Abstract
The demonstration of tyrosine kinase inhibitors (TKIs) in treating chronic myeloid leukemia (CML) has heralded a new era in cancer therapeutics. However, a small population of cells does not respond to TKI treatment, resulting in minimal residual disease (MRD); even the most potent TKIs fail to eradicate these cells. These MRD cells serve as a reservoir to develop resistance to therapy. Why TKI treatment is ineffective against MRD cells is not known. Growth factor signaling is implicated in supporting the survival of MRD cells during TKI treatment, but a mechanistic understanding is lacking. Recent studies demonstrated that an elevated c-Fos and Dusp1 expression as a result of convergent oncogenic and growth factor signaling in MRD cells mediate TKI resistance. The genetic and chemical inhibition of c-Fos and Dusp1 renders CML exquisitely sensitive to TKIs and cures CML in both genetic and humanized mouse models. We identified these target genes using multiple microarrays from TKI-sensitive and -resistant cells. Here, we provide methods for target validation using in vitro and in vivo mouse models. These methods can easily be applied to any target for genetic validation and therapeutic development.
Keywords: Cancer Research, Issue 143, tyrosine kinase inhibitors, chronic myeloid leukemia, minimal residual disease, tyrosine kinase, leukemic stem cells, oncogene addiction, therapy resistance, bone marrow transplant, mouse models
Introduction
Constitutive tyrosine kinase activity of BCR-ABL1 fusion oncogene causes CML, which provides a rationale to target the kinase activity by small molecule inhibitors. The success of TKIs in treating CML patients revolutionized the concept of targeted therapy1,2. Subsequently, anti-kinase therapy as precision medicine was developed for several other malignancies, including solid tumors. So far, more than thirty kinase inhibitors have been approved by the United State FDA for treating various malignancies. While TKI treatment is very effective in suppressing the disease, it is not curative. Besides, a small population of cancer cells persists during the treatment: the MRD3,4,5. Even patients who showed complete remission are left with MRD, which eventually results in relapse if not continuously suppressed. Therefore, the eradication of MRD cells is needed to achieve a durable or curative response. CML represents a valuable paradigm for defining the concept of precision medicine, mechanisms of oncogenesis, rational target-directed therapeutics, disease progression, and drug resistance. However, even today, the mechanism driving TKI-induced cell death in cancer cells is not fully understood, nor why MRD cells (comprised of leukemic stem cells [LSCs]) are intrinsically resistant to TKIs4,6. Nonetheless, the phenomenon of “oncogene dependence” to mutant kinase oncoprotein is implicated in TKI efficacy where the acute inhibition of targeted oncogene by TKIs causes an oncogenic shock that leads to a massive proapoptotic response or quiescence in cell context-dependent manner6,7,8,9. However, the mechanistic underpinning of oncogene dependence is lacking. Recent studies have implicated that growth factor signaling abrogates oncogene dependence and consequently confers resistance to TKI therapy10,11,12. Therefore, to gain insight into the mechanism of oncogene dependence, we performed whole-genome expression profiling from BCR-ABL1 addicted and nonaddicted cells (grown with growth factors), which revealed that c-Fos and Dusp1 are critical mediators of oncogene addiction13. The genetic deletion of c-Fos and Dusp1 is synthetic lethal to BCR-ABL1-expressing cells and the mice used in the experiment did not develop leukemia. Moreover, the inhibition of c-Fos and DUSP1 by small molecule inhibitors cured BCR-ABL1-induced CML in mice. The results show that the expression levels of c-Fos and Dusp1 define the apoptotic threshold in cancer cells, such that lower levels confer drug sensitivity while higher levels cause resistance to therapy13.
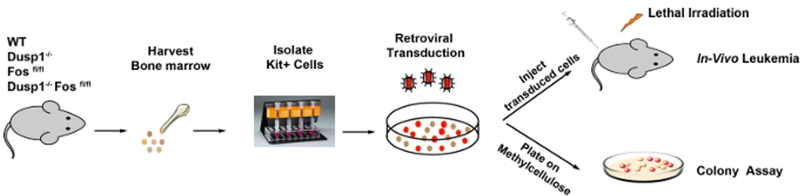
To identify the genes driving the oncogene dependence, we performed several whole-genome expression profiling experiments in the presence of growth factor and a TKI (imatinib) using both mouse- and CML patient-derived cells (K562). These data were analyzed in parallel with CML patient data sets obtained from CD34+ hematopoietic stem cells before and after treatment with imatinib. This analysis revealed three genes (a transcription factor [c-Fos], dual specificity phosphatase 1 [Dusp1], and an RNA-binding protein [Zfp36]) which are commonly upregulated in TKI-resistant cells. To validate the significance of these genes in conferring drug resistance, we carried out step-by-step in vitro and in vivo analysis. The expression levels of these genes were confirmed by real-time qPCR (RT-qPCR) and western blotting in drug-resistant cells. Further, cDNA overexpression and knockdown by shRNA hairpins of c-Fos, Dusp1, and Zfp36 revealed that elevated c-Fos and Dusp1 expressions are sufficient and necessary to confer TKI resistance. Therefore, we performed an in vivo validation using mouse models with c-Fos and Dusp1 only. For the genetic validation of c-Fos and Dusp1, we created ROSACreERT-inducible c-Fosfl/fl mice (conditional knockout)14 and crossed them with Dusp1−/− (straight knockout)15 to make ROSACreERT2-c-Fosfl/flDusp1−/− double-transgenic mice. Bone marrow-derived c-Kit+ cells (from c-Fosfl/fl-, Dusp1−/−-, and c-Fosfl/flDusp1−/−) expressing BCR-ABL1 were analyzed in vitro in a colony-forming unit (CFU) assay, and in vivo by bone marrow transplantation in lethally irradiated mice, to test the requirement of c-Fos and Dusp1 alone or together in leukemia development. Likewise, the chemical inhibitions of c-Fos by DFC (difluorinated curcumin)16 and Dusp1 by BCI (benzylidene-3-(cyclohexylamino)-2,3-dihydro-1H-inden-1-one)17 were tested in vitro and in vivo using BCR-ABL1-expressing, bone marrow-derived c-Kit+ cells from the wild-type (WT) mouse. To confirm the requirement of c-Fos and Dusp1 in leukemic stem cells, we utilized a CML mouse model where BCR-ABL1 was specifically induced in its stem cells by doxycycline (Tet-transactivator expresses under murine stem cell leukemia (SCL) gene 3’ enhancer regulation)18,19. We used bone marrow Lin−Sca+c-Kit+ (LSK) cells from these mice in an in vivo transplant assay. Furthermore, we established phopsho-p38 levels and the expression of IL-6 as pharmaco-dynamic biomarkers for Dusp1 and c-Fos inhibition, respectively, in vivo. Finally, to extend the study for human relevance, patient-derived CD34+ cells (equivalent to the c-Kit+ cells from mice) were subjected to long-term in vitro culture-initiating cell assays (LTCIC) and an in vivo humanized mouse model of CML20,21. The immunodeficient mice were transplanted with CML CD34+ cells, followed by drug treatment and analysis of human leukemic cell survival.
In this project, we develop methods for target identification and validations using both genetic and chemical tools, using different preclinical models. These methods can be successfully applied to validate other targets developing chemical modalities for therapeutic development.
Protocol
All animal experiments were carried out according to the guidelines of the Institutional Animal Care and Use Committee (IACUC) at Cincinnati Children’s Hospital Medical Center (CCHMC). Human specimens (Normal BM and that from CML (p210-BCR-ABL+) leukemia) were obtained through Institutional Review Board-approved protocols (Institutional Review Board: Federalwide Assurance #00002988 Cincinnati Children’s Hospital Medical Center) and donor-informed consent from CCHMC and the University of Cincinnati.
1. Real-time qPCR Analysis
Isolate total RNA from BaF3 cells growing in RPMI supplemented with 10% FBS and 10% WEHI spent culture medium as a source of interleukin-3 (IL-3).
Harvest the cells at logarithmic phase (99% alive) from treated (IL-3 and imatinib) as well as from untreated samples, and centrifuge them at 435 × g for 3 min. The health and the growth stages of the cells are critical as their gene expression profiles can change dramatically.
Isolate RNA by purifying total RNA22. Quantify the total RNA; then, subject it to DNase treatment23. Convert equal amounts of DNA-free RNA (2 μg) to cDNA with reverse transcriptase24.
Perform qPCRs with the gene-specific primers (Table 1). Carry out PCRs in 20 μL reactions in 0.2 mL, thin-walled PCR tubes with optical caps, using a 1× polymerase mix (see Table of Materials), 0.5 μM of each primer, 1 μL of DNA, and water. Place the reaction in the thermocycler with the following cycle: Step 1, 95 °C for 10 min; Step 2, 95 °C for 15 s; Step 3 at 60 °C for 1 min; Step 4, repeat steps 2–3 40 times. Perform all PCRs in triplicates and normalize the real-time data to the β-actin expression before plotting.
