Abstract
This study investigated the effect of maternal care on adolescent ethanol consumption, sensitivity to ethanol-induced hypnosis, as well as gonadal hormones and γ-aminobutyric acid type-A (GABAA) systems. Long Evans rat dams were categorized by maternal licking/grooming (LG) frequency into High- and Low-LG mothers. Both female and male offspring from Low-LG rats demonstrated a greater sensitivity to ethanol-induced hypnosis in the loss-of-righting-reflex test at ethanol doses of 3.0 and 3.5 g/kg during late-adolescence (postnatal Day 50) but not at mid-adolescence (postnatal Day 42). However, we found no effect of maternal care on consumption of a 5% ethanol solution in a two-bottle choice test. We further investigated the association between the observed variations in sensitivity to ethanol-induced hypnosis and baseline hormonal levels in males. In male offspring from Low-LG mothers compared to High-LG mothers, baseline plasma corticosterone and progesterone levels were higher. GABAA α1 and δ subunit expressions were also higher in the cerebral cortex of Low-LG males but lower in the cerebellar synaptosomal fraction. Early environmental influences on adolescent sensitivity to ethanol-induced hypnosis, consumption, and preference may be mediated by gonadal hormones and possibly through GABAergic functions.
Keywords: age-difference, corticosteroid, GABAA receptors, maternal care, progesterone, sex-difference, testosterone
1 |. INTRODUCTION
Alcohol abuse is a socioeconomic issue in the United States, particularly in adolescents (Office of the Surgeon, National Institute on Alcohol, Alcoholism, Substance, & Mental Health Services, 2007). In a 2015 national survey, 10% of eighth graders and 35% of twelfth graders reported drinking during the past 30 days, while 5% of eighth graders and 17% of twelfth graders reported binge drinking within the past 2 weeks (Johnston, Patrick, Richard, Jerald, & John, 2016). Annually, such risky underage drinking behaviors result in more than 189,000 emergency room visits, 4,300 deaths among underage youth, and $24 billion in economic cost (Sacks, Gonzales, Bouchery, Tomedi, & Brewer, 2015). These disturbing statistics, together with the adverse short- and long-term consequences associated with adolescent drinking (Spear, 2016) emphasize the urgent need to address adolescent alcohol use in our society. Understanding the factors that may promote adolescent alcohol consumption may be a wise approach to addressing this adolescent-drinking problem.
Ethanol consumption and sensitivity to ethanol-induced hypnosis may be interrelated. Rodent studies have demonstrated that genetically modified mice that consume more ethanol are less sensitive to its hypnotic effect (Naassila, Ledent, & Daoust, 2002; Thiele et al., 2000). Conversely, adolescents are known to be less sensitive to ethanol-induced motor-impairing and aversive effects, and are also likely to consume more ethanol during binge episodes compared to adults (Holstein, Spanos, & Hodge, 2011; Logue, Chein, Gould, Holliday, & Steinberg, 2014; Schramm-Sapyta et al., 2010; Serlin & Torregrossa, 2015; Spear, 2013). Collectively, this evidence suggests that attenuated sensitivity to ethanol-induced hypnosis may be associated with increased ethanol consumption. Therefore, along with understanding factors that modulate adolescent ethanol consumption, it is important to understand how such factors may also influence sensitivity to ethanol-induced hypnosis.
Early-life experiences may modulate adolescent alcohol consumption. In humans, early-life adversity, particularly during the first few years of life, is associated with early onset of problematic drinking in adolescence, as well as alcohol dependence in early adulthood (see Enoch, 2011 for review). Likewise, rodent models have demonstrated that early-life maternal deprivation, a critical component of early-life experiences, influences ethanol consumption (see Nylander & Roman, 2013 for review). However, the results in the rodent models are inconsistent, partly due to variations in their maternal separation model protocols (see Nylande r& Roman, 2013 for review). Therefore, a reliable model of natural variations in maternal care is needed to unravel inconsistent results in studies examining the influence of early-life experience on ethanol consumption. The impact of early-life maternal care on adolescent ethanol consumption, in particular, ought to be investigated.
Early-life maternal care alters endocrine function and reproductive development by programming the functions of the hypothalamic-pituitary-gonadal (HPG) and hypothalamic-pituitary-adrenal (HPA) axes (Cameron, 2011; Cameron, Shahrokh, et al., 2008). In rats, maternal care consists of many behaviors such as nursing, licking and grooming (LG), incubating, and retrieving pups (Stern, 1996). Of these behaviors, LG has been shown to demonstrate the most variability among Long Evans dams (Champagne, Francis, Mar, & Meaney, 2003). Manipulations that interfered with the frequency of this behavior have clearly showed that reductions in LG influence neural development (Moore, Dou, & Juraska, 1992). Such manipulations include the use of a brush to stroke artificially-reared pups, which clearly demonstrated that decreasing the frequency of the tactile component of maternal LG is sufficient to offset negative behavioral and physiological effects of maternal deprivation. (Chatterjee et al., 2007; Jutapakdeegul, Casalotti, Govitrapong, & Kotchabhakdi, 2003; Lenz et al., 2008; Levy, Melo, Galef, Madden, & Fleming, 2003; Suchecki, Rosenfeld, & Levine, 1993). These include important negative effects on the HPA axis (Jutapakdeegul et al., 2003). The LG behavior is also associated with variations in the neuroendocrine system as compared to female offspring of high LG (High-LG) dams, female offspring of low LG (Low-LG) dams have higher progesterone and luteinizing hormone levels during proestrus (Cameron, Del Corpo et al., 2008), as well as higher corticosterone and 17-β-estradiol levels (Amorim et al., 2011; Cameron, 2011; Cameron, Shahrokh et al., 2008). Maternal care also influences the timing of reproductive maturity. Vaginal opening, an indicator of female sexual maturation in rats, also occurs earlier in Low-LG compared to High-LG females (Borrow, Levy, Soehngen, & Cameron, 2013). However, while the programming of female rats’ reproductive development and endocrine function by early-life maternal care is relatively well characterized, less is known about how the male endocrine function is affected.
Understanding the effect of maternal care on adolescent male endocrine function could provide insight into how maternal care influences sensitivity to ethanol-induced hypnosis. Sensitivity to ethanol-induced hypnosis is significantly modulated by endogenous levels of steroids including corticosterone, gonadal hormones such as progesterone and testosterone, and their metabolites (such as allopregnanolone) (Dazzi et al., 2002; Helms, Rossi, & Grant, 2012; Khisti, VanDoren, O’Buckley, & Morrow, 2003; Morrow, Porcu, Boyd, & Grant, 2006; Sanna et al., 2004). Ethanol-induced hypnotic, anticonvulsant, and sedative effects correlate with ethanol-induced brain allopregnanolone levels (Khisti et al., 2003; VanDoren et al., 2000). Both human and rodent studies report that testosterone may influence sensitivity to ethanol-induced hypnosis and ethanol consumption (Apter & Eriksson, 2003; Eriksson, Kaprio, Pulkkinen, & Rose, 2005; Pettersson, Ellsinger, Sjoberg, & Bjorntorp, 1990; Vetter-O’Hagen, Sanders, & Spear, 2011). Likewise, increased corticosterone levels, either induced by stress (see Meyer, Long, Fanselow, & Spigelman, 2013; Spanagel, Noori, & Heilig, 2014 for review) or exogenously administered (Fahlke, Engel, Eriksson, Hard, & Soderpalm, 1994; Fahlke, Hård, & Hansen, 1996; Fahlke, Hård, Hansen, Eriksson, & Engel, 1995), enhance ethanol consumption and alter ethanol-induced hypnosis (Fernández et al., 2016; Parker, Ponicsan, Spencer, Holmes, & Johnson, 2008). Moreover, certain ethanol use-related behaviors are sex-specific, partly due to sex-differences in gonadal hormone levels (Almeida et al., 1998). Gonadal hormone levels also vary across the female estrous cycle (Haim, Shakhar, Rossene, Taylor, & Ben-Eliyahu, 2003) and mediate estrous cycle-dependent variations in ethanol-use related behaviors (Carroll, Lustyk, & Larimer, 2015; Epstein et al., 2006; Ford, Eldridge, & Samson, 2002). Thus, altering endogenous levels of male progesterone, testosterone, and corticosterone may be a mechanism by which, maternal care modulates sensitivity to ethanol-induced hypnosis.
Both ethanol and neuroactive steroids induce hypnosis primarily by potentiating γ-aminobutyric acid type A (GABAA) receptor functions (Kumar et al., 2009). The GABAergic system is the primary mediator of fast inhibition within the central nervous system (Szabadi, 2009). A fully functional GABAA receptor complex is pentameric, consisting of a selection of five from approximately nineteen known subunits; α (1–6), β (1–3), γ (1–3), δ, ε, ρ, and θ (1–3). The subunit composition of a GABAA receptor complex may determine various characteristics of the receptor such as its location within the brain, cellular localization (synaptic or extrasynaptic), physiological functions (phasic or tonic inhibition), and its kinetic and pharmacological properties (agonist and antagonists) (see reviews Kumar et al., 2009; Lobo & Harris, 2008). For instance, while α1, β1, β2, β3, and γ1 subunit-containing GABAA receptor complexes are located throughout the brain, α2, α3, α4, α5, α6, γ2, and δ subunit-containing receptors are more confined to certain brain areas (Pirker, Schwarzer, Wieselthaler, Sieghart, & Sperk, 2000). Also, while synaptic GABA release triggers phasic currents by activation of low-affinity synaptic γ2-containing GABAA receptors, extrasynaptic δ-containing receptors mediate tonic current and are activated by low GABA concentrations that spill over to the extrasynaptic space (Brickley & Mody, 2012; Carver & Reddy, 2013). Of particular interest for the present study, the GABAA α1 and δ subunit-containing receptors reportedly play significant roles in mediating the effects of sedative-hypnotic drugs such as ethanol and neuroactive steroids (Blednov, Jung et al., 2003; Helms et al., 2012; Orser, 2006; Smith, Shen, Gong, & Zhou, 2007; Zheleznova, Sedelnikova, & Weiss, 2008). Additionally, both the cerebellum and cerebral cortex are strongly associated with ethanol-induced hypnosis (Kashiwabuchi et al., 1995; Kumar et al., 2012). Interestingly, natural variations in maternal LG regulate GABAA receptor subunit genes and protein expressions in a brain region- and receptor subunit-dependent manner (Caldji, Diorio, & Meaney, 2003). These results collectively suggest an association between maternal care and ethanol-induced hypnosis via variations in GABAA receptor expression.
Based on these findings, we hypothesized that Low-LG will result in increased adolescent ethanol consumption and will modulate sensitivity to ethanol-induced hypnosis. Furthermore, we also hypothesized that these behavioral effects will be mediated, at least in part, by higher gonadal hormone levels in Low-LG rats that will emerge at late-adolescence due to accelerated gonadal maturation. To test these hypotheses, we employed a well-established model of characterizing natural variations in early-life maternal care (Champagne et al., 2003; Popoola, Borrow, Sanders, Nizhnikov, & Cameron, 2015) to investigate the influence of maternal care on ethanol use-related behaviors during mid-adolescence (PND 42) and late-adolescence (PND 50). Experiment 1 assessed the influence of maternal care on adolescent ethanol consumption and preference, and sensitivity to ethanol-induced hypnosis in female rats, while experiment 2 assessed these behaviors in male rats. Experiment 3, further examined male offspring by characterizing maternal care-related differences in plasma testosterone, progesterone, and corticosterone levels during mid-adolescence and late adolescence. Experiment 4 was based on a maternal care-related difference in sensitivity to ethanol-induced hypnosis at PND 50 that we observed in Experiment 2. Thus, in this experiment we examined differences in GABAA receptor α1 and δ subunit expressions in the cerebral cortex and cerebellum of experiment-naïve adolescent males as a potential contributor to variations in sensitivity to ethanol-induced hypnosis.
