Abstract
Normal hematopoiesis requires the accurate orchestration of lineage-specific patterns of gene expression at each stage of development, and epigenetic regulators play a vital role. Disordered epigenetic regulation has emerged as a key mechanism contributing to hematological malignancies. Histone deacetylases (HDACs) are a series of key transcriptional cofactors that regulate gene expression by deacetylation of lysine residues on histone and nonhistone proteins. In normal hematopoiesis, HDACs are widely involved in the development of various lineages. Their functions involve stemness maintenance, lineage commitment determination, cell differentiation and proliferation, etc. Deregulation of HDACs by abnormal expression or activity and oncogenic HDAC-containing transcriptional complexes are involved in hematological malignancies. Currently, HDAC family members are attractive targets for drug design, and a variety of HDAC-based combination strategies have been developed for the treatment of hematological malignancies. Drug resistance and limited therapeutic efficacy are key issues that hinder the clinical applications of HDAC inhibitors (HDACis). In this review, we summarize the current knowledge of how HDACs and HDAC-containing complexes function in normal hematopoiesis and highlight the etiology of HDACs in hematological malignancies. Moreover, the implication and drug resistance of HDACis are also discussed. This review presents an overview of the physiology and pathology of HDACs in the blood system.
Keywords: Histone deacetylases, Hematopoiesis, Hematological malignancy, HDAC inhibitor, Drug resistance
Introduction
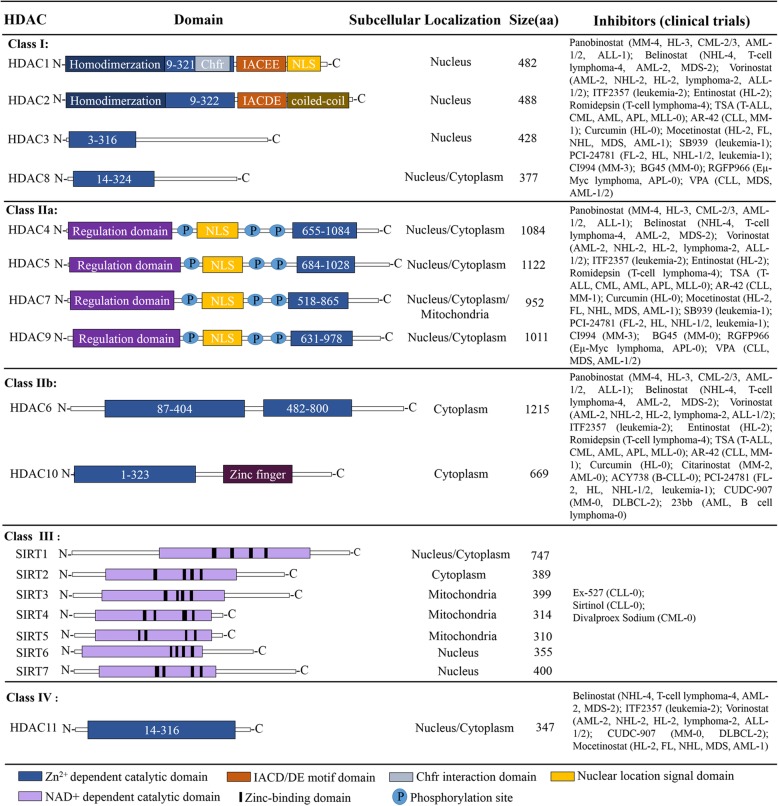
Epigenetic modifications play an indispensable role in the expression of hematopoietic lineage-specific genes. HDAC (histone deacetylase) and HAT (histone acetyltransferase) are two opposite classes of epigenetic modification enzymes. Generally, acetylation mediated by HATs opens the chromatin and allows gene transcription, whereas HDACs have a repressive effect on gene expression by deacetylating lysine residues on histone tails [1, 2]. Mammalian HDACs consist of 18 highly conserved genes [3], and they are divided into Class I (HDAC1, HDAC2, HDAC3, HDAC8), Class IIa (HDAC4, HDAC5, HDAC7, HDAC9), Class IIb (HDAC 6, HDAC 10), Class III (sirt1-sirt7) and Class IV (HDAC11) on the basis of phylogenetic analysis and sequence similarity to yeast factors (Fig. 1) [4]. Class I members are ubiquitously expressed with predominant nuclear localization [5, 6]. Furthermore, they are composed of approximately 400 amino acids and contain an N-terminal catalytic domain. Their catalytic domain is similar to a pocket and consists of two adjacent histidine residues, two aspartic acid residues and one tyrosine residue with Zn2+ ions as the core [7]. Class II members are more selectively expressed and can shuttle actively between the nucleus and cytoplasm [5, 6]. There are 600–1200 amino acids with an N-terminal regulatory domain that mediates the interaction with tissue-specific transcription factors and corepressors in class IIa HDACs. Importantly, there are two or three conserved serine residues in the class IIa N-terminal domain that can be phosphorylated by kinases, such as protein kinase D (PKD), which determines the nuclear export of class IIa HDACs [8, 9]. Class IIb members HDAC6 and HDAC10 contain another catalytic domain and an ubiquitin-binding zinc finger domain at the C-terminal region, respectively [7, 8]. The sirtuin family (SIRT1–7) of deacetylases represents class III, but they are functionally unrelated to HDACs; their deacetylase activity depends on NAD+ rather than on Zn2+-dependent enzymes [7]. As the smallest member of the HDAC family, HDAC11 is predominantly located in the nucleus, and more than 80% of the amino acid sequence is assigned to its catalytic domain [10].
Fig. 1.
Classification of HDAC family
As a series of epigenetic regulatory molecules, HDACs are involved in multiple aspects of hematopoiesis, such as stemness maintenance and lineage commitment. Abnormal expression or activity of HDACs and abnormal interactions between HDACs and transcription factors (TFs)/cofactors interfere with downstream gene regulatory networks, culminating in many different types of malignant hematopoietic diseases, such as lymphoma and leukemia [11]. Currently, HDAC inhibitors (HDACis) have been widely used as single agents or in combination with other chemotherapeutics in tumor treatment. However, therapy resistance remains a key problem. In this review, we focus on recent findings on the role of HDACs and their complexes in regulating gene expression during hematopoiesis or hematological malignancies, followed by a summary of combination therapy strategies and resistance mechanisms of hematological malignancies to HDACis.
HDACs in hematopoiesis
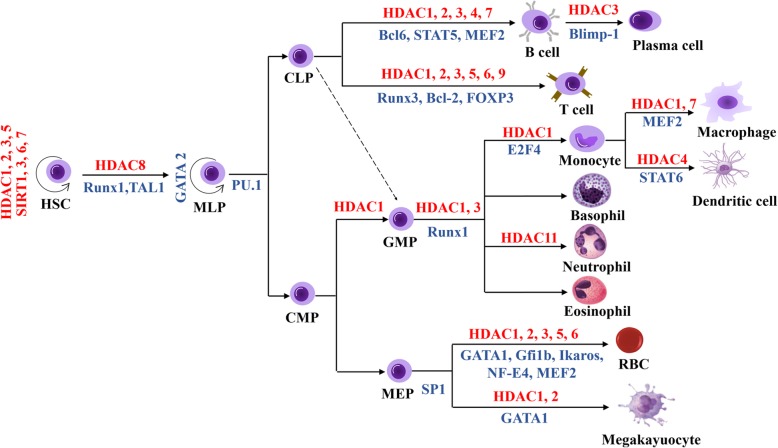
HDACs are extensively involved in multilineage development, including the hematopoietic stem cell (HSC)-progenitor lineage, granulocyte-monocyte lineage, erythropoietic lineage and lymphoid lineage (Fig. 2). During hematopoiesis, HDACs participate in the formation of a variety of transcriptional complexes where the reciprocal regulation between HDACs and TFs or other cofactors regulate histone acetylation levels, TF activity and functions of transcriptional complexes, which in turn modulate expression of various hematopoietic-related genes [12–17] (Table 1).
Fig. 2.
Schematic representation of the main HDACs and HDAC-related TFs involved in hematopoietic lineage commitment.
Table 1.
HDAC-TF complexes in normal and malignant hematopoietic cells and their functions
| TF(s) | Complex components | Cell type or disease | Function |
|---|---|---|---|
| GATA1 | GATA1-acHDAC1 | MEL cells | Promoting β-globin expression and erythroid commitment |
| GATA1-HDAC3/HDAC4/HDAC5 | COS cells | May repress GATA1 target genes and inhibit erythroid cell differentiation | |
| pERK-HDAC5-GATA1-EKLF | Human erythroblast | Inhibiting the transcription of globin genes | |
| GATA-1-Scl/TAL1-ET02-HDACs | G1E-ER-GATA1 cells | Participating in chromosomal translocation in AML | |
| GATA2 | GATA2-HDAC3/HDAC5 | COS cells | Repressing the transcriptional activity of GATA2 |
| GATA3 | GATA3-Tbet-HDAC3/HDAC5 | Jurkat cells | Regulating lymphocyte homing |
| EKLF | EKLF-HDAC1/Sin3A | K562 cells | Inhibiting β-globin gene expression |
| PU.1 | PU.1-HDAC1/Sin3A | MEL cells | Inhibiting β-globin gene expression |
| PU.1-Eto2- Sin3A-HDAC2 | AML cells | Inhibiting myeloid differentiation genes, such as Mcsfr and Gmcsfr | |
| Ikaros | Ikaros-GATA1-FOG1-HDAC1/NuRD | Mouse erythroid cells | Inhibiting γ-globin gene expression |
| Gfi | Gfi-1-G9a-HDAC1 | HL60 cells | Repressing the expression of p21Cip/WAF1 |
| Gfi1b-CoREST-LSD1-HDAC 1/2 | MEL cells | Committing hematopoietic differentiation | |
| GFI1B | GFI1B-LSD1-RCOR1-HDAC1/2 | Megakaryoblasts | Controlling megakaryoblast proliferation and differentiation |
| NF-E4 | NF-E4 - HDAC1 | K562 cells | Inhibiting γ-globin gene expression |
| E2F4 | E2F4-RBL2-HDAC1-BRM (SWI/SNF) | Human monocytes | Repressing pluripotency stem cell factors in human monocytes |
| Runx1 | Runx1-HDAC1/HDAC3 | Human macrophages | Negatively regulating granulocyte formation |
| Runx1-Eto2-Sin3A-HDAC2 | AML cells | Inhibiting myeloid differentiation genes, such as Mcsfr and Gmcsfr | |
| RUNX1/T1 | RUNX1/RUNX1T1-HDACs-DNMTs | t(8;21) AML cells | Inducing leukemogenesis |
| RUNX1/RUNX1T1-ETO-Sin3A-HDAC2 | AML cells | Causing aberrant repression of late differentiation genes | |
| MEF2 | MEF2A/D-HDAC1/HDAC7 | Human macrophages | Repressing the transcription of c-Jun |
| MEF2-HDAC9 | K562 cells | Activating γ-globin gene a and inducing HbF synthesis | |
| MEF2C-HDAC7 | Human lymphoma | Silencing lineage-inappropriate genes in pro–B cells | |
| TCF/LEF1 | TCF/LEF1-SIRT6 | Mouse stem cells | Inhibiting Wnt target genes and maintaining HSC homeostasis |
| Blimp-1 | HDAC1/HDAC2 | pre-B cells | Inhibiting c-myc transcription |
| STAT5 | HDAC3-LSD1-STAT5 | pro-B cells | Promoting the maturation of B cells |
| Bach2 | Bach2-HDAC3-NCoR1/NCoR2-Rif1 | Mature B cells | Increasing the deacetylation of Blimp-1 gene |
| BCL6 | BCL6/SMRT-HDAC3 | Human DLBCL cells | Establishing GC responses |
| BCL6-HDAC4 | Spleen B cells | Blocking B cell development and inducing uncontrolled cell proliferation | |
| BCL6-HDAC9 | Mouse B-NHL cells | Deacetylating BCL6 and upregulating proliferation and survival genes | |
| NF-κB | Sirt1-p300- NF-κB | Mouse T cells | Inhibiting transcription of Bclaf1 |
| AML1 | AML1-ETO-NCoR-mSin3-HDACs(1,2,3) | t(8;21) AML cells | Repressing AML1-mediated transactivation and activating leukemogenesis |
| AML1-ETO-HDAC1/NCoR-SMRT | t(8;21) AML cells | Repressing AML1-mediated transactivation and activating leukemogenesis | |
| AML1-MTG16-HDAC1/3 | t(16; 21) AML cells | Participating in nucleolar targeting | |
| TEL-AML1-HDACs | t(12;21) ALL cells | Repressing AML1 target genes | |
| AML1-MDS1-EVI1-CtBP1-HDAC1 | MDS/CML/AM cells | Repressing gene transcription and inducing leukemia in mice | |
| PML | PML-RARα-NCoR-Sin3-Ski/Sno-HDACs | t(15;17) APL cells | Inhibiting Rb and TRβ-mediated silencing and inducing leukemogenesis |
| PLZF | RARα-PLZF-HDAC1/NCoR-Sin3 | t(11;17) APL cells | Impairing C/EBPα function and contributing to differentiation arrest in APL |
HSCs and progenitor lineage differentiation
HDAC1 and HDAC2 are essential regulators of HSC formation and homeostasis. Simultaneous deletion of HDAC1 and HDAC2 results in the loss of HSCs and, consequently, early hematopoietic progenitors, which are associated with the deregulated expression of genes linked to stem cell survival and maintenance, such as Dmkn, Nurcks1 and Tpt1 [18]. HDAC1 exhibits dynamic expression changes in cell lineage specification during hematopoiesis. Its expression is relatively low in human and mouse CD34+ hematopoietic progenitor cells (HPCs) and mature myeloid cells (monocytes and granulocytes). HDAC1 transcripts are moderately expressed in committed progenitors, erythroblasts and peripheral blood T lymphocytes. The unique expression pattern of HDAC1 is subject to regulation by hematopoietic transcription factors. For instance, HDAC1 transcription is repressed by GATA2 and C/EBP during common myeloid progenitor (CMP) differentiation into myeloid cells, especially granulocytes, and is activated by GATA1 and Sp1 during CMP differentiation into erythro-megakaryocytic cells [19, 20]. Specifically, knockdown of HDAC1 promotes hematopoietic progenitor differentiation toward the myeloid lineage with an increase in granulocyte/macrophage colonies and a reduction in the numbers and sizes of CFU-E and BFU-E, suggesting a role for HDAC1 in lineage commitment determination [6].
Class I HDAC3 was identified as a negative regulator in normal human HSC expansion. In vitro, HDAC3 silencing by HDACi-VA promoted CD34+ cell expansion without affecting differentiation potential [21]. Conditional deletion of HDAC3 in mice increased stem cells and early progenitor cells and blocked progress toward lymphoid-primed multipotential progenitor (LMPP) cells and lymphoid lineages, in which the loss of HDAC3 could impair S phase progression of multipotential progenitor (MPP) cells and, in turn, hinder cell DNA replication [22]. HDAC3 regulates the development of HSCs by interacting with HSC or HPC-specific TFs. For example, HDAC3 cooperates with Ncor2 to repress the fos-vegfd cascade by modulating the acetylation level in the fos promoter region, thereby mediating HSC formation [23]. HDAC3 directly interacts with GATA2 to inhibit GATA2–dependent targeting genes, which may be achieved by modifying the acetylation status of GATA2 in HPCs [24]. Another class I member HDAC8 is highly expressed in LT-HSCs, MPP and LMPP cells. HDAC8 plays a pivotal role in the maintenance and functional integrity of LT-HSC by deacetylating p53 [25].
