Abstract
Study Objective:
We aimed to assess the feasibility of a text messaging intervention by determining the proportion of emergency department (ED) patients who responded to prompted home blood pressure (BP) self-monitoring and had persistent hypertension. We also explored the effect of the intervention on systolic blood pressure (SBP) over time.
Methods:
We conducted a randomized, controlled trial of ED patients with expected discharge to home with elevated BP. Participants were identified by automated alerts from the electronic health record. Those who consented received a BP cuff to take home and enrolled in the 3-week screening phase. Text responders with persistent hypertension were randomized to control or weekly prompted BP self-monitoring and healthy behavior text messages.
Results:
Among the 104 patients enrolled in the ED, 73 reported at least one home BP over the 3-week run-in (screening) period. 55/73 reported a home BP>=140/90, and were randomized to SMS Intervention (n=28) or Control (n=27). The intervention group had significant SBP reduction over time with a mean drop of 9.1 mm Hg (95% CI 1.1 to 17.6).
Conclusions:
The identification of ED patients with persistent hypertension using home BP self-monitoring and text messaging was feasible. The intervention was associated with a decrease in SBP likely to be clinically meaningful. Future studies are needed to further refine this approach and determine its efficacy.
Keywords: Hypertension, Emergency Medicine, Self-Monitoring, Clinical Trial
Introduction
Hypertension is the most prevalent modifiable cardiovascular risk factor,1–5 with treatment reducing cardiovascular disease and all-cause mortality.5 Hypertension is common in the U.S. affecting 78 million adults.6 Hypertension control remains well below the Healthy People 2020 goal.7 While blood pressure (BP) control needs improvement in the overall U.S. adult population, uncontrolled hypertension is even more common among the uninsured and working age Americans.6, 8–10 New approaches to hypertension treatment are needed that focus on these difficult to reach populations in order to achieve health equity.
Currently, there are 136 million emergency department (ED) visits per year – nearly all have at least one BP measured and recorded. About 20% of working age Americans had an ED visit in the last year11 and the uninsured and Medicaid beneficiaries are high volume ED users. Even though these high-risk patient groups are generally not presenting to the ED for hypertension, the ED visit provides a unique opportunity to engage them in chronic disease management. However, there is some concern that ED BP readings may be falsely elevated due to pain or anxiety of the ED visit.12
In this age of electronic health records and mobile health, it may be possible for the ED to become an active partner in efficient chronic disease management by programming the electronic health record (EHR) to identify hypertensive patients and dispense a mobile health behavioral intervention. Text messaging offers an appealing option for behavioral interventions, given its popularity in underserved populations, low cost, ease of adoption, scalability, and ability to reach people in real-time yet remain flexible and convenient.13
In this context, we designed Reach Out ED---a pilot trial of an ED-based, mobile health, multicomponent, health theory-based, behavioral intervention to reduce BP for future testing in a large scale, randomized controlled trial. The overarching aim was to develop an automated, low human resource, ED-based intervention to improve blood pressure in an at-risk population. A key barrier to ED-based interventions is determining patient eligibility for such an intervention. Thus, our primary objective was to determine the feasibility of our intervention. Specifically, we sought to determine the proportion of ED patients who, after discharge to home, responded to prompted BP self-monitoring and the proportion of responses with persistently elevated BP over 140/90 mm Hg. Our secondary objective was to explore the effect of the Reach Out intervention on blood pressure over time.