2 |. METHODS
2.1 |. Animals
Adult Long Evans rats were obtained from Charles River Laboratories (Wilmington, MA). Upon arrival, they were pair-housed in standard Plexiglas cages (22 × 23 × 45 cm) with wood shavings as bedding and allowed to habituate for 2 weeks in our colony before mating. The colony was maintained at a controlled temperature (22 ± 1 °C). These adult animals were mated to produce the subjects that were used in these experiments. A male and two females were housed together for 7 days, after which, the male was separated from the females while the females remained pair-housed for another 7 days. Females were then single-caged until they gave birth. The colony room was maintained in a 10–14 hr light-dark cycle with lights on at 0900 hr. The date of birth was recorded as Postnatal PND 0. No environmental enrichment or nesting material was provided as this has been previously reported to reverse certain long-lasting effects of early-life experiences (Francis, Diorio, Plotsky, & Meaney, 2002). Except during experiments, food and water were available ad libitum. All experiments were conducted in compliance with the National Institute of Health guidelines for laboratory animal use and protocols were approved by the Institutional Animal Care and Use Committee at Binghamton University.
2.2 |. Maternal care observation
Each dam was observed for maternal care behavior, which was scored, every 3 min, for 75 min (25 scores) in each of the five daily sessions from PND 1 through PND 6 totaling to 750 (5 sessions × 25 scores for 6 days) observations per litter. Licking and grooming (LG) was scored by well-trained observers as initially described by Myers, Brunelli, Shair, Squire, & Hofer (1989) and adapted by our laboratory as previously reported (Borrow et al., 2013; Popoola et al., 2015; Prior, Meaney, & Cameron, 2013). Daily observation sessions occurred three times during the light cycle (1030, 1300, and 1700 hr) and twice during the dark cycle (0700 and 2000 hr).
Among maternal care behaviors that were observed, phenotypes were classified in this study based on LG because variations in LG has been found to be more profoundly associated with neural, endocrine, and behavioral development as compared to other behaviors (Champagne et al., 2003; Levine, 1994; Uriarte, Breigeiron, Benetti, Rosa, & Lucion, 2007). Moreover, LG behavior has also been reported to be most variable across litters compared to other behaviors (Pettersson et al., 1990). Dams were classified by comparing their LG frequency scores (averaged across observations) to the cohort average as previously described (Champagne et al., 2003). Litters with LG frequency scores greater or less than one standard deviation from the cohort mean were classified as High- or Low-LG litters, respectively. Litters were neither culled nor sexed, and were left undisturbed until PND 7. At weaning on PND 21, pups were sexed and same-sex siblings were pair-housed in standard sized Plexiglas cages (22 × 23 × 45 cm) with wood shavings as bedding in a holding room maintained on a 12–12 hr light-dark cycle. Light-on was at midnight except for ethanol consumption studies when light-on was at 0800 hr. Like the dams, pups were not provided with environmental enrichment but had ad libitum access to food and water. No more than two animals per sex and per litter were used in each treatment group.
2.3 |. Monitoring female estrous cycle
To monitor the female estrous cycle for Experiment 1, vaginal lavage was conducted by flushing the vagina with saline (0.9% sodium chloride in water) via a dropper glass pipette inserted into the vagina, and collecting the flush as previously described (Borrow & Cameron, 2017). The flush was transferred to a microscope slide and observed under light microscope with 10× objective for cellular populations that characterize the four estrous cycle stages in rats (i.e., proestrus, estrus, metestrus, and diestrus) (Marcondes, Bianchi, & Tanno, 2002). The vaginal lavage procedure took approximately 3–5 s per rat and was conducted between 0800 and 0900 hr daily.
2.4 |. Two-bottle choice test for ethanol consumption and preference ratio
Twenty-two male and sixteen female adolescent rats were tested for ethanol consumption and preference ratio using the intermittent two-bottle choice with faded sucrose paradigm. Testing started on approximately PND 29–35, with age-at-onset similarly distributed between groups. Animals underwent a total of twelve 18-hr drinking sessions (1500–0900 hr the next day) that occurred three times a week (every other day with the weekend off) for 4 weeks. During each session, animals were single-housed in standard size cages and had ad libitum access to lab chow and two drinking bottles: one containing 5% (v/v) ethanol solution in water and the other containing water. The ethanol solution was sweetened with 1% sucrose during week 1 and 0.5% sucrose during week 2, while there was no sweetener during weeks 3 and 4. This faded sweetening paradigm was adapted from previous experiments (Fabio, Macchione, Nizhnikov, & Pautassi, 2015; Samson, 1986), because it effectively enhances ethanol acceptance in rodent ethanol consumption models (Carnicella, Ron, & Barak, 2014; Samson, 1986; Simms et al., 2008). Rats were weighed before every drinking session and drinking bottles were weighed before and after.
Ethanol consumed (g/kg) was calculated as follows: (ethanol solution consumed * 0.04) ÷ body weight. Ethanol preference-to-water ratio (%) was calculated as follow: (weight of ethanol solution consumed ÷ total (water and ethanol) solution consumed) * 100.
2.5 |. Loss of righting reflex test for sensitivity to ethanol-induced hypnosis
Seventy-three male and seventy-eight female adolescent rats were tested at PND 42 (mid adolescence) or PND 50 (late adolescence) for sensitivity to ethanol-induced hypnosis using the loss of righting reflex (LORR) paradigm as previously described (Nizhnikov, Popoola, & Cameron, 2016). Subjects received a 3.0 or 3.5 g/kg dose of 20% v/v ethanol solution in 0.9% saline via intraperitoneal (i.p.) administration. They were then observed in their cage until they lost the capacity to return to their four paws when placed on their backs (i.e., righting reflex). Subjects were then maintained on their backs in a v-shaped trough until they regained their righting reflex and demonstrated it three times within 60 s. The latency to LORR was defined as the duration between ethanol administration and LORR, while the duration between the loss and regaining of righting reflex was designated as the LORR duration.
Immediately following regaining of righting reflex, trunk blood was collected in an EDTA-coated vaccutainer (BD, Franklin Lakes, NJ). Plasma was then separated from the blood by centrifugation at 4 °C and 1500g for 15 min. Blood ethanol concentration (BEC) was analyzed using an AM1 alcohol analyzer (Analox Instruments, Lunenburg, MA).
2.6 |. Hormone analyses and GABAA subunit expressions
We examined the potential contributions of GABAA receptor subunit expressions and dysregulation of the endocrine system, to differences in ethanol consumption and sensitivity to the hypnotic effect of ethanol, between High- and Low-LG adolescent male offspring.
2.7 |. Testosterone analysis
Trunk blood was collected from 34 experiment-naïve adolescent male rats at either mid-adolescence (PND 42) or late-adolescence (PND 50) rat. Plasma was separated as described above and stored at −20 °-C until further processing. Plasma testosterone levels were quantified by radioimmunoassay (RIA) using a Packard Cobra II Autogamma Counter (Perlin Elmer’ Waltham, MA) and a 125I RIA double antibody kit obtained from MP Biomedicals, (Solon, OH) according to manufacturer instructions. The assay specificity was 100% and the inter-assay coefficient of variance was 6.99%.
2.8 |. Corticosterone analysis
Plasma corticosterone levels were quantified directly from plasma, using corticosterone enzyme-linked immunosorbent assay (ELISA) kit (ADI-900–097) purchased from ENZO Life Sciences, Inc. (Farmingdale, NY) and according to the manufacturer’s instructions. The intra-assay and inter-assay coefficients of variance were 7.71% and 13.18%, respectively.
2.9 |. Progesterone analysis
Plasma progesterone levels were quantified using a125I RIA double antibody kit obtained from MP Biomedicals (Solon, OH) with a specificity of 100%. The inter-assay coefficient of variance was 5.54%.
2.10 |. Western blot analysis
Brains were collected from previously undisturbed late-adolescence (PND 50) rats, flash frozen in 2-methyl butane (Sigma–Aldrich, St. Louis, MO) on dry ice immediately after collection and stored at −80 °C until micro-dissection. Using L.W. Swanson’s atlas (3rd Edition, Elsevier Inc., 2004) as a guide, the somatosensory cortex, and cerebellum were dissected from the harvested brains at −20 °C using a cryostat (Leica Biosystems, Buffalo Grove, IL). Due to the small size of the somatomotor area of the cerebral cortex harvested, only whole-cell homogenate was prepared from the cerebro-cortical tissue. However, because of the relatively larger size of the cerebellum one-half of the cerebellum was used for the whole-cell fraction, while synaptosomal fraction was prepared from the other half of the cerebellum. The synaptosomal fraction consisted of the neuronal membrane located at the synaptic bouton, thus allowing for the analysis of receptor composition at the synapse. This fraction contains both synaptic and extrasynaptic membrane components (Whittaker, Michaelson, & Kirkland, 1963, 1994).
To prepare a whole cell homogenate, the tissue sample was homogenized with an XL-2000 series sonicator (Qsonica LLC, Newtown, CT) in homogenization buffer (10 g Sodium dodecyl sulfate (SDS), 2 ml 0.5M EDTA, 10 ml 1M Tris, and 1L dH2O) five times for 10 s with a minimum of 15 min intervals. Synaptosomal fractions were prepared as previously described (Santerre, Gigante, Landin, & Werner, 2014). Briefly, tissue was gently homogenized in an isotonic glucose solution (0.1 glucose in 1 ml PBS solution), followed by centrifugation at 1000g for 20 min. The supernatant was collected and spun at 12,000g for 20 min and the precipitate, which is the synaptosomal fraction, was re-suspended in 500 μl of PBS for approximately 30 sec. The protein content of each sample homogenate was quantified using a Pierce Bicinchoninic acid (BCA) protein assay kit (Thermoscientific, Rockford, IL) according to manufacturer’s instructions. Subsequently, 50 ug of protein per sample were loaded into precast tris-glycine electrophoresis gels (Invitrogen, Carlsbad, CA) and run for 120–150 min at 125 V and 500 mA. Proteins were transferred onto a polyvinyl difluoride (PVDF) membrane (included in the kit) for 7 min, using the iBlot gel dry-transfer equipment and transfer kit (Invitrogen, Carlsbad, CA). Membranes were blocked overnight in ~10 ml of bovine serum albumin (BSA) (1 g BSA per 100 ml TBS-1% Tween) and probed with anti-GABAA receptor α1 and δ (Novus Biologicals, Littleton, CO) subunit proteins primary antibodies (α1–1:1,000 and δ−1:500). Membranes were subsequently incubated in goat anti-rabbit (1:7,500) secondary antibody (Thermoscientific, Waltham, MA). Proteinbands were detected with enhanced chemiluminescence (GE Healthcare, Piscataway, NJ) and visualized by exposure to photographic film (Bioexpress, Kaysville, UT). β-actin (1:10,000; Millipore, Lake Temecula, CA) was used to control for equivalent protein quantity loading across gel wells and samples. Antibodies were diluted in blocking buffer.
