Abstract
Objective:
In 2007, Medicare established ultrasound screening guidelines to identify patients at risk for abdominal aortic aneurysm (AAA). The purpose of this study was to evaluate AAA diagnosis rates and compliance with screening during 10 years (2007–2016) of the Screen for Abdominal Aortic Aneurysms Very Efficiently Act implementation within a regional health care system.
Methods:
A retrospective chart review of all patients screened for AAA from 2007 to 2016 within a regional Veterans Affairs health care system was conducted. Screening criteria were men 65 to 75 years of age who smoked a minimum of 100 cigarettes in their lifetime. An AAA was defined as a maximum aortic diameter ≥3 cm. A comparison was made of the AAA diagnosis rate and clinical adherence rate of screening criteria between the first 5 years and total years evaluated. AAA-related mortality was identified by using terminal diagnosis notes or autopsy reports. All data were recorded by August 31, 2017.
Results:
A total of 19,649 patients (70.7 ± 4.8 years of age, mean ± standard deviation) were screened from January 1, 2007, to December 31, 2016. There were 9916 new patients screened from 2012 to 2016. A total of 1232 aneurysms (6.3% total patients) were identified during the 10-year period. The overall AAA diagnosis rate has declined from 7.2% in the first 5 years to 6.3% in 10 years (13.5% decrease; P < .01). There were 66 patients found with AAA ≥5.5 cm (5.3% of AAAs), and 54 of these patients received successful elective repair. A total of 2321 patients died (11.8%) and 6 deaths were suspected AAA ruptures (0.03%) within the analysis period. A total of 3680 patients screened (18.7%) did not meet screening criteria: 593 patients were <65 years of age, 3087 patients were >75 years of age, and 59 patients were women. This rate has declined from 28.2% within the first 5 years to 18.7% overall in 10 years (33.7% decrease; P < .01). The compliance of screened patients using screening criteria improved significantly from 61.7% in 2007 to 92.4% in 2016 (P < .01). The overall compliance rate since implementation of the screening program during the past 10 years is 81.3%.
Conclusions:
The overall 10-year rate of AAA diagnosis is 6.3%. There are more smaller aneurysms (3.0–4.4 cm) detected and fewer large AAAs ≥5.5 cm in the last 5 years compared with the first 5 years of the screening program. The overall AAA-related mortality rate of all screened patients is 0.03%. There were 54 patients with AAA ≥5.5 cm who underwent successful elective repair resulting from the AAA screening program. The overall compliance of screened patients using screening criteria improved significantly from 61.7% in 2007 to 81.3% since implementation of the screening program during the past 10 years.
Keywords: Abdominal aortic aneurysm, Ultrasound, Screening
The implementation of abdominal aortic aneurysm (AAA) screening has substantially reduced AAA rupture rates in the older male population. The randomized AAA screening trials1–4 showed remarkable benefits with reducing AAA-related mortality, and population screening programs in England5 and Sweden6 observed greater cost-effectiveness and improved life-adjusted years with AAA screening. Long-term data in England from the Multicentre Aneurysm Screening Study (MASS) even revealed a significant reduction in allcause mortality with a one-time screening for AAA.7 However, long-term data on the effectiveness of AAA screening in the United States are limited.
In 2007, the U.S. Congress enacted the Screen for Abdominal Aortic Aneurysms Very Efficiently (SAAAVE) Act into U.S. law. This law directed Medicare to establish ultrasound screening guidelines to identify patients at risk for AAA. This law ushered in a new era of AAA screening in the United States. Yet, Medicare guidelines for AAA screening were cumbersome and confusing, which led to unsuccessful participation of many potentially eligible patients. In its initial roll out of the SAAAVE Act, Medicare included only patients new to Medicare and who followed the “Welcome to Medicare” package guidelines. This led to less than <1% of total eligible Medicare patients screened.8 In 2007, the Department of Veterans Affairs (VA) launched a more inclusive AAA screening campaign than the Medicare SAAAVE program. All veterans already within its system were invited who met screening criteria (any male veteran between 65 and 75 years of age with a history of cigarette smoking was eligible for screening). We first reported the 1-year8 and 5-year9 outcomes of a regional AAA screening program (2007–2011), in which higher AAA detection rates and clinicians’ noncompliance with screening guidelines were found compared with AAA screening randomized trials. This study aimed to add further follow-up of the AAA screening program. The purpose of this study was to evaluate AAA diagnosis rates and compliance with screening during 10 years (2007–2016) of the SAAAVE Act implementation within a regional health care system.
