Abstract
Purpose:
Combining anti-GD2 (disialoganglioside) monoclonal antibody with GM-CSF, IL2 and isotretinoin is now FDA-approved for high-risk neuroblastoma (NB) minimal residual disease (MRD) therapy. The humanized anti-GD2 antibody conjugated to IL2 (hu14.18-IL2) has clinical activity in NB and is more effective in NB-bearing mice than antibody and cytokine given separately. We therefore evaluated the safety, tolerability and anti-tumor activity of hu14.18-IL2 given with GM-CSF and isotretinoin in a schedule similar to standard MRD therapy.
Patients and Methods:
Hu14.18-IL2 was given at the recommended phase II dose of 12 mg/m2/d on days 4-6 of a 28-day cycle with GM-CSF (250 mg/m2/dose, days 1-2 and 8-14) and isotretinoin (160 mg/m2/day, days 11-25). Tolerability was determined based on the number of unacceptable toxicities observed. Response was evaluated separately for patients with disease measurable by standard radiologic criteria (stratum 1), and for patients with disease evaluable only by I123-metaiodobenzylguanidine (I123-MIBG) scan and/or bone marrow histology (stratum 2).
Results:
Fifty-two patients with recurrent or refractory NB were enrolled, 51 were evaluable for toxicity and 45 were evaluable for response. Four patients had unacceptable toxicities, well below the protocol-defined rule for tolerability. Other Grade 3 and 4 non-hematologic toxicities were expected and reversible. No responses were seen in stratum 1 (n=14). In stratum 2 (n=31), 5 objective responses were confirmed by central review (3 complete, 2 partial).
Conclusions:
Hu14.18-IL2 given in combination with GM-CSF and isotretinoin is safe and tolerable. Patients with MIBG and/or bone marrow only disease had a 16.1% response rate, confirming activity of the combination.
INTRODUCTION
Neuroblastoma is a disease characterized by its clinical and biologic heterogeneity with half of all patients presenting with high-risk disease features including wide spread dissemination and/or adverse tumor specific biology1. While roughly 70% of children with high-risk neuroblastoma respond initially to intensive multimodal induction and consolidation therapy, half will eventually die from recurrent disease, most often due to residual, refractory disease that is undetectable, but present at the end of frontline therapy2. The differentiating agent isotretinoin has historically been used to treat this minimal residual disease (MRD) 3, 4. More recently an immunotherapy strategy has been studied, using a monoclonal antibody (mAb) to target the disialonganglioside GD2 which is highly expressed by neuroblastomas and has restricted distribution in normal tissue 5–7. A randomized trial conducted by the Children’s Oncology Group demonstrated superior outcome for those who received treatment with the chimeric monoclonal anti-GD2 antibody ch14.18 (dinutuximab) combined with alternating cycles of granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin 2 (IL2) in addition to isotretinoin therapy compared to treatment with isotretinoin alone 8. This combination is now the standard of care for treatment of MRD in high-risk neuroblastoma patients in North America9.
Hu14.18-IL2 is an immunocytokine consisting of the humanized 14.18 anti-GD2 mAb linked to IL210. Preclinical data suggest activity of hu14.18-IL2 is mediated by activation of antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity via the binding of hu14.18-IL2 to GD2 on the tumor cell surface, followed by binding to Fc receptors on effector cells along with activation of NK and T cells via IL2 receptor binding 10–12. The immunocytokine has activity in neuroblastoma mouse models 13 and has been shown in mice to have superior activity compared to a mixture of separate monoclonal antibody and IL214, 15. Phase I testing of hu14.18-IL2 in pediatric patients demonstrated biologic activity and clinical tolerability at a maximal tolerated dose of 12 mg/m2/day for 3 days16. Dose limiting toxicities included hypotension and allergic reactions. Phase II testing of Hu14.18-IL2 showed tolerability and promising activity in the MRD setting17.
Given the preclinical and clinical activity of hu14.18-IL2, we postulated that substituting this immunocytokine in place of ch.14.18 and IL2 in the now standard MRD regimen might improve high-risk neuroblastoma patient outcomes. Hu14.18-IL2 has only been tested previously as a single agent and therefore the primary objectives of this study were to evaluate the safety, tolerability and anti-tumor activity of hu14.18-IL2 given in combination with GM-CSF and isotretinoin in a schedule similar to current, standard MRD therapy8.
PATIENTS AND METHODS
The National Cancer Institute was the sponsor of this phase II single arm trial [Clinicaltrials.gov trial number: ] which ran from September 26, 2011 – August 17, 2012. Hu14.18-IL2 was supplied collaboratively by the National Cancer Institute Biological Resources Branch (NCI-BRB, Frederick, MD) via a cooperative research and development agreement, currently held by Apeiron Biologics (Vienna, Austria). The study was conducted by the Children’s Oncology Group (COG) following ethical principles of the Declaration of Helsinki. Each COG site’s Institutional Review Board approved the study. Patients and/or legal guardians provided written informed consent with assent obtained as appropriate prior to patient enrollment.