Final optical density (OD) was computed per sample as a ratio of target protein OD to β-actin OD (designated as normalized OD) and expressed as a percentage of the average normalized OD from Low-LG adolescent males used as the control ([Protein OD/Protein β-Actin]/[Average {Control OD/Control β-Actin}] *100).
2.11 |. Statistical analysis
Ethanol consumption and preference-to-water ratio were analyzed by repeated measure ANOVA in three intake stages based on changes in the sweetener concentration of the ethanol solution (i.e., week 1 [1% sucrose], week 2 [0.5%sucrose], and week 3–4 [0%sucrose]). To examine estrous cycle-related variation in ethanol consumption and preference-to-water ratio, females’ ethanol consumption and preference-to-water ratio at each estrous cycle stage across the 4-week testing period were averaged within subjects. These data were then analyzed using a repeated measure one-way ANOVA. Sensitivity to ethanol-induced LORR and BEC at awakening were analyzed using three-way (maternal care group X age X ethanol dose) fixed factor ANOVA. This study aimed at examining age-dependent emergence of maternal care-mediated differences in ethanol sensitivity. Therefore, a follow-up one-way ANOVA within age for each ethanol dose was performed when the three-way ANOVA revealed significant effects and/or interactions. Similarly, plasma testosterone, progesterone, and corticosterone levels were analyzed using two-way ANOVAs (maternal care group × age), followed by one-way ANOVAs at each age. Additionally, pairwise Pearson’s correlation analyses were conducted between progesterone, corticosterone, and testosterone within ages. GABAA receptor subunit expressions were analyzed within brain areas using one-way ANOVAs. A p-value less than 0.05 was considered significant, while a p-value less than 0.1 was reported as a trend toward significance. All analyses were conducted using SPSS (IBM, Armonk, NY) version 20.
3 |. RESULTS
3.1 |. Experiment 1: Effects of maternal care on adolescent female ethanol-use behaviors
3.1.1 |. Ethanol consumption and preference ratio
A repeated measure one-way ANOVA of female ethanol consumption and preference-to-water ratio revealed no significant effects of phenotype. However, there was a significant main effect of time (F(2, 13) = 4.275, p < 0.05) on ethanol consumption on week 1 as ethanol consumed was significantly lower on Day 3 compared to Day 1 in both phenotypes (both p < 0.05) (See Figure 1). A repeated measure ANOVA of averaged ethanol consumption and preference-to-water ratio across estrous cycle stages also revealed that the female estrous cycle had no significant influence on ethanol consumption patterns or preference-to-water ratio (data not shown).
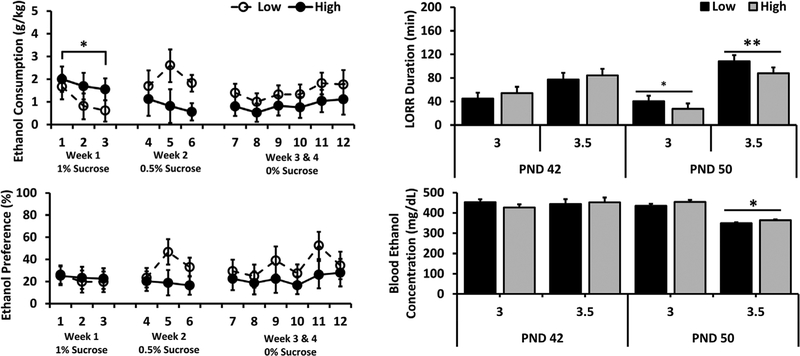
FIGURE 1.
Adolescent High- and Low-LG female 5% ethanol consumption (top left), 5% ethanol preference (bottom left), acute 3.0 or 3.5 g/kg ethanol induced LORR duration (top right), and blood ethanol concentration at awakening (bottom right). Data displayed as group Mean ± S.E.M. (*p < 0.05; **p < 0.01) (n = 8/group for ethanol consumption and preference; n = 8–12/group for LORR)
3.1.2 |. Sensitivity to ethanol-Induced hypnosis
A three-way ANOVA of LORR duration revealed a significant main effect of dose (F(1, 77) = 76.176, p < 0.001) with greater LORR duration at 3.5 g/kg compared to 3.0 g/kg, and a significant main interaction between phenotype and age (F(1, 77) = 5.137, p < 0.05). Follow-up one-way ANOVAs between LG groups revealed a significant difference between High-LG and Low-LG following both 3.0 g/kg (p < 0.05) and 3.5 g/kg (p < 0.01) doses at PND 50, but no difference at PND 42. Compared to Low-LG female adolescents, High-LG females demonstrated significantly shorter LORR duration at both doses on PND 50 (see Figure 1).
Three-way ANOVA of BEC at awakening revealed a significant main effect of age (F(1, 77) = 19.431, p < 0.001) with greater BECs at PND 42 versus PND 50 and dose (F(1, 77) = 16.805, p < 0.005) with lower BECs at 3.5 g/kg compared to 3.0 g/kg. A significant interaction between age and dose (F(1, 77) = 23.881, p < 0.001) was also observed. A follow-up one-way ANOVA revealed significantly higher BEC in High-LG (p < 0.05) compared to Low-LG at 3.5 g/kg dose on PND 50 (see Figure 1).
3.2 |. Experiment 2: effects of maternal care on adolescent male ethanol-use behaviors
3.2.1 |. Ethanol consumption and preference
Repeated measure one-way ANOVAs of male ethanol consumption and preference-to-water ratio revealed no significant effects of phenotype on male ethanol consumption or preference-to-water ratio. However, there was a significant main effect of time on ethanol consumption on week 1 (F(2, 19) = 5.850, p < 0.05) as ethanol consumed was significantly lower on Day 3 compared to Day 1 (See Figure 2).
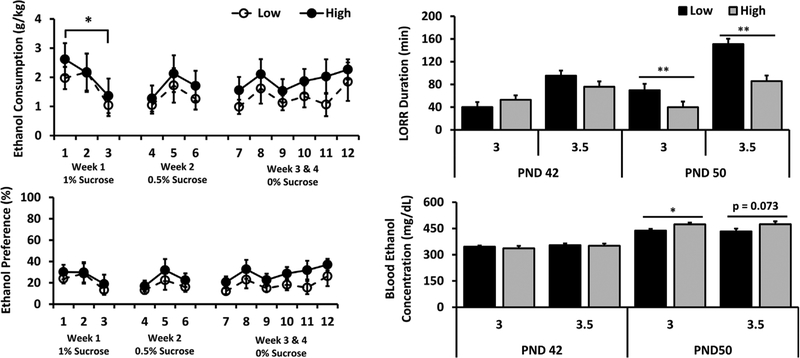
FIGURE 2.
Adolescent High-LG and Low-LG male 5% ethanol consumption (top left), 5% ethanol preference (bottom left), acute 3.0 or 3.5 g/kg ethanol induced LORR duration (top right), and blood ethanol concentration at awakening (bottom right). Data displayed as group Mean ± S.E.M. (*p < 0.05; **p < 0.01) (n = 10–12/group for ethanol consumption and preference; n = 8–10/group for LORR)
3.2.2 |. Sensitivity to ethanol-Induced hypnosis
Acute ethanol-induced LORR duration was assessed in a different set of High- and Low-LG adolescent male offspring on PND 42 and PND 50 at 3.0 or 3.5 g/kg ethanol dose. A three-way ANOVA (2 phenotypes × 2 ages × 2 doses) revealed a significant main effect of phenotype (F(1,63) = 6.397, p < 0.05) with shorter LORR duration in High-LG compared to Low-LG offspring, and dose (F(1, 63) = 29.437, p < 0.001) with greater LORR duration at 3.5 g/kg compared to 3.0 g/kg as expected. We also observed a trend toward a significant effect of age (F(1, 63) = 3.822, p = 0.056) with a tendency toward significantly higher LORR duration at PND 50 compared to PND 42. There was also a significant interaction between phenotype and age (F(1, 63) = 4.480, p < 0.5) and a tendency toward a significant interaction between phenotype and dose (F(1, 63) = 3.072, p = 0.09). Follow-up one-way ANOVAs within ages and doses revealed no significant effect of maternal care at PND 42 irrespective of dose. However, High-LG PND 50 males demonstrated significantly shorter LORR durations at both 3.0 g/kg (p < 0.01) and 3.5 g/kg (p < 0.05) ethanol doses compared to Low-LG males (see Figure 2).
Analyses of BEC at awakening, using a three-way ANOVA, revealed a significant effect of age (F(1, 72) = 25.093, p < 0.001) with higher BECs at PND 50 compared to PND 42, and phenotype (F(1, 72) = 7.543, p < 0.01) with higher BECs in High- group versus Low-LG. A follow-up one-way ANOVA revealed that on PND 50, High-LG males had higher BECs than Low-LG males at 3.0 g/kg ethanol dose (p < 0.05) and a tendency toward higher BECs at 3.5 g/kg ethanol dose (p = 0.073) (see Figure 2).
3.3 |. Experiment 3: Age-related effects of maternal care on endocrine function
3.3.1 |. Corticosterone
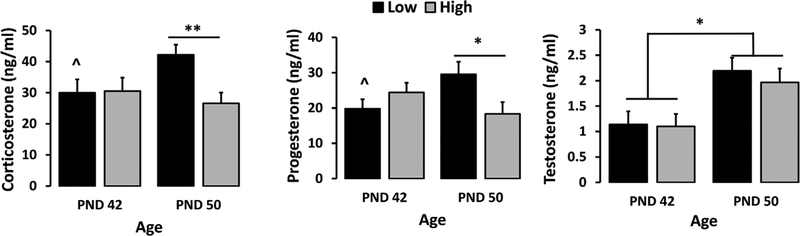
A two-way ANOVA (2 phenotypes × 2 ages) revealed no significant effect of maternal care or age on adolescent male plasma corticosterone levels. However, there was a significant interaction between phenotype and age (F(1, 33) = 4.667, p < 0.05). This interaction was mediated by a significant main effect of phenotype at PND 50 (p < 0.01) with higher plasma corticosterone levels in Low-LG offspring compared to High-LG. Corticosterone levels at PND 42 were not different between phenotypes. Furthermore, plasma corticosterone levels were higher on PND 50 compared to PND 42 (F(1, 16) = 4.525, p = 0.05) in Low-LG offspring but did not differ between ages in High-LG offspring (see Figure 3).
FIGURE 3.
Plasma corticosterone (left), progesterone (middle), and testosterone (right) in experiment-naïve adolescent High- and Low-LG males at PND 42 and PND 50. Data displayed as group Mean ± S.E.M. (*p < 0.05; **p < 0.01) (n = 7–10/group)
3.3.2 |. Progesterone
A two-way ANOVA revealed no significant main effect of maternal care or age on adolescent male plasma progesterone levels, but there was a significant interaction between phenotype and age (F(1, 33) = 7.988, p < 0.01). This interaction was driven by a significant main effect of phenotype at PND 50 (F(1, 14) = 5.272, p < 0.05) with higher plasma progesterone levels in Low-LG offspring compared to High-LG. Plasma progesterone levels at PND 42 were not different between phenotypes. Additionally, plasma progesterone levels were higher on PND 50 compared to PND 42 (F(1, 16) = 7.003, p < 0.05) in Low-LG offspring but did not differ between ages in High-LG offspring (see Figure 3).