METHODS
A retrospective review of patients screened for AAA was performed under an approved protocol by the Institutional Review Board at the Veterans Affairs Northern California Healthcare System (VANCHCS). Patients’ informed consent was not required for this study as a waiver of informed consent was granted from the Institutional Review Board. The AAA screening criteria were male veterans 65 to 75 years of age who smoked at least 100 cigarettes during their lifetime and women 50 years or older with a family history of aneurysmal disease. Patients who met inclusion criteria were sent invitations from the radiology department to participate in AAA screening. Electronic alerts in the patient’s electronic medical record (EMR) also notified primary care physicians when eligible patients in their clinics were due for AAA screening referral.
On completion of an encounter visit for AAA screening, the visit is associated with a billing code specific for AAA screening. A list of patients screened for AAA from January 1, 2007, through December 31, 2016, was generated for this study. Age, maximum aortic diameter, AAA prevalence, clinician’s adherence to AAA screening criteria, and elective AAA repair referrals with repair type (open and endovascular) were evaluated. An aneurysm was defined as having a maximum abdominal aortic diameter of 3.0 cm or greater. A radiologist verified all aortic measurements and reported findings in the EMR to the nearest 0.1 cm. Clinicians’ adherence was evaluated by number of inappropriate screenings and timely referrals made to the vascular surgery clinic for detected AAA ≥5.5 cm in maximum diameter. AAA-related mortality was identified by using terminal diagnosis notes or autopsy reports. All data were recorded by August 31, 2017.
A statistical univariate analysis was performed on collected data. Continuous variables were reported as mean ± standard deviation; a two-tailed t-test was used to compare continuous variables, and a χ2 test was used to compare data proportions. Survival estimates after AAA screening of patients with normal aortas and AAA were calculated using the AAA screening date and date of death or the censored date of August 31, 2017. A P value <.05 was considered statistically significant.
RESULTS
A total of 19,649 patients (70.7 ± 4.8 years of age, mean ± standard deviation) were screened from January 1, 2007, to December 31, 2016. There were 9898 new patients screened from 2012 to 2016. There were 23 women and 19,626 men screened. The race distribution (Table I) of screened patients was as follows: white, 66.4% (n = 13,038); unknown/declined to state, 17.1% (n = 3352); black/African American, 11.1% (n = 2189); Asian or Pacific Islander, 3.7% (n = 728); and American Indian, 1.1% (n = 220). There were 3.7% (n = 723) of patients who identified as Hispanic, 83.4% (n = 16,389) who identified as non-Hispanic, and 12.9% (n = 2537) unknown/declined to state Hispanic origin. The average follow-up period for patients after AAA screening was 5.4 ± 2.9 years (range, 0–10.7 years) from date of screening to the censored date of August 31, 2017. A total of 2321 patients (11.8% total) died within the analysis period.
Table I.