Patient Eligibility
Patients with recurrent or refractory neuroblastoma (age 12 months to 30 years) were eligible. For patients with persistent tumor after frontline therapy (primary refractory disease), a biopsy demonstrating viable tumor was required. Patients were required to have tumor imaging and a bone marrow evaluation done within 21 days of enrollment and were required to have either measurable tumor by CT or MRI, defined as a minimum of 20 mm in at least 1 dimension, a metaiodobenzylguanidine (MIBG) scan with uptake in a minimum of one site or a bone marrow aspirate or biopsy with tumor cells seen on routine morphology in at least one sample. Eligibility required performance status, organ function, recovery from prior therapy and life expectancy the same as required in the prior hu14.18-IL2 Phase II study17. Patients who had previously received therapeutic anti-GD2 antibody were eligible provided that they did not have progressive disease or a severe allergic reaction during their previous treatment. Exclusion criteria included patients with central nervous system tumor involvement, patients requiring chronic immunosuppression and patients with a prior history of ventilator support related to lung injury, due to toxicities seen in the Phase II trial. Patients were also excluded if they had symptomatic pleural effusions or ascites, had major surgery within 2 weeks of enrollment or had organ allografts (including bone marrow).
Protocol therapy
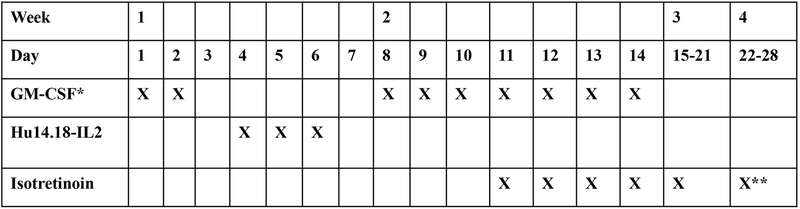
A treatment course was 28 days in length (Figure 1). GM-CSF was given subcutaneously at a dose of 250 mg/m2 on days 1 through 2 and days 8 through 14. Hu14.18-IL2 (12 mg/m2/dose) was administered on an inpatient basis as a four-hour intravenous infusion on days 4 through 6. Patients received indomethacin (0.5 mg/kg/dose) every 6 hours while receiving the immunocytokine. Isotretinoin was given at standard dosing (160 mg/m2/day, divided twice daily) for 14 days starting on day 11. GM-CSF was not given concurrently with Hu14.18-IL2 due to concern of neurotoxicity seen in a prior study when GM-CSF and IL2 were given concurrently (but not seen when IL2 and GM-CSF were given sequentially) 18, 19.
Figure 1.
Treatment course schema. GM-CSF (250 mg/m2 subcutaneously) was given on days 1 through 2 and days 8 through 14. Hu14.18-IL2 (12 mg/m2/dose) was given as a four-hour intravenous infusion on days 4 through 6. Isotretinoin (160 mg/m2/day, divided twice daily) was given for 14 days starting on day 11.
*Hold GM-CSF 24 hours prior and 24 hours after hu14.18-IL2
**Stop on day 24
Disease evaluations were done every two courses and response was determined using the International Neuroblastoma Response Criteria20. Treatment could be continued for a response of stable disease (SD) or better for up to 4 courses. After 4 courses of treatment, patients with at least a partial response (PR) could continue treatment for up to a total of 10 courses, provided that they did not have disease progression or drug intolerance.
Toxicity and Evaluation of Response
Toxicities were graded by the NCI Common Toxicity Criteria (v4.0). A dose limiting toxicity (DLT) for hu14.18-IL2 was defined as any ≥Grade 3 toxicity, with certain reversible exceptions identified in the phase I study 16. Hu14.18-IL2 treatment was held for a DLT and restarted at 50% the previous dose once toxicity resolved. DLTs for GM-CSF and isotretinoin were defined standardly. GM-CSF was held if the total white blood cell count was >50,000/μL but this was not considered a toxicity.
All patients who completed ≥2 courses of combination therapy or who had an event (relapse or progressive disease) at any time in the study were evaluable for inclusion in the response analysis. All responses were confirmed by independent radiology and pathology review.
Response for patients with measurable disease (stratum 1) was determined using the Response Evaluation Criteria in Solid Tumors (RECIST). Response for patients with evaluable disease (MIBG avid and/or bone marrow disease only, stratum 2) was determined as follows:
MIBG Response.
Patients graded locally with complete response (CR) or partial response (PR) by MIBG were scored by central review using Curie scoring 21–24. CR was defined by complete resolution of all MIBG avid lesions. PR was defined as a reduction in MIBG avid lesions so that the relative Curie score (Curie score at response evaluation/baseline Curie score) was between 0.2 and 0.5.
Bone Marrow Response:
For patients who entered with bone marrow disease (neuroblastoma identified in the bone marrow aspirate and/or biopsy by the local pathologist using standard histology), CR was defined as no tumor cells detectable by morphology and immunocytologic analysis on two subsequent bilateral bone marrow aspirates/biopsies done ≥3 weeks apart. Progressive disease (PD) was defined as ≥25% tumor in the marrow and a doubling in the percentage of tumor. Stable disease (SD) was defined as persistence of disease that does not meet criteria for CR or PR.
