Abstract
Aims
Hypoglycaemia, in patients with Type 2 diabetes (T2D) is associated with an increased risk for cardiovascular (CV) events. In EMPA-REG OUTCOME, the sodium-glucose co-transporter-2 inhibitor empagliflozin reduced the risk of CV death by 38% and heart failure hospitalization (HHF) by 35%, while decreasing glycated haemoglobin (HbA1c) without increasing hypoglycaemia. We investigated CV outcomes in patients with hypoglycaemia during the trial and the impact of hypoglycaemia on the treatment effect of empagliflozin.
Methods and results
About 7020 patients with T2D (HbA1c 7–10%) were treated with empagliflozin 10 or 25 mg, or placebo and followed for median 3.1 years. The relationship between on-trial hypoglycaemia and CV outcomes, and effects of empagliflozin on outcomes by incident hypoglycaemia [HYPO-broad: symptomatic hypoglycaemia with plasma glucose (PG) ≤70 mg/dL, any hypoglycaemia with PG <54 mg/dL, or severe hypoglycaemia, and HYPO-strict: hypoglycaemia with PG <54 mg/dL, or severe hypoglycaemia] was investigated using adjusted Cox regression models with time-varying covariates for hypoglycaemia and interaction with treatment. HYPO-broad occurred in 28% in each group and HYPO-strict in 19%. In the placebo group, hypoglycaemia was associated with an increased risk of HHF for both HYPO-broad [hazard ratio (HR, 95% confidence interval, CI) 1.91 (1.25–2.93)] and HYPO-strict [1.72 (1.06–2.78)]. HYPO-broad (but not HYPO-strict) was associated with an increased risk of myocardial infarction (MI) [HR 1.56 (1.06–2.29)]. Empagliflozin improved CV outcomes, regardless of occurrence of hypoglycaemia (P-for interactions >0.05).
Conclusion
In this post hoc exploratory analysis, hypoglycaemia was associated with an increased risk of HHF and MI. Hypoglycaemia risk was not increased with empagliflozin and incident hypoglycaemia did not attenuate its cardio-protective effects.
Keywords: Type 2 diabetes, Hypoglycaemia, Heart failure, Cardiovascular disease, Hospitalization, Mortality
Introduction
Hypoglycaemia is a common complication of diabetes treatment, which has been associated with an increased risk for vascular events and mortality, both in a clinical trial setting1–5 and in population studies.6–8 Individuals at higher risk include those with advanced disease characteristics, e.g. longstanding Type 2 diabetes (T2D), poor renal function, and on a polypharmaceutical treatment regimen, especially with sulphonylureas and/or insulin. Several potential mechanisms link acute hypoglycaemia with a deleterious impact on the cardiovascular (CV) system, including adrenergic activation, autonomic dysfunction, tachycardia, bradycardia, platelet aggregation, and hypokalaemia. These may increase arrhythmogenicity, promote a pro-thrombotic state, increase myocardial ischaemia, and/or have a detrimental haemodynamic effect.9,10 Hypoglycaemia is usually associated with increased risk of sudden death and acute vascular events, but less is known about any association between hypoglycaemia and heart failure (HF) events. However, some studies have linked insulin use with HF risk,11 conceivably through an increase in hypoglycaemia or sodium retention burden. Furthermore, hypoglycaemia has also been associated with prolonged hospitalizations,12,13 and thus, the range of consequences associated with hypoglycaemia has a major impact on societal healthcare costs.
In the EMPA-REG OUTCOME® trial, the sodium-glucose co-transporter-2 (SGLT2) inhibitor empagliflozin, a drug used in T2D to reduce glycated haemoglobin (HbA1c), did not increase the frequency of hypoglycaemic adverse events even when used in combination with insulin.14 The trial further showed that empagliflozin, in patients with T2D and CV disease, reduced major adverse CV events (MACE) by 14%, CV mortality by 38%, hospitalization for HF (HHF) by 35%, and the composite of CV mortality or HHF by 34%.14 Notably, this was the first time that a glucose-lowering agent had been shown to reduce adverse CV events in a large CV outcome trial in high-risk patients with T2D and established CV disease.
The purposes of this post hoc analysis were to investigate the relationship between CV, mortality, and HHF outcomes with preceding hypoglycaemic events and to assess if hypoglycaemia impacted the cardioprotective effects of empagliflozin.
Methods
Study design
The study design of the EMPA-REG OUTCOME® trial (NCT01131676) has been previously described.14,15 In summary, the study included patients from 42 countries with T2D (with HbA1c 7.0–9.0% for drug-naïve patients and 7.0–10.0% for those on stable glucose-lowering therapy), established CV disease, and estimated glomerular filtration rate (eGFR) ≥30 mL/min/1.73 m2. Patients were randomized to empagliflozin 10 mg, empagliflozin 25 mg, or placebo once daily in addition to standard of care. Background glucose-lowering therapy was to remain unchanged for the first 12 weeks after randomization, although intensification was permitted if the patient had a confirmed fasting glucose level of more than 240 mg/dL and a dose reduction or discontinuation of background medication could occur in case of medical necessities. After Week 12 and throughout the trial adjustments of the glucose-lowering therapy was left at the discretion of the investigator according to local guidelines. Investigators were encouraged to monitor blood glucose and HbA1c, and use additional medication for glycaemic control (except SGLT-2 inhibitors) according to applicable standard of care throughout the trial, independent of study treatment assignment that remained masked.
