RNA N6-methyladenosine-modified genes exhibit biased subgenome fractionation, and their coevolutionary relationship with transposable elements is mediated by genomic duplication in maize (Zea mays).
Abstract
RNA N6-methyladenosine (m6A) modification is the most abundant form of RNA epigenetic modification in eukaryotes. Given that m6A evolution is associated with the selective constraints of nucleotide sequences in mammalian genomes, we hypothesize that m6A evolution can be linked, at least in part, to genomic duplication events in complex polyploid plant genomes. To test this hypothesis, we presented the maize (Zea mays) m6A modification landscape in a transcriptome-wide manner and identified 11,968 m6A peaks carried by 5,893 and 3,811 genes from two subgenomes (maize1 and maize2, respectively). Each of these subgenomes covered over 2,200 duplicate genes. Within these duplicate genes, those carrying m6A peaks exhibited significant differences in retention rate. This biased subgenome fractionation of m6A-methylated genes is associated with multiple sequence features and is influenced by asymmetric evolutionary rates. We also characterized the coevolutionary patterns of m6A-methylated genes and transposable elements, which can be mediated by whole genome duplication and tandem duplication. We revealed the evolutionary conservation and divergence of duplicated m6A functional factors and the potential role of m6A modification in maize responses to drought stress. This study highlights complex interplays between m6A modification and gene duplication, providing a reference for understanding the mechanisms underlying m6A evolution mediated by genome duplication events.
N6-Methyladenosine (m6A) is an internal, prevalent RNA modification and has been identified in the RNA of mammals (Adams and Cory, 1975), insects (Levis and Penman, 1978), yeast (Clancy et al., 2002), and plants, such as Arabidopsis (Arabidopsis thaliana; Zhong et al., 2008) and maize (Zea mays; Nichols, 1979). The m6A modification is formed by m6A methyltransferase “writer” proteins (e.g. methyltransferase-like 3 [METTL3], METTL14, and Wilms tumor1-associating protein in mammalian cells; Bokar et al., 1997; Liu et al., 2014; Ping et al., 2014), recognized by “reader” proteins (e.g. YT512-B Homology domain proteins; Xu et al., 2014, 2015), and removed by “eraser” proteins (m6A demethylases; e.g. fat mass and obesity-associated protein and alkylated DNA repair protein AlkB homolog 5 [ALKBH5]; Jia et al., 2011; Zheng et al., 2013), thus forming an epitranscriptomic system of RNA methylations directly analogous to the well-studied reversible DNA and histone modifications (Wang and He, 2014). Loss of function of core components of the m6A modification system in mammals has demonstrated that m6A modification affects multiple aspects of RNA metabolism, including stability (Wang et al., 2014b), translation efficiency (Shi et al., 2017), nuclear export (Roundtree et al., 2017b), and alternative splicing (Zhao et al., 2014). In Arabidopsis, the disruption of N6-adenosine-methyltransferase MT-A70-like (METTL3 homolog), methyltransferase MT-A70 family protein (METTL14 homolog), and the FK506-binding protein 12 interacting protein 37 (FIP37, Wilms tumor1-associating protein homolog) leads to early embryonic lethality (Vespa et al., 2004; Zhong et al., 2008; Růžička et al., 2017), and the depletion of ALKBH10B (At4g02940, ALKBH5 homolog) effects Arabidopsis floral translation (Duan et al., 2017). Additionally, two YT512-B Homology-domain proteins (EVOLUTIONARILY CONSERVED C-TERMINAL REGION2 [ECT2] and ECT3) function as m6A readers and control developmental timing and morphogenesis and trichome morphology (Arribas-Hernández et al., 2018; Scutenaire et al., 2018; Wei et al., 2018). These pioneering biochemical and genetic researches shed light on the functional roles of RNA m6A modification, which constitutes an important regulatory mechanism in RNA biology (Roundtree et al., 2017a; Yang et al., 2018).
Recently, with the development of m6A sequencing (m6A-seq) technologies, an increasing number of m6A methylome comparison studies have begun to unravel the evolution of this important posttranscriptional modification (Dominissini et al., 2012, 2013). The evolutionary conservation of m6A modifications was detected within two yeast species (Saccharomyces mikatae and Saccharomyces Cerevisiae; Schwartz et al., 2013); across two accessions of Arabidopsis (Can-0 and Hen-16; Luo et al., 2014); and between human (Homo sapiens), chimpanzee (Pan troglodytes), and rhesus (Macaca mulatta; Ma et al., 2017). The transcriptome-wide comparison of m6A modifications from human, chimpanzee, and rhesus revealed that m6A evolution is associated with the selective constraints of DNA sequences and occurs in parallel with expression evolution of m6A-methylated genes (Ma et al., 2017). Yet virtually nothing is known about the evolution of m6A modification after genome duplications in plant evolution.