Disease Burden Assessment
A secondary aim of the study was to describe the disease burden of the stratum 2 subjects. This was done through centralized Curie scoring of each patient’s baseline MIBG scan. In addition, treating institutions described baseline bone marrow disease following bilateral bone marrow aspirates and biopsies based on the following scale: score “0” if no tumor seen in any of the enrollment specimens; score “1” if less than 10% tumor cells seen in all specimens but some tumor cells seen in at least one enrollment specimen; score “2” if greater than or equal to 10% tumor cells seen in at least one enrollment specimen.
Immunologic Monitoring (Serum hu14.18-IL2 levels, sIL2R, anti-hu14.18-IL2 antibody)
Peripheral blood samples were obtained prior to the first dose of the hu14.18-IL2 immunocytokine and at 3 subsequent timepoints, for each cycle of hu14.18-IL2 treatment from all patients providing consent. Serum ELISA assays determined the peak hu14.18-IL2 serum levels achieved with each intravenous infusion during the 3 courses of hu14.18-IL2, as previously described 23–25. Serum obtained following each course of therapy was also assayed by bridging ELISA for the generation of an antibody response to the hu14.18-IL2 immunocytokine, as previously described16. The peak anti-immunocytokine antibody was graded as negative, intermediate, or positive based on optical density (O.D. units). Serum soluble IL2 receptor (sIL2R) alpha levels were determined using the Human CD25/IL2 R alpha DuoSet ELISA Kit (R&D Systems, Minneapolis, MN, catalog number DY223), following manufacturer’s instructions.
Statistical considerations
Activity of hu14.18-IL2 combined with GM-CSF and isotretinoin was evaluated separately within each patient stratum. Safety and tolerability were evaluated in the overall cohort using a two-stage design as described below.
The primary objectives of this study were to evaluate safety and tolerability as well as efficacy. The primary endpoints, respectively, were the occurrence of an unacceptable DLT and occurrence of a best overall response of CR, very good partial response (VGPR), or PR. A two-stage stopping rule was used to monitor for an excessive number of unacceptable DLTs. “Unacceptable” was defined as a requirement for pressor and/or ventilator support due to acute vascular leak syndrome or Grade 3 or 4 neurotoxicity with MRI evidence of new CNS thrombi, infarction, or bleeding. The combination therapy was considered intolerable if there was ≥2 unacceptable toxicities in the first 10 patients or ≥7 unacceptable toxicities in the first 30 patients. This rule had a 91% power and type I error of 0.098 under the null hypothesis that the probability of having an unacceptable DLT is <5% and the alternative is >28%.
Responders were defined as evaluable patients who demonstrated a best overall response of CR, VGPR, or PR. Response was analyzed separately within stratum 1 and stratum 2 patients using two separate Simon’s two-stage rules. For stratum 1, if 1 or more patient responded out of the first 12 evaluable patients, 9 more evaluable patients were enrolled. If 2 or more patients out of 21 total evaluable patients responded, the regimen was considered effective. The rule for stratum 1 has 90% power to detect a true response rate of 20% (under a null hypothesis of 1%) with a Type 1 error rate of 0.017. For stratum 2, if 2 or more patients responded out of the first 15 evaluable patients, 15 more evaluable patients were enrolled. If 5 or more patients out of 30 total evaluable stratum 2 patients responded, the regimen was considered effective. The rule for stratum 2 has 92% power to detect a true response rate of 28% (under a null hypothesis of 7%) with a Type 1 error rate of 0.048.
Median baseline disease burden between responders and non-responders was compared using a Wilcoxon rank sum test. Mixed models with a compound symmetry covariance structure were built with data from courses 1 and 2 to test for differences [by stratum, responder status (among stratum 2 patients), and prior anti-GD2 therapy] in peak serum hu14.18-IL2 levels on days 4 and 6 post-infusion. The dependent variable was serum hu14.18-IL2 level and the independent variables included time point (categorical variable with four levels, one for each of the two peak time points across two courses, ordered chronologically), variable of interest, and an interaction term. Similarly, mixed models were constructed for serum IL2 receptor levels at various time points [prior to hu14.18-IL2 and 2, 3 and 7 days following the first day (day 4) of immunocytokine treatment] across courses in order to assess in vivo immune activation, in comparison to baseline (prior to hu14.18-IL2, using contrasts) and by responder status (stratum 2 patients only). Fisher’s exact test was used to evaluate the association between response and development of anti-hu14.18-IL2 antibody among stratum 2 patients.
RESULTS
Patient characteristics
Fifty-two eligible patients were enrolled, 16 in stratum 1 and 36 in stratum 2. Characteristics of these patients are shown in Table 1. The 16 patients in stratum 1 received a total of 43 treatment courses (median, 3 courses) and the 36 patients in stratum 2 received a total of 120 courses (median, 2 courses).
Table 1:
Patient Characteristics by Stratum
| Stratum 1 | Stratum 2 | All | |
|---|---|---|---|
| Enrolled | 16 | 36 | 52 |
| Eligible | 16 | 36 | 52 |
| Evaluable for toxicity | 15 | 36 | 51 |
| Evaluable for response | 14 | 31 | 45 |
| Median age at diagnosis (range), years | 3.8 (1.7-17.4) | 5.0 (2.1-21.7) | 4.6 (1.7-21.7) |
| Median age at enrollment (range), years | 6.9 (3.4-21.4) | 6.9 (3.4-21.4) | 8.3 (2.5-26.9) |
| MYCN amplified:nonamplified:unknown | 2:7:7 | 1:25:10 | 3:32:17 |
| Prior antibody therapy: | 3 | 8 | 11 |
| • Ch14.18 | 3 | 3 | 6 |
| • 3F8 | 0 | 3 | 3 |
| • Hu14.18K322A | 0 | 2 | 2 |
Tolerability
Fifty-one patients were evaluable for toxicity. The one inevaluable patient withdrew consent prior to receiving treatment. Table 2 shows the Grade 3 or greater toxicities experienced over all treatment courses.