Some class II and class III HDAC members are also involved in HSC homeostasis and aging. Class IIa HDAC5 has been shown to negatively regulate HSC homing by regulating p65 deacetylation [26]. Class III (SIRT1-SIRT7) members have been implicated in protecting HSCs from aging [27]. Specifically, SIRT1 maintains aged HSC homeostasis by promoting nuclear localization and activation of FOXO3 and negatively regulating mTOR signaling [27–29]. Downregulation of SIRT3 in stem cells is associated with the aging of HSCs by regulating the global acetylation landscape of mitochondrial proteins and increasing ROS. While upregulation of SIRT3 can rescue functional defects in aged HSCs [30]. SIRT6 maintains HSC homeostasis by interacting with TCF/LEF1 and inhibiting the transcription of Wnt target genes via deacetylating H3K56ac [31]. SIRT7 maintains aged HSCs and prevents myeloid differentiation by repressing NRF1 (a key regulator of mitochondrial genes) and PFSmt (a mitochondrial protein folding stress factor) [32]. In addition, SIRT1 plays a critical role in promoting the mutation acquisition of CML, an age-dependent malignancy. Inhibition of SIRT1 sensitizes leukemic stem cells to imatinib treatment and blocks the acquisition of resistant BCR-ABL mutations by altering the function of DNA repair machineries in CML cells, and reduces the error-prone repair activity of DNA damage [33]. Further understanding of sirtuins in HSC aging and malignancy may provide novel treatment strategies to deter hematological aging and improve the treatment of hematological malignancies.
Granulocyte-monocyte lineage terminal differentiation
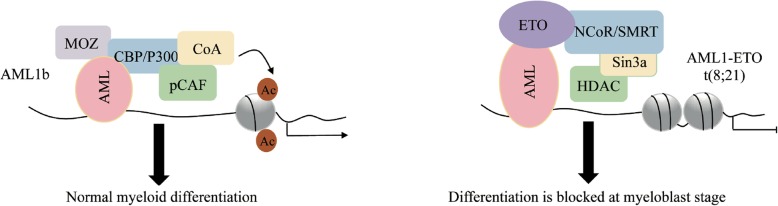
In the bone marrow, granulocyte-monocyte terminal differentiation generally arises from upstream granulocyte-monocyte progenitors (GMPs), which have the potential to differentiate into granulocytes, dendritic cells (DCs) and monocytes/macrophages (Fig. 2) [34–36]. Class I and II HDACs are crucial for the proliferation and differentiation of bone marrow-derived monocytes (BMDMs) into macrophages and DC cells. For instance, under the treatment of TSA (a class I and II HDAC inhibitor), the amplification of murine myeloid progenitors is blocked and, in turn, differentiate into an elongated morphology of mixed M1/M2 profile, instead of the normal pancake-like shape of M1 inflammatory macrophages [37]. In addition, TFs associated with HDAC complexes shows dynamic changes during premonocyte to monocyte differentiation. For example, the differentiation-associated cell cycle exit induces E2F1 replacement with E2F4 at the PARP1 promoter and the assembly of an E2F4-RBL2-HDAC1-BRM (SWI/SNF) repressor complex which reduces PARP1 transcription and represses of pluripotent transcription factors such as POU5F1, SOX2, and NANOG [38]. Moreover, HDAC1 and HDAC7 have been implicated in macrophage terminal differentiation. Both of them interact with MEF2A/D heterodimers on the c-Jun promoter, thereby repressing the transcription of c-Jun, which is important for the development of the monocyte/macrophage lineage [39]. HDAC1 and HDAC3 are implicated in granulopoiesis. When Runx1 is phosphorylated by Src kinase, the reduced interaction of Runx1-HDAC1/HDAC3 is relevant to increased DNA affinity and the induction of granulopoiesis [40]. Class IIa HDAC4 can be recruited to the Arg1 promoter region, which leads to a reduction in the acetylation of both histone 3 and STAT6 proteins and subsequent transcriptional activation of Arg1, resulting in monocyte and CD8α(+) conventional DC differentiation [41]. Importantly, in some case, the aberrant recruitment of HDAC complexes by the oncofusion TFs to key hematopoietic genes is critical for leukemic transformation. For instance, in AML, AML1-ETO and RARalpha-PLZF fusions recruit NCoR/SMRT-HDAC1/3, while lose their binding ability with P300/MOZ/pCAF/CoA present at normal cells, thus reducing histone acetylation and producing repressive chromatin organization by HDACs, which results in gene transcriptional repression responsible for hematopoietic differentiation and, potentially, activation of a subset of other genes, including those for macrophage colony-stimulating factor and BCL-2 [42] (Fig. 3).
Fig. 3.
A model highlights component transformation in transcriptional complex is critical for leukemic tranformation.
HDACs are able to regulate the transcription of granulocyte-macrophage colony-stimulating factor (MCSFR), granulocyte colony-stimulating factor (GMCSFR) and cytokines to control the development and activation of granulocyte-monocyte cells [34–36]. For instance, Runx1 and PU.1 independently interact with the ETO2-SIN3A-HDAC2 corepressor complex coactivate MCSFR and GMCSFR expression [43]. Additionally, class IV HDAC11 is gradually increased from promyelocytes to neutrophils differentiation. Knockout of HDAC11 in mice showed the expansion of maturing neutrophils and increases in TNF-α and IL-6 [2]. Importantly, several studies have shown that cytokines, including IL-2, IL-12, TNF-α and GM-CSF, regulate the innate and adaptive immune system and enhance immunity against FL, NHL and CLL [44]. The involvement of HDACs in cytokines transcription regulation in neutrophils propose a combination application of HDACis and cytokines, which potentially contributes to an enhanced tumor immuneresponse.
Erythrocyte lineage terminal differentiation
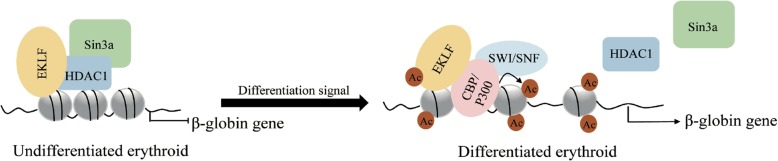
Terminal erythroid differentiation begins with proerythroblasts, which subsequently undergo sequential mitoses, transforming into basophilic, polychromatic, and orthochromatic erythroblasts and enucleating into reticulocytes [45] (Fig. 2). The distinct interaction pattern between HDACs and erythroid-specific TFs plays an important regulatory role in erythropoiesis. HDAC1 and/or HDAC2 are the basic components of the SIN3A, NuRD and CoREST corepressor complexes [46, 47]. HDAC1/Sin3A can be recruited by EKLF to inhibit β-globin expression in undifferentiated EBHX11L cells, while this complex can be converted to the EKLF-p300/CBP-SWI/SNF complex and promote β-globin expression during the differentiation of EBHX11L cells to a primitive erythroid phenotype [48] (Fig. 4). Similarly, the PU.1-MeCP2-HDAC/mSIN3A complex inhibits the β-globin gene in undifferentiated MEL cells, and this complex is dissociated from the β-globin gene region during erythroid differentiation of MEL cells [49]. Interestingly, the acetylation state of the HDAC1/NURD complex plays a dual role during erythropoiesis. On the one hand, the HDAC1/NURD-GATA1 complex inhibits GATA2 expression by exerting deacetylation activity. On the other hand, p300-mediated acetylation of HDAC1 converts the NuRD complex from a repressor to an activator, which recruits GATA1 to promote β-globin expression and erythroid commitment [6, 50, 51]. Furthermore, the Gfi1b-LSD1-CoREST-HDAC1/2 complex is recruited to the c-myc promoter, leading to histone H3 hypoacetylation and c-myc transcriptional repression. C-myc repression is required for the arrest of the cell cycle and the initiation of erythroid differentiation [52].
Fig. 4.
A model of CBP/P300 and HDAC component patterns determines the transcriptional function of TF in erythroleukemia cell differentiation.
Moreover, HDAC and erythroid-specific TF interactions are critical for the regulation of the γ-globin gene. For instance, the Ikaros-GATA1-FOG1-HDAC1/NuRD complex is required for silencing the human γ-gene during γ- to β-globin switching [53]. Diminished binding of acetylated NF-E4 to HDAC1 showed activation of γ-globin and inhibition of β-globin in fetal erythroid cells [54]. In addition, inhibition of HDAC3-NCoR (nuclear receptor corepressor) complex activity by the HDAC3-specific inhibitor SCFAD caused displacement of this complex from the γ-globin gene region with the recruitment of RNA polymerase II and upregulation of histones H3 and H4 acetylation status [55]. In contrast, HDAC9 might be recruited by MEF2 (myocyte enhancer factor 2) to the γ-globin gene promoter to mediate γ-globin activation and HbF synthesis during erythroid maturation of K562 cells [56]. Identification of HDACs-containing complex in association with globin gene switching may provide more molecular targets for intervening β-globin gene disorders.
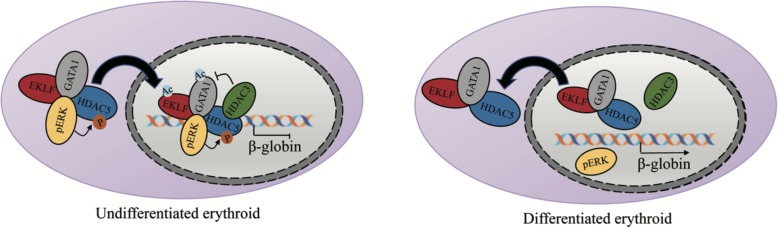
The function of class II HDACs that shuttle other proteins between the cytoplasm and nucleus is critical for erythropoiesis. For instance, Watamoto et al. found that although HDAC5 didn’t have deacetylation activity, it could shuttle GATA1 and EKLF from the cytoplasm to the nucleus via the formation of an erythroid-specific HDAC complex composed of HDAC5, GATA1, EKLF and ERK. Within the complex, the levels of p-ERK determine the shuttling activity of HDAC5. HDAC5 regulates the deacetylation levels of GATA1 and EKLF indirectly via the recruitment of HDAC3 to the complex [4, 5]. During erythroid maturation, HDAC5, GATA1 and EKLF remain associated, but the levels of pERK sharply decrease, which inhibits γ-globin expression [4] (Fig. 5). Furthermore, erythropoietin signaling induces the phosphorylation of HDAC5 via PKD, which promotes the dissociation of HDAC5 from GATA1 and GATA1 acetylation. Mice lacking HDAC5 showed resistance to anemic challenge, enhanced progenitor entry into the erythroid lineage and accelerated erythroid maturation in response to erythropoietin [9]. HDACs are also involved in the enucleation process of erythroid terminal differentiation. To date, HDAC6 and HDAC2 have been shown to play a role in chromatin condensation and enucleation. The former could promote enucleation via deacetylation of mDia2 (mammalian Diaphanous-related formin) and formation of the contractile actin ring (CAR). While HDAC2 specifically enhances enucleation but not differentiation or proliferation, where potential mechanism still needs to be further investigated [57, 58].
Fig. 5.
A model of class II HDAC interaction patterns in erythroid differentiation.
Lymphocyte lineages terminal differentiation
The terminal differentiation of lymphoid lineages originates from common lymphoid progenitors (CLPs), which can be further divided into all-lymphoid progenitors (ALPs) and B cell-biased lymphoid progenitors (BLPs). ALPs retain the potential to generate B cells, T cells, natural killer cells and lymphoid dendritic cells, whereas BLPs are biased toward pre-B cells and immature B cells, and the latter migrate to the spleen to complete their maturation [59–61] (Fig. 2).
B cell
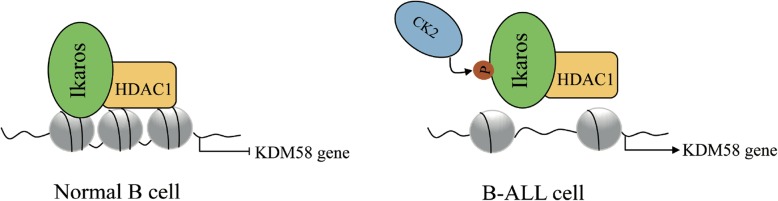
In early B cell progenitors, simultaneous deletion of HDAC1 and HDAC2 resulted in a dramatic block in B cell development at the pre–B cell stage by inducing p21 and p57 expression, accompanied by G1 arrest and apoptosis induction [62]. During terminal B cell development, Blimp-1 represses c-myc via recruiting HDAC1 and HDAC2. In mature B cells, although the loss of both HDAC1 and HDAC2 has no effect on viability, the cells fail to proliferate and undergo apoptosis [63, 64]. Similarly, in Eμ-myc-driven B cell lymphomas, the ablation of HDAC1 and HDAC2 prevents Eμ-myc tumorigenesis by decreasing proliferation and inducing apoptosis [65]. TFs have been found to mediate cross-talk between co-regulators in B cell development. For instance, the normal recruitment of HDAC1 by Ikaros is critical for the repression of the demethylase KDM58. In B-ALL, Casein kinase 2 (CK2)-mediated phosphorylation of Ikaros decreases HDAC1 recruitment to the KDM58 gene, which enhances KDM58 expression and leukogenesis [66, 67] (Fig. 6). For pregerminal center (GC) B cell differentiation, BCL6 RD2 domain-dependent recruitment of HDAC2 mediates repression of the trafficking receptors S1pr1 and Gpr183, contributing to the clustering of B cells within follicles [68]. Conditional knockdown of HDAC3 in early progenitor B cells of mice resulted in impaired B cell maturation and a defect in VDJ recombination [69]. Specifically, HDAC3 is implicated in different complexes at different B cell differentiation stages. For example, in pro-B cells, HDAC3 was identified as a component of the STAT5a-LSD1 complex, where it plays dual roles in determining the activation or repression of STAT5a [70]. In mature B cells, Bach2 recruits the HDAC3-NCoR1/NCoR2-Rif1 complex to repress Prdm1 transcription by deacetylating histone H3-K9, impeding the terminal differentiation of B cells into plasma cells [71].
Fig. 6.
A model of TF modification affects the recruitment of HDAC to the promoter.
HDAC-BCL6 complexes are implicated in the pathogenesis of lymphoma. For example, in normal GC B cells, CREBBP-regulated/active enhancers are counter regulated by the BCL6-SMRT-HDAC3 complex through a poised H3K27 deacetylation. However, in follicular lymphoma (FL) and diffuse large B cell lymphoma (DLBCL), CREBBP mutations disable its acetylation and result in unopposed deacetylation by the BCL6-SMRT-HDAC3 complex at enhancers of B cell signal transduction and immune response genes, thus promoting lymphomagenesis [72, 73]. In B cell non-Hodgkin lymphoma (B-NHL), aberrant expression of the HDAC9-BCL6 complex contributes to lymphomagenesis by altering pathways involved in proliferation and survival, as well as modulating BCL6 activity and p53 tumor suppressor function [74]. In contrast, as a corepressor partner of BCL6, HDAC4 plays an important role in suppressing leukemogenesis in complexes with BCL6 that recruit HDAC4 to repress oncogenes [75]. For example, in miR-155-induced pre-B cell leukemia/lymphoma, miR-155 directly targets HDAC4 and causes disruption of the HDAC4-BCL6 complex activity, resulting in derepression of BCL6 targets that block B cell development at an immature B cell stage and induce uncontrolled cell proliferation [76]. Hence, the interactions with different HDACs could confer different functional properties to TFs.