Methods
Study Design
Briefly, Reach Out ED was a randomized feasibility study. We enrolled hypertensive patients meeting eligibility at the University of Michigan Health System ED, which at the time had an approximate yearly patient volume of 70,000 adult patients per year.1 We prospectively identified patients using EHR-based automatic alert system that notified the study team members to the presence of potentially eligible patients. These were programmed in the EPIC (EPIC systems, Madison, Wisconsin, USA) EHR using Best Practice Alerts, that automatically sent a page to a study team member and placed the visit ID in a research inbasket within EPIC. We have previously used automated EHR alerts to identify eligible research patients in real time in the ED.14, 15 Following initial recruitment, we randomized those persistently hypertensive after 3 weeks into either the text messaging intervention or standard care. The primary objective was to determine the proportion of ED patients who, after discharge to home, respond to text reminders with their home assessed BP and the proportion of responses with persistently elevated BP (over 140/90 mm Hg). We have included the study protocol and consent form in the supplemental material. We report these results in accordance with the CONSORT extension for pilot and feasibility trials and the relevant checklist is also included in the supplemental material.16
Study Population
Adult ED patients were eligible if they had a documented systolic BP ≥160 mm Hg or a diastolic BP ≥100 mm Hg, were likely to be discharged from the ED, and possessed a mobile phone with text-messaging available. We excluded patients who were critically ill, otherwise unable to give informed consent, incarcerated/ institutionalized residents, pregnant, or had a pre-existing condition that made follow-up for 4 months unlikely. All materials and text-messages were created in English; thus participants were excluded if they could not read English. Since patients were initially entered into a screening phase and randomized after responding during this three-week period, the study personnel who were conducting recruitment were blinded to treatment group assignment.
Randomization
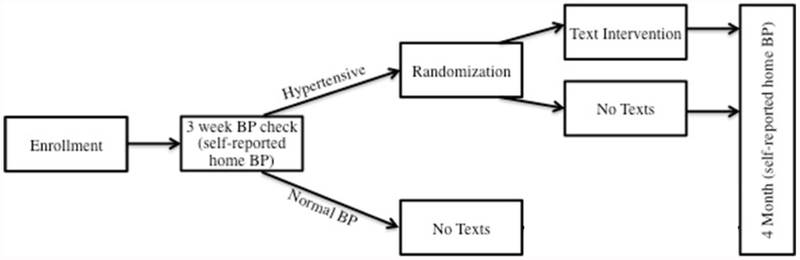
Once enrolled, but prior to randomization, participants underwent a screening phase to determine whether they had persistent hypertension (Figure 1) defined as BP ≥140/90, based on the prevailing definition at the time the study was designed. The goal of this screening phase was to enrich the population receiving the intervention by allowing the design to focus on participants who were willing to respond and had persistent elevated BP. During the three-week screening phase, participants received weekly text messages that solely requested their BP. Each week the text-messaging system made 3 attempts to prompt the participant to text back his/her BP. Participants who responded at least one time, and had persistent hypertension (any reported measurement of systolic BP ≥140 or a diastolic BP ≥90) were randomized. Participants who either did not respond at all, or did not report a qualifying BP were not randomized. The screening phase allowed the study to focus resources on participants most likely to benefit. Randomly permuted blocks of 4 and 6 were generated at randomization.org by WJM and assignments were made for up to 150 participants. At the time of initial enrollment, the study staff recruiting participants did not have access to the randomization assignment. When participants in the screening phase met the eligibility criteria for text messaging response and persistent hypertension, they were initiated on either the intervention or control pathway by the project manager.
Figure 1:
Overview of Study Design
Study Interventions
All participants enrolled in the study were given an automatic SureLife 860211 wrist BP cuff, American Heart Association brochures about hypertension, and received a text message one day/week for three weeks prompting the participant to text in his/her BP. During the subsequent 3 months, the intervention group received healthy behavior text messages and weekly reminders to text back their BP. The healthy behavior text messages addressed the most important lifestyle interventions to reduce BP: salt reduction, increased fruit and vegetable intake and increased physical activity (see appendix for example).1, 17–19 In addition to these generic health messages, targeted text messages based on whether the subject took an antihypertensive medication and had a primary care physician were provided. For example, for participants taking antihypertensive medications, text messages also addressed medication adherence (e.g., pillboxes, schedules, etc.)20 Participants received weekly text message prompts to check and text their BP back. A tailored message comparing their recent BP to their enrollment BP was then sent back to the subject. The control group received no further text messages and were instructed to follow up with their primary care doctor for treatment. All participants received a text message three months after randomization requesting a final self-reported BP (figure 1). Additional details regarding the theory behind the intervention and the content and procedure for the text messaging are provided in the supplemental material.