Table 2:
Treatment related grade 3 and greater toxicities
| Adverse Event N(%)* | Stratum 1** (15 patients) | Stratum 2*** (36 patients) |
|---|---|---|
| No adverse events | 4 (26.7) | 3 (8.3) |
| Anemia | 4 (26.7) | 3 (8.3) |
| Febrile neutropenia | 0 | 1 (2.8) |
| Nausea | 0 | 2 (5.6) |
| Vomiting | 0 | 1 (2.8) |
| Fever | 2 (13.3) | 11 (30.6) |
| Pain | 3 (20.0) | 14 (38.9) |
| Allergic reaction | 0 | 2 (5.6) |
| Catheter related infection | 0 | 2 (5.6) |
| Enterocolitis infectious | 1 (6.7) | 0 |
| Infections and infestations – Other, specify | 1 (6.7) | 1 (2.8) |
| Lung infection | 0 | 1 (2.8) |
| Upper respiratory infection | 0 | 1 (2.8) |
| Alanine aminotransferase increased | 0 | 12 (33.3) |
| Aspartate aminotransferase increased | 0 | 9 (25.0) |
| Blood bilirubin increased | 5 (33.3) | 9 (25.0) |
| GGT increased | 0 | 3 (8.3) |
| Lymphocyte count decreased | 1 (6.7) | 7 (19.4) |
| Neutrophil count decreased | 0 | 4 (11.1) |
| White blood cell decreased | 1 (6.7) | 2 (5.6) |
| Platelet count decreased | 3 (20.0) | 8 (22.2) |
| Weight gain | 0 | 1 (2.8) |
| Anorexia | 0 | 2 (5.6) |
| Dehydration | 0 | 1 (2.8) |
| Hypercalcemia | 1 (6.7) | 0 |
| Hyperglycemia | 3 (20.0) | 2 (5.6) |
| Hypertriglyceridemia | 0 | 1 (2.8) |
| Hypoalbuminemia | 1 (6.7) | 1 (2.8) |
| Hypocalcemia | 1 (6.7) | 3 (8.3) |
| Hypokalemia | 3 (20.0) | 5 (13.9) |
| Hyponatremia | 1 (6.7) | 0 |
| Hypophosphatemia | 2 (13.3) | 3 (8.3) |
| Depressed level of consciousness | 0 | 1 (2.8) |
| Hypoxia | 2 (13.3) | 4 (11.1) |
| Pleural effusion | 2 (13.3) | 0 |
| Pneumonitis | 0 | 1 (2.8) |
| Respiratory failure | 0 | 1 (2.8) |
| Erythroderma | 0 | 1 (2.8) |
| Pruritus | 0 | 1 (2.8) |
| Rash maculo-papular | 1 (6.7) | 2 (5.6) |
| Hypotension | 2 (13.3) | 4 (11.1) |
| Capillary leak syndrome | 4 (26.7) | 4 (11.1) |
N = number of unique subjects experiencing a specific toxicity (% of stratum)
Disease measurable by standard radiographic criteria
Disease evaluable only by MIBG and/or bone marrow histology
The combination therapy met the protocol definition for tolerability with ≤7 unacceptable toxicities in the first 30 evaluable patients. In total there were four unacceptable DLTs. One patient developed hypoxia and respiratory distress due to capillary leak during course 2 and required mechanical ventilation for 24 hours. Two patients had Grade 4 hypotension secondary to capillary leak, one during course 1 and one during course 2, and both required pressor support for 24 hours. A fourth patient developed Grade 5 pulmonary edema during course 2 that was attributed definitely to disease progression and possibly due to treatment. No Grade 3 or 4 neurologic toxicities were reported.
Response
The best overall responses for all evaluable stratum 1 and stratum 2 patients are shown in Table 3. Two of the 16 patients in stratum 1 were not evaluable for response. One patient withdrew consent prior to treatment and one had an unacceptable DLT during course 2 and was taken off protocol therapy. Of the 14 evaluable patients in stratum 1, there were no responders. Two subjects were enrolled beyond the first stage of the trial due to reported responses not centrally reviewed in real time and ultimately not confirmed.
Table 3:
Best overall response by treatment stratum
| Stratum | Evaluable patients | Total Responses | CR | PR | SD | PD |
|---|---|---|---|---|---|---|
| 1 | 14 | 0 | 0 | 0 | 7 | 7 |
| 2 | 31 | 5 | 3 | 2 | 9* | 17 |
Includes patient with CR that could not be confirmed by central review
Five of the 36 patients in stratum 2 were not evaluable for response. One patient came off protocol therapy after course 1 due to refusal of further therapy. Four patients did not complete at least two courses of therapy due to unacceptable or other Grade 4 DLTs. The protocol planned to enroll 30 patients evaluable for response in stratum 2; a 31st patient was enrolled into stratum 2 before it was recognized that a 30th patient had met evaluability criteria. Five patients out of the first 30 evaluable patients responded to treatment as confirmed by central review, meeting protocol-defined activity criteria, for an overall response rate of 16.1% (95% Wilson confidence interval; 7.1%, 32.6%). The statistical criterion for activity required at least 5 responders in stratum 2, and this boundary was met.