Materials
| Name | Company | Catalog Number | Comments |
|---|---|---|---|
| Biological Materials | |||
| RPMI | Cellgro (corning) | 15-040-CV | |
| DMEM | Cellgro (corning) | 15-013-CV | |
| IMDM | Cellgro (corning) | 15-016-CVR | |
| RetroNectin Recombinant Human Fibronectin Fragment | Takara | T100B | |
| MethoCult GF M3434 (Methylcellulose for Mouse CFU) | Stem Cell | 3434 | |
| MethoCult H4434 Classic (Methylcellulose for Human CFU) | Stem Cell | 4434 | |
| 4-Hydroxytamoxifen | Sigma | H6278 | |
| Recombinant Murine SCF | Prospec | CYT-275 | |
| Recombinant Murine Flt3-Ligand | Prospec | CYT-340 | |
| Recombinant Murine IL-6 | Prospec | CYT-350 | |
| Recombinant Murine IL-7 | Peprotech | 217-17 | |
| DFC | LKT Laboratories Inc. | D3420 | |
| BCI | Chemzon Scientific | NZ-06-195 | |
| Imatinib | LC Laboratory | I-5508 | |
| Curcumin | Sigma | 458-37-7 | |
| NDGA | Sigma | 500-38-9 | |
| Penn/Strep | Cellgro (corning) | 30-002-CI | |
| FBS | Atlanta biological | S11150 | |
| Trypsin EDTA 1× | Cellgro (corning) | 25-052-CI | |
| 1× PBS | Cellgro (corning) | 21-040-CV | |
| L-Glutamine | Cellgro (corning) | 25-005-CL | 5 mg/mL stock in water |
| Puromycin | Gibco (life technologies) | A11138-03 | |
| HEPES | Sigma | H7006 | |
| Na2HPO4.7H2O | Sigma | S9390 | |
| Protamine sulfate | Sigma | P3369 | 5 mg/mL stock in water |
| Trypan Blue solution (0.4%) | Sigma | T8154 | |
| DMSO | Cellgro (corning) | 25-950-CQC | |
| WST-1 | Roche | 11644807001 | |
| 0.45 μM acro disc filter | PALL | 2016-10 | |
| 70 μm nylon cell stariner | Becton Dickinson | 352350 | |
| FICOL (Histopaque 1083) (polysucrose) | Simga | 1083 | |
| PBS | Corning | 21040CV | |
| LS Columns | Miltenyi | 130-042-401 | |
| Protease Inhibitor Cocktail | Roche | CO-RO | |
| Phosphatase Inhibitor Cocktail 2 | Sigma | P5762 | |
| Nitrocullulose Membrane | Bio-Rad | 1620115 | |
| SuperSignal West Dura Extended Duration Substrate (chemiluminiscence substrate) | Thermo Scientific | 34075 | |
| CD5 | eBioscience | 13-0051-82 | |
| CD11b | eBioscience | 13-0112-75 | |
| CD45R (B220) | BD biosciences | 553092 | |
| CD45.1-FITC | eBioscience | 11-0453-85 | |
| CD45.2-PE | eBioscience | 12-0454-83 | |
| hCD45-FITC | BD Biosciences | 555482 | |
| Anti-Biotin-FITC | Miltenyi | 130-090-857 | |
| Anti-7-4 | eBioscience | MA5-16539 | |
| Anti-Gr-1 (Ly-6G/c) | eBioscience | 13-5931-82 | |
| Anti-Ter-119 | eBioscience | 13-5921-75 | |
| Ly-6 A/E (Sca1) PE Cy7 | BD | 558612 | |
| CD117 APC | BD | 553356 | |
| BD Pharm Lyse | BD | 555899 | |
| BD Cytofix/Cytoperm (Fixing and permeabilization solution) | BD | 554714 | |
| BD Perm/Wash (permeabilization and wash solution for phospho flow) | BD | 554723 | |
| phospho p38 | Cell Signaling Technologies | 4511S | |
| total p38 | Cell Signaling Technologies | 9212 | |
| Mouse IgG control | BD | 554121 | |
| Alexa Flour 488 conjugated | Invitrogen | A-11034 | |
| Calcium Chloride | Invitrogen | K278001 | |
| 2× HBS | Invitrogen | K278002 | |
| EDTA | Ambion | AM9261 | |
| BSA | Sigma | A7906 | |
| Blood Capillary Tubes | Fisher | 22-260-950 | |
| Blood Collection Tube | Giene Bio-One | 450480 | |
| Newborn Calf Serum | Atlanta biological | S11295 | |
| Erythropoiein | Amgen | 5513-267-10 | |
| human SCF | Prospec | CYT-255 | |
| Human IL-3 | Prospec | CYT-210 | |
| G-SCF | Prospec | CYT-220 | |
| GM-CSF | Prospec | CYT-221 | |
| MyeloCult (media for LTCIC assay) | Stem Cell Technologies | 5100 | |
| Hydrocortisone Sodium Hemisuccinate | Stem Cell Technologies | 7904 | |
| MEM alpha | Gibco | 12561-056 | |
| 1/2 cc Lo-Dose u-100 insulin syringe 28 G1/2 | Becton Dickinson | 329461 | |
| Mortor pestle | Coor tek | 60316 and 60317 | |
| Isoflorane (Isothesia TM) | Butler Schien | 29405 | |
| SOC | New England Biolabs | B90920s | |
| Ampicillin | Sigma | A0166 | 100 mg/mL stock in water |
| Bacto agar (agar) | Difco | 214050 | |
| Terrific broth | Becton Dickinson | 243820 | |
| Agarose | Genemate | E-3119-500 | |
| Doxycycline chow | TestDiet.com | 52662 | modified RMH1500, Autoclavable 5LK8 with 0.0625% Doxycycline |
| Tamoxifen | Sigma | T5648 | |
| Iodonitrotetrazolium chloride | Sigma | 110406 | |
| Kits | |||
| Dneasy Blood & tissue kit | Qiagen | 69506 | |
| GoTaq Green (taq polymerase with Green loadign dye) | Promega | M1722 | |
| miRNeasy Mini Kit (RNA isolation kit) | Qiagen | 217084 | |
| DNA Free Dnase Kit (DNAse treatment for RT PCR) | Ambion, Life Technologies | AM1906 | |
| Superscript III First Strand Synthesis (reverse transcriptase for cDNA synthesis) | Invitrogen | 18080051 | |
| SYBR Green (taq polymerase mix with green interchalating dye for qPCR) | Bio-Rad | 1725270 | |
| CD117 MicroBead Kit | Miltenyi | 130-091-224 | |
| Human Long-Term Culture Initiating Cell Assay | Stemp Cell Technologies | ||
| Instruments | |||
| NAPCO series 8000 WJ CO2 incubator | Thermo scientific | ||
| Swing bucket rotor cetrifuge 5810R | Eppendorf | ||
| TC-10 automated cell counter | Bio-RAD | ||
| C-1000 Thermal cycler | Bio-RAD | ||
| Mastercycler Real Plex 2 | Eppendorf | ||
| ChemiDoc Imaging System (imaging system for gels and western blots) | Bio-RAD | 17001401 | |
| Hemavet (boold counter) | Drew-Scientific | ||
| LSR II (FACS analyzer) | BD | ||
| Fortessa I (FACS analyzer) | BD | ||
| FACSAriaII (FACS Sorter) | BD | ||
| Magnet Stand | Miltenyi | ||
| Irradiator | J.L. Shepherd and Associates, San Fernando CA | Mark I Model 68A | source Cs 137 |
| Mice | |||
| ROSACreERT2 | Jackson Laboratory | ||
| Scl-tTA | Dr. Claudia Huettner’s lab | ||
| BoyJ | mouse core facility at CCHMC | ||
| C57Bl/6 | Jackson Laboratory | ||
| NSGS | mouse core facility at CCHMC | ||
| ROSACreERT2/c-Fosfl/fl Dusp1−/− | Made in house | ||
| ROSACreERT2/c-Fosfl/fl | Made in house | ||
| Cells | |||
| BaF3 | Gift from George Daley, Harvard Medical School, Boston | ||
| WEHI | Gift from George Daley, Harvard Medical School, Boston | ||
| CML-CD34+ and Normal CD34+ cells | University Hospital, University of Cincinnati |
2. Western Blotting
NOTE: Whole-cell extracts were prepared by adding 250 μL of 1× lysis buffer as described in Kesarwani et al.13 supplemented with a protease inhibitor cocktail, and phosphatase inhibitor cocktail 2.
Harvest five million BaF3 cells at logarithmic phase (99% alive) from treated (IL-3 and imatinib) as well as from untreated BaF3 cells by centrifugation at 435 × g for 3 min at 4 °C. Lyse the cells in the lysis buffer (250 μL) by pipetting up and down 1×, followed by a 5 min incubation at 80 °C. Sonicate the lysate to break down all the DNA with 5–6 pulses of 5 s each in a cold room at 4 °C.
Transfer the lysate to a 1.5 mL tube and centrifuge it at 16,000 × g for 5 min to remove any insoluble material. The extract can be stored at −80 °C until use. Heat samples to 80 °C for 5 min in a heat block before loading. Load 10 μL of the sample using a gel-loading tip on 10% SDS-PAGE gel.
