Abstract
In vertebrates, the development of the entire nervous system is triggered by signals from a powerful “organizing” region of the early embryo during gastrulation. This phenomenon - neural induction - was originally discovered and given conceptual definition by experimental embryologists working with the amphibian embryo. The molecular circuitry underlying neural induction, also performed in amphibians, demonstrated that elimination of ongoing TGFβ signaling in the ectoderm is the hallmark of anterior neural-fate acquisition. This observation is the basis of the “default” model of neural induction. Endogenous neural inducers are secreted proteins that act to inhibit TGFβ ligands in the dorsal ectoderm. In the ventral ectoderm, where the signaling ligands escape the inhibitors, non-neural fate is induced. Inhibition of the TGFβ pathway has been recently demonstrated to be sufficient to directly induce neural fate in mammalian embryos and pluripotent mouse and human embryonic stem cells. The molecular process that delineates neural from non-neural ectoderm is evolutionarily conserved across a broad range in the evolutionary tree. Our understanding of the role of signaling pathways (FGF, Wnt, and TGFβ) in neural induction in vertebrate embryos is now being facilitated by the availability of mouse and human embryonic stem cells.
Neural Induction and early patterning in vertebrates
In all vertebrates, the fertilized egg divides to generate a blastocyst (or blastula). Three different territories called embryonic germ layers: ectoderm, mesoderm and endoderm, emerge in the blastula. In the amphibian embryo, where the dorsal (D) and ventral (V) sides of the embryo are specified during fertilization, each germ layer has a distinct D-V polarity and is fated to generate different tissues as the embryo matures (Animation 1). Subsequently, during gastrulation, the primitive ectoderm, or epiblast, covers the outside of the embryo and forms different tissue derivatives depending on position along the embryonic D-V axis. The central nervous system derives from the most dorsal region of the ectoderm, which thickens and flattens after gastrulation to form the neural plate. During subsequent neurula stages, the plate rolls into a tube, separates from the overlying epidermis, and goes on to form the brain at the anterior, and spinal cord at the posterior end. At the opposite ventral side, most of the remaining ectoderm forms epidermis. The neural crest forms where the dorsal and ventral boundaries meet at the edge of the neural plate. This progenitor cell population detaches, and migrates throughout the embryo to form most of the peripheral nervous system, cranium, and cartilage of branchial arches. Ectodermal cells at the most anterior edge of the neural-epidermal boundary give rise to placodal areas that will form sensory organs - such as the ear and nose - as well as some cranial sensory ganglia (Figure 1). At the start of gastrulation, cells from any part of the ectoderm can still develop as either epidermis or neural tissue, but by the end of gastrulation commitment has occurred1. These events are characteristic of all vertebrates although timing and geometry vary. Thus the first step in the establishment of the nervous system in vertebrates involves the partition of the ectoderm into epidermal and neural primordia during gastrulation.
Figure 1: Fate map of the anterior border of the neural plate in Xenopus embryos.
Schematic of dorsal-anterior (head-on) view of a Xenopus neurula (the ventral side is up, and the dorsal side is down). Fate map of the anterior neural plate97. Different colors highlight different fates.
Lessons from experimental embryology
The Mangold and Spemann experiments
The fundamental insight into how the neural plate is established came from the famous experiment of Mangold and Spemann, in which tissue from the dorsal blastopore lip (located in the dorsal mesoderm) of an early newt gastrula was grafted to the ventral side of a second embryo2. The host embryo developed a second set of dorsal axial structures on the ventral side, including a well-organized second nervous system. This experiment suggested that signals from the dorsal lip region, which became known to amphibian embryologists as “Spemann’s organizer”, were responsible for diverting nearby ectoderm to a neural fate (Animation 2). In normal development, cells of the organizer involute into the embryo during gastrulation, giving rise to dorsal structures in the mesoderm such as muscle and the notochord that underlie the future neural plate. Lineage tracing experiments3 demonstrated that while the entire mesodermal derivative of the secondary axis was derived from the progeny of the grafted cells, the entire nervous system, with the exception of the floor plate, was derived from the host, confirming that signals from the organizer caused ventral ectodermal cells, that normally would have given rise to epidermis, to convert instead to neural fate. These results were also reproduced in fish by Oppenheimer, where grafting pieces of organizer (called the shield in fish) were able to induce a secondary axis in the host fish4, 5. Analogous grafting experiments carried out in the chick and the mouse embryos (where the organizer is called the node) led to similar results6, 7, highlighting the evolutionary conservation of the “organizer” as source of signal(s) that is sufficient to generate the entire nervous system.
Development of the animal cap explants and assays
The organizer graft experiments subsequently led to an early form of tissue culture, where the ectoderm of the blastula, called the animal cap, was explanted and cultured in simple pond water. By itself, the isolated animal cap only formed epidermal tissue1 (Animation 3), but when recombined with explants derived from another portion of the embryo, the same explant generated other cell types. Mesodermal derivatives, for example, arose in animal cap explants after exposure to early endoderm while neural tissue arose after exposure to dorsal mesoderm of different ages, including organizer tissue8, 9. This work demonstrated the remarkable potential of animal cap cells to form an array of mesodermal and ectodermal derivatives, depending on the inductive interactions that were encountered over the course of early development. In addition, these experiments reinforced the view from the organizer transplant experiments that the ectoderm forms epidermis as a default state. The obvious line of experiments that followed was to substitute the inducing tissue (the vegetal pole in mesoderm induction, and the organizer for neural induction) with cocktails of extracts or factors that would elicit an inductive response from the animal cap followed by a morphological and molecular diagnostic of the induced fate.
Decades after the discovery of the organizer, however, the identification of the molecules underlying neural induction remained elusive. Limitations in existing techniques thwarted biochemical approaches to identify the endogenous inducers, while the animal cap tended to non-specifically convert to neural fate in response to a variety of materials, often from rather exotic sources (such as guinea pig bone marrow, blue jay liver, boiled dead organizer). More unexpected and surprising was the fact that simple cell dissociation of the animal cap led to conversion of cells from epidermal to neural fate directly, without previous or concomitant induction of mesoderm (Animation 4). To explain these results, neural inducers were proposed to be widely distributed and under negative control in the animal cap by factors that could be lost by dissociation, but the nature of either the inducer or its inhibitor remained undefined. Thus, many decades after the discovery of the organizer, the study of neural induction had reached a virtual impasse.
Lessons from molecular embryology
The field of embryonic induction was invigorated in the early 1990s by the introduction of modern molecular techniques to complement classical experimental embryology in Xenopus. Conversion of the animal cap cells into mesodermal or neural tissue could now be unambiguously scored using fate-specific molecular markers to diagnose induced cell types, and potential inducers could be tested as purified peptide growth factors or by microinjection of synthetic RNA. These approaches soon led to the discovery that physiological amounts of polypeptide growth factors of the FGF and TGFβ family were sufficient to impose mesodermal fates in animal caps. For example, when treated with increasing thresholds of Activin, a member of the TGFβ family, animal caps responded by forming ventral, lateral and dorsal mesoderm (including the organizer) 10. Work in a variety of vertebrate model systems has irrevocably established the pivotal role of these growth factors in the formation of mesodermal and organizer tissues in the embryo11. Animal caps also formed some neural tissue when treated with high concentrations of a mesodermal inducer such as Activin, suggesting that neural tissue was also induced by growth factor action. However, neural induction in this case was likely to be indirect. Experimentally, the difference between indirect versus direct neural induction in animal cap explants can be assessed by examining tissue specific markers, where direct induction is characterized by the expression of neural markers (NCAM) in the absence of mesodermal/organizer-specific molecular markers (brachyury, goosecoid). Expression of both markers, however, is a strong indication of an indirect cascade where one signaling factor induces responding cells to release additional inducing factors, a phenomenon that continues to confounding inducer studies today (see below). It was only when the field of mesodermal induction turned to the study of the Activin receptor, one of the first TGFβ family receptors cloned in mammals12 and in Xenopus13, that the nature of direct neural inducers began to emerge.
Molecular basis of neural induction
To ask if Activin signaling was necessary for mesoderm induction, and to validate the in vivo relevance of these findings, a synthetic RNA encoding a dominant negative mutant form of the Activin receptor (DN-ActRIIB) was engineered to challenge the inducing activity of Activin. Like many dominant-negative mutants, DN-ActRIIB is now known to be broad acting, and capable of blocking all TGFβ ligands, including Nodals, BMPs, and GDFs (i.e. much of the TGFβ pathway shown in Figure 3). When expressed in early embryos, DN-ActRIIB completely inhibited endogenous mesoderm induction, in line with the idea that TGFβ signaling through the ActRIIB receptor is necessary for mesoderm induction in vivo. DN-ActRIIB expression in animal caps also completely blocked mesoderm induction by Activin. Unexpectedly, however, when expressed alone in control animal caps in simple pond water (i.e. not exposed to any ligand), it led to a strong conversion of fate directly from epidermal to neural (Animation 5), in the absence of neural-inducing signals from Spemann’s organizer.
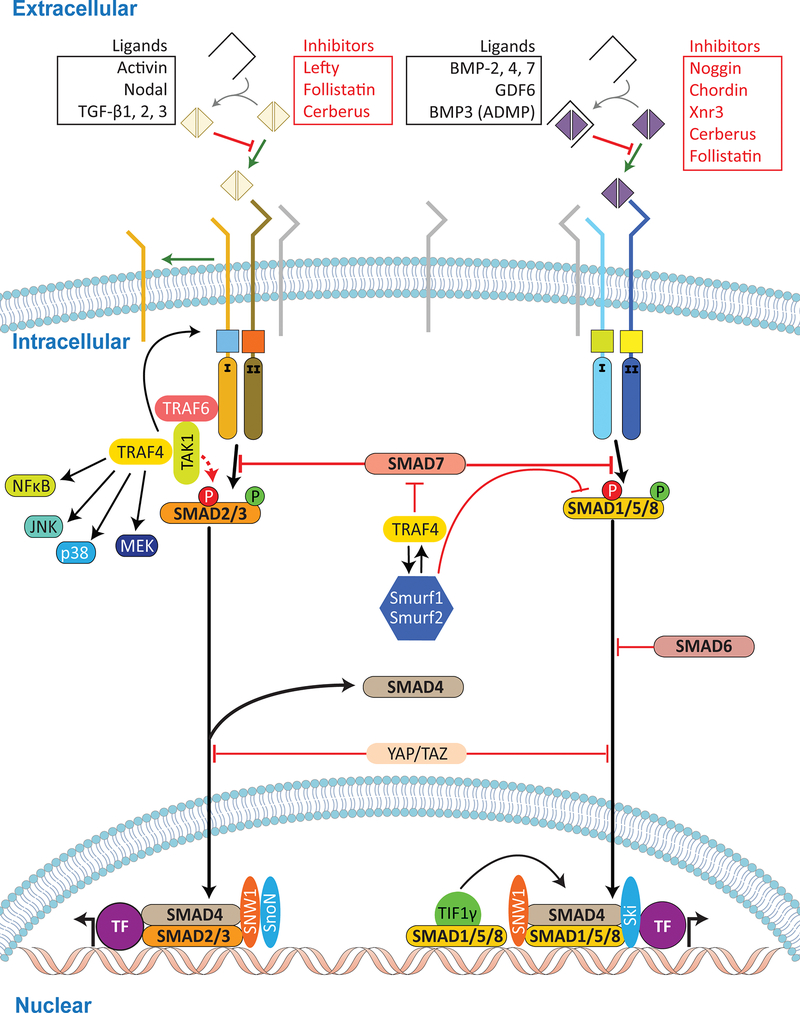
Figure 3: The TGFβ pathway.
