Abstract
Background
The efficacy of azathioprine (AZA) and mycophenolate mofetil (MMF) for interstitial lung disease (ILD) has been described, but mainly in connective tissue disease-associated ILD. The objective of this study was to evaluate the effect of AZA and MMF on lung function and prednisone dose in myositis-related ILD (M-ILD).
Methods
In this retrospective study, patients with M-ILD seen at Johns Hopkins and treated with AZA or MMF and no other steroid-sparing agents were included. Linear mixed-effects models adjusted for sex, age, antisynthetase antibody, and smoking status were used to compare the change in FVC % predicted, diffusing capacity of the lungs for carbon monoxide (Dlco) % predicted, and prednisone dose.
Results
Sixty-six patients with M-ILD were treated with AZA and 44 with MMF. At treatment initiation, mean FVC % predicted and Dlco % predicted were significantly lower in the AZA group than in the MMF group. In both groups, FVC % predicted improved and the prednisone dose was reduced over 2 to 5 years; however, for Dlco % predicted, only the AZA group improved. The adjusted model showed no significant difference in posttreatment FVC % predicted or Dlco % predicted between groups (mean difference of 1.9 and –8.2, respectively), but a 6.6-mg lower dose of prednisone at 36 months in the AZA group. Adverse events were more frequent with AZA than MMF (33.3% vs 13.6%; P = .04).
Conclusions
In M-ILD, AZA treatment was associated with improved FVC % predicted and Dlco % predicted, and lower prednisone dose. Patients treated with MMF had improved FVC % predicted and lower prednisone dose. After 36 months, patients treated with AZA received a lower prednisone dose than those treated with MMF.
Key Words: antisynthetase syndrome, azathioprine, interstitial lung disease, mycophenolate mofetil, myositis
Abbreviations: AE, adverse event; AS, antisynthetase syndrome; AZA, azathioprine; CHP, chronic hypersensitivity pneumonitis; CTD-ILD, connective tissue disease-associated interstitial lung disease; CYC, cyclophosphamide; Dlco, diffusing capacity of the lungs for carbon monoxide; IIM, idiopathic inflammatory myopathy; ILD, interstitial lung disease; JH, Johns Hopkins; MAA, myositis-associated autoantibody; M-ILD, myositis-related interstitial lung disease; MMF, mycophenolate mofetil; MSA, myositis-specific autoantibody; SLS, Scleroderma Lung Study
Interstitial lung disease (ILD) is a common complication of idiopathic inflammatory myopathies (IIMs) that results in high morbidity and mortality.1, 2, 3 Up to 69% of patients with IIM may initially present with lung disease alone, which makes diagnosis and treatment challenging.4, 5, 6, 7 The frequency of autoantibody positivity in patients with IIM is significant and ranges from 50% to 78%.8, 9 These antibodies have been classified into myositis-specific autoantibodies (MSAs) and myositis-associated autoantibodies (MAAs), which has allowed a better clinical categorization of IIMs.10 For example, antisynthetase syndrome (AS), which is associated with autoantibodies to specific tRNA synthetase proteins, is increasingly recognized as an underlying cause of ILD,11 which can be present in 35% to 90% of these patients depending on the type of AS antibody12, 13, 14 When myositis-related ILD (M-ILD) is left untreated, it is often progressive and potentially fatal, with a reported 5-year mortality ranging from 9% to 40%.15, 16, 17 Immunosuppression is the most frequent treatment strategy in these patients, but there have been few studies with small sample sizes.18, 19, 20, 21, 22, 23, 24, 25 As there have been no randomized controlled trials comparing the efficacy of different immunosuppression strategies, observational studies provide the best available evidence.
Common first-line steroid-sparing immunosuppression agents include mycophenolate mofetil (MMF) and azathioprine (AZA), but the efficacy and safety of these agents have been described in a very limited fashion, mainly in patients with connective tissue disease-associated ILD (CTD-ILD). MMF has often been used either as the first steroid-sparing medication or in the setting of intolerance or adverse events (AEs) with AZA.18, 21, 26, 27, 28, 29, 30 To date, there is only one study that compares MMF and AZA in patients with CTD-ILD. Oldham et al28 described pulmonary function stability and drug tolerability in 54 patients treated with AZA and 43 treated with MMF. However, an important limitation of this study was the inclusion of patients who had been concomitantly using other immunosuppressive agents, which in turn precludes an accurate evaluation of the specific benefit of AZA or MMF as monotherapy. Also, their benefits in M-ILD have been shown in small case series only.31 Therefore, our objective was to evaluate the effect of AZA and MMF on lung function and prednisone dose in myositis-related ILD.
Methods
We performed an electronic medical chart review of patients evaluated at the Johns Hopkins (JH) Myositis Center and JH ILD Clinic from July 2007 to March 2017. Patients had been prospectively enrolled in either a myositis or ILD institutional review board-approved natural history study. Patients with myositis-related (MSA and MAA) ILD who had received AZA or MMF without additional steroid-sparing agents to treat their ILD were included. Patients with myositis but no ILD were excluded. Similarly, patients who had been concomitantly receiving other immunosuppressive agents except corticosteroids were excluded. ILD was defined as the presence of interstitial lung infiltrates on high-resolution CT imaging after a multidisciplinary discussion among pulmonologists, rheumatologists and thoracic radiologists. Demographic information (age, sex, race, and smoking status), IIM subtype, prednisone dose at baseline and at follow-up visits, cancer, and mortality were also collected. Pulmonary function was serially measured and included spirometry and diffusing capacity of the lungs for carbon monoxide (Dlco) determination by single-breath carbon monoxide uptake.