Resolve the proteins at 150 V until the reference dye reaches the bottom of the gel. Transfer the protein to nitrocellulose membranes by an electrophoretic transfer at 1 amp for 1 h. After the transfer, block the membranes in 1× Tris-buffered saline + 0.1% Tween 20 (TBST) with 5% non-fat milk for 1 h and probe overnight at 4 °C with the appropriate antibodies at a 1:1,000 dilution.
Wash the membranes 3× to 5× with TBST for 10 min each; then, probe with an HRP-conjugated appropriate secondary antibody (1:5,000 dilution) at room temperature for 1 h. The amount of TBST used depends on the container size. Make sure to submerge the membrane in TBST completely. Wash the secondary antibody 3× for 5 min each with TBST.
Develop the blot using chemiluminescent substrate reagent. Mix equal volumes of the substrate and the buffer from the two bottles, right before use. Add the substrate to the top of the membrane to cover the whole membrane with a 1 mL pipette and incubate for 1 min. The blots should be imaged within 1–10 min of adding the substrate.
Using blunt forceps, carefully place the membrane on the platform of the imaging system (see Table of Materials), avoiding much of the liquid. Select a live view and choose blots and chemiluminiscence from the menu. Using manual acquisition, acquire multiple exposures at different time points. These files can be visualized and quantified using imaging software.
3. Generation of Knockout Mice
Cross c-Fosfl/fl mice with ROSACreERT2 mice to generate ROSACreERT2c-Fosfl/fl mice13. To create the double-knockout (KO) mice, breed ROSACreERT2c-Fosfl/fl mice with Dusp1−/− mice to generate ROSACreERT2c-Fosfl/fl Dusp1−/− mice13. Genotype the mice by tail clips followed by PCR, as described below.
- Genotyping:
- To prepare the DNA, clip a tiny portion of the tail with scissors and put it into a 1.5 mL tube. Cauterize the tail with heated scissors. Add 200 μL of 50 mM NaOH to the clipped tail in the tube and incubate at 95 °C in a heat block for 1 h.
- Vortex the tube well and, then, neutralize by adding 50 μL of 1 M Tris HCl pH 6.8 and vortex again. Centrifuge the tube at maximum speed for 1 min.
- Carry out PCRs in 20 μL reactions in 0.2 mL, thin-walled PCR tubes using 1× Taq polymerase master mix containing dye, 0.5 μM of each primer, 1 μL of DNA, and water. Place the tubes in the thermocycler and run the appropriate cycles as shown in Table 2.
- Run the PCR product on 2% agarose gel and image using a gel documentation system.
4. Isolation of c-Kit+ Cells from Bone Marrow
-
Sacrifice the mice according to institutional guidelines.
NOTE: Here, the mice were exposed to carbon dioxide (CO2) at an according to the AVMA recommended flow rate until death was determined by the cessation of respiration and heartbeat, followed by cervical dislocation.
Harvest the legbones from the mouse — two femurs, two tibias, two iliacs. Clean off all tissues from the bones by scraping away with a scalpel. Put the leg bones in cold 1× PBS on ice — 5 mL in a 15 mL tube. Pour the contents of the tube into a mortar and crush it with a pestle.
Filter the cell suspension through a 70 μm cell strainer into a 50 mL screw cap tube with a 10 mL pipette attached, using a pipette. Wash the mortar and pestle multiple times with cold 1× PBS, collecting and adding cell suspension to the 50 mL tube. Spin down bone marrow at 1,200 × g at 4 °C for 5 min. Remove the supernatant via a vacuum with a glass pipette attached. Suspend the cell pellet in 5 mL of 1× PBS with a pipette.
With a pipette, slowly add suspended bone marrow to the top of 5 mL of room temperature (the temperature is critical) polysucrose, taking great care not to disturb the interface. Spin at 400 × g at room temperature for 20 min with the brake turned off.
Collect the cloudy total mono-nuclear cell (TMNC) interface with a pipette and put it into a new 15 mL tube. Bring the volume up to 10 mL with 1× PBS for the wash, taking 20 μL for counting, and spin at 1,200 × g at 4 °C for 5 min. Discard the supernatant and save the pellet on ice for step 4.6.1.
- Perform c-Kit+ cell isolation.
- Follow a microbead kit protocol for the c-Kit+ cell isolation. Resuspend the cell pellet in 80 μL of 1× PBS + EDTA + BSA to 107 cells. To the cell suspension, add 20 μL of CD117 MicroBeads/107 total cells. Mix well by vortexing and incubate for 10 min at 4 °C. Bring the volume up to 10 mL by adding 1× PBS + EDTA + BSA and spin at 1,200 × g at 4 °C for 5 min.
- Remove the supernatant via a vacuum and suspend the cell pellet in 500 μL/108 total cells in 1× PBS + EDTA + BSA. Using the magnet stand with the magnetic column attached, add 3 mL of 1× PBS + EDTA + BSA and allow it to flow through via gravity into a 15 mL collection tube.
- Once the first buffer addition has finished passing through the column, add the cell suspension to the column, allowing it to flow through via gravity into the 15 mL collection tube. This flowthrough is the undesired cell fraction.
- Wash the column 3 times with 3 mL 1× PBS + EDTA + BSA each time, allowing it to flow through via gravity into the collection tube. Remove the column from the magnet and place onto the top of a 15 mL tube.
- Add 5 mL of 1× PBS + EDTA + BSA and immediately flush it with the plunger provided with the column. Remove the plunger and repeat the flush with an additional 5 mL of 1× PBS + EDTA + BSA. Count the cells in the total volume using standard methods.
- Spin down the cells at 1,200 × g at 4 °C for 5 min. Remove the supernatant and resuspend the pellet in complete IMDM (IMDM + 10% FBS + IL-6 [10 ng/mL] + mSCF [50 ng/mL] + FLT3 ligand [20 ng/mL] + IL-3 [10 ng/mL]) for culture. Culture these cells overnight in 2 × 106 cells/1 mL of cell IMDM complete media by placing them in an incubator at 37 °C and 5% CO2.
5. Transduction
- Retroviral plasmid transfection
- Freshly thaw HEK293 cells the afternoon before in a water bath set to 37 °C. Spin the cryotube containing the HEK cells at 435 × g at room temperature for 3 min. Remove the supernatant via a vacuum and suspend the cell pellet in 1 mL of DMEM supplemented with 10% FBS, penicillin, streptomycin, and glutamine.
- Count the cells and add the appropriate volume to a 10 cm treated dish (~4 × 106 HEK293T cells). Add 14 mL of the DMEM 10 media to the dish and mix it well by sliding up and down for an equal distribution. Place the dish in a 37 °C, 5% CO2 incubator overnight.
- On the next morning, check the confluence of the cells under an inverted light microscope (50% or more works well).
- Combine the DNA (desired retroviral plasmid), PclEco (packaging plasmid), 2 M CaCl2, and H20 in a 1.5 mL tube (= 500 μL) using a micropipette in the following amounts: 7.5 μg of DNA (BCR-ABL1-YFP), 7.5 μg of pCLeco, 62 μL of 2 M CaCl2, and water to a final volume of 500 μL. Add 500 μL of 2× HBS to another 1.5 mL tube.
- Add the DNA mix dropwise to the 1.5 mL tube containing 500 μL of 2× HBS (280 mM NaCl, 100 mM HEPES, and 1.5 mM Na2HPO4) while mixing. Achieve this by setting a vortex to the lowest speed and hold the HBS tube on it for constant mixing while adding the transfection mix.
- Allow the transfection mix to incubate for 20–30 min at room temperature. During the incubation, add 25 nM chloroquine to the HEK cells and place them back in the incubator. After the incubation, add the transfection mix dropwise to the HEK cells and, then, gently swirl the media to mix the transfection mix throughout the plate.
- Incubate the cells with the mix for ~8 h in a 37 °C, 5% CO2 incubator. Change the media on these cells by very slowly adding 14 mL of fresh DMEM 10 media. Pipetting slowly against the wall of the dish helps to prevent any stripping of the HEK cells. Incubate the cells overnight in the 37 °C, 5% CO2 incubator.
- Collect the viral supernatant with a 10 mL syringe, draw the supernatant into the syringe, and attach the 0.45 μm syringe filter. Plunge the viral supernatant into a new collection tube. Take the first collection of viral supernatant ~16 h later.
- Fibronectin viral concentration and transduction
- Add 2 mL of a 0.1 mg recombinant human fibronectin fragment in 1× PBS to untreated 6-well culture plates. Prepare as much fibronectin as needed, which depends on the number of cells and genotypes. One well can sufficiently transduce 10 million c-Kit+ cells. Incubate the plate overnight at 4 °C.
- The next day, remove the fibronectin from the wells with a pipette, pipette 2 mL of sterile 2% BSA in 1× PBS to block the plate, and incubate for 30 min at room temperature. Remove the BSA via a vacuum and wash thoroughly by pipetting 2 mL of 1× PBS then remove it via a vacuum.
- Immediately add freshly collected and filtered (at 0.45 μm) viral supernatant up to 5 mL. Centrifuge at 435 × g at 32 °C for 2 h. Remove the supernatant via a vacuum and repeat this step with additional freshly collected viral supernatant and spin again. Remove the supernatant via a vacuum and wash the wells by adding 2 mL of 1× PBS; then, remove it via a vacuum.