More than 30 different TGFβ ligands are encoded in the vertebrate genome. They include members of BMPs, GDFs, Activins, Nodal, and TGFβs, all of which activate the TGFβ pathway. The activity of these ligands is regulated by a large number of secreted inhibitory factors (Table 1) that inhibit TGFβ signaling extracellularly. Upon secretion, homo- or hetero-dimers of TGFβ ligands that escape inhibition bind to TGFβ receptors at the cell membrane. Ligands act as morphogens exerting diverse cellular responses based on the levels and duration of signaling. Dimeric TGFβ ligands bind type II receptors that phosphorylate and activate type I receptors in a hetero-tetrameric complex. Receptor activation, in turn, leads to the propagation of signaling by at least two pathways involving Smad (in the canonical pathway) or Traf/TGFβ-Activated-Kinase-1 (TAK1, in the non-canonical pathway).
The “default model” model of neural induction
That neural tissue is induced by cell dissociation or by expression of DN-ActRIIB were disparate observations with one common denominator: they both made sense if traditional thinking about neural induction was inverted. In this revised view, the default fate for animal caps would not be epidermal but anterior neural. Ongoing signals in the explants repress the natural tendency of the cells to become neural, by inducing the epidermal fate. When this signaling was interrupted (by either expression of DN-ActRIIB or by cell dissociation), cells assumed a forebrain fate. The model proposed furthermore that neural inducers from the organizer might work by locally antagonizing these epidermal inducing signals, allowing dorsal ectoderm to follow its “default” anterior neural fate. These considerations led to the formulation of a new model of neural induction called the default model14, 15, which was initially controversial since it implies that vertebrate embryonic cells will become nerve cells of the forebrain unless told otherwise16. However, subsequent work identified both the nature of endogenous epidermal-inducing signal, as well as the organizer-derived inhibitors of this signal.
Epidermal induction
The nature of the epidermal inducing signals was revealed by experiments in which dissociated animal cap cells were treated with purified proteins. Since animal cap cells are neuralized upon dissociation, candidate factors could be tested for the ability to suppress neuralization and restore epidermal specification, thus replacing endogenous signals lost on dispersion. Treating these cells with Activin blocked neuralization, but it did so by inducing mesoderm17. However, another member of the TGFβ superfamily, BMP4, not only suppressed neuralization, but also proved to be a potent epidermal inducer (Animation 6). Significantly, the dominant-negative Activin receptor not only blocks signaling by BMP4, but also by related molecules, BMP2 and BMP7. These also happen to be epidermal inducers in this assay18. The expression pattern of the BMPs is in accord with their proposed role as neural inhibitors: BMP4 RNA is found throughout the ectoderm at the start of gastrulation, subsequently disappearing from the prospective neural plate19–21. Epidermal differentiation is also blocked in animal caps after inhibiting endogenous BMP signaling using dominant-negative BMP receptors21–23, dominant negative BMP4 or BMP7 ligands24, or antisense BMP4 RNA22, suggesting further that the BMP family are essential epidermalizing factors in vivo.
Endogenous neural inducers
Three independent approaches in Xenopus led to the identification of the endogenous neural inducers. The first was based on screening cDNA libraries for the embryonic inducing activity of cDNA libraries. This led to the discovery of the first bonafide endogenous direct neural inducer: noggin25. The second involved isolating organizer-specific genes. This led to the identification of chordin26. Finally, testing the activity of candidate TGFβ inhibitors led to the characterization of follistatin. All three genes are secreted proteins, specifically expressed in the organizer, and with direct neural-inducing ability. This established that the organizer tissue was indeed the source of signals that could induce neural tissue. At the time, the fact that one of them, follistatin, was a known extracellular inhibitor of a few TGFβ ligands, was in agreement with the default model27.
Convergence and reconciliation for neural induction
The identification of noggin, chordin and follistatin localized in the organizer led at first to the search for receptors that could instructively transduce their activity during neural induction. However, biochemical characterization of these neural inducers established that they are all potent extracellular inhibitors of TGFβ family signaling (the different arms of the TGFβ pathway are shown in Figure 3). They bind with high affinity to the ligands, thus preventing them from activating their cognate receptors28, 29. These observations suggested that high morphogen thresholds of BMP signaling on the ventral side of the ectoderm promote epidermal fate, whereas on the dorsal side BMP signaling is kept low by organizer generated BMP inhibitors, thus promoting a neural fate (Figure 2). There is now an extensive list of secreted TGFβ inhibitors, with some that are expressed in the organizer that have been isolated from a variety of species (Table 1). Every member of that list that has been tested in the animal cap assay has been shown to act as a direct neural inducer. In addition to these natural inhibitors, a number of small molecules that block the different branches of the TGFβ signaling have been characterized (Table 2). As with endogenous inhibitors, they have been shown to act as direct neural inducers when tested in the context of animal cap explants or in mammalian pluripotent stem cells, as discussed below.
Figure 2: Schematic of graded BMP activity in the gastrula and neurula ectoderm.
(A) A schematic fate map of the early gastrula shows the approximate positions of the future neural plate, border region and epidermis, viewed from the dorsal side. The cement gland and sensory placodes forms in the anterior border region mid-dorsally, while the neural crest arises more laterally. Diffusible antagonists produced in the organizer region of the mesoderm, including noggin, chordin, and follistatin, result in a graded distribution of BMP signaling in the neighboring ectoderm. The relative position of epidermis (EP), neural plate (NP), organizer (O, in blue), cement gland (CG), and neural crest (NC) are shown. Sensory placodes form at various positions in the border region but are not shown here for simplicity (B) Correlation with neurula fate map shown in Figure 1.
Table 1:
Secreted inhibitors of the BMP pathway
| Gene | Inhibits | Species | Gastrula Expression1 | Features-Comments | Reference |
|---|---|---|---|---|---|
| Chordin | BMP-2,4,7 | Mouse | Node (m) | 26 | |
| Xenopus | Organizer (x,z) | 98 | |||
| Zebrafish | Node and rostral mesen-doderm (c) | 28 | |||
| Chicken | 99 | ||||
| CHL/chordin-like | BMP-4,5,6 | Mouse | No | 3 CR-domains | 100 |
| Noggin | BMP-2,4,7 | Mouse | Node (m) | 3-noggin-like genes found in zebrafish | 25 |
| GDF-5 | Xenopus | Organizer (x,z) | 101 | ||
| zebrafish | Axial mesendoderm (c) | 102 | |||
| 103 | |||||
| 104 | |||||
| 105 | |||||
| Follistatin | BMP-2,4,7,11 | Mouse | node (m) | 106 | |
| GDF-8,11 | Xenopus | organizer (x) | 107 | ||
| Activin | Chick | node, mesendoderm, caudal neural plate (c) | 108 | ||
| 109 | |||||
| 105 | |||||
| FSRP proteins: | BMP-2,6,7 | Mouse | FLRG: e7.0 by Northern (m) | follistatin related | 110 |
| FLRG, Flik | Activin | Chicken | Flik-1: node (c) | 111 | |
| 112 | |||||
| 113 | |||||
| 114 | |||||
| Cerberus | BMP-4 | Xenopus | Anterior endoderm (x) | 115 | |
| xNr-1,2 | Mouse (Cer1) | Anterior visceral endoderm (m) | 116 | ||
| Wnt-8 | Chicken | 117 | |||
| Hypoblast, Ant. Endoderm, Prechordal plate (c) | 118 | ||||
| 119 | |||||
| Coco | BMP-4 | Xenopus | Gradient from animal to vegetal | cerberus/dan related | 120 |
| Activin | Strongest expression in ectoderm | ||||
| xNr-1 | |||||
| Wnt-8 | |||||
| Dan | BMP-2,4,7 | Mouse | No | 121 | |
| GDF-5,6,7 | Xenopus | No | 119 | ||
| 122 | |||||
| 123 | |||||
| Caronte | BMP-4,7 | Chicken | Mesoderm flanking the node | 124 | |
| 125 | |||||
| Lefty1 | Nodal | Mouse | notochord/midline (Lefty1; m,c) | 126, 127 | |
| Lefty2 | Chicken | mesoderm (Lefty2; m,c) | |||
| Dante | ND | Mouse | Node | No full-length cDNA reported | 122 |
| PRDC | ND | Mouse | ND | Cerberus/Dan-like | 128 |
| Drm/Gremlin | BMP-2,4 | Mouse | No | 119 | |
| Xenopus | No | 129 | |||
| 122 | |||||
| Neuralin-1 | BMP-4,5 | Mouse | Emerging neural plate | 3 CR-domains | 130 |
| TGF-β1,2 | 100 | ||||
| CTGF | BMP-4 | Xenopus | Weak expression | 1 CR-domain | 131 |
| TGF-β1 | |||||
| Kielin | ND | Xenopus | Axial mesoderm | 27 CR-domains | 132 |
| TSG | BMP-4 | Xenopus | Ventral region (x) | Reported to act as both an antagonist and an agonist of BMP signaling | 133 |
| Mouse | ND | 134 | |||
| 135 | |||||
| 136 | |||||
| 137 | |||||
| Amnionless | ND | Mouse | Visceral endoderm | 1 CR-domain | 138 |
| 1 TM-domain | |||||
| CRIM-1 | ND | Mouse | No | 6 CR-domains | 139 |
| 1 IGFBP motif | |||||
| 1 TM-domain | |||||
| Nell family | ND | Mouse (NELL1,2) | No | Multiple CR-domains. Multiple EGF-domains. Some contain TM-domains | 140 |
| Chicken | ND | 141 | |||
| 142 | |||||
| Xnr3 | BMP-4 | Xenopus | organizer | Nodal-related gene | 143 |
| Sclerostin/SOS T | BMP-5,6 | Mouse | No | 144, 145 | |
| 146 | |||||
| Sclerostin-like | ND | Mouse | ND | 145 | |
| Jiraiya | BMPRII | Xenopus | Dorsal Ectoderm | 147 | |
| Cross Veinless 2 | BMP4, 5, 7 | Xenopus, Mouse, Drosophila | Primitive Streak, Precardiac mesoderm, Tailbud | 5CR domains, 1VWD domain, 1 TIL domain. | 148–150 |
| Reported to act as both an antagonist and an agonist of BMP signaling | |||||
| xNorrin | Xnr1 | Xenopus | Oocyte to late blastula | Cystein knot domain | 151, 152 |
| BMP4 | Zebrafish | Animal pole | Binds to Fzd-4 and acts as a WNT ligand | ||
| Fzd-4/Lrp | Chick | ||||
| Mouse | |||||
| Human |
Expression as measured by RNA localization.
