Abstract
Background: Home-based video visits were provided over one year as a supplement to in-person care for pediatric type 1 diabetes (T1D) patients with suboptimal glycemic control. We hypothesized that the intervention would be feasible and satisfactory for the target population and would significantly improve hemoglobin A1c (HbA1c) levels and completion of recommended quarterly diabetes clinic visits.
Methods: This was a nonrandomized clinical trial. Fifty-seven patients aged 3–17 years with known T1D and HbA1c ≥8% (64 mmol/mol) were recruited to receive the intervention. The study population was 49% adolescent (13–17 years old) and 58% publicly insured patients. Video visits were scheduled every 4, 6, or 8 weeks depending on the HbA1c level. HbA1c levels as well as frequencies of clinic visits and of diabetes-related emergency department (ED) and hospital encounters were compared before and after the study.
Results: Thirty participants completed 12 months of video visits. The study cohort demonstrated significant improvement in mean HbA1c in both intention-to-treat (N = 57) analysis (10.8% [95 mmol/mol] to 10.0% [86 mmol/mol], P = 0.01) and per-protocol (N = 30) analysis (10.8% [95 mmol/mol] to 9.6% [81 mmol/mol], P = 0.004). Completion of ≥4 annual diabetes clinic visits improved significantly from 21% at baseline to 83% during the study period for the entire cohort, P < 0.0001. The frequency of diabetes-related ED and hospital encounters did not change significantly.
Conclusions: Home-based video visits are a feasible supplement to in-person care for children and adolescents with T1D and suboptimal glycemic control and can successfully improve HbA1c levels and adherence to recommended frequency of care in this high-risk clinical population.
Keywords: Home-based telemedicine, Pediatric type 1 diabetes, Remote data-sharing, Glycemic control
Introduction
Management of type 1 diabetes (T1D) has advanced substantially in the last two decades. More effective insulin preparations,1 insulin pumps,2 continuous glucose monitoring (CGM),3 and hybrid closed-loop systems4 are facilitating closer monitoring and better glycemic control5–7 for patients who have access to them. Simultaneously, evidence linking outpatient diabetes care, hemoglobin A1c (HbA1c) levels, and long-term complications has led to evidence-based guidelines for treatment during childhood and adolescence.8,9 Despite these advancements, the majority of pediatric T1D patients are failing to achieve recommended HbA1c levels10 and remain at high risk for morbid and costly complications later in life.
While recommended quarterly clinic visits are sufficient for a subset of T1D patients, the Diabetes Control and Complications Trial11 and multiple single-center studies12–15 have demonstrated that more frequent contact with providers improves glycemic control for high-risk patients. However, more frequent in-person visits are difficult to achieve. Undersupply of pediatric diabetes specialists,16 an expanding number of youth with T1D,17 and poor reimbursement for multidisciplinary services in an office setting18 limit clinic appointments. For patients and families, the additional missed school hours, parental time away from work, and transportation expenses are a significant burden.19 Fortunately, recent innovations in diabetes technology and connected health20–22 as well as increasing availability of smart devices23 have made remote patient monitoring and telemedicine encounters feasible for T1D.
Previously published studies of video visits for pediatric T1D are few and primarily include trials of video-based behavioral therapy by mental health professionals and trials of video-based physician visits replacing in-person visits for patients with limited access. Studies in the former category have delivered frequent behavioral therapy to pediatric patients with T1D and demonstrated improved glycemic control after 3 months.14,15 Studies in the latter category have shown no improvement in glycemic control after 1–2 years, but have demonstrated high patient satisfaction and reported time and cost savings.24–26 We investigated the utility of supplementing in-person care with frequent home-based video visits for a population of pediatric T1D patients with suboptimal glycemic control over the course of one year. We hypothesized that this intervention would be feasible and satisfactory for patients and that it would increase the overall frequency of diabetes care and significantly improve glycemic control during the study period.
Methods
Study design and recruitment
This was a nonrandomized clinical trial in which all participants received the study intervention. Patients were recruited at the University of California, Davis (UCD), Pediatric Diabetes Clinic between November 27, 2017, and February 2, 2018. Inclusion criteria were (1) age 1–17 years, (2) diagnosis of T1D >3 months prior (to ensure baseline HbA1c did not reflect prediagnosis glucose levels), (3) HbA1c level ≥8% (64 mmol/mol) at time of enrollment, (4) access to the internet using a device with video and audio capability, and (5) ability to connect the patient's home glucose meter—as well as insulin pump and/or CGM, if applicable—to an internet-capable device through Bluetooth or physical cable. The only exclusion criterion was need for English language interpretation due to difficulty in securing interpretation services remotely. Written informed consent was obtained from each patient's parent or guardian, and patients 8–17 years of age provided assent to participate. The UCD Institutional Review Board approved this study and it was registered with ClinicalTrials.gov (registration number NCT03374462).
