS among patients that received vosaroxin compared to placebo[60]. Likewise, although there has been some enthusiasm for the use of clofarabine in the refractory setting, the CLASSIC I trial showed no difference in median OS for intermediate-dose cytarabine alone or in combination with a single dose of clofarabine in patients ≥ 55 years old with relapsed and refractory AML[61]. An international phase III study of 381 patients with relapsed or refractory AML who were treated with elacytarabine versus investigator choice of 7 different salvage regimens (HiDAC, MEC, FLAG/FLAG-Ida, LDAC, HMA, hydroxyurea, or best supportive care) found no difference in median OS in the elacytarabine arm compared to the control arm (3.5 months vs. 3.3 months, p=0.96); there was also no difference in the CR rate in the elacytarabine arm compared to the control arm (23% vs. 21%)[62]. Importantly, a subgroup analysis also revealed no significant differences in outcome among any of the treatment options in the investigator choice arm[62]. While no subgroup analysis for PRD was included, the overall short survivals with all salvage arms makes it unlikely that a particular approach was associated with markedly better outcomes.
Hypomethylating agents
The HMAs azacitidine and decitabine are frequently used to treat patients with PRD, especially those patients who are older or less fit or who are also refractory to intensive salvage chemotherapy. Although most prospective randomized studies of HMAs have been conducted in the frontline setting[63–65], several smaller studies have also suggested that patients with PRD benefit from treatment with HMAs. A retrospective analysis of 47 patients with relapsed or refractory AML who were treated with azacitidine found that 21% achieved a CR; median OS was 9 months[66]. Another study of 130 patients over the age of 50 years with refractory or relapsed AML who received azaciditine as part of a “compassionate use” program in France found similar outcomes with a CR/CRi rate of 17% and median OS of 8.4 months[67]. Although the reported CR rates with HMAs in the relapsed/refractory settings are widely variable (3.6%−21%), partial responses (PR) or stabilization of disease may also provide a clinical benefit or improvement in quality of life[59]. HMAs also have the benefit of significantly less toxicity compared with intensive salvage regimens and are typically given in the outpatient setting. Available data make a compelling argument for their use, especially in the context of patients not expected to bridge to transplant.
Allogeneic hematopoietic stem cell transplant
Allogeneic HSCT is critically important for fit patients with primary refractory AML as salvage chemotherapy alone is not sufficient for long-term disease control. A possible exception are the rare PRD patients with core-binding factor AML who potentially can experience long-term disease control with HiDAC-based salvage and post-remission therapy. A study of 150 patients with PRD who were treated on a recent SWOG study (S0106) found that the 4-year OS rate was only 4% for the 86 patients who did not undergo allogeneic HSCT compared to 48% for those subjects who underwent transplantation[68]. A similar study of patients with PRD who were treated at the M.D. Anderson Cancer Center found that the median OS of patients who received salvage chemotherapy alone was 2.9 months and 3-year OS was only 2%, compared to 39% for patients who underwent an up-front allogeneic HSCT (p<0.001)[69]. Thus, all patients with PRD who are eligible for allogeneic HSCT should be referred to a transplant center with expeditious completion of HLA typing and initiation of a donor search.
Consideration for immediate allogeneic HSCT is reasonable for eligible patients with PRD, especially if a well-matched donor has been identified, as reported 3-year OS rates for patients who undergo allogeneic HSCT with active AML are in the range of 14–39%[58,69–76]. A review of registry data from the Center for International Blood and Marrow Transplant Research (CIBMTR) from 1440 subjects with PRD specifically who underwent allogeneic HSCT found a 5 year OS rate of 21% (95% CI, 19–23%)[76]. The preferred preparative regimen for patients with active AML at the time of transplantation varies by center. At our institution, we are currently using a regimen containing clofarabine and busulfan based on promising results reported in several small studies. Among 71 subjects included in a prospective phase II study of patients with relapsed and/or refractory AML who underwent myeloablative allogeneic HSCT with clofarabine and busulfan conditioning, the 2-year OS rate was 26% while the non-relapse mortality at 2 years was 25%[73]. Of note, the 2-year event-free survival (EFS) was significantly better among the subgroup of patients with PRD compared to those with relapsed AML (2-year EFS 34% vs 8%, p<0.01) and there was also a trend toward improved OS in this group (2-year OS 34% vs. 24%, p=0.09)[73].
Because a number of studies have found that a lower bone marrow blast percentage prior to allogeneic HSCT is associated with significantly improved outcomes after transplantation[71,72,77–81], at our institution we typically offer salvage therapy to patients with PRD prior to proceeding with allogeneic HSCT in an attempt to maximally decrease the burden of disease prior to transplant. It should be noted, however, that because CR rates to salvage are low, salvage may largely select for a lower risk transplant population. Although a randomized trial to determine whether salvage improves overall survival as compared to direct allogeneic transplant is logistically challenging, indeed this question remains unanswered. The SIERRA trial (NCT02665065) will address this question by randomizing older patients with either PRD or relapsed/refractory AML to salvage chemotherapy followed by conventional allogeneic transplant vs. immediate reduced intensity transplant using a novel radioimmunotherapeutic preparative regimen.
Several scoring systems have been developed to predict outcomes for patients who undergo allogeneic HSCT with active AML[70,74], including the model of Todisco et al. developed specifically for patients with PRD. These authors found that age > 60, ≥ 25% bone marrow blasts at the time of transplant, > 2 prior cycles of chemotherapy, and intermediate-2 or adverse cytogenetics by ELN criteria were independently associated with an increased risk of death after transplant[74]. Subjects with 0–1 risk factors had a 3-year OS rate of 32%, while those with 2 risk factors had a 3-year OS of 10% and those with 3–4 risk factors had a 3-year OS of only 3%[74]. Overall, allogeneic HSCT with active AML is feasible and should be considered in select patients. The use of predictive scoring systems may help identify those patients who are most likely to benefit from transplantation with active disease[70,74].
NEW AND EMERGING THERAPIES
Given the poor prognosis of patients with PRD, we encourage consideration of enrollment on a clinical trial whenever feasible, especially those who have either failed or are not candidates for salvage chemotherapy and allogeneic HSCT. Molecular testing by PCR and/or next-generation sequencing (NGS) techniques should be performed for all patients with PRD in order to evaluate for the presence of specific mutations for which oral small molecule inhibitors have been developed, namely FLT3, IDH1, and IDH2 mutations. Several new AML therapies that have either recently been approved or are currently being investigated in clinical trials are discussed here.