Class IIa HDAC7 plays a physiological role in the lineage commitment of B cell progenitors. Conditional deletion of HDAC7 in mouse pro–B cells showed a block at the pro–B to pre–B cell transition, accompanied by severe lymphopenia in peripheral organs and pro-B cell lineage promiscuity. HDAC7 specifically interacts with the transcription factor MEF2C in pro-B cells, and the HDAC7-MEF2C complex is involved in silencing lineage-inappropriate genes, ensuring correct B cell differentiation. In particular, HDAC7 is frequently underexpressed in pro-B-ALL and Burkitt lymphoma. Ectopically expressed HDAC7 interacts with the MEF2C-HDAC3-SMRT complex and suppresses c-Myc expression in both MEF2C- and self-catalytic activity–dependent manners [61, 77, 78]. Conversely, HDAC7 appears to overexpressed in pre-B-ALL t (9;22), B-ALL t (8;14), ALL t (12;21) and T-ALL (Fig. 7), which suggest different expression patterns for HDACs in different hematological malignancies. In addition, HDAC6 is suggested to play a role in the regulation of immunogenicity in CLL and MM. The HDAC6 inhibitor ACY1215 or HDAC6 specific silencing resulted in the downregulation of PD-L1 in primary B cells isolated from CLL patients and restoration of CD4:CD8 ratio [79]. Similarly, the HDAC6 inhibitor ACY241 significantly reduces the frequency of CD138+ MM cells, CD4 + CD25 + FoxP3+ regulatory T cells, and decreases expression of PD1/PD-L1 on CD8+ T cells in bone marrow cells from myeloma patients [80]. More recently, the combination of HDAC6 inhibitor and anti-PD-L1 antibody can trigger cytotoxic T lymphocytes and NK cell-mediated MM cell killing. A combination of HDAC6 inhibitor, anti-PD1 and lenalidomide further enhanced the anti-MM immune response of MM cells induced by HDAC6 inhibition [81]. Although some HDACis such as Panobinostat has been shown to exert toxic effects on lymphocytes while several studies showed a stimulation of CD8+ T-cells activation and function upon pan-HDACi treatment [82]. These studies suggest that reasonable selection of HDACi is critical for immunotherapy in hematological malignancies.
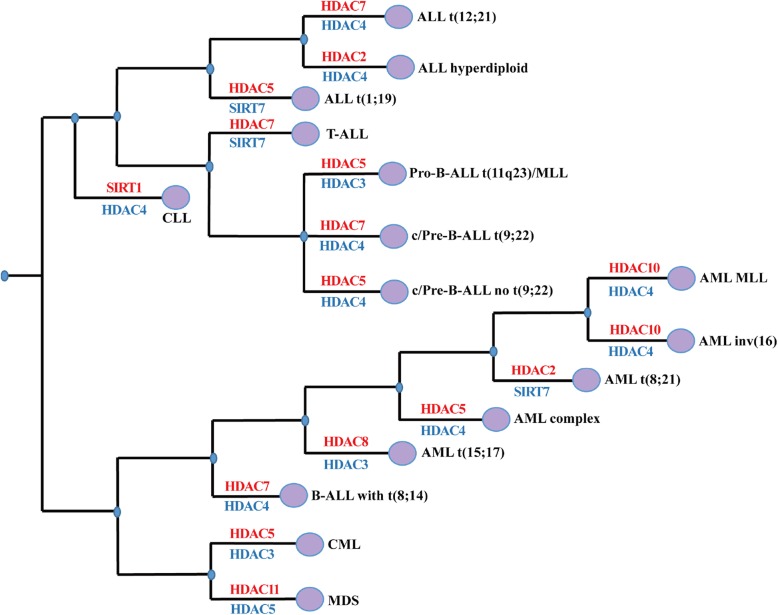
Fig. 7.
Abnormal gene expression of HDACS in different hematological malignancies.
T cell
During T cell development, HDAC1 and HDAC2 are essential for maintaining CD4 lineage integrity by inhibiting Runx3-CBFβ complexes that induce CD8 lineage programs in CD4+ T cells. Loss of HDAC1 and HDAC2 at the early stages of T cell differentiation result in a severe reduction in thymocyte numbers due to a block in cell cycle progression at the pre-TCR stage. Loss of these cells during late T cell development leads to reduced numbers of peripheral T cells and the appearance of CD4+ CD8+ T cells (TH) [83]. Similarly, Dovey et al. found that disruption of the HDAC1/2-Sin3A-NuRD complex by deletion of HDAC1 and HDAC2 in T cells of neonatal mice resulted in a marked reduction in thymocyte cellularity, a block in double-negative (DN) to double-positive (DP) transition, and a failure to proliferate in response to T cell receptor (TCR) signaling. Moreover, HDAC1/2 haploinsufficiency in mice causes a lethal pathology by T cell lymphomas with global histone acetylation and chromosomal instability [84], indicating an essential role for HDAC1/2 in the development of mature T cell populations and in maintaining genome stability.
HDAC3 is required for multiple stages in T cell development, including CD4 and CD8 lineage commitment, positive selection and peripheral T cell maturation. Specifically, HDAC3-deficient DP thymocytes fail to induce the CD4-lineage program and accelerate the redirection of MHC class II-restricted thymocytes to the CD8 lineage prior to positive selection with an increase in histone acetylation of CD8-lineage genes, such as Runx3 and Patz1 [85]. HDAC3 is required for positive selection of thymocytes. T cell development in CD2-icre HDAC3 conditional knockout (cKO) mice was blocked at positive selection due to a failure to downregulate RORγt (retinoic acid-related orphan receptor) and upregulate Bcl-2, which led to few CD4 and CD8 T cells. ChIP assays revealed that HDAC3 directly deacetylates histones to inhibit RORγt gene expression. The deletion of RORγt and transgenic expression of Bcl-xl corrects the positive selection defect in HDAC3-cKO mice [86]. HDAC3 is also required for T cell development from DN stage 4 into the early CD4/CD8 DP stage. The deletion of HDAC3 in the DN stage of thymocyte development by Lck-Cre-transgene caused a significant impairment at the CD8 immature single-positive (ISP) stage and the CD4/CD8 (DP) stage. When HDAC3−/− mice were crossed with Bcl-xl-, Bcl2-, or TCRβ-expressing transgenic mice, CD4 and CD8 SP cells were partially rescued [87]. The NKAP-HDAC3 complex is required for post-thymic T cell maturation. The majority of HDAC3-deficient naïve T cells are recent thymic emigrants (RTEs), which cannot become long-lived naïve T cells. HDAC3-deficient peripheral T cells have a defect in TNF licensing after TCR/CD28 stimulation [88]. Moreover, the HDAC3-NCoR1/2-SMRT complex is essential for the normal development and suppressive functions of thymic and peripheral FOXP3+ T regulatory cells (Tregs) via association with FOXP3. The enzymatic activity of HDAC3 can be enhanced by NCoR1 or NCoR2/SMRT, which, in turn, deacetylates histone H3 at the IL-2 promoter and inhibits IL-2 transcription in FOXP3+ Tregs [89]. Therapy with pan HDACi such as TSA or SAHA can stimulate the thymic production of FOXP3+ Tregs and promote the peripheral conversion of murine and human T cells into Tregs [90].
Class IIa HDAC7 plays a role in life/death decisions in thymic T cell development. HDAC7 is exported from the nucleus by PKD during positive selection in thymocytes, and it regulates genes mediating the coupling between TCR engagement and downstream events that determine cell survival. Thymocytes lacking HDAC7 are inefficiently positively selected due to a severely shortened lifespan and exhibit a truncated repertoire of TCR Ja segments [91, 92]. Class IIa HDAC5 is implicated in Treg homeostasis. HDAC5−/− mice showed reduced suppressive function and a decrease in Foxp3 in Tregs. CD4+ T cells lacking HDAC5 impair the ability of T effector cells to convert into induced Tregs. CD8+ T cells missing HDAC5 have a reduced ability to produce the cytokine of IFN-γ [93]. Class IV HDAC11 serves as a negative regulator of the T effector cell phenotype and function. T cells lacking HDAC11 show increased proliferation and proinflammatory cytokine production, such as IL-2 and IFN-γ, and inhibited tumor progression in murine lymphoma [94]. Specifically, HDAC11 and HDAC6 physically interact with each other and are simultaneously recruited to the IL-10 gene in antigen-presenting cells (APCs), where HDAC6 and HDAC11 act as a transcription activator and repressor of IL-10 expression, respectively [95]. Their dynamic interaction and the dynamic changes in the expression of IL-10 are suggested to explain the intrinsic plasticity of APCs in determining T cell activation versus T cell tolerance.
The clinical implications of HDACis in malignant hematopoiesis
Aberrant expression of HDACs is linked to hematological malignancies, such as leukemias and lymphomas (Fig. 7). Specifically, the overexpression of HDAC5 and HDAC7 is associated with ALL, CML and AML. Low expression of HDAC4 is widespread in ALL, CML and AML [96–98]. To date, little is known about the mutation status and copy number alteration of HDACs in malignant hematopoiesis. However, by analyzing the TCGA database, we found that HDAC1, 4, 7 exhibited both gene mutations and CNAs in DLBCL, suggesting a key etiology for these HDACs (Table 2). However, their pathogenic mechanisms still need to be further investigated.
Table 2.
HDACs with mutations or abnormal copy numbers in hematological malignancies
| Gene mutations | Copy number alteration (CNA) | |||||||
|---|---|---|---|---|---|---|---|---|
| Disease | Gene | Mutation number | Case number with mutation | Percentage (total number) | Cytoband | Type of CNA | Case number with CNA | Percentage (total number) |
| AML | HDAC4 | 2 | 2 | 0.3% (622) | NA | NA | NA | NA |
| HDAC7 | NA | NA | NA | 12q13.11 | DEL (deletion) | 1 | 0.5% (191) | |
| CLL | HDAC4 | 1 | 1 | 0.2% (506) | NA | NA | NA | NA |
| DLBCL | HDAC1 | 1 | 1 | 0.7% (135) | 1p35.2-p35.1 | DEL (deletion) | 1 | 2.1% (48) |
| HDAC4 | 3 | 2 | 1.5% (135) | 2q37.3 | AMP (amplifications) | 1 | 2.1% (48) | |
| HDAC7 | 1 | 1 | 0.7% (135) | 12q13.11 | AMP (amplifications) | 2 | 4.2% (48) | |
| DEL (deletion) | 1 | 2.1% (48) | ||||||
| MM | HDAC7 | 1 | 1 | 0.5% (205) | NA | NA | NA | NA |
| NHL | HDAC7 | 1 | 1 | 7.1% (14) | NA | NA | NA | NA |
Notes: NA Not applicable; All data come from the TCGA database
Furthermore, HDACs are critical for the optimal oncogenic activity of leukemia fusion proteins. For example, AML1-ETO, PML-RARα and RARα-PLZF cause transcriptional repression of genes responsible for hematopoietic differentiation via recruitment of HDAC1/3, thus contributing substantially to leukemogenesis [99–107]. Given that the expression and activity of HDACs are closely related to the etiology of hematological malignancies, HDACs are hot targets for clinical drug development.
The application of HDACis in malignant hematopoiesis
HDACis represent a class of cytostatic agents that interfere with the function of HDACs and are able to directly or indirectly regulate gene expression by inducing acetylation of histones or nonhistone proteins, involving cell-cycle arrest, promotion of differentiation or apoptosis and have different kinetics and activities depending on their chemical structures (Fig. 8). Generally, normal cells are often less sensitive to HDACis than tumor cells, and many HDAC inhibitors are undergoing extensive clinical evaluation as single agents and in combination with other chemotherapeutics [108, 109]. To date, panobinostat and belinostat have received FDA approval for the treatment of MM and NHL respectively. In addition, panobinostat, belinostat, romidepsin, entinostat and mocetinostat are in phase I, II or III clinical trials alone or in combination with other drugs for the treatment of other hematological malignancies (Fig. 1 and Table 3). Although, hydroxamate-based HDACis attract much attention in development of HDACi inhibitors, based on their remarkable zinc chelating capability. Nevertheless, it should be noted that some pan-HDACis, like romidepsin, panobinostat and vorinostat, display adverse effects, such as poor oral absorption, metabolic and pharmacokinetic problems because of glucuronidation, sulfation and enzymatic hydrolysis that lead to a short in vivo half-life [109]. Moreover, hydroxamate group can give rise to multiple off-target and mutagenic effects resulting from the coordination of other metalloenzymes, leading to undesirable adverse effects, such as nausea, thrombocytopenia, anemia and other metabolic issues, which may limit their clinical applications and promote the development of a new class of HDAC isoform-selective antagonists with reducing adverse effects [7, 110].
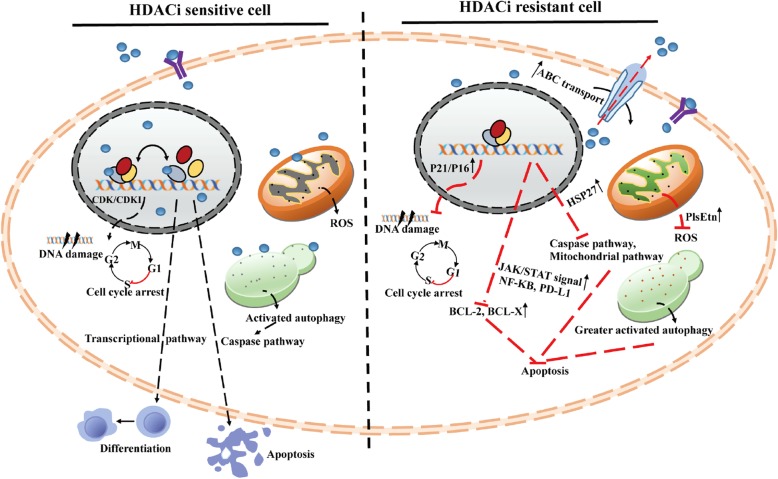
Fig. 8.
Sensitivity and resistance mechanisms of hematological malignancies to HDACis.
Table 3.