Statistical Analyses
Baseline characteristics of patients with AZA and MMF were compared by Student t-tests or χ2 tests for continuous and categorical variables, respectively. The primary outcomes were the change in FVC % predicted and Dlco % predicted of patients with M-ILD who received treatment with AZA and MMF. Prednisone dose and drug AEs were also analyzed as secondary outcomes. Unadjusted linear mixed-effects models were used to compare the FVC % predicted and Dlco % predicted of patients treated with AZA and MMF. Time was treated as a continuous variable after a reasonable linear relationship was demonstrated between FVC % predicted and time. The mixed-effects model was used with random intercept and with independent correlation structure to account for repeated measurements from the same subject. This correlation structure was chosen on the basis of the exploratory data analysis from the autocorrelation function. No specific pattern was discovered that could indicate other types of correlation structure. Patients who were switched from MMF to AZA (or vice versa) were considered to be receiving one drug until an alternative drug was initiated and thereafter they were included in the other drug group. Adjusted linear mixed-effects models were used to compare the difference in FVC % predicted, Dlco % predicted, and prednisone dose from the time of initiation of treatment over time up to 60 months. Models were adjusted for age, sex, antisynthetase antibody, and smoking history. To illustrate these analyses, we plotted the mean FVC % predicted during treatment for each group as well as the difference in FVC % predicted in patients treated with AZA and MMF. Data on AEs is reported as the numbers of participants who experienced the AE at a moderate or severe level. Multiple AEs in the same patient were counted separately. If a patient had to be switched to a different agent because of AEs, these were included in the event analysis for the first drug. If the patient later experienced new AEs associated with the second agent, they were included in the event analysis for the second drug. Stata version 12.1 (StataCorp) was used for the analysis. The study protocol followed the standards norms of the Helsinki declaration and was approved by the JH Institutional Review Board (00007454, 00023365, and 00076981).
Results
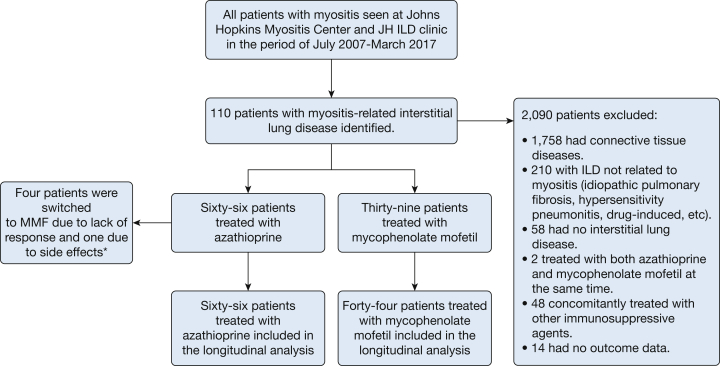
We identified 2,200 patients with myositis who were evaluated at the JH Myositis Center or JH ILD Clinic and provided consent to collect data. Of these patients, 110 were included in the analysis: 66 were treated with AZA and 44 with MMF (Fig 1). Patient cohort characteristics are summarized in Table 1. The mean age of subjects receiving AZA was 48.5 (SD 13.2) years, and that of patients receiving MMF was 52.5 (SD 11.6) years. Almost 70% of the AZA group and 75% of the MMF group were female. Forty-two percent of the AZA group and 50% of the MMF group were white (Table 1). Eighty-two percent of the patients met criteria for dermatomyositis or polymyositis, but only 67% had elevated creatine kinase levels. More patients receiving AZA had a Jo-1 antibody (53% vs. 15.9%; P < .0001). The AZA group had worse baseline lung function than the MMF group (respectively: FVC % predicted, 58.4% vs 72%; percent-predicted total lung capacity, 58.3% vs 69.9%; and Dlco % predicted, 54.5% vs 66.6%) and its members were receiving higher doses of prednisone at baseline (28.4 vs 18.1 mg, respectively). Only five patients switched drugs (Fig 1). Mean FVC % predicted, Dlco % predicted, and prednisone dose can be found in e-Table 1.
Figure 1.
STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) figure of patients included in the study. *Drug washout interval ranged from 3.5 to 24 months in four patients. ILD = interstitial lung disease; JH = Johns Hopkins; MMF = mycophenolate mofetil.
Table 1.