FLT3 inhibitors
FLT3-ITD mutations occur in approximately 23% of adult AML, while mutations in the tyrosine kinase domain (TKD), most commonly FLT3-D835, are found in about 7%[82,83]. Both types of mutations cause constitutive kinase activation [84,85], but FLT3-ITD mutations in particular are associated with a significantly worse prognosis due to relatively high rates of relapse[86,87]. Additionally, the presence of a high FLT3-ITD:WT allelic ratio or certain ITD insertion regions (e.g. Beta-1 sheet of tyrosine kinase 1 domain) predict higher rates of PRD[83,88]. Because refractory and/or relapsed FLT3-ITD+ AML seldom responds durably to salvage chemotherapy[89], we recommend that that these patients be referred for evaluation for enrollment on a clinical trial of a FLT3 inhibitor when possible.
A number of FLT3 inhibitors have been investigated, including the multi-kinase inhibitors sorafenib, lestaurtinib, and midostaurin as well as the more potent and FLT3-selective inhibitors crenolanib, gilteritinib (ASP2215), and quizartinib (AC220). A phase I/II study of sorafenib in combination with idarubicin and intermediate-dose cytarabine, which primarily included patients with newly diagnosed AML, found that 18/19 subjects (95%) with FLT3-ITD mutations achieved CR[90,91]. Although this suggested that the addition of sorafenib could potentially decrease the incidence of PRD in patients with FLT3-ITD mutations, subsequent randomized trials that evaluated sorafenib versus placebo in combination with standard “7+3” found that sorafenib increased toxicity but did not improve OS[92,93]. By contrast, the addition of midostaurin to standard induction and consolidation chemotherapy for newly-diagnosed FLT3-mutated patients under age 60 did not increase toxicity and was associated with a statistically significant improvement in OS compared to placebo (51.4% vs. 44.3 at 4 years, respectively; HR 0.78, p=0.009)[94]. A randomized phase III study to compare crenolanib versus midostaurin when given in combination with standard induction chemotherapy (NCT03258931) will be underway soon.
While none of the multi-kinase inhibitors has had substantial, durable activity in relapsed/refractory patients, the clinical activity of the more potent, selective inhibitors have been promising, even as single agents. Among 169 patients with relapsed and/or refractory FLT3-mutated AML who received gilteritinib on a phase I/II dose-escalation study at a dose of at least 80 mg daily, 43% had elimination of all circulating or extramedullary blasts and reduction in marrow blasts to < 5%, with variable peripheral recovery and very modest toxicity[95]. The median duration of response to gilteritinib at these doses was 20 weeks with a median OS of 31 weeks (range 1.7–61 weeks)[95]. Pivotal phase III randomized trials comparing gilteritinib (NCT02421939) and quizartinib (QuANTUM-R; NCT02039726) to salvage chemotherapy for relapsed and refractory FLT3-mutated AML have been conducted. The QuANTUM-R study demonstrated an improvement in OS with quizartinib monotherapy compared to salvage chemotherapy in patients with relapsed/refractory FLT3-ITD-mutated AML (HR 0.76, 95% CI 0.58–0.98). U.S. Food and Drug Administration (FDA) review of gilteritinib and quizartinib is either underway or expected soon. FLT3 inhibitors therefore appear likely to soon become the standard of care for patients with PRD who have a FLT3 mutations. Studies combining FLT3 inhibitors with other active agents for relapsed/refractory AML may improve upon these responses and survival, and such studies are either underway or planned[96,97].
Currently, if a patient with refractory FLT3-ITD+ AML is unable to enroll on a clinical trial evaluating a selective FLT3 inhibitor, a reasonable salvage option is the combination of azacitidine and sorafenib[98]. A phase II study of this combination in 43 patients (93% of whom had FLT3-ITD mutations) found a CR/CRi rate of 43% (16/43 subjects) with a median duration of response of 2.3 months (range 1–14.3 months) and median OS of 6.2 months[98]. Of note, although transplant can be performed in substantial numbers of relapsed and refractory patients following response to FLT3 inhibitors, post-transplant relapse rates remain high[99]. Therefore, when feasible, we recommend restarting the FLT3 inhibitor following donor engraftment.
Ivosidenib and enasidenib
Somatic point mutations in isocitrate dehydrogenase (IDH) 1 or IDH2 occur relatively frequently in AML and result in gain-of-function enzymatic activity leading to the conversion of alpha-ketoglutarate to a new metabolite, (R)-2-hydroxyglutarate (2-HG)[100] [101,102]. 2-HG accumulates in cells, leading to a variety of metabolic and epigenetic changes that result in a block in cellular differentiation and promote leukemogenesis[103–105]. Several oral inhibitors of the mutant IDH enzymes have been developed, including ivosidenib which selectively inhibits mutant IDH1 and enasidenib which targets mutant IDH2. In patients with relapsed and/or refractory AML with IDH2 mutations, a phase I/II study of enasidenib found that 40.3% (71/176) of subjects had an objective response including 19.3% (34/176) who achieved a CR, with a median OS of 9.3 months[106]. Likewise, a phase I study of ivosidenib found a CR/CRi rate of 30.4% (38/125) with a median duration of response of 8.2 months in patients with relapsed and/or refractory IDH1-mutant AML who received a dose of 500mg daily[107]. Of note, grade 3–4 IDH-inhibitor-associated differentiation syndrome was observed in 3–5% of patients in both studies, and managed with corticosteroids and/or temporary holding of the drug in some instances[106,107]. Based on these studies, the FDA approved enasidenib in August 2017 and is currently reviewing a new drug application for ivosidenib for the treatment of relapsed and/or refractory AML with mutations in IDH2 and IDH1, respectively.
CPX-351
CPX-351 is a novel formulation of cytarabine and daunorubicin in a liposomal carrier at a fixed 5:1 molar ratio, which in vitro studies have suggested generates maximal synergy of the two drugs[108]. CPX-351 was recently approved by the FDA for the treatment of newly diagnosed AML that is either therapy-related or secondary to prior myelodysplastic syndrome (MDS). This approval was based on the results of a phase III randomized controlled trial of CPX-351 versus standard “7+3” in 309 older patients (ages 60–75) with previously untreated secondary AML. On this trial, patients treated with CPX-351 had a superior CR/CRi rate (47.7% vs 33.3%, p=0.016) and an improved median OS (9.56 vs. 5.95 months, p=0.005)[109]. Although this frontline study is promising, currently there are insufficient data to recommend the use of CPX-351 over other cytotoxic regimens in the context of PRD.
Venetoclax
Venetoclax is an oral small molecule inhibitor of the anti-apoptotic protein BCL-2 and is currently FDA-approved for the treatment of chronic lymphocytic leukemia[110]. Venetoclax has received breakthrough therapy designation from the FDA for the treatment of patients with newly diagnosed AML who are older or ineligible for intensive induction in combination with HMA or LDAC. This was based on promising preliminary results from several studies, including a phase Ib dose-escalation study of venetoclax in combination with azacitidine or decitabine in patients ≥ 65 years old with previously untreated AML (NCT02203773) which demonstrated a CR/CRi rate of 61% (35/57)[111,112]. Randomized phase III studies are ongoing (NCT02993523 and NCT03069352). If these high response rates are confirmed then it is possible that the use of venetoclax could lead to a reduction in induction failure rates in the elderly population that is at high risk for PRD. Of note, the activity of venetoclax in the relapsed and/or refractory setting appears to be much more modest, with objective response rates around 20%, although the available data is limited[113,114]. Thus, the role of venetoclax in the setting of disease that is refractory to induction chemotherapy remains to be defined.