Species expression domains are described as follows: (m) mouse; (c) chicken; (x) Xenopus laevis; (f) zebrafish; ND: not determined
Table 2:
Small molecules shown to block different branches of the TGFβ signaling
| Small Molecule Inhibitors | Target Receptors | In vitro concentration | Reference |
|---|---|---|---|
| SB431542 | ALK4, 5, 7 | 10 μM | 153 |
| A083–01 | ALK4, 5, 7 | 0.5 μM | 154 |
| Dorsomorphin | ALK2, 3, 6 (non-specific: VEGFR, AMP Kinase | 1–2 μM | 155 |
| LDN-193189 | ALK2, 3, 6 (non-specific: VEGFR, AMP Kinase | 100 nM | 156 |
| DMHI | ALK2, 3, 6 (Highly specific) | 0.5–5 μM | 157 |
Animal cap cells pass through a competence period when they respond to Activin/Nodal signaling by forming mesendodermal derivatives, followed by a second competence period when they become epidermis in response to BMP signaling. In the default model, therefore, a neural fate ensues only when animal cap cells avoid both mesoderm and epidermal inducing signals. Perhaps this explains why co-inhibition of both SMAD1/5/8 and SMAD2/3 branches of the canonical pathway induces a neural fate more potently than each alone30 in a manner similar to DN-ActRIIB, which interferes with both Activin/Nodal and BMP signaling.
Evolutionary conservation of molecular circuitry underlying neural induction
Inhibition of ongoing TGFβ signaling to delineate neural and non-neural ectoderm has been conserved evolutionarily. In the fruitfly Drosophila for example, short gastrulation (sog) is a homolog of the organizer-specific BMP-inhibitor chordin. Sog was identified in a systematic screen for genes involved in patterning the Drosophila embryo along the D-V axis31. As in vertebrates, the dorsal and ventral regions of the ectoderm of the Drosophila embryos generate different fates. However, as the embryonic axis is flipped in Arthropods compared to Chordates, the epidermis forms in the dorsal regions, while the neural tissue arises from a ventral position. Nonetheless, the molecular circuitry involving inhibition of BMP in segregating dorsal from ventral ectoderm operates in precisely the same manner as in vertebrates32, 33. Drosophila components of the BMP signaling branch of the TGFβ pathway, including ligands, receptors and inhibitors such as Sog generate an activity gradient of Dpp, a BMP-like ligand, from high dorsal to low ventral, thus specifying epidermal and neural tissue, respectively34. Indeed, Sog has been shown to directly promote neuroectoderm specification in blastoderm drosophila embryos by inhibiting the anti-neurogenic and dorsalizing activity of Dpp35. This acitivity of Sog is also shared by other annelids, such as spider and beetles36. Similarly, inhibition of HrBMPb, the ascidian homolog of BMP, is required for induction of rostral neural lineages in sea squirts (urochordates) and its overexpression results in a fate switch of the presumptive neural cells to epidermal lineages37. A notable exception to this rule is found in Acorn worms (hemichordates), which lack both, an organized CNS, as well as segregation of the ectoderm into neurogenic and epidermal territories. Exposure of these embryos to exogenous BMPs does not repress neural markers, and conversely, BMP knockdown does not promote neuralization, even though it has a role in D-V patterning in these embryos38. Taken together, these observations perhaps suggest that D-V patterning by the BMP pathway is an ancient mechanism that evolved early in metazoans and was subsequently utilized by many metazoans which have a CNS as a means of establishing different ectodermal fates in the early embryo36. The conservation of this neural induction mechanism has been also observed in mammalian embryos and has now been demonstrated in human embryonic stem cells as well (see below).
Molecular redundancy in neural induction
As with most signaling pathways, the BMP patterning system that underlies neural induction in vertebrates is notable for extensive redundancy in gene function that has made loss-of-function approaches problematic (Table 1). Thus, genetic tests of the putative neural inducers in other species were initially unimpressive since mutations that eliminate only one of these inhibitors tend to have relatively mild phenotypes on their own. For example, a loss-of-function mutation in Zebrafish chordin (the chordino mutant) causes only a reduction in the size of the neural plate while mouse embryos that lack just one of the BMP antagonists, chordin or noggin by knockout mutations have a relatively normal nervous system. However, the full potential of these antagonists becomes apparent when several of them are removed at the same time. For example, a complete loss of neural tissue is observed when all three BMP antagonists - i.e. chordin, follistatin and noggin - are simultaneously targeted using morpholinos, both in Xenopus 39 and Zebrafish40. Similarly, in mouse embryos loss of noggin and chordin alone has no severe phenotypes, while the double noggin/chordin mutant lacks all anterior neural structures41. Conversely, multiple BMP ligands are required for epidermal differentiation: at least three of the four BMPs, BMP2/4/7, need to be disrupted by morpholinos in Xenopus embryos to expand the neural plate, but even then, some ventral epidermal tissue remains. Thus in vivo, neural induction may depend on multiple ligands and inhibitors as a means to ensure robustness in BMP signaling during early patterning of the embryo.
FGF signaling and neural induction
Differential BMP signaling fulfills the expectation of an instructive mechanism for determining why neural tissue forms in one place in the embryo but not another42. The default model, however, leaves open the possibility that other factors are involved in neural induction, including those operating in a more permissive fashion to alter the competence of the ectoderm both spatially and temporally. The best evidence for a factor in this category are ligands of the FGF and IGF family, both of which bind to tyrosine kinase receptors and signal via the MAPK cascade. Significantly, FGF signaling has also been shown to inhibit BMP signaling in the early embryo by several mechanisms, thus potentially influencing the response of tissue to the activity of the BMP inhibitors produced by the organizer during neural induction. FGF signaling, for example, can promote phosphorylation of the linker domain and degradation of SMAD1, thereby reducing the efficacy of BMP signaling43. FGF signaling can also inhibit BMP activity indirectly, by inducing the expression of a protein called Zeb2, a zinc-finger homeodomain protein also known as SIP1 (and Zfhx1b), which binds to and represses the transcriptional activity of the SMAD protein44. For much of the neural plate, the role of FGF signaling is likely to be minor, since neural induction by the BMP inhibitors occurs readily in Xenopus in the absence of FGF signaling45. As discussed in the next section, this has also been shown to be the case in mammalian pluripotent cells.
Neural induction and early neural patterning
The early neural plate is already specified to form different parts of the nervous system as it arises following neural induction. For example, the wider part of the neural plate at the anterior end of the embryo will form brain tissue, while the narrow part posteriorly will form the spinal cord. A complex set of inductive signals generated from different parts of the organizer as well as neighboring epidermis is known to pattern the neural plate into different regions along the embryonic axes. Strikingly, in the absence of these additional signals, neural tissue induced by inhibiting BMP signaling leads to anterior forebrain-like tissue as a default state, while more posterior regions of the nervous system requires additional Wnt, FGF and Retinoic Acid signaling46–48. Thus, a hallmark of the default model is that ectoderm will form neural tissue with forebrain character in the absence of instructive signals. More posterior regions of the nervous system such as spinal cord are thought to be induced in two steps, by inhibiting BMPs, followed by a posteriorizing signal, even if both steps are mediated by the same factor such as FGF.
Neural Induction in Mammalian Embryonic Stem Cells (mESCs)
About thirty years ago, mESCs were derived from the blastocysts of the pre-implantation mouse embryo49, 50. These cells provided the functional in vitro definition of ESCs: unlimited proliferation (self-renewal) with retention of the capacity to differentiate into cells from each of the three embryonic germ layers - ectoderm, mesoderm, and endoderm - (pluripotency). The formal test of ES cell pluripotency was provided by the ability to contribute significantly to all tissues in the morula aggregation assay51. This advance provided the technical means to manipulate the mouse germline, and formally demonstrated that mESCs, reintroduced into the context of implantation development, were able to give rise to all cells of the embryo. Even more stringently, mESCs have been shown to generate entire mice in the tetraploid embryo complementation assay52.
Human embryonic stem cells (hESCs) were derived thereafter from human blastocysts. These hESC lines demonstrated the hallmark characteristics of self-renewal and pluripotency. While the gold standard pluripotency assays of morula aggregation or tetraploid embryo complementation are ethically impermissible using human cells, hESCs have passed all the standard tests for pluripotency including embryoid body (EB) formation teratoma assays and contribution to the embryonic germ layers of the mouse embryo53.
The cardinal translational promise of stem cell biology is that these cells can be used to generate novel in vitro models of intractable and poorly understood diseases and potentially for regenerative cell replacement strategies. From a developmental perspective however, mouse and human ESCs provide an in vitro platform to test hypotheses and investigate mechanisms controlling embryonic fate determination. For mouse this system is a complement to in vivo approaches, however for human it constitutes the only experimental window into early human embryogenesis. As with Xenopus pluripotent animal cap cells, a composite picture of the necessity and sufficiency of TGFβ/BMP inhibition for neural induction in mammalian pluripotent cells is also emerging.