Study intervention
The study intervention consisted of home-based video visits with a pediatric endocrinologist as a supplement to quarterly in-person visits at the UCD Pediatric Diabetes Clinic. Video visits took place every 4 weeks (for patients with HbA1c >12% or >108 mmol/mol), 6 weeks (for patients with HbA1c 10%–12% or 86–108 mmol/mol), or 8 weeks (for patients with HbA1c <10% or <86 mmol/mol). Visit frequency was assigned at enrollment and again after 6 months based on the most recent HbA1c value. Video visits were conducted using an HIPAA-compliant videoconferencing platform27 and included the provider, patient, and parent or guardian, with the exception of patients ≥16 years of age whose parents gave consent for solo visits with the provider. A single pediatric endocrinologist conducted all video visits, eliminating any interprovider variability in the clinical approach. Participants uploaded data from their diabetes devices before each visit using secure internet platforms that were compatible with their home computers, tablets, or mobile phones.28–32 The research team assisted with the initial setup of these platforms and provided support for any technical issues during the study. Each video visit included discussion of interval health events and patient or family concerns, review of shared glucose data and insulin dose information, and provision of recommendations by the physician, including changes in behavior and/or insulin dose adjustments. This content was equivalent to the physician portion of in-person diabetes clinic visits, except that a detailed physical examination could not be performed.
Data collection and analysis
Baseline data were collected from the electronic health record (EHR) at enrollment. These included demographic and insurance information, HbA1c values over the previous year, current use of insulin pump and/or CGM, and frequency of diabetes clinic visits and diabetes-related emergency department (ED) and hospital encounters during the prior year. Patients and their parents were also surveyed regarding their recollection of diabetes-related ED and hospital encounters during the previous year to capture any encounters not listed in our EHR. During each video visit, participants were asked about any ED or hospital encounters since the last visit and any technical issues encountered while uploading diabetes data or using the video program. At 6 and 12 months, participants were surveyed regarding their experiences (see Supplementary Table S1 for a complete list of survey questions and responses). At 12 months, we administered an additional survey instrument that has been published and validated for assessment of telemedicine diabetes care.33 Survey responses were elicited from patients and parents together in most cases—with parents typically providing the final answers—and from patients alone for those participants ≥16 years of age who engaged in independent video visits with the provider.
The dates of participants' visits to the UCD Pediatric Diabetes Clinic during the study period, HbA1c levels measured at those visits, and dates of any ED or hospital encounters during the study period were abstracted from the EHR. The records for ED and hospital encounters—those reported by participants or discovered in the EHR—were reviewed by a pediatric endocrinologist to determine which were diabetes related and which met criteria for diabetic ketoacidosis (DKA) according to published guidelines.34 All participants who withdrew from the study consented to have their EHR data collected for the year post-enrollment.
HbA1c values were measured using point-of-care devices with an upper measurement limit of 14% (130 mmol/mol), therefore values >14% (>130 mmol/mol) were designated as 14.5% (135 mmol/mol) for our numerical HbA1c analyses. The designation of 14.5% (135 mmol/mol) was chosen to separate these values minimally from measurements of 14.0% (130 mmol/mol), knowing that this trimming of higher values would likely underestimate any improvement in HbA1c observed during the study. To evaluate HbA1c changes over time, the date of each HbA1c measurement was identified by the number of months it occurred after enrollment, rounded to the nearest integer. To characterize changes in HbA1c throughout the study period, mean HbA1c was calculated at quarterly intervals by grouping HbA1c values measured during each quarter (1–3, 4–6, 7–9, and 10–12 months after enrollment) for the entire cohort and for active versus withdrawn participants. Finally, we employed a linear mixed-effects model35 to evaluate the impact of the intervention on glycemic control over time, where HbA1c values at enrollment and each quarter were the dependent variables and time was the primary independent variable. Baseline characteristics—including demographics, insurance status, distance from clinic, and use of insulin pumps and CGMs—were explored through this model for any association with HbA1c. Variables that were associated with HbA1c in our cohort—age (P = 0.001), race (P = 0.003), and pump use (P = 0.06)—were included as covariates in the final model (Table 3). Random effects at the patient level were also included in the model to account for correlation of multiple measurements over time.