Gemtuzumab ozogamicin
Although the interesting history of gemtuzumab ozogamicin (GO), an antibody-drug conjugate that consists of an antibody targeting CD33 linked to a calicheamicin antibiotic, has been reviewed elsewhere[115], GO is now back on the market after being granted full FDA approval in September 2017 for both newly diagnosed and relapsed and/or refractory, CD33-positive AML. The approval for relapsed and/or refractory disease is based on the MyloFrance-1 study which included patients with AML in first relapse only who were treated with GO 3mg/m2 on days 1, 4, and 7. A CR or CRi was achieved in 33% subjects and median relapse-free survival was 11 months[116]. On this study, patients were required to have a prior first remission of at least 3 months, as patients with chemorefractory AML seldom respond to GO monotherapy. Still, combination salvage regimens that contain GO might show better outcomes and should be studied in PRD[58].
Novel immunotherapeutic approaches
Immune checkpoint inhibitors targeting PD-1 and CTLA-4 have revolutionized the treatment of many solid malignancies, but have thus far appeared less promising as monotherapy for relapsed and refractory AML. However, both nivolumab and ipilimumab can occasionally induce remissions in patients with AML who relapse after allogeneic HSCT, and ongoing trials are evaluating the role of checkpoint blockade in combination with chemotherapy (NCT02768792) and HMAs (NCT02397720)[117–119]. The role of checkpoint inhibitors in combination therapy for PRD specifically remains to be evaluated.
Alternative antibody-based immunotherapy approaches may be more promising for AML, including flotetuzumab, which is a humanized dual-affinity molecule that recognizes and redirects T cells to target cells expressing CD123, the alpha chain of the interleukin-3 (IL-3) receptor[120]. CD123 is expressed by a majority of AML cells as well as normal immature hematopoietic cells [121]. A preliminary analysis of a phase I dose-escalation study in patients with relapsed and/or refractory AML and MDS demonstrated that flotetuzumab has an acceptable tolerability and safety profile[120]. The most common adverse events were infusion reactions and cytokine release syndrome (CRS). Of 14 subjects who received a dose of at least 500 ng/kg/day and had available response data, the ORR was 43% (6/14 subjects), with a CR/CRi rate of 28% (4/14 subjects)[120].
Tagraxofusp (SL-401) is another biologic agent targeting CD123 that consists of diphtheria toxin conjugated to interleukin 3 (IL-3)[122]. Preliminary data from 17 subjects with AML and blastic plasmacytoid dendritic cell neoplasm (BPDCN) enrolled in the lead-in phase of a study of tagraxofusp demonstrated feasibility and safety, although two BPDCN patients experienced capillary leak syndrome as a dose-limiting toxicity (DLT)[122]. No dose-limiting toxicity was identified for the AML patients, however, and dose escalation is still on-going (NCT02113982)[122]. Another study is evaluating tagraxofusp in combination with azacitidine for relapsed and refractory AML (NCT03113643).
With regard to cellular therapies, the past year has seen tremendous advances for the treatment of B-cell malignancies, with the FDA approval of tisagenlecleucel (Kymriah) and axicabtagene ciloleucel (Yescarta) for acute lymphoblastic leukemia and large B-cell lymphomas. Chimeric antigen receptor (CAR) T cell therapy for AML, however, has lagged behind, which is at least partially related to the lack of a truly AML-specific target, as most AML surface molecules are also expressed by normal hematopoietic stem and progenitor cells (HSPCs) and/or myeloid progenitor cells[123]. Budde et al. recently presented preliminary safety and feasibility results from a first-in-human trial of CD123-specific CAR T cells in patients with AML and BPDCN [124]. Surprisingly, myeloablation was not observed[124]. Of the 6 subjects in the AML cohort, all of whom had relapsed and refractory disease after allogeneic HSCT, 2 subjects had a CR or CRi, 1 subject achieved a morphologic leukemia-free state, and 2 subjects had stable disease[124]. Although these data are preliminary, they illustrate the transformative potential of cellular therapies for PRD.
APPROACH TO PRIMARY REFRACTORY AML
All patients with PRD who are candidates for allogeneic HSCT should be referred to a transplant center and undergo expedited HLA typing and donor identification, as HSCT represents the best chance for long-term disease-free survival[125]. A decision for direct allogeneic versus salvage chemotherapy as an interim step should be made expeditiously so as to not lose a window for immediate transplant, should this be a possibility. All patients should undergo molecular testing via PCR and/or NGS, which not only provides important prognostic information but might also reveal the presence of a targetable mutation in FLT3, IDH1, or IDH2.
For fit patients who are not candidates for a molecularly targeted agent, the decision of whether to attempt to achieve a CR with salvage chemotherapy prior to proceeding with allogeneic HSCT is not straightforward; risks and benefits of both options need to be weighed carefully. Because a lower bone marrow blast percentage has been associated with improved outcomes following allogeneic HSCT and because pre-transplant testing and donor identification takes time, we typically offer patients salvage chemotherapy. If the patient has not previously received high-dose cytarabine, then a combination regimen such as MEC is reasonable. For patients who still have persistent disease following salvage chemotherapy, the likelihood of achieving a CR with additional salvage chemotherapy is low[126]. In this case, proceeding directly to an allogeneic HSCT if a donor has been identified or enrolling the patient on a clinical trial is ideal.
For patients with PRD who are elderly or have a poor performance status following induction chemotherapy, we typically treat with azacitidine or decitabine if the patient is not enrolled on a trial or otherwise eligible for targeted agents. We are also more likely to consider a HMA in patients with mutations in TP53[51]. If a response is obtained then we continue the HMA indefinitely as long as the patient has a continued clinical benefit or, if the performance status has improved, may consider proceeding to a RIC allogeneic HSCT, particularly if the patient has entered CR or CRi. While our institution does not have a defined maximum age for allogeneic transplantation, successful transplant outcomes in patients over 70 years old are uncommon and require very careful patient selection. Frail patients with PRD and those with poor performance status may benefit more from supportive/palliative therapy and/or low-dose Ara-C or HMA than transplant.
Our current approach to the treatment of primary refractory AML is summarized in Figure 1.
Figure 1. Approach to management of primary refractory AML.