Similarities and differences in the pluripotent state of mouse and human ESCs
Both mouse and human ESCs express identical embryonic transcription factors (TFs) such as OCT4 (POU5F1), SOX2 and NANOG during pluripotency, and form teratomas as xenografts54. However, there are also important differences—in signaling requirements, X-chromosome status, and growth characteristics—between human and mouse ESCs, which can be explained by the hypothesis that mESCs represent an earlier stage of development than hESCs.
Mouse ESCs require LIF, BMP, and WNTs for the maintenance a naïve (or “ground”) state of pluripotency55. Treatment with FGFs or WNT inhibition induces a conversion of mESCs to epiblast stem cells (EpiSCs) that self-renew and maintain pluripotency, but acquire the gene expression signature of post-implantation epiblast cells56, 57. This suggests that these signaling pathways may also contribute to the transition from naïve to primed pluripotency in vivo. Mouse EpiSCs have distinct signaling requirements—Activin/Nodal and FGF—compared to mESCs and display the same phenotype when derived directly from both pre- and post-implantation embryos.
Human ESCs on the other hand are dependent on Activin/Nodal and FGF signaling for maintenance of pluripotency, similar to EpiSCs not mESCs, even though they are derived from an equivalent embryonic source as mESCs: the inner cell mass of pre-implantation blastocysts58–60. Human ESCs are therefore considered to represent a more advanced stage of pluripotency than mESCs and are closer to EpiSCs in their developmental potential54, 59. Based on functional assays, it seems likely - though not formally proven - that the pluripotent cells of Xenopus animal caps are closer to the primed pluripotent state of mEpiSCs and hESCs than to the naïve state of mESCs since they can give rise to all the germ layer derivatives in the absence of priming61–63. Somatic cells reprogrammed to a pluripotent state, called induced pluripotent stem cells (iPSCs), share identical signaling properties for maintenance and differentiation to the ESCs of the species from which they were derived. Hence, mouse miPSCs require LIF, BMP and WNT for pluripotency, while human iPSCs require Activin/Nodal and FGF signaling (Figure 4)64–66.
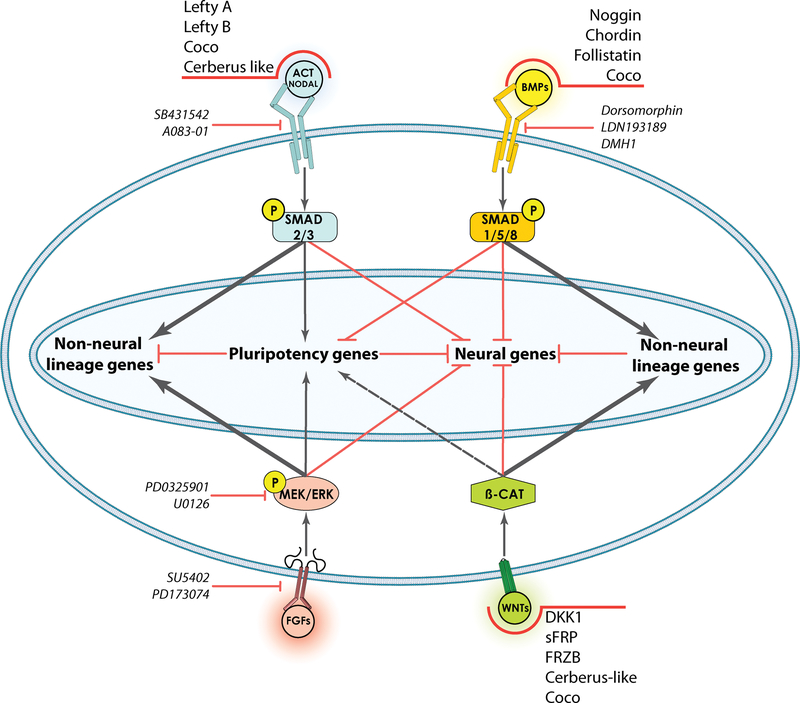
Figure 4: Signaling pathways involved in pluripotency and induction of neural fate in hESCs by a “default” mechanism.
Schematic showing that the three pathways mediating pluripotency in primed pluripotent cells, i.e. Activin/Nodal-SMAD2/3, FGF-MEK and WNT-β-catenin may repress neural fate directly and indirectly via pluripotency genes like NANOG. In addition, all these pathways can promote alternate non-neural fates at higher thresholds of signaling, as denoted by thick lines. These non-neural fates in turn also repress neural fate genes. Small molecule inhibitors of TGFβ and BMP signaling by secreted proteins (such as Lefty and Noggin) or small molecules (SB431542 and Dorsomorphin) are sufficient to convert pluripotent hESCs to a neural fate 86. Hence the state of pluripotency requires overcoming of the default neural state. Arrows represent activation (shown as proportional to the thickness of the lines) while hatches represent inhibition. Dotted lines denote postulated mechanisms from evidence in non-human systems.
Neural induction in mouse ESCs/EpiSCs and the role of FGF signaling
Neural induction paradigms in ESCs have evolved over the past decade from culturing ESCs as EBs in serum- and retinoic acid- containing medium to co-culturing ESCs with cell lines possessing neural-inducing activity, and now to defined culture conditions utilizing some combinations of growth factors or small molecules. We will only discuss the latter two protocols, as they are most informative with respect to the default model. A screen of feeder cell lines identified a bone marrow-derived stromal cell line could strongly promote neuronal differentiation from mESCs without concomitant mesoderm induction. The nature of this stromal cell-derived neural inducing activity (SDIA) remains unknown but could be blocked by BMP4, which promoted an epidermal fate in mESCs. This study was therefore among the first to provide evidence suggesting that the same signaling mechanisms determining the fate of pluripotent Xenopus animal cap cells may be conserved in mammalian ESCs as well. Subsequently, it was shown that mESCs cultured under defined low-density conditions—mimicking the dissociated Xenopus animal cap experiments—promoted their conversion into nestin-expressing neural precursors. In this paradigm, inhibition of BMP signaling with Noggin or Cerberus enhanced the appearance of neural colonies, as did Smad4 knockout mESCs, which are resistant to TGFβ/BMP signaling. A role for FGF in neuralization of mESCs was also suggested in this study, as well in separate studies utilizing defined media conditions in monolayer mESC cultures where the FGF pathway was either stimulated or repressed. These studies, however, did not resolve whether FGF was acting directly or indirectly as a neuralizing factor.
Although not known at the time, these observations can be easily reconciled by the fact that mESCs require FGF signaling to progress to a primed state of pluripotency i.e. the epiblast-like EpiSC state (also referred to as ‘primitive ectoderm’ in some of these studies), before they acquire the competence for neural induction56, 67, 68. Hence, FGF signaling conceivably regulates the competence of mESCs for germ layer differentiation, rather than neural induction per se68, 69. In fact, FGF signaling has recently been shown to inhibit rather than promote neural induction in EpiSCs, as would be expected from the default model69, 70. In addition, small molecule inhibitors of TGFβ/BMP signaling promote rapid neural commitment from EpiSCs under defined conditions, providing direct evidence for the validity of the default model in the mouse system71. It is worth noting that FGF signaling can directly inhibit SMAD signaling by promoting the degradation of SMAD1 via linker phosphorylation, as has been suggested in animal caps, but this role has not been directly tested in the paradigms above43. Thus, the requirements for FGF may be largely explained by its role in the transition of mESCs to EpiSCs, which are then primed for differentiation and hence resemble the pluripotent cells of the Xenopus animal cap more closely.
Other well characterized feeder- and serum- free protocols have also been developed for neural induction from mESCs that do not involve exogenous FGF signaling. For example, exposure of low-density mESC monolayer cultures to a sonic hedgehog inhibitor, led to the generation of telencephalic neurons that recapitulated the temporal hierarchy of in vivo cortical development72. In this context, inhibition of sonic hedgehog prevented ventral patterning of the nascent neural progenitors while the low density culture condition promoted neuralization in a manner evocative of the Xenopus animal cap dissociation experiments. Similarly, embryoid body differentiation with small molecule inhibitors of TGFβ and WNT signaling also recapitulated major spatial and temporal milestones of cortical development and generated functional neurons with forebrain identities73, 74. While the use of a TGFβ inhibitor falls in line with default neural differentiation, the WNT inhibitor in this paradigm likely facilitates the transition of mESCs to EpiSCs, as discussed above. Indeed, inhibition of endogenous WNT signaling in mESCs has been shown to readily promote their conversion to EpiSCs57. Furthermore, exogenous BMP4 completely abolished neural induction in this setting, supporting the default model’s tenet that inhibition of both Activin/Nodal-SMAD2/3 and BMP-SMAD1/5/8 signaling are necessary for neural induction30.
Neural induction in hESCs/iPSCs and the role of FGF signaling
Not surprisingly, many of the same protocols that have been used for neural induction in mESCs have also been adapted for neural differentiation of hESCs. Since hESCs do not survive as single cells, most early studies have used embryoid body differentiation approaches in the absence of exogenous factors. This approach showed that hESCs preferentially differentiate into anterior (forebrain) neural derivatives, presumably reflecting a default pathway in the absence of exogenous signaling75, 76. A role for endogenous FGF signaling was suggested as a requirement for neural induction in these studies, since small molecule inhibitors of FGF signaling reduced the number of cells expressing PAX677, 78. However, it is important to note that FGF or FGF inhibitors were not added in the initial four days of differentiation before the appearance of PAX675, 78, 79. This leaves open the possibility that FGF signaling is not directly promoting neural induction in these experiments, but rather has a survival and/or proliferative role in the early neuroepithelium. In support of this idea, exogenous FGF appeared to increase the size of neural colonies without changing the efficiency of neural induction76. In addition, neuralized hESCs displayed low levels of BMP-SMAD1/5/8 signaling, presumably due to the high level expression of several soluble BMP antagonists such as Noggin, Follistatin, and Gremlin as well intracellular inhibitors of BMP signaling such SMAD6 and ZEB2 (SIP1/ZFHX1B). Several other EB based protocols regularly include Noggin in serum-free medium to promote neuralization of hESCs80, 81. Together, these studies suggest that in the absence of exogenous morphogens, hESC colonies take on a neural fate of anterior character in line with the default model.