Table 3.
Change in Mean HbA1c for Study Cohort Adjusted for Patient-Level Factors
| Time | Change in mean HbA1c from enrollment | 95% Confidence interval | P |
|---|---|---|---|
| 1–3 Months | −0.74% | −1.21 to −0.27% | 0.002 |
| −8.4 mmol/mol | −14.9 to −3.7 mmol/mol | ||
| 4–6 Months | −0.63% | −1.10 to −0.15% | 0.01 |
| −7.3 mmol/mol | −12.0 to −2.5 mmol/mol | ||
| 7–9 Months | −0.49% | −0.98 to −0.01% | 0.05 |
| −5.9 mmol/mol | −10.8 to −1.1 mmol/mol | ||
| 10–12 Months | −0.79% | −1.25 to −0.32% | 0.001 |
| −8.9 mmol/mol | −14.5 to −4.2 mmol/mol |
Results of the linear mixed-effects model, including age, race, and insulin pump use as covariates.
Results
Study population
Fifty-seven patients enrolled in the study. Participants were predominantly non-Hispanic White/Caucasian and publicly insured (Table 1). Almost half were adolescents, most lived >50 km away from the UCD Pediatric Diabetes Clinic, and more than half had HbA1c levels ≥10% (86 mmol/mol) at enrollment. The majority had been diagnosed with diabetes 1–5 years before study enrollment. Fifty-three percent of enrolled participants completed 12 months in the study. Among the 27 participants who withdrew from the study, 13 exited before receiving the intervention, and the vast majority (23 participants) withdrew due to trouble scheduling and completing video visits within the assigned time frame. Three patients withdrew due to loss of internet- or video-capable devices, and one was withdrawn by the treating endocrinologist due to new mental health symptoms requiring urgent attention. Baseline characteristics were compared for participants who withdrew versus those who completed the study, including demographic characteristics, HbA1c, use of insulin pumps and CGMs, and frequency of diabetes care encounters (clinic, ED, and hospital) during the year prior to enrollment. None of these variables differed significantly between groups except CGM use, which was more common among those who completed the study (47%) than among those who withdrew (15%), P = 0.01.
Table 1.
Study Population Characteristics (N = 57)
| N (%) | |
|---|---|
| Age in years | |
| 3–7 | 7 (12) |
| 8–12 | 22 (39) |
| 13–17 | 28 (49) |
| Sex | |
| Female | 25 (44) |
| Male | 32 (56) |
| Race | |
| Asian | 2 (4) |
| Black/African American | 7 (12) |
| White/Caucasian | 41 (72) |
| More than one race | 3 (5) |
| Unknown/not reported | 4 (7) |
| Ethnicity | |
| Hispanic/Latino | 6 (11) |
| Not Hispanic/Latino | 51 (89) |
| Insurance | |
| Public | 33 (58) |
| Private | 24 (42) |
| Distance in kilometers to clinic | |
| ≤50 | 24 (42) |
| 51–100 | 15 (26) |
| 101–200 | 7 (12) |
| >200 | 11 (19) |
| Duration of diabetes | |
| ≤1 year | 3 (5) |
| 1–5 years | 32 (56) |
| ≥5 years | 22 (39) |
| HbA1c at enrollment | |
| 8.0%–9.9% (64–85 mmol/mol) | 25 (46) |
| 10.0%–11.9% (86–107 mmol/mol) | 14 (26) |
| 12.0%–13.9% (108–129 mmol/mol) | 6 (11) |
| ≥14% (≥130 mmol/mol) | 9 (17) |
HbA1c = hemoglobin A1c.
Feasibility and satisfaction
During participants' first video visits, 18% reported difficulty using the video application and 20% reported difficulty sharing diabetes data remotely. Among subsequent video visits, patient-reported issues occurred 7% of the time for the video application and 17% of the time for remote data sharing. Of the 30 participants who completed the study, 97% were very satisfied and 3% were somewhat satisfied with home-based video visits. The most frequently cited benefits were improved self-monitoring of blood glucose and improved access to the care team between clinic visits, each of which was endorsed by 93% of completing participants. Detailed survey response information is available in Supplementary Table S1. Responses to the validated diabetes telemedicine survey administered at study completion33 were likewise overwhelmingly positive. All participants at 12 months agreed or strongly agreed with the statements, “I believe the doctor understood my blood sugar situation during the video visit” and “I believe that a video visit is good for achieving good control of my diabetes.” For complete information on survey questions and responses for this instrument, see Supplementary Fig. S1.