3.3.3 |. Testosterone
A two-way ANOVA of adolescent male plasma testosterone levels revealed a significant main effect of age (F(1, 33) = 11.782, p < 0.01) with plasma testosterone levels significantly higher at PND 50 compared to PND 42. A follow-up one-way ANOVA revealed no significant main effect of maternal care on plasma testosterone levels or significant interaction between age and maternal care (see Figure 3).
3.3.4 |. Correlation analyses
We also assessed associations between male adolescent plasma levels of progesterone, corticosterone, and testosterone by conducting pairwise linear correlation analyses. We found a significant positive correlation between plasma corticosterone and progesterone levels at PND 50 (r = 0.698, p < 0.005). No additional significant correlations were observed (see Table 1).
TABLE 1.
R values from pairwise correlation analyses between plasma corticosterone, progesterone, and testosterone levels in experiment-naïve adolescent males at PND 42 and PND 50
| Corticosterone | Testosterone | Progesterone | ||||
|---|---|---|---|---|---|---|
| Age (PND) | 42 | 50 | 42 | 50 | 42 | 50 |
| Corticosterone | 1 | 1 | ||||
| Testosterone | −0.10 | −0.11 | 1 | 1 | ||
| Progesterone | 0.36 | 0.698** | −0.31 | −0.28 | 1 | 1 |
p < 0.005
3.4 |. Experiment 4: Effects of maternal care on cerebral cortical and cerebellar GABAA receptor subunit expressions
We further assessed GABAA receptor α1 and δ subunit expressions within the cerebral cortex and cerebellum at PND 50 as these subunits and brain regions reportedly play important roles in modulating sensitivity to ethanol-induced hypnosis (Kumar et al., 2009).
3.4.1 |. Cerebral cortex
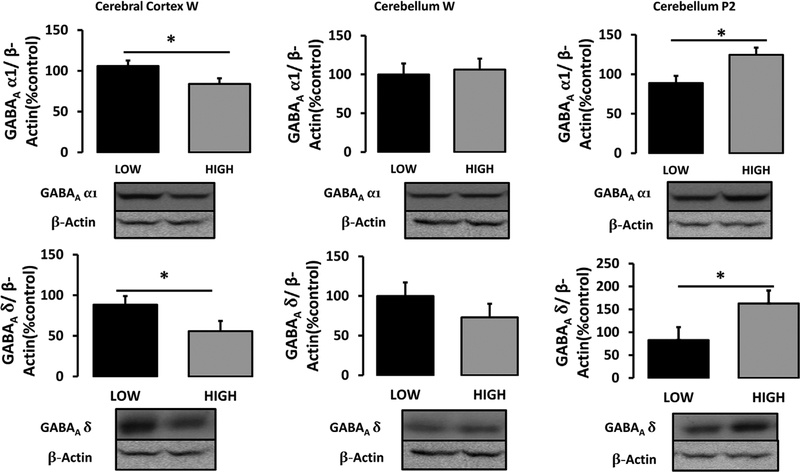
A one-way ANOVA within the cerebral cortex revealed a significant effect of maternal care on α1 (F(1, 14) = 5.279, p < 0.05) and δ (F(1, 14) = 7.042, p < 0.05) subunit expressions. Both α1 and δ subunits were expressed significantly more in Low-LG adolescent males compared to their High-LG counterparts (see Figure 4).
FIGURE 4.
GABAA α1 (top) and δ (bottom) subunit expressions in whole-cell fractions of the cerebral cortex (left) and cerebellum (middle), and synaptosomal fraction of the cerebellum (right) of experiment-naïve PND 50 adolescent High- and Low-LG male rats. Data displayed as group Mean ± S.E.M. (*p < 0.05) (n = 7–8/group)
3.4.2 |. Cerebellum.
A one-way ANOVA in cerebellar whole-cell fractions revealed no significant differences in α1 or δ subunit expressions. However, there were significant differences in both α1 (F(1, 14) = 7.907, p < 0.05) and δ (F(1, 14) = 6.25, p < 0.05) subunit expressions in synaptosomal fractions. These significant differences were due to greater synaptic α1 and δ subunit expressions in High-LG compared to Low-LG male rats (see Figure 4).
4 |. DISCUSSION
This study investigated the associations between natural variations in early-life maternal care, measured by LG frequency, and patterns of ethanol consumption and ethanol sensitivity during adolescence. We hypothesized that Low-LG would result in increased adolescent ethanol consumption, and would modulate sensitivity to ethanol-induced hypnosis. Surprisingly, we found no significant LG-related differences in ethanol consumption or preference-to-water ratio. However, Low-LG males and females were more sensitive to ethanol-induced hypnosis during late- but not mid-adolescence at both doses tested. We also hypothesized that natural variations in LG-related differences in sensitivity to ethanol-induced hypnosis may be associated with differences in plasma gonadal hormone levels and GABAA receptor subunit expressions, as these have been shown to be influenced by LG (Caldji et al., 2003; Cameron, Del Corpo et al., 2008). Hence, we measured adolescent plasma steroids and brain GABAA receptor protein subunits expression in experiment-naïve males. Baseline plasma progesterone and corticosterone levels were positively correlated and both were higher in Low-LG compared to High-LG males during late-adolescence but not mid-adolescence. Importantly, only Low-LG animals demonstrated a progesterone surge at late-adolescence compared to mid-adolescence. Baseline plasma testosterone surged at late-adolescence in both High- and Low-LG groups but did not differ between groups at either ages. Lastly, during late-adolescence, Low-LG males expressed more GABAA α1 and δ subunits in the cerebral cortex, but less in the cerebellar synaptosomal fraction compared to their High-LG counterparts. Nevertheless, higher progesterone and corticosteroid plasma levels in Low-LG males may have influenced brain GABAA subunit expressions and contributed to an increase in sensitivity to ethanol-induced hypnosis in this phenotype.
4.1 |. Ethanol consumption and preference
Consistent with existing experimental evidence suggesting that maternal deprivation does not influence adolescent’s ethanol consumption (Daoura, Haaker, & Nylander, 2011; Lancaster, 1998; Peñasco, Mela, López-Moreno, Viveros, & Marco, 2015), we found that natural variation in maternal-LG is not associated with ethanol consumption patterns in adolescent rats. However, some studies have reported associations between early-life maternal separation and increased ethanol consumption in male rodents. For example, some studies conducted with male mice reported increased oral ethanol self-administration, as well as lever pressing for ethanol (Cruz, Quadros, da Planeta, & Miczek, 2008; García-Gutiérrez et al., 2016) in adolescence, following early-life maternal Article 02: Untitled separation. Genetic-related differences in species (mice vs. rats) may, at least in part, explain the inconsistence between these mice studies and ours (see Leeman et al., 2010 for review; Rhodes, Best, Belknap, Finn, & Crabbe, 2005; Yoneyama, Crabbe, Ford, Murillo, & Finn, 2008). Differences in consumption assessment paradigms between studies may also constitute an additional source of variation between our findings in males and certain previously published reports. Operant self-administration as employed in the mice studies (Cruz et al., 2008; García-Gutiérrez et al., 2016) measures motivation (June & Gilpin, 2010), while our two-bottle choice paradigm better measures ethanol preference (Rhodes et al., 2005). Moreover, a previous study (Hulin, Amato, Porter, Filipeanu, & Winsauer, 2011) reported that the two paradigms, (i.e., ethanol self-administration in the home cage versus operant chamber), produced very different ethanol intake patterns. Nevertheless, our results corroborate some existing results suggesting that reduced maternal care may not affect adolescent ethanol consumption and preference in rats.
4.2 |. Age-dependent ethanol-induced hypnosis
Herein, we also report increased ethanol-induced sensitivity to hypnosis in low maternal LG recipients during late- but not mid-adolescence. The impact of early-life environment on alcohol-induced behaviors is currently receiving increased attention (Fernandez et al., 2014; Pautassi, Nizhnikov, Fabio, & Spear, 2012 see Sinha, 2008 for review). Our observation is consistent with previously reported greater sensitivity to ethanol-induced locomotor activation (at 1.25 g/kg dose) and motor suppression (at 2.5 g/kg dose) in maternal deprived rats (Fernandez et al., 2014). Additionally, increased sensitivity to the rewarding and locomotor-related effects of other drugs such as methamphetamine (Hensleigh & Pritchard, 2014; Pritchard, Hensleigh, & Lynch, 2012), cocaine (Gracia-Rubio et al., 2016; Kikusui, Faccidomo, & Miczek, 2005), and nicotine (Dalaveri, Nakhaee, Esmaeilpour, Mahani, & Sheibani, 2017) have all been associated with maternal deprivation. However, Pautassi and colleagues (Pautassi et al., 2012) found no effect of maternal deprivation on ethanol-induced locomotion plausibly due to the complications associated with the Maternal Separation (MS) model (see Nylander & Roman, 2013 for detailed review of the MS model). Therefore, our findings corroborate and extend existing evidence that low early-life maternal care may be associated with increased sensitivity to motor-related drug effects, particularly ethanol-induced hypnosis.
Based on existing evidence (Naassila et al., 2002; Thiele et al., 2000), we expected maternal care to modulate both ethanol-induced hypnosis and ethanol intake-behaviors. Yet, only ethanol-induced hypnosis was affected. This unexpected finding reflects the distinction between the mechanisms mediating the two behaviors. Ethanol’s neurobehavioral effects differ by dose, blood concentration, and brain area (Gessa, Muntoni, Collu, Vargiu, & Mereu, 1985; Quintanilla, 1999; see Wallner, Hanchar, & Olsen, 2006 for review). Moreover, the effects of maternal care vary by brain area (Caldji et al., 2003). Therefore, the possibility that variations in maternal care differentially altered brain areas mediating ethanol consumption and ethanol-induced hypnosis could explain why the previously reported concordance between ethanol consumption and sensitivity to ethanol-induced hypnosis was not found in our study.
The late adolescence emergence of LG-related differences in sensitivity to ethanol-induced hypnosis between High- and Low-LG groups was also interesting. The consequences of variations in early-life maternal care are not always delayed until late adolescence (Fernandez et al., 2014; Pautassi et al., 2012; Pena, Neugut, Calarco, & Champagne, 2014; Pena, Neugut, & Champagne, 2013), although some effects are (Holland, Ganguly, Potter, Chartoff, & Brenhouse, 2014; Kaufman et al., 2007; Tsuda, Yamaguchi, & Ogawa, 2011). Such delay may be due to the contributions of peri-adolescent endocrine-related changes as we discuss in more detail below. Therefore, our study corroborates and extends existing evidence that certain behavioral differences that are associated with natural variations in maternal care may emerge in late-adolescence as do some endocrine hormones.