Inhibition of Activin/Nodal-SMAD2/3 signaling has also been shown to be a prerequisite for neuroectodermal differentiation of hESCs either as EBs or monolayer cultures82–85. Combining the classical observations made in Xenopus animal cap explants with these studies in hESCs, a feeder-free protocol for direct neural differentiation of hESCs utilizing small molecule inhibitors of Activin/Nodal-SMAD2/3 and BMP-SMAD1/5/8 signaling demonstrated rapid and high efficiency conversion to neural fate (>80% of cells)86. This system was made additionally tractable, and brought closer to the Xenopus animal cap assay by use of a Rho-associated kinase inhibitor, which confers survival on hESCs as single cells87 permitting single cell plating of hESCs/hiPSCs and differentiation in adherent culture conditions. As expected from the default model, the neuralized cells were of anterior identity in this paradigm, expressing the forebrain transcription factors (TFs) OTX2 and FOXG1. The primitive neuroepithelia could subsequently be patterned into multiple regional CNS derivatives, including midbrain, floor plate, and spinal cord. This dual-SMAD inhibition paradigm has now been adapted for chemically defined media as well as EB-based hESC and hiPSC differentiation protocols88, 89. It is worth noting that while exogenous FGF was used during neural induction in the original protocol, it has since been shown that FGF signaling directly inhibits induction of the neural determinant PAX690. This is in line with the inhibitory role of FGF in neural induction of EpiSCs derived from mouse embryos70. Interestingly, the inhibitory effect in hESCs was found to be restricted to a limited window, as continued FGF inhibition in the presence of TGFβ/BMP inhibition promoted a peripheral nervous system fate. Together these studies provide the strongest evidence so far that the molecular mechanism underlying neural fate specification in hESCs is conserved from Xenopus and conforms to the default model (Figure 4).
Other protocols have also been developed for neural induction in hESCs/hiPSCs and show a requirement for TGFβ inhibition. The SFEB protocol described above has also been adapted for hESCs and like in mESCs, has been shown to recapitulate major spatial and temporal milestones during cortical development and generate forebrain precursors74. While this protocol uses inhibition of Activin/Nodal-SMAD2/3 and WNT signaling in hESCs but not BMP-SMAD1/5/8 inhibition, addition of exogenous BMP did inhibit neural induction, which again points to the activity of endogenous BMP inhibition91. Use of WNT inhibitors in this system probably serve to prevent posteriorizing signaling and prevent non-neural differentiation92, 93.
Downstream mechanisms of default neural induction in mouse and human ESCs
The ability to generate purified populations of neuralized ESCs in vitro combined with use of gain-of-function and loss-of-function approaches has permitted scrutiny of the mechanisms operating downstream of TGFβ inhibition by which pluripotent cells undergo neural conversion. Inhibition of Activin/Nodal-SMAD2/3 down-regulates NANOG and promotes expression of ZEB2, a SMAD binding protein85. In pluripotent cells, ZEB2 limits the mesoderm inducing effects of Activin/Nodal signaling and is repressed directly by NANOG and OCT4. Once upregulated, ZEB2 promotes neuroectodermal differentiation of EpiSCs and hESCs. In addition, TGFβ/BMP-SMAD1/5/8 inhibition also promotes expression of a COUP-TFII (NR2F2), which is among the earliest TFs expressed during neural differentiation of hESCs90, 94. In pluripotent hESCs, OCT4 and the OCT4-induced microRNA mir-302 regulate expression of NR2F2 by transcriptional and post-transcription mechanisms, respectively, whereas in the differentiating neuroectoderm, NR2F2 directly represses OCT4 expression and promotes expression of other neural specific markers.
BMP-SMAD1/5/8 inhibition also contributes to neuroectodermal differentiation through several other mechanisms. First, it promotes the specificity of neural induction by inhibiting induction of non-neural germ layers such as trophectoderm, mesoderm, and non-neural ectoderm90. Indeed, inhibition of BMP signaling together with down-regulation of OCT4 is a prerequisite for neuroectodermal specification in hESCs95. Second, inhibition of BMP signaling may serve to stabilize the neural fate by maintaining expression of shared pluripotency and neural genes such as SOX290. Third, absence of BMP signaling promotes expression of cell-intrinsic neural determinants, such as the zinc finger TF, ZNF521, which is necessary and sufficient for neural induction in hESCs as well as EpiSCs91. Lastly, BMP inhibition may also promote acquisition of anterior neural fate, as neural induction protocols, which involve inhibition of Activin/Nodal-SMAD2/3, in the absence of BMP inhibitors, appear to adopt a more posterior neural identity in both EpiSCs and hESCs83, 84.
As mentioned above, FGF signaling maintains pluripotency in EpiSCs and hESCs. It is thought that FGF-MEK-ERK directly regulates NANOG expression in hESCs, but not EpiSCs70, 96. Hence one way FGF inhibition may contribute to neural induction is by down-regulating pluripotency TFs thereby permitting expression of the default neural program. In addition, early during differentiation FGF-MEK-ERK signaling has been shown to directly repress expression of the neural determinant TF PAX6 in hESCs as well as EpiSCs70, 90. Furthermore, inhibition of FGF signaling promotes rapid induction of the forebrain and midbrain enriched homeobox TF OTX2 in hESCs. OTX2 in turn directly binds to the PAX6 promoter and enhances its expression in hESCs90.
Conclusions and Perspectives
The default model provides a molecular explanation for the rich foundation of observations made in early embryology experiments, which is revealed in the embryo by the local inhibition of global inhibitors of neural fate. This double negative still appears to be the most persuasive explanation for observations from in vivo and in vitro assays of neural specification from fly to human. However, many open questions remain. To what extent does a default mechanism or inhibition of an inhibitor repeat itself during nervous system development? For example, what is the default positional identity within the nervous system? What determines the timing of double inhibitory events? When does it end? How is the dynamic aspect of signaling and signal inhibition regulated at the network level? How is TGFβ inhibition integrated in the hierarchical network of signaling that occurs concomitantly or immediately after neural induction to precisely establish temporal and positional identity - and therefore cellular diversity - in the nervous system? From an evolutionary point of view why should the nervous system be the default cellular identity? What advantage did this confer at the root of metazoan taxonomy? Future work in comparative developmental biology and evolution in diverse systems will begin to furnish responses to some of these questions.
Animation 1: Rotation and partition of Xenopus gastrula fate map
(Top) Animal pole, (Bottom) Vegetal pole. Ectoderm in the animal pole is depicted in blue. Light blue is ventral ectoderm that will give rise to the epidermis. Dark blue is dorsal ectoderm from which the neural plate will emerge. The boundary between ventral and dorsal ectoderm will give rise to sensory placodes on the anterior portion and neural crest in the medio-lateral edge. Mesoderm is in the equatorial region and depicted in green (ventral), orange (lateral), and red (dorsal/organizer). The organizer tissue arches at 60o (with 30o on each side of the midline). Endoderm in the vegetal pole is depicted in yellow. After a rotation the schematic is deconstructed to reveal the inside blastocoel cavity in the animal pole, highlighting the existence of ventral, lateral, and dorsal subdivisions in the mesodermal ring (pink, brown and red). Green cells attached to the endoderm on the dorsal side are bottle cells that demarcate the first site of involution when gastrulation movements begin.
Animation 2: Schematic animation and simplified graphic version of the organizer graft experiment (Spemann and Mangold experiment2)
This experiment was originally performed in the newt, but here cartooned in Xenopus embryos. The color code of the fate map (animation 1) has been maintained. A piece of dorsal mesoderm (red) when substituted for the ventral piece (green) induces a complete secondary axis in the tadpole.
Animation 3: Intact animal cap explants
Schematic rotation of a Xenopus blastula (made of ~4000 cells). Animal pole is shown in brown, and vegetal pole in yellow. Removing an explant from the top (future ectoderm), by microsurgery, reveals the blastocoel cavity. When cultured in neutral medium (equivalent of pond water) the explanted tissue (animal cap) rounds up and closes to give rise solely to epidermal (ventral ectoderm) fate.
Animation 4: Dissociated animal caps
Same explants as in animation 3, but upon removal cells of the explants are dissociated by simple elimination of Calcium and Magnesium from the neutral culture medium, so that they can no longer communicate and exchange signals. Surprisingly, these cells will not become epidermal, as when kept together, but rather become telencephalic neurons.
Animation 5: Inhibition of TGFβ signaling in animal cap explants
Animal cap explants removed from embryos that express a dominant negative Activin receptor (DN-ActRIIB, red cells) that blocks TGFβ signaling. (Embryos were microinjected at 2-cell stage with a synthetic mRNA encoding the mutant receptor and injected embryos are cultured until blastula stage). In this case explanted animal caps also differentiate to telencephalic cells, rather than staying epidermal as their uninjected sibling controls (animation 3).
Animation 6
Exposure of dissociated animal cap cells to physiological doses of purified TGFβ ligands such as BMPs inhibits their conversion to neuronal telencephalic fate and instead revert the cells back to their original induction of epidermis.
In the canonical pathway, a type I receptor propagates the signal by phosphorylating serine residues located at the C-terminus of receptor-Smads (R-Smads). Two groups of R-Smads transduce signals: R-Smads 2/3 (from Activins/Nodals and TGFβ1/2/3) and R-Smad1/5/8 (from BMP2/4/7 and some GDFs). R-Smads are part of a trimeric complex with a common mediator Smad - called co-Smad4 - that translocates to the nucleus to regulate transcription via transcription factors. As in the extracellular space, a series of inhibitors influences input from TGFβ signaling inside the cell at multiple levels. At the membrane level, co-receptors, such as Bambi, EGF-CFCs, and Tomoregulins regulate the activity and selectivity of TGFβ receptor transduction. Downstream of receptor activation inhibitory influences on R-Smads occurs by linker-phosphorylation via MAPK, GSK3β and CDKs, providing connection between TGFβ and other signaling pathways. TGFβ signaling itself also has the ability to phosphorylate R-Smad linker. Linker phosphorylation leads to either degradation via the ubiquitination by Smurf1/2, or changes in R-Smad specificity of gene regulation. Smad6 and Smad7 provide another level of inhibition. Smad6 acts in a BMP-dependent manner to compete with Smad4 binding and inhibit nuclear translocation of Smad1/5/8, while Smad7 acts in a ligand-independent manner to inhibit the pathway at multiple levels, including downstream of the activated type I receptor. Finally, dephosphorylation of the C-terminal end of R-Smads, by phosphatases such as small C-terminal domain phosphatases (SCPs), has also been shown to down-regulate the signal. The YAP/TAZ complex regulates Smad nuclear translocation and connects to the Hippo pathway.
