Abstract
Background:
Assessing vitamin A status in populations remains a high public health priority for low- and middle-income countries. However, analytical difficulties with serum retinol measurements persist in international laboratories. Nearly all participants in a Centers for Disease Control and Prevention external quality assessment program use HPLC to measure serum retinol, but round-to-round results failing to meet acceptable criteria suggest the need to provide a straightforward stable HPLC ultraviolet (UV) method that can be adopted by these laboratories to improve performance. We present a protein precipitation HPLC-UV method that measures serum retinol below the deficiency cutoff value (<0.7 μmol/L or 20 μg/dL) that is suitable for low- and middle-income countries and uses commercially available materials.
Methods:
Serum (25 μL) added to retinyl acetate was precipitated with acetonitrile (125 μL) to extract retinol. Solvent-based calibration solutions required no extraction. Calibration used either single-point (50 μg/dL) or multipoint solutions (0.52–100 μg/dL). C18 column (4.6 × 100 mm) and acetonitrile with 0.1% triethylamine/water (83/17, v/v) as isocratic mobile phase (1.1 mL/min), achieved baseline separation (7 minutes).
Results:
With only 25 μL of serum, the limit of detection was 0.52 μg/dL. Single- and multipoint calibration generated equivalent results. Over several years, between-run imprecision was ≤7.1% in multiple quality-control materials. Overall mean (CV) method bias for NIST-certified reference materials (e-series) was −0.2% (5.8%). Maximally, 180 samples were processed within 24 h.
Conclusions:
This method was robust and stable over years and accurately measured serum retinol with low-volume samples. Thus, it may be of interest to low- and middle-income countries and to pediatric and finger stick applications.
Interest in population vitamin A status has remained strong in the international community as evidenced by the inclusion of retinol or its surrogate, retinol-binding protein (RBP), in multiple large-scale national nutritional surveys (1-3). In most cases vitamin A status via assay of liver biopsy tissue (best indicator) cannot be conducted; thus, the World Health Organization recommends direct serum retinol measurements as a surrogate to assess prevalence and severity of vitamin A deficiency in populations (4). Low-resource laboratories sometimes prefer using nonchromatographic methods, such as immunoassays to measure RBP as a proxy, to assess vitamin A status. To better interpret RBP, measuring a subset of samples for both retinol and RBP is recommended (5). A Centers for Disease Control and Prevention (CDC)-operated laboratory external quality assessment program, VITAL-EQA, for several nutritional indicators has revealed both the extent of interest and analytical problems encountered by laboratories measuring serum retinol (approximately 20 laboratories per round). Nearly 50% of the data reported in the 2016–2017 rounds did not meet the program minimum criterion for bias (<±12% bias), and 35% of the data did not meet the program minimum criterion for imprecision (<7.1% CV) (6-8). To address ongoing analytical problems and to help build international laboratory capacity for direct retinol analysis, we developed and present a low serum volume HPLC-UV method. The method has been used since 2006 at the CDC Global Micronutrient Laboratory to conduct routine analysis and for target value assignments on the serum materials used for the VITAL-EQA program. This method can be adapted in laboratory settings with basic HPLC resources to generate accurate retinol data in support of long-term population studies on vitamin A status.
METHODS
Chemicals and reagents
The following chemicals were used: retinol (PN R7632) and retinyl acetate (PN R4632) from Sigma-Aldrich, ethanol from Pharmco-Aaper, acetonitrile (ACN)from Burdick and Jackson, and triethylamine from Fisher Scientific Inc. Purified water (18 MΩ) was obtained from a water purification system by Aqua Solutions Inc. Serum certified standard reference materials (SRM 968) and a 13-cis-retinol supplemented serum were purchased from NIST. Three levels of in-house QC materials were prepared with unmodified serum from US blood banks pooled to obtain sufficient volume (Solomon Park Research Institute and Tennessee Blood Services).
Equipment and supplies
The HPLC-UV system consisted of a Waters 2690 Alliance, a Waters 2996 photodiode array detector (fixed @ 325 nm), Empower 3 software (Waters Corporation). Centrifugation was conducted with a microcentrifuge (Hermle-Labortechnik). The HPLC column was a Hypersil Gold aQ polar endcapped column, 100 mm × 4.6 mm, C18, 3-μm particle size (Thermo Electron Corporation).