In plants, genome duplications (GDs), including whole genome duplications (WGDs), segmental duplications, tandem duplications (TDs), and translocated duplications, generate a source of specific genomic context (i.e. duplicated regions) as a dominant force of plant genome evolution (Freeling, 2009). Following GD events, duplicate genes were subjected to different levels of purifying selection, a proportion of which were lost in a process known as fractionation. There are also many duplicate genes retained in the genome as paralog pairs, in which the individual genes may be subfunctionalized (partitioning and sharing the original gene function) and/or neofunctionalized (gaining novel functions; Panchy et al., 2016). Both the bias in fractionation and the functional divergence of duplicate genes have been reported to be associated with differences in DNA methylation, rates of movement of transposable elements (TEs), gene expression, and posttranscriptional regulation (Wang et al., 2014a, 2015; Panchy et al., 2016; Cheng et al., 2018). Therefore, GDs provide a source of specific genomic context that may have profound influences on transcriptional regulation and posttranscriptional regulation. This raises the question of whether, and to what degree, the evolution of m6A modification is mediated by GD events in complex polyploid plant genomes.
To investigate this question, we performed deep m6A-seq on the leaf tissue of maize and explored the patterns of m6A evolution in maize. Maize underwent a recent WGD event after its divergence from the lineage that gave rise to sorghum (Sorghum bicolor) ∼5–12 million years ago (Swigonova et al., 2004). Since that time, the two subgenomes in maize experienced a variety of changes (e.g. chromosomal rearrangements and gene conversion; Schnable et al., 2011) and were combined into a diploid genome (Wei et al., 2007; Schnable et al., 2011). Because of this unusual evolutionary history, together with the availability of high quality of the 2maize B73 reference genome (Jiao et al., 2017) and the sorghum reference genome (Paterson et al., 2009; McCormick et al., 2018), we selected maize as a model crop system to study the evolutionary implications of m6A modification in the context of GD events. Using the transcriptome-wide map of m6A generated from our study, we made an effort to address some topics of conceptual importance to understanding the evolutionary characteristics of m6A in maize. For example, do GD events contribute to the evolutionary novelty of m6A modification? How is the evolution of m6A modification associated with the expression divergence (ED) of duplicate genes? How do m6A modification and TEs experience some coevolutionary process following WGD in maize, or do they at all? Our answers to these questions are provided by examining the coordination patterns of RNA m6A modification with gene duplication, evolutionary divergence, gene expression, and TE accumulation.
RESULTS
Transcriptome-Wide Mapping of m6A in Maize
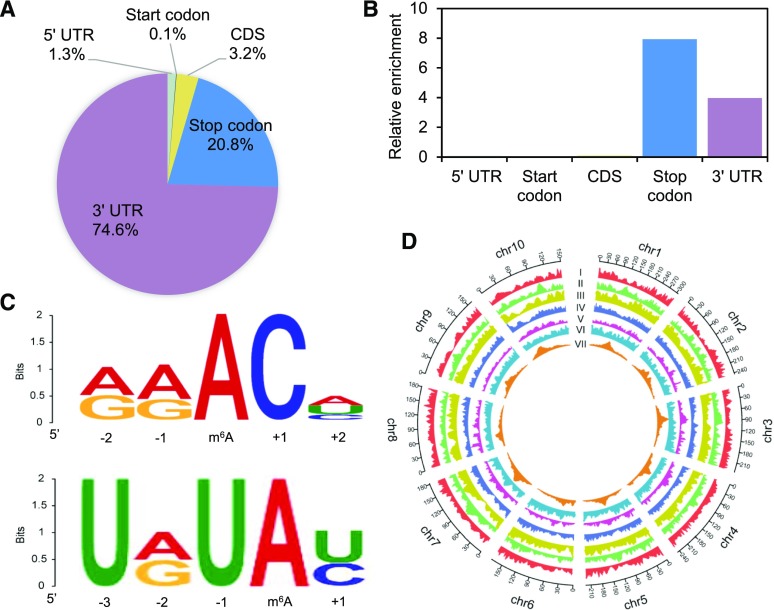
To obtain the transcriptome-wide m6A map in maize, a series of m6A-immunoprecipitation (IP) and the matched input (non-IP control) libraries was constructed and sequenced (Supplemental Table S1). This series included leaf tissue of maize B73 and Han21 seedlings under both well-watered (WW) and drought-stressed (DS) conditions, with three biological replicates each. Raw sequencing reads were processed to discard adaptor sequences and low-quality bases using the Trimmomatic v0.36 tool (Bolger et al., 2014). The resulting reads from maize B73 and Han21 samples were aligned to the maize B73 reference genome (B73_RefGen_v4) and Han21 pseudogenome, respectively, using Tophat v2.1.1 (Kim et al., 2013). To build the Han21 pseudogenome, single nucleotide polymorphisms (SNPs) between B73 and Han21 were identified by aligning Han21 RNA sequencing (RNA-seq) data to the maize B73 reference genome (B73_RefSeq_v4) using STAR (Dobin et al., 2013), followed by SNP calling using the genome analysis toolkit (GATK) UnifiedGenotyper (McKenna et al., 2010). A total of 94,761 SNPs within transcribed regions were used to replace the corresponding nucleotides in the maize B73 reference genome to generate a pseudoreference genome for Han21. Read distribution analysis showed that the reads from m6A-IP samples are highly accumulated around the stop codon and within 3′-untranslated regions (3′-UTRs) in all experimental conditions (Supplemental Fig. S1). We detected 8,224–11,134 m6A peaks in each individual biological replicate (Supplemental Fig. S2). For each experimental condition (one inbred line under one environmental condition), highly confident peaks were identified, referring to the previous study (Yoon et al., 2017). In brief, by intersecting peak regions in a pairwise fashion among all three replicates, regions that overlap in at least two of three replicates were designated as high-confidence m6A peak regions. Strong correlations were observed for the abundance of confident peaks between biological replicates (Supplemental Fig. S3). Confident m6A peaks from different experimental conditions were further merged into a unique set of m6A peaks. As a result, a total of 11,968 unique m6A peaks with high confidence were finally detected from 11,219 maize genes (Supplemental Table S2), accounting for an average of ∼1.07 m6A peaks within transcription units from each gene. The m6A peaks in maize are abundant in 3′-UTRs (74.6% of m6A peaks), near stop codons (20.8%), and coding sequences (3.2%; Fig. 1A). Enrichment analysis showed that the stop codon was the most enriched segment, representing ∼8-fold enrichment, followed by the 3′-UTR (∼4-fold enrichment; Fig. 1B). Similar distribution patterns of m6A peaks were also observed in the separate analysis of m6A-seq data from B73 and Han21 (Supplemental Fig. S4). At the genome level, 11,219 m6A-methylated genes (i.e. genes whose transcripts carry m6A peaks; abbreviated as m6A genes) were unevenly distributed across each chromosome (Fig. 1D).