Baseline Characteristics of Patients With Myositis-Related Interstitial Lung Disease Treated With Azathioprine and Mycophenolate Mofetil
| Characteristic | Azathioprine (n = 66) | Mycophenolate Mofetil (n = 44) | P Value |
|---|---|---|---|
| Age at onset, mean ± SD, y | 48.5 ± 13.2 | 52.5 ± 11.6 | .106 |
| Women, No. (%) | 46 (69.7) | 33 (75) | .758 |
| Smoking history, No. (%)a | < .0001 | ||
| Never | 35 (53) | 10 (22.7) | |
| Ever smokerb | 21 (31.8) | 2 (4.5) | |
| Race, No. (%)c | .03 | ||
| White | 28 (42.4) | 22 (50) | |
| Black | 31 (47) | 11 (25) | |
| Other | 5 (7.6) | 3 (6.8) | |
| MSA, No. (%) | |||
| Anti-Jo-1 | 35 (53) | 7 (15.9) | < .0001 |
| Anti-PL-7 | 8 (12.1) | 5 (11.4) | .858 |
| Anti-PL-12 | 8 (12.1) | 2 (4.5) | .176 |
| Anti-EJ | 2 (3) | 0 (0) | .486 |
| Anti-OJ | 3 (4.5) | 0 (0) | .335 |
| Anti-SRP5 | 0 (0) | 2 (4.5) | .082 |
| Anti-Mi-2 | 1 (1.5) | 4 (9.1) | .053 |
| Anti-Mi-2b | 1 (1.5) | 3 (6.8) | .138 |
| Anti-Mi-2a | 0 (0) | 1 (2.3) | .147 |
| Anti-MDA5 | 1 (1.5) | 7 (15.9) | .039 |
| Anti-Nxp2 | 1 (1.5) | 2 (4.6) | .392 |
| MAA, No. (%) | |||
| Anti-Ro52 | 22 (33.3) | 9 (20.5) | .009 |
| Anti-Ro | 8 (12.1) | 6 (13.7) | .931 |
| Anti-Nt5c | 0 (0) | 1 (2.3) | .451 |
| Anti-PMS | 3 (4.5) | 11 (25) | .005 |
| Anti-PM75 | 3 (4.5) | 7 (15.9) | .079 |
| Anti-PM100 | 2 (3) | 8 (18.2) | .02 |
| Anti-SAE1 | 0 (0) | 1 (2.3) | .147 |
| Anti-U1-RNP | 2 (3) | 2 (4.5) | .894 |
| Anti-U2-RNP | 1 (1.5) | 0 (0) | .658 |
| Anti-U3-RNP | 0 (0) | 1 (2.3) | .219 |
| Anti-RNAP | 1 (1.5) | 0 (0) | .412 |
| Anti-HMGCR | 0 (0) | 1 (2.3) | .219 |
| Inflammatory myopathy subtype, No. (%)d | .525 | ||
| DM | 40 (60.6) | 26 (59.1) | |
| PM | 16 (24.2) | 8 (18.2) | |
| FVC % predicted, mean ± SD | 58.4 ± 19.1 | 72 ± 22.2 | .003 |
| TLC % predicted, mean ± SD | 58.3 ± 17.8 | 69.9 ± 19.7 | .011 |
| Dlco % predicted, mean ± SD | 54.5 ± 21.3 | 66.6 ± 26.2 | .021 |
| Prednisone dose, mean ± SD, mg | 28.4 ± 18.7 | 18.1 ± 12.2 | .002 |
| Malignancy, No. (%) | 1 (1.5) | 2 (4.5) | .458 |
| Deaths, No. (%) | 7 (10.6) | 2 (4.5) | .151 |
Dlco = diffusion capacity of the lungs for carbon monoxide; DM = dermatomyositis; MAA = myositis-associated autoantibody; MSA = myositis-specific autoantibody; PM = polymyositis; TLC = total lung capacity.
Ten pack-years or more.
Data were available only for 56 patients in the azathioprine group and for 12 in the mycophenolate mofetil group.
Data were available only for 64 patients in the azathioprine group and for 36 in the mycophenolate mofetil group.
Data were available only for 56 patients in the azathioprine group and for 34 in the mycophenolate mofetil group.
Mixed-Model Analysis
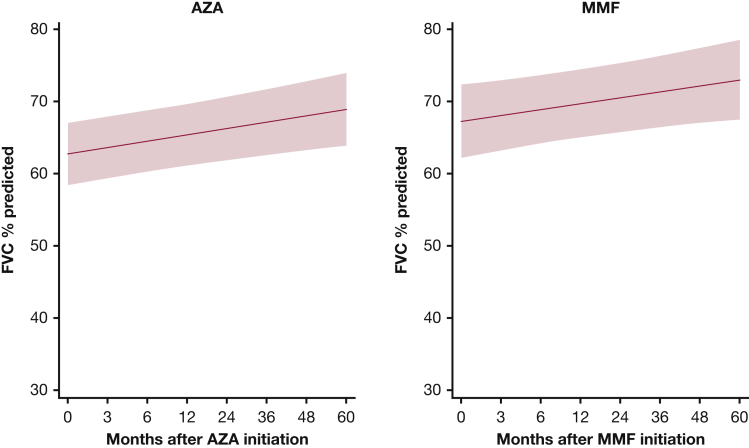
Overall, there was no statistically significant difference in FVC % predicted between the AZA and MMF groups at any time point (Fig 2, e-Table 2). However, both therapies significantly improved FVC % predicted at 24 months (AZA, 3.6% [P = .001]; MMF, 3.3% [P = .021]) (Fig 3, e-Table 3). The adjusted model also estimated that baseline FVC % predicted was 3.4% lower for patients treated with AZA compared with MMF (P = .375). This difference decreased over time and becomes 2.2% at 24 months (P = .496) (e-Table 2).
Figure 2.
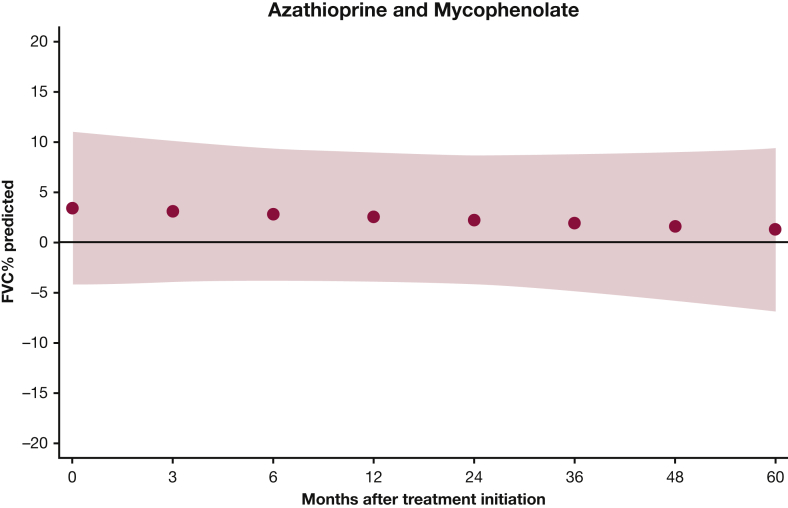
Mixed-effects model estimates for FVC % predicted over time for patients with myositis-related interstitial lung disease treated with azathioprine and mycophenolate mofetil (MMF). P = .6744. Red circles above the x axis indicate that the mean FVC % predicted was higher for the MMF group compared with the azathioprine group. The red shaded area represents the 95% CI.