Study Endpoints/Outcome
The primary study endpoint was the proportion of ED patients who, after discharge to home, responded to prompted BP self-monitoring and had persistent hypertension defined as BP ≥ 140/90 mm Hg. Amongst those meeting the primary endpoint who were then randomized to the next phase, the secondary endpoint was self-reported SBP four months from the time of enrollment. The primary endpoint was chosen to assess study feasibility.
Sample Size and Statistical Analysis
We defined the maximum sample size as 150. We planned to accrue until the end of the academic year related to resource availability even if the maximum sample size was not reached. Our primary aim was to determine if the proportion of participants remaining hypertensive within the three-week screening phase was at least 33% based on our belief that this would be a reasonable yield for distribution of BP monitoring devices. The 95% confidence interval for a one-sample proportion (33%) of a total sample size of 150 is 25.5% - 40.5% (binomial method without continuity correction). Therefore, if the observed proportion was greater than or equal to 25.5% we would conclude that the primary hypothesis of the trial (that proportion of participants with persistent hypertension measured within 3 weeks is at least 33%) has been achieved within a reasonable degree of certainty. For the secondary analyses, we calculated the mean change in BP, along with the 95% confidence interval for the change from the baseline randomization phase measures to final measurement for the intervention and control groups. We did not compare the means or conduct a hypothesis test on them. The BP change analyses were intended to assess whether the intervention was in a zone of promise. We did not define this formally a priori but in general we believed that a reduction in SBP of around 3-5 mm Hg would be likely to be clinically meaningful based on past cardiovascular trials.21 As such, estimating whether our intervention was potentially consistent with this magnitude of effect was the intent of these analyses. 21 We used the median of the up to three home BP measurements during the screening phase as the baseline BP in qualifying participants.
Post Hoc and Graphical Analyses
A large proportion of the final outcomes were missing. We addressed this through graphical exploration of the data and using a last observation carried forward approach. First, we graphed the change from ED initial SBP from the initial visit in the ED, to the median of the screening phase, and finally to the end of study. Second, we graphed each recorded SBP (baseline, final, or subject reported), for each participant by week of the study. This depicted how many readings were at markedly high or low levels. Third, for subjects with a missing final visit, we imputed the value by taking the last recorded blood pressure from the study and carrying it forward – last observation carried forward (LOCF). For example, a subject with an ED SBP of 247, who did not respond to any texts, would have 247 entered as the final measurement for zero change. We estimated the means and standard errors for the treatment and control groups. It is important to note, that no LOCF imputations were used for the graphical analyses reported above. Finally, we stratified the cohort by whether the subjects were taking 1 or more BP medicines at the time of initial enrollment and estimated the mean change in SBP and standard errors by treatment and control groups as well. Given the small sample size, we only conducted this stratified analysis using the LOCF, imputed population.
Safety and Adverse Event Tracking
Education during initial enrollment included a warning that patients should contact their clinician directly or call 911 if they have any urgent questions or health problems that should be addressed before their next scheduled visit. Participants self-reporting a weekly BP >180/110 were sent an automated message advising immediate contact with a doctor to have their BP checked as they are at high risk. Additionally, any participants who spontaneously sent in messages, (such as questions or comments), received an immediate automated message advising them that if they have questions they should contact their doctor, or in an emergency call 911. We used a study specific adverse event reporting plan. We only collected and reported serious adverse events that were definitely, probably, or possibly related to the study (e.g. ED visit from cuff injury).
Human Subjects Protection
The protocol was approved by the University of Michigan Medical School Institutional Review Board (IRBMED) with approval number HUM00091668. Written informed consent was obtained from all participants.