Calibration
Calibration can be performed with either a single-point calibration forced through zero or a multipoint calibration curve with no weighting. Quantification was performed with peak height for ease of integration. The calibrators were nonextracted solvent-based solutions containing retinol and internal standard (retinyl acetate). The retinol stock calibration solution was prepared by mixing pure retinol (either purchased at >97.5% purity or purified via HPLC purification to >97.5% purity) with ACN/ethanol (50/50, v/v), approximate concentration 30 μg/mL. The retinyl acetate internal standard solution was prepared by mixing pure retinyl acetate in 100% ethanol, approximate concentration 90 μg/dL. Purity and concentration of both solutions were assessed chromatographically and spectrophotometrically at 325 nm, [absorption coefficients (E1%1cm) retinol 1850 dL/g · cm and retinyl acetate 1550 dL/g · cm] (9, 10). For a single-point calibration curve, a concentration near 50 μg/dL was targeted by mixing a 25-μL aliquot of retinol (absorbance of 0.093) with a 50-μL aliquot of retinyl acetate (absorbance of 0.13). Multipoint calibration curves were prepared in the same manner, ranging from 0.52 μg/dL (limit of detection) to 100 μg/dL.
Sample preparation and instrumental analysis
For each specimen, a 50-μL aliquot of the retinyl acetate internal standard solution was placed into a 500-μL microcentrifuge tube, followed by a well-mixed 25-μL aliquot of sample (serum or water blank), followed by a 125-μL aliquot of 100% ACN (see Fig. 1 in the Data Supplement that accompanies the online version of this article at http://www.jalm.org/content/vol4/issue1). The solution was vigorously mixed either manually (30 s) or on a mechanical vortexer (10 s) and centrifuged for at least 3 min at 25 °C, 212g RCF. Alternatively, the solution may be left to gravimetrically settle for ≥5 min if a centrifuge is not available. An aliquot (50–75 μL) of the supernatant was transferred to an HPLC vial and placed in a 20 °C compartment for analysis. An aliquot (15 μL) of each sample extract was injected onto the C18 column (at ambient temperature) with a mobile phase of ACN with 0.1% triethylamine/water (83/17, v/v) at isocratic flow rate of 1.1 mL/min and 6–7 min run time. Once the column equilibrated and stabilized, the separation between the 2 compounds was around 2.2 min (retention time retinol = 4.1 min, retinyl acetate = 6.3 min). A typical run during a standard work day included a calibration curve, a blank, up to 180 unknowns, and 3 levels of QC before and after unknowns. Carryover was assessed by analyzing a blank after the highest calibrator and analyzing the QCs from high to low concentration.
RESULTS
This straightforward protein precipitation HPLC-UV method, continuously employed by the CDC laboratory for over a decade, produced highly consistent retinol results over time (Table 1). Nine different QC pools produced in-house and analyzed in duplicate in >30 analytical runs obtained on average close agreement with the CDC target value (range, 98% to 104%) and low average within-run (range, 0.8% to 7.1%) and between-run (range, 3.0% to 7.1%) imprecision. NIST-certified reference materials analyzed in ≥90 analytical runs obtained on average close agreement with the NIST target value (range, 98% to 102%) and low average within-run (range, 2.4% to 5.9%) and between-run (range, 5.0% to 6.8%) imprecision. The method also maintained an overall 1.1% average bias compared to NIST on NIST Round Robin challenges over 12 years with annual biases (10 samples per year) ranging between −5.3% to +6.1% (9 of the 12 years had biases of <5%).
Table 1.
Analytical performance of a low-volume protein precipitation HPLC-UV method for measuring serum retinol.