- Add the c-Kit+ mouse cells that were cultured overnight (step 4.6.6) to the viral coated wells by mixing the cell suspensions well and pipetting them into the now viral concentrated fibronectin plate. Spin at 1,200 × g at 25 °C for 90 min. Put the cells in a 37 °C, 5% CO2 incubator.
- 48 h posttransduction, pellet the cells by centrifugation at 1,200 × g at 4 °C for 5 min and resuspend them in FACS buffer #1 (1× PBS + 0.5% BSA) to 6 × 106/mL. Determine the percentage of BCR-ABL1 positive cells by measuring the percentage of YFP positive using flow cytometry.
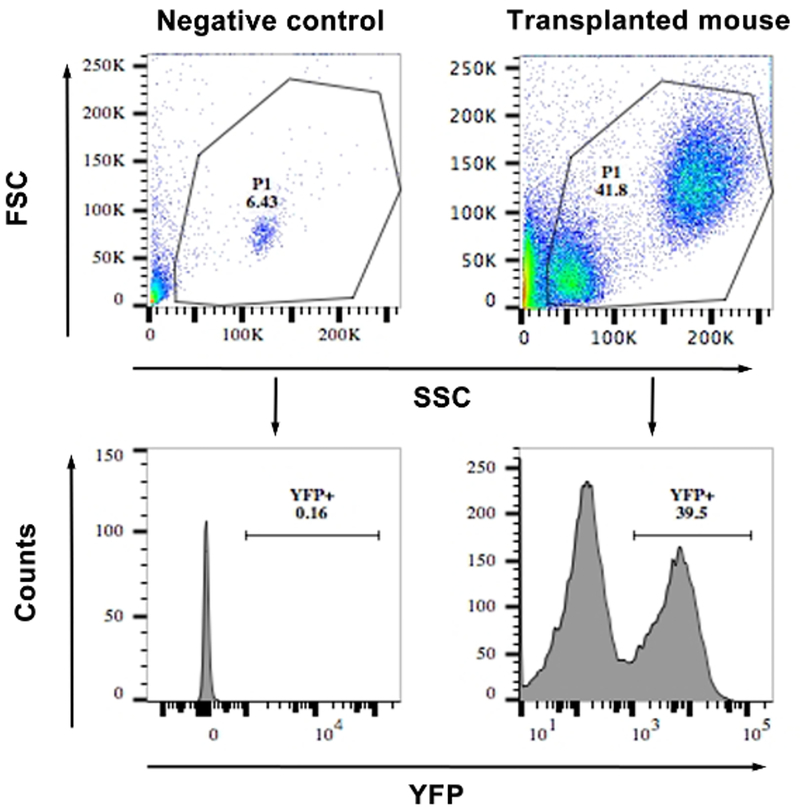
- Acquire events with the standard procedure. First, gate the live cells based on forward and side scatter. Then, gate the YFP positive live cells excluding the YFP-negativecells determined by negative control (Figure 4).
Figure 4: FACS showing the frequency of YFP+ cells from the blood of a transplanted and a WT mouse (negative control).
The top panels show a scatter plot of the cells and the gating. The lower panels show histograms of YFP vs. side scatter. Cells with an MFI of >103 were considered YFP+. Similar gating was used for bone marrow cells after transduction for calculating the percentage transduction.
6. Colony-forming UnitAssays
Thaw the methylcellulose with cytokines for mouse at room temperature for 1–2 h. Aliquot 3 mL of methylcellulose in a FACS tube using a 5 mL syringe without a needle. Sort the YFP-positive cells on a cell sorter.
Spin the cells down and suspend the pellet in IMDM complete media. Count the cells to be plated and dilute them to obtain 5,000 YFP-positive cells in 100 μL of IMDM complete media.
Add 100 μL of the cell suspension containing 5,000 YFP-positive cells and the appropriate inhibitors to 3 mL of methylcellulose (see Table of Materials). Vortex on high to mix it for 10–30 s.
After most of the air bubbles have escaped, use a 1 mL syringe to plate 1 mL of media in three replicate 3 cm plates by ejecting the media on one side and slowly tilting the plate in a circular motion to spread evenly.
The final concentration of the inhibitors to use are imatinib at 3 μM, DFC at 0.2 μM, and BCI at 0.5 μM, alone or in combinations. Wherever appropriate, delete c-Fos by plating the cells with 4-hydroxytamoxifen (1 μg/mL) added to the methylcellulose.
After plating, put the plates in a large Petri dish with one open dish containing 2 mL of sterile water in the middle. Cover the large Petri dish and carefully incubate at the 37 °C, 5% CO2 incubator. Record the colony numbers after one week of plating by counting the total number of colonies under a microscope.
Analyze a few colonies from appropriate plates for c-Fos deletion by PCR. Collect the colonies by sucking them with a P1000 tip. Dislodge the cells in 500 μL of 1× PBS by washing the tip in the PBS multiple times. Spin the cell suspensions at 6,000 × g for 1 min and extract the DNA from the cell pellet using a genomic DNA isolation kit.
Use the genomic DNA for PCR using the primers mFos-FP3 and mFos-RP5, as described in Table 2. Analyze the PCR products on a 2% agarose gel and image using a gel documentation system.
For a graphic presentation of the colonies, stain the colonies, using Iodonitrotetrazolium chloride. Dissolve 10 mg of the Iodonitrotetrazolium chloride in 10 mL of water to obtain a 1 mg/mL solution. Filter sterilize the solution using a Luer lock with a 0.2 μm filter attached to a 10 mL syringe under sterile conditions in a tissue culture hood.
In the tissue culture hood, add 100 μL of staining solution dropwise around the CFU plate; be careful not to slide or disturb colonies. Incubate the plates overnight in a 37 °C, 5% CO2 cell culture incubator. The colonies will turn dark reddish brown. Using a white background in the sterile tissue culture hood, take photos of the stained CFU plate.
7. Transplantation and Mortality Assay
Lethally irradiate the mice with split radiation using a 137 Cs-based Gamma-ray at a dosage of 7.0 Gy followed by 4.75 Gy (0.5 Gy/min), 3 h apart before the transplant.
Transplant 4 × 104 unsorted YFP-positive (calculated based on the percentage of YFP) cells with 3 × 105 normal bone marrow cells as a carrier into each mouse through tail vein injection.
After one week of transplantation, determine the engraftment by analyzing the YFP-positive cells from the peripheral blood using FACS.
- Perform a tail vein bleed and FACS analysis.
- Warm the cage of mice under a heat lamp with caution not to overheat or burn them.
- Restrain a mouse in a mouse restrainer and nick the tail vein with a sterile blade. Gently milk the tail from anterior to posterior, allowing a blood drop to accumulate. Collect the blood with a heparinized capillary tube until it is filled (collect around 75 – 100 μL). Plunge the blood from the filled capillary tube into a blood collection tube containing EDTA and mix well.
- Lyse the RBCs by adding 30–40 μL of blood to 3 mL of 1× RBC lysis buffer (150 mM ammonium chloride, 10 mM potassium bicarbonate, and 0.13 mM EDTA). Mix well, inverting the tube multiple times. Incubate at room temperature for 10–15 min. Spin at 1,200 × g at 4 °C for 5 min. Remove the supernatant via a vacuum and suspend the pellet in 2 mL of 1× PBS for a wash.
- Spin at 1,200 × g at 4 °C for 5 min. Remove the supernatant and suspend it in FACS buffer #1. Carry out FACS analysis as described above in step 5.2.5–5.2.66. Short-term store the remaining blood in the collection tube, which can be used for various other purposes, at 4 °C.
Transplanted mice that showed 10–40% YFP-positive cells in the peripheral blood were used for the experiment. Mice that had less than 2% of YFP-positive cells were excluded from the study.
Prepare tamoxifen at 20 mg/mL in corn oil at 60 °C with intermittent vortexing until completely dissolved, and store it at 4 °C in the dark for the duration of the treatment. After one week of transplantation, delete the c-Fosfl/fl allele by injecting tamoxifen (100 mg/kg in corn oil) intraperitoneally (i.p.) into the mice, every day for three consecutive days. It’s important that the mice are weighed to determine how much tamoxifen to inject. After the tamoxifen treatment (where appropriate), group the mice for drug treatments (n = 5/group).
Monitor the mice for leukemia progression and survival and determine the leukemic burden (YFP-positive cells by FACS) weekly up to eight weeks in surviving mice.
Analyze the blood for c-Fos deletion wherever appropriate, as described above in section 6. Record the number of surviving mice every day and plot the survival curve at the end of the experiment using a graphing software.
8. Transgenic Mice Model of BCR-ABL1 Leukemia
- LSK isolation
- Maintain the Scl-tTA transgenic mice on doxycycline chow to prevent an expression of BCR-ABL1. Four weeks before the transplant, take the mice off the doxycycline chow for BCR-ABL1 expression.
- Isolate TMNCs from Scl-tTA transgenic mice as described above in section 4. Suspend the TMNCs in FACS buffer #2 (1× PBS + 0.5% BSA + 2 mM EDTA) to obtain 106 cells/mL.