Figure 1.
Overview of m6A methylome in maize. Fractions (A) and relative enrichment of m6A peaks (B) in five nonoverlapping transcript segments: 5′ UTRs, start codons (200-nucleotide window centered on the translational start sites), coding sequences (CDS), stop codons (200-nucleotide window centered on the translational stop codons), and 3′ UTRs. C, The motif on the top represents the canonical RRACH motif within 90.6% m6A peaks. The motif on the bottom is the enriched URUAY motif. D, The landscape of m6A genes and distribution of genomic features across ten chromosomes (chr) of the maize genome. From outside to inside, each track represents frequency of m6A genes (I), mean gene length (II), mean guanine-cytosine (GC) content (III), mean exon lengths (IV), mean intron lengths (V), mean exon number (VI), and (VII) mean distance to adjacent gene (VII); larger distances are associated with centromeric sequences.
We observed that 90.6% of 11,968 m6A peaks contain the canonical motif RRACH (where R represents A/G, A is m6A, and H represents A/C/U) in maize (Fig. 1C; Supplemental Table S3). As shown in Supplemental Table S3, this proportion is comparable to those (80.6%, 92.3% and 81.2%) estimated using m6A peaks generated from three recent Arabidopsis m6A methylome studies (Luo et al., 2014; Shen et al., 2016; Anderson et al., 2018). These 11,968 m6A peaks were further scanned for enriched motifs using the MEME suite (http://meme-suite.org/index.html; Bailey et al., 2009). As expected, the RRACH motif is significantly enriched within maize m6A peaks (Supplemental Fig. S5). We also noted an interesting motif, URUAY (where Y represents C/U; Fig. 1C), which can also be detected from m6A peaks from each replicate sample (Supplemental Fig. S6). The URUAY motif has recently been regarded as a plant-specific consensus motif recognized by m6A reader protein ECT2 (Wei et al., 2018). Indeed, as shown in Supplemental Figure S5, this URUAY motif is also enriched within m6A peaks generated from three recent Arabidopsis m6A methylome studies (Luo et al., 2014; Shen et al., 2016; Anderson et al., 2018). By using another commonly used motif enrichment analysis software, the HOMER suite (v4.10; http://homer.ucsd.edu/homer; Heinz et al., 2010), enriched URUAY motif can also be detected from maize and Arabidopsis m6A data used in our study (Supplemental Fig. S5). Dot-blot assay was also performed to validate the specificity of m6A antibody for the URUAY motif (Supplemental Fig. S7).
The transcriptome-wide m6A map in maize is provided for the benefit of the readers in the future analysis. An overview of the transcriptome-wide m6A map supported by JBrowse (Buels et al., 2016) and downloadable Browser Extensible Data format files may be accessed in the Maize Epigenetic Data Browser (MEDB), which is publicly available at http://bioinfo.nwafu.edu.cn/MaizeBrowse/index.html.
m6A Genes Exhibit Distinct Sequence Features from Non-m6A Genes
To identify sequence features that may associate with m6A modification, we first tested the Pearson correlation coefficient (PCC) between the frequency of m6A genes and different sequence features (gene length, exon length, exon number, GC content, intron length, and gene distance) along the maize genome in a sliding window of 100 adjacent genes (Supplemental Table S4). The statistical significance of PCC was assessed using 10,000 permutation tests; in each, 11,219 m6A genes were randomly selected from the maize B73 genome annotation, and a new PCC value was calculated for generating a background distribution. The corresponding P value was calculated as P = (1 + N)/10,000; here, N is the number of PCCs in the background distribution, which exceeds the PCC for the original data. A P value <0.0001 denotes none of the 10,000 permutation tests exceeding the PCC for the original data. We observed that the frequency of m6A genes was slightly positively correlated with exon length (PCC = 0.06; P = 0.0955; Supplemental Fig. S8) but significantly positively correlated with gene length (PCC = 0.33; P value < 0.0001) and exon number (PCC = 0.57; P < 0.0001; Fig. 2, A and B; Supplemental Fig. S8). In addition, the frequency of m6A genes was significantly negatively correlated with GC content (PCC = −0.16; P = 0.0002) and gene distance (PCC = −0.36; P < 0.0001; Fig. 2, C and D; Supplemental Fig. S8).
