Abstract
Hypoglycemia remains a major barrier to the achievement of target levels of glycemic control for most individuals with insulin-dependent type 1 diabetes (T1D). Both the loss of β cells and an accompanying defect in the α cell response to hypoglycemia predispose patients with T1D to the development of low blood glucose. Increased glucose variability, exposure to hypoglycemia, and impaired awareness of hypoglycemia all contribute to increased risk of experiencing severe hypoglycemia, which is explained by progressive impairment in epinephrine secretion and autonomic symptom generation in response to hypoglycemia leading to defective glucose counterregulation and hypoglycemia unawareness that characterize hypoglycemia-associated autonomic failure (HAAF). Interruption of HAAF requires interfering with the mechanisms of brain adaptation to low blood glucose that affect central glucose sensing and the autonomic response to hypoglycemia, or avoidance of hypoglycemia that may allow for eventual recovery of counterregulatory and autonomic symptom responses. Strategies for hypoglycemia avoidance that include continuous glucose monitoring may reduce, but do not eliminate, clinically significant hypoglycemia, with ongoing counterregulatory defects and impaired awareness of hypoglycemia. Complete avoidance of hypoglycemia can be achieved following pancreatic islet transplantation and allows for the restoration of counterregulatory and autonomic symptom responses that evidences the potential for reversing HAAF in T1D.
Keywords: type 1 diabetes, hypoglycemia-associated autonomic failure, glucose counterregulation, hypoglycemia unawareness
Introduction
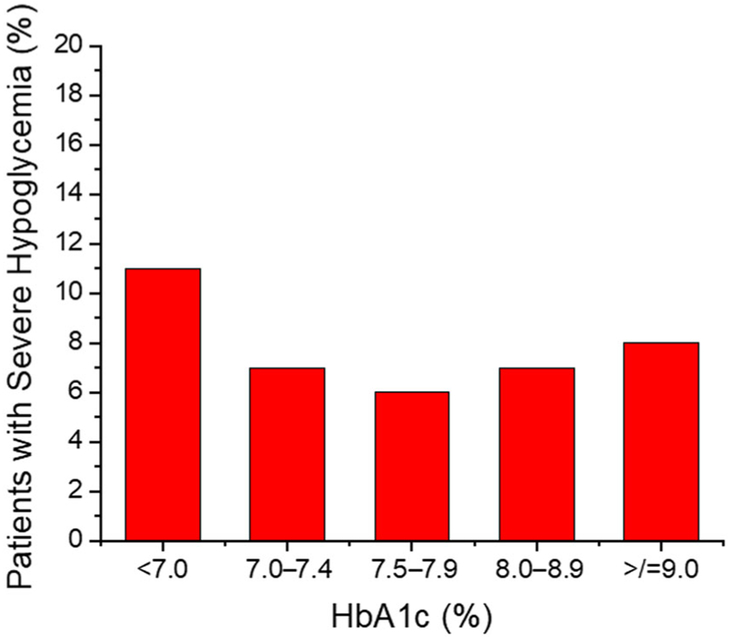
Type 1 diabetes (T1D) is caused by autoimmune destruction of the pancreatic islet β cells that are responsible for insulin production and secretion that normally regulates blood glucose in a narrow homeostatic range. Inadequate control of hyperglycemia can lead to the development of diabetic retinopathy, nephropathy, and neuropathy, which are leading causes of blindness, kidney failure, and nontraumatic amputation in the United States. On the other hand, episodes of hypoglycemia can be acutely life-threatening and represent a major barrier to the achievement of adequate glycemic control for most patients with insulin-dependent diabetes, both those with T1D and advanced type 2 diabetes.1 The American Diabetes Association treatment guidelines recommend that adults with T1D target glycosylated hemoglobin (HbA1c) levels < 7.0% unless there is a reason, such as significant hypoglycemia or hypoglycemia unawareness, to set a higher target of <8.0%.2 However, even with HbA1c < 7.0% the residual risk for cardiovascular and all-cause mortality in patients with T1D remains more than twice that in nondiabetics,3 with the lowest mortality rates seen with HbA1c ≤ 6.5%.4 Unfortunately, despite tremendous advances in the technology available for insulin delivery and glucose monitoring and their use over the past decade, glycemic control has worsened in adults with T1D in the United States receiving specialized diabetes care with currently only 21% achieving the recommended HbA1c level <7.0%.5 In addition, 7% reported experiencing a severe hypoglycemic event resulting in seizure or loss-of-consciousness in the prior 3 months, including 11% of those with HbA1c < 7.0%, 7% of those with HbA1c in the range 7.0 to <9.0%, and 8% of those with HbA1c ≥ 9.0% (Fig. 1).5 Thus, current recommendations to set a higher HbA1c target for patients with significant hypoglycemia or hypoglycemia unawareness2 are unlikely to impact the burden of severe hypoglycemia in T1D. Moreover, the targeting of higher HbA1c levels is often not acceptable to patients striving to avoid or mitigate chronic diabetic complications.
Figure 1.
Proportion of patients with type 1 diabetes (T1D) experiencing a severe episode of hypoglycemia resulting in seizure or loss-of-consciousness in the prior 3 months according to most recent HbA1c as reported in the T1D Exchange Registry and Clinic Network of specialized diabetes care practices in the United States. Data are from Foster et al.5
The American Diabetes Association and the Endocrine Society define the occurrence of severe hypoglycemia as an event associated with loss-of-consciousness or requiring third-party assistance for recovery.6 Severe episodes of hypoglycemia are life-threatening, fear of such episodes distressing, and the cumulative effects of recurrent hypoglycemia impair neurocognitive function. Diabetes-related death is the most frequent cause of mortality among patients under 30 years of age,7,8 and while severe hypoglycemia is documented only in ~12% of diabetes-related deaths, this is likely an underrepresentation due to the presence of twice as many unexplained diabetes-related deaths. Not uncommonly, young people with T1D are found “dead-in-bed,”9,10 an unfortunate consequence of likely severe hypoglycemia inducing brain death11 or fatal cardiac arrhythmia.12 In fact, patients reporting an episode of severe hypoglycemia experience a 3.4-fold increase in mortality over the subsequent 5 years.13 Clearly, there is a need to further understand the mechanisms contributing to hypoglycemia in T1D in order to advance treatment approaches that may realize the benefits of near-normal glycemic control without the accompanying risk for severe hypoglycemia. In order to set the stage for future mechanistic investigation of hypoglycemia in T1D, this paper aims to review (1) mechanisms for the development of hypoglycemia in T1D, (2) mechanisms for hypoglycemia-associated autonomic failure (HAAF) in T1D, (3) hypoglycemia avoidance and counterregulatory responses in T1D, and (4) pancreatic islet transplantation for reversal of HAAF in T1D.