4.3 |. Adolescent male hormone levels
A surge in hormone and neuroactive steroid levels is characteristic of transitioning from adolescence to adulthood (Grota, 1971; Sisk & Zehr, 2005; Vetter-O’Hagen & Spear, 2012). We and others have already demonstrated that early-life environmental factors alter the timing of the hormonal surge and their associated physiological characteristics, such as menarche and vaginal opening in female rats (Allsworth, Weitzen, & Boardman, 2005; Borrow et al., 2013; see Cameron, 2011 for review; Foster, Hagan, & Brooks-Gunn, 2008). However, the effect of maternal care on adolescent male hormonal changes has not been previously characterized. Therefore, in this study we only investigated changes in plasma steroid levels associated with variation in maternal LG frequency in adolescent males. Hence, the present study is the first to show greater plasma progesterone and corticosterone levels in male rats at PND 50 compared to PND 42 in the Low-LG group, but not the High-LG. Early-life stress accelerates the development of adult-like neurobehavioral characteristics (Bath, Manzano-Nieves, & Goodwill, 2016; Callaghan & Richardson, 2014; Gee et al., 2013). It has been shown that reduced maternal care levels can be a stressor that can be ameliorated by increased tactile stimulation (stroking) similar to maternal LG (Chatterjee et al., 2007; Levy et al., 2003; Suchecki et al., 1993; van Oers, de Kloet, Whelan, & Levine, 1998). Therefore, it is possible that lower levels of maternal care may also act as an early-life stressor, as we observed an acceleration in the occurrence of peri-adolescent hormonal maturation.
We observed an increase in sensitivity to ethanol-induced hypnosis at PND 50 compared to PND 42 in the Low-LG group. This corresponds with the finding that ethanol sensitivity increases as rats transition from adolescence to adulthood (Silveri & Spear, 1998). Also, steroid metabolites of corticosterone and progesterone positively modulate sensitivity to ethanol-induced hypnosis (see Helms et al., 2012 for review). Therefore, higher sensitivity to ethanol-induced hypnosis in Low- compared to High-LG males on PND 50 can be attributed, at least in part, to the early surge in progesterone and corticosterone plasma levels in Low-LG, but not High-LG, males. In addition, we verified the previously reported positive correlation between progesterone and corticosterone (Andersen, Martins, D’Almeida, Bignotto, & Tufik, 2005; Hueston & Deak, 2014; Vetter-O’Hagen & Spear, 2012) that we found only in PND 50 male rats. While this positive correlation was expected, as corticosterone is synthesized from progesterone (Helms et al., 2012), our study further suggests that this correlation is established late in adolescence.
Surprisingly, there was no difference in plasma testosterone levels between Low- and High-LG groups on either PND 42 or 50, while both groups showed a significant testosterone increase across time as expected (Grota, 1971; Vetter-O’Hagen & Spear, 2012). This implies that natural variations in maternal LG may not be associated with differences in baseline plasma testosterone levels at these ages. Furthermore, testosterone levels did not correlate with either progesterone or corticosterone plasma levels on either PND 42 or 50, which validates existing reports (Andersen et al., 2005; Vetter-O’Hagen & Spear, 2012). Moreover, while progesterone is a precursor for both testosterone and corticosterone, testosterone can be directly synthesized from pregnenolone, totally bypassing progesterone, unlike corticosterone (Helms et al., 2012). Therefore, our results corroborate existing evidence of associations between early-life experiences and the timing of peri-adolescent progesterone and corticosterone surges. Additionally, our results also suggest that testosterone may not be as influential as other steroids in modulating sensitivity to ethanol-induced hypnosis in adolescent male rats at the ages tested in the present study.
4.4 |. Brain GABAA receptor subunit expressions
The GABAA system is a principal ethanol target in the brain that contributes significantly to mediating ethanol-induced hypnosis (Davies, 2003; Kumar et al., 2009) and the development of alcoholism (Enoch, 2008). Maternal-LG is known to alter brain GABAA receptor subunits expressions (Caldji et al., 2003). We were interested in the expression of GABAA α1 subunit due to several research findings that have associated the α1 subunit expression with variations in sensitivity to ethanol-induced hypnosis (Blednov, Jung et al., 2003; Blednov, Walker et al., 2003; Kumar et al., 2012). We were also interested in δ subunit expression because it is mostly expressed in extrasynaptic GABAA receptors (Kumar et al., 2009), which are primary sites at which neuroactive steroids modulate ethanol sensitivity (Helms et al., 2012). This study is the first to find increased cerebral-cortical, but decreased cerebellar (in the synaptosomal fraction), GABAA α1 and δ subunit protein expressions in Low-LG compared to High-LG males. This finding is consistent with previous reports of brain region-specific associations between maternal care and GABAA receptor subunit expression (Caldji, Diorio, Anisman, & Meaney, 2004; Caldji et al., 2003). This finding also corroborates our recently reported association between increased cortical GABAA α1 and δ subunit expressions and increased sensitivity to ethanol-induced hypnosis (Popoola, Nizhnikov, & Cameron, 2017). In addition, we revealed the first reported evidence of associations between variations in maternal care and GABAA receptor subunit expressions in brain regions that are associated with the execution and coordination of motor activity (Hanchar, Dodson, Olsen, Otis, & Wallner, 2005; Saeed, 2006). This observation corroborates and extends previously reported associations between variations in maternal care and GABAA receptor subunit expressions in the limbic system (Caldji et al., 2004; Caldji et al., 2003; Hsu et al., 2003; León Rodríguez & Dueñas, 2013).
Increased GABAA receptor subunit expressions in the cerebral cortex of Low-LG males, as we observed, suggest a positive relationship between GABAA receptor expression and sensitivity to ethanol-induced hypnosis (Carlson, Bohnsack, & Morrow, 2016; Carlson, Bohnsack, Patel, & Morrow, 2016; Carlson, Kumar, Werner, Comerford, & Morrow, 2013; Kumar et al., 2012; Kumar et al., 2010). The difference in GABAA receptor subunit expression between Low- and High-LG males may be related to differences in circulating steroid levels. Research has shown that corticosterone, progesterone, and progesterone metabolites modulate GABAA receptor gene expression and alter the expression of selective subunits (Orchinik, Weiland, & McEwen, 1994, 1995). Nevertheless, these observed lower GABAA α1 and δ subunit expressions in the cerebellar synaptosomal fraction in the Low-LG group compared to the High-LG group, and the lack of a difference between groups in cerebellar whole-cell fraction, were unexpected. These expression patterns within the cerebellum suggest that while existing evidence implicates cerebellar GABAergic receptor expression in ethanol-induced locomotor activity (Hanchar et al., 2005; Saeed, 2006), additional factors may contribute to determining sensitivity to ethanol-induced hypnosis.
Studies have reported that ethanol exposure increase the levels of progesterone metabolites allopregnanolone (VanDoren et al., 2000) and alter GABAA α1 subunit expression in the cerebral cortex (Kumar et al., 2012). These ethanol-induced effects have further been reported to modulate ethanol-induced hypnosis (Kumar et al., 2012; VanDoren et al., 2000). There may be some differences in ethanol effects on both the brain and endocrine system that we have not identified in our animal model as our tissue and plasma were collected in experiment-naïve animals. These ethanol-induced effects could have impacted sensitivity to ethanol-induced hypnosis. Therefore, future experiments should investigate LG-related differences in hormonal levels and GABAA receptor subunit expressions following ethanol challenge as potential factors contributing to LG-related differences in sensitivity to ethanol-induced hypnosis.
4.5 |. Study limitations
Despite the important findings of this study, it has certain limitations. We did not examine how manipulating maternal care would impact our observed LG-related behavioral and molecular differences, and thus we cannot conclude that variations in maternal care was solely responsible for such behavioral and molecular differences. Hence, this study only suggests an association between variations in LG and differences in sensitivity to ethanol-induced hypnosis but does not reveal a causal relationship. In addition, this study examined associations between maternal care and both hormonal levels and GABAA receptor subunit protein expressions only in males while the behavioral assessments were in both sexes. We primarily focused on males in this study because we have previously examined LG-related differences in hormonal regulation, GABAA receptor expression and their behavioral, as well as physiological implications in female rats (Borrow et al., 2013; Borrow & Cameron, 2017; Cameron, 2011; Cameron, Del Corpo et al., 2008). Nevertheless, the GABAA receptor protein subunits expression in the cerebellum in females, has not yet been investigated and constitutes a limitation of the present study. Lastly, the analysis of GABAA receptor subunit expressions in the entire cerebellum in the present study lacked specificity with respect to cerebellar sub-regions. Future investigations should focus on protein expressions within targeted cerebellar sub-regions and cell types that play specific roles in ethanol-induced motor activity.
5 |. CONCLUSION
This study demonstrates that in rats, sensitivity to ethanol-induced hypnosis is associated with natural variations in maternal-LG frequency in both sexes during late adolescence. Interestingly, this effect on sensitivity to ethanol-induced hypnosis was not correlated with variations in ethanol intake, suggesting that the two behaviors are mediated by independent systems. We also revealed that progesterone and corticosterone surged in Low-LG but not High-LG male rats at PND 50, which may contribute to the increased sensitivity to ethanol-induced hypnosis in the Low-LG group at this age. Lastly, we revealed novel brain-region specific associations between variations in maternal-LG frequency and GABAA receptor subunit expressions in regions that are associated with motor activity and in patterns that partly corroborate group differences in sensitivity to ethanol-induced hypnosis. Collectively, these findings provide a novel insight into the potential regulatory impact of early-life maternal care on sensitivity to ethanol-induced hypnosis and the neurophysiological factors that may mediate such impact in males. In addition, our findings emphasize a need to consider natural variations in maternal care in research when possible, as it could underlie significant variability in neurobehavioral observations.
ACKNOWLEDGEMENT
The authors will like to acknowledge Lynda Ruffini for her assistance in running the RIAs in this study and Dr. Sarah Laszlo for her consultation concerning statistical analyses. This project was funded by a NIAAA grant to the Developmental Exposure Alcohol Research Center at Binghamton University (P50AA017823 to LPS) and a NIAAA grant R21AA023072 to NMC.