Results
Characteristics of cohort
During the 7-month enrollment period between October 2014 and April 2015, over 9,300 patients with elevated BP were identified through the EHR-based automatic alerts. A total of 1,908 of these had data on eligibility abstracted. Of these, 169 were approached and 104 patients enrolled (64%). The enrolled cohort was primarily white, insured, and had a history of hypertension (Table 1). Follow up of the last participant occurred in August of 2015. Enrollment ended prior to the recruitment of 150 participants since it was the end of the academic year and this was a preplanned criterion for termination of recruitment.
Table 1: Characteristics of participants at baseline.
Characteristics of each of the groups, based on enrollment status and group assignment. N = count SD = standard deviation N/A = not applicable. * (all participants selecting other wrote in a form of private insurance).
| Control (N=27) | Intervention (N=28) | Enrolled, Not Randomized (N=49) | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 50 | 12 | 49 | 13 | 48 | 13 |
| N | percent | N | percent | N | percent | |
| Female | 9 | 33% | 15 | 54% | 30 | 61% |
| Hispanic | 2 | 7% | 0 | 0% | 3 | 6% |
| Race | ||||||
| Asian | 0 | 0% | 0 | 0% | 1 | 2% |
| Black / African American | 5 | 19% | 7 | 25% | 12 | 24% |
| White | 21 | 78% | 20 | 71% | 32 | 65% |
| Other, N/A or not disclosed | 0 | 0% | 1 | 4% | 5 | 10% |
| Desired text frequency | ||||||
| Once every other day | 16 | 59% | 12 | 43% | 27 | 55% |
| Once per day | 9 | 33% | 14 | 50% | 18 | 37% |
| Twice per day | 2 | 7% | 2 | 7% | 4 | 8% |
| Health Insurance (multiple types could be selected) | ||||||
| Private | 17 | 63% | 19 | 68% | 22 | 45% |
| Medicaid | 2 | 7% | 3 | 11% | 11 | 22% |
| Medicare | 4 | 15% | 3 | 11% | 9 | 18% |
| Uninsured | 0 | 0% | 0 | 0% | 2 | 4% |
| Other (all wrote in a form of private insurance) | 7 | 26% | 6 | 21% | 13 | 27% |
| Routine place for primary medical care | 22 | 81% | 28 | 100% | 44 | 90% |
| Previous diagnosis of hypertension | 20 | 74% | 21 | 75% | 35 | 71% |
| Prior hospitalization for hypertension | 4 | 15% | 2 | 7% | 6 | 12% |
| Prior medication for hypertension | 13 | 48% | 17 | 61% | 26 | 53% |
| Current number of hypertension medications taking | ||||||
| 0 | 18 | 67% | 12 | 43% | 25 | 51% |
| 1 | 4 | 15% | 10 | 36% | 14 | 29% |
| 2 | 1 | 4% | 6 | 21% | 4 | 8% |
| 3 | 1 | 4% | 0 | 0% | 3 | 6% |
| 4 | 1 | 4% | 0 | 0% | 2 | 4% |
| 5 | 2 | 7% | 0 | 0% | 0 | 0% |
| Smoke cigarettes | 6 | 22% | 7 | 25% | 3 | 6% |
| Most commonly used communication method | ||||||
| In person conversations | 5 | 19% | 6 | 21% | 16 | 33% |
| Internet/social media | 1 | 4% | 2 | 7% | 3 | 6% |
| Other | 1 | 4% | 0 | 0% | 1 | 2% |
| Phone calls | 13 | 48% | 10 | 36% | 18 | 37% |
| Text messages | 7 | 26% | 10 | 36% | 11 | 22% |
Proportion responding with persistent hypertension: Primary endpoint results
A total of 73 of the 104 enrolled participants responded to at least one text during the screening phase representing 70% (95% CI 60-78%) of our cohort. For our primary endpoint, 55 out of 104 enrolled patients (53%, 95% CI 43 to 62%) responded and were hypertensive; this exceeded our pre-defined minimum threshold of 25.5%. No participants reported any adverse effects attributable to the study protocol.