Hypoglycaemic events—categories and definitions
Hypoglycaemic episodes during study follow-up were investigator-reported and for this analysis categorized as either HYPO-broad, defined as a symptomatic hypoglycaemic adverse event with plasma glucose (PG) ≤70 mg/dL, a hypoglycaemic adverse event with PG <54 mg/dL, or a severe hypoglycaemic adverse event; or HYPO-strict defined as a hypoglycaemic adverse event with PG <54 mg/dL, or a severe hypoglycaemic adverse event. A severe hypoglycaemic event was defined as requiring the assistance of another person to actively administer carbohydrate, glucagon, or other resuscitative actions.
Outcomes
The primary outcome of the EMPA REG OUTCOME trial and in the current study was time to first occurrence of 3P-MACE [three-point major adverse CV events; a composite of CV death, non-fatal myocardial infarction (MI), and non-fatal stroke]. Additional outcomes studied were CV death, fatal or non-fatal MI, fatal or non-fatal stroke, all-cause mortality, non-CV mortality, and HHF. Definition of HHF and other outcomes has been reported,14,16 and all CV outcome events, HF hospitalizations, and CV deaths were prospectively adjudicated by blinded Clinical Events Committees.14,15
Statistical analysis
Based on the previously reported consistency of effect,14,16 analyses were performed on the pooled empagliflozin dose groups vs. placebo. Baseline characteristics were reported as percentages for categorical variables and means and standard deviations for continuous variables.
Time to first hypoglycaemic adverse event (for HYPO-broad and -strict) was analysed with a Cox proportional-hazards model, with study group, age, sex, baseline body mass index, baseline HbA1c level, baseline eGFR, and geographic region as factors. In addition, Kaplan–Meier estimates are presented. Data for patients who did not have an event were censored on the last day they were known to be free of the outcome.
To investigate the relationship between hypoglycaemia and CV outcomes, any hypoglycaemic adverse events prior to a CV event, or respectively censoring for the CV event, were considered. Cox regression models were employed, adjusting for age, gender, baseline body mass index categories, baseline HbA1c categories, baseline eGFR categories, geographical region, treatment, a time-varying covariate for hypoglycaemic events and interaction of treatment, and a time-varying covariate for hypoglycaemic events. An extended Cox regression model also included terms for the following baseline covariates: prior coronary artery disease, prior peripheral artery disease, history of ischaemic/haemorrhagic stroke, baseline albuminuria, time since diagnosis of T2D, insulin at baseline, prior cardiac failure, smoking status at baseline, history of atrial fibrillation, and prior intake of sulfonylurea or glinide. In an attempt to characterize temporal relationship between hypoglycaemia and CV complications, we also defined analysis for HYPO-broad and -strict for CV-, mortality-, and HHF events occurring within a 90-day time-window following the hypoglycaemic event.
All analyses were conducted following a modified intent-to-treat (ITT) approach in patients treated with at least one dose of study drug. Each patient who did not have an event was censored on the last day they were known to be free of the outcome. All analyses were performed on a nominal two-sided α = 0.05 without adjustment for multiplicity. Statistical analyses were performed using SAS® version 9.4.
Results
Study population and effects on glycated haemoglobin and hypoglycaemia
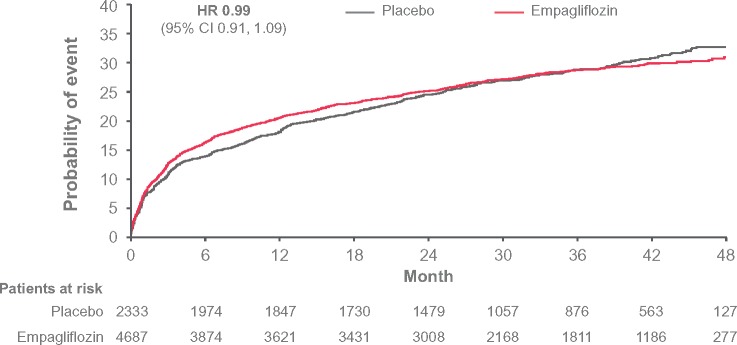
Over the 3.1 years median follow-up, hypoglycaemia rates were comparable between empagliflozin and placebo treatment groups. Out of 7020 participants, 1964 (28.0%), experienced an episode of HYPO-broad [empagliflozin 27.9%, incidence rate (IR) per 100 patient-years 12.3; placebo: 28.2%, IR 12.4; Figure 1], whereas 1321 (18.8%) experienced an episode of HYPO-strict (empagliflozin 18.8%, IR 7.5; placebo 18.9%, IR 7.6, Supplementary material online, Figure S1). There was no difference between the treatment groups [hazard ratio (HR, 95% confidence interval, CI) 0.99 (0.91–1.09), P = 0.91, and HR 0.99 (0.88–1.11), P = 0.84, respectively]. This was despite a significant but modest reduction in HbA1c with empagliflozin [after 12 weeks adjusted mean differences (95% CI) vs. placebo in HbA1c were −0.54% (−0.58 to −0.49) with empagliflozin 10 mg and −0.60% (−0.64 to –0.55) with empagliflozin 25 mg; at Week 94, the adjusted mean differences vs. placebo in HbA1c were −0.42% (−0.48 to −0.36) and −0.47% (−0.54 to −0.41), respectively, and at Week 206, they were −0.24% (−0.40 to −0.08) and −0.36% (−0.51 to −0.20). Severe hypoglycaemia, analysed with the modified ITT approach, occurred in 1.8% (43/2333) in the placebo group (6.5 per 1000-patient years at risk) and 1.6% (73/4687) in the empagliflozin group (5.4 per 1000-patient years at risk), respectively, with no between-group differences.