Figure 3.
FVC % predicted in patients with myositis-related interstitial lung disease treated with azathioprine and mycophenolate mofetil. The red shaded area represents the 95% CI. AZA = azathioprine. See Figure 1 legend for expansion of other abbreviation.
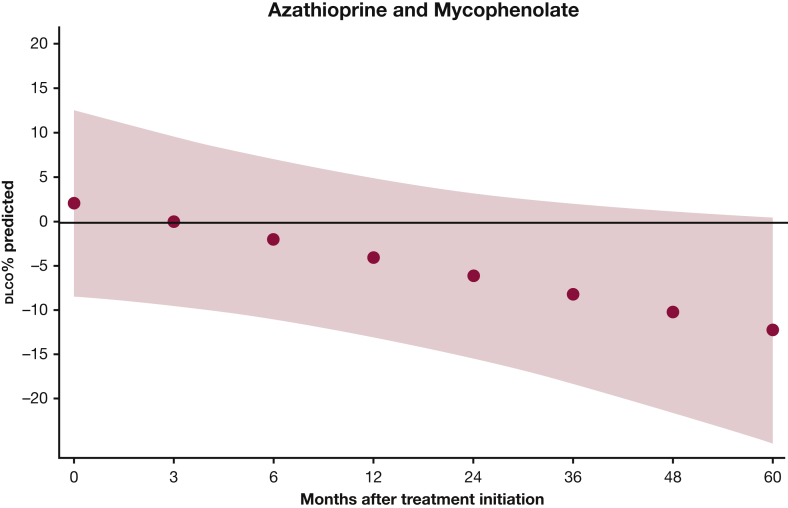
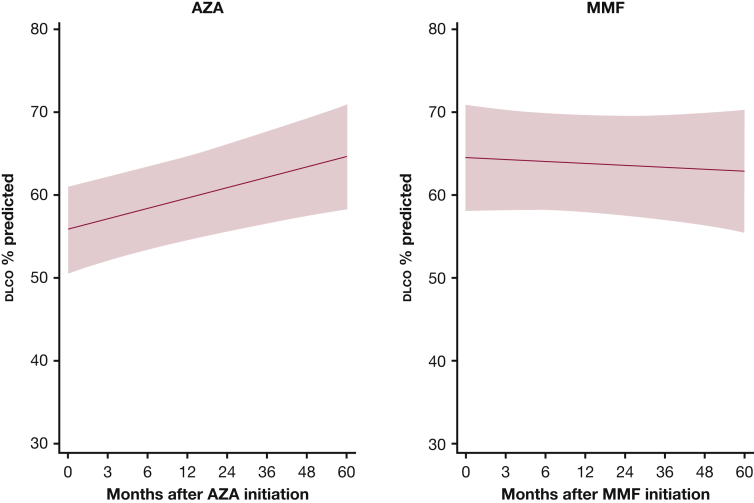
For Dlco % predicted, there was a statistically significant improvement for AZA (P = .002), but not for MMF (P = .657), at 24 months. Our model estimates that there was no statistically significant difference in Dlco % predicted between the two groups at 24 months (P = .192) (e-Table 2, Fig 4). However, Dlco % predicted increased over time in the AZA group and slightly decreased in the MMF group, making the difference between the two groups more pronounced at 48 months (–10.3%) and 60 months (–12.3%), although it did not reach statistical significance (e-Table 2, Fig 5).
Figure 4.
Mixed-effects model estimates for Dlco (diffusing capacity of the lungs for carbon monoxide) % predicted over time for patients with myositis-related interstitial lung disease treated with AZA and MMF. P = .1202. Red circles above the x axis indicate that the mean Dlco % predicted is higher in the MMF group compared with the AZA group; values below the x axis indicate that the mean Dlco % predicted is lower in the MMF group compared with the AZA group. The red shaded area represents the 95% CI. See Figure 1 and 3 legends for expansion of abbreviations.
Figure 5.
Dlco % predicted in patients with myositis-related interstitial lung disease treated with azathioprine and mycophenolate mofetil. The red shaded area represents the 95% CI. See Figure 1, 3, and 4 legends for expansion of abbreviations.