Race distribution of screened patients (N = 19,649)
| Race | No. (%) |
|---|---|
| White | 13,038 (66.4) |
| Black/African American | 2189 (11.1) |
| Asian/Pacific Islander | 728 (3.7) |
| American Indian | 220 (1.1) |
| Hispanic | 122 (0.6) |
| Unknown | 3352 (17.1) |
| Hispanic | 723 (3.7) |
| Non-Hispanic | 16,389 (83.4) |
| Declined to state | 2537 (12.9) |
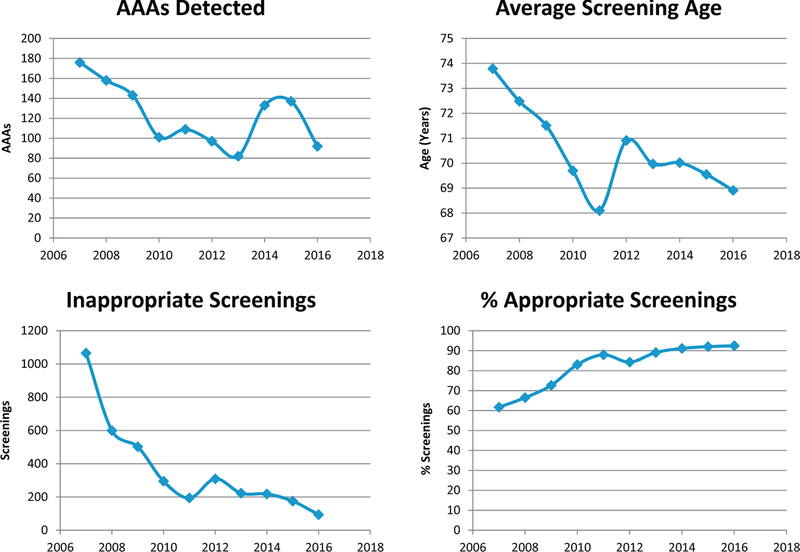
The average age of screened patients decreased from 73.8 ± 4.1 years in 2007 to 68.9 ± 3.8 years in 2016. A total of 3674 patients screened (18.7%) did not meet age screening criteria; 593 patients were <65 years of age and 3081 patients were >75 years of age. This rate has declined from 28.2% within the first 5 years to 10.3% in the last 5 years (36.5 % decrease; P < .01). In addition, the rate of appropriately screened patients based on screening criteria rose significantly from 61.7% in 2007 to 92.4% in 2016 (P < .01). The overall compliance rate since implementation of the screening program during the past 10 years was 81.3%. Fig 1 summarizes these data.
Fig 1.
The 10-year data on detected abdominal aortic aneurysms (AAAs), the average screening age enrolled each year, the number of inappropriate screenings outside screening criteria per year, and the percentage of appropriate screenings that met screening criteria per year.
A total of 1232 aneurysms (6.3% of total patients) were identified within the 10-year analysis (Table II). The overall AAA diagnosis rate has declined from 7.2% in the first 5 years to 5.5% in the last 5 years (21.5% decrease; P < .01). In addition, more smaller aneurysms (3.0–4.4 cm) were detected (87.2%) from screening in the last 5 years of the AAA screening program compared with the first 5 years (79.2%) of the study (P < .01). However, there were fewer larger aneurysms (4.5–5.4 cm) detected in the last 5 years (8.7%) than in the first 5 years (14.4%) of the study (P < .01).
Table II.
Summary of 10-year abdominal aortic aneurysm (AAA) screening data
| 2007–2011 (n = 9747), No. (%) | 2012–2016 (n = 9902), No. (%) | P value | 2007–2016 (N = 19,649), No. (%) | |
|---|---|---|---|---|
| Inconclusive | 168 (1.7) | 403 (4.1) | <.01 | 571 (2.9) |
| Normal | 7732 (79.3) | 6908 (69.8) | <.01 | 14,640 (74.5) |
| Ectatic (2.5–2.9 cm) | 1160 (11.9) | 2050 (20.7) | <.01 | 3210 (16.3) |
| AAAs ≥3.0 cm | 687 (7.0) | 541 (5.5) | <.01 | 1228 (6.2) |
| 3.0–4.4 cm | 544 (79.2) | 472 (87.2) | <.01 | 1016 (5.2) |
| 4.5–5.4 cm | 99 (14.4) | 47 (8.7) | <.01 | 146 (0.7) |
| ≤5.5 cm | 44 (6.4) | 22 (4.1) | 0.07 | 66 (0.3) |
There were 281 AAA patients (22.8% AAA patients, 1.4% total screened patients) who died within the analysis period. There were six suspected AAA ruptures (2.1% AAA patients, 0.03% total screened patients) within the analysis period. Cause of mortality was categorized into cardiopulmonary, cancer, neurologic, other causes, unknown, and suspected ruptures. These data are summarized in Table III.