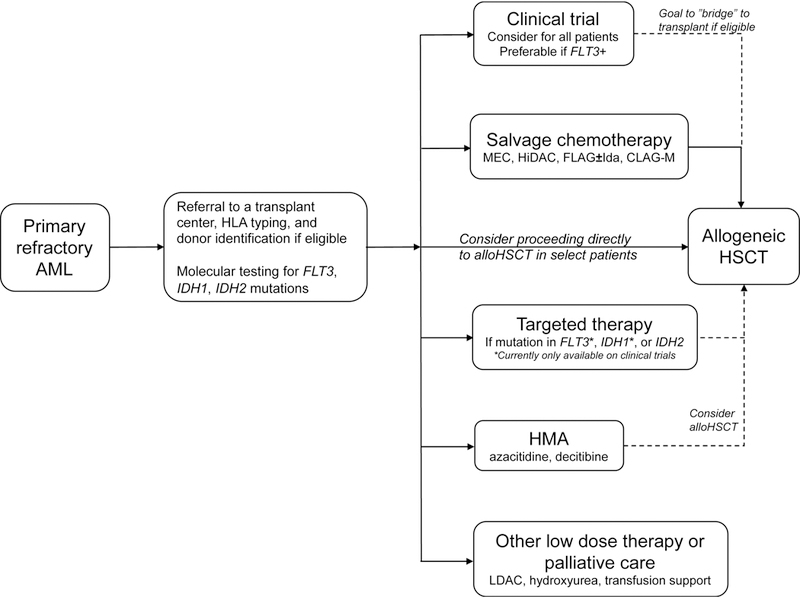
Our approach to primary refractory AML takes into account patient eligibility for allogeneic HSCT and salvage chemotherapy, clinical trial availability, and molecular mutation status.
Abbreviations: AML, acute myeloid leukemia; HLA, human leukocyte antigen; FLT3-ITD, fms-like tyrosine kinase 3-internal tandem duplication; HSCT, hematopoietic stem cell transplantation; HMA, hypomethylating agent; LDAC, low-dose cytarabine
CONCLUSION
Primary refractory AML represents a continued challenge in clinical management. Risk factors for PRD include an advanced age, elevated WBC count at diagnosis, complex or monosomal karyotype, the presence of a TP53 mutation, inv(3q), and an increased time to blast clearance after the initiation of induction chemotherapy. Treatment options for PRD include salvage chemotherapy, allogeneic HSCT, non-intensive therapies such as a HMA or LDAC, molecularly targeted agents, and enrollment on clinical trials. Allogeneic transplantation represents the best chance for long-term survival, either immediately after declaring refractoriness to induction or after salvage therapy.
Table 1.
Risk factors for primary refractory AML.
| Age > 60 years |
| Cytogenetics |
| Adverse risk karyotype |
| inv(3)(q21q26)/t(3;3)(q21;q26) |
| Monosomal karyotype |
| TP53 mutation |
| High WBC count at diagnosis |
| Secondary AML High allelic ratio FLT3-ITD:WT ratio |
| Blast clearance |
| Nadir marrow biopsy results |
| Time to peripheral blast clearance |
| Poor performance status |
Abbreviations: TP53, tumor protein p53; WBC, white blood cell; AML, acute myeloid leukemia; FLT3-ITD, fms-like tyrosine kinase 3-internal tandem duplication; WT, wild-type
Acknowledgements:
C.M. is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number TL1TR001880. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
DISCLOSURE STATEMENT
C.M. reports no conflicts of interest. A.E.P. has served as a consultant or a member of an advisory board for the following: AbbVie, Actinium Pharmaceuticals, Arog Pharmaceuticals, Astellas, Daiichi Sankyo, Novartis, Pfizer, and Seattle Genetics.
REFERENCES
- 1.Thol F, Schlenk RF, Heuser M, Ganser A. How I treat refractory and early relapsed acute myeloid leukemia. Blood 2015;126:319–327. [DOI] [PubMed] [Google Scholar]
- 2.Burnett AK, Russell NH, Hills RK, et al. . A randomized comparison of daunorubicin 90 mg/m2 vs 60 mg/m2 in AML induction: results from the UK NCRI AML17 trial in 1206 patients. Blood 2015;125:3878–3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez HF, Sun Z, Yao X, et al. . Anthracycline dose intensification in acute myeloid leukemia. New England Journal of Medicine 2009;361:1249–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Löwenberg B, Ossenkoppele GJ, van Putten W, et al. . High-dose daunorubicin in older patients with acute myeloid leukemia. New England Journal of Medicine 2009;361:1235–1248. [DOI] [PubMed] [Google Scholar]
- 5.Dohner H, Estey E, Grimwade D, et al. . Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2016. [DOI] [PMC free article] [PubMed]
- 6.Estey E Why are there so few randomized trials for patients with primary refractory acute myeloid leukemia? Best Pract Res Clin Haematol 2016;29:324–328. [DOI] [PubMed] [Google Scholar]
- 7.Ravandi F, Cortes J, Faderl S, et al. . Characteristics and outcome of patients with acute myeloid leukemia refractory to 1 cycle of high-dose cytarabine-based induction chemotherapy. Blood 2010;116:5818–5823; quiz 6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheson BD, Bennett JM, Kopecky KJ, et al. . Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. Journal of Clinical Oncology 2003;21:4642–4649. [DOI] [PubMed] [Google Scholar]
- 9.Walter RB, Kantarjian HM, Huang X, et al. . Effect of complete remission and responses less than complete remission on survival in acute myeloid leukemia: a combined Eastern Cooperative Oncology Group, Southwest Oncology Group, and MD Anderson Cancer Center Study. Journal of Clinical Oncology 2010;28:1766–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ossenkoppele GJ, Schuurhuis GJ. MRD in AML: it is time to change the definition of remission. Best Practice & Research Clinical Haematology 2014;27:265–271. [DOI] [PubMed] [Google Scholar]
- 11.Ravandi F Primary refractory acute myeloid leukaemia–in search of better definitions and therapies. British journal of haematology 2011;155:413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Xie H, Wood BL, et al. . Relation of clinical response and minimal residual disease and their prognostic impact on outcome in acute myeloid leukemia. Journal of Clinical Oncology 2015;33:1258–1264. [DOI] [PubMed] [Google Scholar]
- 13.Freeman SD, Virgo P, Couzens S, et al. . Prognostic relevance of treatment response measured by flow cytometric residual disease detection in older patients with acute myeloid leukemia. Journal of Clinical Oncology 2013;31:4123–4131. [DOI] [PubMed] [Google Scholar]
- 14.Terwijn M, van Putten WL, Kelder A, et al. . High prognostic impact of flow cytometric minimal residual disease detection in acute myeloid leukemia: data from the HOVON/SAKK AML 42A study. Journal of Clinical Oncology 2013;31:3889–3897. [DOI] [PubMed] [Google Scholar]
- 15.Walter RB, Gooley TA, Wood BL, et al. . Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. Journal of Clinical Oncology 2011;29:1190–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jourdan E, Boissel N, Chevret S, et al. . Prospective evaluation of gene mutations and minimal residual disease (MRD) in patients with core binding factor acute myeloid leukemia (CBF-AML). Blood 2013:blood-2012–2010-462879. [DOI] [PubMed]
- 17.Ivey A, Hills RK, Simpson MA, et al. . Assessment of minimal residual disease in standard-risk AML. New England Journal of Medicine 2016;374:422–433. [DOI] [PubMed] [Google Scholar]
- 18.Balsat M, Renneville A, Thomas X, et al. . Postinduction minimal residual disease predicts outcome and benefit from allogeneic stem cell transplantation in acute myeloid leukemia with NPM1 mutation: a study by the acute leukemia French Association Group. Journal of Clinical Oncology 2016:JCO 20162067 1875. [DOI] [PubMed] [Google Scholar]