- Deplete the TMNCs for lineage-positive cells by adding 10 μL of lineage cell detection cocktail biotin (biotin-conjugated monoclonal antibodies against CD5 [rat IgG2a], CD11b [rat IgG2a], CD45R [B220] [rat IgG2a], Anti-7–4 [rat IgG2a], Anti-Gr-1 [Ly-6G/C, rat IgG2a], and Anti-Ter-119 [rat IgG2b]) per 1 × 106 cells. Incubate the cells at 4 °C for 10 min in the dark, followed by a wash with 5 mL of FACS buffer #2, and spin at 1,200 × g at 4 °C for 5 min. Aspirate the supernatant completely and suspend the cells in 400 μL of FACS buffer #2.
- Add 5 μL of anti-biotin antibody-FITC conjugate, 1.2 μL of Ly-6A/E (Sca1)PECy7, and 2.4 μL of CD117 (c-Kit) APC antibodies for up to 108 million cells. Incubate in the dark at room temperature for 10 min. Wash by bringing the volume up to 5 mL with FACS buffer #2; then, centrifuge at 1,200 × g at 4 °C for 5 min. Remove the supernatant and suspend the cell pellet in 500 μL of FACS buffer #2.
- Filter the cell suspension into a blue filter-cap FACS tube.
- Gate live cells using forward and side scatter. Then, select the lin− cells that showed MFI (mean fluorescence intensity of less than 102) to get LSK cells c-Kit+ and Sca1+ on a biexponential plot from the Lin− parent, and sort them (Figure 7C).
- Collect the sorted cells and centrifuge them at 1,200 × g at 4 °C for 5 min.
- Transplantation
- Lethally irradiate the BoyJ mice with a split radiation dosage of 7.0 Gy followed by 4.75 Gy after 3 h, before the transplant.
- Inject 3 × 103 to 5 × 103 BCR-ABL1-LSK cells with 0.3 × 106 helper bone marrow cells from WT BoyJ mice via tail vein (i.v.) into the irradiated BoyJ mice.
- Four weeks posttransplantation, analyze the recipient mice for CD45.1 and CD45.2. Obtain the TMNC from the peripheral blood by tail vein bleeding as described in step 7.4. Resuspend the cells in 100 μL of FACS buffer. Incubate the cells with 1 μL each of the CD45.1-FITC and CD45.2-PE antibody at room temperature for 1 h.
- Wash the cells by bringing up the volume to 3 mL with FACS buffer and spin at 1,200 × g at 4 °C for 5 min. Remove the supernatant and resuspend it in 200 μL of 1× PBS.
- Analyze the percentage on CD45.1 and CD45.2 by first gating the live cells based on forward and side scatter, followed by gating FITC-and PE-positive cells based on the unstained sample.
- Group the mice into four groups (n = 6 per group). Start the treatment with imatinib (75 mg/kg 2× daily) alone and in combination with DFC + BCI (both drugs were given at a dose of 10 mg/kg 2× daily).
- Likewise, treat other groups with combinations of imatinib (75 mg/kg) + curcumin (150 mg/kg) + BCI (10 mg/kg) and imatinib (75 mg/kg) + NDGA (100 mg/kg) + BCI (10 mg/kg) for three months, 2× a day, by injected i.p.
- Analyze the mice 1× a month for six months for leukemic chimerism by determining the percentage of CD45.2 positive cells in the peripheral blood by tail vein bleeding as described in step 7.4.
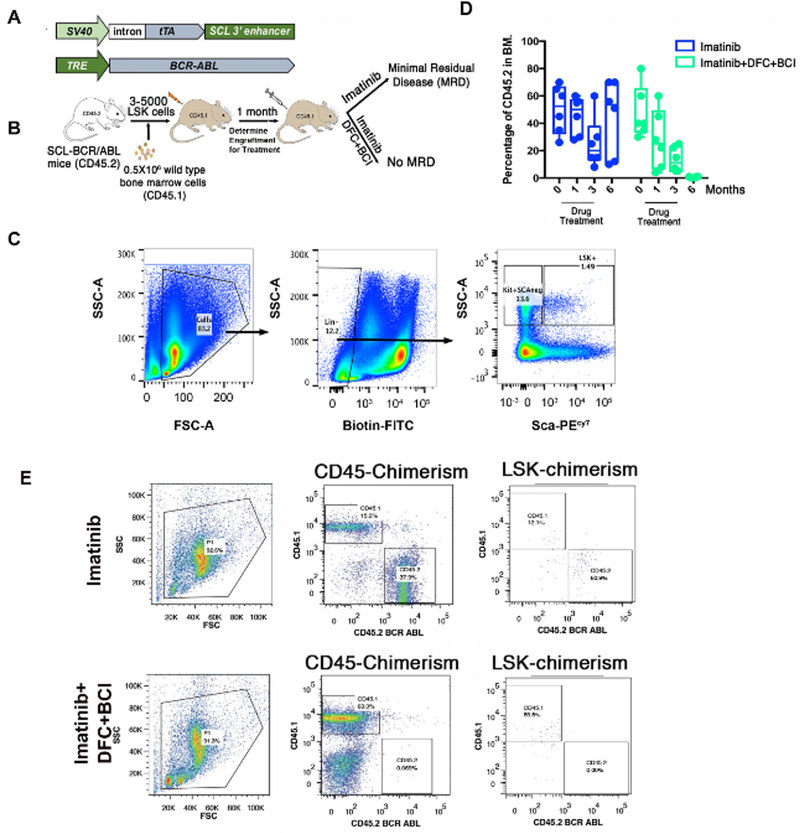
Figure 7: c-Fos and Dusp1 are required for the survival of LSCs.
(A) Schematic of the transgene used in transgenic mice expressing BCR-ABL1 in stem cells. (B) Experimental design for studying the effects of Dusp1 and c-Fos inhibition by DFC and BCI in vivo. (C) FACS plots of the swing gating strategy used for sorting LSK cells for transplantation. (D) Percentage of the BCR-ABL1 (determined by the percentage of CD45.2 from donor mice) positive cells in mice during and posttreatment with inhibitors. (E) FACS scatter plots showing the CD45 chimerism in live cells, as well as in the LSK cell compartment. Please note the lack of CD45.2 in total and in the LSK compartment in treated mice. This figure was adapted with permission from Kesarwani et al.13.
9. In Vivo Evaluation of BCI and DFC Activity
- Phospho-p38 analysis
- Take three leukemic mice (8–12 weeks old) that were transplanted with BCR-ABL1-expressing c-Kit+ cells, as described in section 7. Inject the mice with BCI (10 mg/kg) by injecting i.p. four weeks posttransplant.
- Measure the levels of phospho-p38 peripheral blood TMNC using the phosphorylation flow cytometry kit according to the supplier’s instructions. Collect the blood from each mouse before and 6 h after the drug injection. Isolate the TMNCs by RBC depletion and fix them as follows.
- Add 4 mL of RBC lysis solution per 100 μL of peripheral blood. Mix and incubate on ice for 5 min to lyse the RBCs. Spin down the cells at 1,200 × g for 5 min at 4 °C and remove the supernatant. Repeat the lysis 1× more.
- After the second lysis step, wash the cell pellets with 1 mL of 2% BSA in PBS. Fix the cells by adding 100 μL of fixation and permeabilization solutions and incubate for 20 min at 4 °C in the dark. Pellet the cell by centrifugation at 1,200 × g for 5 min at 4 °C.
- Wash the pellets with 1 mL of 1× permeabilization wash. Block the cells by adding 300 μL of 2% BSA in permeabilization wash buffer at room temperature for 20 min. Divide the cells into three equal aliquots of 100 μL each (~1 ×106).
- To each aliquot of fixed cells add 1 μL of total p-38 antibody, 1 μL of phospho-p38 antibody, or 1 μL of isotype IgG control, respectively, and incubate overnight at 4 °C.
- Wash the cells 1× with 5 mL of 2% BSA in PBS and incubate with secondary antibody (1 μL of Alexa Fluor-488 conjugated) for 1 h at room temperature. Wash the cells 1× again and suspend them in 200 μL of 1× PBS.
- Acquire the data by FACS and analyze them by flow cytometry analysis software.
- Deduct the MFI of the IgG control from the MFI of the experimental samples. The MFI values of phospho-p38 are then normalized to that of total p-38 to determine the phospho-p38 levels after the BCI injection.
- Quantitative gene expression analysis of c-Fos target genes
- Take three leukemic mice (8–12 weeks old), that were transplanted with BCR-ABL1-expressing c-Kit+ cells as described in section 7. Collect the peripheral blood from each mouse and then inject the mice with 10 mg/kg each of DFC + BCI.
- Collect the peripheral blood 6 h after the drug injection. Isolate TMNCs by RBC depletion, as described above. Pellet the TMNCs and suspend them in a phenol-based lysis buffer (see Table of Materials).
10. Long-term Culture-Initiating Cell Assay
NOTE: An LTCIC assay was performed as described previously25.