Utilization of Text Messaging
During the 3-week screening phase, 43 participants texted BP measurements for three weeks, 25 texted BPs for two weeks, and 7 texted a BP one of the weeks only. Within the treatment group receiving weekly text prompted self-monitoring, we observed a uniform distribution of text responses with the most frequent number of text responses 6 out of the 12 weeks (Figure 3).
Figure 3: Distribution of BP responses in the intervention group.
Within the intervention group, the number of weeks that a blood pressure was texted back in response to the system prompt.
Change in SBP over time: Secondary endpoint results
We illustrate the change in SBP or loss to follow up over the course of the study for all 104 participants in Figure 4. We indicate whether the patient reported taking BP medications at baseline and show how much the BP changed from screening to randomization, and from randomization to the final visit. Very few self-reported blood pressures were over the threshold to prompt a warning to urgently see a doctor (Figure 5).
Figure 4: Blood Pressure Trends and Early Drop-out Over Study Period.
Each of the 104 patients grouped from left to right by those who did not qualify for randomization, the intervention group and the control group. Within each group participants are arranged from left to right in order of highest ED SBP. Red bars depict the change in SBP from ED visit to median screening phase SBP for patients taking BP medications; the blue bars represent this change for patients not taking BP medications. For patients who never returned any texts, the circles represent the ED SBP for patients taking BP medications and the triangles represent each subject who was not taking BP medications. The narrower, yellow bars represent the change in SBP from the screening phase to the final visit for the intervention group. Subject 73 is an example of a case where the SBP was higher at the final visit. The narrower, purple bars represent the change from screening phase to end of study for the control group. Some subjects with median SBP lower than 140 from the screening phase depicted above were randomized. In one case, a participant had a diastolic BP over 90, in the other cases at least one SBP measurement was 140 or above.
Figure 5 – All study blood pressures over time.
All systolic blood pressures, by week of study. Week 1 is the baseline in the emergency department, Weeks 2-4 are the screening period, Weeks 5-16 are weekly text messaging-based responses in the intervention group, and Week 17 is the final in-person follow up visit. Patients who were not eligible (due to SBP under 140) or did not respond to texts are indicated with a plus sign, the intervention group is indicated with triangles, and the control group with circles.
The intervention group had significant SBP reduction over time with a mean drop of 9.1 mm Hg (95% CI 1.1 to 17.6) (Table 2). The mean drop for the control group was lower, but substantial at 6.6 mm Hg (95% CI −2.4 to 15.6), although the confidence interval for this change crossed zero. When we repeated the analysis using the LOCF imputation procedure, we observed similar drops in blood pressure over time across the groups. The stratified analyses using the LOCF data, demonstrated that current BP medication use may be important, as the control group without current medication had almost no change in BP, whereas the control and intervention subjects on medication had drops of 11.2 and 9.5 mm Hg respectively.
Table 2: Blood pressure changes: baseline versus final visit.
Results of blood pressure change analyses. Any negative numbers represent an increase in blood pressure. LOCF = Last Observation Carried Forward, SBP = Systolic Blood Pressure, mm Hg = millimeters of mercury.
| Systolic blood pressure reduction (final minus baseline) with final visit | ||||
|---|---|---|---|---|
| N | Mean (mm Hg) | Std. Error of Mean | 95% confidence interval | |
| Intervention | 14 | 9.1 | 4.1 | 1.1 to 17.1 |
| Control | 16 | 6.6 | 4.6 | −2.4 to 15.6 |
| Systolic blood pressure reduction (final minus baseline) LOCF | ||||
| N | Mean (mm Hg) | Std. Error of Mean | 95% confidence interval | |
| All randomized | ||||
| Intervention | 28 | 8.7 | 2.8 | 3.2 to 14.2 |
| Control | 27 | 4.1 | 3 | −1.8 to 10 |
| No reported BP meds | ||||
| Intervention | 28 | 7.7 | 3.5 | 0.8 to 14.6 |
| Control | 27 | 0.5 | 3.2 | −5.8 to 6.8 |
| Reported BP meds | ||||
| Intervention | 28 | 9.5 | 4.2 | 1.3 to 17.7 |
| Control | 27 | 11.2 | 3.5 | 4.3 to 18.1 |
Adverse Events
We did not observe any serious adverse events during the course to the study that met the definition in our pre-specified IRB-approved safety reporting plan.