Funding information
National Institute on Alcohol Abuse and Alcoholism, Grant numbers: R21AA023072, P50AA017823
REFERENCES
- Allsworth JE, Weitzen S, & Boardman LA (2005). Early age at menarche and allostatic load: Data from the Third National Health and Nutrition Examination Survey. Annals of Epidemiology, 15(6), 438–444. https://doi.org./10.1016/j.annepidem.2004.12.010 [DOI] [PubMed] [Google Scholar]
- Almeida OF, Shoaib M, Deicke J, Fischer D, Darwish MH, & Patchev VK (1998). Gender differences in ethanol preference and ingestion in rats. The role of the gonadal steroid environment. Journal of Clinical Investigation, 101(12), 2677–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorim JP, Chuffa LG, Teixeira GR, Mendes LO, Fioruci BA,Martins OA, … Martinez M (2011). Variations in maternal care alter corticosterone and 17beta-estradiol levels, estrous cycle and folliculo-genesis and stimulate the expression of estrogen receptors alpha and beta in the ovaries of UCh rats. Reproductive Biology and Endocrinology, 9(1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen ML, Martins PJ, D’Almeida V, Bignotto M, & Tufik S (2005). Endocrinological and catecholaminergic alterations during sleep deprivation and recovery in male rats. Journal of Sleep Research, 14(1), 83–90. https://doi.org./10.1111/j.1365-2869.2004.00428.x [DOI] [PubMed] [Google Scholar]
- Apter SJ, & Eriksson CJ (2003). The effect of alcohol on testosterone concentrations in alcohol-preferring and non-preferring rat lines. Alcoholism: Clinical and Experimental Research, 27(7), 1190–1193. https://doi.org./10.1097/01.alc.0000075832.83254.81 [DOI] [PubMed] [Google Scholar]
- Bath KG, Manzano-Nieves G, & Goodwill H (2016). Early life stress accelerates behavioral and neural maturation of the hippocampus in male mice. Hormones and Behavior, 82, 64–71. https://doi.org./10.1016/j.yhbeh.2016.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Jung S, Alva H, Wallace D, Rosahl T, Whiting PJ, & Harris RA (2003). Deletion of the alpha1 or beta2 subunit of GABAA receptors reduces actions of alcohol and other drugs. Journal of Pharmacology and Experimental Therapeutics, 304(1), 30–36. https://doi.org./10.1124/jpet.102.042960 [DOI] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Alva H, Creech K, Findlay G, & Harris RA (2003). GABAA receptor alpha 1 and beta 2 subunit null mutant mice: Behavioral responses to ethanol. Journal of Pharmacology and Experimental Therapeutics, 305(3), 854–863. https://doi.org./10.1124/jpet.103.049478 [DOI] [PubMed] [Google Scholar]
- Borrow AP, & Cameron NM (2017). Maternal care and affective behavior in female offspring: Implication of the neurosteroid/GABAergic system. Psychoneuroendocrinology, 76, 29–37. [DOI] [PubMed] [Google Scholar]
- Borrow AP, Levy MJ, Soehngen EP, & Cameron NM (2013). Perinatal testosterone exposure and maternal care effects on the female rat’s development and sexual behaviour. Journal of Neuroendocrinology, 25(6), 528–536. https://doi.org./10.1111/jne.12035 [DOI] [PubMed] [Google Scholar]
- Brickley SG, & Mody I Extrasynaptic GABAA receptors: Their function in the CNS and implications for disease. Neuron, 73(1), 23–34. https://doi.org./10.1016/j.neuron.2011.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Anisman H, & Meaney MJ (2004). Maternal behavior regulates Benzodiazepine/GABAA receptor subunit expression in brain regions associated with fear in BALB/c and C57BL/6 mice. Neuropsychopharmacology, 29(7), 1344–1352. [DOI] [PubMed] [Google Scholar]
- Caldji C, Diorio J, & Meaney MJ (2003). Variations in maternal care alter GABA(A) receptor subunit expression in brain regions associated with fear. Neuropsychopharmacology, 28(11), 1950–1959. https://doi.org./10.1038/sj.npp.1300237 [DOI] [PubMed] [Google Scholar]
- Callaghan BL, & Richardson R (2014). Early emergence of adult-like fear renewal in the developing rat after chronic corticosterone treatment of the dam or the pups. Behavioral Neuroscience, 128(5), 594–602. https://doi.org./10.1037/bne0000009 [DOI] [PubMed] [Google Scholar]
- Cameron N, Del Corpo A, Diorio J, McAllister K, Sharma S, & Meaney MJ (2008). Maternal programming of sexual behavior and hypothalamic-Pituitary-Gonadal function in the female rat. PLoS ONE, 3(5), e2210 https://doi.org./10.1371/journal.pone.0002210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron N (2011). Maternal programming of reproductive function and behavior in the female rat. [Review]. Frontiers in Evolutionary Neuroscience, 3(10), https://doi.org./10.3389/fnevo.2011.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron NM, Shahrokh D, Del Corpo A, Dhir SK, Szyf M, Champagne FA, & Meaney MJ (2008). Epigenetic programming of phenotypic variations in reproductive strategies in the rat through maternal care. Journal of Neuroendocrinology, 20(6), 795–801. [DOI] [PubMed] [Google Scholar]
- Carlson SL, Bohnsack JP, & Morrow AL (2016). Ethanol regulation of synaptic GABAA alpha4 receptors is prevented by protein kinase a activation. Journal of Pharmacology and Experimental Therapeutics, 357(1), 10–16. https://doi.org./10.1124/jpet.115.230417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SL, Bohnsack JP, Patel V, & Morrow AL (2016). Regulation of extrasynaptic GABAA alpha4 receptors by ethanol-Induced protein kinase A, but not protein kinase C activation in cultured rat cerebral cortical neurons. Journal of Pharmacology and Experimental Therapeutics, 356(1), 148–156. https://doi.org./10.1124/jpet.115.228056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SL, Kumar S, Werner DF, Comerford CE, & Morrow AL (2013). Ethanol activation of protein kinase a regulates GABA(A) α1 receptor function and trafficking in cultured cerebral cortical neurons. The Journal of Pharmacology and Experimental Therapeutics, 345(2), 317–325. https://doi.org./10.1124/jpet.112.201954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Ron D, & Barak S (2014). Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol, 48(3), 243–252. https://doi.org./10.1016/j.alcohol.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll HA, Lustyk KB, & Larimer ME (2015). The relationship between alcohol consumption and menstrual cycle: A review of the literature. Archives of women’s mental health, 18(6), 773–781. https://doi.org./10.1007/s00737-015-0568-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CM, & Reddy DS (2013). Neurosteroid interactions with synaptic and extrasynaptic GABA(A) receptors: Regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacology, 230(2), 151–188. https://doi.org./10.1007/s00213-013-3276-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Francis DD, Mar A, & Meaney MJ (2003). Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiology and Behavior, 79(3), 359–371. [DOI] [PubMed] [Google Scholar]
- Chatterjee D, Chatterjee-Chakraborty M, Rees S, Cauchi J, de Medeiros CB, & Fleming AS (2007). Maternal isolation alters the expression of neural proteins during development: ‘Stroking’ stimulation reverses these effects. Brain Research, 1158, 11–27. https://doi.org./10.1016/j.brainres.2007.04.069 [DOI] [PubMed] [Google Scholar]
- Cruz FC, Quadros IM, da Planeta CS, & Miczek KA (2008). Maternal separation stress in male mice: Long-term increases in alcohol intake. Psychopharmacology, 201(3), 459–468. https://doi.org./10.1007/s00213-008-1307-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalaveri F, Nakhaee N, Esmaeilpour K, Mahani SE, & Sheibani V (2017). Effects of maternal separation on nicotine-induced conditioned place preference and subsequent learning and memory in adolescent female rats. Neuroscience Letters, 639, 151–156. https://doi.org./10.1016/j.neulet.2016.11.059 [DOI] [PubMed] [Google Scholar]
- Daoura L, Haaker J, & Nylander I (2011). Early environmental factors differentially affect voluntary ethanol consumption in adolescent and adult male rats. Alcoholism: Clinical and Experimental Research, 35(3), 506–515. https://doi.org./10.1111/j.1530-0277.2010.01367.x [DOI] [PubMed] [Google Scholar]
- Davies M (2003). The role of GABA(A) receptors in mediating the effects of alcohol in the central nervous system. Journal of Psychiatry and Neuroscience, 28(4), 263–274. [PMC free article] [PubMed] [Google Scholar]
- Dazzi L, Serra M, Seu E, Cherchi G, Pisu MG, Purdy RH, & Biggio G (2002). Progesterone enhances ethanol-induced modulation of mesocortical dopamine neurons: Antagonism by finasteride. Journal of Neurochemistry, 83(5), 1103–1109. https://doi.org./10.1046/j.1471-4159.2002.01218.x [DOI] [PubMed] [Google Scholar]
- Enoch M-A (2008). The role of GABA(A) receptors in the development of alcoholism. Pharmacology, Biochemistry, and Behavior, 90(1), 95–104. https://doi.org./10.1016/j.pbb.2008.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch M-A (2011). The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology, 214(1), 17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein EE, Rhines KC, Cook S, Zdep-Mattocks B, Jensen NK, & McCrady BS (2006). Changes in alcohol craving and consumption by phase of menstrual cycle in alcohol dependent women. Journal of Substance Use, 11(5), 323–332. https://doi.org./10.1080/14659890500419717 [Google Scholar]
- Eriksson CJ, Kaprio J, Pulkkinen L, & Rose RJ (2005). Testosterone and alcohol use among adolescent male twins: Testing between-family associations in within-family comparisons. Behavior Genetics, 35(3), 359–368. https://doi.org./10.1007/s10519-005-3228-x [DOI] [PubMed] [Google Scholar]
- Fabio MC, Macchione AF, Nizhnikov ME, & Pautassi RM (2015). Prenatal ethanol increases ethanol intake throughout adolescence, alters ethanol-mediated aversive learning, and affects mu but not delta or kappa opioid receptor mRNA expression. European Journal of Neurosci, 41(12), 1569–1579. https://doi.org./10.1111/ejn.12913 [DOI] [PubMed] [Google Scholar]
- Fahlke C, Engel JA, Eriksson CJ, Hard E, & Soderpalm B (1994). Involvement of corticosterone in the modulation of ethanol consumption in the rat. Alcohol, 11(3), 195–202. [DOI] [PubMed] [Google Scholar]
- Fahlke C, Hård E, & Hansen S (1996). Facilitation of ethanol consumption by intracerebroventricular infusions of corticosterone. Psychopharmacology, 127(1), 133–139. https://doi.org./10.1007/BF02805986 [DOI] [PubMed] [Google Scholar]
- Fahlke C, Hård E, Hansen S, Eriksson CJP, & Engel JA (1995). Consequence of long-term exposure to corticosterone or dexamethasone on ethanol consumption in the adrenalectomized rat, and the effect of type I and type II corticosteroid receptor antagonists. Psychopharmacology, 117(2), 216–224. https://doi.org./10.1007/BF02245190 [DOI] [PubMed] [Google Scholar]
- Fernandez M, Fabio MC, Nizhnikov ME, Spear NE, Abate P, & Pautassi RM (2014). Maternal isolation during the first two postnatal weeks affects novelty-induced responses and sensitivity to ethanol-induced locomotor activity during infancy. Developmental Psychobiology, 56(5), 1070–1082. https://doi.org./10.1002/dev.21192 [DOI] [PubMed] [Google Scholar]
- Fernández MS, Fabio MC, Miranda-Morales RS, Virgolini MB, De Giovanni LN, Hansen C, … Pautassi RM (2016). Age-related effects of chronic restraint stress on ethanol drinking, ethanol-induced sedation, and on basal and stress-induced anxiety response. Alcohol, 51, 89–100. https://doi.org./10.1016/j.alcohol.2015.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Eldridge JC, & Samson HH (2002). Ethanol consumption in the female Long–Evans rat: A modulatory role of estradiol. Alcohol, 26(2), 103–113. [DOI] [PubMed] [Google Scholar]
- Foster H, Hagan J, & Brooks-Gunn J (2008). Growing up fast: Stress exposure and subjective “weathering” in emerging adulthood. Journal of Health and Social Behavior, 49(2), 162–177. https://doi.org./10.1016/S0741-8329(01)00203-8 [DOI] [PubMed] [Google Scholar]