Mechanisms for the development of hypoglycemia in T1D
The risk of experiencing a severe hypoglycemic episode increases with the duration of disease, being three times greater with more than 15 years compared with less than 5 years of disease duration.14 This increased risk with longer disease duration is related to the progressive development of compromised physiologic defense mechanisms against a falling plasma glucose concentration in the setting of therapeutic hyperinsulinemia. By 15 years of disease duration, most patients with T1D have developed near-total loss of functioning β cells (C-peptide negative),15 and so have lost any autoregulatory capacity to turn off endogenous insulin secretion during the development of low blood glucose. T1D is also associated with the development of an intrinsic defect in α cell glucagon secretion in response to hypoglycemia, whereas α cell responsiveness to nutrient stimulation, such as by amino acids, remains intact.16 Importantly, the glucagon response to hypoglycemia is already markedly impaired at the onset of T1D.17 Normally, a reduction in insulin and an increase in glucagon delivered to the liver together increase endogenous (primarily hepatic) glucose production (EGP) to circumvent the development of hypoglycemia, and in the absence of this response individuals with T1D are predisposed to experiencing low blood glucose.
The intrinsic defect in α cell glucagon secretion in response to hypoglycemia may be explained by the loss of a paracrine signal from neighboring β cells that insulin secretion is turning off since insulin secretion reciprocally regulates glucagon secretion in nondiabetic humans.18 Whether this paracrine signal is mediated by an intraislet decrement of insulin itself, or is mediated by the zinc associated with insulin molecules, gamma-aminobutyric acid (GABA) released by β cells, or by direct contact between islet β and α cells remains debated. Superimposed is the potential for autonomic neural regulation of these reciprocal islet cell responses to hypoglycemia. Sympathetic innervation of the islet may contribute to the inhibition of insulin secretion by activation of α2-adrenergic receptors on β cells, and to the stimulation of glucagon secretion by activation of β2-adrenergic receptors on α cells.19 Early sympathetic islet neuropathy has been shown to develop as a consequence of the islet inflammation that leads to the development of T1D and results in a marked loss of islet sympathetic nerves in individuals with short-duration T1D that may contribute to the defect in activating glucagon release during hypoglycemia.20 Additionally, α cells in the diseased islets of T1D downregulate the expression of multiple genes important for α cell identity, which likely also contributes to the functional defect in response to hypoglycemia.21
In the absence of islet cell responses to hypoglycemia, central recognition of low blood glucose by the brain further activates both the sympathetic and parasympathetic branches of the autonomic nervous system (ANS), and epinephrine secretion and autonomic symptom generation become critical to increase EGP through β2-adrenergic activation of hepatocytes and alert the individual to ingest food.22 While epinephrine has been shown to stimulate glucagon secretion independent of hypoglycemia,23 individuals with T1D and an intact epinephrine response to hypoglycemia still have an absent glucagon response,24 suggesting that the intrinsic α cell defect cannot be overcome by β2adrenergic activation. Also, parasympathetic innervation of the islet during hypoglycemia normally augments glucagon secretion and stimulates pancreatic polypeptide secretion through activation of M3 muscarinic receptors on α and pancreatic polypeptide cells, respectively.19 However, individuals with T1D and an intact pancreatic polypeptide response to hypoglycemia still cannot augment the absent glucagon response.24 A similar α cell defect is observed with exercise in T1D, where there is loss of the increase in glucagon that normally stimulates EGP to match the increased rate of glucose disposal in working muscles, and this despite increasing levels of circulating epinephrine in response to exercise.25 Thus, an intrinsic defect in diabetic islets appears as the dominant factor preventing the α cell to respond to hypoglycemia in T1D.
Unfortunately, the centrally mediated autonomic responses to hypoglycemia are attenuated by recurrent exposure to even subclinical episodes of mild hypoglycemia and lead to the development of hypoglycemia unawareness in T1D, also known as HAAF.26 In HAAF, there is marked impairment of epinephrine secretion in response to hypoglycemia that leads to an inability to increase EGP (defective glucose counterregulation), as well as the lack of autonomic symptom generation (hypoglycemia unawareness) that leaves the individual physiologically defenseless against the development of hypoglycemia. In addition to the attenuation of these sympathoadrenal responses to hypoglycemia, the pancreatic polypeptide response to hypoglycemia that serves as a marker of parasympathetic activation of the islet is markedly reduced in HAAF,24 and the pituitary—adrenal response to hypoglycemia that includes increases in growth hormone and cortisol secretion is blunted.27 Additional factors that are not required for the development of HAAF but can contribute to defective glucose counterregulation and hypoglycemia unawareness in the T1D patient with multiple diabetes-associated complications include components of systemic autonomic failure, such as gastroparesis28 and cardiovascular autonomic neuropathy29,30 and the use of pharmacologic β-adrenergic blockade.31,32 Moreover, a common genetic variant of the β2-adrenergic receptor associated with reduced agonist-mediated EGP is also associated with significantly increased risk for severe hypoglycemia in T1D.33 Importantly, the presence of impaired awareness of hypoglycemia in T1D is associated with a sixfold increased risk of experiencing severe hypoglycemia,34,35 and this increased risk may be as high as 20-fold with unawareness.34
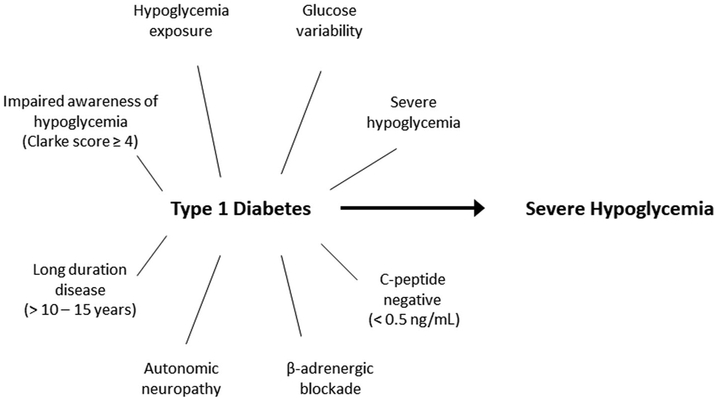
Of the population with T1D, ~20% are estimated to have impaired awareness of hypoglycemia,35 and ~32% have experienced an episode of severe hypoglycemia in the prior year.34,35 It is important to note that not all who have experienced a severe hypoglycemia event have hypoglycemia unawareness, but patients with impaired awareness of hypoglycemia who have also experienced a severe hypoglycemia event are at substantially increased risk of experiencing future severe hypoglycemia (Fig. 2) and makeup just under 10% of the population with T1D.35 Moreover, over half of all episodes of severe hypoglycemia are experienced by ~5% of individuals with T1D.34 Clinically, individuals with T1D who experience mild hypoglycemia most often are more likely to remain asymptomatic and also have a significantly increased risk of experiencing severe hypoglycemia.36 An additional risk factor for experiencing severe hypoglycemia is imparted by glucose variability,32,37,38 which can also be expressed as glycemic lability when accounting for the time intervals during which glucose levels fluctuate.39 Considering these interrelated manifestations of “brittle” T1D, a recently proposed definition for problematic hypoglycemia is experiencing two or more episodes of severe hypoglycemia in the past year, or one episode associated with impaired awareness of hypoglycemia, extreme glycemic lability, or major fear and maladaptive behavior.40 Simple clinical tools are available that can reproducibly quantitate impaired awareness of hypoglycemia (Clarke score), hypoglycemia severity (HYPO score), and glycemic lability (Lability Index),41 and should be included in the prospective evaluation of interventions aimed at ameliorating hypoglycemia in T1D.