Figure 1.
Time to first HYPO-broad: symptomatic hypoglycaemia adverse event with plasma glucose ≤70 mg/dL, or hypoglycaemia adverse event with plasma glucose <54 mg/dL, or severe hypoglycaemia adverse event. Hazard ratio from Cox regression model. Based on Kaplan–Meier estimates; hazard ratio based on a Cox proportional-hazards model, with study group, age, sex, baseline body mass index, baseline glycated haemoglobin level, baseline estimated glomerular filtration rate, and geographic region as factors. HR, hazard ratio.
Insulin was used at baseline in 48.2% of patients and sulphonylurea in 42.8% and overall, introduction of post-baseline medication for glycaemic control was more frequent in the placebo group (e.g. sulphonylurea in 7.0% of the placebo group and 3.8% in the empagliflozin group) with also a greater use of insulin (11.5% placebo vs. 5.8% empagliflozin) and larger insulin doses at study end (Supplementary material online, Tables S1 and S2).
Baseline characteristics of patients with and without hypoglycaemia as defined by HYPO-broad or HYPO-strict are provided in Table 1 and Supplementary material online, Table S2. Patients with episodes of hypoglycaemia, had a longer history of T2D, a higher prevalence of albuminuria and retinopathy, and more frequently received insulin (and at higher doses), statins, and angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers. There were no differences in the pattern of baseline characteristics between the empagliflozin and placebo groups by hypoglycaemia identified using either criterion.
Table 1.
Baseline characteristics of participants in EMPA-REG OUTCOME with and without HYPO-broad
| Participants with HYPO-broad |
Participants without HYPO-broad |
|||
|---|---|---|---|---|
| Empagliflozin (n = 1307) | Placebo (n = 657) | Empagliflozin (n = 3380) | Placebo (n = 1676) | |
| Age (years) | 63.5 (8.5) | 63.8 (8.3) | 63.0 (8.6) | 63.0 (9.0) |
| Male | 922 (70.5) | 462 (70.3) | 2414 (71.4) | 1218 (72.7) |
| eGFR, MDRD (mL/min/1.73 m2) | 71.7 (21.7) | 69.2 (20.7) | 75.1 (21.5) | 75.6 (20.9) |
| 30 to <60 | 391 (29.9) | 234 (35.6) | 800 (23.7) | 367 (21.9) |
| <30 | 6 (0.5) | 4 (0.6) | 15 (0.4) | 2 (0.1) |
| UACR (mg/g), median (IQR) | 21.2 (7.1–90.2) | 22.5 (8.0–109.2) | 16.8 (6.2–64.5) | 16.8 (6.2–62.8) |
| UACR (mg/g) | ||||
| <30 | 728 (55.7) | 357 (54.3) | 2061 (61.0) | 1025 (61.2) |
| 30–300 | 392 (30.0) | 198 (30.1) | 946 (28.0) | 477 (28.5) |
| >300 | 168 (12.9) | 97 (14.8) | 341 (10.1) | 163 (9.7) |
| Missing | 19 (1.5) | 5 (0.8) | 32 (0.9) | 11 (0.7) |
| HbA1c (%) | 8.1 (0.8) | 8.1 (0.8) | 8.1 (0.9) | 8.1 (0.9) |
| Diabetes duration (years) | ||||
| ≤1 | 9 (0.7) | 7 (1.1) | 119 (3.5) | 45 (2.7) |
| >1 to 5 | 84 (6.4) | 38 (5.8) | 628 (18.6) | 333 (19.9) |
| >5 to 10 | 254 (19.4) | 115 (17.5) | 921 (27.2) | 456 (27.2) |
| >10 | 960 (73.5) | 497 (75.6) | 1712 (50.7) | 842 (50.2) |
| BMI (kg/m2) | 30.7 (5.4) | 30.6 (5.0) | 30.6 (5.2) | 30.7 (5.3) |
| SBP/DBP (mmHg) | 137 (18)/75 (10) | 136 (19)/75 (11) | 135 (17)/77 (10) | 136 (17)/77 (10) |
| Background medications | ||||
| Insulin | 969 (74.1) | 483 (73.5) | 1283 (38.0) | 652 (38.9) |
| Daily insulin dose | 69.4 (49.7) | 71.2 (57.2) | 62.4 (47.2) | 60.3 (44.6) |
| Metformin | 929 (71.1) | 452 (68.8) | 2530 (74.9) | 1282 (76.5) |
| Sulfonylurea | 496 (37.9) | 232 (35.3) | 1518 (44.9) | 760 (45.3) |
| Any antihypertensives | 1249 (95.6) | 620 (94.4) | 3198 (94.6) | 1602 (95.6) |
| ACE inhibitor/ARB | 1087 (83.2) | 537 (81.7) | 2712 (80.2) | 1331 (79.4) |
| Statins | 1070 (81.9) | 532 (81.0) | 2560 (75.7) | 1241 (74.0) |
| Pre-existing conditions | ||||
| Prior stroke | 258 (19.7) | 138 (21.0) | 826 (24.4) | 415 (24.8) |
| Prior MI | 572 (43.8) | 291 (44.3) | 1618 (47.9) | 792 (47.3) |
| Heart failure | 116 (8.9) | 70 (10.7) | 346 (10.2) | 174 (10.4) |
| Retinopathy | 403 (30.8) | 221 (33.6) | 620 (18.3) | 302 (18.0) |
Data are expressed as n (%) and continuous parameters reported as mean (standard deviation) unless otherwise stated. Patients were treated with ≥1 dose of study drug; those with/without a hypoglycaemic AE were determined at the time of CV event/censoring (time at risk not considered).