| Trueness |
Imprecision |
||||||
|---|---|---|---|---|---|---|---|
| Materiala | Period in use | Runsb (n) | Targetc, μg/dL |
Agreement with target, % |
Between-run SD |
Within-rund CV, % |
Between-run CV, % |
| 2006 Low QC | 2006–2013 | 130 | 22.2 | 98.0 | 1.6 | 2.2 | 6.8 |
| 2006 Mid QC | 133 | 45.6 | 98.4 | 3.0 | 2.4 | 6.3 | |
| 2006 High QC | 133 | 78.0 | 98.4 | 5.2 | 0.8 | 6.2 | |
| 2014 Low QC | 2014–2017 | 100 | 45.0 | 102 | 3.2 | 4.3 | 6.4 |
| 2014 Mid QC | 96 | 49.5 | 104 | 4.5 | 5.4 | 7.1 | |
| 2014 High QC | 99 | 108 | 102 | 8.7 | 7.1 | 6.6 | |
| 2017 Low QC | 2017+ | 31 | 27.2 | 100 | 1.3 | 3.2 | 3.8 |
| 2017 Mid QC | 31 | 42.6 | 99 | 1.6 | 2.2 | 3.0 | |
| 2017 High QC | 31 | 57.0 | 100 | 2.0 | 2.5 | 3.0 | |
| SRM 968c Level 1 | 2006–2011 | 100 | 84.1 | 101 | 5.8 | 2.4 | 6.8 |
| SRM 968c Level 2 | 98 | 48.4 | 102 | 2.5 | 4.4 | 5.0 | |
| SRM 968e Level 1 | 2011–2017+ | 90 | 34.1 | 100 | 2.0 | 5.9 | 5.8 |
| SRM 968e Level 2 | 91 | 48.2 | 101 | 2.8 | 4.5 | 5.9 | |
| SRM 968e Level 3 | 90 | 64.7 | 98 | 3.7 | 3.2 | 5.8 | |
QC pools prepared in-house; SRMs purchased from the NIST (https://www.nist.gov/). Transitions between 1 QC lot to the next QC lot were concurrently analyzed in a minimum of 20 runs. The previous lot was used for pass/fail while the new lot was being value assigned.
Each analytical run consisted of duplicate results for QC pools, 1 at the start of the run and 1 at the end of the run. Analytical runs with SRMs consisted of varying amounts of replication (n ≥ 1).
Target value for QC pools assigned on the basis of 20 characterization runs (n = 40 results per pool). Target values for SRMs assigned by NIST. Agreement with target was based on the number of runs shown in the table. SDs were based on pair means per run for QC pools and for individual results for NIST SRMs since SRMs were included at varying frequencies from run to run.
Within-run imprecision calculated with n ≥ 10 results for QC pools and n ≥ 4 for SRMs from 1 analytical run. Between-run imprecision data were based on the number of runs shown in the table, namely on duplicate measurements for QC pools and in most cases on singlicate measurements for SRMs; some runs contained additional replicates of SRMs.
The calibration curve produced linear results from the limit of detection (0.52 μg/dL) to 300 μg/dL (11). Although the peak heights observed with this method were approximately 20 times lower than those of a more complex CDC liquid–liquid extraction method on 100 μL of serum (9), the sensitivity was sufficient to distinguish normal and deficient serum retinol concentrations (<20 μg/dL or <0.7 μmol/L). Before 2017, we used a 50 μg/dL single-point calibration forced through zero. In 2017, we added additional calibration points and expanded the curve to 100 μg/dL. Both approaches showed equivalent results (2.2% difference, Pearson correlation r = 0.9996).
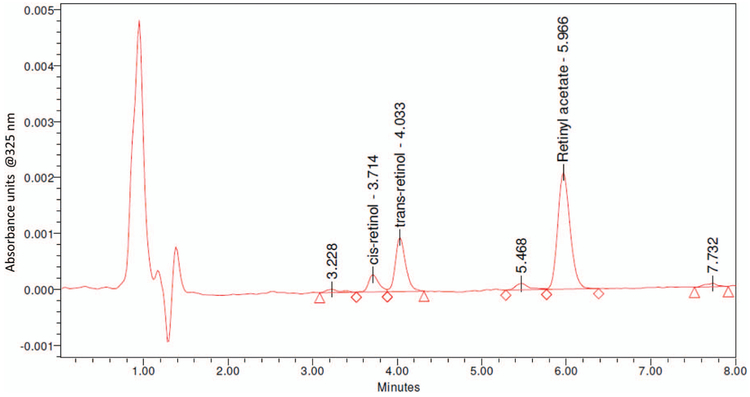
Before 2013, a C12 HPLC column maintained at 35 °C was used for this method until it was no longer manufactured. The replacement C18 Hypersil Gold aQ column (presented here), maintained at room temperature, baseline-separated retinol and retinyl acetate, had stable run-to-run retention times (retinol: 4.1 ± 0.2 min, retinyl acetate: 6.3 ± 0.3 min, n = 12 runs), and low column backpressures (approximately 690 psi). Equivalency between the columns was established (0.2% concentration difference between them). Both columns separated unknown impurities and isomers (13-cis- and trans-retinol) while maintaining run times within 6–7 min, which we observed from a 13-cis-retinol-supplemented serum included in a NIST quality assurance challenge (Fig. 1). To date, the column life for the C18 column exceeded 1100 injections. Previously, we observed the original C12 column life was 500–1500 injections. Additionally, we observed that column life may be extended by flushing the column daily with 100% ACN after completing a run. Column replacement is suggested when the peak quality declines or the peak separation degrades (see Fig. 2 in the online Data Supplement).