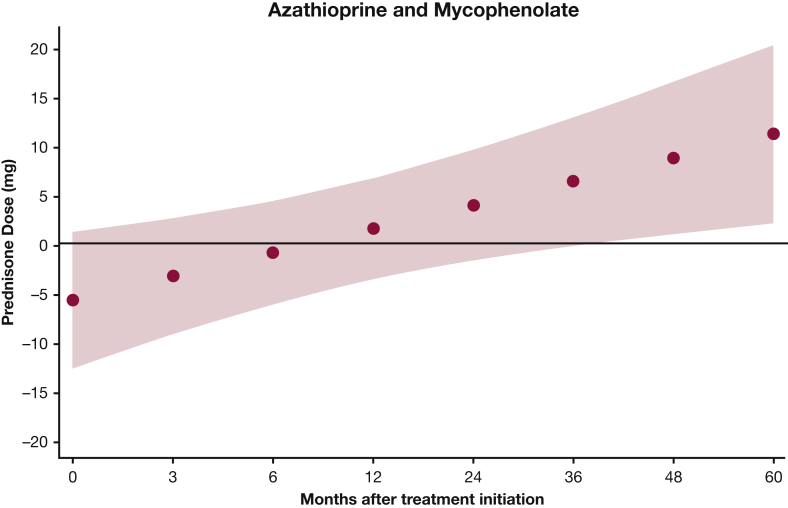
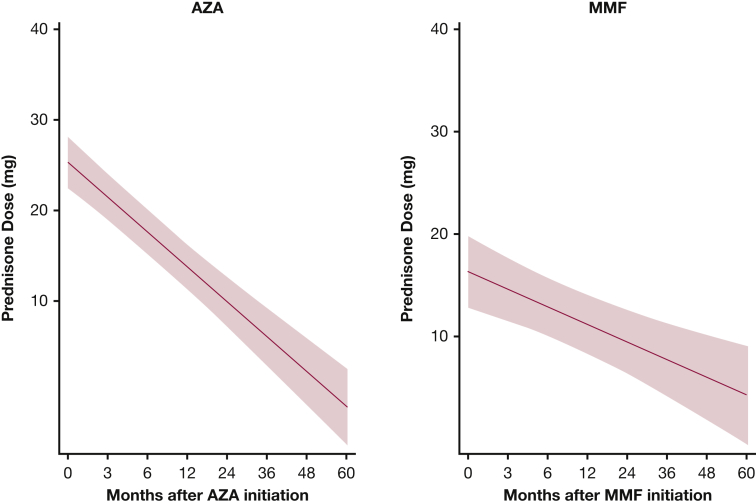
Our model estimates that there was no statistically significant difference in mean prednisone dose between the two treatments at 24 months (e-Table 2, Fig 6). However, this difference became significant at 36 months after treatment, when the prednisone dose was 6.6 mg lower in the AZA group (e-Table 2), albeit this analysis included fewer than 20 patients in each group. Also, the mean prednisone dose significantly decreased by 15.5 mg at 24 months after AZA initiation (P < .001) and by 6.9 mg after MMF (P < .001) (Fig 7).
Figure 6.
Mixed-effects model estimates for prednisone dose over time for patients with myositis-related interstitial lung disease treated with AZA and MMF. P = .023. Red circles below the x axis indicate that the mean prednisone dose was lower in the MMF group compared with the AZA group; values above the x axis indicate that the mean prednisone dose was higher in the MMF group. The red shaded area represents the 95% CI. See Figure 1 and 3 legends for expansion of abbreviations.
Figure 7.
Prednisone dose in patients with myositis-related interstitial lung disease treated with AZA and MMF. The red shaded area represents the 95% CI. See Figure 1 and 3 legends for expansion of abbreviations.
Exploratory subgroup analysis of treatment effect for patients treated with AZA showed that men had a higher FVC % predicted in comparison with women over time (P = .016), but no difference based on the presence of AS antibody was observed. In contrast, in patients treated with MMF, the presence of an AS antibody was associated with a lower FVC % predicted over time (P = .003), but no difference based on sex was found.
For a sensitivity analysis, we analyzed the data after excluding the five patients who were switched from AZA to the MMF group (Fig 1), and the outcome in the data was unchanged for FVC % predicted and Dlco % predicted. The only difference occurred in the prednisone outcome (P value became nonsignificant). Notably, the drug washout interval ranged from 3.5 to 24 months for four patients; only one was immediately switched from AZA to MMF.
Adverse Events
AEs are listed in Table 2. Overall, there was a higher rate of AEs with AZA than MMF (P = .04), with drug discontinuation occurring in 17% of subjects receiving AZA compared with 7.5% of subjects receiving MMF (P = .16). AEs between the two medications are similar except for transaminitis, which was more common with AZA (P = .04). The majority of AEs were tolerated without having to discontinue therapy except for one patient, who was switched to MMF. Dose reduction was accomplished without discontinuation in two subjects taking AZA and none with MMF.
Table 2.
Adverse Drug Events of Patients With Myositis-Related Interstitial Lung Disease Treated With Azathioprine and Mycophenolate Mofetil
| Adverse Event | Azathioprine (n = 66) |
Mycophenolate Mofetil (n = 44) |
All |
|---|---|---|---|
| No. (%) | No. (%) | No. (%) | |
| Transaminitis | 10 (15.2)a | 1 (2.3) | 11 (10)b |
| Nausea | 3 (4.5)a | 1 (2.3) | 4 (3.6) |
| Leukopenia | 3 (4.5) | 1 (2.3) | 4 (3.6) |
| Pancytopenia | 1 (1.5) | 0 | 1 (0.9) |
| Pneumonia | 1 (1.5) | 0 | 1 (0.9) |
| Rash | 1 (1.5) | 0 | 1 (0.9) |
| Insomnia | 0 | 1 (2.3) | 1 (0.9) |
| Abdominal pain | 0 | 1 (2.3) | 1 (0.9) |
| Diarrhea | 2 (3) | 0 | 2 (1.8) |
| Other | 1 (1.5) | 1 (2.3) | 2 (1.8) |
| Total | 22 (33.3) | 6 (13.6) | 28 (25.5)b |
These patients experienced both nausea and transaminitis.
P = .04.
Discussion
Our observational study of patients with M-ILD presents the largest data set on patients with M-ILD and describes the use of two therapies that are often considered first-line after corticosteroids. Strengths of the study include the number of patients included, diverse antibody profile of MSA and MAA (including the largest number of AS patients reported in the literature), and the inclusion of important outcomes of pulmonary function. Another relevant strength of this study is the evaluation of the specific effect of AZA and MMF, as patients who had been receiving other immunosuppressant drugs were excluded. We believe this distinction is very important to avoid a possible overestimation of the effect of AZA or MMF.