Grow a mouse fibroblast cell line MS-5 in MEMα to confluency in a T175 tissue culture flask. Trypsinize the cells as described above for HEK293T. Suspend the cells in MEMα at 2 × 106 cells/mL. Take 10 × 106 cells in a 50 mL conical tube and irradiate at 80 Gy. Dilute the cells to 0.5 × 106 cells/mL in MEMα supplemented with 10% FBS (M10) media. Plate 2 mL per well in a 12-well plate and incubate it in a 37 °C, 5% CO2 incubator overnight.
On the next day, prepare the hydrocortisone sodium hemisuccinate by dissolving 2.5 mg in 5 mL of MEMα (1 M solution). Filter-sterilize the hydrocortisone solution using a 0.2 μm syringe filter in biosafety hood. Dilute the hydrocortisone by serial dilution in MEM to obtain a 100 μM solution. Add 250 μL of this to 25 mL of human long-term culture medium (HLTM) (see Table of Materials) just prior to use, to achieve a final concentration of 1 μM.
Spin down the CML-CD34+ and UCP3 TMNCs and suspend them in the HLTM by brief vortexing, and determine the counts by a hemocytometer.
Vacuum off the M10 media from the 12-well plate seeded with stroma cells and replace it with the 1 mL of patient cell (CML-CD34+ [10,000] and UCP3 TMNCs [1 × 106]) suspension in HLTM. Use three wells per drug combination per cell type.
Dilute the drugs stocks to 2× of the required concentration in HLTM. Add 1 mL of the media with drugs to the above-mentioned cells to obtain a 1× drug concentration. Replace one-half of the media with freshly prepared HLTM every week for five weeks.
At the end of the six-week LTC-IC assay period, collect nonadherent cells into a tube from the cultures. Trypsinize adherent cells and combine them with the corresponding nonadherent cells.
Wash the cells and assay for CFUs in methylcellulose medium containing 30% fetal calf serum, erythropoietin (3 units/mL), SF (50 ng/mL), and IL-3(20 ng/mL), IL-6 (20 ng/mL), G-CSF (20 ng/mL), and granulocyte/macrophage colony-stimulating factor (GM-CSF) (20 ng/mL). Determine the counts by a hemocytometer.
Plate 5 × 104 cells in three replicates. Freeze down the remaining cells in 20% FBS and 10% DMSO. Arrange the plates in a large Petri dish with one open dish containing water in the middle. Cover the large Petri dish carefully and incubate it in a 37 °C, 5% CO2 incubator for two weeks.
-
Score the colonies two weeks later. In some cases, only part of the harvest is assayed for CFUs). Calculate the total number of LTCIC present in the initial cell suspension by extrapolating based on the CFUs obtained from part of the cell harvest. The average CFU output per LTC-IC is 8.
NOTE: For example, if initially 1 × 106 cells were plated in a well, after five weeks, 0.5 × 106 cells were harvested, and for CFU, 5 × 104 cells were plated and 50 CFUs were obtained. This equals 500 CFUs/1 million plated cells. Divide this number by 8 to get the LTCIC, which is 62.5.
11. Humanized Mouse Model Using CML CD34+ Cells
Sublethally irradiate the 8 week-old NOD scid gamma mice, expressing human IL3, GM-CSF and SCF (NSGS), with a single dose of 7.0 Gy (700 rads) with 50 rads/min.
Inject 3 × 106 CD34+ selected cells from BCR-ABL1-positive CML chronic phase patients into the irradiated mice by intravenous injection.
After two weeks of transplantation, determine leukemic engraftment in bone marrow by FACS using mouse- and human-specific antibodies against CD45.
- Perform a FACS analysis.
- Take the bone marrow aspirate from the femurs of the transplanted mice. Lyse RBCs using RBC lysis buffer as described above and collect the TMNCs by centrifugation. Wash the pellet 1× with cold 1× PBS.
- Block the cells with human FcR block and mouse FcR block followed by staining with anti-human CD45 FITC and anti-mouse CD45 APCCy7 overnight at 4 °C. Perform the FACS analysis on LSRII and analyze the data using flow cytometry analysis software.
Group the mice into four different cohorts (n = 6/group) for the treatment with vehicle, imatinib (75 mg/kg), DFC + BCI (both at 10 mg/kg), or imatinib (75 mg/kg) + DFC + BCI (both at 10 mg/kg). Dilute the stock drugs in 1× PBS (vehicle) and administer them by injecting them i.p. 2× daily.
Treat the mice for six weeks and determine the leukemic burden every two weeks, until week eight after the transplantation as described above in 11.4.
Representative Results
Oncogene addiction has been implicated in the therapeutic efficacy of TKIs. However, the mechanisms driving the oncogene dependence are not understood. We performed multiple unbiased gene expression analyses to identify the genetic component involved in orchestrating the addiction. These analyses revealed the upregulation of three genes, c-Fos, Dusp1, and Zfp36, in cancer cells that are not dependent on oncogenic signaling for survival and, thus, are insensitive to TKI treatment. The downregulation of c-Fos and Dusp1 by shRNA-mediated knockdown is sufficient to restore drug sensitivity in otherwise TKI-resistant cells.
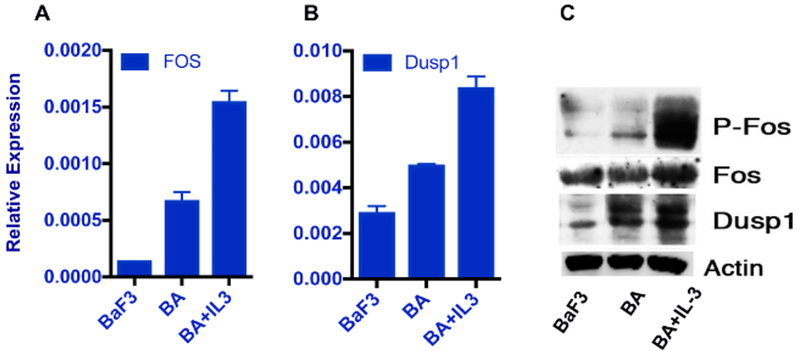
To validate the role of c-Fos and Dusp1 as the therapeutic target in leukemia, their overexpression was first confirmed using BaF3 cells expressing the BCR-ABL1 oncogene. Growth factor signaling in the BaF3-BA cells abrogates oncogene dependence; as a consequence, they do not respond to TKI treatment. Quantitative expression analysis by RT-qPCR and western blotting confirmed that both c-Fos and Dusp1 are induced by BCR-ABL1 and their expression is further augmented by the growth factor IL-3 (Figure 1A–1C).
Figure 1: c-Fos and Dusp1 are overexpressed in response to growth factor IL-3.
qPCR analysis of an overexpression of (A) c-Fos and (B) Dusp1 in cytokine-treated BaF3-BA cells. The relative expression was determined after normalization to β-actin. The error bars represent the standard deviation from three replicate samples. (C) Western blot analysis of BaF3-BA cells treated with IL-3 and probed with c-Fos total or P-c-Fos and Dusp1 antibody. Anti-actin antibody was used as loading control. This figure was adapted with permission from Kesarwani et al.13.
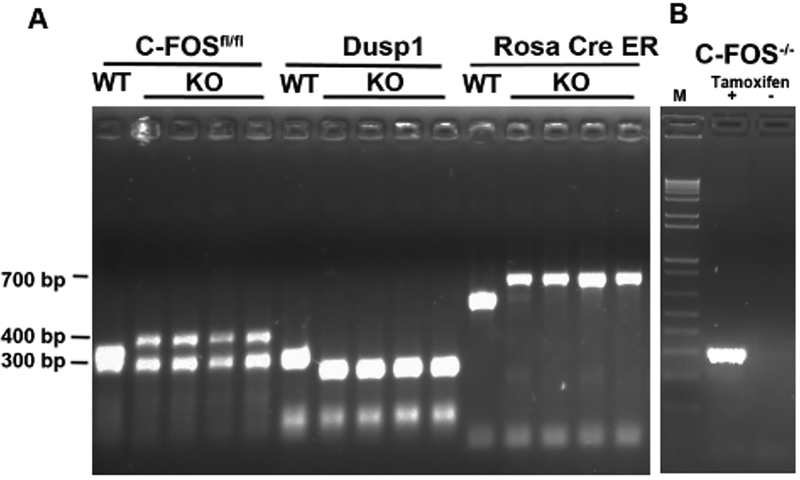
To study the role of c-Fos and Dusp1 in vivo, mice lacking c-Fos (conditional mouse model c-Fosfl/fl with Rosa26-CreERT2) or Dusp1 (straight KO Dusp1−/−) and mice lacking both c-Fos and Dusp1 (Rosa26-CreERt2-Fosfl/fl/Dusp1−/−) were developed. These mice were genotyped by PCR using genomic DNA from the tail, and the PCR easily distinguished the c-Fosfl/fl, Dusp1 KO from the WT mice (Figure 2A). The induced deletion of c-Fos by tamoxifen treatment was confirmed by PCR, by the appearance of a band compared to no band in mice not treated with tamoxifen (Figure 2B).
Figure 2: Genotyping of c-Fosfl/flDusp1 and Rosa cre-ER mice.
(A) PCR analysis of tail DNA. Expected bands are from WT or from the c-Fosfl/fl-carrying CreER transgene and Dusp1 KO mice. The band sizes are indicated on the left. (B) PCR analysis of a smaller band after the deletion of c-Fos with tamoxifen treatment. M = 1 kb+ DNA Ladder.