Figure 2.
The correlation of m6A genes with multiple gene features. A to D, Correlations between the frequency of m6A genes and mean gene length, mean exon number, mean GC content, and mean distance to adjacent gene. E to G, Comparison of gene features (exon number, GC content, and intron length) among different m6A gene types. Genes are divided into three categories, according to the number of m6A sites per gene. Statistical analysis was conducted using Student’s t test. **P < 0.001. H, Density plot of gene length for three gene categories.
Complementary to the correlation analysis using contiguous windows, we further performed statistical analysis using sequence features from individual genes. Maize genes were split into three groups according to the corresponding number of m6A peaks from our study: low-m6A genes (10,559 genes carrying only one m6A peak), high-m6A genes (660 genes carrying at least two m6A peaks), and non-m6A genes (28,105 genes carrying no m6A peak; Supplemental Table S2). Compared with non-m6A genes, m6A genes (both low-m6A genes and high-m6A genes) had significantly more exons (Fig. 2E), lower GC content (Fig. 2F), and longer introns (Fig. 2G). The mean gene length is in the order of high-m6A genes (11,796 bp) > low-m6A genes (7,271 bp) > non-m6A genes (2,849 bp; Fig. 2H). These results may indicate that longer genes tend to have a higher probability of containing the m6A peaks.
The aforementioned statistical analysis using contiguous windows and individual genes was also performed on 10,604 and 10,085 m6A genes determined from m6A-seq data sets of B73 and Han21, separately. We found that there is no substantial difference between the statistical results for the two inbred lines (Supplemental Figs. S9 and S10). This result is as expected, as a substantial number of m6A genes (9,470) were overlapped in both maize inbred lines (Supplemental Fig. S11A). We observed that significant differences exist between 1,134 B73-specific and 615 Han21-specific m6A genes in the distribution of GC content and average intron length (Supplemental Fig. S11B). Next, we examined whether there are significant differences in nucleotide sequence variation between m6A genes from these two inbred lines (Han21 and B73). Comparison analysis revealed that the SNP density in m6A genes is significantly lower than that in non-m6A genes (Supplemental Fig. S12).
Overall, these results suggest that m6A modification in maize is correlated with multiple sequence features, including gene length, exon number, intron length, GC content, and SNP density.
Biased Fractionation of m6A Genes Exists between Two Subgenomes in Maize
The maize genome underwent its most recent WGD shortly after divergence from sorghum (Schnable et al., 2011). After the WGD event, one copy of many duplicate gene pairs in maize was lost (fractionated), leaving the other one as a singleton. Because of the biased gene fractionation, the loss of duplicate genes is uneven between two maize subgenomes (maize1 and maize2), so that the maize1 subgenome retains more genes than the maize2 subgenome (Brohammer et al., 2018). In human and yeast, the evolutionary consequences of gene duplication are associated with DNA methylation (Keller and Yi, 2014), gene expression (Gout et al., 2010), as well as posttranslational modification (Amoutzias et al., 2010). Considering that m6A modification is correlated with multiple sequence features (Fig. 2; Supplemental Fig. S11) and has a role in regulating gene expression (Yue et al., 2015; Roignant and Soller, 2017; Roundtree et al., 2017a), we raised the question of whether m6A modification might also be linked to duplicate gene retention.