HDACis in combination with other anticancer agents in phase I/II/III clinical trials
| HDACis | Combination(s) | Cancer(s) | Clinical trial |
|---|---|---|---|
| Vorinostat | Sorafenib | AML, APL, MDS | I |
| Carfilzomib | B-cell lymphoma | I | |
| Zolinza | Lymphoma or Leukemia | I/II | |
| Azacitidine | AML,MDS | II | |
| Temozolomide | AML | II | |
| Rituximab | Lymphoma | II | |
| Decitabine | AML, ALL, CLL, Lymphoma | I | |
| MDS | II | ||
| Alisertib | Lymphoma | I | |
| Alvocidib | AML, CML, ALL | I | |
| Isotretinoin | APL,AML, Lymphoma | I | |
| Idarubicin | AML, CML, MDS | I | |
| Idarubicin, Cytarabine | AML, MDS | II | |
| Sorafenib, bortezomib | AML | I/II | |
| Cytarabine, Decitabine | AML, MDS | I | |
| AMG655, Bortezomib | Lymphoma | I | |
| Lenalidomide, Azacitidine | CML, MDS | II | |
| Lenalidomide, Dexamethasone | MM | I | |
| Bortezomib, Dexamethasone | MM | II | |
| Gemtuzumab, Ozogamicin, Azacitidine | AML | I/II | |
| Tacrolimus, Cyclosporine, Methotrexate | CML, AML, Lymphoma | II | |
| Panobinostat | Carfilzomib | MM | I/II |
| Bortezomib | T-cell lymphoma, MM | II, I | |
| Everolimus | Lymphoma | I/II | |
| Lenalidomide | HL, MM | II, I | |
| Placebo | HL | III | |
| Melphalan | MM | I/II | |
| Cytarabine | Leukemia, NHL | I | |
| 5-Azacytidine | AML, MDS, CMML | I | |
| Decitabine | AML, MDS | I/II | |
| Everolimus | Lymphoma | I/II | |
| Imatinib mesylate | Leukemia | I | |
| Lenalidomide, Dexamethasone | MM | II | |
| Carfilzomib, Dexamethasone | MM | I | |
| Bortezomib, Dexamethasone | MM | II | |
| Bortezomib, Placebo | MM | III | |
| Dexamethasone, MLN9708 | MM | II | |
| Dexamethasone, Lenalidomide, Bortezomib | MM | I | |
| Cytarabine, Daunorubicin | AML, MDS | I | |
| Ifosfamide, Mesna, Carboplatin, Etoposide, Pegfilgrastim | HL | I/II | |
| Belinostat | Carfilzomib | Peripheral T-cell lymphoma, NHL, DCBCL, FL | I |
| Rituximab | Lymphoma | II | |
| Idarubicin | AML | I/II | |
| Bortezomib | AML,ALL,MDS,CML | I | |
| VPA | Decitabine | AML, MDS | II |
| 5-azacytidine | AML, MDS | II | |
| Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, Prednisone | DLBCL | I/II | |
| Romidepsin | Gemcitabine, dexamethasone and cisplatin | DCBCL | I |
| 5-azacitidine | Relapsed/refractory lymphoid maligancies | I/II | |
| Mocetinostat | Brentuximab vedotin (SGN-35) | HL | I/II |
| Azacitidine | MDS, AML | I/II | |
| AR42 | Decitabine | AML | I |
| Pomalidomid | MM | I | |
| Entinostat | Sorafenib tosylate | AML | I |
| 4-PBA | Azacitidine | AML, MDS | I |
| SB939 | Azacitidine | Hematologic Malignancies, MDS | I |
Acquirement of the crystal structure of a given HDAC isoform combined with kinetic studies may contribute to overcome the structural homology between HDACs. For example, the availability of crystal structures of unique catalytic channels, such as catalytic domains CD1 and CD2 of HDAC6 and an acetate release channel of HDAC8, provided a unique strategy to develop their selective antagonists [111]. For the entrance ring area of catalytic channel, isoform selectivity could be achieved by designing zinc-binding groups bearing substituents that make specific interactions into the foot pocket (HDAC1–3) or into the lower pocket (class IIa HDACs) of a given isoform. For example, replacement of serine 107 by tyrosine in HDAC3 leads to a spatially restricted foot pocket that can be exploited to develop antagonists selective for class I HDACs [112]. Furthermore, the choice of surface-binding motifs that make specific interactions with the external characteristic grooves of the desired isoform, or targeting specific surfaces between HDACs and interacting partners that are critical for efficient deacetylase activity, such as the Ins (1,4,5,6) P4 binding site for HDAC1–3 or the CCHC zinc-binding motif for class IIa HDACs, might contribute to gain selectivity [113]. Finally, subtle structural differences in the hydrophobic active site channel have been exploited toward selective inhibitor design. Specifically, favorable interactions with a unique sub-pocket in the hydrophobic active site channel led to the creation of HDAC8-selective inhibitors [7, 114].
In addition, overcoming tumor heterogeneity and drug resistance promotes the development of combination treatment for HDACis. For example, a phase I study of vorinostat with decitabine-treated R/R AML patients who had mixed lineage leukemia (MLL) demonstrated a 35% composite complete response (CRC) rather than a 17% overall response rate (ORR) of vorinostat monotherapy [115]. Hence, combining HDACis with other chemotherapeutic agents is considered to be an effective way to enhance tumor drug sensitivity by improving the cellular efficacy and toxicity of HDACis to tumor cells [116–125] (Table 3 and Table 4). To date, the different mechanisms of HDACis combined with chemotherapeutic agents such as topoisomerase inhibitors, platinum-based chemotherapeutics, proteasome inhibitors, tyrosine kinase pathway inhibitors and epigenetic modifiers for advanced or drug-resistant hematological malignancies include (1) acetylating histones and inducing p21-CDK-mediated cell cycle arrest; (2) inducing apoptosis by regulating the expression of pro- and antiapoptotic genes through the intrinsic or extrinsic pathway; (3) inducing DNA damage and oxidative stress; (4) activating BTK (in CLL) or inhibiting ERK (in MM) and AKT (in CML) signaling pathways; and (5) regulating the expression of drug resistance-related molecules, such as downregulating BCR-ABL and upregulating Bim in hematological malignancies and downregulating CD44 in multiple myeloma (MM), NF-κB in ALL, γ-catenin in CML, and BRCA1, CHK1 and RAD51 in AML [126–145]. Specific combination strategies and their corresponding mechanisms are summarized in Table 3 [146–155]. Moreover, two-phase I clinical trials were carried out to assess the DNA methyltransferase (5-azacitidine) and HDACi (phenylbutyrate) for the treatment of hematological malignancies. A combination of BCL6 inhibitor (RI-BPI) with HDAC inhibitor (HDI) enhanced RI-BPI killing of primary human DLBCL cells in vitro [156, 157]. These studies suggest that the combination of HDACis with HDAC-interacting molecule inhibitors, such as TF inhibitors, chromatin remodeling molecule inhibitors or histone/DNA-modifying co-regulator inhibitors, is a potential combination strategies for hematological malignancies. However, optimizing combination scheduling and doses are necessary for avoiding pharmacological antagonism. For example, the combination of Vorinostat, Bortezomib and Pegylated liposomal doxorubicin (PLD) is suffering from the withdraw in phase I trials of MM. Since whole blood proteasome activity assays demonstrated a potential impact of Vorinostat on the chymotryptic-like activity of the proteasome [158].
Table 4.
Mechanisms of HDACis combined with other agents in treating malignant hematopoiesis at preclinical settings
| HDACis | Combination(s) | Cancer(s) | Mechanism(s) |
|---|---|---|---|
| Vorinostat (SAHA) | Bortezomib | Relapsed/refractory MM | Increasing p21 and cleaved PARP expression |
| T-ALL | Inhibiting NF-κB signaling | ||
| Carfilzomib or Bortezomib | Relapsed/refractory B cell lymphomas | Decreasing NF-κB activation and increasing Bim levels | |
| Rituximab | Lymphoma/leukemia | Increasing in p21 and acetylation of histone H3 leading to cell cycle arrest | |
| ABT-737 | DLBCL | Inhibiting binding of BH3-only modulators and proapoptotic activators | |
| MG-132 | Imatinib-resistant CML | Increasing intracellular ROS and repressing BCR-ABL expression | |
| S116836 | Imatinib-resistant CML | Repressing antiapoptosis proteins Mcl-1 and XIAP, promoting Bim expression and mitochondrial damage | |
| BI2536 | Imatinib-resistant CML | Triggering pronounced mitochondrial dysfunction, generating reactive oxygen species (ROS) and DNA damage | |
| KW-2449 | Imatinib-resistant CML / AML | Inhibiting Bcr/Abl and inducing ROS and DNA damage | |
| ABT-737 | Emu-myc lymphomas | Repressing BCR-ABL expression | |
| Idarubicin + Cytarabine | Advanced AML or Aza–resistant MDS | Generating reactive oxygen species (ROS) | |
| Panobinostat (LBH589) | Bortezomib | Relapsed/refractory TCL | Increasing acetylation of HSP90, downregulating mitogen-activated protein kinase pathway signaling |
| Carfilzomib | Relapsed/refractory MM | Inhibiting p97, HDAC or PI3Kα | |
| Ibrutinib | Relapsed/refractory MM | Generating ROS and inactivating ERK1/2 | |
| ABT-199 | Ibrutinib-resistant CLL | Reducing BTK/mutated BTK protein and signaling | |
| Ponatinib or Imatinib | AML | Upregulating Bim expression | |
| Everolimus | Imatinib-resistant CML | Forcing histone acetylation and decreasing BCR-ABL and AKT signaling | |
| Everolimus | HL/NHL | Activating the caspase pathway, inhibiting STAT5 and STAT6 phosphorylation, GLUT1 and mTOR | |
| Romidepsin | Rituximab | Rituximab-resistant BL | Decreasing phosphorylated STAT3 binding to the MyD88 promotor |
| ExPBNK | BL | Reducing p38 MAPK phosphorylation and enhancing MICA/B expression | |
| Ara-C | AML | Enriching Myc- and HOXA9-regulated gene pathways and inducing cell cycle arrest and DNA damage | |
| ATRA | APL | Inducing p21-mediated cell-cycle arrest and the expression of MDR1 | |
| Gemcitabine, cisplatin and dexamethasone | DLBCL | Reducing LMP1 and c-myc expression | |
| Belinostat | Vincristine or Paclitaxel | DLBCL | Inducing mitosis arrest and apoptosis |
| Bortezomib | AML / ALL | Inhibiting NF-κB signaling and upregulating Bim expression | |
| Entinostat | Sorafenib | Refractory/relapsed AML | Inhibiting HOXA9, MEIS1 and FLT3 |
| KW-2449 | Imatinib-resistant CML / AML | Inhibiting Bcr/Abl, inducing ROS and DNA damage | |
| Valproic acid | Decitabine | AML or MDS I/II | Inducing cell cycle arrest, DNA damage and apoptosis |
| TRAIL/Apo2L | TRAIL/Apo2L-resistant CML | Increasing DR4 and DR5 expression | |
| ABT-737 | Emu-myc lymphomas | Restricting Bcl-2 and Bcl-XL | |
| Chloroquine (CQ) | AML | Inducing RASSF1A expression and inhibiting autophagy | |
| MGCD0103 | Cytarabine or daunorubicin | AML | Inducing DNA damage and apoptosis |
| Brentuximab vedotin | Relapsed/refractory HL | N/A | |
| Azacitidine | High-risk MDS or AML | Increasing p15 and caspase-3 expression | |
| AR-42 | Decitabine | M5 subtype-AML | Elevating miR-199b expression |
| Lenalidomide | Lenalidomide-resistant MM | Upregulating miR-9-5p, downregulating IGF2BP3 and CD44 | |
| Depsipeptide | ATRA | APL | Upregulating of MDR1 and inducing p21-mediated cell cycle arrest |
| SBHA | ABT-737 | Relapsed/refractory MM | Upregulating Bim expression and disabling cytoprotective autophagy |
| JSL-1 | Imatinib | Imatinib-resistant CML | Inhibiting γ-catenin |
| Sodium phenylbutyrate | Azacitidine | AML or MDS | Reducing endoplasmic reticulum (ER) stress and ablating CHOP protein |
Notes: NA Not applicable
NIH clinical trial database: www.clinicaltrials.gov. (These trials have been completed or are in active).
Drug resistance mechanisms
Although HDACis play a tremendous role in improving patient survival and symptom control, in most cases, hematological malignancy cells develop drug resistance to HDACis, resulting in malignant phenotype regeneration and maintenance. Resistant-related proteins and abnormal in epigenetic or genetic factors and pathways are implicated in resistance to HDACis, including drug efflux, target status, chromatin alteration, upregulation of oxidative stress response mechanism, defects in proapoptotic pathways, and upregulation of antiapoptotic signals/stimuli (Fig. 8). For instance, SAHA induced multidrug resistance-related ABC transporter genes (MDR1, BCRP, MRP7, and MRP8) in leukemia cells. Overexpression of these cellular pumps has side effects on broad-spectrum drug resistance and cell intake. Changing the permeability proprieties of HDACis, adjusting the sequence of treatment or adopt nano-packaging materials may improve the efficacy of HDACis [159]. Furthermore, HSP72, as the most overexpressed protein in CTCL cell lines, induces chemoresistance against SAHA and VPA by suppressing the activation of caspase-3/8/9 and the mitochondrial pathway of Bcl-2 and reducing HDACi-induced histone H3 acetylation [160]. Highly elevated peroxisomes protect vorinostat-resistant lymphoma cells from ROS damage via two antioxidant mechanisms: (1) upregulating catalase and (2) increasing the levels of plasmalogens (PlsEtn) and related genes (such as GNPAT, FAR1 and FAR2) [161]. High levels of HSPA1A is associated with VPA resistance in lymphoid neoplasms. Inhibition of HSPA1A by KNK-437 could resensitize cells to VPA-induced apoptosis [162]. Furthermore, the phosphoproteins MAPKAPK2, ACTB, HSP90AA1 and HSP90AB1 were considered resistance hubs in VPA-resistant AML cell lines [163].
Altered levels of antiapoptotic proteins drive resistance against HDACi-mediated apoptosis. Specifically, it has been observed that increased JAK/STAT signaling negatively affects HDACi-induced death of CTCL cells and that high levels of phosphorylated STAT 3 is correlated with a lack of response to vorinostat [164]. Consistently, elevated levels of the antiapoptotic proteins BCL-2 and Bcl-xL show strong resistance to vorinostat-induced apoptosis in DLBCL cell lines [119]. Similarly, NF- κB upregulation confers Hodgkin’s lymphoma (HL) cell resistance to MGCD0103 and panobinostat by interfering with apoptosis [165]. Moreover, significant induction of NF-κB and its upstream regulator PD-L1 were found to be related to the resistance of myelodysplastic syndrome (MDS) and AML to LBH-589 therapy [166]. CDK inhibitors also act as key resistance-inducing factors. P21 and p27 have sustained overexpression in DLBCL, inhibiting cell cycle arrest and cell death induced by PXD101 [167]. Overexpression of p21 and p16 induces G1 arrest, increases SAHA- and depsipeptide-induced antiapoptotic genes and decreases proapoptotic genes in acute T cell leukemia cells [168]. Upregulation of p21 in acute promyelocytic leukemia (APL) cells attenuates HDACi-induced DNA damage and cell cycle arrest, which is correlated with DNA repair [167].
The generation of ROS is one of the key mechanisms by which HDACis induce cell death in malignant cells. However, the increased expression of vorinostat-induced antioxidant genes, such as glutathione (GSH), glutamate cysteine ligase (GCL) and superoxide dismutase (SOD), is related to HDACi resistance in advanced AML and MDS [169, 170]. In addition, properly activated autophagy promotes apoptosis in HDACi-treated cells. Conversely, a study showed that excessive activation of autophagy is necessary to protect the vorinostat-resistant lymphoma cell line and DLBCL cell line from apoptosis. HDAC6 deacetylation of HSP90 mediates chaperone complex assembly, and excessive autophagy to remove accumulated misfolded/aggregated proteins is considered a protective mechanism of autophagy against vorinostat-resistant cells [171]. To date, remarkably little is known about HDACi-induced multidrug resistance at single-cell levels. Elucidation of resistant mechanisms using a series of single-cell sequencing may contribute to overcome tumor heterogeneity for HDACi-resistance.
Conclusion
As key deacetyltransferase subunits of multiprotein complexes, they regulate histone affinity for DNA and chromatin accessibility to their cognate binding proteins by compaction of DNA/histone complexes. Their biochemical and molecular characterization significantly affects the deacetyltransferase activity of HDAC-containing complexes. Importantly, the catalytic/noncatalytic and histone/nonhistone effects of HDACs on hematopoietic cells confer their ability to regulate a variety of cellular events in normal and malignant hematopoiesis. HDAC actions are gene or environment specific during hematopoiesis: (1) Different genes regulated by the same HDAC require the recruitment of different coregulators. For instance, HDAC1 has been found in at least three multiprotein complexes, including Sin3, CoREST and NuRD complexes. (2) One HDAC can act as a coactivator or corepressor on different genes and utilize different domains to act on interacting proteins. For instance, HDAC1-containing NuRD/MeCP1 corepressor complexes play an important role in GATA-1-mediated repression of target genes (i.e., GATA-2, γ-globin, c-myc, c-kit and Hes1), which are all required for the proliferation of hematopoietic progenitors. However, during GATA-1-mediated activation of the β-globin gene, the HDAC1/NuRD/MeCP1 complex is still recruited to the GATA-1 sites of the β-globin locus. (3) HDACs act as multifunctional regulators of transcription complex activity. For instance, HDAC1 can be acetylated by histone acetyltransferase p300. Acetylated HDAC1 not only loses its deacetylase activity but also inhibits the deacetylase activity of HDAC2, thereby downregulating the overall deacetylase activity of HDAC1/2-containing complexes, including the NuRD complex.