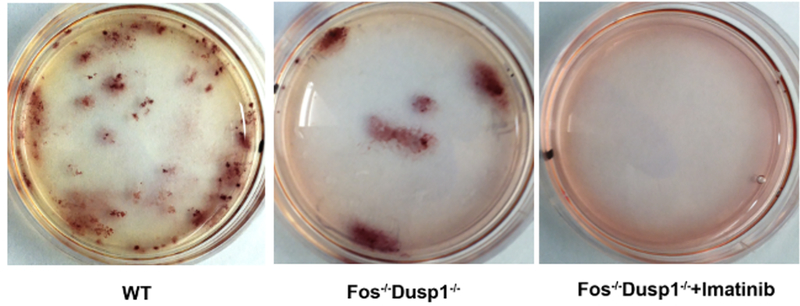
To test the role of c-Fos and Dusp1, bone marrow-derived c-Kit+ cells from the WT, Dusp1−/−, c-Fosfl/fl, and Dusp1−/−/c-Fosfl/fl were isolated and transduced with MSCV-BCR-ABL1-Ires-YFP retroviruses; a schematic of the procedure is shown in Figure 3. YFP+ expressing (used as a surrogate marker for BCR-ABL1 expression) were sorted by FACS (Figure 4) for further in vitro (CFU) and in vivo assays. The results show that the deletion of c-Fos and Dusp1 alone inhibited CFU numbers (<50%). Interestingly, cells lacking both c-Fos and Dusp1 were significantly compromised in their colony-forming ability, and imatinib treatment wiped out all CFUs, suggesting that the loss of c-Fos and Dusp1 is synthetically lethal to BCR-ABL1 expression (Figure 5).
Figure 3:
Schematic of the transduction and transplantation model.
Figure 5: Representative plates from CFU plating.
The plates show the colonies stained with iodonitrotetrazolium chloride.
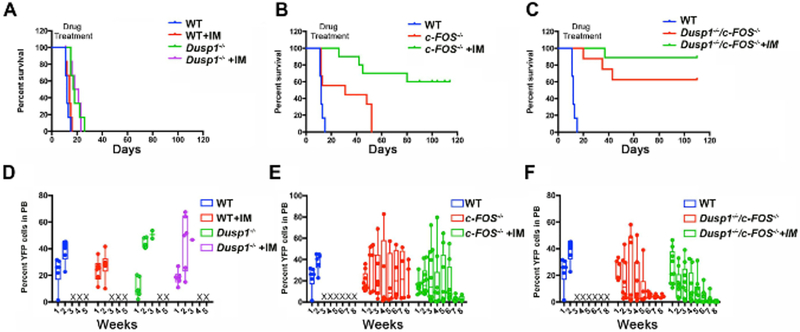
The in vitro CFU assays enable researchers to quickly test the phenotype of the KO and activity of the small molecule inhibitors. However, further in vivo analysis will confirm the validity of the targets. For in vivo validation, we first utilized a quick transduction transplantation model1 where BCR-ABL1 causes mortality within two to three weeks. Fifty thousand YFP-positive cells, along with 300,000–500,000 bone marrow-derived mononuclear cells from the normal mouse, were injected into lethally irradiated mice to induce CML. The mice carrying the c-Fosfl/fl cells or the c-Fosfl/flDusp1−/− cells were injected with tamoxifen after 10 days of transplantation to delete the c-Fos. Mice that received either WT or Dusp1−/−cells developed leukemia and succumbed to death within two to four weeks, while the deletion of c-Fos alone showed a significant delay in disease development, and TKI treatment saved almost 50% of the mice from death. Interestingly, the mice transplanted with cells lacking both c-Fos and Dusp1 survived longer, and the TKI treatment cured all mice of the CML (Figure 6A–6C). The mice that survived progressively lost the YFP+ cells, as shown in c-Fos and double KO when treated with imatinib (Figure 6D–6F), suggesting the clearance of BCR-ABL1 positive cells.
Figure 6: c-Fos and Dusp1 deficiencies compromise leukemia development.
(A-C) Survival curves of mice transplanted with c-Kit+ BCR-ABL1 cells from WT, Dusp1−/−c-Fos−/−, and c-Fos−/− Dusp1−/− mice. (D-E) Percentage of YFP+ cells as determined by FACS in the peripheral blood of the transplanted mice drawn every week posttransplant. The levels of YFP increase and the mice succumb to death as in WT and Dusp1 KO. The mice that survive progressively lose the YFP+ cells as shown in c-Fos and double KO when treated with imatinib. This figure was adapted with permission from Kesarwani et al.13.
Given that CML is a stem cell disease, and given the inability of retroviruses to target the primitive and quiescent hematopoietic cells, it is imperative to test whether targeting c-Fos and Dusp1 would be sufficient to eliminate the leukemic stem cells. Tetracycline-inducible BCR-ABL1 transgenic mice where tTA is expressed by an Scl enhancer, which is specifically expressed in hematopoietic stem cells, have been previously utilized to study the role of target genes in LSCs. The tetracycline-inducible BCR-ABL1 transgenic mice (Figure 7A–7B) were utilized to investigate the role of c-Fos and Dusp1 in LSCs. The LSK cells from these mice were sorted using FACS and injected into the recipient mice (Figure 7C). After one month of transplantation, leukemic engraftment was scored, followed by drug treatment. The leukemic burden and levels of LSK were determined every month for six months. Mice treated with imatinib alone showed a suppression of leukemia but were left with the MRD. Therefore, treatment discontinuation, as observed in the clinic, resulted in disease relapse. In contrast, mice treated with a combination of drugs imatinib + DFC (c-Fos inhibitor) + BCI (Dusp1 inhibitor) completely eradicated the leukemic cells, and the mice were cured of CML (Figure 7D and 7E).
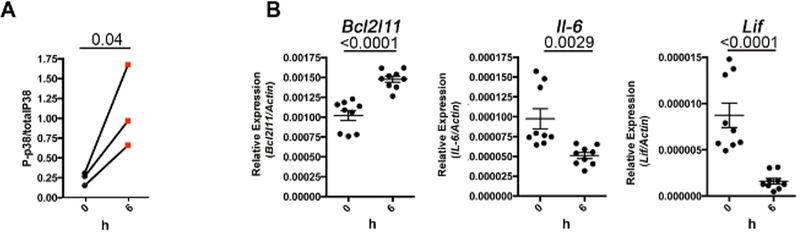
To establish the pharmaco-dynamic read-out for c-Fos and Dusp1 inhibitors and to study their on-target effect, phospho-p38 levels (a surrogate marker for Dusp1 inhibition) were measured and the expression of c-Fos-regulated genes, such as IL-6, bcl2l11, and Lif, was quantified. We and others have established that the inhibition of Dusp1 results in the activation of p38 (measured by the increased phosphorylation of p38)13. Phospo-p38 levels were measured by FACS in whole-blood cells isolated from drug-treated mice. As expected, the phospho-p38 levels increased (Figure 8A) and the expression of c-Fos-regulated genes (IL-6, bcl2l11, and Lif) were downregulated in drug-treated mice (Figure 8B). These results suggest that the inhibitors inhibit their target and the survival of mice is due to the inhibition of c-Fos and Dusp1.
Figure 8: In vivo activity of DFC and BCI.
(A) Line graph showing the levels of phospho-p38 after BCI treatment. (B) RT-qPCR showing the expression of the c-Fos target genes in peripheral blood of the mice after DFC treatment. The dots represent three replicates from each mouse (n = 3). The number above the graphs represent the p-value calculated by Student’s t-test. This figure was adapted with permission from Kesarwani et al.13.
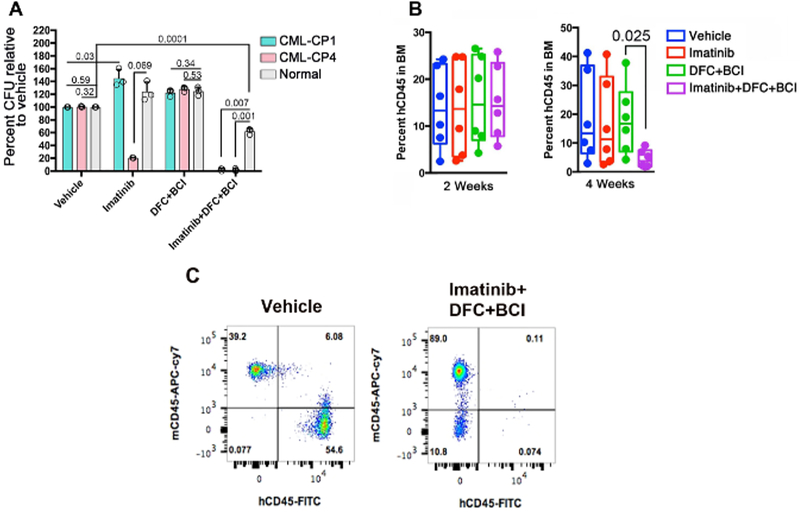
For human relevance, the efficacy of c-Fos and Dusp1 inhibitors were tested using patient samples in in vitro (LTC-IC) and in vivo assays (mouse xenografts). The treatment with DFC + BCI is not effective on leukemic stem cells. However, a combination of DFC + BCI + imatinib selectively eradicated the leukemic stem cells in both assays while sparing normal stem cells (Figure 9A–9C). These results establish that c-Fos and Dusp1 are essential for leukemic transformation, and an elevated expression of these genes abrogates oncogene dependence resulting in disease relapse and drug-resistance. Therefore, a combinatorial targeting of c-Fos and Dusp1 along with driver oncogene will be more effective and, perhaps, a curative strategy for many cancers. We anticipate that the methods described here can generally be applied for additional target identification and validation.