Care utilization
Annual diabetes clinic visits (in-person+video) for all participants increased from a mean of 3.1 at enrollment to a mean of 7.9 at study completion (P < 0.001) (Table 2). The proportion of our entire study population (N = 57) achieving the recommended 4+ visits per year9 also increased significantly from 21% at baseline to 83% (P < 0.0001) during the study period. This finding was even more pronounced among patients who completed the study (N = 30), 100% of whom achieved 4+ visits per year during the study compared with 23% of the same group who had achieved this during the year before study enrollment. Among patients who withdrew from the study, clinic attendance also improved, with the proportion achieving 4+ visits per year increasing from 19% at baseline to 63% one year later. Our cohort also demonstrated significant increases in insulin pump use (44%–53%, P = 0.03) and CGM use (32%–51%, P = 0.002) during the study period. The proportions of participants experiencing diabetes-related ED encounters, DKA hospitalizations, and other diabetes-related hospitalizations did not change significantly during the study.
Table 2.
Diabetes Care Before and During the Study Period for the Entire Study Cohort (N = 57)
| Year before study | Study period | P | |
|---|---|---|---|
| Mean diabetes clinic visits (SD) | 3.1 (1.0) | 7.9 (4.1) | <0.001 |
| Total diabetes clinic visits | |||
| 0 | — | 1 (2%) | <0.0001 |
| 1 | 1 (2%) | 1 (2%) | |
| 2 | 15 (26%) | 2 (4%) | |
| 3 | 29 (51%) | 6 (11%) | |
| 4+ | 12 (21%) | 47 (83%) | |
| In-person visits | |||
| 0 | — | 1 (2%) | 0.004 |
| 1 | 1 (2%) | 1 (2%) | |
| 2 | 15 (26%) | 7 (12%) | |
| 3 | 29 (51%) | 22 (39%) | |
| 4+ | 12 (21%) | 26 (46%) | |
| Video visits | |||
| 0 | 57 (100%) | 13 (23%) | — |
| 1 | — | 10 (18%) | |
| 2 | — | 17 (30%) | |
| 3 | — | 11 (19%) | |
| 4+ | — | 6 (11%) | |
| Insulin pump use | 25 (44%) | 30 (53%) | 0.03 |
| Continuous glucose monitor use | 18 (32%) | 29 (51%) | 0.002 |
| Diabetes emergency department visits | |||
| 0 | 48 (84%) | 51 (89%) | 0.8 |
| 1 | 9 (16%) | 5 (9%) | |
| 2 | — | 1 (2%) | |
| Diabetic ketoacidosis hospitalizations | |||
| 0 | 50 (88%) | 51 (89%) | 1 |
| 1 | 7 (12%) | 5 (9%) | |
| 2 | — | 1 (2%) | |
| Other diabetes hospitalizations | |||
| 0 | 54 (95%) | 53 (93%) | 1 |
| 1 | 2 (4%) | 4 (7%) | |
| 2 | 1 (2%) | — | |
P values calculated by paired t-test, McNemar's test, or Wilcoxon signed-rank test, as appropriate.