ACE, angiotensin-converting enzyme; AE, adverse event; ARB, angiotensin-receptor blocker; BMI, body mass index; CV, cardiovascular; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; HYPO-broad, any symptomatic hypoglycaemic AE with PG ≤70 mg/dL, a hypoglycaemic AE with PG <54 mg/dL, or a severe hypoglycaemic AE (requiring assistance regardless of PG level); IQR, interquartile range; MDRD, modification of diet in renal disease; MI, myocardial infarction; PG, plasma glucose; SBP, systolic blood pressure; UACR, urine albumin-to-creatinine ratio.
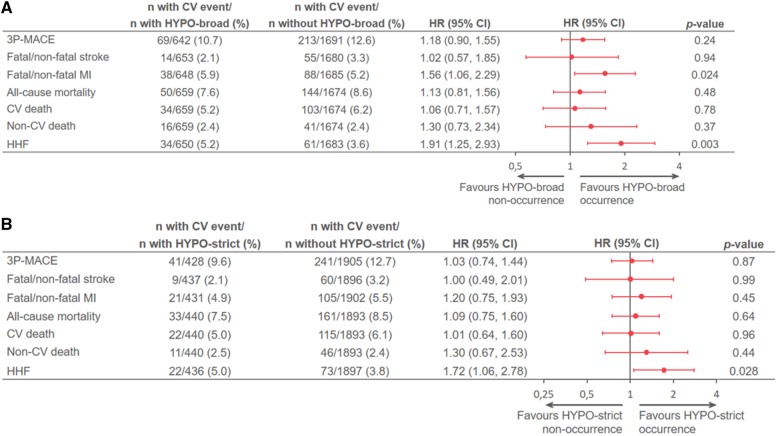
Relationship between hypoglycaemia and cardiovascular, mortality, and heart failure hospitalization outcomes in the placebo group
Of all participants with a first 3P-MACE event in the placebo group [n = 282 (12.1%)], 69 had a preceding HYPO-broad event to the subsequent 3P-MACE, whereas 213 had 3P-MACE with no preceding hypoglycaemic event (Figure 2A). Correspondingly, HYPO-strict preceded 3P-MACE in 41 subjects and there were 241 that had a first 3P-MACE without preceding HYPO-strict (Figure 2B). There was no statistically significant increased risk associated with occurrence of 3P-MACE with preceding hypoglycaemia regardless of classification (HYPO-broad or HYPO-strict) [HR vs. no HYPO-broad: 1.18 (0.90–1.55), P = 0.24; HR vs. no HYPO-strict: 1.03 (0.74–1.44), P = 0.87]. Similar neutral association was observed for stroke and all mortality outcomes. However, preceding hypoglycaemia was associated with an increased risk of HHF using both the HYPO-broad [HR 1.91 (1.25–2.93); P = 0.003] (Figure 2A) and HYPO-strict [HR 1.72 (1.06–2.78); P = 0.028] (Figure 2B) definitions, as well as an increased risk of fatal and non-fatal MI using the HYPO-broad definition [HR 1.56 (1.06–2.29); P = 0.024] (Figure 2A), but not using the HYPO-strict definition (Figure 2B). Using the extended model that included additional baseline covariates, the increased risk for HHF in the placebo group using the HYPO-broad definition [HR 1.72 (1.11–2.66); P = 0.015] remained, whereas others associations were attenuated [e.g. HR for preceding HYPO-broad vs. no HYPO-broad for MI 1.45 (0.98–2.15), P = 0.06; Supplementary material online, Table S4].
Figure 2.
(A) Relationship between occurrence vs. non-occurrence of HYPO-broad and outcomes in the placebo group of EMPA-REG OUTCOME. (B) Relationship between occurrence vs. non-occurrence of HYPO-strict and outcomes in the placebo group of EMPA-REG OUTCOME. Based on a Cox regression model with terms for age, sex, baseline body mass index categories, baseline HbA1c categories, baseline estimated glomerular filtration rate categories, geographical region, treatment, time-varying covariate of HYPO-broad or HYPO-strict, interaction of treatment, and time-varying covariate of HYPO-broad or HYPO-strict. Patients were treated with ≥1 dose of study drug; only for presentation of patient numbers, those with/without HYPO-broad or HYPO-strict were determined at the time of CV event/censoring. *P value for interaction. 3P-MACE, three-point major adverse cardiovascular event (CV death, non-fatal MI, or non-fatal stroke); AE, adverse event; BMI, body mass index; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; HHF, hospitalization for heart failure; HYPO-broad: time to first symptomatic hypoglycaemic AE with PG ≤70 mg/dL, any hypoglycaemic AE with PG <54 mg/dL, or a severe hypoglycaemic AE (requiring assistance regardless of PG level); HYPO-strict, any hypoglycaemic AE with PG <54 mg/dL, or a severe hypoglycaemic AE (requiring assistance regardless of PG level); MI, myocardial infarction; PG, plasma glucose.