The characteristics of the 5 responders in stratum 2 are described in Table 4. Two patients (4 and 37) had prolonged responses and were alive with no evidence of disease 5 years from the completion of therapy. Both of these patients ended protocol therapy with a partial response and received radiation to residual MIBG avid sites. Patient 48 had a complete response after 2 courses of treatment and received a total of 10 courses of treatment. He remained in a complete response with no further therapy until relapse was detected on a surveillance MIBG scan done 5 years after completion of therapy. Of the two other responders, one (patient 24) had a complete response after 2 courses of therapy but then had progressive disease at the end of 8 courses. The other (patient 50) received a total of 10 courses of therapy and was in a complete remission at the end of treatment but relapsed 3 months later. In addition to these 5 responders confirmed by central review, there was a sixth patient scored as a complete responder by the treating institution. This patient (52) was reported to have bone marrow involvement only at study entry. The patient received 4 courses of protocol therapy with no further treatment and remained in remission 5 years from the completion of therapy. This patient did not have baseline bone marrow samples available for central review and thus could not be considered a responder.
Table 4:
Response Details for the 5 stratum 2 responders
| Patient ID | Prior AB* | Baseline Curie Score | Baseline BM** score | Treatment Courses | Best Response | Time to event (months)*** |
|---|---|---|---|---|---|---|
| 4 | No | 8 | 2 | 10 | PR | No event**** |
| 24 | No | 1 | 1 | 8 | CR | 8 |
| 37 | No | 9 | 1 | 10 | PR | No event**** |
| 48 | No | 0 | 2 | 10 | CR | 70***** |
| 50 | No | 4 | 2 | 10 | CR | 13 |
AB = anti-GD2 antibody
BM = bone marrow
Time to progression from start of therapy
Patient received XRT after completing protocol therapy, no evidence of disease 5 years off therapy
Relapse diagnosed on surveillance MIBG done 5 years off therapy
There were no responses seen in the eleven patients who received prior anti-GD2 antibody, although the patient mentioned above whom was judged a CR by the treating institution, but not confirmed by central review did receive prior dinutuximab.
To better understand which stratum 2 patients responded to therapy, baseline disease burden was described using the baseline Curie score and a qualitative bone marrow score. Responders had a lower baseline median Curie score (4, range 0-9), compared to non-responders (10, range 0-25) but this difference was not statistically significant (p=0.1395). The median baseline bone marrow score was 2 for responders vs. 1 for non-responders (p=0.1243).
Immunologic Correlates
There was no significant difference between peak hu14.18-IL2 serum levels on days 4 and 6 between stratum 1 and stratum 2 patients, between responders and non-responders among stratum 2 patients, or between patients who received prior anti-GD2 antibody and those who did not. Serum IL2 receptor levels were significantly increased compared to baseline (course 1 day 4, p<0.0001) at all time points except course 2 day 4 and course 3 day 4. Serum IL2 receptor concentration did not correlate with response.
The generation of an anti-immunocytokine antibody level was positive or intermediate in 12 of the 26 stratum 2 patients evaluable for response and with serum available for testing. There was no association of anti-immunocytokine antibody response with prior mAb treatment. Three of the 5 responders were within the positive or intermediate anti-IC group. The data in Table 5 suggest there is no correlation of anti-IC with response. A single patient (a non-responder) developed a strong anti-immunocytokine antibody following course 1, which effectively neutralized detection of circulating hu14.18-IL2 (levels <10% of predicted) in the 3 subsequent courses.
Table 5:
Development of anti-hu14.18-IL2 antibody in relation to response
| Test | Factor | N | Number of responders | p-value |
|---|---|---|---|---|
| Anti-IC | Negative Positive or intermediate |
14 12 |
2 (14.3%) 3 (25.0%) |
0.6348 |
| Anti-IC | Negative Positive |
14 5 |
2 (14.3%) 1 (20.0%) |
1.0000 |
| Anti-IC | Negative or Intermediate Positive |
21 5 |
4 (19.0%) 1 (20.0%) |
1.0000 |
The peak anti-IC level for each of 26 patients evaluable for response with serum available by bridging-ELISA method was characterized as: negative (<0.5 OD); intermediate (0.5 −2.0 OD); or positive (>2.0 OD).
Discussion
This study demonstrates antitumor activity of hu14.18-IL2 in combination with GM-CSF and isotretinoin in patients with relapsed/refractory neuroblastoma with disease evaluable by MIBG or bone marrow examination. Five of 31 evaluable patients had a confirmed objective response, three of which were durable 5 years or more after the completion of therapy. These results are similar to what was seen in the single agent phase II trial (5 complete responders out of 23 evaluable patients)17 and confirm the anti-tumor activity of Hu14.18-IL2. The results also suggest that the addition of GM-CSF and isotretinoin was neither synergistic nor antagonistic in regards to activity, although the study was not designed to make this direct comparison.