Table III.
Mortality summary of abdominal aortic aneurysm (AAA) patients (N = 281)
| Cause of death | No. (%) | AAA diameter, cm (mean ± SD) |
|---|---|---|
| Cardiopulmonary | 86 (30.6) | 4.0 ± 0.9 |
| CHF | 42 (49.4) | 4.0 ± 0.9 |
| COPD | 19 (22.3) | 4.1 ± 1.0 |
| MI | 12 (14.1) | 3.7 ± 0.8 |
| Other | 13 (15.2) | 3.7 ± 0.8 |
| Cancer | 74 (26.4) | 3.9 ± 1.0 |
| Lung cancer | 31 (41.9) | 3.8 ± 0.7 |
| Prostate cancer | 9 (12.2) | 4.0 ± 0.8 |
| Other cancer | 34 (45.9) | 4.0 ± 1.3 |
| Neurologic disorders | 15 (5.3) | 4.2 ± 0.9 |
| Stroke | 7 (46.7) | 4.0 ± 1.2 |
| Dementia | 6 (40.0) | 4.6 ± 0.7 |
| Other | 2 (13.3) | 4.2 ± 0.9 |
| Infection, bleeding, trauma, other | 11 (3.9) | 3.7 ± 0.8 |
| Renal failure | 6 (2.1) | 4.1 ± 1.0 |
| Suspected aortic rupture | 6 (2.1) | 5.7 ± 1.3 |
| Unknown | 83 (29.6) | 3.9 ± 1.0 |
CHF, Chronic heart failure; COPD, chronic obstructive pulmonary disease; MI, myocardial infarction; SD, standard deviation.
There were 66 patients with detected AAA ≥5.5 cm (5.4% of all AAAs; Table IV). There were 44 patients (6.4% AAAs) with AAA ≥5.5 cm in the first 5 years and 22 patients (4.1% AAAs) in the last 5 years (P = .07). Of these 66 patients, only 62 patients received vascular surgery consultation. Three patients refused vascular consultation and one patient died before the scheduled vascular appointment; this person may have died of a suspected AAA rupture as reported previously.9 Within the 10-year period, there were 54 elective repair cases, 42 within the VA and 12 outside the VA (Table V). Within the VA, we can confirm that there were 34 endovascular aneurysm repair (EVAR) cases and eight open repairs. Last, 25 patients screened ≥5.5 cm died within the 10-year period.
Table IV.
Summary of detected abdominal aortic aneurysms (AAAs) ≥5.5 cm (N = 66)
| Average AAA diameter (6.4 ± 1.0 cm) | No. (%) |
|---|---|
| Vascular surgeon referral after detection | 62 (93.9) |
| Refused vascular consultation | 3 (4.5) |
| Died before vascular consultation | 1 (1.5) |
| Patients died within 10-year period | 25 (37.9) |
| Patients died of suspected rupture | 1 (2.2) |
Table V.
Abdominal aortic aneurysm (AAA) elective repairs (N = 54)
| Type | No. (%) |
|---|---|
| Repairs at non-VA facility | 12 (22.2) |
| Repairs at VA facility | 42 (77.8) |
| EVAR | 34 (81) |
| Open | 8 (19) |
EVAR, Endovascular aneurysm repair; VA, Veterans Affairs.