Glycemic control
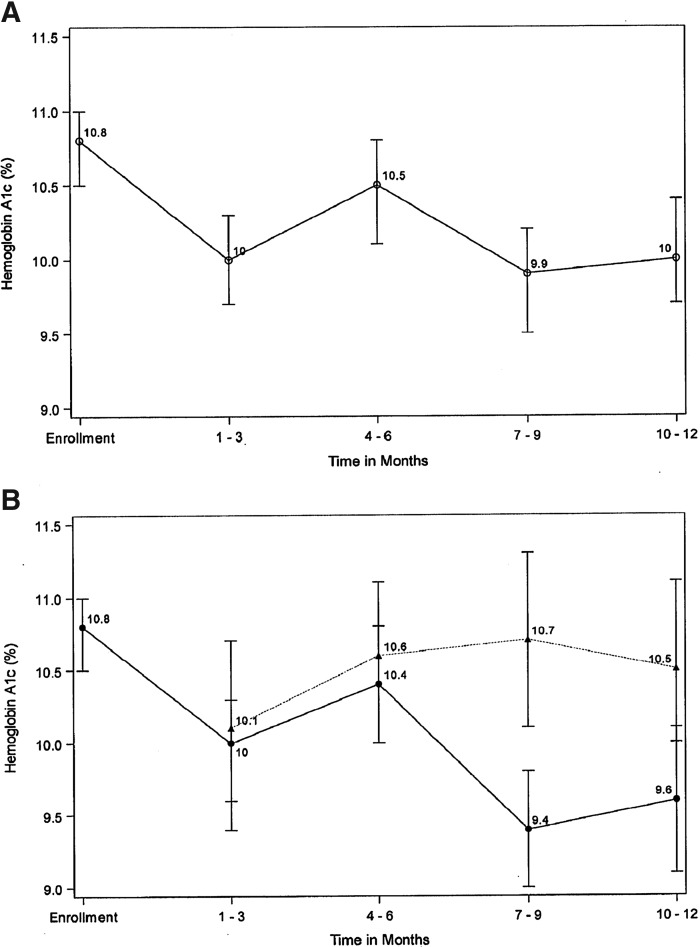
Mean HbA1c for the study population improved from 10.8% (95 mmol/mol) at baseline to 10.0% (86 mmol/mol) at 10–12 months, and participants who completed 12 months of video visits experienced an even greater decline to 9.6% (81 mmol/mol) (Fig. 1). These changes were both statistically significant in the mixed-effects model (Table 3), with P = 0.001 for the entire cohort and P = 0.004 (not shown in Table 3) for those who completed the study. Graphic (Fig. 1) and tabular (Table 3) depictions of HbA1c changes over time demonstrate that the study population experienced a dramatic decline in HbA1c by the first quarter of the study, after which HbA1c values increased transiently and then decreased again by the end of the study. This pattern held true for the entire cohort (Fig. 1A) and for the subset of participants who completed 12 months of video visits (Fig. 1B). Mean HbA1c for withdrawn subjects and participating subjects remained similar at 1–3 and 4–6 months, but diverged significantly by 7–9 months of the intervention (Fig. 1B).
FIG. 1.
Mean HbA1c ± SEM at quarterly intervals for (A) entire cohort and (B) participating subjects (circles, solid line) versus withdrawn subjects (triangles, dotted line). HbA1c, hemoglobin A1c.
Discussion
This nonrandomized clinical trial demonstrates that home-based video visits can improve glycemic control and adherence to recommended outpatient care among high-risk pediatric patients with T1D. Our results are both statistically significant and clinically meaningful, including a decrease in HbA1c of 0.8% (9 mmol/mol) among the entire cohort (P = 0.001) and 1.2% (14 mmol/mol) among participants who completed the study (P = 0.004). The most recent comparable trials of video visits for pediatric T1D by Wood et al.26 and Reid et al.25 demonstrated improvement in mean annual outpatient visits from 2.0 to 2.9 and 2.6 to 3.5, respectively, as well as high rates of satisfaction, but no significant changes in HbA1c after 1 year. In comparison, our intervention demonstrated much higher mean visit frequency (7.9 annual visits) during the study period and significant HbA1c reduction. Given evidence that frequent phone contact11,12 and frequent behavioral therapy14,15 can improve glycemic control in T1D, our intervention's impact on HbA1c was likely due in part to the high frequency of encounters.
Our nonrandomized design cannot separate the intervention's effect from any expected changes in glycemic control over time. However, recent data demonstrate that HbA1c values typically rise during childhood and adolescence36 rather than demonstrating a regression to the mean, so it is unlikely that the observed improvements were due to biologic or environmental forces external to the intervention. It is also unlikely that improvements in endogenous pancreatic function—as seen during the honeymoon period—played a role in our results because only three study subjects had a diabetes duration of ≤1 year, and these patients did not show significant improvement in their HbA1c values during the study.
The finding that substantial glycemic improvement occurred during the first quarter of the study was unexpected. This initial decrease in HbA1c by 1–3 months was observed both in active participants and those who had already withdrawn (Fig. 1B), suggesting that the change may not have been entirely due to video visits. We hypothesize that subjects who withdrew during the first quarter experienced a transient increase in diabetes awareness resulting from conversations with the research team and use of the apps provided at enrollment. However, by 7–9 months, mean HbA1c values for participating and withdrawn subjects diverged sharply, suggesting that ongoing improvement in HbA1c was due to the intervention itself.