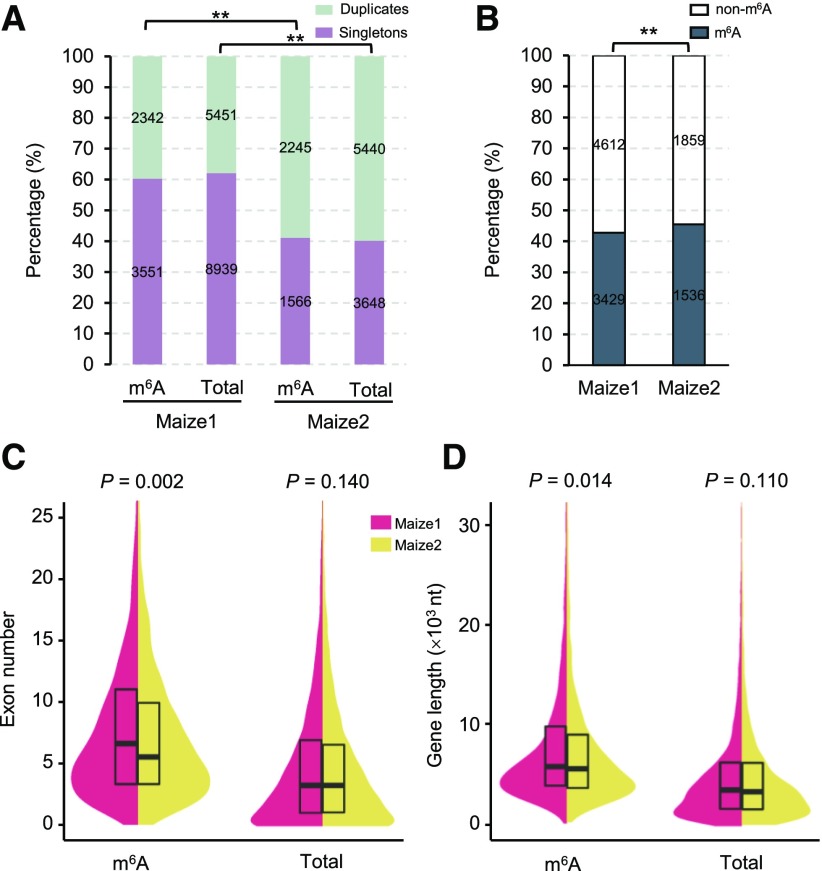
We first examined the duplication status of m6A genes retained after the most recent WGD event. Genes in maize1 and maize2 were identified by performing syntenic analysis between the maize B73 reference genome and the sorghum reference genome (Brohammer et al., 2018). Among 11,219 m6A genes, 5,893 and 3,811 were annotated as maize1 and maize2 genes, respectively (Supplemental Table S2). The singleton-duplicate ratio of m6A genes in maize1 (1:0.66; 3,551 vs. 2,342) is significantly higher than that of m6A genes in maize2 (1:1.43; 1,566 vs. 2,245; χ2 test; P < 0.001), which is consistent with the trend in total subgenome genes (Fig. 3A). The significant difference in singleton-duplicate ratio between maize1 and maize2 was also apparent when m6A genes in tandem duplication were not involved (Supplemental Fig. S13). Notably, the frequency of m6A genes in maize2 singletons is significantly higher than that in maize1 singletons (Fig. 3B). These divergences of m6A genes between two subgenomes are most likely the evolutionary consequence of biased subgenome fractionation. The biased fractionation of m6A singletons between two subgenomes may associate with multiple sequence features. As shown in Figure 3, C and D, singletons carrying m6A peaks (m6A singletons) in maize1 have significantly more exons and longer nucleotides than those in maize2 (Student’s t test; P < 0.05), but these features were not significantly different between total maize1 singletons and maize2 singletons. These suggest that the biased fractionation of m6A genes may be relative to gene length.
Figure 3.
Evolutionary influences on RNA m6A methylome bias between two maize subgenomes. A, Comparison of singleton-duplication ratio of m6A genes and total subgenome genes in maize1 and maize2. Statistical analysis was conducted using the χ2 test. **P < 0.001. B, Comparison of m6A gene frequency in maize1 singletons and maize2 singletons. Statistical analysis was conducted using the χ2 test. **P < 0.001. C, D, Comparison of exon number and gene length of m6A singletons in maize1 and maize2. Statistical analysis was conducted using Student’s t test.
We then compared the evolutionary rate (ω) of m6A genes from maize1 and maize2 subgenomes. The evolutionary rates, ratio of nonsynonymous substitution (Ka)/synonymous sites (Ks), of genes in maize were estimated using interspecific comparisons with putatively orthologous sequences between maize and sorghum. The ω values of m6A genes were significantly higher than those of non-m6A genes, and Ks values of m6A genes were considerably lower than those of non-m6A genes (Student’s t test; P < 0.001; Supplemental Fig. S14). This is indicative of higher evolutionary rate and less evolutionary time of m6A genes. We also observed that the evolutionary rate of m6A singletons in maize1 was significantly lower than that of m6A singletons in maize2, but the evolutionary rate of non-m6A singletons was not significantly different between maize1 and maize2 (Table 1). These findings indicated that m6A singletons in maize1 have experienced a higher intensity of purifying selection than those in maize2. This asymmetric purifying selection may have an influence on biased fractionation of m6A singletons. In contrast, m6A duplicates in maize1 and maize2 evolved under similar levels of purifying selection, but the evolutionary rate of non-m6A duplicates in maize1 was significantly lower than that of non-m6A duplicates in maize2 (Table 1). This indicated that m6A modification could associate with the divergence of evolutionary rate between duplicate genes in two subgenomes.
Table 1. Comparison of evolutionary rates (ω) of m6A singletons and m6A duplicates, and non-m6A singletons and non-m6A duplicates in maize1 and maize2, respectively.
Dashes indicate no data.
| Comparison | m6A Singletons | m6A Duplicates | P valuea | Non-m6A Singletons | Non-m6A Duplicates | P valuea |
|---|---|---|---|---|---|---|
| Maize1 | 0.2485 ± 0.1560 | 0.2256 ± 0.1617 | < 0.0001 | 0.2372 ± 0.7533 | 0.2088 ± 0.1625 | 0.0108 |
| Maize2 | 0.2598 ± 0.1631 | 0.2283 ± 0.1568 | < 0.0001 | 0.2330 ± 0.1734 | 0.2146 ± 0.1632 | < 0.0001 |
| P valuea | 0.0101 | 0.2861 | – | 0.4050 | 0.0244 | – |
Statistical analysis was conducted using the Student’s t test.