Discussion
In this pilot trial of an ED-based, mobile health, multicomponent, health theory-based, behavioral intervention to reduce BP, we found that ED recruitment of patients who later had persistently elevated blood pressure is feasible. We found that 53% of participants who enrolled had persistent hypertension during the 3 weeks after their ED visit. Our findings show the feasibility of automated, real-time EHR alerts to identify possibly eligible ED patients, and confirms the feasibility of the recruitment strategy and text-prompted BP self-monitoring to assess subject eligibility. These findings were instrumental in the successful NIH funding of a larger scale Phase II trial evaluating a multi-component text messaging intervention for patients with elevated blood pressures in the ED. Our post hoc analyses demonstrated potential heterogeneity of SBP trajectory following the ED visit based on whether the participants reported being on BP medications at time of initial enrollment. In our follow up study, we plan to use this as a stratification variable at the time of randomization and we will hopefully gain better understanding regarding the different prognosis for patients with and without prior antihypertensive treatment.
Our findings suggest that the ED can be a valuable partner in hypertension screening particularly among the working age population who can be hard-to-reach and derive substantial benefit from hypertension control. Our automated alerts identified over 9000 potentially eligible participants in 7 months. Furthermore, of those approached over 50% agreed to enrollment in the screening phase of our trial. Additionally, we found that about one-half of participants who were enrolled in the ED had persistent hypertension defined as ≥ 140/90. If current definitions of hypertension were used, the proportion of participants with persistent hypertension would likely increase. Our findings are concordant with a single center observational study in an urban ED that found that 51% of hypertensive patients remained hypertensive 1 week after their ED visit.22 There are many competing demands on the ED workforce many of which outweigh chronic disease management. Thus, Reach Out was designed with this in mind. With its automated patient identification via EHR, if the Reach Out intervention was effective the ED workforce would only need to dispense a BP cuff. This practical approach increases the possibility of future dissemination and implementation if future studies confirm this approach can meaningfully reduce BP.
Little data exists to guide the management of ED patients with asymptomatic hypertension. While guidelines recommend BP screening,23 the guidance for management of asymptomatic hypertension in the ED is based on consensus opinion which varies widely from no intervention, referral for outpatient follow up or initiation of antihypertensives.24 We found a reductions in blood pressure over time in both the Reach Out intervention and control groups; however only the intervention group confidence interval excluded zero change or worsening.
The use of weekly prompted BP self-monitoring for both study inclusion and as a component of the intervention is novel. We found variable adherence to returning text messages in our treatment group, despite using an enrichment strategy to increase the likelihood of including patients who would be willing to respond. Mobile health interventions to reduce BP have shown promise, but are limited by short duration of follow-up, data on the optimal intervention components and delivery, and absence of rigorous clinical trial design.25 The Reach Out pilot and its future randomized trial will fill some of these scientific gaps.