Figure 9: Role of c-Fos and DUSP1 inhibition in a human patient sample.
(A) Percentage of CFUs determined by the LTC-IC assay from two CML patients, and a healthy donor treated with vehicle or the indicated drug combinations. (B) Percentage of human leukemic cells (hCD45) in the bone marrow of NSGS mice at week 2 (left) and week 4 (right) of the treatment. (C) This panel shows representative FACS plots showing a dramatic reduction of hCD45 cells in the drug-treated mice while sparing the mouse cells shown as mCD45. This figure was adapted with permission from Kesarwani et al.13.
Discussion
For the bulk of cancer cells, the therapeutic response to TKI is mediated by a blockade of tyrosine-kinase-oncoprotein signals to which the tumor is addicted. However, relatively little is known about how a minority of cancer cells contributing to MRD escape the oncogene dependence and therapy4. Recent studies revealed that growth factor signaling mediates drug resistance in both leukemia and solid organ tumors. This suggests that various molecular mechanisms might underlie intrinsic resistance10,11,12. To understand the mechanism and identify gene targets for therapeutic development, we performed comprehensive whole-genome expression profiles using mouse (BaF3) and human (K562) cells. Primary samples from leukemic mice or CML patients were not used because we suspected that, as primary bone-marrow samples are significantly heterogeneous, they will mask the identity of relevant genes/targets. Even the cells purified by antibody-mediated sorting (stem progenitor cells) exhibit significant heterogeneity. Therefore, a purified cell line is probably better suited for target identification to avoid any undesired complexity imposed by genetic heterogeneity. The whole-genome expression analysis identified that c-Fos, Dusp1, and Zfp36 are commonly upregulated in drug-resistant cells. Once the targets are identified, their validation becomes a critical part to recognize their role in the given genetic context. Using cDNA overexpression and genetic knockdown studies in BaF3 cells revealed that the inhibition of c-Fos and Dusp1 is sufficient and necessary for effective TKI sensitivity. Perhaps one of the most critical steps in target validation is to validate the relevance of identified genes/targets in primary samples from both mouse and human. In this protocol, we provide step by step utilization of multiple genetic models and provide better mechanistic insights for the further refinement of drug/target evaluation.
A quick screening at the cellular level utilizing CFUs or the LTCIC assay helps researchers to test multiple drug combinations, as well as concentrations, quickly before going to more time-consuming transplant methods. Given that CML is a stem cell disease, and given the inability of retroviruses to target the primitive and quiescent hematopoietic cells, it is imperative to test using models that directly address their role in LSC survival. To address this, we utilized a tetracycline-inducible BCR-ABL transgenic mouse where tTA is expressed by an Scl enhancer. Nonetheless, genetic models are deficient in recapitulating the human disease. Therefore, it is necessary to test primary patient samples in humanized mouse models. However, to be able to do a long-term study for the detection of MRD over time, a stable xenograft is required which, depending on the mice and cell types, may vary. After four months of transplant, the human CD34+ cells were depleted even without the drug treatment, which is a limitation in most humanized mouse models. Better humanized mouse models are being developed for testing the human cells in mice26. We strongly recommend determining the on-target activity of the inhibitors as any off-target effect may cause toxicity to normal cells, limiting its further application. However, if the downstream target is not known, different approaches should be utilized. For example, identifying the drug-resistant mutants using random mutagenesis of the target protein can directly address whether the inhibitor is on target27.
The protocol presented here provides a comprehensive method for target identification and validation using multiple in vitro and in vivo model systems. These methods can be easily adapted to other targets and cancer types. Further mechanistic studies on identified targets and refinement in drug targeting may help in developing better therapeutic modalities for effective and/or curative treatment.
Supplementary Material
Acknowledgements
The authors are thankful to G. Q. Daley for providing the BaF3 and WEHI cells and T. Reya for the MSCV-BCR-ABL-Ires-YFP constructs. The authors are thankful to M. Carroll for providing the patient samples from the CML blast crisis. This study was supported by grants to M.A. from the NCI (1RO1CA155091), the Leukemia Research Foundation and V Foundation, and from the NHLBI (R21HL114074-01).
Footnotes
Disclosures
The authors have nothing to disclose.
Video Link
The video component of this article can be found at https://www.jove.com/video/58194/
References
- 1.Daley GQ, Van Etten RA, & Baltimore D Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 247, 824–830 (1990). [DOI] [PubMed] [Google Scholar]
- 2.Druker BJ, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nature Medicine. 2, 561–566 (1996). [DOI] [PubMed] [Google Scholar]
- 3.Mahon FX, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncology. 11, 1029–1035 (2010). [DOI] [PubMed] [Google Scholar]
- 4.O’Hare T, Zabriskie MS, Eiring AM, & Deininger MW Pushing the limits of targeted therapy in chronic myeloid leukaemia. Nature Reviews Cancer. 12, 513–526 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Rousselot P, et al. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood. 109, 58–60 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Krause DS, & Van Etten RA Tyrosine kinases as targets for cancer therapy. The New England Journal of Medicine. 353, 172–187 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Sharma SV, & Settleman J Exploiting the balance between life and death: targeted cancer therapy and “oncogenic shock”. Biochemical Pharmacology. 80, 666–673.(2010). [DOI] [PubMed] [Google Scholar]
- 8.Sharma SV, & Settleman J Oncogene addiction: setting the stage for molecularly targeted cancer therapy. Genes & Development. 21, 3214–3231 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Weinstein IB Cancer. Addiction to oncogenes--the Achilles heal of cancer. Science. 297, 63–64 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Corbin AS, et al. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. The Journal of Clinical Investigation. 121, 396–409 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Straussman R, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 487, 500–504 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson TR, et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature. 487, 505–509 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kesarwani M, et al. Targeting c-FOS and DUSP1 abrogates intrinsic resistance to tyrosine-kinase inhibitor therapy in BCR-ABL-induced leukemia. Nature Medicine. 23, 472–482 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, et al. c-fos regulates neuronal excitability and survival. Nature Genetics. 30, 416–420 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Dorfman K, et al. Disruption of the erp/mkp-1 gene does not affect mouse development: normal MAP kinase activity in ERP/MKP-1-deficient fibroblasts. Oncogene. 13, 925–931 (1996). [PubMed] [Google Scholar]
- 16.Padhye S, et al. Fluorocurcumins as cyclooxygenase-2 inhibitor: molecular docking, pharmacokinetics and tissue distribution in mice. Pharmaceutical Research. 26, 2438–2445 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molina G, et al. Zebrafish chemical screening reveals an inhibitor of Dusp6 that expands cardiac cell lineages. Nature Chemical Biology. 5, 680–687 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang B, et al. Effective targeting of quiescent chronic myelogenous leukemia stem cells by histone deacetylase inhibitors in combination with imatinib mesylate. Cancer Cell. 17, 427–442 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koschmieder S, et al. Inducible chronic phase of myeloid leukemia with expansion of hematopoietic stem cells in a transgenic model of BCR-ABL leukemogenesis. Blood. 105, 324–334 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Abraham SA, et al. Dual targeting of p53 and c-MYC selectively eliminates leukaemic stem cells. Nature. 534, 341–346 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L, et al. Activation of p53 by SIRT1 inhibition enhances elimination of CML leukemia stem cells in combination with imatinib. Cancer Cell. 21, 266–281 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiagen-miRNAeasy kit. https://www.qiagen.com/us/shop/sample-technologies/rna/mirna/mirneasy-mini-kit/#resources. (2018).
- 23.DNA-free DNA removal kit. https://www.thermofisher.com/order/catalog/product/AM1906?gclid=EAIaIQobChMIg4D-_ZvC2wIVx5-zCh00Cg8fEAAYASAAEgIv6vD_BwE&s_kwcid=AL!3652!3!264318446624!b!!g!!&ef_id=V7SO5AAAAck3ba89:20180607185004:s. (2018).
- 24.SuperScript™ III First-Strand Synthesis System. https://www.thermofisher.com/order/catalog/product/18080051. (2018).
- 25.Human Long Term Culture Initiating Cell Itc-ic Assay. https://www.stemcell.com/human-long-term-culture-initiating-cell-ltc-ic-assay.html. (2018).
- 26.Abarrategi A, et al. Modeling the human bone marrow niche in mice: From host bone marrow engraftment to bioengineering approaches. Journal of Experimental Medicine. 215, 729–743 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kesarwani M, Huber E, Kincaid Z, & Azam M A method for screening and validation of resistant mutations against kinase inhibitors. Journal of Visualized Experiments. (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.