The previous phase II results suggested that hu14.18-IL2 was more effective in patients with a smaller disease burden, as no responses were seen in patients with measurable disease 17. This was also seen in the current study with no confirmed responders seen in stratum 1. To further address the question of the correlation between response and disease burden, this study used baseline Curie and bone marrow scores as surrogate measures of disease burden. Neither statistically impacted response, although there was a suggestion that patients with a lower disease burden by Curie score (i.e. those with fewer MIBG avid sites) were more likely to respond to therapy than those with a larger MIBG-avid disease burden. The median degree of bone marrow involvement was actually greater for responders than non-responders. This may not be a true trend but may be related to the wide cut off values set to semi-quantitatively measure bone-marrow disease (all patients with greater than or equal to 10% tumor cells seen in at least one enrollment specimen were considered to have the same amount of marrow disease in our analysis).
The primary objective of this study was to evaluate the tolerability of combining hu14.18-IL2 with GM-CSF and isotretinoin in a schedule similar to the way dinutuximab is now given to high-risk neuroblastoma patients for minimal residual disease maintenance therapy as per the completed Children’s Oncology Group Phase III trial ANBL0032 8.We knew there may be risk of increased neurologic toxicity because of dose limiting toxicity seen in a Phase Ib /II study where GM-CSF and IL2 were given concurrently to adults with renal cell cancer 18, 19. For this reason, similar to what was tolerable in the renal cell carcinoma study, we dosed GM-CSF and the immunocytokine sequentially and did not see any Grade 3 or 4 neurologic toxicities in this neuroblastoma trial. There were 4 “unacceptable” toxicities observed in the current study, one of which was more likely due to progressive disease than treatment. The other 3 unacceptable toxicities resolved 24 hours after stopping immunocytokine and were similar to what was described in the single agent phase II trial 17. The other Grade 3 and 4 toxicities observed were consistent with those previously reported for hu14.18-IL2 and for anti-GD2 mAb plus IL2. These results suggest that the addition of GM-CSF and isotretinoin did not change the toxicity profile of hu14.18-IL2.
Evidence for immune activation was seen as treatment induced increases in serum sIL2R levels, but the magnitude of these was not correlated with tumor response. None of the responders received prior antibody but it is not clear whether this is a direct correlation or the result of small sample size. One patient previously treated with dinutuximab, with bone-marrow-only disease at study entry, who could not be considered a responder due to lack of material for central review, did have a reported (by local institutional histological review of bone marrow aspirates and biopsies) durable complete response in the bone marrow after receiving 2 courses of treatment in this study. This observation is consistent with, but does not prove the speculation that prior dinutuximab exposure did not prevent activity, but this question needs to be studied on a larger scale. Clearly this is an important consideration for future studies given the widespread use of anti-GD2 antibodies in newly diagnosed neuroblastoma patients. Data about prior antibody therapy were not collected on the prior hu14.18-IL2 trials so this cannot be used to help draw conclusions.
Despite the anti-tumor activity and relative tolerability of the combination of hu-14.18-IL2 with GM-CSF and isotretinoin in this study, the overall response rate in this study was less than 20% and substantial toxicities were still observed. Even though the chimeric version of this immunocytokine (ch14.18-IL2) shows far superior antitumor activity in neuroblastoma-bearing mice over the combination of equivalent doses of ch14.18 plus IL2 as separate molecules 14, it is difficult to compare the clinical results from this phase II study with published phase II trials of ch14.18 mAb, with or without GM-CSF or IL2 26 and be convinced that any one regimen is superior to the other, in part due to differences in patient population, eligibility criteria, and other variables that prevent direct comparisons. Given the durability of responses seen in some stratum 2 relapsed/refractory patients, and the smaller baseline tumor burden in patients that have responded, hu14.18-IL2 might also be considered as possible MRD treatment of relapsed patients who have achieved a second objective response. Other anti-GD2-based approaches are also being pursued. The recent reports from COG of what appears to be greater efficacy seen when dinutuximab is combined with irinotecan, temozolomide and GM-CSF27, and from St. Jude with a similar approach combining chemotherapy with a separate humanized anti-GD2 mAb (hu14.18K322A)28, suggest this approach deserves additional focus, in other combinations and with other anti-GD2-based agents. Finally, recent preclinical data have demonstrated potent in vivo anti-tumor efficacy, in mice with large GD2 tumors, when intratumoral hu14.18-IL2 is combined with local low-dose radiotherapy29 and even greater potency when anti-CTLA4 checkpoint blockade is added30. This regimen induces both innate and adaptive anti-tumor immune activity in these tumor-bearing mice. Clinical testing of intratumoral hu14.18-IL2 with local low-dose radiotherapy and checkpoint blockade is being initiated in the treatment of GD2 expressing melanomas; this approach with hu14.18-IL2 may be worthy of clinical investigation in GD2 expressing pediatric tumors. As the incorporation of anti-GD2 immunotherapy into combination therapy approaches proceeds it will be helpful to be evaluating correlative biomarkers to potentially help predict which patients may be most likely to benefit from which therapeutic approach31.
TRANSLATIONAL RELEVANCE.