This work has several important limitations. Our results only apply to participants who are expected to be discharged to home from the ED and thus cannot be extrapolated to participants who were admitted to the hospital. There were several participants with missing data for the final measurement of SBP, although the focus of this study was to determine how many participants would respond to text requests for their BP and remain hypertensive and therefore be eligible for randomization. The greater loss to follow-up in the control arm informed the design of our follow up study. Specifically, in our ongoing Phase II trial we provide patient incentives for follow up, and request self-monitored blood pressures from all participants in all arms of the trial. In our pilot, all potentially eligible participants were not approached for enrollment. However, times of research assistant availability was varied and should therefore reflect the overall ED population at a suburban academic ED. We limited our enrollment to an academic year, as the pilot study had limited funding and we utilized college students gaining academic credit as our primarily recruiters. In addition, patients seek care for different reasons in diverse settings and our study was conducted at a single center in one community. For our secondary analysis, we used a last observation carried forward approach to missing data. This may be conservative; although it is possible that subjects who dropped out had improving or worsening blood pressure so it is not clear the direction of bias or noise this approach is introducing. The application of the LOCF imputation resulted in a difference in means for both groups that were smaller with wider confidence intervals, yet still was within a promising zone for the treatment group. Given the methods we used to tailor our text messages, we focused on an English speaking population only. In addition, we did not systematically assess whether our intervention induced ED visits that did not result in a change in hypertension management – although ED utilization of participants will be monitored in our follow up trial. We used wrist cuffs, to address patient preference and limit the need to size upper arm cuffs. Wrist cuffs may not be as accurate as upper arm cuffs, however it is unclear whether they would be systematically over or under estimating arterial BP; in addition, we use the cuff over time within patient and that could mitigate the influence of this potential problem. We did not collect individual data on self-efficacy or medication adherence. Our cohort was majority white, and almost entirely insured, which may limit generalizability to other populations. Finally, within this feasibility study, we did not collect data regarding the initiation of new medications or dosage changes. In our ongoing clinical trial, we plan to routinely query participants regarding the timing and frequency of changes in their medications, along with assessing medication adherence.
Conclusions
In conclusion, weekly prompted BP self-monitoring is feasible and can identify ED patients with persistent hypertension who may benefit from a hypertension intervention. Further research is needed to determine the efficacy of the ED-based, mobile health, multicomponent, health theory-based, behavioral intervention to reduce BP.
Supplementary Material
Figure 2:
Participant Screening and Flow
Acknowledgments
WJM, LS, and DB conceived the study, designed the trial, and obtained research funding. WJM, LS, and MD supervised the conduct of the trial and data collection. DD, SO,VG and MD undertook recruitment of patients and managed the data, including quality control. WJM provided statistical advice on study design and analyzed the data. WJM drafted the manuscript, and all authors contributed substantially to its revision. WJM takes responsibility for the paper as a whole
Financial Support
This study was supported in part by the University of Michigan Cardiovascular Center Inaugural award (Skolarus) and by the University of Michigan Department and Emergency Medicine through PI discretionary funds. In addition, the University of Michigan Undergraduate Research Opportunity Program provided partial funding. Finally, analytic work was supported by the National Institutes of Health, National Institute of Minority Health and Disparities R01-MD011516.
Footnotes
Data Access and Responsibility
This study was registered on clincialtrials.gov concurrent with initiation of enrollment () in fall of 2014. Dr. Meurer and Dr. Skolarus had full access to the data and vouch for its integrity. The final de-identified analytical dataset is available from Dr. Skolarus and Dr. Meurer contingent upon execution of a data use agreement in accordance with requirements of the University of Michigan.
Potential Conflicts of Interest
The investigators have no conflicts of interest to report.