The generalizability of our findings is limited by our use of a single provider for video visits, and this raises the possibility that the intervention's effect was at least partially due to the provider rather than the video modality or frequency of care. However, a significant portion of care for all participants (mean of 3.3 in-person visits during the study period) was delivered by their continuity endocrinologists. For 10 of our 57 subjects, the in-person endocrinologist was the same provider who delivered video visits, and these patients demonstrated a mean HbA1c improvement of only 0.2% (2 mmol/mol)—from 10.7% (93 mmol/mol) at enrollment to 10.5% (91 mmol/mol) at study completion. This suggests that the benefits of our intervention were not due to the effectiveness of the video provider specifically. However, it raises the possibility that the addition of a second care provider can be helpful in this population, and this hypothesis should be explored in future studies.
This nonrandomized trial was designed primarily to explore the feasibility of frequent home-based video visits for a high-risk clinical population. We anticipated that the time commitment required for frequent visits as well as the need to utilize specialized software might make our intervention untenable for a subset of the population, and it appears that these factors did contribute to our low retention rate. However, participants who completed the study reported high satisfaction despite the time commitment and technology hurdles. The frequency of reported video problems also decreased greatly after participants' first visits (from 18% to 7%), suggesting habituation to the video software. The fact that frequency of data-sharing glitches did not decrease much (from 20% to 18%) reflects the difficulty inherent in using certain software platforms as well as insurance-mandated changes in devices that many participants experienced mid-study.
One factor that likely mitigated the burden of frequent visits was that the time investment for video visits was much less than for in-person visits. Our study participants estimated the median number of minutes required for data sharing at 5 (range 1–60), for a video visit at 30 (range 15–60), and for an in-person visit at 240 (range 120–2880), including transit to and from the clinic (Supplementary Table S1). Video visits were offered at a variety of times between 8 am and 5 pm and could be completed at a variety of locations, including the home, school, parent's workplace, or two locations simultaneously such as the patient connecting from home and the parent connecting from work. This flexibility made video visits easier to complete than in-person visits and facilitated participation by secondary caregivers, including those unable to miss work, and stepparents or grandparents who were not attending in-person visits. The involvement of additional caregivers has been linked to improved glycemic control37 and may be another reason that HbA1c levels improved in this study.
Two unexpected positive findings—improved in-person clinic attendance and high initiation of pump and CGM use—are worthy of discussion. We were concerned at the study's onset that participants might defer in-person visits while engaging in video visits, but in fact, adherence to quarterly in-person visits improved during the study. We suspect this was due to participants' greater prioritization of diabetes care during the study and perception of easier access to the care team, as reflected in survey responses. Unfortunately, data from our electronic medical record could not reliably identify cancelations and no-shows, so it is unclear how much of this increased care frequency was due to a higher scheduling rate versus a higher completion rate for scheduled encounters.
Increased use of insulin pumps and CGMs during the study appeared to be a by-product of improved glucose self-monitoring and ability to meet payer requirements (e.g., 4+ glucose checks per day) among patients who had desired these devices previously. Patients' decisions to adopt the devices may also have stemmed from greater comfort with diabetes technology as a result of the intervention. In addition, the latest generation of CGM products—including those that do not require calibration by finger-stick blood glucose measurements—became commercially available during our study period,38 which may have increased patient desire to acquire CGM devices. The rates of pump and CGM use in our study population at the conclusion of the study were similar to those observed in our clinic's overall patient population, at ∼50% each.
Our study had multiple limitations, including a nonrandomized design, small sample size, and high attrition rate. Participants who withdrew did not differ from those who completed the study on any measured baseline characteristics except CGM use. The fact that participants who remained in the study were more likely to use CGMs is unsurprising because the most prevalent CGM29 allows easy data sharing. However, it is possible the groups differed in other ways—such as comfort with technology or family structure—that were not captured in our data. In addition, our small sample size precludes any subanalyses to explore the influence of patient characteristics (such as age) on outcomes. Finally, the observed increases in insulin pump use, CGM use, and frequency of in-person care are potential confounders, and a randomized trial is needed to determine if these were likely a result of the intervention or unrelated.