The temporal relationship analysis was limited by few number of hypoglycaemic events followed by an outcome event within 90 days which precluded robust outcome-association analysis, but generally the pattern remained as observed in the primary analysis, e.g. HR for preceding HYPO-broad for HHF 1.71 (0.96–3.04), P = 0.07 and HR for preceding HYPO-strict for MI 1.91 (1.02–3.57), P = 0.04 (Supplementary material online, Table S5).
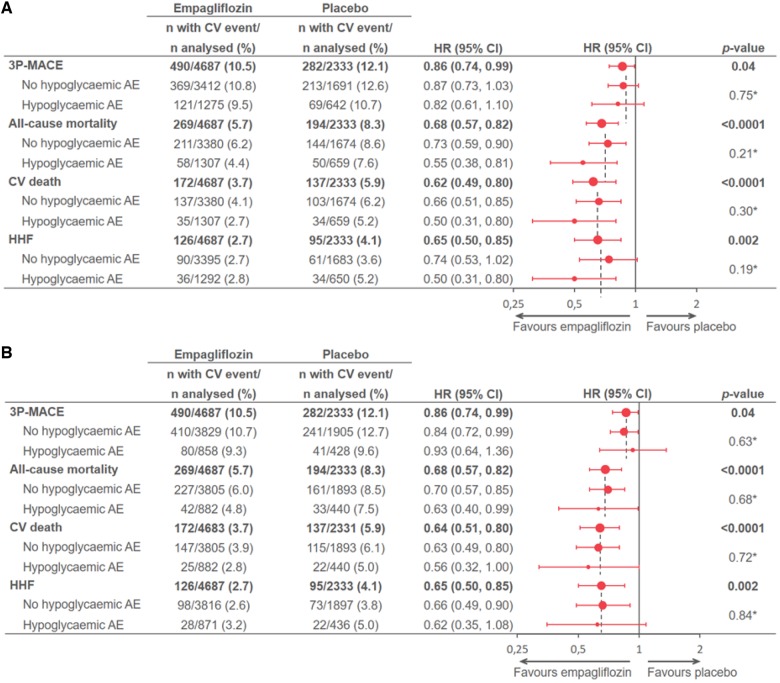
Empagliflozin treatment effects on cardiovascular, mortality, and heart failure hospitalization outcomes by occurrence of hypoglycaemia
There were no differences in proportion of individuals that experienced hypoglycaemia (HYPO-broad or HYPO-strict) between the treatment groups. Empagliflozin treatment, however, significantly reduced 3P-MACE, CV death, all-cause mortality, and HHF, all consistent with the previously reported magnitude of effects,14,15 regardless of the occurrence or severity of the hypoglycaemia (P-values for all interactions >0.05) (Figure 3A and B).
Figure 3.
(A) Effect of empagliflozin on outcomes in the total population and by occurrence of HYPO-broad in EMPA-REG OUTCOME. (B) Effect of empagliflozin on outcomes in the total population and by occurrence of HYPO-strict in EMPA-REG OUTCOME. Based on a Cox regression model with terms for age, sex, baseline body mass index categories, baseline glycated haemoglobin categories, baseline estimated glomerular filtration rate categories, geographical region, treatment, time-varying covariate of HYPO-broad or HYPO-strict, interaction of treatment, and time-varying covariate of HYPO-broad or HYPO-strict. Patients were treated with ≥1 dose of study drug; only for presentation of patient numbers, those with/without HYPO-broad or HYPO-strict were determined at the time of cardiovascular event/censoring. *P value for interaction. 3P-MACE, three-point major adverse cardiovascular event (CV death, non-fatal MI, or non-fatal stroke); AE, adverse event; BMI, body mass index; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; HHF, hospitalization for heart failure; HYPO-broad, time to first symptomatic hypoglycaemic AE with PG ≤70 mg/dL, any hypoglycaemic AE with PG <54 mg/dL, or a severe hypoglycaemic AE (requiring assistance regardless of PG level); MI, myocardial infarction; PG, plasma glucose.
Discussion
In this post hoc analysis and exploratory analysis from the EMPA-REG OUTCOME trial, hypoglycaemia was associated with an increased risk of MI and HHF but no other investigated CV outcomes. Furthermore, hypoglycaemia risk was not increased with empagliflozin and incident hypoglycaemia did not attenuate its cardio-protective effects.
The observation of a positive association between preceding hypoglycaemia and CV events (e.g. MACE and mortality) has been observed in population-based studies6,7 and previous other CV outcome trials involving subjects with T2D and CV disease,1–4,17–19 although not in all trials.20 Also, previous reports mainly found an association with severe hypoglycaemia but not non-severe combined with severe hypoglycaemia, which is at variance with the current results, which used a more moderate hypoglycaemia definition. The magnitude of association of effect in our trial for MI, with an HR of 1.56 (1.06–2.29) for the broader hypoglycaemia classification, was, however, far less than, for example, the HR 3.53 (2.41–5.17) that was observed in ADVANCE for mortality.1 These differences may be attributed to different populations and distinct treatment regimens and approaches tested in the two trials. Since EMPA-REG OUTCOME was not a trial to test intensive glycaemic strategies, together with the inherent low risk of hypoglycaemia with SGLT-2 inhibitors, as also confirmed in CV outcomes trials completed to date,21 we observed few severe episodes of hypoglycaemia. Consequently, although we could not assess any association between preceding severe hypoglycaemia and CV outcome-events, it is possible the results would have differed in a population more susceptible to hypoglycaemia. Another important consideration that has been debated intensively is whether there is any causality between hypoglycaemia and outcomes events, or whether hypoglycaemia is merely a marker of frailty and high risk.20,22,23 Recent studies suggest that there is a temporal association between hypoglycaemia and outcomes with the highest risk of CV events and all-cause mortality occurring early (e.g. during the first weeks and months following a severe hypoglycaemic episode) and that these risks decline during the following months, yet remain higher also in long-term follow-up.4,7 Owing to the limited number of hypoglycaemic events, the present trial cannot provide robust support for or against this hypothesis.