Epigenetic changes that occur during the development of hematopoietic malignancies are reversible and amenable to pharmacological intervention. The abnormal activity and expression of HDACs or the occurrence of aberrant composition in HDAC-containing transcriptional complexes could lead to malignant hematopoiesis via hyperproliferation and/or blocks in differentiation. However, the molecular basis for hematopoietic transformation, malignant development and drug resistance by HDACs are still largely unclear. For example, although it has been found that the activity of HDACs is regulated through posttranslational modifications, i.e., CBP/p300, and HDAC1 gene expression is regulated by the C/EBP family, GATA1 and Sp1, the misregulation in HDAC expression by multiple layers of regulation mechanisms, such as a given mutation or epigenetic modifier, are largely unknown. In some cases, the function of oncogenic TFs and fusion proteins is reliant on direct interactions with HDAC-containing complexes. For example, transcriptional and differential repression of several transcriptional fusion proteins with key roles in the progression of acute leukemias, such as AML1/ETO, STAT5/RARa, and PLZF/RARa fusion proteins, is mediated by the aberrant recruitment of corepressor complexes in the N-CoR/mSin3/HDAC1 complex. Hence, many HDAC–TF/cofactor interaction surfaces represent compelling therapeutic targets.
In hematopoietic systems, HDACis have shown synergistic or additive effects with numerous chemotherapeutic agents, such as proteasome inhibitors, hormonal therapy, tyrosine kinase inhibitors, DNA-hypomethylating agents, and immune checkpoint inhibitors, in preclinical and clinical settings. HDACis recently emerged as promising immunomodulatory drugs, like TMP195 and ACY241, via modulation of immune cell phenotypes or expression of immune checkpoints [80, 172]. Immune checkpoint inhibitors have revolutionized the treatment of hematological malignancies. Their combination with HDACis may be considered a major breakthrough in the treatment of hematological malignancies. Furthermore, more current research efforts are focused on developing HDAC isoform-selective inhibitors to improve toxicity against specific cancer types and overcome drug resistance or off-target effects. The availability of crystal structures of HDAC isoforms may provide a major contribution to understanding isoform selectivity.
Given that epigenetic regulation of HDACs globally affects the gene regulatory network, an ensemble of key hematopoietic HDACs has been identified. Future studies need to identify more regulatory factors that dysregulate HDAC expression and determine how their misexpression contributes to the pathogenesis of hematological malignancies. Identification of more structures of HDAC isoforms and epigenetic mechanisms, thereby targeting specific HDAC, HDAC pathways and HDAC-TF/cofactor interactions may represent ideal strategies to treat malignant hematopoiesis.
Acknowledgements
Not applicable.
Abbreviations
- ALL
Acute lymphocytic leukemia
- ALPs
All-lymphoid progenitors
- AML MLL
Acute myeloid leukemia with a Mixed Lineage Leukemia
- AML
Acute myeloid leukemia
- APCs
Antigen-presenting cells
- APL
Acute promyelocytic leukemia
- BFU-E
Burst-forming unit-erythroid
- BLPs
B cell-biased lymphoid progenitors
- BMDMs
Bone marrow-derived monocytes
- B-NHL
B cell non-Hodgkin lymphoma
- CAR
Contractile actin ring
- CFU-E
Colony-forming unit-erythroid
- CK2
Casein kinase 2
- cKO
Conditional knockout
- CLL
Chronic lymphocytic leukemia
- CLP
Common lymphoid progenitor
- CML
Chronic myeloid leukemia
- CMP
Common myeloid progenitor
- CRc
Composite complete response
- CTCL
Cutaneous T-cell lymphoma
- DCs
Dendritic cells
- DLBCL
Diffuse large B cell lymphoma
- DN
Double-negative
- DP
Double-positive
- FL
Follicular lymphoma
- GC
Germinal center
- GCL
Glutamate cysteine ligase
- GMCSFR
Granulocyte colony-stimulating factor
- GMP
Granulocyte-monocyte progenitor
- GSH
Glutathione
- HAT
Histone acetyltransferase
- HD
Hodgkin disease
- HDACis
Histone deacetylase inhibitors
- HDACs
Histone deacetylases
- HL
Hodgkin’s lymphoma
- HPCs
Hematopoietic progenitor cells
- HSC
Hematopoietic stem cell
- ISP
Immature single-positive
- LMPP
Lymphoid-primed multipotential progenitor
- MCSFR
Granulocyte-macrophage colony-stimulating factor
- MDS
Myelodysplastic syndrome
- MEP
Megakaryocyte- erythrocyte progenitor
- MLP
Multilineage progenitor
- MM
Multiple myeloma
- NHL
Non-Hodgkin lymphoma
- ORR
Overall response rate
- PlsEtn
Plasmalogens
- RORγt
Retinoic acid-related orphan receptor
- RTEs
Recent thymic emigrants
- SOD
Superoxide dismutase
- TCR
T cell receptor
- TF
Transcription factor
- Tregs
T regulatory cells
Authors’ contributions
ZW and PW designed this study. ZW and PW drafted the manuscript. JL, ZW and PW revised this manuscript. PW drew the figures. All authors read and approved the final manuscript.
Funding
This work was supported by the grants from National Key Research and Development Program of China (2108YFA0107800); National Natural Science Foundation of China [Grant numbers 81920108004, 81770107, 81470362 and 81702722]; National Postdoctoral Program for Innovative Talents [Grant number BX201700292]; Natural Science Foundation of Hunan Province [Grant number 2018JJ3703]; Science and Technology Key Project of Hunan Province [Grant number 2018SK21212]; Fundamental Research Funds for the Central Universities of Central South University [Grant number 2018zzts386].
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All of the authors are aware of and agree to the content of the paper and their being listed as a co-author of the paper.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
3/12/2020
After the publication of this work [1], the authors note that there are no figure legends in published paper.
Contributor Information
Zi Wang, Email: zhongnanwangzi@126.com.
Jing Liu, Email: jingliucsu@hotmail.com.
References
- 1.Greco TM, Yu F, Guise AJ, Cristea IM. Nuclear import of histone deacetylase 5 by requisite nuclear localization signal phosphorylation. Mol Cell Proteomics. 2011;10:M110.004317. doi: 10.1074/mcp.M110.004317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sahakian E, Chen J, Powers JJ, Chen X, Maharaj K, Deng SL, Achille AN, Lienlaf M, Wang HW, Cheng F, et al. Essential role for histone deacetylase 11 (HDAC11) in neutrophil biology. J Leukoc Biol. 2017;102:475–486. doi: 10.1189/jlb.1A0415-176RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah RR, Koniski A, Shinde M, Blythe SA, Fass DM, Haggarty SJ, Palis J, Klein PS. Regulation of primitive hematopoiesis by class I histone deacetylases. Dev Dyn. 2013;242:108–121. doi: 10.1002/dvdy.23906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varricchio L, Dell'Aversana C, Nebbioso A, Migliaccio G, Altucci L, Mai A, Grazzini G, Bieker JJ, Migliaccio AR. Identification of NuRSERY, a new functional HDAC complex composed by HDAC5, GATA1, EKLF and pERK present in human erythroid cells. Int J Biochem Cell Biol. 2014;50:112–122. doi: 10.1016/j.biocel.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watamoto K, Towatari M, Ozawa Y, Miyata Y, Okamoto M, Abe A, Naoe T, Saito H. Altered interaction of HDAC5 with GATA-1 during MEL cell differentiation. Oncogene. 2003;22:9176–9184. doi: 10.1038/sj.onc.1206902. [DOI] [PubMed] [Google Scholar]
- 6.Wada T, Kikuchi J, Nishimura N, Shimizu R, Kitamura T, Furukawa Y. Expression levels of histone deacetylases determine the cell fate of hematopoietic progenitors. J Biol Chem. 2009;284:30673–30683. doi: 10.1074/jbc.M109.042242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Micelli C, Rastelli G. Histone deacetylases: structural determinants of inhibitor selectivity. Drug Discov Today. 2015;20:718–735. doi: 10.1016/j.drudis.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Parra M, Verdin E. Regulatory signal transduction pathways for class IIa histone deacetylases. Curr Opin Pharmacol. 2010;10:454–460. doi: 10.1016/j.coph.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Delehanty LL, Bullock GC, Goldfarb AN. Protein kinase D-HDAC5 signaling regulates erythropoiesis and contributes to erythropoietin cross-talk with GATA1. Blood. 2012;120:4219–4228. doi: 10.1182/blood-2011-10-387050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yanginlar C, Logie C. HDAC11 is a regulator of diverse immune functions. Biochim Biophys Acta Gene Regul Mech. 1861;2018:54–59. doi: 10.1016/j.bbagrm.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Prasad P, Ronnerblad M, Arner E, Itoh M, Kawaji H, Lassmann T, Daub CO, Forrest AR, Lennartsson A, Ekwall K. High-throughput transcription profiling identifies putative epigenetic regulators of hematopoiesis. Blood. 2014;123:e46–e57. doi: 10.1182/blood-2013-02-483537. [DOI] [PubMed] [Google Scholar]
- 12.Fujiwara T, Lee HY, Sanalkumar R, Bresnick EH. Building multifunctionality into a complex containing master regulators of hematopoiesis. Proc Natl Acad Sci U S A. 2010;107:20429–20434. doi: 10.1073/pnas.1007804107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen GY, Osada H, Santamaria-Babi LF, Kannagi R. Interaction of GATA-3/T-bet transcription factors regulates expression of sialyl Lewis X homing receptors on Th1/Th2 lymphocytes. Proc Natl Acad Sci U S A. 2006;103:16894–16899. doi: 10.1073/pnas.0607926103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duan Z, Zarebski A, Montoya-Durango D, Grimes HL, Horwitz M. Gfi1 coordinates epigenetic repression of p21Cip/WAF1 by recruitment of histone lysine methyltransferase G9a and histone deacetylase 1. Mol Cell Biol. 2005;25:10338–10351. doi: 10.1128/MCB.25.23.10338-10351.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saleque S, Kim J, Rooke HM, Orkin SH. Epigenetic regulation of hematopoietic differentiation by Gfi-1 and Gfi-1b is mediated by the cofactors CoREST and LSD1. Mol Cell. 2007;27:562–572. doi: 10.1016/j.molcel.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 16.van Oorschot R, Hansen M, Koornneef JM, Marneth AE, Bergevoet SM, van Bergen M, van Alphen FPJ, van der Zwaan C, Martens JHA, Vermeulen M, et al. Molecular mechanisms of bleeding disorderassociated GFI1B(Q287*) mutation and its affected pathways in megakaryocytes and platelets. Haematologica. 2019;104:1460–1472. doi: 10.3324/haematol.2018.194555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kong S, Kim SJ, Sandal B, Lee SM, Gao B, Zhang DD, Fang D. The type III histone deacetylase Sirt1 protein suppresses p300-mediated histone H3 lysine 56 acetylation at Bclaf1 promoter to inhibit T cell activation. J Biol Chem. 2011;286:16967–16975. doi: 10.1074/jbc.M111.218206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heideman MR, Lancini C, Proost N, Yanover E, Jacobs H, Dannenberg JH. Sin3a-associated Hdac1 and Hdac2 are essential for hematopoietic stem cell homeostasis and contribute differentially to hematopoiesis. Haematologica. 2014;99:1292–1303. doi: 10.3324/haematol.2013.092643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwasaki H, Mizuno S, Arinobu Y, Ozawa H, Mori Y, Shigematsu H, Takatsu K, Tenen DG, Akashi K. The order of expression of transcription factors directs hierarchical specification of hematopoietic lineages. Genes Dev. 2006;20:3010–3021. doi: 10.1101/gad.1493506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamamura K, Ohishi K, Katayama N, Yu Z, Kato K, Masuya M, Fujieda A, Sugimoto Y, Miyata E, Shibasaki T, et al. Pleiotropic role of histone deacetylases in the regulation of human adult erythropoiesis. Br J Haematol. 2006;135:242–253. doi: 10.1111/j.1365-2141.2006.06275.x. [DOI] [PubMed] [Google Scholar]
- 21.Elizalde C, Fernandez-Rueda J, Salcedo JM, Dorronsoro A, Ferrin I, Jakobsson E, Trigueros C. Histone deacetylase 3 modulates the expansion of human hematopoietic stem cells. Stem Cells Dev. 2012;21:2581–2591. doi: 10.1089/scd.2011.0698. [DOI] [PubMed] [Google Scholar]
- 22.Summers AR, Fischer MA, Stengel KR, Zhao Y, Kaiser JF, Wells CE, Hunt A, Bhaskara S, Luzwick JW, Sampathi S, et al. HDAC3 is essential for DNA replication in hematopoietic progenitor cells. J Clin Invest. 2013;123:3112–3123. doi: 10.1172/JCI60806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei Y, Ma D, Gao Y, Zhang C, Wang L, Liu F. Ncor2 is required for hematopoietic stem cell emergence by inhibiting Fos signaling in zebrafish. Blood. 2014;124:1578–1585. doi: 10.1182/blood-2013-11-541391. [DOI] [PubMed] [Google Scholar]
- 24.Ozawa Y, Towatari M, Tsuzuki S, Hayakawa F, Maeda T, Miyata Y, Tanimoto M, Saito H. Histone deacetylase 3 associates with and represses the transcription factor GATA-2. Blood. 2001;98:2116–2123. doi: 10.1182/blood.v98.7.2116. [DOI] [PubMed] [Google Scholar]