Figure 2.
Risk factors for experiencing severe hypoglycemia in patients with type 1 diabetes.
Mechanisms for HAAF in T1D
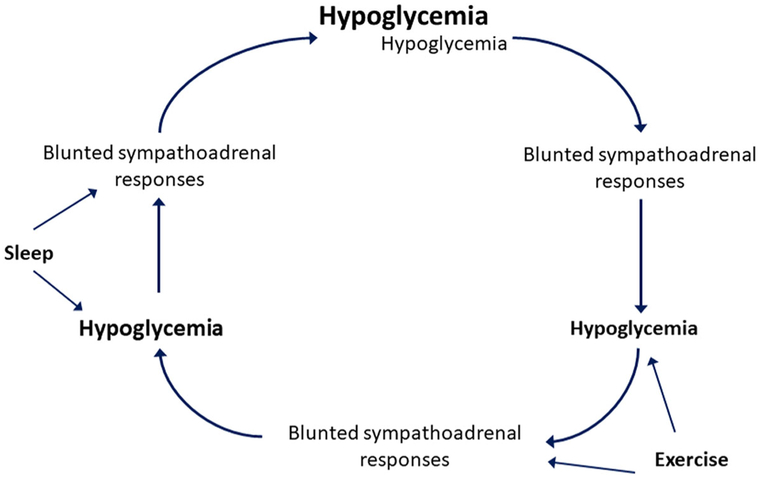
The counterregulatory defects in response to hypoglycemia observed in HAAF can be elicited experimentally in nondiabetic humans with exposure to moderate insulin-induced hypoglycemia in the range of 50–58 mg/dL (2.8–3.2 mmol/L).42 These defects involve not only a diminished magnitude of response, but also a shifting of the glycemic threshold (i.e., the glucose level that elicits the response) for each counterregulatory hormone and symptom response to lower plasma glucose concentrations. Thus, repeated exposure to hypoglycemia progressively blunts the sympathoadrenal responses to defend against a subsequent episode of hypoglycemia, setting up a vicious cycle of hypoglycemia begets hypoglycemia in T1D (Fig. 3).43 While the pituitary—adrenal responses to hypoglycemia are also impaired in HAAF,27 it is the loss of epinephrine and not growth hormone or cortisol that becomes critical for the development of defective glucose counterregulation.44 Defective glucose counterregulation in T1D predisposes such individuals to the development of hypoglycemia during exercise and up to 24 h after completing exercise that has recently been reviewed.45 Hypoglycemia occurring antecedent to exercise further impairs counterregulatory responses to defend against the development of low blood glucose during exercise,46 and exercise itself blunts counterregulatory responses to subsequent hypoglycemia,47 feeding the cycle of progressive impairment in glucose counterregulation. Similarly, counterregulatory responses are also further blunted during sleep, which itself impairs hypoglycemia symptom recognition.48 Thus, exercise-associated and nocturnal hypoglycemia both contribute importantly to the development of HAAF in T1D.
Figure 3.
Vicious cycle of hypoglycemia begets hypoglycemia in patients with type 1 diabetes (T1D). Sympathoadrenal responses refer to an increase in epinephrine secretion and the generation of autonomic symptoms that are critical for defense against hypoglycemia in T1D. Adapted from Cryer.1
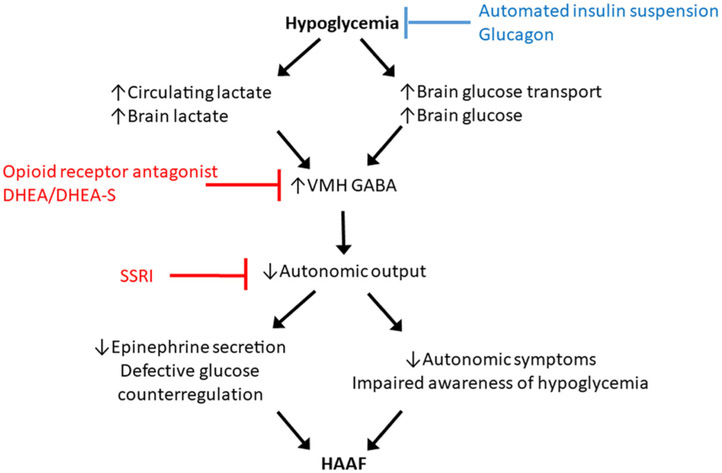
The blunting of counterregulatory responses and lowering of the glycemic thresholds required for activation are best explained by central adaptation to low blood glucose. This central adaptation of the brain to tolerate lower peripheral concentrations of glucose may be mediated through increased expression of brain glucose transporters allowing for enhanced cerebral utilization of glucose, a shift in brain fuel utilization to alternative substrates such as lactate49 (circulating levels of which increase during insulin-mediated hypoglycemia), or some combination of these mechanisms as has recently been reviewed.50 While such adaptation is undoubtedly beneficial for tolerating prolonged fasting, this becomes maladaptive in the setting of insulin-induced hypoglycemia where the window is lost for correcting the development of even further reduction in blood glucose before cognitive function is affected and results in the experience of severe hypoglycemia. The mechanisms for central adaptation converge on altered brain glucose sensing in the ventromedial hypothalamus (VMH) leading to increased GABA that impairs activation of the ANS required for the counterregulatory response against low blood glucose (Fig. 4). Indeed, pharmacologic activation of the GABAA receptors has been shown to blunt the counterregulatory response to hypoglycemia in nondiabetic humans.51
Figure 4.
Mechanisms for brain adaptation to hypoglycemia leading to hypoglycemia-associated autonomic failure (HAAF). In red are strategies for interrupting HAAF based on central signaling pathways. In blue are strategies for preventing/reversing HAAF based on hypoglycemia avoidance.
One interventional approach for HAAF is to interrupt the consequence of brain adaptation to hypoglycemia impairing autonomic activation of the counterregulatory response (Fig. 4). Studies suggest that endogenous opiates can module the counterregulatory responses to hypoglycemia and may play a role in the development of HAAF through opioid receptor expression in the VMH.52 Administration of the opioid receptor antagonist naloxone during experimental insulin-induced hypoglycemia prevents the development of counterregulatory defects in response to a subsequent episode of hypoglycemia in nondiabetic humans53 and in subjects with T1D.54 However, in subjects with T1D and impaired awareness of hypoglycemia, 4 weeks of administration of the opioid receptor antagonist naltrexone had no effect on glucose counterregulatory hormone or symptom responses in a randomized clinical trial.55 Importantly, the clinical trial participants had long-standing T1D that, as will be discussed later, may require a longer period of intervention in order to mediate the reversal of established HAAF.