Fig. 1. Typical HPLC chromatogram for a serum sample containing approximately 42 μg/dL trans-retinol.
Baseline separation of cis- and trans-retinol and unknown impurities.
DISCUSSION
This assay has been in use for retinol value assignments for a CDC external quality assessment program for over a decade and has demonstrated great accuracy and long-term stability. Compared to other HPLC methods, we believe that it has many features that make it valuable for low-resource laboratories performing vitamin A status assessment: namely, simplicity of no liquid–liquid extraction, optional single-point calibration, low sample volume, speed and throughput, HPLC column flexibility, excellent trueness and sensitivity, and acceptable precision (9, 12-17). If the column described is unavailable, other columns were tested and found to provide good separation between trans-retinol and the internal standard within 10 min including C8, C12, C18, pentafluorophenyl, and phenyl hexyl columns offered by Waters, Agilent Technologies, Supelco, and Thermo Fisher Scientific (data not included).
Given the uncertainties with measuring RBP as a surrogate indicator for vitamin A status, a direct measure of retinol in serum is preferred (4, 5, 18), particularly if used in conjunction with other biomarkers as recommended by the World Health Organization (4, 19). Although most methods, including serum retinol or stable isotope techniques that provide whole-body vitamin A status (20), are not useful as measures of vitamin A status in individuals, they can be useful for determining population prevalence of vitamin A deficiency (4). The streamlined serum retinol method presented here can be adopted in most low-resource laboratories, uses readily available supplies, and can be successfully used to support long-term longitudinal studies that assess vitamin A status.
Supplementary Material
IMPACT STATEMENT.
This straightforward HPLC ultraviolet method will benefit low-resource laboratories performing retinol status assessment for population surveillance. It uses commercially available analytical instrumentation, supplies, and protein precipitation on minimal serum volume. The accuracy of this method can be verified by use of certified reference materials.
Acknowledgments:
Bridgette M. Toombs (formally Haynes) provided laboratory assistance for data presented in this manuscript.
Disclaimer: The findings and conclusions in this study are those of the authors and do not necessarily represent the views of the US Department of Health and Human Services, or the US Centers for Disease Control and Prevention. Use of trade names and commercial sources is for identification only and does not constitute endorsement by the US Department of Health and Human Services, or the US Centers for Disease Control and Prevention.
Glossary
- UV
ultraviolet
- RBP
retinol-binding protein
- CDC
Centers for Disease Control and Prevention
- ACN
acetonitrile
- SRM
standard reference material
Footnotes
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest: Employment or Leadership: M. Chaudhary-Webb, Centers for Disease Control and Prevention. Consultant or Advisory Role: None declared. Stock Ownership: None declared. Honoraria: None declared. Research Funding: None declared. Expert Testimony: None declared. Patents: None declared.
Role of Sponsor: No sponsor was declared.
REFERENCES
- 1.UNICEF. Nepal National Micronutrient Status Survey— 2016. https://www.unicef.org/nepal/media/1206/file (Accessed February 2019).
- 2.Uganda Bureau of Statistics. Uganda Demographic and Health Survey 2016. http://www.ubos.org/onlinefiles/uploads/ubos/pdf%20documents/Uganda_DHS_2016_KIR.pdf (Accessed February 2019).
- 3.National Statistical Office. Malawi Micronutrient Survey Key Indicators Report 2015–16. https://dhsprogram.com/pubs/pdf/FR319/FR319m.pdf (Accessed February 2019).