- 25.Hua WK, Qi J, Cai Q, Carnahan E, Ayala Ramirez M, Li L, Marcucci G, Kuo YH. HDAC8 regulates long-term hematopoietic stem-cell maintenance under stress by modulating p53 activity. Blood. 2017;130:2619–2630. doi: 10.1182/blood-2017-03-771386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang X, Guo B, Liu S, Wan J, Broxmeyer HE. Neutralizing negative epigenetic regulation by HDAC5 enhances human haematopoietic stem cell homing and engraftment. Nat Commun. 2018;9:2741. doi: 10.1038/s41467-018-05178-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roth M, Wang Z, Chen WY. Sirtuins in hematological aging and malignancy. Crit Rev Oncog. 2013;18:531–547. doi: 10.1615/critrevoncog.2013010187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leko V, Varnum-Finney B, Li H, Gu Y, Flowers D, Nourigat C, Bernstein ID, Bedalov A. SIRT1 is dispensable for function of hematopoietic stem cells in adult mice. Blood. 2012;119:1856–1860. doi: 10.1182/blood-2011-09-377077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rimmele P, Bigarella CL, Liang R, Izac B, Dieguez-Gonzalez R, Barbet G, Donovan M, Brugnara C, Blander JM, Sinclair DA, Ghaffari S. Aging-like phenotype and defective lineage specification in SIRT1-deleted hematopoietic stem and progenitor cells. Stem Cell Reports. 2014;3:44–59. doi: 10.1016/j.stemcr.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown K, Xie S, Qiu X, Mohrin M, Shin J, Liu Y, Zhang D, Scadden DT, Chen D. SIRT3 reverses aging-associated degeneration. Cell Rep. 2013;3:319–327. doi: 10.1016/j.celrep.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H, Diao D, Shi Z, Zhu X, Gao Y, Gao S, Liu X, Wu Y, Rudolph KL, Liu G, et al. SIRT6 controls hematopoietic stem cell homeostasis through epigenetic regulation of Wnt signaling. Cell Stem Cell. 2016;18:495–507. doi: 10.1016/j.stem.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Wrighton KH. Stem cells: SIRT7, the UPR and HSC ageing. Nat Rev Mol Cell Biol. 2015;16:266–267. doi: 10.1038/nrm3981. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z, Yuan H, Roth M, Stark JM, Bhatia R, Chen WY. SIRT1 deacetylase promotes acquisition of genetic mutations for drug resistance in CML cells. Oncogene. 2013;32:589–598. doi: 10.1038/onc.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33:657–670. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 35.Das Gupta K, Shakespear MR, Iyer A, Fairlie DP, Sweet MJ. Histone deacetylases in monocyte/macrophage development, activation and metabolism: refining HDAC targets for inflammatory and infectious diseases. Clin Transl Immunology. 2016;5:e62. doi: 10.1038/cti.2015.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ostuni R, Natoli G, Cassatella MA, Tamassia N. Epigenetic regulation of neutrophil development and function. Semin Immunol. 2016;28:83–93. doi: 10.1016/j.smim.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Cabanel M, Brand C, Oliveira-Nunes MC, Cabral-Piccin MP, Lopes MF, Brito JM, de Oliveira FL, El-Cheikh MC, Carneiro K. Epigenetic control of macrophage shape transition towards an atypical elongated phenotype by histone Deacetylase activity. PLoS One. 2015;10:e0132984. doi: 10.1371/journal.pone.0132984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wisnik E, Ploszaj T, Robaszkiewicz A. Author correction: Downregulation of PARP1 transcription by promoter-associated E2F4-RBL2-HDAC1-BRM complex contributes to repression of pluripotency stem cell factors in human monocytes. Sci Rep. 2018;8:5764. doi: 10.1038/s41598-018-23162-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aude-Garcia C, Collin-Faure V, Bausinger H, Hanau D, Rabilloud T, Lemercier C. Dual roles for MEF2A and MEF2D during human macrophage terminal differentiation and c-Jun expression. Biochem J. 2010;430:237–244. doi: 10.1042/BJ20100131. [DOI] [PubMed] [Google Scholar]
- 40.Leong WY, Guo H, Ma O, Huang H, Cantor AB, Friedman AD. Runx1 phosphorylation by Src increases trans-activation via augmented stability, reduced histone Deacetylase (HDAC) binding, and increased DNA affinity, and activated Runx1 favors Granulopoiesis. J Biol Chem. 2016;291:826–836. doi: 10.1074/jbc.M115.674234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Quan, Wei Jianyang, Zhong Limei, Shi Maohua, Zhou Pan, Zuo Shengkai, Wu Kang, Zhu Mingjiang, Huang Xi, Yu Ying, Zhang Hui, Yin Huiyong, Zhou Jie. Cross Talk between Histone Deacetylase 4 and STAT6 in the Transcriptional Regulation of Arginase 1 during Mouse Dendritic Cell Differentiation. Molecular and Cellular Biology. 2014;35(1):63–75. doi: 10.1128/MCB.00805-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gelmetti V, Zhang J, Fanelli M, Minucci S, Pelicci PG, Lazar MA. Aberrant recruitment of the nuclear receptor corepressor-histone deacetylase complex by the acute myeloid leukemia fusion partner ETO. Mol Cell Biol. 1998;18:7185–7191. doi: 10.1128/mcb.18.12.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu Z, Gu X, Baraoidan K, Ibanez V, Sharma A, Kadkol S, Munker R, Ackerman S, Nucifora G, Saunthararajah Y. RUNX1 regulates corepressor interactions of PU.1. Blood. 2011;117:6498–6508. doi: 10.1182/blood-2010-10-312512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Houot R, Kohrt H, Goldstein MJ, Levy R. Immunomodulating antibodies and drugs for the treatment of hematological malignancies. Cancer Metastasis Rev. 2011;30:97–109. doi: 10.1007/s10555-011-9274-3. [DOI] [PubMed] [Google Scholar]
- 45.Hu J, Liu J, Xue F, Halverson G, Reid M, Guo A, Chen L, Raza A, Galili N, Jaffray J, et al. Isolation and functional characterization of human erythroblasts at distinct stages: implications for understanding of normal and disordered erythropoiesis in vivo. Blood. 2013;121:3246–3253. doi: 10.1182/blood-2013-01-476390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jian W, Yan B, Huang S, Qiu Y. Histone deacetylase 1 activates PU.1 gene transcription through regulating TAF9 deacetylation and transcription factor IID assembly. FASEB J. 2017;31:4104–4116. doi: 10.1096/fj.201700022R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahringer J. NuRD and SIN3 histone deacetylase complexes in development. Trends Genet. 2000;16:351–356. doi: 10.1016/s0168-9525(00)02066-7. [DOI] [PubMed] [Google Scholar]
- 48.Chen X, Bieker JJ. Stage-specific repression by the EKLF transcriptional activator. Mol Cell Biol. 2004;24:10416–10424. doi: 10.1128/MCB.24.23.10416-10424.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suzuki M, Yamada T, Kihara-Negishi F, Sakurai T, Oikawa T. Direct association between PU.1 and MeCP2 that recruits mSin3A-HDAC complex for PU.1-mediated transcriptional repression. Oncogene. 2003;22:8688–8698. doi: 10.1038/sj.onc.1207182. [DOI] [PubMed] [Google Scholar]
- 50.Gregory GD, Miccio A, Bersenev A, Wang Y, Hong W, Zhang Z, Poncz M, Tong W, Blobel GA. FOG1 requires NuRD to promote hematopoiesis and maintain lineage fidelity within the megakaryocytic-erythroid compartment. Blood. 2010;115:2156–2166. doi: 10.1182/blood-2009-10-251280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang T, Jian W, Luo Y, Fu X, Noguchi C, Bungert J, Huang S, Qiu Y. Acetylation of histone deacetylase 1 regulates NuRD corepressor complex activity. J Biol Chem. 2012;287:40279–40291. doi: 10.1074/jbc.M112.349704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamamoto R, Kawahara M, Ito S, Satoh J, Tatsumi G, Hishizawa M, Suzuki T, Andoh A. Selective dissociation between LSD1 and GFI1B by a LSD1 inhibitor NCD38 induces the activation of ERG super-enhancer in erythroleukemia cells. Oncotarget. 2018;9:21007–21021. doi: 10.18632/oncotarget.24774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bottardi S, Ross J, Bourgoin V, Fotouhi-Ardakani N, Affarel B, Trudel M, Milot E. Ikaros and GATA-1 combinatorial effect is required for silencing of human gamma-globin genes. Mol Cell Biol. 2009;29:1526–1537. doi: 10.1128/MCB.01523-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao Q, Cumming H, Cerruti L, Cunningham JM, Jane SM. Site-specific acetylation of the fetal globin activator NF-E4 prevents its ubiquitination and regulates its interaction with the histone deacetylase, HDAC1. J Biol Chem. 2004;279:41477–41486. doi: 10.1074/jbc.M405129200. [DOI] [PubMed] [Google Scholar]
- 55.Mankidy R, Faller DV, Mabaera R, Lowrey CH, Boosalis MS, White GL, Castaneda SA, Perrine SP. Short-chain fatty acids induce gamma-globin gene expression by displacement of a HDAC3-NCoR repressor complex. Blood. 2006;108:3179–3186. doi: 10.1182/blood-2005-12-010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muralidhar SA, Ramakrishnan V, Kalra IS, Li W, Pace BS. Histone deacetylase 9 activates gamma-globin gene expression in primary erythroid cells. J Biol Chem. 2011;286:2343–2353. doi: 10.1074/jbc.M110.115725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ji P, Yeh V, Ramirez T, Murata-Hori M, Lodish HF. Histone deacetylase 2 is required for chromatin condensation and subsequent enucleation of cultured mouse fetal erythroblasts. Haematologica. 2010;95:2013–2021. doi: 10.3324/haematol.2010.029827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li X, Mei Y, Yan B, Vitriol E, Huang S, Ji P, Qiu Y. Histone deacetylase 6 regulates cytokinesis and erythrocyte enucleation through deacetylation of formin protein mDia2. Haematologica. 2017;102:984–994. doi: 10.3324/haematol.2016.161513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cobaleda C, Busslinger M. Developmental plasticity of lymphocytes. Curr Opin Immunol. 2008;20:139–148. doi: 10.1016/j.coi.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 60.Barneda-Zahonero B, Roman-Gonzalez L, Collazo O, Mahmoudi T, Parra M. Epigenetic regulation of B lymphocyte differentiation, transdifferentiation, and reprogramming. Comp Funct Genomics. 2012;2012:564381. doi: 10.1155/2012/564381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Azagra A, Roman-Gonzalez L, Collazo O, Rodriguez-Ubreva J, de Yebenes VG, Barneda-Zahonero B, Rodriguez J, Castro de Moura M, Grego-Bessa J, Fernandez-Duran I, et al. in vivo conditional deletion of HDAC7 reveals its requirement to establish proper B lymphocyte identity and development. J Exp Med. 2016;213:2591–2601. doi: 10.1084/jem.20150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamaguchi T, Cubizolles F, Zhang Y, Reichert N, Kohler H, Seiser C, Matthias P. Histone deacetylases 1 and 2 act in concert to promote the G1-to-S progression. Genes Dev. 2010;24:455–469. doi: 10.1101/gad.552310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu J, Angelin-Duclos C, Greenwood J, Liao J, Calame K. Transcriptional repression by blimp-1 (PRDI-BF1) involves recruitment of histone deacetylase. Mol Cell Biol. 2000;20:2592–2603. doi: 10.1128/mcb.20.7.2592-2603.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ying HY, Su ST, Hsu PH, Chang CC, Lin IY, Tseng YH, Tsai MD, Shih HM, Lin KI. SUMOylation of Blimp-1 is critical for plasma cell differentiation. EMBO Rep. 2012;13:631–637. doi: 10.1038/embor.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pillonel V, Reichert N, Cao C, Heideman MR, Yamaguchi T, Matthias G, Tzankov A, Matthias P. Histone deacetylase 1 plays a predominant pro-oncogenic role in emu-myc driven B cell lymphoma. Sci Rep. 2016;6:37772. doi: 10.1038/srep37772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang H, Song C, Ding Y, Pan X, Ge Z, Tan BH, Gowda C, Sachdev M, Muthusami S, Ouyang H, et al. Transcriptional regulation of JARID1B/KDM5B histone Demethylase by Ikaros, histone Deacetylase 1 (HDAC1), and casein kinase 2 (CK2) in B-cell acute lymphoblastic leukemia. J Biol Chem. 2016;291:4004–4018. doi: 10.1074/jbc.M115.679332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song C, Pan X, Ge Z, Gowda C, Ding Y, Li H, Li Z, Yochum G, Muschen M, Li Q, et al. Epigenetic regulation of gene expression by Ikaros, HDAC1 and casein kinase II in leukemia. Leukemia. 2016;30:1436–1440. doi: 10.1038/leu.2015.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang C, Gonzalez DG, Cote CM, Jiang Y, Hatzi K, Teater M, Dai K, Hla T, Haberman AM, Melnick A. The BCL6 RD2 domain governs commitment of activated B cells to form germinal centers. Cell Rep. 2014;8:1497–1508. doi: 10.1016/j.celrep.2014.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stengel KR, Barnett KR, Wang J, Liu Q, Hodges E, Hiebert SW, Bhaskara S. Deacetylase activity of histone deacetylase 3 is required for productive VDJ recombination and B-cell development. Proc Natl Acad Sci U S A. 2017;114:8608–8613. doi: 10.1073/pnas.1701610114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nanou A, Toumpeki C, Lavigne MD, Lazou V, Demmers J, Paparountas T, Thanos D, Katsantoni E. The dual role of LSD1 and HDAC3 in STAT5-dependent transcription is determined by protein interactions, binding affinities, motifs and genomic positions. Nucleic Acids Res. 2017;45:142–154. doi: 10.1093/nar/gkw832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tanaka H, Muto A, Shima H, Katoh Y, Sax N, Tajima S, Brydun A, Ikura T, Yoshizawa N, Masai H, et al. Epigenetic regulation of the Blimp-1 gene (Prdm1) in B cells involves Bach2 and histone Deacetylase 3. J Biol Chem. 2016;291:6316–6330. doi: 10.1074/jbc.M116.713842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pasqualucci L, Dominguez-Sola D, Chiarenza A, Fabbri G, Grunn A, Trifonov V, Kasper LH, Lerach S, Tang H, Ma J, et al. Inactivating mutations of acetyltransferase genes in B-cell lymphoma. Nature. 2011;471:189–195. doi: 10.1038/nature09730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jiang Y, Ortega-Molina A, Geng H, Ying HY, Hatzi K, Parsa S, McNally D, Wang L, Doane AS, Agirre X, et al. CREBBP inactivation promotes the development of HDAC3-dependent lymphomas. Cancer Discov. 2017;7:38–53. doi: 10.1158/2159-8290.CD-16-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gil VS, Bhagat G, Howell L, Zhang J, Kim CH, Stengel S, Vega F, Zelent A, Petrie K. Deregulated expression of HDAC9 in B cells promotes development of lymphoproliferative disease and lymphoma in mice. Dis Model Mech. 2016;9:1483–1495. doi: 10.1242/dmm.023366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lemercier C, Brocard MP, Puvion-Dutilleul F, Kao HY, Albagli O, Khochbin S. Class II histone deacetylases are directly recruited by BCL6 transcriptional repressor. J Biol Chem. 2002;277:22045–22052. doi: 10.1074/jbc.M201736200. [DOI] [PubMed] [Google Scholar]
- 76.Sandhu SK, Volinia S, Costinean S, Galasso M, Neinast R, Santhanam R, Parthun MR, Perrotti D, Marcucci G, Garzon R. Croce CM: miR-155 targets histone deacetylase 4 (HDAC4) and impairs transcriptional activity of B-cell lymphoma 6 (BCL6) in the emu-miR-155 transgenic mouse model. Proc Natl Acad Sci U S A. 2012;109:20047–20052. doi: 10.1073/pnas.1213764109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barneda-Zahonero B, Collazo O, Azagra A, Fernandez-Duran I, Serra-Musach J, Islam AB, Vega-Garcia N, Malatesta R, Camos M, Gomez A, et al. The transcriptional repressor HDAC7 promotes apoptosis and c-Myc downregulation in particular types of leukemia and lymphoma. Cell Death Dis. 2015;6:e1635. doi: 10.1038/cddis.2014.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Matthews SA, Liu P, Spitaler M, Olson EN, McKinsey TA, Cantrell DA, Scharenberg AM. Essential role for protein kinase D family kinases in the regulation of class II histone deacetylases in B lymphocytes. Mol Cell Biol. 2006;26:1569–1577. doi: 10.1128/MCB.26.4.1569-1577.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Powers John J., Maharaj Kamira K., Sahakian Eva, Xing Limin, PerezVillarroel Patricio, Knox Tessa, Quayle Steven, Jones Simon S, Villagra Alejandro, Sotomayor Eduardo M., Pinilla-Ibarz Javier. Histone Deacetylase 6 (HDAC6) As a Regulator of Immune Check-Point Molecules in Chronic Lymphocytic Leukemia (CLL) Blood. 2014;124(21):3311–3311. [Google Scholar]