Another approach targeting the central mechanisms of HAAF involves administration of the adrenal steroid dehydroepiandrosterone (DHEA). DHEA and its sulfated metabolite DHEA-sulfate have anti-GABA effects and may block GABA in the VMH and consequently have been shown to prevent the induction of HAAF by experimental hypoglycemia in nondiabetic humans.56 In addition, selective serotonin reuptake inhibitors (SSRIs) amplify the central output of the ANS and have also been shown to mitigate a reduction in the counterregulatory response to hypoglycemia by antecedent hypoglycemia in nondiabetic humans57 and in individuals with T1D.58 To date, approaches targeting the interruption of HAAF by administration of adrenal steroids or SSRIs to reverse counterregulatory defects in patients with long-standing T1D and hypoglycemia unawareness have not been reported.
Hypoglycemia avoidance and counterregulatory responses in T1D
Reversing HAAF in T1D requires the avoidance of hypoglycemia in order to shift the glycemic thresholds for activation of sympathoadrenal responses to hypoglycemia back toward the normal range for the plasma concentration of glucose (Fig. 4). Strict avoidance of hypoglycemia in T1D complicated by hypoglycemia unawareness has been shown to improve counterregulatory epinephrine and autonomic symptom responses and consequently reestablish the awareness of hypoglycemia.59-62 These early proof-of-concept studies involved patients with rather short disease duration who at the time were receiving “conventional” insulin therapy regimens, and so implementation of what is now standard “intensive” basal-bolus insulin therapy led to a paradoxical increase in HbA1c of 0.5% or more associated with the prevention of hypoglycemia and improvement of impaired counterregulatory epinephrine and autonomic symptom responses after only 1–3 months of intervention.59-62 More recently, studies involving patients with long-standing T1D and hypoglycemia unawareness have demonstrated that the implementation of educational programs can improve hypoglycemia awareness and reduce the frequency of experiencing severe hypoglycemia events after 6 months of intervention.63,64 In the HypoCOMPaSS trial, the educational intervention was combined with randomized assignment to receive insulin delivery by either multidose injection (MDI) or continuous subcutaneous insulin infusion (or “pump”) therapy and glucose monitoring by self-monitoring of blood glucose (SMBG) with or without adjunctive continuous glucose monitoring (CGM), and comparable improvement in hypoglycemia awareness and reduction in severe hypoglycemia events was observed regardless of insulin delivery or glucose monitoring strategy.64 Importantly, a modest improvement in autonomic symptoms was reported after 6 months in the HypoCOMPaSS clamp substudy.65 Thus, present glycemic control strategies for strict hypoglycemia avoidance should include basal-bolus insulin delivery using currently available insulin analogs administered by either MDI or pump therapy in conjunction with frequent (at least four times daily) SMBG and/or CGM.
While education in and implementation of intensive insulin therapy represents an important initial strategy in the management of those with T1D affected with HAAF, despite such intensive attention to glycemic control, some patients remain unaware of hypoglycemia and continue to experience severe hypoglycemia. CGM is increasingly being used as a tool to help avoid hypoglycemia in T1D without requiring elevation of average glycemic as indicated by the HbA1c. While CGM assignment was not independently associated with improvement in problematic hypoglycemia in the HypoCOMPaSS trial, compliance with device use in the CGM arm was low.64 Studies in patients with long-standing T1D and hypoglycemia unawareness support that high adherence to the use of CGM over at least a 4-month period is associated with a reduction in hypoglycemia frequency and severity without increasing the HbA1c.66-69 However, these studies of CGM implementation in patients experiencing problematic hypoglycemia, while reducing, have not demonstrated the elimination of severe hypoglycemic events,66-69 and hypoglycemia awareness has improved in some68 but not all66,67,69 reports. This may be explained by the incomplete reduction of time spent with hypoglycemia with CGM intervention not being sufficient to restore defective glucose counterregulation68 or hypoglycemia symptom awareness.67 Continued exposure to hypoglycemia likely accounts for a lack of improvement in the epinephrine response and only small differences in autonomic symptoms during hypoglycemic when compared with euglycemic clamp experiments after 6 and 18 months of CGM intervention.68 Nevertheless, the use of CGM has been associated with reductions in glucose variability that likely contribute to the reduced risk for experiencing severe hypoglycemia events.67,69 Thus, the use of CGM may help patients with long-standing T1D improve hypoglycemia awareness and/or glucose variability and experience less severe hypoglycemia, but physiologic defenses against the development of hypoglycemia remained compromised, and so the near-complete adherence to CGM use appears necessary to reduce clinically significant hypoglycemia.70
A limitation to instituting hypoglycemia avoidance by CGM is the dependence on the patient to respond appropriately to device alerts (vibration) and alarms in order to ingest carbohydrate and/or decrease or suspend insulin delivery in order to prevent or correct low blood glucose, which is particularly challenging during sleep. Elimination of nocturnal hypoglycemia may be particularly amenable to automated suspension of insulin delivery with a sensor-augmented pump71 or hybrid closed-loop pump; however, studies to date evaluating these advanced insulin delivery systems have largely excluded from participation subjects with hypoglycemia unawareness or a recent history of experiencing severe hypoglycemia. Whether automated suspension of insulin delivery following a hypoglycemia prediction algorithm may allow for sufficient avoidance of hypoglycemia in patients with long-standing T1D complicated by hypoglycemia unawareness to reverse HAAF and lead to clinically meaningful improvement of glucose counterregulation is currently under investigation (ClinicalTrials.gov #). Finally, with the recent development of soluble preparations of glucagon, there is the possibility to provide either automated72 or patient-directed administration of glucagon73,74 to prevent or correct low blood glucose, but again have yet to be investigated specifically for the potential to reverse HAAF. Future work is required in patients with T1D and hypoglycemia unawareness that incorporate approaches to glucagon administration with automated suspension of insulin delivery.
Pancreatic islet transplantation for reversal of HAAF in T1D
For patients with T1D and hypoglycemia unawareness who do not respond to the adoption of advanced technologies for hypoglycemia avoidance and continue to experience impaired awareness of hypoglycemia complicated by severe hypoglycemia events, consideration should be given to whole pancreas or isolated islet transplantation.40,75 Pancreas and islet transplantation are the only interventions that have been shown to restore both glucose counterregulation and hypoglycemia symptom recognition in patients with long-standing T1D, and consequently provide complete protection against the development of severe hypoglycemia events, while at the same time enabling maintenance of the near-normal levels of glucose control required to prevent chronic complications of diabetes.76 Mild hypoglycemia has been reported by some whole pancreas transplant recipients in the postprandial setting,77 however, whether this is the same reactive hypoglycemia that can occur with a native pancreas or may be related to delayed inhibition of insulin secretion due to denervation of the transplanted pancreas78-80 is not known.