There have been only a few studies comparing MMF and AZA in CTD-ILD. Oldham et al28 described AZA as associated with a significant yearly increase in FVC % predicted of 1.53 (95% CI, 0.19-2.87; P = .025) and in Dlco % predicted of 4.91 (95% CI, 1.53-8.3; P = .004), whereas MMF was associated with a nonsignificant yearly decline in FVC % predicted of –0.56 (95% CI, –1.55 to 0.43; P = .27) and Dlco % predicted of –2.1 (95% CI, –4.62 to 0.42%; P = .1) in a study of 97 patients. An important limitation of this study was the inclusion of 13 patients who had been concomitantly receiving tacrolimus and seven patients who had been receiving immunoglobulins, which precludes an evaluation of the specific benefit of AZA or MMF as monotherapy. The largest clinical trials examining, to date, the efficacy of immunosuppression in the treatment of CTD-ILD are the Scleroderma Lung Studies I and II. Scleroderma Lung Study (SLS) I found that cyclophosphamide (CYC) was significantly associated with stability to modest improvement in FVC % predicted.23 However, CYC was associated with increased AEs.23 SLS II showed that FVC % predicted improved at 24 months in both the MMF and CYC groups, with a significantly lower number of subjects discontinuing MMF due to significant AEs.32 Another study looking at the treatment of chronic hypersensitivity pneumonitis (CHP) with MMF or AZA demonstrated that both treatments stabilized FVC % predicted and improved Dlco % predicted.33 Similarly, various small studies have examined the use of MMF in CTD-ILD,18, 21, 28 the largest of which examined 125 subjects with CTD-ILD.18 In this study, use of MMF was associated with FVC % predicted and Dlco % predicted stability in the subgroup with usual interstitial pneumonia, and improvement in those without this pattern. Moreover, MMF was also associated with significantly lower corticosteroid dose.18 Another retrospective study of 46 subjects with polymyositis/dermatomyositis-ILD demonstrated that therapy with CYC, AZA, and MMF had similar results when used for initial steroid-sparing therapy with improvement in dyspnea, pulmonary function stabilization, and a significant steroid dose reduction.21 Worth noting is that they reported a more favorable response in patients treated with MMF who were anti-Jo-1-positive, suggesting that this subgroup may have a better response to the drug. In general, less frequent monitoring and a favorable tolerability profile have been reported for MMF in CTD-ILD. However, larger studies are necessary to confirm this hypothesis.
Our treatment discontinuation rate was 17% in the AZA group and 7.5% in the MMF group, whereas the study on CHP presented lower rates of discontinuation (10.5% in the AZA group and 5.8% in the MMF group).33 Potential explanations for this difference include possible inherent higher AEs in patients with M-ILD given its frequent multiorgan involvement; the smaller sample size in the CHP study; or higher doses of AZA and MMF used to treat M-ILD, as we often try to achieve an MMF dosage of 3 g/d and an AZA dosage of 2 mg/kg/d to obtain maximal effect, which may result in greater toxicity and AEs. This might also explain why our discontinuation rates are similar to the study by Fischer et al18 and Tashkin et al32 but higher than the study by Oldham et al28 in patients with CTD-ILD. Fischer et al18 report a 10% discontinuation rate in patients taking MMF, whereas Oldham et al28 report only 5% in the MMF group and 13% in the AZA group. Sixty-five percent of the patients achieved a 3-g dose per day of MMF in the study by Fischer et al,18 whereas the median dose was 2 g/d in the study by Oldham et al.28 Morisset et al33 also demonstrated a reported AE rate of 15.8% with AZA and 13.7% with MMF,33 whereas our study report an AE rate of 33.3% with AZA and 13.6% with MMF. Possible explanations for this difference include a higher detection/reporting rate of AEs, given that even though up to 33.3% had reported AEs, most did not require dose reduction or drug discontinuation. However, this explanation does not account for the fact that our study still has higher discontinuation rates. Another likely possibility is that our patients usually present with a multisystem disease compared with CHP, which is, for the most part, thought to be a single-organ disease. Medications such as AZA and MMF are systemic therapies that, even though thought to help the underlying pulmonary disease, may have unintended consequences in other organ systems, causing intolerable AEs. This theory is supported by the fact that in SLS II, a similar rate of AEs was reported and there was a very similar MMF discontinuation rate (7.9% in SLS II and 7.5% in our study).27 Last, it is also possible that physicians prescribing these medications were more likely to report AEs and more likely to discontinue or change therapy compared with the Morisset et al33 study in CHP, especially considering the systemic and multiorgan involvement of the disease. Despite the rates of AEs, therapy with AZA and MMF was overall well tolerated and allowed for corticosteroid dose reduction.
There are a number of limitations to our study including the observational nature, indication bias, variable and limited follow-up of subjects over time which may overestimate or underestimate a beneficial drug effect, lack of systematic collection of adverse effects, and insufficient number of subjects included to perform subgroup analyses examining how medications may affect various disease subtypes or antibodies. Also, a direct comparison of two drugs retrospectively might not have accounted for the systematic differences in the cohorts in spite of using the mixed-model adjusted analysis. Even though we adjusted our model for the presence of antisynthetase antibodies, the role of a particular antibody in the response to therapy should be evaluated in larger studies. Our findings require further investigation and need to be confirmed with prospective studies; however, clinical trials in patients with rare conditions require multicenter collaborations and prolonged follow-up. Thus, in the meantime, these data may be helpful to guide therapy in patients with myositis-related interstitial lung disease.