Superior anti-tumor activity is seen in mice with microscopic amounts of neuroblastoma when treated with a fusion protein consisting of IL2 linked to an anti-GD2 monoclonal antibody (the hu14.18-IL2 immunocytokine) than when treated with the same amount of anti-GD2 monoclonal antibody and IL2 given as separate agents. Phase I and II studies have defined a predictable toxicity profile and showed anti-neuroblastoma activity of hu14.18-IL2 in children with non-bulky relapsed/refractory neuroblastoma. Anti-GD2 monoclonal antibody (dinutuximab) is now standardly used in North America in combination with IL2, GMCSF and isotretinoin as minimal residual disease therapy for high-risk neuroblastoma patients. In this trial we used the hu14.18-IL2 immunocytokine in place of dinutuximab and IL2, in combination with GMCSF and isotretinoin for patients with relapsed/refractory neuroblastoma. We found that this combination is tolerable and that anti-neuroblastoma activity is seen for patients with non-bulky disease. Further clinical study of hu14.18-IL2 is being pursued.
Acknowledgement of Research Support:
This work was supported by:
Alex’s Lemonade Stand Foundation: P. Sondel, J. Hank and J. Maris
The St. Baldrick’s Foundation: P. Sondel and J. Hank
CA032685: P. Sondel, J. Hank and J. Gan
CA87025: P. Sondel, J. Hank and J. Gan
CA166105: P. Sondel, J. Hank and J. Gan
CA 180886: J. Park
CA180899: C.Van Ryn and A. Naranjo
CA197078: P. Sondel and J. Hank
CA220500: J. Maris
UL1TR000427: P. Sondel and J. Hank
1TL1RR025013-01: P. Sondel and J. Hank
The Stand Up To Cancer – St. Baldrick’s Pediatric Dream Team Translational Research Grant (SU2C-AACR-DT1113). Stand Up to Cancer is a division of the Entertainment Industry Foundation. Research grants are administered by the American Association for Cancer Research, the Scientific Partner of SU2C.: P. Sondel, J. Hank, J. Maris and J. Park
Abbreviation list:
- GD2
disialoganglioside
- NB
neuroblastoma
- MIBG
metaiodobenzylguanidine
- MRD
minimal residual disease
- GMCSF
granulocyte-macrophage colony-stimulating factor
- IL2
interleukin 2
- mAb
monoclonal antibody
- ADCC
antibody-dependent cellular cytotoxicity
- CR
complete response
- PR
partial response
- SD
stable disease
- PD
progressive disease
- DLT
dose limiting toxicity
- sIL2R
serum soluble IL2 receptor
- VGPR
very good partial response
Footnotes
The authors declare no potential conflict of interest.
References:
- 1.Irwin MS, Park JR. Neuroblastoma: paradigm for precision medicine. Pediatr Clin North Am. 2015;62: 225–256. [DOI] [PubMed] [Google Scholar]
- 2.Pinto NR, Applebaum MA, Volchenboum SL, et al. Advances in Risk Classification and Treatment Strategies for Neuroblastoma. J Clin Oncol. 2015;33: 3008–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matthay KK, Reynolds CP, Seeger RC, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children’s oncology group study. J Clin Oncol. 2009;27: 1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N Engl J Med. 1999;341: 1165–1173. [DOI] [PubMed] [Google Scholar]
- 5.Gilman AL, Ozkaynak MF, Matthay KK, et al. Phase I study of ch14.18 with granulocyte-macrophage colony-stimulating factor and interleukin-2 in children with neuroblastoma after autologous bone marrow transplantation or stem-cell rescue: a report from the Children’s Oncology Group. J Clin Oncol. 2009;27: 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu AL, Uttenreuther-Fischer MM, Huang CS, et al. Phase I trial of a human-mouse chimeric anti-disialoganglioside monoclonal antibody ch14.18 in patients with refractory neuroblastoma and osteosarcoma. J Clin Oncol. 1998;16: 2169–2180. [DOI] [PubMed] [Google Scholar]
- 7.Ozkaynak MF, Sondel PM, Krailo MD, et al. Phase I study of chimeric human/murine anti-ganglioside G(D2) monoclonal antibody (ch14.18) with granulocyte-macrophage colony-stimulating factor in children with neuroblastoma immediately after hematopoietic stem-cell transplantation: a Children’s Cancer Group Study. J Clin Oncol. 2000;18: 4077–4085. [DOI] [PubMed] [Google Scholar]
- 8.Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363: 1324–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozkaynak MF, Gilman AL, London WB, et al. A Comprehensive Safety Trial of Chimeric Antibody 14.18 With GM-CSF, IL-2, and Isotretinoin in High-Risk Neuroblastoma Patients Following Myeloablative Therapy: Children’s Oncology Group Study ANBL0931. Front Immunol. 2018;9: 1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillies SD, Reilly EB, Lo KM, Reisfeld RA. Antibody-targeted interleukin 2 stimulates T-cell killing of autologous tumor cells. Proc Natl Acad Sci U S A. 1992;89: 1428–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buhtoiarov IN, Neal ZC, Gan J, et al. Differential internalization of hu14.18-IL2 immunocytokine by NK and tumor cell: impact on conjugation, cytotoxicity, and targeting. J Leukoc Biol. 2011;89: 625–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hank JA, Surfus JE, Gan J, et al. Activation of human effector cells by a tumor reactive recombinant anti-ganglioside GD2 interleukin-2 fusion protein (ch14.18-IL2). Clin Cancer Res. 1996;2: 1951–1959. [PubMed] [Google Scholar]