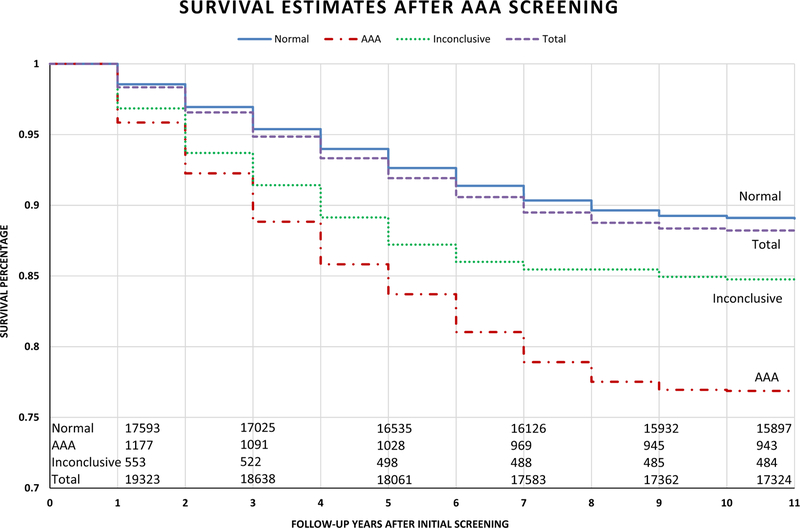
Patients screened normal had higher survivability on average than those diagnosed with AAAs during the 10-year period (89.1% vs 76.8%; P < .01; Fig 2). Those patients with inconclusive scans were observed in this study and had a survivability of 84.8%. A subanalysis of AAA patients in 2007 showed that 34.7% of patients died with an average follow-up of 5.0 years, and the 65.3% of patients (percentage censored in survival analysis) who remained alive had an average follow-up of 10.2 years. There were 76 patients with AAA (6.2% of total AAAs) who had at least 10 years of follow-up after AAA screening.
Fig 2.
Survival analysis of patients screened within the 10-year period with a mean ± standard deviation follow-up of 5.4 ± 2.9 years (range, 0–10.7 years) and a censor rate of 65.3% patients remaining alive at the end of analysis. Patients screened normal lived significantly longer than patients with detected abdominal aortic aneurysm (AAA; P < .01).
DISCUSSION
This retrospective study reports the 10-year outcomes of an ongoing regional VA screening program for AAA. The study continues the work previously done of a 5-year outcomes study of the same AAA screening program.9 The importance of this study is the addition of long-term AAA screening data within the United States. Locally, the results of this study and the ongoing efforts of the VANCHCS AAA screening program have created a robust database of the northern California veterans population.
Our data show that more patients with detected AAAs are in the 3.0- to 4.4-cm range, substantially more than other groups, especially the reduction of larger aneurysms ≥5.5 cm found by screening. We reported in a previous study of the surveillance of small aneurysms that these patients are most at risk for follow-up failure from physicians who are unfamiliar with AAA surveillance guidelines as 65.1% of patients successfully had an appropriate follow-up imaging study.10 We recommended automatic ordering of follow-up imaging studies for all patients with detected AAA. However, we suspected that this could become a developing institutional problem within the next decade as this AAA cohort of patients (3.0–4.4 cm) grows and the number of large aneurysms (≥5.5 cm) decreases. Increased surveillance examinations could become a financial and labor burden on radiology departments. A cost-analysis study on the feasibility of including more surveillance examinations of these small AAAs could determine overall financial burdens for a medical institution. However, we suspect that with cardiovascular risk factor management, smoking cessation and statin intervention in addition to continued surveillance will still be the treatment of choice for most physicians in small AAA management. Identifying a biomarker that would target a subset of screening patients more at risk for AAA development to reduce cost and workload burdens is also warranted.
Patients with large AAA ≥5.5 cm detected from AAA screening typically receive elective repair. There were 65% of patients with AAA ≥5.5 cm in this study who received elective AAA repair. At the VA, vascular surgeons opted for EVAR more than for open repair (34 vs 8 cases). This is consistent with the literature as EVAR is typically preferred by vascular surgeons for elective AAA repair.11
The average screening age of patients within the 10-year period has decreased from 73.8 ± 4.1 years in 2007 to 68.9 ± 3.8 years in 2016. We predicted from our 5-year outcome study that this would occur as greater awareness of AAA screening guidelines by primary care physicians improved over time. As the screening program matures, we expect younger patients to be screened as eligible patients will have thus turned 65 years, making detected AAAs smaller in diameter and less frequent in diagnosis. From a quality assurance perspective, there has been a substantial improvement of eligible screened patients from 61.7% in 2007 to 92.4% in 2016. However, we observed throughout this study that the regional population of eligible veterans who meet AAA screening criteria is slowly becoming exhausted and may reflect the observed decreasing average screening age. As the VANCHCS AAA screening program continues to function, we predict the average age will continue to become closer to 65 years but never reach an exact average of 65 years.