Patients with T1D who have undergone islet transplantation are protected from clinically significant hypoglycemia for the duration of islet graft function, even if low-dose insulin therapy is required to maintain normoglycemia. Islets are transplanted via portal vein infusion resulting in intrahepatic engraftment that has recently been reviewed.81 In T1D recipients of intrahepatic islet transplants, there is recovery of the physiologic islet cell responses to insulin-induced hypoglycemia whereby endogenous insulin secretion is appropriately suppressed and glucagon secretion is partially restored.82,83 The partial α cell response is best explained by and consistent with the less than normal mass of islets that survives engraftment based on measures of β cell secretory capacity.84,85 Importantly, the glucagon response to hypoglycemia is activated at a normal glycemic threshold,86 that with the normal decrease in endogenous insulin secretion supports intact paracrine signaling from neighboring β cells to activate α cells in transplanted islets. Reinnervation of intrahepatic islets by the sympathetic nervous system has been demonstrated in a rat model, where sympathetic nerves follow hepatic arterioles into islets during revascu-larization and engraftment.87 During liver perifusion experiments 3 months after portal vein delivery of islets in the rat model, stimulation of the sympathetic nerves entering the liver decreased insulin secretion evidencing neural inhibition of insulin secretion from intrahepatic islets,87 similar to the neural regulation of insulin secretion in the native pancreas. Indeed, unlike for a denervated whole pancreas transplant, where there is impaired inhibition of insulin secretion, recipients of intrahepatic islet transplantation do not experience postprandial hypoglycemia.88
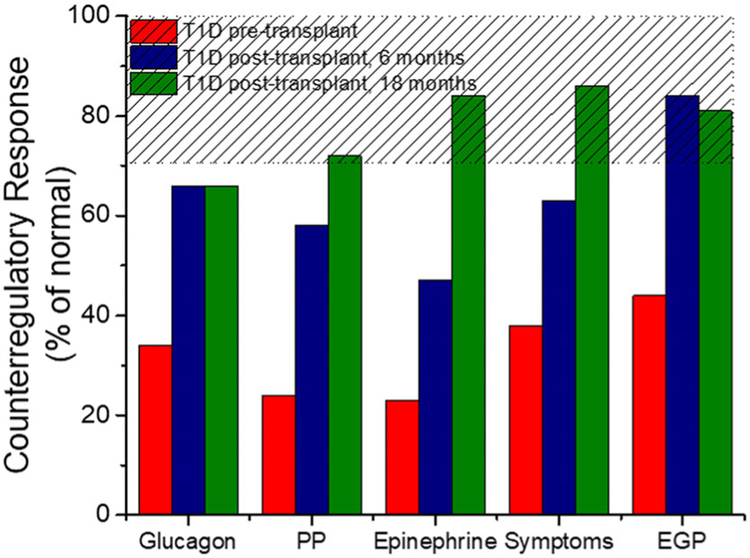
In addition to the restoration of appropriate islet cell responses to insulin-induced hypoglycemia, patients with long-standing T1D demonstrate normalization of the glycemic thresholds for activation of epinephrine and autonomic symptom responses by 6 months following islet transplantation.86 Longitudinal study of the same patients with T1D and hypoglycemia unawareness from before to after islet transplantation indicates the partial glucagon response seen at 6 months remains intact at 18 months, and the epinephrine response that is only partially improved at 6 months becomes fully normalized by 18 months, with a similar effect seen for pancreatic polypeptide secretion and autonomic symptom generation (Fig. 5).89 The additional improvement in the pancreatic polypeptide, epinephrine, and autonomic symptom responses from 6 to 18 months is best explained by the longer duration of hypoglycemia avoidance as CGM measures document essentially no time spent with hypoglycemia and significant reductions in glucose variability.89 Thus, the recovery of ANS function in those most severely affected by HAAF may require complete avoidance of hypoglycemia and resolution of glycemic lability for more than a 6-month period.
Figure 5.
Recovery of counterregulatory responses to insulin-induced hypoglycemia following islet transplantation in patients with type 1 diabetes (T1D) and hypoglycemia unawareness prior to transplant. Response measures are taken from the final hour of a hypoglycemic clamp and expressed as a percentage of normally derived measures from a nondiabetic control group using data reported by Rickels et al.89 The hashed area gives the range of responses that were both significantly greater than pretransplant and not statistically different than normal.
Since glucose counterregulation is best defined by the increase in EGP that is ultimately required to circumvent the development of low blood glucose, it is important to note from these studies of islet transplantation that partial recovery of the glucagon response together with partial improvement of the epinephrine response to insulin-induced hypoglycemia at 6 months following transplantation is already associated with complete restoration of the EGP response.82 Moreover, a study involving islet transplant recipients requiring insulin to support partial islet graft function reported a modest recovery of glucagon and epinephrine secretion with partial restoration of the EGP response to insulin-induced hypoglycemia that was associated with clinical protection from hypoglycemia.90 CGM measures have demonstrated similar reductions in mean glucose, glucose variability, and time spent with clinically significant hypoglycemia (<54—60 mg/dL (3.0–3.3 mmol/L)) relative to T1D for both insulin-independent and -requiring islet recipients.91-93 That the islet graft is responsible for these improvements in CGM measures of glycemic control is supported by their significant correlation with measures of islet β cell graft function.94,95 Thus, even partial improvements in glucose counterregulation and hypoglycemia symptom recognition likely work in concert with reduced glucose variability to provide the robust protection from hypoglycemia afforded by islet transplantation.
Conclusions
Hypoglycemia contributes substantially to the morbidity and mortality of patients with T1D, especially with longer disease duration and near-total β cell loss. Dependence on exogenous insulin and an intrinsic defect in α cell glucagon secretion in response to hypoglycemia predisposes patients with T1D to experiencing low blood glucose. The central recognition of hypoglycemia by the brain that is necessary for epinephrine secretion and autonomic symptom generation becomes attenuated by repeated exposure to hypoglycemia leading to the syndrome of HAAF or hypoglycemia unawareness that substantially increases the risk of experiencing severe hypoglycemia events. Several strategies are targeting the interruption of brain adaptation to low glucose in order to preserve the ANS response to defend against hypoglycemia, but these approaches have not been shown to reverse counterregulatory defects in patients with long-standing T1D and hypoglycemia unawareness. Improvements in hypoglycemia awareness and reductions in severe hypoglycemia events can be observed with intensive attention to glycemic control that includes the avoidance of hypoglycemia and may benefit from the adoption of CGM. However, to date, technologic approaches to hypoglycemia avoidance have not restored physiologic defenses against the development of low blood glucose, suggesting an insufficient reduction of hypoglycemia and/or glucose variability that may require the incorporation of automated suspension of insulin delivery and/or glucagon administration. Pancreatic islet transplantation addresses both the loss of β cells and intrinsic α cell defect in T1D, where intrahepatic islet grafts appropriately respond to hypoglycemia and restore normal glucose counterregulation. Islet transplantation is the only intervention to date that has demonstrated the complete reversal of HAAF in patients with long-standing T1D and hypoglycemia unawareness and suggests that near-complete avoidance of hypoglycemia and blood glucose stability for more than a 6-month period may be required to restore physiologic defense mechanisms against the development of severe hypoglycemia.