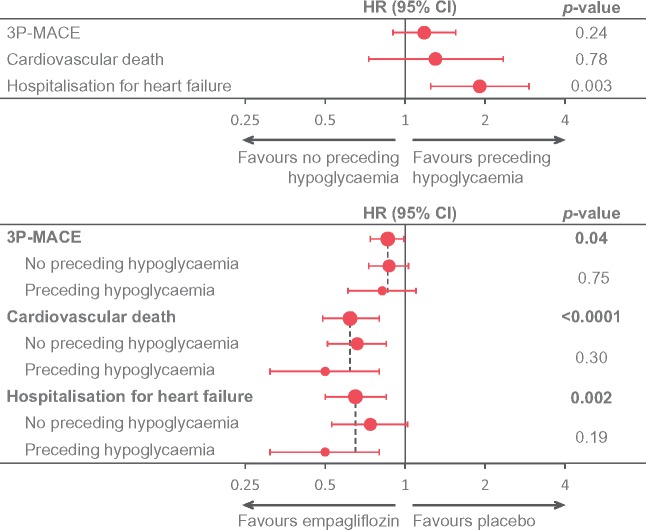
A novel observation from this analysis that needs further confirmation is the increased risk for HHF associated with preceding hypoglycaemia (Take home figure). It is possible, as suggested by others, that acute hypoglycaemia might have a deleterious impact on an already vulnerable myocardium (as a result of autonomic activation, electrophysiological alterations, haemodynamic changes, and/or effects via the coagulation system).9,10,24 However, another possibility is that greater use of insulin and at higher doses in the placebo group over time could be playing a role. Insulin treatment is known to induce weight gain, sodium, and fluid retention,25 all associated with deleterious vascular effects26 and in some HF trials, is associated with worse outcomes.11 While insulin treatment may just be a marker of severity of disease (i.e. confounder by indication), there exist some mechanistic data to suggest that insulin may adversely affect myocardial contractility mediated by β2-adrenergic receptor activation pathways.27 Although our expanded analysis that included an adjustment for insulin use at baseline confirmed an association of increased risk of hypoglycaemia with HHF, we did not adjust for the insulin-dose increase in the placebo group over time: this remains to be further explored.
Take home figure.
The key observations of this article. Upper panel: relationship between occurrence vs. non-occurrence of hypoglycaemia and select outcomes in the placebo group of EMPA-REG OUTCOME. Lower panel: Effect of empagliflozin on select outcomes in the total population and by occurrence of hypoglycaemia in EMPA-REG OUTCOME. Hypoglycaemia defined as symptomatic hypoglycaemia with plasma glucose ≤70 mg/dL, any hypoglycaemia with PG <54 mg/dL, or severe hypoglycaemia.
Another area for further research in CV outcome trials not analysed in the present study, given the observation of an association between preceding hypoglycaemia and MI risk, is to test the clinical hypothesis whether it could be an association between hypoglycaemia and coronary revascularization procedures, as shown in registry studies.28
The observation of a consistent benefit of empagliflozin on CV, mortality, and HHF outcomes regardless of occurrence of hypoglycaemia is reassuring and is consistent with several other subanalyses.14,16 The present post hoc analysis, confirms its treatment effect on reducing CV mortality and HHF before and after occurrence of hypoglycaemia. These efficacy and safety findings are supportive of the European Society of Cardiology (ESC) HF guideline and other diabetes treatment algorithms,29,30 suggesting that empagliflozin should be considered in patients with T2D and CV disease. These data also support the treatment algorithm of European Association for the Study of Diabetes (EASD)/American Diabetes Association (ADA) consensus recommendation for choice of therapies even outside of the context of patients with established CV disease, when there is a compelling need to avoid hypoglycaemia,31 as well as the ESC/EASD clinical practice guidelines that underscores that attention should be paid to avoidance of hypoglycaemia whilst achieving glycaemic goals in an individualized manner.32
In conclusion, in this exploratory post hoc analysis, hypoglycaemia was associated with an increased risk of HHF and MI but no other outcomes. Hypoglycaemia risk was not increased with empagliflozin and incident hypoglycaemia did not attenuate its cardio-protective effects.
Supplementary Material
Acknowledgements
The authors thank the investigators, co-ordinators, and patients who participated in this trial. They acknowledge Matt Smith, PhD CMPP, and Giles Brooke, PhD CMPP, from Envision Scientific Solutions for graphical support (Forest Plots and KM curves), supported financially by Boehringer Ingelheim.
Funding
This work was supported by the Boehringer Ingelheim and Eli Lilly and Company. Boehringer Ingelheim was involved in the design and conduct of the study; collection, analysis, and interpretation of data; and preparation of this manuscript. Eli Lilly’s involvement was limited to co-funding of the study.