Compared with other national screening programs, there are two key differences with inclusion criteria between VA, England, and Sweden national AAA screening programs. First, the VA screens patients who are all male smokers, whereas the England and Sweden programs included nonsmokers and smokers. Second, the VA screening age criterion is 65 to 75 years. National AAA screening programs in England5 and Sweden6 have relaxed their screening criteria to allow any men at least 65 years of age regardless of smoking status to participate in screening because the inclusion of additional patients was still cost-effective. These inclusion criteria differences may explain why the AAA detection rate is higher in the VA screening program than the reported detection rates in Europe. The VA in the future could potentially follow the footsteps of its European counterparts and allow the screening criteria to be more inclusive, whereby all male veterans at least 65 years of age regardless of smoking status are eligible for AAA screening. We suspect that AAA detection rates will significantly decline in the veteran population with the inclusion of all men at least 65 years of age. A costutility analysis for future implementation of these screening criteria at the VA is warranted.
The survival estimates show that AAA patients have higher mortality than patients who screened normal at the 10-year mark. Although this study cannot conclude that AAA screening reduces all-cause mortality, the effectiveness of AAA screening even at 10 years still benefits patients long term. The final analysis of the MASS trial revealed that the average life expectancy of patients with detected AAA is 11 years.7 This study cannot verify the 11-year life expectancy for detected AAA within our screening population, but a majority of AAA patients (65.7%) screened in 2007 had an average follow-up of 10.2 years. We suspect that these patients will outlive their projected 11-year life expectancy, given a longer analysis period of 15 to 20 years. Whether these new data could potentially amend current surveillance guidelines will require further study.
There are some limitations to this study. First, because of the retrospective nature of this study, data could be analyzed only to test for associations and to report on AAA screening outcomes, not causations. Second, the patients in the study were a homogeneous cohort of mostly male veterans of a single large institution in northern California. Although patients in this study may be reflective of the general AAA screening population on the basis of screening criteria, veterans have higher AAA detection rates than in the randomized trials9 and are typically known to be more at risk for cardiovascular disease.12 Third, many vascular technologists and radiologists participate in the AAA screening program, so we cannot confirm consistency in measuring the aortic diameters and initiating clinic encounters in the EMR. There could also be some instances in which the radiologist may code incorrectly for the AAA screening encounter, which generates an incorrect billing code and thus under-reports the actual number of AAA screenings for this study. Hence, our study accounts only for those patients who have the correct coding for AAA screening. Last, the true response rate from screening invitations was difficult to measure. We identified an 84% response rate during the first year of screening in 2007.8 This response rate is similar to the overall MASS trial response rate of 80%1 and matches the overall Sweden AAA screening program response rate of 84%.6 Calculating the actual number of screening invitations in the subsequent years of the VA screening program is challenging because many patients were reinvited for screening if initial visits were not completed. Therefore, we speculate that our screening program’s true response rate from invitation is approximately 80%. This could suggest that our AAA-related mortality rate is under-reported because some of those patients not accepting an invitation for screening may have undiagnosed aneurysms that are at risk for rupture.
CONCLUSIONS
The overall 10-year rate of AAA diagnosis is 6.3%. There are more smaller aneurysms (3.0–4.4 cm) detected and fewer large AAAs ≥5.5 cm in the last 5 years compared with the first 5 years of the screening program. The overall AAA-related mortality of all screened patients is 0.03%. There were 54 patients with AAA ≥5.5 cm who underwent successful elective repair resulting from the AAA screening program. The overall compliance of screened patients using screening criteria improved significantly from 61.7% in 2007 to 81.3% since implementation of the screening program during the past 10 years.