This study illustrates that supplemental, home-based video visits are feasible and satisfactory for pediatric T1D patients with suboptimal glycemic control; video visits can be achieved without sacrificing in-person encounters; and they can lead to significant improvements in HbA1c and frequency of outpatient diabetes care in this clinical population. Future randomized trials are needed to more robustly measure the utility of this intervention, examine any differences in effectiveness based on demographic and clinical factors, and better characterize secondary benefits such as caregiver involvement, time savings, and access to diabetes technology.
Supplementary Material
Authors' Contributions
S.S.C. conceptualized and designed the study, analyzed the data, drafted the initial manuscript, and reviewed and revised the manuscript. J.P.M. and N.S.G. assisted critically with the study design and data analysis and reviewed and revised the manuscript. L.Q. performed statistical analysis of the data and reviewed and revised the manuscript. H.S.S., A.M.R., S.T.C., and V.A.T. collected the data, assisted with analysis of the data, and reviewed and revised the manuscript. All authors approved the final manuscript as submitted.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Details of this study's recruitment and data after 6 months—including retention, satisfaction, care frequency, and glycemic control—were previously published.39
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This research was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR001860 and linked award KL2 TR001859.
Supplementary Material
References
- 1. Lal RA, Buckingham B, Maahs DM: Advances in care for insulin-requiring patients without closed loop. Diabetes Technol Ther 2018;20(S2):S285–S291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hofer S, Meraner D, Koehle J: Insulin pump treatment in children and adolescents with type 1 diabetes. Minerva Pediatr 2012;64:433–438 [PubMed] [Google Scholar]
- 3. Haviland N, Walsh J, Roberts R, Bailey TS: Update on clinical utility of continuous glucose monitoring in type 1 diabetes. Curr Diab Rep 2016;16:115. [DOI] [PubMed] [Google Scholar]
- 4. Dadlani V, Pinsker JE, Dassau E, Kudva YC: Advances in closed-loop insulin delivery systems in patients with type 1 diabetes. Curr Diab Rep 2018;18:88. [DOI] [PubMed] [Google Scholar]
- 5. Sherr JL, Hermann JM, Campbell F, et al. : Use of insulin pump therapy in children and adolescents with type 1 diabetes and its impact on metabolic control: comparison of results from three large, transatlantic paediatric registries. Diabetologia 2016;59:87–91 [DOI] [PubMed] [Google Scholar]
- 6. DeSalvo DJ, Miller KM, Hermann JM, et al. : Continuous glucose monitoring and glycemic control among youth with type 1 diabetes: International comparison from the T1D Exchange and DPV Initiative. Pediatr Diabetes 2018;19:1271–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Faulds ER, Zappe J, Dungan KM: Real-world implications of hybrid close loop (HCL) insulin delivery system. Endocr Pract 2019;25:477–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. American Diabetes Association: 13. Children and adolescents: standards of Medical Care in Diabetes-2019. Diabetes Care 2019;42(Suppl 1):S148–S164 [DOI] [PubMed] [Google Scholar]
- 9. Pihoker C, Forsander G, Fantahun B, et al. : ISPAD Clinical Practice Consensus Guidelines 2018: the delivery of ambulatory diabetes care to children and adolescents with diabetes. Pediatr Diabetes 2018;19 Suppl 27:84–104 [DOI] [PubMed] [Google Scholar]
- 10. Clements MA, Foster NC, Maahs DM, et al. : Hemoglobin A1c (HbA1c) changes over time among adolescent and young adult participants in the T1D exchange clinic registry. Pediatr Diabetes 2016;17:327–336 [DOI] [PubMed] [Google Scholar]
- 11. Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, et al. : The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 12. Maldonado MR, D'Amico S, Rodriguez L, et al. : Improved outcomes in indigent patients with ketosis-prone diabetes: effect of a dedicated diabetes treatment unit. Endocr Pract 2003;9:26–32 [DOI] [PubMed] [Google Scholar]
- 13. Franklin VL, Waller A, Pagliari C, Greene SA: A randomized controlled trial of Sweet Talk, a text-messaging system to support young people with diabetes. Diabet Med 2006;23:1332–1338 [DOI] [PubMed] [Google Scholar]