Further analysis of m6A duplicates showed that the evolutionary time (Ks) was significantly different from duplicate gene pairs with different m6A patterns: non-m6A pattern (neither of two partners carrying m6A peaks), diverged-m6A (DM) pattern (one partner had m6A peaks while the other did not), and identical-m6A (IM) pattern (both of two partners carrying m6A peaks). Duplicate genes with non-m6A pattern had the significantly highest level of Ks values, followed by duplicate genes with DM and IM patterns (Student’s t test; P < 0.001; Supplemental Fig. S15). Gene transposition could cause the separation of syntenic duplicates into two singletons. Protein sequence comparison between the maize and sorghum genome identified 198 and 108 pairs of transposed singletons in maize1 and maize2, respectively (Supplemental Table S2). We observed that transposed gene pairs had a significantly higher proportion of divergence of m6A status than syntenic duplicate gene pairs without transposition (χ2 test; P < 0.001; Supplemental Fig. S16). These observations indicate that m6A modification divergence in young duplicate pairs was smaller than that in older duplicate pairs, and gene transposition could enhance the extent of m6A divergence between duplicate pairs.
Coevolutionary Consequences of m6A Methylome and TE Influence Duplicate Retention and Expression Divergence
We then explored whether there was an association between m6A modification and gene expression in maize. Similar to that for m6A-seq, a series of RNA-seq libraries was constructed using leaf tissue of maize B73 and Han21 seedlings under both WW and DS conditions, with three biological replicates each (Supplemental Table S1). For each of these RNA-seq libraries, the expression abundance of maize genes was estimated in terms of fragments per kilobase per million (FPKM). We observed that the expression abundance of m6A genes was significantly higher than that of non-m6A genes (Fig. 4A), and the singleton-duplicate ratio of m6A genes (1:0.71; 6,329 vs. 4,520) was significantly lower than that of non-m6A genes (1:0.34; 18,523 vs. 6,234; χ2 test; P < 0.001), reflecting an overall higher retention rate for duplicate genes methylated by m6A (Fig. 4B). The association between m6A modification and gene expression was also revealed by the differential expression abundance between m6A duplicates and singletons in maize (Supplemental Fig. S17).
Figure 4.
RNA m6A modification enhanced gene stability and contributed to duplicate retention. A, Comparison of expression abundance between m6A genes and non-m6A genes. B, Comparison of ratios of duplicates to singletons in m6A genes and non-m6A genes. C, Comparison of expression abundance divergency between IM pattern duplicates and DM pattern duplicates. D, Comparison of expression abundance between two DM duplicate partners, methylated partner (MP) and nonmethylated partner (Non-MP). In A, C, and D, box plots range from the first (Q1) to the third quartile (Q3) of the distribution and represent the interquartile range (IQR). A line across the box indicates the median. The whiskers are lines extending from Q1 and Q3 to end points that are defined as the most extreme data points within Q1 − 1.5 × IQR and Q3 + 1.5 × IQR, respectively. Statistical analysis was conducted using Student’s t test. **P < 0.001. In B, statistical analysis was conducted using the χ2 test. **P < 0.001.
With the m6A-seq and RNA-seq data at hand, we were interested in whether m6A modification divergence associated with ED for duplicate genes. For a duplicate gene pair (G1, G2), the ED was calculated using the following formula: ED = (E1 − E2)/(E1 + E2), where E1 and E2 denote the mean expression level of G1 and G2, respectively. The ED of m6A genes with DM pattern was significantly higher than that of genes with IM pattern (Fig. 4C). For genes with DM pattern, the methylated partners exhibited a higher level of gene expression than nonmethylated partners (Fig. 4D). These findings suggested that m6A modification was more likely to occur on actively transcribed genes and could be associated with the retention rate and ED of duplicate gene pairs.
Previous studies have reported that the frequency of TEs, which is associated with local genomic stability, also affects the expression of their neighboring genes (Sahebi et al., 2018). This prompted us to investigate whether there is an evolutionary interplay between m6A and TEs. In our data, we observed that m6A genes are closer to TEs than non-m6A genes (Fig. 5A), and the frequency of TE-related genes (gene loci overlap with TE loci) carrying m6A peaks (61.1%; 6,850/11,219) was significantly higher than that of non-m6A genes (57.3%; 16,118/28,090; χ2 test; P < 0.01; Fig. 5B). Moreover, by comparing the evolutionary rates of m6A genes with those of non-m6A genes, significantly higher ω values were observed from m6A genes (Fig. 5C), and ω values of TE-related m6A genes were significantly higher than those of non-TE-related m6A genes (Fig. 5D). These evidences suggest that, after WGD in maize, genes with m6A modification had undergone relaxed selection, which is accompanied by the gathering of TEs close to genes; such tendencies would be indicative of coevolution between m6A genes and TEs.