- 19.Grimwade D, Freeman SD. Defining minimal residual disease in acute myeloid leukemia: which platforms are ready for “prime time”? ASH Education Program Book 2014;2014:222–233. [DOI] [PubMed] [Google Scholar]
- 20.Slovak ML, Kopecky KJ, Cassileth PA, et al. . Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood 2000;96:4075–4083. [PubMed] [Google Scholar]
- 21.Byrd JC, Mrózek K, Dodge RK, et al. . Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood 2002;100:4325–4336. [DOI] [PubMed] [Google Scholar]
- 22.Breems DA, Van Putten WL, De Greef GE, et al. . Monosomal karyotype in acute myeloid leukemia: a better indicator of poor prognosis than a complex karyotype. Journal of Clinical Oncology 2008;26:4791–4797. [DOI] [PubMed] [Google Scholar]
- 23.Walter RB, Othus M, Burnett AK, et al. . Resistance prediction in AML: analysis of 4601 patients from MRC/NCRI, HOVON/SAKK, SWOG and MD Anderson Cancer Center. Leukemia 2015;29:312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grimwade D, Hills RK, Moorman AV, et al. . Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood 2010;116:354–365. [DOI] [PubMed] [Google Scholar]
- 25.Grimwade D, Walker H, Oliver F, et al. . The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood 1998;92:2322–2333. [PubMed] [Google Scholar]
- 26.Mrózek K, Marcucci G, Nicolet D, et al. . Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia. Journal of Clinical Oncology 2012;30:4515–4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papaemmanuil E, Gerstung M, Bullinger L, et al. . Genomic classification and prognosis in acute myeloid leukemia. New England Journal of Medicine 2016;374:2209–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Testoni N, Borsaru G, Martinelli G, et al. . 3q21 and 3q26 cytogenetic abnormalities in acute myeloblastic leukemia: biological and clinical features. Haematologica 1999;84:690–694. [PubMed] [Google Scholar]
- 29.Charrin C, Belhabri A, Treille-Ritouet D, et al. . Structural rearrangements of chromosome 3 in 57 patients with acute myeloid leukemia: clinical, hematological and cytogenetic features. The Hematology Journal 2002;3:21–31. [DOI] [PubMed] [Google Scholar]
- 30.Lugthart S, Gröschel S, Beverloo HB, et al. . Clinical, molecular, and prognostic significance of WHO type inv (3)(q21q26. 2)/t (3; 3)(q21; q26. 2) and various other 3q abnormalities in acute myeloid leukemia. Journal of Clinical Oncology 2010;28:3890–3898. [DOI] [PubMed] [Google Scholar]
- 31.Medeiros BC, Othus M, Fang M, Roulston D, Appelbaum FR. Prognostic impact of monosomal karyotype in young adult and elderly acute myeloid leukemia: the Southwest Oncology Group (SWOG) experience. Blood 2010;116:2224–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kayser S, Zucknick M, Dohner K, et al. . German-Austrian Acute Myeloid Leukemia Study, Group: Monosomal karyotype in adult acute myeloid leukemia: prognostic impact and outcome after different treatment strategies. Blood 2012;119:551–558. [DOI] [PubMed] [Google Scholar]
- 33.Perrot A, Luquet I, Pigneux A, et al. . Dismal prognostic value of monosomal karyotype in elderly patients with acute myeloid leukemia: a GOELAMS study of 186 patients with unfavorable cytogenetic abnormalities. Blood 2011;118:679–685. [DOI] [PubMed] [Google Scholar]
- 34.Löwenberg B, Pabst T, Vellenga E, et al. . Cytarabine dose for acute myeloid leukemia. New England Journal of Medicine 2011;364:1027–1036. [DOI] [PubMed] [Google Scholar]
- 35.Muluneh B, Buhlinger K, Deal AM, et al. . A Comparison of clofarabine-based (GCLAC) and cladribine-based (CLAG) salvage chemotherapy for relapsed/refractory AML. Clin Lymphoma Myeloma Leuk 2018;18:e13–e18. [DOI] [PubMed] [Google Scholar]
- 36.Wierzbowska A, Robak T, Pluta A, et al. . Cladribine combined with high doses of arabinoside cytosine, mitoxantrone, and G-CSF (CLAG-M) is a highly effective salvage regimen in patients with refractory and relapsed acute myeloid leukemia of the poor risk: a final report of the Polish Adult Leukemia Group. Eur J Haematol 2008;80:115–126. [DOI] [PubMed] [Google Scholar]
- 37.Jaglal MV, Duong VH, Bello CM, et al. . Cladribine, cytarabine, filgrastim, and mitoxantrone (CLAG-M) compared to standard induction in acute myeloid leukemia from myelodysplastic syndrome after azanucleoside failure. Leuk Res 2014;38:443–446. [DOI] [PubMed] [Google Scholar]
- 38.Holowiecki J, Grosicki S, Giebel S, et al. . Cladribine, but not fludarabine, added to daunorubicin and cytarabine during induction prolongs survival of patients with acute myeloid leukemia: a multicenter, randomized phase III study. J Clin Oncol 2012;30:2441–2448. [DOI] [PubMed] [Google Scholar]
- 39.Wierzbowska A, Wawrzyniak E, Siemieniuk-Rys M, et al. . Concomitance of monosomal karyotype with at least 5 chromosomal abnormalities is associated with dismal treatment outcome of AML patients with complex karyotype - retrospective analysis of Polish Adult Leukemia Group (PALG). Leuk Lymphoma 2017;58:889–897. [DOI] [PubMed] [Google Scholar]
- 40.Bacher U, Kern W, Schnittger S, Hiddemann W, Haferlach T, Schoch C. Population-based age-specific incidences of cytogenetic subgroups of acute myeloid leukemia. Haematologica 2005;90:1502–1510. [PubMed] [Google Scholar]
- 41.Bertoli S, Bories P, Béné MC, et al. . Prognostic impact of day 15 blast clearance in risk-adapted remission induction chemotherapy for younger patients with acute myeloid leukemia: long-term results of the multicenter prospective LAM-2001 trial by the GOELAMS study group. Haematologica 2014;99:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liso V, Albano F, Pastore D, et al. . Bone marrow aspirate on the 14th day of induction treatment as a prognostic tool in de novo adult acute myeloid leukemia. Haematologica 2000;85:1285–1290. [PubMed] [Google Scholar]
- 43.Kern W, Haferlach T, Schoch C, et al. . Early blast clearance by remission induction therapy is a major independent prognostic factor for both achievement of complete remission and long-term outcome in acute myeloid leukemia: data from the German AML Cooperative Group (AMLCG) 1992 Trial. Blood 2003;101:64–70. [DOI] [PubMed] [Google Scholar]