- 13.Neal ZC, Yang JC, Rakhmilevich AL, et al. Enhanced activity of hu14.18-IL2 immunocytokine against murine NXS2 neuroblastoma when combined with interleukin 2 therapy. Clin Cancer Res. 2004;10: 4839–4847. [DOI] [PubMed] [Google Scholar]
- 14.Lode HN, Xiang R, Dreier T, Varki NM, Gillies SD, Reisfeld RA. Natural killer cell-mediated eradication of neuroblastoma metastases to bone marrow by targeted interleukin-2 therapy. Blood. 1998;91: 1706–1715. [PubMed] [Google Scholar]
- 15.Lode HN, Xiang R, Varki NM, Dolman CS, Gillies SD, Reisfeld RA. Targeted interleukin-2 therapy for spontaneous neuroblastoma metastases to bone marrow. J Natl Cancer Inst. 1997;89: 1586–1594. [DOI] [PubMed] [Google Scholar]
- 16.Osenga KL, Hank JA, Albertini MR, et al. A phase I clinical trial of the hu14.18-IL2 (EMD 273063) as a treatment for children with refractory or recurrent neuroblastoma and melanoma: a study of the Children’s Oncology Group. Clin Cancer Res. 2006;12: 1750–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shusterman S, London WB, Gillies SD, et al. Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: a Children’s Oncology Group (COG) phase II study. J Clin Oncol. 2010;28: 4969–4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hotton KM, Khorsand M, Hank JA, et al. A phase Ib/II trial of granulocyte-macrophage-colony stimulating factor and interleukin-2 for renal cell carcinoma patients with pulmonary metastases: a case of fatal central nervous system thrombosis. Cancer. 2000;88: 1892–1901. [PubMed] [Google Scholar]
- 19.Schiller JH, Hank JA, Khorsand M, et al. Clinical and immunological effects of granulocyte-macrophage colony-stimulating factor coadministered with interleukin 2: a phase IB study. Clin Cancer Res. 1996;2: 319–330. [PubMed] [Google Scholar]
- 20.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11: 1466–1477. [DOI] [PubMed] [Google Scholar]
- 21.Matthay KK, Edeline V, Lumbroso J, et al. Correlation of early metastatic response by 123I-metaiodobenzylguanidine scintigraphy with overall response and event-free survival in stage IV neuroblastoma. J Clin Oncol. 2003;21: 2486–2491. [DOI] [PubMed] [Google Scholar]
- 22.Yanik GA, Parisi MT, Shulkin BL, et al. Semiquantitative mIBG scoring as a prognostic indicator in patients with stage 4 neuroblastoma: a report from the Children’s oncology group. J Nucl Med. 2013;54: 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albertini MR, Ranheim EA, Hank JA, et al. A pilot trial of hu14.18-IL2 in patients with completely resectable recurrent stage III or stage IV melanoma. Annual Meeting of the American Society of Clinical Oncology (ASCO 2014), Chicago, IL, USA, May 30-June 3, 2014. J Clin Oncol. 2014;32 (5s): (Abstract 9044, Poster). [Google Scholar]
- 24.King DM, Albertini MR, Schalch H, et al. Phase I clinical trial of the immunocytokine EMD 273063 in melanoma patients. J Clin Oncol. 2004;22: 4463–4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albertini MR, Yang RK, Ranheim EA, et al. Pilot trial of the hu14.18-IL2 immunocytokine in patients with completely resectable recurrent stage III or stage IV melanoma. Cancer Immunol Immunother. 2018;67: 1647–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seidel D, Shibina A, Siebert N, et al. Disialoganglioside-specific human natural killer cells are effective against drug-resistant neuroblastoma. Cancer Immunol Immunother. 2015;64: 621–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mody R, Naranjo A, Van Ryn C, et al. Irinotecan-temozolomide with temsirolimus or dinutuximab in children with refractory or relapsed neuroblastoma (COG ANBL1221): an open-label, randomised, phase 2 trial. Lancet Oncol. 2017;18: 946–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Federico SM, McCarville MB, Shulkin BL, et al. A Pilot Trial of Humanized Anti-GD2 Monoclonal Antibody (hu14.18K322A) with Chemotherapy and Natural Killer Cells in Children with Recurrent/Refractory Neuroblastoma. Clin Cancer Res. 2017;23: 6441–6449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris ZS, Guy EI, Francis DM, et al. In Situ Tumor Vaccination by Combining Local Radiation and Tumor-Specific Antibody or Immunocytokine Treatments. Cancer Res. 2016;76: 3929–3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris ZS, Guy EI, Werner LR, et al. Tumor-Specific Inhibition of In Situ Vaccination by Distant Untreated Tumor Sites. Cancer Immunol Res. 2018;6: 825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voeller J, Sondel PM. Advances in Anti-GD2 Immunotherapy for Treatment of High-Risk Neuroblastoma. Journal of Pediatric Hematology-Oncology. 2019;41:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]