Figure 5.
Evidence for coevolution of m6A genes and TEs. A, Comparison of distance to the nearest TEs between m6A genes and non-m6A genes. B, Comparison of TE frequency between m6A genes and non-m6A genes. C, Comparison of evolutionary rates between m6A genes and non-m6A genes. D, Comparison of evolutionary rates between TE-related m6A genes and non-TE-related m6A genes. E, Comparison of frequency of m6A methylation between tandem duplicated (TD) genes and non-TD genes. F, Comparison of frequencies of DM pattern and IM pattern between TD clusters and WGD pairs. G, Comparison of distance to the nearest TEs between m6A TD genes and m6A non-TD genes. H, Comparison of frequency of TEs between m6A TD genes and m6A non-TD genes. In A, C, D, and G, box plots range from the Q1 to Q3 of the distribution and represent the IQR. A line across the box indicates the median. The whiskers are lines extending from Q1 and Q3 to end points that are defined as the most extreme data points within Q1 − 1.5 × IQR and Q3 + 1.5 × IQR, respectively. Statistical analysis was conducted using Student’s t test. *P < 0.05; **P < 0.001. In B, E, F, and H, statistical analysis was conducted using the χ2 test. *P < 0.05; **P < 0.001.
The coevolution between m6A genes and TEs was also exemplified by evolutionary analysis of TD genes methylated by m6A. In total, we obtained 4,448 TD genes from 1,758 TD clusters identified by Kono et al. (2018). We observed that both Ka and Ks values of m6A and non-m6A TD genes are significantly higher than those of non-TD genes. However, ω values for m6A TD genes are significantly lower than those of m6A non-TD genes, and there is no significant difference between ω values for non-m6A TD genes and non-m6A non-TD genes (Supplemental Table S5). These findings suggest that although both m6A and non-m6A genes involved in TD events have had a higher substitution rate than those not involved, m6A genes have been under stronger selective constraint during TD events than non-m6A genes. After that, we found the frequency of m6A in TD genes is significantly lower than that in non-TD genes (Fig. 5E), and the ratio of DM pattern to IM pattern in TD clusters (2.04:1; 228 vs. 112) was significantly higher than that observed in WGD duplicates (0.56:1; 990 vs. 1,765; Fig. 5F). Remarkably, m6A TD genes were significantly less distant from TEs than non-TD genes (Fig. 5G), and 65.9% (323/490) of m6A TD genes were TE-related genes. This ratio was significantly lower in non-TD genes (60.8%; 6,527/10,729; Fig. 5H). These results suggest that the evolutionary scenario of m6A TD genes is accompanied by a preferential accumulation of TEs during the process of TD events.
Evolution of m6A Functional Factors and Their Influences on Hypomethylation of Transcripts of Drought-Stress Response Genes
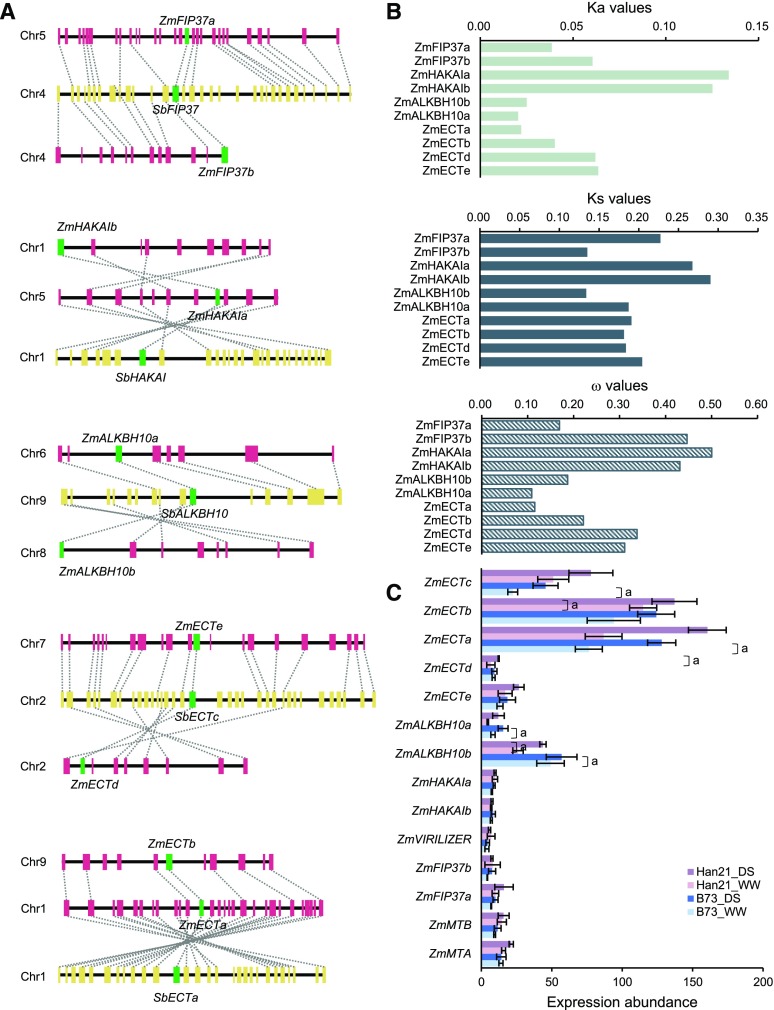
Phylogenetic analysis identified the functional counterparts of the m6A methyltransferases (N6-adenosine-methyltransferase MT-A70-like, methyltransferase MT-A70 family protein, FIP37, putative ortholog of human KIAA1429 [VIRILIZER], and the E3 ubiquitin ligase hakai [HAKAI]), demethylases (ALKBH10B), and binding proteins (ECT2, ECT3, and ECT4) in maize (Supplemental Fig. S18). According to the phylogenetic tree and genomic coordinates, five homologous pairs formed by the WGD event were identified: ZmFIP37a/ZmFIP37b, ZmHAKAIa/ZmHAKAIb, ZmECTa/ZmeECTb, ZmECTd/ZmECTe, and ZmALKBH10a/ZmALKBH10b (Fig. 6A). For ZmHAKAIa/ZmHAKAIb and ZmECTd/ZmECTe, their corresponding partners showed very similar evolutionary rates, as reflected by Ka, Ks, and ω (Fig. 6B). In contrast, for the other three duplicate pairs, their corresponding partners exhibited notably distinct evolutionary rates, which appeared to exhibit different strengths of purifying selection. These results indicate that evolutionary conservation and divergence of m6A functional factors may be mediated by WGD.