Acknowledgment
M.R.R. is supported in part by Public Health Services Research Grant R01 DK091331.
Footnotes
Competing interests
M.R.R. receives research support from Xeris Pharmaceuticals.
Statement
Portions of this article were presented at the National Institute of Diabetes and Digestive and Kidney Diseases meeting “the Autonomic Nervous System: Role in the Regulation of Peripheral Metabolism and Pathophysiology of Metabolic Disease” in Bethesda, MD, on September 20–21, 2018.
References
- 1.Cryer PE 2008. The barrier of hypoglycemia in diabetes. Diabetes 57: 3169–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes Association. 2018. 6. Glycemic targets: standards of medical care in diabetes—2018. Diabetes Care 41: S55–S64. [DOI] [PubMed] [Google Scholar]
- 3.Lind M et al. 2014. Glycemic control and excess mortality in type 1 diabetes. N. Engl. J. Med 371: 1972–1982. [DOI] [PubMed] [Google Scholar]
- 4.Stadler M et al. 2014. Mortality and incidence of renal replacement therapy in people with type 1 diabetes mellitus—a three decade long prospective observational study in the Lainz T1DM cohort. J. Clin. Endocrinol. Metab 99: 4523–4530. [DOI] [PubMed] [Google Scholar]
- 5.Foster NC et al. 2019. State of type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol. Ther 21: 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seaquist ER et al. 2013. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. J. Clin. Endocrinol. Metab 98: 1845–1859. [DOI] [PubMed] [Google Scholar]
- 7.Skrivarhaug T et al. 2006. Long-term mortality in a nationwide cohort of childhood-onset type 1 diabetic patients in Norway. Diabetologia 49: 298–305. [DOI] [PubMed] [Google Scholar]
- 8.Feltbower RG et al. 2008. Acute complications and drug misuse are important causes of death for children and young adults with type 1 diabetes—results from the Yorkshire Register of diabetes in children and young adults. Diabetes Care 31: 922–926. [DOI] [PubMed] [Google Scholar]
- 9.Tattersall RB & Gill GV. 1991. Unexplained deaths of type-1 diabetic-patients. Diabet. Med 8: 49–58. [DOI] [PubMed] [Google Scholar]
- 10.Tanenberg RJ, Newton CA & Drake AJ. 2010. Confirmation of hypoglycemia in the “dead-in-bed” syndrome, as captured by a retrospective continuous glucose monitoring system. Endocr. Pract 16: 244–248. [DOI] [PubMed] [Google Scholar]
- 11.Auer RN 2004. Hypoglycemic brain damage. Metab. Brain Dis 19: 169–175. [DOI] [PubMed] [Google Scholar]
- 12.Tu E, Twigg SM & Semsarian C. 2010. Sudden death in type 1 diabetes: the mystery of the ‘dead in bed’ syndrome. Int. J. Cardiol 138: 91–93. [DOI] [PubMed] [Google Scholar]
- 13.Mccoy RG et al. 2012. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care 35: 1897–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heller SR et al. 2007. Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia 50: 1140–1147. [DOI] [PubMed] [Google Scholar]
- 15.Tsai EB et al. 2006. The rise and fall of insulin secretion in type 1 diabetes mellitus. Diabetologia 49: 261–270. [DOI] [PubMed] [Google Scholar]
- 16.Gerich JE et al. 1973. Lack of glucagon response to hypoglycemia in diabetes—evidence for an intrinsic pancreatic alpha cell defect. Science 182: 171–173. [DOI] [PubMed] [Google Scholar]
- 17.Arbelaez AM et al. 2014. Blunted glucagon but not epinephrine responses to hypoglycemia occurs in youth with less than 1 yr duration of type 1 diabetes mellitus. Pediatr. Diabetes 15: 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooperberg BA & Cryer PE. 2010. Insulin reciprocally regulates glucagon secretion in humans. Diabetes 59: 2936–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thorens B 2014. Neural regulation of pancreatic islet cell mass and function. Diabetes Obes. Metab 16(Suppl. 1): 87–95. [DOI] [PubMed] [Google Scholar]
- 20.Mundinger TO et al. 2016. Human type 1 diabetes is characterized by an early, marked, sustained, and islet-selective loss of sympathetic nerves. Diabetes 65: 2322–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brissova M et al. 2018. α Cell function and gene expression are compromised in type 1 diabetes. Cell Rep. 22: 2667–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cryer PE, Davis SN & Shamoon H. 2003. Hypoglycemia in diabetes. Diabetes Care 26: 1902–1912. [DOI] [PubMed] [Google Scholar]
- 23.Gerich JE et al. 1976. Studies on mechanism of epinephrine-induced hyperglycemia in man—evidence for participation of pancreatic glucagon-secretion. Diabetes 25: 65–71. [DOI] [PubMed] [Google Scholar]
- 24.White NH et al. 1985. Plasma pancreatic-polypeptide response to insulin-induced hypoglycemia as a marker for defective glucose counterregulation in insulin-dependent diabetes-mellitus. Diabetes 34: 870–875. [DOI] [PubMed] [Google Scholar]
- 25.Mallad A et al. 2015. Exercise effects on postprandial glucose metabolism in type 1 diabetes: a triple-tracer approach. Am. J. Physiol. Endocrinol. Metab 308: E1106–E1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cryer PE 2013. Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N. Engl. J. Med 369: 362–372. [DOI] [PubMed] [Google Scholar]
- 27.Cryer PE 2005. Mechanisms of hypoglycemia-associated autonomic failure and its component syndromes in diabetes. Diabetes 54: 3592–3601. [DOI] [PubMed] [Google Scholar]
- 28.Aleppo G et al. 2017. Reported gastroparesis in adults with type 1 diabetes (T1D) from the T1D Exchange clinic registry. J. Diabetes Complications 31: 1669–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoeldtke RD et al. 1982. Reduced epinephrine secretion and hypoglycemia unawareness in diabetic autonomic neuropathy. Ann. Intern. Med 96: 459–462. [DOI] [PubMed] [Google Scholar]
- 30.Meyer C et al. 1998. Effects of autonomic neuropathy on counterregulation and awareness at hypoglycemia in type 1 diabetic patients. Diabetes Care 21: 1960–1966. [DOI] [PubMed] [Google Scholar]
- 31.Popp DA et al. 1984. Oral propranolol and metoprolol both impair glucose recovery from insulin-induced hypoglycemia in insulin-dependent diabetes mellitus. Diabetes Care 7: 243–247. [DOI] [PubMed] [Google Scholar]
- 32.Weinstock RS et al. 2016. Risk factors associated with severe hypoglycemia in older adults with type 1 diabetes. Diabetes Care 39: 603–610. [DOI] [PubMed] [Google Scholar]
- 33.Rokamp KZ et al. 2018. Impact of genetic polymorphism in the β2-receptor gene on risk of severe hypoglycemia in patients with type 1 diabetes. J. Clin. Endocrinol. Metab 103: 2901–2908. [DOI] [PubMed] [Google Scholar]