The non-canonical TGFβ pathway is not as well understood, however, type II TGFβ receptors have been shown to signal through the Traf/TAK1 proteins. TAK1, in turn, activates JNK, p38, and MEK and the NF-κβ pathway. As TAK1 can also be activated by a variety of cytokines, the Wnt pathway, and the MAPK pathway, it provides yet another integration site for crosstalk amongst different signaling pathways.
Supplementary Material
Bibliography
- 1.Holtfreter J, and Hamburger V Amphibians. In analysis of Development, , B. H., Weiss P and Hamburger V, eds. Philadelphia: W. B. Saunders Company; 1955:230–296. [Google Scholar]
- 2.Spemann H, Mangold H. The induction of embryonic predispositions by implantation of organizers foreign to the species. Arch Mikrosk Anat En. 1924;100:599–638. [Google Scholar]
- 3.Gimlich RL, Cooke J. Cell lineage and the induction of second nervous systems in amphibian development. Nature. 1983;306:471–473. [DOI] [PubMed] [Google Scholar]
- 4.Oppenheimer JM. Transplantation experiments on developing teleosts (Fundulus and Perca). Journal of Experimental Zoology. 1936;72:409–437. [Google Scholar]
- 5.Oppenheimer JM. The Development of Transplanted Fragments of Fundulus Gastrulae. Proc Natl Acad Sci U S A. 1953;39:1149–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beddington RS. Induction of a second neural axis by the mouse node. Development. 1994;120:613–620. [DOI] [PubMed] [Google Scholar]
- 7.Waddington CH. The Epigenetics of Brids. Cambridge: Cambridge University Press; 1952. [Google Scholar]
- 8.Nieuwkoop PD. New Experiments on the Activation and Organization of the Central Nervous System in Amphibians. Anat Rec. 1951;111:453–454. [Google Scholar]
- 9.Slack JM, Forman D. An interaction between dorsal and ventral regions of the marginal zone in early amphibian embryos. Journal of embryology and experimental morphology. 1980;56:283–299. [PubMed] [Google Scholar]
- 10.Kurth T, Meissner S, Schackel S, et al. Establishment of mesodermal gene expression patterns in early Xenopus embryos: the role of repression. Developmental dynamics : an official publication of the American Association of Anatomists. 2005;233:418–429. [DOI] [PubMed] [Google Scholar]
- 11.Schier AF. Nodal morphogens. Cold Spring Harb Perspect Biol. 2009;1:a003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathews LS, Vale WW. Expression cloning of an activin receptor, a predicted transmembrane serine kinase. Cell. 1991;65:973–982. [DOI] [PubMed] [Google Scholar]
- 13.Kintner CaB A Ah. Neural Induction. Developmental Neuroscience. Elsevier Press; In press 2011. [Google Scholar]
- 14.Hemmatibrivanlou A, Melton DA. A Truncated Activin Receptor Inhibits Mesoderm Induction and Formation of Axial Structures in Xenopus Embryos. Nature. 1992;359:609–614. [DOI] [PubMed] [Google Scholar]
- 15.Hemmati-Brivanlou A, Melton DA. Inhibition of activin receptor signaling promotes neuralization in Xenopus. Cell. 1994;77:273–281. [DOI] [PubMed] [Google Scholar]
- 16.HemmatiBrivanlou A, Melton D. Vertebrate embryonic cells will become nerve cells unless told otherwise. Cell. 1997;88:13–17. [DOI] [PubMed] [Google Scholar]
- 17.Wilson PA, Hemmati-Brivanlou A. Induction of epidermis and inhibition of neural fate by Bmp-4. Nature. 1995;376:331–333. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki A, Kaneko E, Ueno N, et al. Regulation of epidermal induction by BMP2 and BMP7 signaling. Dev Biol. 1997;189:112–122. [DOI] [PubMed] [Google Scholar]
- 19.Fainsod A, Steinbeisser H, De Robertis EM. On the function of BMP-4 in patterning the marginal zone of the Xenopus embryo. Embo J. 1994;13:5015–5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemmati-Brivanlou A, Thomsen GH. Ventral mesodermal patterning in Xenopus embryos: expression patterns and activities of BMP-2 and BMP-4. Developmental genetics. 1995;17:78–89. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt JE, Suzuki A, Ueno N, et al. Localized BMP-4 mediates dorsal/ventral patterning in the early Xenopus embryo. Dev Biol. 1995;169:37–50. [DOI] [PubMed] [Google Scholar]
- 22.Sasai Y, Lu B, Steinbeisser H, et al. Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature. 1995;377:757. [DOI] [PubMed] [Google Scholar]
- 23.Xu RH, Kim J, Taira M, et al. A dominant negative bone morphogenetic protein 4 receptor causes neuralization in Xenopus ectoderm. Biochemical and biophysical research communications. 1995;212:212–219. [DOI] [PubMed] [Google Scholar]
- 24.Hawley SH, Wunnenberg-Stapleton K, Hashimoto C, et al. Disruption of BMP signals in embryonic Xenopus ectoderm leads to direct neural induction. Genes & development. 1995;9:2923–2935. [DOI] [PubMed] [Google Scholar]
- 25.Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–840. [DOI] [PubMed] [Google Scholar]
- 26.Sasai Y, Lu B, Steinbeisser H, et al. Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994;79:779–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hemmatibrivanlou A, Kelly OG, Melton DA. Follistatin, an Antagonist of Activin, Is Expressed in the Spemann Organizer and Displays Direct Neuralizing Activity. Cell. 1994;77:283–295. [DOI] [PubMed] [Google Scholar]
- 28.Piccolo S, Sasai Y, Lu B, et al. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86:589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zimmerman CM, Mathews LS. Activin receptors: cellular signalling by receptor serine kinases. Biochemical Society symposium. 1996;62:25–38. [PubMed] [Google Scholar]
- 30.Chang C, Harland RM. Neural induction requires continued suppression of both Smad1 and Smad2 signals during gastrulation. Development. 2007;134:3861–3872. [DOI] [PubMed] [Google Scholar]
- 31.Zusman SB, Sweeton D, Wieschaus EF. short gastrulation, a mutation causing delays in stage-specific cell shape changes during gastrulation in Drosophila melanogaster. Dev Biol. 1988;129:417–427. [DOI] [PubMed] [Google Scholar]
- 32.Eldar A, Dorfman R, Weiss D, et al. Robustness of the BMP morphogen gradient in Drosophila embryonic patterning. Nature. 2002;419:304–308. [DOI] [PubMed] [Google Scholar]
- 33.Holley SA, Jackson PD, Sasai Y, et al. A conserved system for dorsal-ventral patterning in insects and vertebrates involving sog and chordin. Nature. 1995;376:249–253. [DOI] [PubMed] [Google Scholar]
- 34.Mizutani CM, Nie Q, Wan FY, et al. Formation of the BMP activity gradient in the Drosophila embryo. Developmental cell. 2005;8:915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biehs B, Francois V, Bier E. The Drosophila short gastrulation gene prevents Dpp from autoactivating and suppressing neurogenesis in the neuroectoderm. Genes & development. 1996;10:2922–2934. [DOI] [PubMed] [Google Scholar]
- 36.Mizutani CM, Bier E. EvoD/Vo: the origins of BMP signalling in the neuroectoderm. Nat Rev Genet. 2008;9:663–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miya T, Morita K, Suzuki A, et al. Functional analysis of an ascidian homologue of vertebrate Bmp-2/Bmp-4 suggests its role in the inhibition of neural fate specification. Development. 1997;124:5149–5159. [DOI] [PubMed] [Google Scholar]
- 38.Lowe CJ, Terasaki M, Wu M, et al. Dorsoventral patterning in hemichordates: insights into early chordate evolution. PLoS biology. 2006;4:e291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khokha MK, Yeh J, Grammer TC, et al. Depletion of three BMP antagonists from Spemann’s organizer leads to a catastrophic loss of dorsal structures. Developmental cell. 2005;8:401–411. [DOI] [PubMed] [Google Scholar]
- 40.Dal-Pra S, Furthauer M, Van-Celst J, et al. Noggin1 and Follistatin-like2 function redundantly to Chordin to antagonize BMP activity. Dev Biol. 2006;298:514–526. [DOI] [PubMed] [Google Scholar]
- 41.Bachiller D, Klingensmith J, Kemp C, et al. The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature. 2000;403:658–661. [DOI] [PubMed] [Google Scholar]
- 42.Levine AJ, Brivanlou AH. Proposal of a model of mammalian neural induction. Dev Biol. 2007;308:247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pera EM, Ikeda A, Eivers E, et al. Integration of IGF, FGF, and anti-BMP signals via Smad1 phosphorylation in neural induction. Genes & development. 2003;17:3023–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheng G, dos Reis M, Stern CD. Churchill, a zinc finger transcriptional activator, regulates the transition between gastrulation and neurulation. Cell. 2003;115:603–613. [DOI] [PubMed] [Google Scholar]
- 45.Wills AE, Choi VM, Bennett MJ, et al. BMP antagonists and FGF signaling contribute to different domains of the neural plate in Xenopus. Dev Biol. 2010;337:335–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cox WG, Hemmati-Brivanlou A. Caudalization of neural fate by tissue recombination and bFGF. Development. 1995;121:4349–4358. [DOI] [PubMed] [Google Scholar]
- 47.Retinoids Maden M. and spinal cord development. Journal of neurobiology. 2006;66:726–738. [DOI] [PubMed] [Google Scholar]
- 48.Niehrs C Head in the WNT: the molecular nature of Spemann’s head organizer. Trends in genetics : TIG. 1999;15:314–319. [DOI] [PubMed] [Google Scholar]
- 49.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. [DOI] [PubMed] [Google Scholar]
- 51.Bradley A, Evans M, Kaufman MH, et al. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature. 1984;309:255–256. [DOI] [PubMed] [Google Scholar]
- 52.Bortvin A, Eggan K, Skaletsky H, et al. Incomplete reactivation of Oct4-related genes in mouse embryos cloned from somatic nuclei. Development. 2003;130:1673–1680. [DOI] [PubMed] [Google Scholar]
- 53.James JL, Stone PR, Chamley LW. The regulation of trophoblast differentiation by oxygen in the first trimester of pregnancy. Human reproduction update. 2006;12:137–144. [DOI] [PubMed] [Google Scholar]
- 54.Hanna JH, Saha K, Jaenisch R. Pluripotency and cellular reprogramming: facts, hypotheses, unresolved issues. Cell. 2010;143:508–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ying QL, Wray J, Nichols J, et al. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo G, Yang J, Nichols J, et al. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development. 2009;136:1063–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.ten Berge D, Kurek D, Blauwkamp T, et al. Embryonic stem cells require Wnt proteins to prevent differentiation to epiblast stem cells. Nature cell biology. 2011;13:1070–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.James D, Levine AJ, Besser D, et al. TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development. 2005;132:1273–1282. [DOI] [PubMed] [Google Scholar]