In conclusion, our observational study showed that the use of AZA was associated with improvement of FVC % predicted and Dlco % predicted, and reduction of prednisone dose. The use of MMF was associated with improvement of FVC % predicted and reduction of prednisone dose, and stabilization of Dlco % predicted in patients with myositis-related ILD over 2 to 5 years. Higher AE rates were seen in the AZA group.
Acknowledgments
Author contributions: S. K. D. is the guarantor of the article and takes responsibility for the integrity of the work as a whole, from inception to published article. J. A. H., L. S., I. P.-F., A. L. M., L. C.-S., and S. K. D. conceived the idea for this manuscript and contributed equally to the conception of this work. J. A. H., I. P.-F., M. C.-D., C. J., J. A., J. J. P., and S. K. D. outlined the questions regarding the effect of AZA and MMF on myositis-related ILD, played an active role in the creation of the manuscript, and drafted the report. J. A. H., L. S., I. P.-F., and M. C.-D. were all directly involved in the acquisition of data for the article. All the authors critically edited the manuscript and approved the final product.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: L. C.-S. has received funding for clinical trials from Corbus Pharmaceuticals, Pfizer, Kezar, CSL Behring, and Novartis. She has also participated on advisory boards for OptionCare, Mallinckrodt, AstraZeneca, and Kezar and received royalties from Inova Diagnostics. None declared (J. A. H., L. S., I. P.-F., M. C.-D., C. J., J. A., J. J. P., S. A., A. L. M., S. K. D.).
Role of sponsors: The sponsors had no role in the conception of this work.
Other contributions: The authors thank the Arricale Family Research Fund for Pediatric Interstitial Lung Disease Pilot and Feasibility Grant, and the Rheumatology Research Foundation and the Huayi and Siuling Zhang Discovery Fund.
Additional information: The e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
Drs Huapaya and Silhan contributed equally to this work.
FUNDING/SUPPORT: Supported by the Arricale Family Research Fund for Pediatric Interstitial Lung Disease Pilot and Feasibility Grant. Rheumatology Research Foundation: K. Bridge Award. The Johns Hopkins Myositis Cohort was supported by the Huayi and Siuling Zhang Discovery Fund.
Supplementary Data
References
- 1.Hallowell R.W., Danoff S.K. Interstitial lung disease associated with the idiopathic inflammatory myopathies and the antisynthetase syndrome: recent advances. Curr Opin Rheumatol. 2014;26(6):684–689. doi: 10.1097/BOR.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 2.Fathi M., Dastmalchi M., Rasmussen E., Lundberg I.E., Tornling G. Interstitial lung disease, a common manifestation of newly diagnosed polymyositis and dermatomyositis. Ann Rheum Dis. 2004;63(3):297–301. doi: 10.1136/ard.2003.006122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solomon J., Swigris J.J., Brown K.K. Myositis-related interstitial lung disease and antisynthetase syndrome. J Bras Pneumol. 2011;37(1):100–109. doi: 10.1590/s1806-37132011000100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tillie-Leblond I., Wislez M., Valeyre D. Interstitial lung disease and anti-Jo-1 antibodies: difference between acute and gradual onset. Thorax. 2008;63(1):53–59. doi: 10.1136/thx.2006.069237. [DOI] [PubMed] [Google Scholar]
- 5.Friedman A.W., Targoff I.N., Arnett F.C. Interstitial lung disease with autoantibodies against aminoacyl-tRNA synthetases in the absence of clinically apparent myositis. Semin Arthritis Rheum. 1996;26(1):459–467. doi: 10.1016/s0049-0172(96)80026-6. [DOI] [PubMed] [Google Scholar]
- 6.Ye S., Chen X.X., Lu X.Y. Adult clinically amyopathic dermatomyositis with rapid progressive interstitial lung disease: a retrospective cohort study. Clin Rheumatol. 2007;26(10):1647–1654. doi: 10.1007/s10067-007-0562-9. [DOI] [PubMed] [Google Scholar]
- 7.Nash P., Schrieber L., Webb J. Interstitial lung disease as the presentation of anti-Jo-1 positive polymyositis. Clin Rheumatol. 1987;6(2):282–286. doi: 10.1007/BF02201038. [DOI] [PubMed] [Google Scholar]
- 8.Li Q., Wang L., Zhu C., Yan M., Zuo X., Zhang H. Profiling autoantibodies in idiopathic inflammatory myopathies. J Immunol. 2018;200(1 suppl) 45.30. [Google Scholar]
- 9.Ghirardello A., Zampieri S., Iaccarino L. [Myositis specific and myositis associated autoantibodies in idiopathic inflammatory myopathies: a serologic study of 46 patients] Reumatismo. 2005;57(1):22–28. doi: 10.4081/reumatismo.2005.22. [article in Italian] [DOI] [PubMed] [Google Scholar]
- 10.Cruellas M.G.P., dos Santos Trindade Viana V., Levy-Neto M., de Souza F.H.C., Shinjo S.K. Myositis-specific and myositis-associated autoantibody profiles and their clinical associations in a large series of patients with polymyositis and dermatomyositis. Clinics. 2013;68(7):909–914. doi: 10.6061/clinics/2013(07)04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen I.J., Jan Wu Y.J., Lin C.W. Interstitial lung disease in polymyositis and dermatomyositis. Clin Rheumatol. 2009;28(6):639–646. doi: 10.1007/s10067-009-1110-6. [DOI] [PubMed] [Google Scholar]
- 12.Grau J.M., Miro O., Pedrol E. Interstitial lung disease related to dermatomyositis: comparative study with patients without lung involvement. J Rheumatol. 1996;23(11):1921–1926. [PubMed] [Google Scholar]