- 14. Lehmkuhl HD, Storch EA, Cammarata C, et al. : Telehealth behavior therapy for the management of type 1 diabetes in adolescents. J Diabetes Sci Technol 2010;4:199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harris MA, Freeman KA, Duke DC: Seeing is believing: using skype to improve diabetes outcomes in youth. Diabetes Care 2015;38:1427–1434 [DOI] [PubMed] [Google Scholar]
- 16. Lee JM, Davis MM, Menon RK, Freed GL: Geographic distribution of childhood diabetes and obesity relative to the supply of pediatric endocrinologists in the United States. J Pediatr 2008;152:331–336 [DOI] [PubMed] [Google Scholar]
- 17. Tuomilehto J: The emerging global epidemic of type 1 diabetes. Curr Diab Rep 2013;13:795–804 [DOI] [PubMed] [Google Scholar]
- 18. Jewett EA, Anderson MR, Gilchrist GS: The pediatric subspecialty workforce: public policy and forces for change. Pediatrics 2005;116:1192–1202 [DOI] [PubMed] [Google Scholar]
- 19. Katz ML, Laffel LM, Perrin JM, Kuhlthau K: Impact of type 1 diabetes mellitus on the family is reduced with the medical home, care coordination, and family-centered care. J Pediatr 2012;160:861–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Drincic A, Prahalad P, Greenwood D, Klonoff DC: Evidence-based mobile medical applications in diabetes. Endocrinol Metab Clin North Am 2016;45:943–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klonoff DC, Kerr D: Digital diabetes communication: there's an app for that. J Diabetes Sci Technol 2016;10:1003–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Neinstein A, Wong J, Look H, et al. : A case study in open source innovation: developing the Tidepool Platform for interoperability in type 1 diabetes management. J Am Med Inform Assoc 2016;23:324–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lindstrom Johnson S, Tandon SD, Trent M, et al. : Use of technology with health care providers: perspectives from urban youth. J Pediatr 2012;160:997–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malasanos TH, Burlingame JB, Youngblade L, et al. : Improved access to subspecialist diabetes care by telemedicine: cost savings and care measures in the first two years of the FITE diabetes project. J Telemed Telecare 2005;11 Suppl 1:74–76 [DOI] [PubMed] [Google Scholar]
- 25. Reid MW, Krishnan S, Berget C, et al. : CoYoT1 clinic: home telemedicine increases young adult engagement in diabetes care. Diabetes Technol Ther 2018;20:370–379 [DOI] [PubMed] [Google Scholar]
- 26. Wood CL, Clements SA, McFann K, et al. : Use of telemedicine to improve adherence to American Diabetes Association Standards in pediatric type 1 diabetes. Diabetes Technol Ther 2016;18:7–14 [DOI] [PubMed] [Google Scholar]
- 27. Vidyo. www.vidyo.com/solutions/healthcare (accessed November1, 2016)
- 28. Carelink. https://carelink.medtronic.com/ (accessed November30, 2017)
- 29. Dexcom Clarity. https://clarity.dexcom.com/ (accessed November30, 2017)
- 30. OneTouch Reveal. https://onetouchreveal.com/ (accessed November30, 2017)
- 31. Accuchek Connect. www.accu-chekconnect.com (accessed November30, 2017)
- 32. TConnect. https://tconnect.tandemdiabetes.com (accessed November30, 2017)
- 33. Fatehi F, Martin-Khan M, Smith AC, et al. : Patient satisfaction with video teleconsultation in a virtual diabetes outreach clinic. Diabetes Technol Ther 2015;17:43–48 [DOI] [PubMed] [Google Scholar]
- 34. Wolfsdorf J, Glaser N, Sperling MA, American Diabetes A: Diabetic ketoacidosis in infants, children, and adolescents: a consensus statement from the American Diabetes Association. Diabetes Care 2006;29:1150–1159 [DOI] [PubMed] [Google Scholar]
- 35. Laird NM, Ware JH: Random-effects models for longitudinal data. Biometrics 1982;38:963–974 [PubMed] [Google Scholar]
- 36. Foster NC, Beck RW, Miller KM, et al. : State of type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol Ther 2019;21:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Markowitz JT, Volkening LK, Laffel LM: Care utilization in a pediatric diabetes clinic: cancellations, parental attendance, and mental health appointments. J Pediatr 2014;164:1384–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martin CT, Criego AB, Carlson AL, Bergenstal RM: Advanced technology in the management of diabetes: which comes first-continuous glucose monitor or insulin pump? Curr Diab Rep 2019;19:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Crossen S, Glaser N, Sauers-Ford H, et al. : Home-based video visits for pediatric patients with poorly controlled type 1 diabetes. J Telemed Telecare 2019:1357633X19828173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.