The authors would like to thank Muling Lin, Betty Tan, and Ashley Schmidt for assisting with data collection.
ARTICLE HIGHLIGHTS.
Type of Research: Retrospective review of a regional Veterans Affairs abdominal aortic aneurysm (AAA) screening program
Key Findings: In 19,649 patients screened, 1232 AAAs (6.3%) were found, with ± AAA-related deaths (0.03% mortality rate); 54 patients with AAA ≥5.5 cm received elective repair. Compliance of screened patients using screening criteria improved significantly from 61.7% in 2007 to 81.3% since implementation of the screening program during the past 10 years.
Take Home Message: A total of 1232 aneurysms (6.3% total patients) were identified, and 54 patients with AAA ≥5.5 cm underwent successful elective repair resulting from the AAA screening program.
Acknowledgments
This material is based on work supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development: Clinical Sciences Research and Development, VA Merit Award I01CX001683.
Footnotes
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States Government.
Author conflict of interest: none.
Presented at the Forty-sixth Annual Symposium of the Society for Clinical Vascular Surgery, Las Vegas, Nev, March 17–21, 2017.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
REFERENCES
- 1.Ashton HA, Buxton MJ, Day NE, Kim LG, Marteau TM, Scott RA, et al. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet 2002;360:1531–9. [DOI] [PubMed] [Google Scholar]
- 2.Lindholt JS, Juul S, Fasting H, Henneberg EW. Screening for abdominal aortic aneurysms: single centre randomised controlled trial. BMJ 2005;330:750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norman PE, Jamrozik K, Lawrence-Brown MM, Le MT, Spencer CA, Tuohy RJ, et al. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ 2004;329:1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scott RA, Wilson NM, Ashton HA, Kay DN. Influence of screening on the incidence of ruptured abdominal aortic aneurysm: 5-year results of a randomized controlled study. Br J Surg 1995;82:1066–70. [DOI] [PubMed] [Google Scholar]
- 5.Davis M, Harris M, Earnshaw JJ. Implementation of the National Health Service Abdominal Aortic Aneurysm Screening Program in England. J Vasc Surg 2013;57:1440–5. [DOI] [PubMed] [Google Scholar]
- 6.Wanhainen A, Hultgren R, Linne A, Holst J, Gottsater A, Langenskiold M, et al. Outcome of the Swedish Nationwide Abdominal Aortic Aneurysm Screening Program. Circulation 2016;134:1141–8. [DOI] [PubMed] [Google Scholar]
- 7.Ashton HA, Gao L, Kim LG, Druce PS, Thompson SG, Scott RA. Fifteen-year follow-up of a randomized clinical trial of ultrasonographic screening for abdominal aortic aneurysms. Br J Surg 2007;94:696–701. [DOI] [PubMed] [Google Scholar]
- 8.Lee ES, Pickett E, Hedayati N, Dawson DL, Pevec WC. Implementation of an aortic screening program in clinical practice: implications for the Screen For Abdominal Aortic Aneurysms Very Efficiently (SAAAVE) Act. J Vasc Surg 2009;49:1107–11. [DOI] [PubMed] [Google Scholar]
- 9.Chun KC, Teng KY, Van Spyk EN, Carson JG, Lee ES. Outcomes of an abdominal aortic aneurysm screening program. J Vasc Surg 2013;57:376–81. [DOI] [PubMed] [Google Scholar]
- 10.Chun KC, Schmidt AS, Bains S, Nguyen AT, Samadzadeh KM, Wilson MD, et al. Surveillance outcomes of small abdominal aortic aneurysms identified from a large screening program. J Vasc Surg 2016;63:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dua A, Kuy S, Lee CJ, Upchurch GR Jr, Desai SS. Epidemiology of aortic aneurysm repair in the United States from 2000 to 2010. J Vasc Surg 2014;59:1512–7. [DOI] [PubMed] [Google Scholar]
- 12.Kazis LE, Miller DR, Clark J, Skinner K, Lee A, Rogers W, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med 1998;158:626–32. [DOI] [PubMed] [Google Scholar]