- 44.Arellano M, Pakkala S, Langston A, et al. . Early clearance of peripheral blood blasts predicts response to induction chemotherapy in acute myeloid leukemia. Cancer 2012;118:5278–5282. [DOI] [PubMed] [Google Scholar]
- 45.Lacombe F, Arnoulet C, Maynadie M, et al. . Early clearance of peripheral blasts measured by flow cytometry during the first week of AML induction therapy as a new independent prognostic factor: a GOELAMS study. Leukemia 2009;23:350–357. [DOI] [PubMed] [Google Scholar]
- 46.Network CGAR. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med 2013;2013:2059–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel JP, Gönen M, Figueroa ME, et al. . Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. New England Journal of Medicine 2012;366:1079–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schlenk RF, Döhner K, Krauter J, et al. . Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. New England Journal of Medicine 2008;358:1909–1918. [DOI] [PubMed] [Google Scholar]
- 49.Rücker FG, Schlenk RF, Bullinger L, et al. . TP53 alterations in acute myeloid leukemia with complex karyotype correlate with specific copy number alterations, monosomal karyotype, and dismal outcome. Blood 2012;119:2114–2121. [DOI] [PubMed] [Google Scholar]
- 50.Yanada M, Yamamoto Y, Iba S, et al. . TP53 mutations in older adults with acute myeloid leukemia. Int J Hematol 2016;103:429–435. [DOI] [PubMed] [Google Scholar]
- 51.Welch JS, Petti AA, Miller CA, et al. . TP53 and Decitabine in Acute Myeloid Leukemia and Myelodysplastic Syndromes. N Engl J Med 2016;375:2023–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gaidzik VI, Schlenk RF, Moschny S, et al. . Prognostic impact of WT1 mutations in cytogenetically normal acute myeloid leukemia: a study of the German-Austrian AML Study Group. Blood 2009;113:4505–4511. [DOI] [PubMed] [Google Scholar]
- 53.Virappane P, Gale R, Hills R, et al. . Mutation of the Wilms’ tumor 1 gene is a poor prognostic factor associated with chemotherapy resistance in normal karyotype acute myeloid leukemia: the United Kingdom Medical Research Council Adult Leukaemia Working Party. Journal of Clinical Oncology 2008;26:5429–5435. [DOI] [PubMed] [Google Scholar]
- 54.Hou H, Lin C, Chou W, et al. . Integration of cytogenetic and molecular alterations in risk stratification of 318 patients with de novo non-M3 acute myeloid leukemia. Leukemia 2014;28:50–58. [DOI] [PubMed] [Google Scholar]
- 55.Karanes C, Kopecky KJ, Head DR, et al. . A phase III comparison of high dose ARA-C (HIDAC) versus HIDAC plus mitoxantrone in the treatment of first relapsed or refractory acute myeloid leukemia: Southwest Oncology Group Study. Leukemia research 1999;23:787–794. [DOI] [PubMed] [Google Scholar]
- 56.Greenberg PL, Lee SJ, Advani R, et al. . Mitoxantrone, etoposide, and cytarabine with or without valspodar in patients with relapsed or refractory acute myeloid leukemia and high-risk myelodysplastic syndrome: a phase III trial (E2995). Journal of Clinical Oncology 2004;22:1078–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Becker PS, Kantarjian HM, Appelbaum FR, et al. . Retrospective comparison of clofarabine versus fludarabine in combination with high-dose cytarabine with or without granulocyte colony-stimulating factor as salvage therapies for acute myeloid leukemia. Haematologica 2013;98:114–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wattad M, Weber D, Döhner K, et al. . Impact of salvage regimens on response and overall survival in acute myeloid leukemia with induction failure. Leukemia 2017. [DOI] [PubMed]
- 59.Orlowski RJ, Mangan JK, Luger SM. Approach to patients with primary refractory acute myeloid leukemia. Curr Opin Hematol 2015;22:97–107. [DOI] [PubMed] [Google Scholar]
- 60.Ravandi F, Ritchie EK, Sayar H, et al. . Vosaroxin plus cytarabine versus placebo plus cytarabine in patients with first relapsed or refractory acute myeloid leukaemia (VALOR): a randomised, controlled, double-blind, multinational, phase 3 study. The Lancet Oncology 2015;16:1025–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Faderl S, Wetzler M, Rizzieri D, et al. . Clofarabine plus cytarabine compared with cytarabine alone in older patients with relapsed or refractory acute myelogenous leukemia: results from the CLASSIC I Trial. Journal of Clinical Oncology 2012;30:2492–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roboz GJ, Rosenblat T, Arellano M, et al. . International randomized phase III study of elacytarabine versus investigator choice in patients with relapsed/refractory acute myeloid leukemia. Journal of Clinical Oncology 2014;32:1919–1926. [DOI] [PubMed] [Google Scholar]
- 63.Dombret H, Seymour JF, Butrym A, et al. . International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 2015;126:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fenaux P, Mufti GJ, Hellström-Lindberg E, et al. . Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. Journal of Clinical Oncology 2009;28:562–569. [DOI] [PubMed] [Google Scholar]
- 65.Kantarjian HM, Thomas XG, Dmoszynska A, et al. . Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. Journal of Clinical Oncology 2012;30:2670–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ivanoff S, Gruson B, Chantepie SP, et al. . 5-Azacytidine treatment for relapsed or refractory acute myeloid leukemia after intensive chemotherapy. Am J Hematol 2013;88:601–605. [DOI] [PubMed] [Google Scholar]
- 67.Itzykson R, Thepot S, Berthon C, et al. . Azacitidine for the treatment of relapsed and refractory AML in older patients. Leuk Res 2015;39:124–130. [DOI] [PubMed] [Google Scholar]
- 68.Litzow MR, Othus M, Cripe LD, et al. . Failure of three novel regimens to improve outcome for patients with relapsed or refractory acute myeloid leukaemia: a report from the Eastern Cooperative Oncology Group. British journal of haematology 2010;148:217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jabbour E, Daver N, Champlin R, et al. . Allogeneic stem cell transplantation as initial salvage for patients with acute myeloid leukemia refractory to high‐dose cytarabine‐based induction chemotherapy. American journal of hematology 2014;89:395–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Duval M, Klein JP, He W, et al. . Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. Journal of Clinical Oncology 2010;28:3730–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Craddock C, Labopin M, Pillai S, et al. . Factors predicting outcome after unrelated donor stem cell transplantation in primary refractory acute myeloid leukaemia. Leukemia 2011;25:808–813. [DOI] [PubMed] [Google Scholar]