- 4.World Health Organization. Serum retinol concentrations for determining the prevalence of vitamin A deficiency in populations WHO/NMH/NHD/MNM/11.3. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization; 2011. [Google Scholar]
- 5.Tanumihardjo S, Russell R, Stephensen C, Gannon B, Craft N, Haskell M, et al. Biomarkers of nutrition for development (BOND)-vitamin A review. J Nutr 2016;146:1816S–48S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fraser C, Petersen P, Libeer J, Ricos C. Proposals for setting generally applicable quality goals solely based on biology. Ann Clin Biochem 1997;34:8–12. [DOI] [PubMed] [Google Scholar]
- 7.Lacher D, Hughes J, Carroll M. Estimate of biological variation of laboratory analytes based on the Third National Health and Nutrition Examination Survey. Clin Chem 2005;51:450–2. [DOI] [PubMed] [Google Scholar]
- 8.Haynes BM, Schleicher RL,Jain RB, Pfeiffer CM. The CDC VITAL-EQA program, external quality assurance for serum retinol, 2003–2006. Clin Chem Acta 2008;390:90–96. [DOI] [PubMed] [Google Scholar]
- 9.Sowell A, Huff D, Yeager P, Caudill S, Gunter E. Retinol, α-tocopherol, lutein/zeaxanthin, 3-cryptoxanthin, lycopene, α-carotene, trans-β-carotene, and four retinyl esters in serum determined simultaneously by reversed-phase HPLC with multiwavelength detection. Clin Chem 1994;40:411–6. [PubMed] [Google Scholar]
- 10.Hulshof P, van Roekel-Jansen T, van de Bovenkamp P, West C. Variation in retinol and carotenoid content of milk and milk products in The Netherlands. J Food Compos Anal 2006;19:67–75. [Google Scholar]
- 11.Walsh JL. Taylor's series and approximation to analytic functions. Bull Am Math Soc 1946;52:572–9. [Google Scholar]
- 12.DeRuyter MGM, De Leenheer AP. Determination of serum retinol (vitamin A) by high-speed liquid chromatography. Clin Chem 1976;22:1593–5. [PubMed] [Google Scholar]
- 13.DeRuyter MGM, De Leenheer AP. Simultaneous determination of retinol and retinyl esters in serum or plasma by reversed-phase high performance liquid chromatography. Clin Chem 1978;24:1920–3. [PubMed] [Google Scholar]
- 14.Hess D, Keller HE, Oberlin B, Bonfanti R, Schuep W. Simultaneous determination of retinol, tocopherols, carotenes and lycopene in plasma by means of high-performance liquid chromatography on reversed phase. Int J Vitam Nutr Res 1991;61:232–8. [PubMed] [Google Scholar]
- 15.Schmidt CK, Brouwer A, Nau H. Chromatographic analysis of endogenous retinoids in tissues and serum. Anal Biochem 2003;315:36–48. [DOI] [PubMed] [Google Scholar]
- 16.Chatzimichalakis PF, Samanidou VF, Papadoyannis IN. Development of a validated liquid chromatography method for the simultaneous determination of eight fat-soluble vitamins in biological fluids after solid-phase extraction. J Chromatogr B 2004;805:289–96. [DOI] [PubMed] [Google Scholar]
- 17.Martinefski MR, Scioscia S, Contin MD, Samassa P, Lucangioli SE, Tripodi VP. A simple microHPLC-UV method for the simultaneous determination of retinol and α-tocopherol in human plasma. Application to intrahepatic cholestasis of pregnancy. Anal Methods 2014;6:3365–9. [Google Scholar]
- 18.Stevens GA, Bennett JE, Hennocq Q, Lu Y, De-Regil LM, Rogers L, et al. Trends and mortality effects of vitamin A deficiency in children in 138 low-income and middle-income countries between 1991 and 2013: a pooled analysis of population-based surveys. Lancet Glob Health 2015;3:e528–36. [DOI] [PubMed] [Google Scholar]
- 19.Talsma EF, Verhoef H, Brouwer ID, Mburu-de Wagt AS, Hulshof PJM, Melse-Boonstra A. Proxy markers of serum retinol concentration, used alone and in combination, to assess population vitamin A status in Kenyan children: a cross-sectional study. BMC Medicine 2015;13:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lietz G, Furr HC, Gannon BM, Green MH, Haskell M, Lopez-Teros V, et al. Current capabilities and limitations of stable isotope techniques and applied mathematical equations in determining whole-body vitamin A status. Food Nutr Bull 2016;37(2 Suppl):S87–103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.