Conflict of interest: D.F. has received personal fees from Boehringer Ingelheim, Novo Nordisk, AstraZeneca, Sanofi, and Merck & Co. S.E.I. has received personal fees from Merck & Co, Janssen, Novo Nordisk, Sanofi, Intarcia, Lexicon, vTv Therapeutics, and AstraZeneca; received personal fees and non-financial support from Boehringer Ingelheim. C.W. has received personal fees from Boehringer-Ingelheim, Genzyme-Sanofi, Eli Lilly and Company, and Janssen. B.Z. has received personal fees from Boehringer Ingelheim, Merck & Co, Novo Nordisk, Sanofi, Eli Lilly and Company, Takeda, AstraZeneca, and Janssen and has received grants from Boehringer Ingelheim, Merck & Co, and Novo Nordisk. O.V., M.M., J.T.G., and O.E.J. are employees of Boehringer Ingelheim.
See page 218 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz730)
References
- 1. Zoungas S, Patel A, Chalmers J, de Galan BE, Li Q, Billot L, Woodward M, Ninomiya T, Neal B, MacMahon S, Grobbee DE, Kengne AP, Marre M, Heller S; Group Advance Collaborative. Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010;363:1410–1418. [DOI] [PubMed] [Google Scholar]
- 2. Bonds DE, Miller ME, Bergenstal RM, Buse JB, Byington RP, Cutler JA, Dudl RJ, Ismail-Beigi F, Kimel AR, Hoogwerf B, Horowitz KR, Savage PJ, Seaquist ER, Simmons DL, Sivitz WI, Speril-Hillen JM, Sweeney ME.. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010;340:b4909.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Origin Trial Investigators Mellbin LG, Rydén L, Riddle MC, Probstfield J, Rosenstock J, Díaz R, Yusuf S, Gerstein HC.. Does hypoglycaemia increase the risk of cardiovascular events? A report from the ORIGIN trial. Eur Heart J 2013;34:3137–3144. [DOI] [PubMed] [Google Scholar]
- 4. Zinman B, Marso SP, Christiansen E, Calanna S, Rasmussen S, Buse JB; Investigators Leader Publication Committee on behalf of the LEADER Trial. Hypoglycemia, cardiovascular outcomes, and death: the LEADER experience. Diabetes Care 2018;41:1783–1791. [DOI] [PubMed] [Google Scholar]
- 5.Writing Group for the DCCT/EDIC Research Group, Orchard TJ, Nathan DM, Zinman B, Cleary P, Brillon D, Backlund JY, Lachin JM.. Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA 2015;313:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hsu PF, Sung SH, Cheng HM, Yeh JS, Liu WL, Chan WL, Chen CH, Chou P, Chuang SY.. Association of clinical symptomatic hypoglycemia with cardiovascular events and total mortality in type 2 diabetes: a nationwide population-based study. Diabetes Care 2013;36:894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lo SC, Yang YS, Kornelius E, Huang JY, Lai YR, Huang CN, Chiou JY.. Early cardiovascular risk and all-cause mortality following an incident of severe hypoglycaemia: a population-based cohort study. Diabetes Obes Metab 2019;21:1878–1885. [DOI] [PubMed] [Google Scholar]
- 8. Lu CL, Shen HN, Hu SC, Wang JD, Li CY.. A population-based study of all-cause mortality and cardiovascular disease in association with prior history of hypoglycemia among patients with type 1 diabetes. Diabetes Care 2016;39:1571–1578. [DOI] [PubMed] [Google Scholar]
- 9. Frier BM, Schernthaner G, Heller SR.. Hypoglycemia and cardiovascular risks. Diabetes Care 2011;34 Suppl 2:S132–S137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nordin C. The proarrhythmic effect of hypoglycemia: evidence for increased risk from ischemia and bradycardia. Acta Diabetol 2014;51:5–14. [DOI] [PubMed] [Google Scholar]
- 11. Cosmi F, Shen L, Magnoli M, Abraham WT, Anand IS, Cleland JG, Cohn JN, Cosmi D, De Berardis G, Dickstein K, Franzosi MG, Gullestad L, Jhund PS, Kjekshus J, Køber L, Lepore V, Lucisano G, Maggioni AP, Masson S, McMurray JJV, Nicolucci A, Petrarolo V, Robusto F, Staszewsky L, Tavazzi L, Teli R, Tognoni G, Wikstrand J, Latini R.. Treatment with insulin is associated with worse outcome in patients with chronic heart failure and diabetes. Eur J Heart Fail 2018;20:888–895. [DOI] [PubMed] [Google Scholar]
- 12. Nirantharakumar K, Marshall T, Kennedy A, Narendran P, Hemming K, Coleman JJ.. Hypoglycaemia is associated with increased length of stay and mortality in people with diabetes who are hospitalized. Diabet Med 2012;29:e445–e448. [DOI] [PubMed] [Google Scholar]
- 13. Budnitz DS, Lovegrove MC, Shehab N, Richards CL.. Emergency hospitalizations for adverse drug events in older americans. N Engl J Med 2011;365:2002–2012. [DOI] [PubMed] [Google Scholar]
- 14. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE.. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 15. Zinman B, Inzucchi SE, Lachin JM, Wanner C, Ferrari R, Fitchett D, Bluhmki E, Hantel S, Kempthorne-Rawson J, Newman J, Johansen OE, Woerle HJ, Broedl UC.. Rationale, design, and baseline characteristics of a randomized, placebo-controlled cardiovascular outcome trial of empagliflozin (EMPA-REG OUTCOME). Cardiovasc Diabetol 2014;13:102.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fitchett D, Butler J, van de Borne P, Zinman B, Lachin JM, Wanner C, Woerle HJ, Hantel S, George JT, Johansen OE, Inzucchi SE.. Effects of empagliflozin on risk for cardiovascular death and heart failure hospitalisation across the spectrum of heart failure risk in the EMPA-REG OUTCOME® trial. Eur Heart J 2018;39:363–370. [DOI] [PubMed] [Google Scholar]