- 80.Bae J, Hideshima T, Tai YT, Song Y, Richardson P, Raje N, Munshi NC, Anderson KC. Histone deacetylase (HDAC) inhibitor ACY241 enhances anti-tumor activities of antigen-specific central memory cytotoxic T lymphocytes against multiple myeloma and solid tumors. Leukemia. 2018;32:1932–1947. doi: 10.1038/s41375-018-0062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tremblay-LeMay R, Rastgoo N, Chang H. Modulating PD-L1 expression in multiple myeloma: an alternative strategy to target the PD-1/PD-L1 pathway. J Hematol Oncol. 2018;11:46. doi: 10.1186/s13045-018-0589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Terranova-Barberio M, Thomas S, Munster PN. Epigenetic modifiers in immunotherapy: a focus on checkpoint inhibitors. Immunotherapy. 2016;8:705–719. doi: 10.2217/imt-2016-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boucheron N, Tschismarov R, Goeschl L, Moser MA, Lagger S, Sakaguchi S, Winter M, Lenz F, Vitko D, Breitwieser FP, et al. CD4(+) T cell lineage integrity is controlled by the histone deacetylases HDAC1 and HDAC2. Nat Immunol. 2014;15:439–448. doi: 10.1038/ni.2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dovey OM, Foster CT, Conte N, Edwards SA, Edwards JM, Singh R, Vassiliou G, Bradley A, Cowley SM. Histone deacetylase 1 and 2 are essential for normal T-cell development and genomic stability in mice. Blood. 2013;121:1335–1344. doi: 10.1182/blood-2012-07-441949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Philips RL, Lee JH, Gaonkar K, Chanana P, Chung JY, Romero Arocha SR, Schwab A, Ordog T, Shapiro VS. HDAC3 restrains CD8-lineage genes to maintain a bi-potential state in CD4(+)CD8(+) thymocytes for CD4-lineage commitment. Elife. 2019;8. [DOI] [PMC free article] [PubMed]
- 86.Philips RL, Chen MW, McWilliams DC, Belmonte PJ, Constans MM, Shapiro VS. HDAC3 is required for the Downregulation of RORgammat during Thymocyte positive selection. J Immunol. 2016;197:541–554. doi: 10.4049/jimmunol.1502529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stengel KR, Zhao Y, Klus NJ, Kaiser JF, Gordy LE, Joyce S, Hiebert SW, Summers AR. Histone Deacetylase 3 is required for efficient T cell development. Mol Cell Biol. 2015;35:3854–3865. doi: 10.1128/MCB.00706-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hsu FC, Belmonte PJ, Constans MM, Chen MW, McWilliams DC, Hiebert SW, Shapiro VS. Histone Deacetylase 3 is required for T cell maturation. J Immunol. 2015;195:1578–1590. doi: 10.4049/jimmunol.1500435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang L, Liu Y, Han R, Beier UH, Bhatti TR, Akimova T, Greene MI, Hiebert SW, Hancock WW. FOXP3+ regulatory T cell development and function require histone/protein deacetylase 3. J Clin Invest. 2015;125:1111–1123. doi: 10.1172/JCI77088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Akimova T, Ge G, Golovina T, Mikheeva T, Wang L, Riley JL, Hancock WW. Histone/protein deacetylase inhibitors increase suppressive functions of human FOXP3+ Tregs. Clin Immunol. 2010;136:348–363. doi: 10.1016/j.clim.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kasler HG, Young BD, Mottet D, Lim HW, Collins AM, Olson EN, Verdin E. Histone deacetylase 7 regulates cell survival and TCR signaling in CD4/CD8 double-positive thymocytes. J Immunol. 2011;186:4782–4793. doi: 10.4049/jimmunol.1001179. [DOI] [PubMed] [Google Scholar]
- 92.Parra M, Kasler H, McKinsey TA, Olson EN, Verdin E. Protein kinase D1 phosphorylates HDAC7 and induces its nuclear export after T-cell receptor activation. J Biol Chem. 2005;280:13762–13770. doi: 10.1074/jbc.M413396200. [DOI] [PubMed] [Google Scholar]
- 93.Xiao H, Jiao J, Wang L, O'Brien S, Newick K, Wang LC, Falkensammer E, Liu Y, Han R, Kapoor V, et al. HDAC5 controls the functions of Foxp3(+) T-regulatory and CD8(+) T cells. Int J Cancer. 2016;138:2477–2486. doi: 10.1002/ijc.29979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Villagra A, Cheng F, Wang HW, Suarez I, Glozak M, Maurin M, Nguyen D, Wright KL, Atadja PW, Bhalla K, et al. The histone deacetylase HDAC11 regulates the expression of interleukin 10 and immune tolerance. Nat Immunol. 2009;10:92–100. doi: 10.1038/ni.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheng F, Lienlaf M, Perez-Villarroel P, Wang HW, Lee C, Woan K, Woods D, Knox T, Bergman J, Pinilla-Ibarz J, et al. Divergent roles of histone deacetylase 6 (HDAC6) and histone deacetylase 11 (HDAC11) on the transcriptional regulation of IL10 in antigen presenting cells. Mol Immunol. 2014;60:44–53. doi: 10.1016/j.molimm.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hagelkruys A, Sawicka A, Rennmayr M, Seiser C. The biology of HDAC in cancer: the nuclear and epigenetic components. Handb Exp Pharmacol. 2011;206:13–37. doi: 10.1007/978-3-642-21631-2_2. [DOI] [PubMed] [Google Scholar]
- 97.New M, Olzscha H, La Thangue NB. HDAC inhibitor-based therapies: can we interpret the code? Mol Oncol. 2012;6:637–656. doi: 10.1016/j.molonc.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barneda-Zahonero B, Parra M. Histone deacetylases and cancer. Mol Oncol. 2012;6:579–589. doi: 10.1016/j.molonc.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang J, Hoshino T, Redner RL, Kajigaya S, Liu JM. ETO, fusion partner in t (8;21) acute myeloid leukemia, represses transcription by interaction with the human N-CoR/mSin3/HDAC1 complex. Proc Natl Acad Sci U S A. 1998;95:10860–10865. doi: 10.1073/pnas.95.18.10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Farooqi AA, Naqvi SK, Perk AA, Yanar O, Tabassum S, Ahmad MS, Mansoor Q, Ashry MS, Ismail M, Naoum GE, Arafat WO. Natural agents-mediated targeting of histone Deacetylases. Arch Immunol Ther Exp. 2018;66:31–44. doi: 10.1007/s00005-017-0488-0. [DOI] [PubMed] [Google Scholar]
- 101.Fu L, Shi J, Liu A, Zhou L, Jiang M, Fu H, Xu K, Li D, Deng A, Zhang Q, et al. A minicircuitry of microRNA-9-1 and RUNX1-RUNX1T1 contributes to leukemogenesis in t (8;21) acute myeloid leukemia. Int J Cancer. 2017;140:653–661. doi: 10.1002/ijc.30481. [DOI] [PubMed] [Google Scholar]
- 102.Liu J, Lu W, Liu S, Wang Y, Li S, Xu Y, Xing H, Tang K, Tian Z, Rao Q, et al. ZFP36L2, a novel AML1 target gene, induces AML cells apoptosis and inhibits cell proliferation. Leuk Res. 2018;68:15–21. doi: 10.1016/j.leukres.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 103.Hoogeveen AT, Rossetti S, Stoyanova V, Schonkeren J, Fenaroli A, Schiaffonati L, van Unen L, Sacchi N. The transcriptional corepressor MTG16a contains a novel nucleolar targeting sequence deranged in t (16; 21)-positive myeloid malignancies. Oncogene. 2002;21:6703–6712. doi: 10.1038/sj.onc.1205882. [DOI] [PubMed] [Google Scholar]
- 104.Stams WA, den Boer ML, Beverloo HB, Kazemier KM, van Wering ER, Janka-Schaub GE, Pieters R. Effect of the histone deacetylase inhibitor depsipeptide on B-cell differentiation in both TEL-AML1-positive and negative childhood acute lymphoblastic leukemia. Haematologica. 2005;90:1697–1699. [PubMed] [Google Scholar]
- 105.Senyuk V, Chakraborty S, Mikhail FM, Zhao R, Chi Y, Nucifora G. The leukemia-associated transcription repressor AML1/MDS1/EVI1 requires CtBP to induce abnormal growth and differentiation of murine hematopoietic cells. Oncogene. 2002;21:3232–3240. doi: 10.1038/sj.onc.1205436. [DOI] [PubMed] [Google Scholar]
- 106.Khan MM, Nomura T, Kim H, Kaul SC, Wadhwa R, Zhong S, Pandolfi PP, Ishii S. PML-RARalpha alleviates the transcriptional repression mediated by tumor suppressor Rb. J Biol Chem. 2001;276:43491–43494. doi: 10.1074/jbc.C100532200. [DOI] [PubMed] [Google Scholar]
- 107.Girard N, Tremblay M, Humbert M, Grondin B, Haman A, Labrecque J, Chen B, Chen Z, Chen SJ, Hoang T. RARalpha-PLZF oncogene inhibits C/EBPalpha function in myeloid cells. Proc Natl Acad Sci U S A. 2013;110:13522–13527. doi: 10.1073/pnas.1310067110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Boumber Y, Younes A, Garcia-Manero G. Mocetinostat (MGCD0103): a review of an isotype-specific histone deacetylase inhibitor. Expert Opin Investig Drugs. 2011;20:823–829. doi: 10.1517/13543784.2011.577737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Benedetti R, Conte M, Altucci L. Targeting histone Deacetylases in diseases: where are we? Antioxid Redox Signal. 2015;23:99–126. doi: 10.1089/ars.2013.5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shen S, Kozikowski AP. Why Hydroxamates may not be the best histone Deacetylase inhibitors--what some may have forgotten or would rather forget? ChemMedChem. 2016;11:15–21. doi: 10.1002/cmdc.201500486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Osko JD, Christianson DW. Methods for the expression, purification, and crystallization of histone deacetylase 6-inhibitor complexes. Methods Enzymol. 2019;626:447–474. doi: 10.1016/bs.mie.2019.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chou CJ, Herman D, Gottesfeld JM. Pimelic diphenylamide 106 is a slow, tight-binding inhibitor of class I histone deacetylases. J Biol Chem. 2008;283:35402–35409. doi: 10.1074/jbc.M807045200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hu E, Chen Z, Fredrickson T, Zhu Y, Kirkpatrick R, Zhang GF, Johanson K, Sung CM, Liu R, Winkler J. Cloning and characterization of a novel human class I histone deacetylase that functions as a transcription repressor. J Biol Chem. 2000;275:15254–15264. doi: 10.1074/jbc.M908988199. [DOI] [PubMed] [Google Scholar]
- 114.Bieliauskas AV, Pflum MK. Isoform-selective histone deacetylase inhibitors. Chem Soc Rev. 2008;37:1402–1413. doi: 10.1039/b703830p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Saygin C, Carraway HE. Emerging therapies for acute myeloid leukemia. J Hematol Oncol. 2017;10:93. doi: 10.1186/s13045-017-0463-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nanavati C, Mager DE. Sequential exposure of Bortezomib and Vorinostat is synergistic in multiple myeloma cells. Pharm Res. 2017;34:668–679. doi: 10.1007/s11095-017-2095-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Holkova B, Kmieciak M, Bose P, Yazbeck VY, Barr PM, Tombes MB, Shrader E, Weir-Wiggins C, Rollins AD, Cebula EM, et al. Phase 1 trial of carfilzomib (PR-171) in combination with vorinostat (SAHA) in patients with relapsed or refractory B-cell lymphomas. Leuk Lymphoma. 2016;57:635–643. doi: 10.3109/10428194.2015.1075019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xue K, Gu JJ, Zhang Q, Mavis C, Hernandez-Ilizaliturri FJ, Czuczman MS, Guo Y. Vorinostat, a histone deacetylase (HDAC) inhibitor, promotes cell cycle arrest and re-sensitizes rituximab- and chemo-resistant lymphoma cells to chemotherapy agents. J Cancer Res Clin Oncol. 2016;142:379–387. doi: 10.1007/s00432-015-2026-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Thompson RC, Vardinogiannis I, Gilmore TD. The sensitivity of diffuse large B-cell lymphoma cell lines to histone deacetylase inhibitor-induced apoptosis is modulated by BCL-2 family protein activity. PLoS One. 2013;8:e62822. doi: 10.1371/journal.pone.0062822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhou W, Zhu W, Ma L, Xiao F, Qian W. Proteasome inhibitor MG-132 enhances histone deacetylase inhibitor SAHA-induced cell death of chronic myeloid leukemia cells by an ROS-mediated mechanism and downregulation of the Bcr-Abl fusion protein. Oncol Lett. 2015;10:2899–2904. doi: 10.3892/ol.2015.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bu Q, Cui L, Li J, Du X, Zou W, Ding K, Pan J. SAHA and S116836, a novel tyrosine kinase inhibitor, synergistically induce apoptosis in imatinib-resistant chronic myelogenous leukemia cells. Cancer Biol Ther. 2014;15:951–962. doi: 10.4161/cbt.28931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dasmahapatra G, Patel H, Nguyen T, Attkisson E, Grant S. PLK1 inhibitors synergistically potentiate HDAC inhibitor lethality in imatinib mesylate-sensitive or -resistant BCR/ABL+ leukemia cells in vitro and in vivo. Clin Cancer Res. 2013;19:404–414. doi: 10.1158/1078-0432.CCR-12-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nguyen T, Dai Y, Attkisson E, Kramer L, Jordan N, Nguyen N, Kolluri N, Muschen M, Grant S. HDAC inhibitors potentiate the activity of the BCR/ABL kinase inhibitor KW-2449 in imatinib-sensitive or -resistant BCR/ABL+ leukemia cells in vitro and in vivo. Clin Cancer Res. 2011;17:3219–3232. doi: 10.1158/1078-0432.CCR-11-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Whitecross KF, Alsop AE, Cluse LA, Wiegmans A, Banks KM, Coomans C, Peart MJ, Newbold A, Lindemann RK, Johnstone RW. Defining the target specificity of ABT-737 and synergistic antitumor activities in combination with histone deacetylase inhibitors. Blood. 2009;113:1982–1991. doi: 10.1182/blood-2008-05-156851. [DOI] [PubMed] [Google Scholar]
- 125.Nieto Y, Valdez BC, Thall PF, Ahmed S, Jones RB, Hosing C, Popat U, Shpall EJ, Qazilbash M, Gulbis A, et al. Vorinostat combined with high-dose gemcitabine, Busulfan, and Melphalan with autologous stem cell transplantation in patients with refractory lymphomas. Biol Blood Marrow Transplant. 2015;21:1914–1920. doi: 10.1016/j.bbmt.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Garcia-Manero G. Can we improve outcomes in patients with acute myelogenous leukemia? Incorporating HDAC inhibitors into front-line therapy. Best Pract Res Clin Haematol. 2012;25:427–435. doi: 10.1016/j.beha.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 127.Ding H, Peterson KL, Correia C, Koh B, Schneider PA, Nowakowski GS, Kaufmann SH. Histone deacetylase inhibitors interrupt HSP90*RASGRP1 and HSP90*CRAF interactions to upregulate BIM and circumvent drug resistance in lymphoma cells. Leukemia. 2017;31:1593–1602. doi: 10.1038/leu.2016.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Harousseau Jean-Luc, Shaughnessy John, Richardson Paul. Multiple Myeloma. Hematology. 2004;2004(1):237–256. doi: 10.1182/asheducation-2004.1.237. [DOI] [PubMed] [Google Scholar]