- 34.Pedersen-Bjergaard U et al. 2004. Severe hypoglycaemia in 1076 adult patients with type 1 diabetes: influence of risk markers and selection. Diabetes Metab. Res. Rev 20: 479–486. [DOI] [PubMed] [Google Scholar]
- 35.Geddes J et al. 2008. Prevalence of impaired awareness of hypoglycaemia in adults with type 1 diabetes. Diabet. Med 25: 501–504. [DOI] [PubMed] [Google Scholar]
- 36.Henriksen MM et al. 2018. Hypoglycemic exposure and risk of asymptomatic hypoglycemia in type 1 diabetes assessed by continuous glucose monitoring. J. Clin. Endocrinol. Metab 103: 2329–2335. [DOI] [PubMed] [Google Scholar]
- 37.Cox DJ et al. 1994. Frequency of severe hypoglycemia in insulin-dependent diabetes mellitus can be predicted from self-monitoring blood glucose data. J. Clin. Endocrinol. Metab 79: 1659–1662. [DOI] [PubMed] [Google Scholar]
- 38.Kilpatrick ES et al. 2007. Relating mean blood glucose and glucose variability to the risk of multiple episodes of hypoglycaemia in type 1 diabetes. Diabetologia 50: 2553–2561. [DOI] [PubMed] [Google Scholar]
- 39.Ryan EA et al. 2004. Assessment of the severity of hypoglycemia and glycemic lability in type 1 diabetic subjects undergoing islet transplantation. Diabetes 53: 955–962. [DOI] [PubMed] [Google Scholar]
- 40.Choudhary P et al. 2015. Evidence-informed clinical practice recommendations for treatment of type 1 diabetes complicated by problematic hypoglycemia. Diabetes Care 38: 1016–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Senior PA et al. 2015. Consistency of quantitative scores of hypoglycemia severity and glycemic lability and comparison with continuous glucose monitoring system measures in long-standing type 1 diabetes. Diabetes Technol. Ther 17: 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heller SR & Cryer PE. 1991. Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia after 1 episode of hypoglycemia in nondiabetic humans. Diabetes 40: 223–226. [DOI] [PubMed] [Google Scholar]
- 43.Cryer PE 1993. Hypoglycemia begets hypoglycemia in IDDM. Diabetes 42: 1691–1693. [DOI] [PubMed] [Google Scholar]
- 44.Boyle PJ & Cryer PE. 1991. Growth-hormone, cortisol, or both are involved in defense against, but are not critical to recovery from, hypoglycemia. Am. J. Physiol 260: E395–E402. [DOI] [PubMed] [Google Scholar]
- 45.Riddell MC et al. 2017. Exercise management in type 1 diabetes: a consensus statement. Lancet Diabetes Endocrinol. 5: 377–390. [DOI] [PubMed] [Google Scholar]
- 46.Galassetti P et al. 2006. Effect of differing antecedent hypoglycemia on counterregulatory responses to exercise in type 1 diabetes. Am. J. Physiol. Endocrinol. Metab 290: E1109–E1117. [DOI] [PubMed] [Google Scholar]
- 47.Sandoval DA et al. 2006. Acute, same-day effects of antecedent exercise on counterregulatory responses to subsequent hypoglycemia in type 1 diabetes mellitus. Am. J. Physiol. Endocrinol. Metab 290: E1331–E1338. [DOI] [PubMed] [Google Scholar]
- 48.Cryer PE 2004. Current concepts: diverse causes of hypoglycemia-associated autonomic failure in diabetes. N. Engl. J. Med 350: 2272–2279. [DOI] [PubMed] [Google Scholar]
- 49.Wiegers EC et al. 2016. Brain lactate concentration falls in response to hypoglycemia in patients with type 1 diabetes and impaired awareness of hypoglycemia. Diabetes 65: 1601–1605. [DOI] [PubMed] [Google Scholar]
- 50.Stanley S, Moheet A & Seaquist ER. 2019. Central mechanisms of glucose sensing and counterregulation in defense of hypoglycemia. Endocr. Rev 40: 768–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hedrington MS et al. 2010. Effects of antecedent GABAA activation with alprazolam on counterregulatory responses to hypoglycemia in healthy humans. Diabetes 59: 1074–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCrimmon RJ 2011. The response to hypoglycemia: a role for the opioid system? J. Clin. Endocrinol. Metab 96: 3357–3359. [DOI] [PubMed] [Google Scholar]
- 53.Leu J et al. 2009. Hypoglycemia-associated autonomic failure is prevented by opioid receptor blockade. J. Clin. Endocrinol. Metab 94: 3372–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vele S et al. 2011. Opioid receptor blockade improves hypoglycemia-associated autonomic failure in type 1 diabetes mellitus. J. Clin. Endocrinol. Metab 96: 3424–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moheet A et al. 2015. Naltrexone for treatment of impaired awareness of hypoglycemia in type 1 diabetes: a randomized clinical trial. J. Diabetes Complications 29: 1277–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mikeladze M et al. 2016. Acute effects of oral dehydroepiandrosterone on counterregulatory responses during repeated hypoglycemia in healthy humans. Diabetes 65: 3161–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Briscoe VJ et al. 2008. Effects of a selective serotonin reuptake inhibitor, fluoxetine, on counterregulatory responses to hypoglycemia in healthy individuals. Diabetes 57: 2453–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Briscoe VJ et al. 2008. Effects of the selective serotonin reuptake inhibitor fluoxetine on counterregulatory responses to hypoglycemia in individuals with type 1 diabetes. Diabetes 57: 3315–3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fanelli CG et al. 1993. Meticulous prevention of hypoglycemia normalizes the glycemic thresholds and magnitude of most of neuroendocrine responses to, symptoms of, and cognitive function during hypoglycemia in intensively treated patients with short-term IDDM. Diabetes 42: 1683–1689. [DOI] [PubMed] [Google Scholar]
- 60.Cranston I et al. 1994. Restoration of hypoglycemia awareness in patients with long-duration insulin-dependent diabetes. Lancet 344: 283–287. [DOI] [PubMed] [Google Scholar]
- 61.Dagogojack S, Rattarasarn C & Cryer PE. 1994. Reversal of hypoglycemia unawareness, but not defective glucose counterregulation, in IDDM. Diabetes 43: 1426–1434. [DOI] [PubMed] [Google Scholar]
- 62.Liu DT, McManus RM & Ryan EA. 1996. Improved counter-regulatory hormonal and symptomatic responses to hypoglycemia in patients with insulin-dependent diabetes mellitus after 3 months of less strict glycemic control. Clin. Invest. Med 19: 71–82. [PubMed] [Google Scholar]
- 63.de Zoysa N et al. 2014. A psychoeducational program to restore hypoglycemia awareness: the DAFNE-HART pilot study. Diabetes Care 37: 863–866. [DOI] [PubMed] [Google Scholar]
- 64.Little SA et al. 2014. Recovery of hypoglycemia awareness in long-standing type 1 diabetes: a multicenter 2 × 2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self-monitoring (HypoCOMPaSS). Diabetes Care 37: 2114–2122. [DOI] [PubMed] [Google Scholar]