- 59.Vallier L, Mendjan S, Brown S, et al. Activin/Nodal signalling maintains pluripotency by controlling Nanog expression. Development. 2009;136:1339–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. Journal of cell science. 2005;118:4495–4509. [DOI] [PubMed] [Google Scholar]
- 61.Dixon JE, Allegrucci C, Redwood C, et al. Axolotl Nanog activity in mouse embryonic stem cells demonstrates that ground state pluripotency is conserved from urodele amphibians to mammals. Development. 2010;137:2973–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scerbo P, Girardot F, Vivien C, et al. Ventx factors function as Nanog-like guardians of developmental potential in Xenopus. PloS one. 2012;7:e36855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Theunissen TW, Costa Y, Radzisheuskaya A, et al. Reprogramming capacity of Nanog is functionally conserved in vertebrates and resides in a unique homeodomain. Development. 2011;138:4853–4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. [DOI] [PubMed] [Google Scholar]
- 65.Nakagawa M, Koyanagi M, Tanabe K, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nature biotechnology. 2008;26:101–106. [DOI] [PubMed] [Google Scholar]
- 66.Park IH, Zhao R, West JA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. [DOI] [PubMed] [Google Scholar]
- 67.Stavridis MP, Lunn JS, Collins BJ, et al. A discrete period of FGF-induced Erk1/2 signalling is required for vertebrate neural specification. Development. 2007;134:2889–2894. [DOI] [PubMed] [Google Scholar]
- 68.Kunath T, Saba-El-Leil MK, Almousailleakh M, et al. FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development. 2007;134:2895–2902. [DOI] [PubMed] [Google Scholar]
- 69.Sterneckert J, Stehling M, Bernemann C, et al. Neural induction intermediates exhibit distinct roles of Fgf signaling. Stem Cells. 2010;28:1772–1781. [DOI] [PubMed] [Google Scholar]
- 70.Greber B, Wu G, Bernemann C, et al. Conserved and divergent roles of FGF signaling in mouse epiblast stem cells and human embryonic stem cells. Cell stem cell. 2010;6:215–226. [DOI] [PubMed] [Google Scholar]
- 71.Najm FJ, Chenoweth JG, Anderson PD, et al. Isolation of epiblast stem cells from preimplantation mouse embryos. Cell stem cell. 2011;8:318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gaspard N, Bouschet T, Hourez R, et al. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature. 2008;455:351–357. [DOI] [PubMed] [Google Scholar]
- 73.Watanabe K, Kamiya D, Nishiyama A, et al. Directed differentiation of telencephalic precursors from embryonic stem cells. Nature neuroscience. 2005;8:288–296. [DOI] [PubMed] [Google Scholar]
- 74.Eiraku M, Watanabe K, Matsuo-Takasaki M, et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell stem cell. 2008;3:519–532. [DOI] [PubMed] [Google Scholar]
- 75.Zhang SC, Wernig M, Duncan ID, et al. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nature biotechnology. 2001;19:1129–1133. [DOI] [PubMed] [Google Scholar]
- 76.Pankratz MT, Li XJ, Lavaute TM, et al. Directed neural differentiation of human embryonic stem cells via an obligated primitive anterior stage. Stem Cells. 2007;25:1511–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zeng H, Guo M, Martins-Taylor K, et al. Specification of region-specific neurons including forebrain glutamatergic neurons from human induced pluripotent stem cells. PloS one. 2010;5:e11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yoo YD, Huang CT, Zhang X, et al. Fibroblast growth factor regulates human neuroectoderm specification through ERK1/2-PARP-1 pathway. Stem Cells. 2011;29:1975–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.LaVaute TM, Yoo YD, Pankratz MT, et al. Regulation of neural specification from human embryonic stem cells by BMP and FGF. Stem Cells. 2009;27:1741–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Itsykson P, Ilouz N, Turetsky T, et al. Derivation of neural precursors from human embryonic stem cells in the presence of noggin. Molecular and cellular neurosciences. 2005;30:24–36. [DOI] [PubMed] [Google Scholar]
- 81.Yao S, Chen S, Clark J, et al. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc Natl Acad Sci U S A. 2006;103:6907–6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vallier L, Rugg-Gunn PJ, Bouhon IA, et al. Enhancing and diminishing gene function in human embryonic stem cells. Stem Cells. 2004;22:2–11. [DOI] [PubMed] [Google Scholar]
- 83.Vallier L, Touboul T, Brown S, et al. Signaling pathways controlling pluripotency and early cell fate decisions of human induced pluripotent stem cells. Stem Cells. 2009;27:2655–2666. [DOI] [PubMed] [Google Scholar]
- 84.Patani R, Compston A, Puddifoot CA, et al. Activin/Nodal inhibition alone accelerates highly efficient neural conversion from human embryonic stem cells and imposes a caudal positional identity. PloS one. 2009;4:e7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chng Z, Teo A, Pedersen RA, et al. SIP1 mediates cell-fate decisions between neuroectoderm and mesendoderm in human pluripotent stem cells. Cell stem cell. 2010;6:59–70. [DOI] [PubMed] [Google Scholar]
- 86.Chambers SM, Fasano CA, Papapetrou EP, et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nature biotechnology. 2009;27:275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Watanabe K, Hamada S, Bianco C, et al. Requirement of glycosylphosphatidylinositol anchor of Cripto-1 for trans activity as a Nodal co-receptor. The Journal of biological chemistry. 2007;282:35772–35786. [DOI] [PubMed] [Google Scholar]
- 88.Boulting GL, Kiskinis E, Croft GF, et al. A functionally characterized test set of human induced pluripotent stem cells. Nature biotechnology. 2011;29:279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boissart C, Nissan X, Giraud-Triboult K, et al. miR-125 potentiates early neural specification of human embryonic stem cells. Development. 2012;139:1247–1257. [DOI] [PubMed] [Google Scholar]
- 90.Greber B, Coulon P, Zhang M, et al. FGF signalling inhibits neural induction in human embryonic stem cells. Embo J. 2011;30:4874–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kamiya N, Mishina Y. New insights on the roles of BMP signaling in bone-A review of recent mouse genetic studies. Biofactors. 2011;37:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fasano CA, Chambers SM, Lee G, et al. Efficient derivation of functional floor plate tissue from human embryonic stem cells. Cell stem cell. 2010;6:336–347. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 93.Menendez L, Yatskievych TA, Antin PB, et al. Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells. Proc Natl Acad Sci U S A. 2011;108:19240–19245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rosa A, Brivanlou AH. microRNAs in early vertebrate development. Cell Cycle. 2009;8. [DOI] [PubMed] [Google Scholar]
- 95.Wang X, Wang J, Huang V, et al. Induction of NANOG expression by targeting promoter sequence with small activating RNA antagonizes retinoic acid-induced differentiation. The Biochemical journal. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen G, Gulbranson DR, Yu P, et al. Thermal stability of fibroblast growth factor protein is a determinant factor in regulating self-renewal, differentiation, and reprogramming in human pluripotent stem cells. Stem Cells. 2012;30:623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Eagleson GW, Harris WA. Mapping of the presumptive brain regions in the neural plate of Xenopus laevis. Journal of neurobiology. 1990;21:427–440. [DOI] [PubMed] [Google Scholar]
- 98.Schulte-Merker S, Lee KJ, McMahon AP, et al. The zebrafish organizer requires chordino. Nature. 1997;387:862–863. [DOI] [PubMed] [Google Scholar]
- 99.Streit A, Lee KJ, Woo I, et al. Chordin regulates primitive streak development and the stability of induced neural cells, but is not sufficient for neural induction in the chick embryo. Development. 1998;125:507–519. [DOI] [PubMed] [Google Scholar]
- 100.Nakayama N, Han CE, Scully S, et al. A novel chordin-like protein inhibitor for bone morphogenetic proteins expressed preferentially in mesenchymal cell lineages. Dev Biol. 2001;232:372–387. [DOI] [PubMed] [Google Scholar]
- 101.Zimmerman LB, De Jesus-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. [DOI] [PubMed] [Google Scholar]
- 102.Furthauer M, Thisse B, Thisse C. Three different noggin genes antagonize the activity of bone morphogenetic proteins in the zebrafish embryo. Dev Biol. 1999;214:181–196. [DOI] [PubMed] [Google Scholar]
- 103.Connolly DJ, Patel K, Cooke J. Chick noggin is expressed in the organizer and neural plate during axial development, but offers no evidence of involvement in primary axis formation. Int J Dev Biol. 1997;41:389–396. [PubMed] [Google Scholar]
- 104.McMahon JA, Takada S, Zimmerman LB, et al. Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev. 1998;12:1438–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chapman SC, Schubert FR, Schoenwolf GC, et al. Analysis of spatial and temporal gene expression patterns in blastula and gastrula stage chick embryos. Dev Biol. 2002;245:187–199. [DOI] [PubMed] [Google Scholar]
- 106.Hemmati-Brivanlou A, Kelly OG, Melton DA. Follistatin, an antagonist of activin, is expressed in the Spemann organizer and displays direct neuralizing activity. Cell. 1994;77:283–295. [DOI] [PubMed] [Google Scholar]
- 107.Nakamura T, Takio K, Eto Y, et al. Activin-binding protein from rat ovary is follistatin. Science. 1990;247:836–838. [DOI] [PubMed] [Google Scholar]