- 129.Gao L, Gao M, Yang G, Tao Y, Kong Y, Yang R, Meng X, Ai G, Wei R, Wu H, et al. Synergistic activity of Carfilzomib and Panobinostat in multiple myeloma cells via modulation of ROS generation and ERK1/2. Biomed Res Int. 2015;2015:459052. doi: 10.1155/2015/459052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bottoni A, Rizzotto L, Lai TH, Liu C, Smith LL, Mantel R, Reiff S, El-Gamal D, Larkin K, Johnson AJ, et al. Targeting BTK through microRNA in chronic lymphocytic leukemia. Blood. 2016;128:3101–3112. doi: 10.1182/blood-2016-07-727750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schwartz J, Niu X, Walton E, Hurley L, Lin H, Edwards H, Taub JW, Wang Z, Ge Y. Synergistic anti-leukemic interactions between ABT-199 and panobinostat in acute myeloid leukemia ex vivo. Am J Transl Res. 2016;8:3893–3902. [PMC free article] [PubMed] [Google Scholar]
- 132.Matsuda Y, Yamauchi T, Hosono N, Uzui K, Negoro E, Morinaga K, Nishi R, Yoshida A, Kimura S, Maekawa T, Ueda T. Combination of panobinostat with ponatinib synergistically overcomes imatinib-resistant CML cells. Cancer Sci. 2016;107:1029–1038. doi: 10.1111/cas.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lemoine M, Derenzini E, Buglio D, Medeiros LJ, Davis RE, Zhang J, Ji Y, Younes A. The pan-deacetylase inhibitor panobinostat induces cell death and synergizes with everolimus in Hodgkin lymphoma cell lines. Blood. 2012;119:4017–4025. doi: 10.1182/blood-2011-01-331421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mondello P, Brea EJ, De Stanchina E, Toska E, Chang AY, Fennell M, Seshan V, Garippa R, Scheinberg DA, Baselga J, et al. Panobinostat acts synergistically with ibrutinib in diffuse large B cell lymphoma cells with MyD88 L265P mutations. JCI Insight. 2017;2:e90196. [DOI] [PMC free article] [PubMed]
- 135.Chu Y, Yahr A, Huang B, Ayello J, Barth M. M SC: Romidepsin alone or in combination with anti-CD20 chimeric antigen receptor expanded natural killer cells targeting Burkitt lymphoma in vitro and in immunodeficient mice. Oncoimmunology. 2017;6:e1341031. doi: 10.1080/2162402X.2017.1341031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yan B, Chen Q, Shimada K, Tang M, Li H, Gurumurthy A, Khoury JD, Xu B, Huang S, Qiu Y. Histone deacetylase inhibitor targets CD123/CD47-positive cells and reverse chemoresistance phenotype in acute myeloid leukemia. Leukemia. 2019;33:931–944. doi: 10.1038/s41375-018-0279-6. [DOI] [PubMed] [Google Scholar]
- 137.Tabe Y, Konopleva M, Contractor R, Munsell M, Schober WD, Jin L, Tsutsumi-Ishii Y, Nagaoka I, Igari J, Andreeff M. Up-regulation of MDR1 and induction of doxorubicin resistance by histone deacetylase inhibitor depsipeptide (FK228) and ATRA in acute promyelocytic leukemia cells. Blood. 2006;107:1546–1554. doi: 10.1182/blood-2004-10-4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.O'Connor OA, Ozcan M, Jacobsen ED, Roncero JM, Trotman J, Demeter J, Masszi T, Pereira J, Ramchandren R, Beaven A, et al. Randomized phase III study of Alisertib or Investigator's choice (selected single agent) in patients with relapsed or refractory peripheral T-cell lymphoma. J Clin Oncol. 2019;37:613–623. doi: 10.1200/JCO.18.00899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Reiman T, Savage KJ, Crump M, Cheung MC, MacDonald D, Buckstein R, Couban S, Piliotis E, Imrie K, Spaner D, et al. A phase I study of romidepsin, gemcitabine, dexamethasone and cisplatin combination therapy in the treatment of peripheral T-cell and diffuse large B-cell lymphoma; the Canadian cancer trials group LY.15 studydagger. Leuk Lymphoma. 2019;60:912–919. doi: 10.1080/10428194.2018.1515937. [DOI] [PubMed] [Google Scholar]
- 140.Amengual JE, Lichtenstein R, Lue J, Sawas A, Deng C, Lichtenstein E, Khan K, Atkins L, Rada A, Kim HA, et al. A phase 1 study of romidepsin and pralatrexate reveals marked activity in relapsed and refractory T-cell lymphoma. Blood. 2018;131:397–407. doi: 10.1182/blood-2017-09-806737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Havas AP, Rodrigues KB, Bhakta A, Demirjian JA, Hahn S, Tran J, Scavello M, Tula-Sanchez AA, Zeng Y, Schmelz M, Smith CL. Belinostat and vincristine demonstrate mutually synergistic cytotoxicity associated with mitotic arrest and inhibition of polyploidy in a preclinical model of aggressive diffuse large B cell lymphoma. Cancer Biol Ther. 2016;17:1240–1252. doi: 10.1080/15384047.2016.1250046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dai Y, Chen S, Wang L, Pei XY, Kramer LB, Dent P, Grant S. Bortezomib interacts synergistically with belinostat in human acute myeloid leukaemia and acute lymphoblastic leukaemia cells in association with perturbations in NF-kappaB and Bim. Br J Haematol. 2011;153:222–235. doi: 10.1111/j.1365-2141.2011.08591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ngamphaiboon N, Dy GK, Ma WW, Zhao Y, Reungwetwattana T, DePaolo D, Ding Y, Brady W, Fetterly G, Adjei AA. A phase I study of the histone deacetylase (HDAC) inhibitor entinostat, in combination with sorafenib in patients with advanced solid tumors. Investig New Drugs. 2015;33:225–232. doi: 10.1007/s10637-014-0174-6. [DOI] [PubMed] [Google Scholar]
- 144.Suraweera A, O'Byrne KJ, Richard DJ. Combination therapy with histone Deacetylase inhibitors (HDACi) for the treatment of Cancer: achieving the full therapeutic potential of HDACi. Front Oncol. 2018;8:92. doi: 10.3389/fonc.2018.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Iacomino G, Medici MC, Russo GL. Valproic acid sensitizes K562 erythroleukemia cells to TRAIL/Apo2L-induced apoptosis. Anticancer Res. 2008;28:855–864. [PubMed] [Google Scholar]
- 146.Davood ZA, Shamsi S, Ghaedi H, Sahand RI, Mojtaba G, Mahdi T, Reza M, Ebrahimi MJ, Miri-Moosavi RS, Boosaliki S, Davood OM. Valproic acid may exerts its cytotoxic effect through rassf1a expression induction in acute myeloid leukemia. Tumour Biol. 2016;37:11001–11006. doi: 10.1007/s13277-016-4985-2. [DOI] [PubMed] [Google Scholar]
- 147.Zhao J, Xie C, Edwards H, Wang G, Taub JW, Ge Y. Histone deacetylases 1 and 2 cooperate in regulating BRCA1, CHK1, and RAD51 expression in acute myeloid leukemia cells. Oncotarget. 2017;8:6319–6329. doi: 10.18632/oncotarget.14062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.von Tresckow B, Diehl V. An update on emerging drugs for Hodgkin lymphoma. Expert Opin Emerg Drugs. 2014;19:215–224. doi: 10.1517/14728214.2014.912277. [DOI] [PubMed] [Google Scholar]
- 149.Liu HB, Mayes PA, Perlmutter P, McKendrick JJ, Dear AE. The anti-leukemic effect and molecular mechanisms of novel hydroxamate and benzamide histone deacetylase inhibitors with 5-aza-cytidine. Int J Oncol. 2011;38:1421–1425. doi: 10.3892/ijo.2011.914. [DOI] [PubMed] [Google Scholar]
- 150.Favreau AJ, McGlauflin RE, Duarte CW. Sathyanarayana P: miR-199b, a novel tumor suppressor miRNA in acute myeloid leukemia with prognostic implications. Exp Hematol Oncol. 2015;5:4. doi: 10.1186/s40164-016-0033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Canella A, Cordero Nieves H, Sborov DW, Cascione L, Radomska HS, Smith E, Stiff A, Consiglio J, Caserta E, Rizzotto L, et al. HDAC inhibitor AR-42 decreases CD44 expression and sensitizes myeloma cells to lenalidomide. Oncotarget. 2015;6:31134–31150. doi: 10.18632/oncotarget.5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chen S, Zhang Y, Zhou L, Leng Y, Lin H, Kmieciak M, Pei XY, Jones R, Orlowski RZ, Dai Y, Grant S. A Bim-targeting strategy overcomes adaptive bortezomib resistance in myeloma through a novel link between autophagy and apoptosis. Blood. 2014;124:2687–2697. doi: 10.1182/blood-2014-03-564534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jin Y, Yao Y, Chen L, Zhu X, Jin B, Shen Y, Li J, Du X, Lu Y, Jiang S, Pan J. Depletion of gamma-catenin by histone Deacetylase inhibition confers elimination of CML stem cells in combination with Imatinib. Theranostics. 2016;6:1947–1962. doi: 10.7150/thno.16139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang QL, Wang L, Zhang YW, Jiang XX, Yang F, Wu WL, Janin A, Chen Z, Shen ZX, Chen SJ, Zhao WL. The proteasome inhibitor bortezomib interacts synergistically with the histone deacetylase inhibitor suberoylanilide hydroxamic acid to induce T-leukemia/lymphoma cells apoptosis. Leukemia. 2009;23:1507–1514. doi: 10.1038/leu.2009.41. [DOI] [PubMed] [Google Scholar]
- 155.Mihailidou C, Papavassiliou AG, Kiaris H. Cell-autonomous cytotoxicity of type I interferon response via induction of endoplasmic reticulum stress. FASEB J. 2017;31:5432–5439. doi: 10.1096/fj.201700152R. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 156.Rudek MA, Zhao M, He P, Hartke C, Gilbert J, Gore SD, Carducci MA, Baker SD. Pharmacokinetics of 5-azacitidine administered with phenylbutyrate in patients with refractory solid tumors or hematologic malignancies. J Clin Oncol. 2005;23:3906–3911. doi: 10.1200/JCO.2005.07.450. [DOI] [PubMed] [Google Scholar]
- 157.Cerchietti LC, Hatzi K, Caldas-Lopes E, Yang SN, Figueroa ME, Morin RD, Hirst M, Mendez L, Shaknovich R, Cole PA, et al. BCL6 repression of EP300 in human diffuse large B cell lymphoma cells provides a basis for rational combinatorial therapy. J Clin Invest. 2010;120:4569–4582. doi: 10.1172/JCI42869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Voorhees PM, Gasparetto C, Moore DT, Winans D, Orlowski RZ, Hurd DD. Final results of a phase 1 study of Vorinostat, Pegylated liposomal doxorubicin, and Bortezomib in relapsed or refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2017;17:424–432. doi: 10.1016/j.clml.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 159.Hauswald S, Duque-Afonso J, Wagner MM, Schertl FM, Lubbert M, Peschel C, Keller U, Licht T. Histone deacetylase inhibitors induce a very broad, pleiotropic anticancer drug resistance phenotype in acute myeloid leukemia cells by modulation of multiple ABC transporter genes. Clin Cancer Res. 2009;15:3705–3715. doi: 10.1158/1078-0432.CCR-08-2048. [DOI] [PubMed] [Google Scholar]
- 160.Fujii K, Suzuki N, Jimura N, Idogawa M, Kondo T, Iwatsuki K, Kanekura T. HSP72 functionally inhibits the anti-neoplastic effects of HDAC inhibitors. J Dermatol Sci. 2018;90:82–89. doi: 10.1016/j.jdermsci.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 161.Dahabieh MS, Ha Z, Di Pietro E, Nichol JN, Bolt AM, Goncalves C, Dupere-Richer D, Pettersson F, Mann KK, Braverman NE, et al. Peroxisomes protect lymphoma cells from HDAC inhibitor-mediated apoptosis. Cell Death Differ. 2017;24:1912–1924. doi: 10.1038/cdd.2017.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fujii K, Suzuki N, Ikeda K, Hamada T, Yamamoto T, Kondo T, Iwatsuki K. Proteomic study identified HSP 70 kDa protein 1A as a possible therapeutic target, in combination with histone deacetylase inhibitors, for lymphoid neoplasms. J Proteome. 2012;75:1401–1410. doi: 10.1016/j.jprot.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 163.Forthun RB, Sengupta T, Skjeldam HK, Lindvall JM, McCormack E, Gjertsen BT, Nilsen H. Cross-species functional genomic analysis identifies resistance genes of the histone deacetylase inhibitor valproic acid. PLoS One. 2012;7:e48992. doi: 10.1371/journal.pone.0048992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fantin VR, Loboda A, Paweletz CP, Hendrickson RC, Pierce JW, Roth JA, Li L, Gooden F, Korenchuk S, Hou XS, et al. Constitutive activation of signal transducers and activators of transcription predicts vorinostat resistance in cutaneous T-cell lymphoma. Cancer Res. 2008;68:3785–3794. doi: 10.1158/0008-5472.CAN-07-6091. [DOI] [PubMed] [Google Scholar]
- 165.Huang R, Zhang X, Min Z, Shadia AS, Yang S, Liu X. MGCD0103 induces apoptosis and simultaneously increases the expression of NF-kappaB and PD-L1 in classical Hodgkin's lymphoma. Exp Ther Med. 2018;16:3827–3834. doi: 10.3892/etm.2018.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liu H, Hu Y, Rimoldi R, Von Hagt C, Khong EWC, Lee N, Flemming S, Spencer A, Dear AE. Epigenetic treatment-mediated modulation of PD-L1 predicts potential therapy resistance over response markers in myeloid malignancies: a molecular mechanism involving effectors of PD-L1 reverse signaling. Oncol Lett. 2019;17:2543–2550. doi: 10.3892/ol.2018.9841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Noack K, Mahendrarajah N, Hennig D, Schmidt L, Grebien F, Hildebrand D, Christmann M, Kaina B, Sellmer A, Mahboobi S, et al. Analysis of the interplay between all-trans retinoic acid and histone deacetylase inhibitors in leukemic cells. Arch Toxicol. 2017;91:2191–2208. doi: 10.1007/s00204-016-1878-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Peart MJ, Smyth GK, van Laar RK, Bowtell DD, Richon VM, Marks PA, Holloway AJ, Johnstone RW. Identification and functional significance of genes regulated by structurally different histone deacetylase inhibitors. Proc Natl Acad Sci U S A. 2005;102:3697–3702. doi: 10.1073/pnas.0500369102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hu Y, Lu W, Chen G, Zhang H, Jia Y, Wei Y, Yang H, Zhang W, Fiskus W, Bhalla K, et al. Overcoming resistance to histone deacetylase inhibitors in human leukemia with the redox modulating compound beta-phenylethyl isothiocyanate. Blood. 2010;116:2732–2741. doi: 10.1182/blood-2009-11-256354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Garcia-Manero G, Yang H, Bueso-Ramos C, Ferrajoli A, Cortes J, Wierda WG, Faderl S, Koller C, Morris G, Rosner G, et al. Phase 1 study of the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid [SAHA]) in patients with advanced leukemias and myelodysplastic syndromes. Blood. 2008;111:1060–1066. doi: 10.1182/blood-2007-06-098061. [DOI] [PubMed] [Google Scholar]
- 171.Dupere-Richer D, Kinal M, Menasche V, Nielsen TH, Del Rincon S, Pettersson F, Miller WH., Jr Vorinostat-induced autophagy switches from a death-promoting to a cytoprotective signal to drive acquired resistance. Cell Death Dis. 2013;4:e486. doi: 10.1038/cddis.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Guerriero JL, Sotayo A, Ponichtera HE, Castrillon JA, Pourzia AL, Schad S, Johnson SF, Carrasco RD, Lazo S, Bronson RT, et al. Class IIa HDAC inhibition reduces breast tumours and metastases through anti-tumour macrophages. Nature. 2017;543:428–432. doi: 10.1038/nature21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.