- 65.Leelarathna L et al. 2013. Restoration of self-awareness of hypoglycemia in adults with long-standing type 1 diabetes hyperinsulinemic-hypoglycemic clamp substudy results from the HypoCOMPaSS trial. Diabetes Care 36: 4063–4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Choudhary P et al. 2013. Real-time continuous glucose monitoring significantly reduces severe hypoglycemia in hypoglycemia-unaware patients with type 1 diabetes. Diabetes Care 36: 4160–4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Beers CA et al. 2016. Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia (IN CONTROL): a randomised, open-label, crossover trial. Lancet Diabetes Endocrinol. 4: 893–902. [DOI] [PubMed] [Google Scholar]
- 68.Rickels MR et al. 2018. Continuous glucose monitoring for hypoglycemia avoidance and glucose counterregulation in long-standing type 1 diabetes. J. Clin. Endocrinol. Metab 103:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heinemann L et al. 2018. Real-time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. Lancet 391: 1367–1377. [DOI] [PubMed] [Google Scholar]
- 70.Lin YK et al. 2019. Impaired awareness of hypoglycemia continues to be a risk factor for severe hypoglycemia despite the use of continuous glucose monitoring system in type 1 diabetes. Endocr. Pract 25: 517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ly TT et al. 2013. Effect of sensor-augmented insulin pump therapy and automated insulin suspension vs standard insulin pump therapy on hypoglycemia in patients with type 1 diabetes: a randomized clinical trial. JAMA 310: 1240–1247. [DOI] [PubMed] [Google Scholar]
- 72.Weisman A et al. 2017. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol. 5: 501–512. [DOI] [PubMed] [Google Scholar]
- 73.Haymond MW et al. 2017. Efficacy and safety of mini-dose glucagon for treatment of non-severe hypoglycemia in adults with type 1 diabetes. J. Clin. Endocrinol. Metab 102: 2994–3001. [DOI] [PubMed] [Google Scholar]
- 74.Rickels MR et al. 2018. Mini-dose glucagon as a novel approach to prevent exercise-induced hypoglycemia in type 1 diabetes. Diabetes Care 41: 1909–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Byrne ML et al. 2015. Outcomes for adults with type 1 diabetes referred with severe hypoglycaemia and/or referred for islet transplantation to a specialist hypoglycaemia service. Horm. Metab. Res 47: 9–15. [DOI] [PubMed] [Google Scholar]
- 76.Rickels MR 2012. Recovery of endocrine function after islet and pancreas transplantation. Curr. Diab. Rep 12: 587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Redmon JB, Teuscher AU & Robertson RP. 1998. Hypoglycemia after pancreas transplantation. Diabetes Care 21: 1944–1950. [DOI] [PubMed] [Google Scholar]
- 78.Elahi D et al. 1991. Islet cell responses to glucose in human transplanted pancreas. Am. J. Physiol 261: E800–E808. [DOI] [PubMed] [Google Scholar]
- 79.Luzi L et al. 1992. Lack of feedback inhibition of insulin-secretion in denervated human pancreas. Diabetes 41:1632–1639. [DOI] [PubMed] [Google Scholar]
- 80.Boden G et al. 1993. Evidence that suppression of insulin-secretion by insulin itself is neurally-mediated. Metabolism 42: 786–789. [DOI] [PubMed] [Google Scholar]
- 81.Rickels MR &Robertson RP. 2019. Pancreatic islet transplantation in humans: recent progress and future directions. Endocr. Rev 40: 631–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rickels MR et al. 2015. Restoration of glucose counterregulation by islet transplantation in long-standing type 1 diabetes. Diabetes 64: 1713–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rickels MR et al. 2005. Islet cell hormonal responses to hypoglycemia after human islet transplantation for type 1 diabetes. Diabetes 54: 3205–3211. [DOI] [PubMed] [Google Scholar]
- 84.Rickels MR et al. 2010. Beta-cell secretory capacity and demand in recipients of islet, pancreas, and kidney transplants. J. Clin. Endocrinol. Metab 95: 1238–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rickels MR et al. 2013. Improvement in β-cell secretory capacity after human islet transplantation according to the CIT07 protocol. Diabetes 62: 2890–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rickels MR et al. 2007. Glycemic thresholds for activation of counterregulatory hormone and symptom responses in islet transplant recipients. J. Clin. Endocrinol. Metab 92: 873–879. [DOI] [PubMed] [Google Scholar]
- 87.Gardemann A et al. 1994. Intraportal transplantation of pancreatic-islets into livers of diabetic rats—reinnervation of islets and regulation of insulin-secretion by the hepatic sympathetic-nerves. Diabetes 43: 1345–1352. [DOI] [PubMed] [Google Scholar]
- 88.Baidal DA et al. 2009. Early metabolic markers of islet allograft dysfunction. Transplantation 87: 689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rickels MR et al. 2016. Long-term improvement in glucose control and counterregulation by islet transplantation for type 1 diabetes. J. Clin. Endocrinol. Metab 101: 4421–4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ang M et al. 2014. Magnitude and mechanisms of glucose counterregulation following islet transplantation in patients with type 1 diabetes suffering from severe hypoglycaemic episodes. Diabetologia 57: 623–632. [DOI] [PubMed] [Google Scholar]
- 91.Kessler L et al. 2002. Reduction of blood glucose variability in type 1 diabetic patients treated by pancreatic islet transplantation. Diabetes Care 25: 2256–2262. [DOI] [PubMed] [Google Scholar]
- 92.Paty BW et al. 2006. Assessment of glycemic control after islet transplantation using the continuous glucose monitor in insulin-independent versus insulin-requiring type 1 diabetes subjects. Diabetes Technol. Ther 8: 165–173. [DOI] [PubMed] [Google Scholar]
- 93.Gorn L et al. 2008. Impact of islet transplantation on glycemic control as evidenced by a continuous glucose monitoring system. J. Diabetes Sci. Technol 2: 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vantyghem MC et al. 2012. Continuous glucose monitoring after islet transplantation in type 1 diabetes: an excellent graft function (β-score greater than 7) is required to abrogate hyperglycemia, whereas a minimal function is necessary to suppress severe hypoglycemia (β-score greater than 3). J. Clin. Endocrinol. Metab 97: E2078–E2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brooks AM et al. 2015. Demonstration of an intrinsic relationship between endogenous C-peptide concentration and determinants of glycemic control in type 1 diabetes following islet transplantation. Diabetes Care 38: 105–112. [DOI] [PubMed] [Google Scholar]