- Francis DD, Diorio J, Plotsky PM, & Meaney MJ (2002). Environmental enrichment reverses the effects of maternal separation on stress reactivity. The Journal of Neuroscience, 22(18), 7840–7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Gutiérrez MS, Navarrete F, Aracil A, Bartoll A, Martínez-Gras I, Lanciego JL, … Manzanares J (2016). Increased vulnerability to ethanol consumption in adolescent maternal separated mice. Addiction Biology, 21(4), 847–858. https://doi.org./10.1111/adb.12266 [DOI] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, … Tottenham N (2013). Early developmental emergence of human amygdala–prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences, 110(39), 15638–15643. https://doi.org./10.1073/pnas.1307893110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L, & Mereu G (1985). Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Research, 348(1), 201–203. 10.1016/0006-8993 [DOI] [PubMed] [Google Scholar]
- Gracia-Rubio I, Valverde O, Martinez-Laorden E, Moscoso-Castro M, Milanés MV, & Laorden ML (2016). Maternal separation impairs cocaine-Induced behavioural sensitization in adolescent mice. PLoS ONE, 11(12), e0167483 https://doi.org./10.1371/journal.pone.0167483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grota LJ (1971). Effect of age and experience on plasma testosterone. Neuroendocrinology, 8(2), 136–143. [DOI] [PubMed] [Google Scholar]
- Haim S, Shakhar G, Rossene E, Taylor AN, & Ben-Eliyahu S (2003). Serum levels of sex hormones and corticosterone throughout4- and 5-day estrous cycles in Fischer 344 rats and their simulation in ovariectomized females. [journal article]. Journal of Endocrinological Investigation, 26(10), 1013–1022. https://doi.org./10.1007/bf03348201 [DOI] [PubMed] [Google Scholar]
- Hanchar HJ, Dodson PD, Olsen RW, Otis TS, & Wallner M (2005). Alcohol-induced motor impairment caused by increased extrasynaptic GABA(A) receptor activity. Nature Neuroscience, 8(3), 339–345. https://doi.org./10.1038/nn1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Rossi DJ, & Grant KA (2012). Neurosteroid influences on sensitivity to ethanol. Frontiers in Endocrinology, 3, 10 https://doi.org./10.3389/fendo.2012.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensleigh E, & Pritchard LM (2014). The effect of early environmental manipulation on locomotor sensitivity and methamphetamine conditioned place preference reward. Behavioural Brain Research, 268, 66–71. https://doi.org./10.1016/j.bbr.2014.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland FH, Ganguly P, Potter DN, Chartoff EH, & Brenhouse HC (2014). Early life stress disrupts social behavior and prefrontal cortex parvalbumin interneurons at an earlier time-point in females than in males. Neuroscience Letters, 566, 131–136. https://doi.org./10.1016/j.neulet.2014.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstein SE, Spanos M, & Hodge CW (2011). Adolescent C57BL/6J mice show elevated alcohol intake, but reduced taste aversion, as compared to adult mice: A potential behavioral mechanism for binge drinking. Alcoholism, Clinical and Experimental Research, 35(10), 1842–1851. https://doi.org./10.1111/j.1530-0277.2011.01528.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu F-C, Zhang G-J, Raol YSH, Valentino RJ, Coulter DA, & Brooks-Kayal AR (2003). Repeated neonatal handling with maternal separation permanently alters hippocampal GABAA receptors and behavioral stress responses. Proceedings of the National Academy of Sciences, 100(21), 12213–12218. https://doi.org./10.1073/pnas.2131679100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueston CM, & Deak T (2014). On the time course, generality, and regulation of plasma progesterone release in male rats by stress exposure. Endocrinology, 155(9), 3527–3537. https://doi.org./10.1210/en.2014-1060 [DOI] [PubMed] [Google Scholar]
- Hulin MW, Amato RJ, Porter JR, Filipeanu CM, & Winsauer PJ (2011). Neurosteroid binding sites on the GABAA receptor complex as novel targets for therapeutics to reduce alcohol abuse and dependence. Advances in Pharmacological Sciences, 2011, 12 https://doi.org./10.1155/2011/926361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L, Patrick OM, Richard M, Jerald B, John S (2016). Monitoring the Future National Survey Results on Drug Abuse 1975–2015: Overview, key findings on adolescent drug use Ann Arbor: Institute for Social Research, The University of Michigan. [Google Scholar]
- June HL, Gilpin NW (2010). Operant Self-Administration Models for Testing the Neuropharmacological Basis of Ethanol Consumption in Rats. Current protocols in neuroscience/editorial board, Crawley Jacqueline N., … [et al. ], Chapter 9, Unit-9.121–26. https://doi.org./10.1002/0471142301.ns0912s51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutapakdeegul N, Casalotti SO, Govitrapong P, & Kotchabhakdi N (2003). Postnatal touch stimulation acutely alters corticosterone levels and glucocorticoid receptor gene expression in the neonatal rat. Developmental Neuroscience, 25(1), 26–33. https://doi.org./10.1159/000071465 [DOI] [PubMed] [Google Scholar]
- Kashiwabuchi N, Ikeda K, Araki K, Hirano T, Shibuki K, Takayama C, … Mishina M (1995). Impairment of motor coordination, Purkinje cell synapse formation, and cerebellar long-term depression in GluR delta 2 mutant mice. Cell, 81(2), 245–252. [DOI] [PubMed] [Google Scholar]
- Kaufman D, Banerji MA, Shorman I, Smith ELP, Coplan JD, Rosenblum LA, & Kral JG (2007). Early-Life stress and the development of obesity and insulin resistance in juvenile bonnet macaques. Diabetes, 56(5), 1382–1386. https://doi.org./10.2337/db06-1409 [DOI] [PubMed] [Google Scholar]
- Khisti RT, VanDoren MJ, O’Buckley T, & Morrow AL (2003). Neuroactive steroid 3α-hydroxy-5α-pregnan-20-one modulates ethanol-induced loss of righting reflex in rats. Brain Research, 980(2), 255–265. https://doi.org./10.1016/S0006-8993(03)02978-0 [DOI] [PubMed] [Google Scholar]
- Kikusui T, Faccidomo S, & Miczek KA (2005). Repeated maternal separation: Differences in cocaine-induced behavioral sensitization in adult male and female mice. Psychopharmacology, 178(2–3), 202–210. https://doi.org./10.1007/s00213-004-1989-1 [DOI] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, & Morrow AL (2009). The role of GABA(A) receptors in the acute and chronic effects of ethanol: A decade of progress. Psychopharmacology, 205(4), 529–564. https://doi.org./10.1007/s00213-009-1562-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Ren Q, Beckley JH, O’Buckley TK, Gigante ED, Santerre JL, … Morrow AL (2012). Ethanol activation of protein kinase a regulates GABA(A) receptor subunit expression in the cerebral cortex and contributes to ethanol-Induced hypnosis. Frontiers in Neuroscience, 6, 44 https://doi.org./10.3389/fnins.2012.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Suryanarayanan A, Boyd KN, Comerford CE, Lai MA, Ren Q, & Morrow AL (2010). Ethanol reduces GABAA alpha1 subunit receptor surface expression by a protein kinase Cgamma-dependent mechanism in cultured cerebral cortical neurons. Molecular Pharmacology, 77(5), 793–803. https://doi.org./10.1124/mol.109.063016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster FE (1998). Sex differences in voluntary drinking by Long Evans rats following early stress. Alcoholism: Clinical and Experimental Research, 22(4), 830–836. [PubMed] [Google Scholar]
- Leeman RF, Heilig M, Cunningham CL, Stephens DN, Duka T, & O’Malley SS (2010). Ethanol consumption: How should we measure it? achieving consilience between human and animal phenotypes. Addiction Biology, 15(2), 109–124. https://doi.org./10.1111/j.1369-1600.2009.00192.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz KM, Graham MD, Parada M, Fleming AS, Sengelaub DR, & Monks DA (2008). Tactile stimulation during artificial rearing influences adult function and morphology in a sexually dimorphic neuromuscular system. Developmental Neurobiology, 68(4), 542–557. https://doi.org./10.1002/dneu.20608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- León Rodríguez DA, & Dueñas Z (2013). Maternal separation during Breastfeeding induces gender-Dependent changes in anxiety and the GABA-A receptor alpha-Subunit in adult wistar rats. PLOS ONE, 8(6), e68010 https://doi.org./10.1371/journal.pone.0068010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S (1994). The ontogeny of the hypothalamic-Pituitary-Adrenal axis. the influence of maternal factorsa. Annals of the New York Academy of Sciences, 746(1), 275–288. https://doi.org./10.1111/j.1749-6632.1994.tb39245.x [DOI] [PubMed] [Google Scholar]
- Levy F, Melo AI, Galef BG Jr., Madden M, & Fleming AS (2003). Complete maternal deprivation affects social, but not spatial, learning in adult rats. Developmental Psychobiology, 43(3), 177–191. https://doi.org./10.1002/dev.10131 [DOI] [PubMed] [Google Scholar]
- Lobo IA, & Harris RA (2008). GABA(A) receptors and alcohol. Pharmacology Biochemistry and Behavior, 90(1), 90–94. https://doi.org./10.1016/j.pbb.2008.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue S, Chein J, Gould T, Holliday E, & Steinberg L (2014). Adolescent mice, unlike adults, consume more alcohol in the presence of peers than alone. Developmental Science, 17(1), 10.1111/desc.1210110.1111/desc.12101https://doi.org./10.1111/desc.12101https://doi.org./10.1111/desc.12101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, & Tanno AP (2002). Determination of the estrous cycle phases of rats: Some helpful considerations. Brazilian Journal of Biology, 62, 609–614. [DOI] [PubMed] [Google Scholar]
- Meyer EM, Long V, Fanselow MS, & Spigelman I (2013). Stress increases voluntary alcohol intake, but does not alter established drinking habits in a rat model of posttraumatic stress disorder. Alcoholism: Clinical and Experimental Research, 37(4), 566–574. https://doi.org./10.1111/acer.12012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CL, Dou H, & Juraska JM (1992). Maternal stimulation affects the number of motor neurons in a sexually dimorphic nucleus of the lumbar spinal cord. Brain Research, 572(1–2), 52–56. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Porcu P, Boyd KN, & Grant KA (2006). Hypothalamic-pituitary-adrenal axis modulation of GABAergic neuroactive steroids influences ethanol sensitivity and drinking behavior. Dialogues in Clinical Neuroscience, 8(4), 463–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MM, Brunelli SA, Shair HN, Squire JM, & Hofer MA (1989). Relationships between maternal behavior of SHR and WKY dams and adult blood pressures of cross-fostered F1 pups. Developmental Psychobiology, 22(1), 55–67. https://doi.org./10.1002/dev.420220105 [DOI] [PubMed] [Google Scholar]
- Naassila M, Ledent C, & Daoust M (2002). Low ethanol sensitivity and increased ethanol consumption in mice lacking adenosine A2A receptors. The Journal of Neuroscience, 22(23), 10487–10493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizhnikov ME, Popoola DO, & Cameron NM (2016). Transgenerational transmission of the effect of gestational ethanol exposure on ethanol use-related behavior. Alcoholism: Clinical and Experimental Research, 40(3), 497–506. https://doi.org./10.1111/acer.12978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylander I, & Roman E (2013). Is the rodent maternal separation model a valid and effective model for studies on the early-life impact on ethanol consumption? Psychopharmacology, 229(4), 555–569. https://doi.org./10.1007/s00213-013-3217-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of the Surgeon, G., National Institute on Alcohol, A., Alcoholism, Substance, A., & Mental Health Services, A (2007). Publications and Reports of the Surgeon General The Surgeon General’s Call to Action To Prevent and Reduce Underage Drinking. Rockville (MD): Office of the Surgeon General (US). [PubMed] [Google Scholar]