- 72.Todisco E, Ciceri F, Oldani E, et al. . The CIBMTR score predicts survival of AML patients undergoing allogeneic transplantation with active disease after a myeloablative or reduced intensity conditioning: a retrospective analysis of the Gruppo Italiano Trapianto Di Midollo Osseo. Leukemia 2013;27:2086–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Magenau J, Westervelt P, Khaled S, et al. . A multicenter trial of myeloablative clofarabine and busulfan conditioning for relapsed or primary induction failure AML not in remission at the time of allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2017;52:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Todisco E, Ciceri F, Boschini C, et al. . Factors predicting outcome after allogeneic transplant in refractory acute myeloid leukemia: a retrospective analysis of Gruppo Italiano Trapianto di Midollo Osseo (GITMO). Bone Marrow Transplant 2017. [DOI] [PubMed]
- 75.Pagel JM, Gooley TA, Rajendran J, et al. . Allogeneic hematopoietic cell transplantation after conditioning with 131I–anti-CD45 antibody plus fludarabine and low-dose total body irradiation for elderly patients with advanced acute myeloid leukemia or high-risk myelodysplastic syndrome. Blood 2009;114:5444–5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weisdorf DJ, Millard HR, Horowitz MM, et al. . Allogeneic transplantation for advanced acute myeloid leukemia: The value of complete remission. Cancer 2017. [DOI] [PMC free article] [PubMed]
- 77.Biggs JC, Horowitz MM, Gale RP, et al. . Bone marrow transplants may cure patients with acute leukemia never achieving remission with chemotherapy. Blood 1992;80:1090–1093. [PubMed] [Google Scholar]
- 78.Kebriaei P, Kline J, Stock W, et al. . Impact of disease burden at time of allogeneic stem cell transplantation in adults with acute myeloid leukemia and myelodysplastic syndromes. Bone marrow transplantation 2005;35:965–970. [DOI] [PubMed] [Google Scholar]
- 79.Oyekunle A, Kröger N, Zabelina T, et al. . Allogeneic stem-cell transplantation in patients with refractory acute leukemia: a long-term follow-up. Bone marrow transplantation 2006;37:45–50. [DOI] [PubMed] [Google Scholar]
- 80.Hemmati PG, Terwey TH, Na IK, et al. . Allogeneic stem cell transplantation for refractory acute myeloid leukemia: a single center analysis of long-term outcome. Eur J Haematol 2015;95:498–506. [DOI] [PubMed] [Google Scholar]
- 81.Liu N, Ning HM, Hu LD, et al. . Outcome of myeloablative allogeneic peripheral blood hematopoietic stem cell transplantation for refractory/relapsed AML patients in NR status. Leuk Res 2015;39:1375–1381. [DOI] [PubMed] [Google Scholar]
- 82.Schnittger S, Schoch C, Dugas M, et al. . Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood 2002;100:59–66. [DOI] [PubMed] [Google Scholar]
- 83.Thiede C, Steudel C, Mohr B, et al. . Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood 2002;99:4326–4335. [DOI] [PubMed] [Google Scholar]
- 84.Kiyoi H, Ohno R, Ueda R, Saito H, Naoe T. Mechanism of constitutive activation of FLT3 with internal tandem duplication in the juxtamembrane domain. Oncogene 2002;21:2555. [DOI] [PubMed] [Google Scholar]
- 85.Yamamoto Y, Kiyoi H, Nakano Y, et al. . Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood 2001;97:2434–2439. [DOI] [PubMed] [Google Scholar]
- 86.Mead AJ, Linch DC, Hills RK, Wheatley K, Burnett AK, Gale RE. FLT3 tyrosine kinase domain mutations are biologically distinct from and have a significantly more favorable prognosis than FLT3 internal tandem duplications in patients with acute myeloid leukemia. Blood 2007;110:1262–1270. [DOI] [PubMed] [Google Scholar]
- 87.Fröhling S, Schlenk RF, Breitruck J, et al. . Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood 2002;100:4372–4380. [DOI] [PubMed] [Google Scholar]
- 88.Kayser S, Schlenk RF, Londono MC, et al. . Insertion of FLT3 internal tandem duplication in the tyrosine kinase domain-1 is associated with resistance to chemotherapy and inferior outcome. Blood 2009;114:2386–2392. [DOI] [PubMed] [Google Scholar]
- 89.Pratz KW, Levis M. How I treat FLT3-mutated AML. Blood 2016:blood-2016–2009-693648. [DOI] [PMC free article] [PubMed]
- 90.Ravandi F, Cortes JE, Jones D, et al. . Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia. J Clin Oncol 2010;28:1856–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ravandi F, Yi CA, Cortes JE, et al. . Final report of phase II study of sorafenib, cytarabine and idarubicin for initial therapy in younger patients with acute myeloid leukemia. Leukemia 2014;28:1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Serve H, Krug U, Wagner R, et al. . Sorafenib in combination with intensive chemotherapy in elderly patients with acute myeloid leukemia: results from a randomized, placebo-controlled trial. J Clin Oncol 2013;31:3110–3118. [DOI] [PubMed] [Google Scholar]
- 93.Röllig C, Serve H, Hüttmann A, et al. . Addition of sorafenib versus placebo to standard therapy in patients aged 60 years or younger with newly diagnosed acute myeloid leukaemia (SORAML): a multicentre, phase 2, randomised controlled trial. The Lancet Oncology 2015;16:1691–1699. [DOI] [PubMed] [Google Scholar]
- 94.Stone RM, Mandrekar SJ, Sanford BL, et al. . Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. New England Journal of Medicine 2017;377:454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Perl AE, Altman JK, Cortes J, et al. . Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: a multicentre, first-in-human, open-label, phase 1–2 study. The Lancet Oncology 2017;18:1061–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Iyer SP, Jethava Y, Karanes C, Eckardt JR, Collins R. Safety study of salvage chemotherapy high-dose Ara-C/mitoxantrone (HAM) and type I FLT3-TKI crenolanib in first relapsed/primary refractory AML (Abstract). Am Soc Hematology; 2016.