- 17. Pieber TR, Marso SP, McGuire DK, Zinman B, Poulter NR, Emerson SS, Pratley RE, Woo V, Heller S, Lange M, Brown-Frandsen K, Moses A, Barner Lekdorf J, Lehmann L, Kvist K, Buse JB; DEVOTE Study Group. DEVOTE 3: temporal relationships between severe hypoglycaemia, cardiovascular outcomes and mortality. Diabetologia 2018;61:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heller SR, Bergenstal RM, White WB, Kupfer S, Bakris GL, Cushman WC, Mehta CR, Nissen SE, Wilson CA, Zannad F, Liu Y, Gourlie NM, Cannon CP; EXAMINE Investigators. Relationship of glycated haemoglobin and reported hypoglycaemia to cardiovascular outcomes in patients with type 2 diabetes and recent acute coronary syndrome events: the EXAMINE trial. Diabetes Obes Metab 2017;19:664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD. ; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139. [DOI] [PubMed] [Google Scholar]
- 20. Standl E, Stevens SR, Armstrong PW, Buse JB, Chan JCN, Green JB, Lachin JM, Scheen A, Travert F, Van de Werf F, Peterson ED, Holman RR; Tecos Study Group. Increased risk of severe hypoglycemic events before and after cardiovascular outcomes in TECOS suggests an at-risk type 2 diabetes frail patient phenotype. Diabetes Care 2018;41:596–603. [DOI] [PubMed] [Google Scholar]
- 21. Inzucchi SE, Zinman B, Wanner C, Ferrari R, Fitchett D, Hantel S, Espadero RM, Woerle HJ, Broedl UC, Johansen OE.. SGLT-2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res 2015;12:90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. International Hypoglycaemia Study Group. Hypoglycaemia, cardiovascular disease, and mortality in diabetes: epidemiology, pathogenesis, and management. Lancet Diabetes Endocrinol 2019;7:385–396. [DOI] [PubMed] [Google Scholar]
- 23. Davis IC, Ahmadizadeh I, Randell J, Younk L, Davis SN.. Understanding the impact of hypoglycemia on the cardiovascular system. Expert Rev Endocrinol Metab 2017;12:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fitzpatrick C, Chatterjee S, Seidu S, Bodicoat DH, Ng GA, Davies MJ, Khunti K.. Association of hypoglycaemia and risk of cardiac arrhythmia in patients with diabetes mellitus: a systematic review and meta-analysis. Diabetes Obes Metab 2018;20:2169–2178. [DOI] [PubMed] [Google Scholar]
- 25. Gans ROB, Bilo HJG, Donker A.. The renal response to exogenous insulin in non-insulin dependent diabetes mellitus in relation to blood pressure and cardiovascular hormonal status. Nephrol Dial Transplant 1996;11:794–802. [DOI] [PubMed] [Google Scholar]
- 26. Nandish S, Bailon O, Wyatt J, Smith J, Stevens A, Lujan M, Chilton R.. Vasculotoxic effects of insulin and its role in atherosclerosis: what is the evidence? Curr Atheroscler Rep 2011;13:123–128. [DOI] [PubMed] [Google Scholar]
- 27. Wang Q, Liu Y, Fu Q, Xu B, Zhang Y, Kim S, Tan R, Barbagallo F, West T, Anderson E, Wei W, Abel ED, Xiang YK.. Inhibiting insulin-mediated β2-adrenergic receptor activation prevents diabetes-associated cardiac dysfunction. Circulation 2017;135:73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johnston SS, Conner C, Aagren M, Smith DM, Bouchard J, Brett J.. Evidence linking hypoglycemic events to an increased risk of acute cardiovascular events in patients with type 2 diabetes. Diabetes Care 2011;34:1164–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 30. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FD, Løchen ML, Löllgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WM; Authors/Task Force Members. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, Rossing P, Tsapas A, Wexler DJ, Buse JB.. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018;41:2669–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ryden L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, Deaton C, Escaned J, Hammes HP, Huikuri H, Marre M, Marx N, Mellbin L, Ostergren J, Patrono C, Seferovic P, Uva MS, Taskinen MR, Tendera M, Tuomilehto J, Valensi P, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Torbicki A, Wijns W, Windecker S, De Backer G, Ezquerra EA, Avogaro A, Badimon L, Baranova E, Betteridge J, Ceriello A, Funck-Brentano C, Gulba DC, Kjekshus JK, Lev E, Mueller C, Neyses L, Nilsson PM, Perk J, Reiner Z, Sattar N, Schachinger V, Scheen A, Schirmer H, Stromberg A, Sudzhaeva S, Viigimaa M, Vlachopoulos C, Xuereb RG.. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J 2013;34:3035–3087. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.