- Orchinik M, Weiland NG, & McEwen BS (1994). Adrenalectomy Selectively Regulates GABAA Receptor Subunit Expression in the Hippocampus. Molecular and Cellular Neuroscience, 5(5), 451–458. 10.1006/mcne.1994.1055 [DOI] [PubMed] [Google Scholar]
- Orchinik M, Weiland NG, & McEwen BS (1995). Chronic exposure to stress levels of corticosterone alters GABAA receptor subunit mRNA levels in rat hippocampus. Molecular Brain Research, 34(1), 29–37. 10.1016/0169-328X(95)00118-C [DOI] [PubMed] [Google Scholar]
- Orser BA (2006). Extrasynaptic GABAA receptors are critical targets for sedative-hypnotic drugs. Journal of Clinical Sleep Medicine, 2(2), S12–S18. [PubMed] [Google Scholar]
- Parker CC, Ponicsan H, Spencer RL, Holmes A, & Johnson TE (2008). Restraint stress and exogenous corticosterone differentially alter sensitivity to the sedative-hypnotic effects of ethanol in inbred long-sleep and inbred short-sleep mice. Alcohol (Fayetteville, N.Y.), 42(6), 477–485. https://doi.org./10.1016/j.alcohol.2008.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Nizhnikov ME, Fabio MC, & Spear NE (2012). Early maternal separation affects ethanol-induced conditioning in a nor-BNI insensitive manner, but does not alter ethanol-induced locomotor activity. Pharmacology, Biochemistry, and Behavior, 100(3), 630–638. https://doi.org./10.1016/j.pbb.2011.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena CJ, Neugut YD, Calarco CA, & Champagne FA (2014). Effects of maternal care on the development of midbrain dopamine pathways and reward-directed behavior in female offspring. European Journal of Neuroscience, 39(6), 946–956. https://doi.org./10.1111/ejn.12479 [DOI] [PubMed] [Google Scholar]
- Pena CJ, Neugut YD, & Champagne FA (2013). Developmental timing of the effects of maternal care on gene expression and epigenetic regulation of hormone receptor levels in female rats. Endocrinology, 154(11), 4340–4351. https://doi.org./10.1210/en.2013-1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñasco S, Mela V, López-Moreno JA, Viveros M-P, & Marco EM (2015). Early maternal deprivation enhances voluntary alcohol intake induced by exposure to stressful events later in life. Neural Plasticity, 2015, 342761 https://doi.org./10.1155/2015/342761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson P, Ellsinger BM, Sjoberg C, & Bjorntorp P (1990). Fat distribution and steroid hormones in women with alcohol abuse. Journal of Internal Medicine, 228(4), 311–316. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, & Sperk G (2000). GABA(A) receptors: Immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience, 101(4), 815–850. [DOI] [PubMed] [Google Scholar]
- Popoola DO, Borrow AP, Sanders JE, Nizhnikov ME, & Cameron NM (2015). Can low-level ethanol exposure during pregnancy influence maternal care? An investigation using two strains of rat across two generations. Physiology and Behavior, 148, 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoola DO, Nizhnikov ME, & Cameron NM (2017). Strain-specific programming of prenatal ethanol exposure across generations. Alcohol, 60, 191–199. https://doi.org./10.1016/j.alcohol.2017.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior KM, Meaney MJ, & Cameron NM (2013). Variations in maternal care associated with differences in female rat reproductive behavior in a group-mating environment. Developmental Psychobiology, 55(8), 838–848. [DOI] [PubMed] [Google Scholar]
- Pritchard LM, Hensleigh E, & Lynch S (2012). Altered locomotor and stereotyped responses to acute methamphetamine in adolescent, maternally separated rats. Psychopharmacology, 223(1), 27–35. https://doi.org./10.1007/s00213-012-2679-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanilla ME (1999). Effect of low doses of ethanol on spontaneous locomotor activity in UChB and UChA rats. Addiction Biology, 4(4), 443–448. https://doi.org./10.1080/13556219971434 [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, & Crabbe JC (2005). Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiology and Behavior, 84(1), 53–63. https://doi.org./10.1016/j.physbeh.2004.10.007 [DOI] [PubMed] [Google Scholar]
- Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, & Brewer RD (2015). 2010 national and state costs of excessive alcohol consumption. American Journal of Preventive Medicine, 49(5), e73–e79. https://doi.org./10.1016/j.amepre.2015.05.031 [DOI] [PubMed] [Google Scholar]
- Saeed DM (2006). Co-modulation of acute ethanol-induced motor impairment by mouse cerebellar adenosinergic A1 and GABAA receptor systems. Brain Research Bulletin, 71(1–3), 287–295. https://doi.org./10.1016/j.brainresbull.2006.09.016 [DOI] [PubMed] [Google Scholar]
- Samson HH (1986). Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcoholism: Clinical and Experimental Research, 10(4), 436–442. [DOI] [PubMed] [Google Scholar]
- Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, & Biggio G (2004). Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. The Journal of Neuroscience, 24(29), 6521–6530. https://doi.org./10.1523/jneurosci.0075-04.%202004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santerre JL, Gigante ED, Landin JD, & Werner DF (2014). Molecular and behavioral characterization of adolescent protein kinase C following high dose ethanol exposure. Psychopharmacology, 231(8), 1809–1820. https://doi.org./10.1007/s00213-013-3267-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, DiFeliceantonio A, Foscue E, Glowacz S, Haseeb N, Wang N, … Kuhn CM (2010). Aversive Effects of Ethanol in Adolescent vs. Adult Rats: Potential causes and implication for future drinking. Alcoholism, Clinical, and Experimental Research, 34(12), 2061–2069. https://doi.org./10.1111/j.1530-0277.2010.01302.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serlin H, & Torregrossa MM (2015). Adolescent rats are resistant to forming ethanol seeking habits. Developmental Cognitive Neuroscience, 16, 183–190. https://doi.org./10.1016/j.dcn.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, & Spear LP (1998). Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcoholism: Clinical and Experimental Research, 22(3), 670–676. https://doi.org./10.1111/j.1530-0277.1998.tb04310.x [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, & Bartlett SE (2008). Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcoholism: Clinical and Experimental Research, 32(10), 1816–1823. https://doi.org./10.1111/j.1530-0277.2008.00753.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R (2008). Chronic stress, drug use, and vulnerability to addiction. Annals of the New York Academy of Sciences, 1141, 105–130. https://doi.org./10.1196/annals.1441.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, & Zehr JL (2005). Pubertal hormones organize the adolescent brain and behavior. Frontiers in Neuroendocrinology, 26(3–4), 163–174. https://doi.org./10.1016/j.yfrne.2005.10.003 [DOI] [PubMed] [Google Scholar]
- Smith SS, Shen H, Gong QH, & Zhou X (2007). Neurosteroid regulation of GABAA receptors: Focus on the α4 and δ subunits. Pharmacology and Therapeutics, 116(1), 58–76. https://doi.org./10.1016/j.pharmthera.2007.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Noori HR, & Heilig M (2014). Stress and alcohol interactions: Animal studies and clinical significance. Trends in Neuro-sciences, 37(4), 219–227. https://doi.org./10.1016/j.tins.2014.02.006 [DOI] [PubMed] [Google Scholar]
- Spear LP (2013). Adolescents and alcohol. Current Directions in Psychological Science, 22(2), 152–157. https://doi.org./10.1177/0963721412472192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP (2016). Consequences of adolescent use of alcohol and other drugs: Studies using rodent models. Neuroscience and Biobehavioral Reviews, 70, 228–243. https://doi.org./10.1016/j.neubiorev.2016.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JM (1996). Somatosensation and maternal care in Norway rats. Advances in the Study of Behavior, 25, 243–294. https://doi.org./10.1016/S0065-3454(08)60335-6 [Google Scholar]
- Suchecki D, Rosenfeld P, & Levine S (1993). Maternal regulation of the hypothalamic-pituitary-adrenal axis in the infant rat: The roles of feeding and stroking. Brain Research. Developmental Brain Research, 75(2), 185–192. [DOI] [PubMed] [Google Scholar]
- Szabadi E (2009). Introduction to neuropsychopharmacology. British Journal of Clinical Pharmacology, 68(6), 965–965. https://doi.org.10.1111/j.1365-2125.2009.03529.x [Google Scholar]
- Thiele TE, Willis B, Stadler J, Reynolds JG, Bernstein IL, & McKnight GS (2000). High ethanol consumption and low sensitivity to ethanol-Induced sedation in protein kinase A-Mutant mice. The Journal of Neuroscience, 20(10), RC75–RC75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda MC, Yamaguchi N, & Ogawa S (2011). Early life stress disrupts peripubertal development of aggression in male mice. Neuroreport, 22(6), 259–263. https://doi.org./10.1097/WNR.0b013e328344495a [DOI] [PubMed] [Google Scholar]
- Uriarte N, Breigeiron MK, Benetti F, Rosa XF, & Lucion AB (2007). Effects of maternal care on the development, emotionality, and reproductive functions in male and female rats. Developmental Psychobiology, 49(5), 451–462. https://doi.org./10.1002/dev.20241 [DOI] [PubMed] [Google Scholar]
- van Oers HJJ, de Kloet ER, Whelan T, & Levine S (1998). Maternal deprivation effect on the infant’s neural stress markers is reversed by tactile stimulation and feeding but not by suppressing corticosterone. The Journal of Neuroscience, 18(23), 10171–10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, & Morrow AL (2000). Neuroactive steroid 3α-Hydroxy-5α-Pregnan-20-One modulates electrophysiological and behavioral actions of ethanol. The Journal of Neuroscience, 20(5), 1982–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen CS, Sanders KW, & Spear LP (2011). Evidence for suppressant effects of testosterone on sex-typical ethanol intake in male Sprague-Dawley rats. Behavioural Brain Research, 224(2), 403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen CS, & Spear LP (2012). Hormonal and physical markers of puberty and their relationship to adolescent-typical novelty-directed behavior. Developmental Psychobiology, 54(5), 523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, & Olsen RW (2006). Low dose acute alcohol effects on GABA(A) receptor subtypes. Pharmacology and Therapeutics, 112(2), 513–528. https://doi.org./10.1016/j.pharmthera.2006.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker VP, Michaelson IA, & Kirkland RJ (1963). The separation of synaptic vesicles from disrupted nervending particles. Biochemical Pharmacology, 12, 300–302. [DOI] [PubMed] [Google Scholar]
- Whittaker VP, Michaelson IA, & Kirkland RJ (1964). The separation of synaptic vesicles from nerve-ending particles (‘synaptosomes’). Biochemical Journal, 90(2), 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, & Finn DA (2008). Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol (Fayetteville, N.Y.), 42(3), 149–160. https://doi.org./10.1016/j.alcohol.2007.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheleznova N, Sedelnikova A, & Weiss DS (2008). α1β2δ, a silent GABAA receptor: Recruitment by tracazolate and neurosteroids. British Journal of Pharmacology, 153(5), 1062–1071. https://doi.org./10.1038/sj.bjp.0707665 [DOI] [PMC free article] [PubMed] [Google Scholar]