References
- 1.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 2.Turnbull F, Blood Pressure Lowering Treatment Trialists C. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–1535. [DOI] [PubMed] [Google Scholar]
- 3.Cheng S, Claggett B, Correia AW, et al. Temporal trends in the population attributable risk for cardiovascular disease: the Atherosclerosis Risk in Communities Study. Circulation. 2014;130:820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berlowitz DR, Ash AS, Hickey EC, et al. Inadequate management of blood pressure in a hypertensive population. The New England journal of medicine. 1998;339:1957–1963. [DOI] [PubMed] [Google Scholar]
- 5.Group SR, Wright JT,, Williamson JD, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. The New England journal of medicine. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011-2012. NCHS data brief. 2013:1–8. [PubMed] [Google Scholar]
- 7.Egan BM, Li J, Hutchison FN, Ferdinand KC. Hypertension in the United States, 1999 to 2012: progress toward Healthy People 2020 goals. Circulation. 2014;130:1692–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris AB, Li J, Kroenke K, Bruner-England TE, Young JM, Murray MD. Factors associated with drug adherence and blood pressure control in patients with hypertension. Pharmacotherapy. 2006;26:483–492. [DOI] [PubMed] [Google Scholar]
- 9.Barron WM. Failed Appointments - Who Misses Them, Why They Are Missed, and What Can Be Done. Primary Care. 1980;7:563–574. [PubMed] [Google Scholar]
- 10.Egan BM, Li J, Small J, Nietert PJ, Sinopoli A. The Growing Gap in Hypertension Control Between Insured and Uninsured Adults: National Health and Nutrition Examination Survey 1988 to 2010. Hypertension. 2014;64:997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gindi RM, Cohen RA, Kirzinger WK. Emergency room use among adults aged 18-64: early release of estimates from the National Health Interview Survey, January–June 2011. National Center for Health Statistics. 2012. [Google Scholar]
- 12.Decker WW, Godwin SA, Hess EP, Lenamond CC, Jagoda AS. Clinical policy: critical issues in the evaluation and management of adult patients with asymptomatic hypertension in the emergency department. Annals of emergency medicine. 2006;47:237–249. [DOI] [PubMed] [Google Scholar]
- 13.Bennett GG. Connecting eHealth with 2-1-1 to reduce health disparities. American journal of preventive medicine. 2012;43:S509–511. [DOI] [PubMed] [Google Scholar]
- 14.Cardozo E, Meurer WJ, Smith BL, Holschen JC. Utility of an automated notification system for recruitment of research subjects. Emergency Medicine Journal. 2010:emj. 2009.081299. [DOI] [PubMed] [Google Scholar]
- 15.Meurer WJ, Smith BL, Losman ED, et al. Real-Time Identification of Serious Infection in Geriatric Patients Using Clinical Information System Surveillance. Journal of the American Geriatrics Society. 2009;57:40–45. [DOI] [PubMed] [Google Scholar]
- 16.Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot and feasibility studies. 2016;2:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. The New England journal of medicine. 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2006;113:e873–923. [DOI] [PubMed] [Google Scholar]
- 19.Graudal N, Jurgens G. The blood pressure sensitivity to changes in sodium intake is similar in Asians, Blacks and Whites. An analysis of 92 randomized controlled trials. Frontiers in physiology. 2015;6:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreuter MW, Wray RJ. Tailored and targeted health communication: strategies for enhancing information relevance. American journal of health behavior. 2003;27 Suppl 3:S227–232. [DOI] [PubMed] [Google Scholar]
- 21.BPLT Trialists’Collaboration. Effects of different regimens to lower blood pressure on major cardiovascular events in older and younger adults: meta-analysis of randomised trials. BMJ. 2008;336:1121–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanabe P, Persell SD, Adams JG, McCormick JC, Martinovich Z, Baker DW. Increased Blood Pressure in the Emergency Department: Pain, Anxiety, or Undiagnosed Hypertension? Annals of Emergency Medicine.51:221–229. [DOI] [PubMed] [Google Scholar]
- 23.Decker WW, Godwin SA, Hess EP, Lenamond CC, Jagoda AS, American College of Emergency Physicians Clinical Policies Subcommittee on Asymptomatic Hypertension in the ED. Clinical policy: critical issues in the evaluation and management of adult patients with asymptomatic hypertension in the emergency department. Ann. Emerg. Med 2006;47:237–249. [DOI] [PubMed] [Google Scholar]
- 24.Wolf SJ, Lo B, Shih RD, Smith MD, Fesmire FM, American College of Emergency Physicians Clinical Policies C. Clinical policy: critical issues in the evaluation and management of adult patients in the emergency department with asymptomatic elevated blood pressure. Ann. Emerg. Med 2013;62:59–68. [DOI] [PubMed] [Google Scholar]
- 25.Burke LE, Ma J, Azar KM, et al. Current Science on Consumer Use of Mobile Health for Cardiovascular Disease Prevention: A Scientific Statement From the American Heart Association. Circulation. 2015;132:1157–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.