- 13.Richards T.J., Eggebeen A., Gibson K. Characterization and peripheral blood biomarker assessment of anti-Jo-1 antibody-positive interstitial lung disease. Arthritis Rheum. 2009;60(7):2183–2192. doi: 10.1002/art.24631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinal-Fernandez I., Casal-Dominguez M., Huapaya J.A. A longitudinal cohort study of the anti-synthetase syndrome: increased severity of interstitial lung disease in black patients and patients with anti-PL7 and anti-PL12 autoantibodies. Rheumatology. 2017;56(6):999–1007. doi: 10.1093/rheumatology/kex021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson C., Connors G.R., Oaks J. Clinical and pathologic differences in interstitial lung disease based on antisynthetase antibody type. Respir Med. 2014;108(10):1542–1548. doi: 10.1016/j.rmed.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Arsura E.L., Greenberg A.S. Adverse impact of interstitial pulmonary fibrosis on prognosis in polymyositis and dermatomyositis. Semin Arthritis Rheum. 1988;18(1):29–37. doi: 10.1016/0049-0172(88)90032-7. [DOI] [PubMed] [Google Scholar]
- 17.Marie I., Hatron P.Y., Dominique S., Cherin P., Mouthon L., Menard J.F. Short-term and long-term outcomes of interstitial lung disease in polymyositis and dermatomyositis: a series of 107 patients. Arthritis Rheum. 2011;63(11):3439–3447. doi: 10.1002/art.30513. [DOI] [PubMed] [Google Scholar]
- 18.Fischer A., Brown K.K., Du Bois R.M. Mycophenolate mofetil improves lung function in connective tissue disease-associated interstitial lung disease. J Rheumatol. 2013;40(5):640–646. doi: 10.3899/jrheum.121043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marie I., Josse S., Hatron P.Y. Interstitial lung disease in anti-Jo-1 patients with antisynthetase syndrome. Arthritis Care Res. 2013;65(5):800–808. doi: 10.1002/acr.21895. [DOI] [PubMed] [Google Scholar]
- 20.Miller S.A., Glassberg M.K., Ascherman D.P. Pulmonary complications of inflammatory myopathy. Rheum Dis Clin North Am. 2015;41(2):249–262. doi: 10.1016/j.rdc.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Mira-Avendano I.C., Parambil J.G., Yadav R. A retrospective review of clinical features and treatment outcomes in steroid-resistant interstitial lung disease from polymyositis/dermatomyositis. Respir Med. 2013;107(6):890–896. doi: 10.1016/j.rmed.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Schnabel A., Reuter M., Biederer J., Richter C., Gross W.L. Interstitial lung disease in polymyositis and dermatomyositis: clinical course and response to treatment. Semin Arthritis Rheum. 2003;32(5):273–284. doi: 10.1053/sarh.2002.50012. [DOI] [PubMed] [Google Scholar]
- 23.Tashkin D.P., Elashoff R., Clements P.J. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354(25):2655–2666. doi: 10.1056/NEJMoa055120. [DOI] [PubMed] [Google Scholar]
- 24.Tazelaar H.D., Viggiano R.W., Pickersgill J., Colby T.V. Interstitial lung disease in polymyositis and dermatomyositis: clinical features and prognosis as correlated with histologic findings. Am Rev Respir Dis. 1990;141(3):727–733. doi: 10.1164/ajrccm/141.3.727. [DOI] [PubMed] [Google Scholar]
- 25.Lega J.-C., Reynaud Q., Belot A., Fabien N., Durieu I., Cottin V. Idiopathic inflammatory myopathies and the lung. Eur Respir Rev. 2015;24(136):216–238. doi: 10.1183/16000617.00002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swigris J.J., Olson A.L., Fischer A. Mycophenolate mofetil is safe, well tolerated, and preserves lung function in patients with connective tissue disease-related interstitial lung disease. Chest. 2006;130(1):30–36. doi: 10.1378/chest.130.1.30. [DOI] [PubMed] [Google Scholar]
- 27.Morganroth P.A., Kreider M.E., Werth V.P. Mycophenolate mofetil for interstitial lung disease in dermatomyositis. Arthritis Care Res. 2010;62(10):1496–1501. doi: 10.1002/acr.20212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oldham J.M., Lee C., Valenzi E. Azathioprine response in patients with fibrotic connective tissue disease-associated interstitial lung disease. Respir Med. 2016;121:117–122. doi: 10.1016/j.rmed.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dheda K., Lalloo U.G., Cassim B., Mody G.M. Experience with azathioprine in systemic sclerosis associated with interstitial lung disease. Clin Rheumatol. 2004;23(4):306–309. doi: 10.1007/s10067-004-0906-7. [DOI] [PubMed] [Google Scholar]
- 30.Weese W.C., Levine B.W., Kazemi H. Interstitial lung disease resistant to corticosteroid therapy: report of three cases treated with azathioprine or cyclophosphamide. Chest. 1975;67(1):57–60. doi: 10.1378/chest.67.1.57. [DOI] [PubMed] [Google Scholar]
- 31.Morisset J., Johnson C., Rich E., Collard H.R., Lee J.S. Management of myositis-related interstitial lung disease. Chest. 2016;150(5):1118–1128. doi: 10.1016/j.chest.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 32.Tashkin D.P., Roth M.D., Clements P.J. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med. 2016;4(9):708–719. doi: 10.1016/S2213-2600(16)30152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morisset J., Johannson K.A., Vittinghoff E. Use of mycophenolate mofetil or azathioprine for the management of chronic hypersensitivity pneumonitis. Chest. 2017;151(3):619–625. doi: 10.1016/j.chest.2016.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.