- 108.Yamashita H, ten Dijke P, Huylebroeck D, et al. Osteogenic protein-1 binds to activin type II receptors and induces certain activin-like effects. J Cell Biol. 1995;130:217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Iemura S, Yamamoto TS, Takagi C, et al. Direct binding of follistatin to a complex of bone-morphogenetic protein and its receptor inhibits ventral and epidermal cell fates in early Xenopus embryo. Proc Natl Acad Sci U S A. 1998;95:9337–9342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shibanuma M, Mashimo J, Mita A, et al. Cloning from a mouse osteoblastic cell line of a set of transforming-growth-factor-beta 1-regulated genes, one of which seems to encode a follistatin-related polypeptide. Eur J Biochem. 1993;217:13–19. [DOI] [PubMed] [Google Scholar]
- 111.Patel K, Connolly DJ, Amthor H, et al. Cloning and early dorsal axial expression of Flik, a chick follistatin-related gene: evidence for involvement in dorsalization/neural induction. Dev Biol. 1996;178:327–342. [DOI] [PubMed] [Google Scholar]
- 112.Hayette S, Gadoux M, Martel S, et al. FLRG (follistatin-related gene), a new target of chromosomal rearrangement in malignant blood disorders. Oncogene. 1998;16:2949–2954. [DOI] [PubMed] [Google Scholar]
- 113.Schneyer A, Tortoriello D, Sidis Y, et al. Follistatin-related protein (FSRP): a new member of the follistatin gene family. Mol Cell Endocrinol. 2001;180:33–38. [DOI] [PubMed] [Google Scholar]
- 114.Tortoriello DV, Sidis Y, Holtzman DA, et al. Human follistatin-related protein: a structural homologue of follistatin with nuclear localization. Endocrinology. 2001;142:3426–3434. [DOI] [PubMed] [Google Scholar]
- 115.Bouwmeester T, Kim S, Sasai Y, et al. Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann’s organizer. Nature. 1996;382:595–601. [DOI] [PubMed] [Google Scholar]
- 116.Piccolo S, Agius E, Leyns L, et al. The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature. 1999;397:707–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Biben C, Stanley E, Fabri L, et al. Murine cerberus homologue mCer-1: a candidate anterior patterning molecule. Dev Biol. 1998;194:135–151. [DOI] [PubMed] [Google Scholar]
- 118.Belo JA, Bouwmeester T, Leyns L, et al. Cerberus-like is a secreted factor with neutralizing activity expressed in the anterior primitive endoderm of the mouse gastrula. Mech Dev. 1997;68:45–57. [DOI] [PubMed] [Google Scholar]
- 119.Hsu DR, Economides AN, Wang X, et al. The Xenopus dorsalizing factor Gremlin identifies a novel family of secreted proteins that antagonize BMP activities. Mol Cell. 1998;1:673–683. [DOI] [PubMed] [Google Scholar]
- 120.Bell E, Muñoz-Sanjuán I, Altmann C, and Brivanlou AH Cell Fate Specification and Competence, a maternal BMP, TGFb and WNT inhibitor. Development. 2003;in press. [DOI] [PubMed] [Google Scholar]
- 121.Ozaki T, Ma J, Takenaga K, et al. Cloning of mouse DAN cDNA and its down-regulation in transformed cells. Jpn J Cancer Res. 1996;87:58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pearce JJ, Penny G, Rossant J. A mouse cerberus/Dan-related gene family. Dev Biol. 1999;209:98–110. [DOI] [PubMed] [Google Scholar]
- 123.Eimon PM, Harland RM. Xenopus Dan, a member of the Dan gene family of BMP antagonists, is expressed in derivatives of the cranial and trunk neural crest. Mech Dev. 2001;107:187–189. [DOI] [PubMed] [Google Scholar]
- 124.Rodriguez Esteban C, Capdevila J, Economides AN, et al. The novel Cer-like protein Caronte mediates the establishment of embryonic left-right asymmetry. Nature. 1999;401:243–251. [DOI] [PubMed] [Google Scholar]
- 125.Yokouchi Y, Vogan KJ, Pearse RV, et al. Antagonistic signaling by Caronte, a novel Cerberus-related gene, establishes left-right asymmetric gene expression. Cell. 1999;98:573–583. [DOI] [PubMed] [Google Scholar]
- 126.Meno C, Saijoh Y, Fujii H, et al. Left-right asymmetric expression of the TGF beta-family member lefty in mouse embryos. Nature. 1996;381:151–155. [DOI] [PubMed] [Google Scholar]
- 127.Meno C, Ito Y, Saijoh Y, et al. Two closely-related left-right asymmetrically expressed genes, lefty-1 and lefty-2: their distinct expression domains, chromosomal linkage and direct neuralizing activity in Xenopus embryos. Genes Cells. 1997;2:513–524. [DOI] [PubMed] [Google Scholar]
- 128.Minabe-Saegusa C, Saegusa H, Tsukahara M, et al. Sequence and expression of a novel mouse gene PRDC (protein related to DAN and cerberus) identified by a gene trap approach. Dev Growth Differ. 1998;40:343–353. [DOI] [PubMed] [Google Scholar]
- 129.Topol LZ, Marx M, Laugier D, et al. Identification of drm, a novel gene whose expression is suppressed in transformed cells and which can inhibit growth of normal but not transformed cells in culture. Mol Cell Biol. 1997;17:4801–4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Coffinier C, Tran U, Larrain J, et al. Neuralin-1 is a novel Chordin-related molecule expressed in the mouse neural plate. Mech Dev. 2001;100:119–122. [DOI] [PubMed] [Google Scholar]
- 131.Abreu JG, Ketpura NI, Reversade B, et al. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol. 2002;4:599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Matsui M, Mizuseki K, Nakatani J, et al. Xenopus kielin: A dorsalizing factor containing multiple chordin-type repeats secreted from the embryonic midline. Proc Natl Acad Sci U S A. 2000;97:5291–5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Oelgeschlager M, Larrain J, Geissert D, et al. The evolutionarily conserved BMP-binding protein Twisted gastrulation promotes BMP signalling. Nature. 2000;405:757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chang C, Holtzman DA, Chau S, et al. Twisted gastrulation can function as a BMP antagonist. Nature. 2001;410:483–487. [DOI] [PubMed] [Google Scholar]
- 135.Ross JJ, Shimmi O, Vilmos P, et al. Twisted gastrulation is a conserved extracellular BMP antagonist. Nature. 2001;410:479–483. [DOI] [PubMed] [Google Scholar]
- 136.Scott IC, Blitz IL, Pappano WN, et al. Homologues of Twisted gastrulation are extracellular cofactors in antagonism of BMP signalling. Nature. 2001;410:475–478. [DOI] [PubMed] [Google Scholar]
- 137.Larrain J, Oelgeschlager M, Ketpura NI, et al. Proteolytic cleavage of Chordin as a switch for the dual activities of Twisted gastrulation in BMP signaling. Development. 2001;128:4439–4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kalantry S, Manning S, Haub O, et al. The amnionless gene, essential for mouse gastrulation, encodes a visceral-endoderm-specific protein with an extracellular cysteine-rich domain. Nat Genet. 2001;27:412–416. [DOI] [PubMed] [Google Scholar]
- 139.Kolle G, Georgas K, Holmes GP, et al. CRIM1, a novel gene encoding a cysteine-rich repeat protein, is developmentally regulated and implicated in vertebrate CNS development and organogenesis. Mech Dev. 2000;90:181–193. [DOI] [PubMed] [Google Scholar]
- 140.Matsuhashi S, Noji S, Koyama E, et al. New gene, nel, encoding a M(r) 93 K protein with EGF-like repeats is strongly expressed in neural tissues of early stage chick embryos. Dev Dyn. 1995;203:212–222. [DOI] [PubMed] [Google Scholar]
- 141.Watanabe TK, Katagiri T, Suzuki M, et al. Cloning and characterization of two novel human cDNAs (NELL1 and NELL2) encoding proteins with six EGF-like repeats. Genomics. 1996;38:273–276. [DOI] [PubMed] [Google Scholar]
- 142.Kuroda S, Tanizawa K. Involvement of epidermal growth factor-like domain of NELL proteins in the novel protein-protein interaction with protein kinase C. Biochem Biophys Res Commun. 1999;265:752–757. [DOI] [PubMed] [Google Scholar]
- 143.Hansen CS, Marion CD, Steele K, et al. Direct neural induction and selective inhibition of mesoderm and epidermis inducers by Xnr3. Development. 1997;124:483–492. [DOI] [PubMed] [Google Scholar]
- 144.Balemans W, Ebeling M, Patel N, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet. 2001;10:537–543. [DOI] [PubMed] [Google Scholar]
- 145.Balemans W, Van Hul W. Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev Biol. 2002;250:231–250. [PubMed] [Google Scholar]
- 146.Brunkow ME, Gardner JC, Van Ness J, et al. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet. 2001;68:577–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Aramaki T, Sasai N, Yakura R, et al. Jiraiya attenuates BMP signaling by interfering with type II BMP receptors in neuroectodermal patterning. Developmental cell. 2010;19:547–561. [DOI] [PubMed] [Google Scholar]
- 148.Ambrosio AL, Taelman VF, Lee HX, et al. Crossveinless-2 Is a BMP feedback inhibitor that binds Chordin/BMP to regulate Xenopus embryonic patterning. Developmental cell. 2008;15:248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Coffinier C, Hudon SE, Lee R, et al. A potent HIV protease inhibitor, darunavir, does not inhibit ZMPSTE24 or lead to an accumulation of farnesyl-prelamin A in cells. The Journal of biological chemistry. 2008;283:9797–9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Conley CA, Silburn R, Singer MA, et al. Crossveinless 2 contains cysteine-rich domains and is required for high levels of BMP-like activity during the formation of the cross veins in Drosophila. Development. 2000;127:3947–3959. [DOI] [PubMed] [Google Scholar]
- 151.Xu S, Cheng F, Liang J, et al. Maternal xNorrin, a canonical Wnt signaling agonist and TGF-beta antagonist, controls early neuroectoderm specification in Xenopus. PLoS biology. 2012;10:e1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ye X, Wang Y, Cahill H, et al. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell. 2009;139:285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Inman GJ, Nicolas FJ, Callahan JF, et al. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Molecular pharmacology. 2002;62:65–74. [DOI] [PubMed] [Google Scholar]
- 154.Tojo M, Hamashima Y, Hanyu A, et al. The ALK-5 inhibitor A-83–01 inhibits Smad signaling and epithelial-to-mesenchymal transition by transforming growth factor-beta. Cancer Sci. 2005;96:791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yu PB, Hong CC, Sachidanandan C, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cuny GD, Yu PB, Laha JK, et al. Structure-activity relationship study of bone morphogenetic protein (BMP) signaling inhibitors. Bioorg Med Chem Lett. 2008;18:4388–4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hao J, Ho JN, Lewis JA, et al. In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chem Biol. 2010;5:245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.