- 97.Swaminathan M, Kantarjian HM, Daver N, et al. . The combination of quizartinib with azacitidine or low dose cytarabine is highly active in patients with FLT3-ITD mutated myeloid leukemias: Interim report of a phase I/II trial (Abstract). Blood 2017;130:723–723. [Google Scholar]
- 98.Ravandi F, Alattar ML, Grunwald MR, et al. . Phase 2 study of azacytidine plus sorafenib in patients with acute myeloid leukemia and FLT-3 internal tandem duplication mutation. Blood 2013;121:4655–4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Levis MJ, Martinelli G, Perl AE, et al. . The benefit of treatment with quizartinib and subsequent bridging to HSCT for FLT3-ITD (+) patients with AML (abstract). American Society of Clinical Oncology; 2014.
- 100.Stein E, Yen K. Targeted differentiation therapy with mutant IDH inhibitors: Early experiences and parallels with other differentiation agents. Ann Rev of Cancer Biol 2017;1:379–401. [Google Scholar]
- 101.Dang L, White DW, Gross S, et al. . Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2009;462:739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ward PS, Patel J, Wise DR, et al. . The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting α-ketoglutarate to 2-hydroxyglutarate. Cancer Cell 2010;17:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Figueroa ME, Abdel-Wahab O, Lu C, et al. . Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell 2010;18:553–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lu C, Ward PS, Kapoor GS, et al. . IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature 2012;483:474–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Losman J-A, Looper RE, Koivunen P, et al. . (R)-2-hydroxyglutarate is sufficient to promote leukemogenesis and its effects are reversible. Science 2013;339:1621–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stein EM, Dinardo CD, Pollyea DA, et al. . Enasidenib in mutant-IDH2 relapsed or refractory acute myeloid leukemia. Blood 2017;130:722–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.DiNardo CD, Stein EM, de Botton S, et al. . Durable Remissions with Ivosidenib in IDH1-Mutated Relapsed or Refractory AML. New England Journal of Medicine 2018;378:2386–2398. [DOI] [PubMed] [Google Scholar]
- 108.Feldman EJ, Lancet JE, Kolitz JE, et al. . First-in-man study of CPX-351: a liposomal carrier containing cytarabine and daunorubicin in a fixed 5: 1 molar ratio for the treatment of relapsed and refractory acute myeloid leukemia. Journal of Clinical Oncology 2011;29:979–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lancet J, Uy G, Cortes J, Newell L, Lin T, Ritchie E. Final results of a phase III randomized trial of CPX-351 versus 7+ 3 in older patients with newly diagnosed high risk (secondary) AML [abstract 7000]. J Clin Oncol 2016;34.
- 110.Davids MS, Letai A. ABT-199: a new hope for selective BCL-2 inhibition. Cancer Cell 2013;23:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wei A, Strickland SA, Roboz GJ, et al. . Safety and Efficacy of Venetoclax Plus Low-Dose Cytarabine in Treatment-Naive Patients Aged≥ 65 Years with Acute Myeloid Leukemia (abstract). Am Soc Hematology; 2016.
- 112.DiNardo CD, Pratz KW, Letai A, et al. . Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol 2018;19:216–228. [DOI] [PubMed] [Google Scholar]
- 113.Konopleva M, Pollyea DA, Potluri J, et al. . Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discovery 2016;6:1106–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.DiNardo CD, Rausch CR, Benton C, et al. . Clinical experience with the BCL2‐inhibitor venetoclax in combination therapy for relapsed and refractory acute myeloid leukemia and related myeloid malignancies. American journal of hematology 2017. [DOI] [PubMed]
- 115.Rowe JM, Löwenberg B. Gemtuzumab ozogamicin in acute myeloid leukemia: a remarkable saga about an active drug. Blood 2013;121:4838–4841. [DOI] [PubMed] [Google Scholar]
- 116.Taksin AL, Legrand O, Raffoux E, et al. . High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: a prospective study of the alfa group. Leukemia 2007;21:66–71. [DOI] [PubMed] [Google Scholar]
- 117.Berger R, Rotem-Yehudar R, Slama G, et al. . Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clinical Cancer Research 2008;14:3044–3051. [DOI] [PubMed] [Google Scholar]
- 118.Albring J, Inselmann S, Sauer T, et al. . PD-1 checkpoint blockade in patients with relapsed AML after allogeneic stem cell transplantation. Bone marrow transplantation 2017;52:317. [DOI] [PubMed] [Google Scholar]
- 119.Davids MS, Kim HT, Bachireddy P, et al. . Ipilimumab for patients with relapse after allogeneic transplantation. New England Journal of Medicine 2016;375:143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Uy GL, Godwin J, Rettig MP, et al. . Preliminary results of a phase 1 study of flotetuzumab, a CD123 x CD3 bispecific DART® protein, in patients with relapsed/refractory acute myeloid leukemia and myelodysplastic syndrome (Abstract). Blood 2017;130:637–637. [Google Scholar]
- 121.Gill S, Tasian SK, Ruella M, et al. . Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor-modified T cells. Blood 2014;123:2343–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sweet KL, Pemmaraju N, Lane AA, et al. . Lead-in stage results of a pivotal trial of SL-401, an interleukin-3 receptor (IL-3R) targeting biologic, in patients with blastic plasmacytoid dendritic cell neoplasm or acute myeloid leukemia (Abstract). Blood 2015;126:3795–3795. [Google Scholar]
- 123.Gill S Chimeric antigen receptor T cell therapy in AML: How close are we? Best Pract Res Clin Haematol 2016;29:329–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Budde L, Song JY, Kim Y, et al. . Remissions of Acute Myeloid Leukemia and Blastic Plasmacytoid Dendritic Cell Neoplasm Following Treatment with CD123-Specific CAR T Cells: A First-in-Human Clinical Trial (Abstract). Blood 2017;130:811–811. [Google Scholar]
- 125.Othus M, Appelbaum FR, Petersdorf SH, et al. . Fate of patients with newly diagnosed acute myeloid leukemia who fail primary induction therapy. Biol Blood Marrow Transplant 2015;21:559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Giles F, O’brien S, Cortes J, et al. . Outcome of patients with acute myelogenous leukemia after second salvage therapy. Cancer 2005;104:547–554. [DOI] [PubMed] [Google Scholar]
- 127.Becker PS, Kantarjian HM, Appelbaum FR, et al. . Clofarabine with high dose cytarabine and granulocyte colony‐stimulating factor (G‐CSF) priming for relapsed and refractory acute myeloid leukaemia. British journal of haematology 2011;155:182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yavuz S, Paydas S, Disel U, Sahin B. IDA-FLAG regimen for the therapy of primary refractory and relapse acute leukemia: a single-center experience. American Journal of Therapeutics 2006;13:389–393. [DOI] [PubMed] [Google Scholar]