Figure 6.
Evolutionary dynamics of genes encoding m6A functional factors. A, Orthologous relationships of genes encoding m6A functional factors between maize and sorghum. Purple boxes indicate maize genes, and yellow boxes indicate sorghum genes. Green boxes represent syntenic orthologs of m6A functional factors. B, Evolutionary rates of maize m6A functional factors compared with their syntenic homologs in sorghum. C, RNA-seq expression profiles of genes encoding m6A functional factors. Data are means ± sd (n = 3, three biological replicates). A, The significance test of differential expression was conducted using Cuffdiff software; false discovery rate (FDR)–adjusted P value ≤0.05.
We performed RNA-seq analysis to quantify the expression abundance of m6A functional factors in the leaf sample of two maize inbred lines’ responses to drought stress. In the drought-sensitive inbred line B73, two ALKBH10 homologs and two ECT2/ECT4 homologs were significantly upregulated by drought stress (Fig. 6C). These expression patterns were also verified by quantitative PCR (qPCR) assay (Supplemental Fig. S19). Meanwhile, the m6A abundance in DS samples was significantly lower than that in WW samples, indicating global m6A hypomethylation induced by drought stress (Supplemental Fig. S20, A and B). For each maize line, differential methylated peaks (DMPs) were identified by comparing the abundance of m6A peaks between DS and WW conditions (Supplemental Table S6). The drought-tolerant Han21 line (2,998) has more DMPs than the drought-sensitive B73 line (386). However, the proportion of hypomethylated DMPs in Han21 (92.0%; 2,758/2,998) is comparable to that in B73 (87.8%; 339/386; Supplemental Fig. S20C). These results may reflect the phenomenon that levels of m6A modification are significantly decreased during drought stress in both inbred lines. Considering the expression patterns of m6A methyltransferase and demethylase genes, the induced expression of m6A demethylase genes most likely contributes to the decrease in m6A methylation during drought stress.
Most DMPs were located in 3′-UTR and stop codon regions in both B73 and Han21 (Supplemental Fig. S21A). A recent article reviewed that the genic locations of m6A peaks play distinct roles in mediating the functional output of m6A modification (Shen et al., 2019). Therefore, we performed gene ontology (GO) enrichment analysis of genes containing DMPs to explore functional characteristics of DMPs in the context of genic location. In B73, the GO terms “protein phosphorylation” and “regulation of apoptotic process” were specifically enriched in genes with DMPs within 3′-UTR, whereas the GO terms “histone exchange” and “cytoplasmic translation” were specifically enriched in genes within DMPs near the stop codon (Supplemental Fig. S21B). In Han21, the GO terms “regulation of transcription” and “protein transport” were specifically enriched in genes with DMPs within 3′-UTR, whereas the GO terms “photoreactive repair” and “siderophore biosynthetic process” were specifically enriched in genes within DMPs near DMPs (Supplemental Fig. S21B). These results revealed that genes containing DMPs in specific genic locations play roles in distinct biological processes in B73 and Han21.
The Han21 line was significantly more tolerant of drought stress than B73, which manifests as dramatic phenotypic differences observed, including increased plant height, alterations in relative water content and water loss rate, and robustness in root development (Supplemental Fig. S22). To understand the potential role of m6A hypomethylation in these phenotypic differences, we performed GO analysis of genes containing hypomethylated m6A peaks in B73 and Han21, respectively (Supplemental Fig. S23). In B73, genes involved in cuticle development, negative regulation of apoptotic signaling pathway, and response to abscisic acid are highly enriched. In Han21, genes were significantly enriched in response to abiotic stress processes, such as cellular response to oxidative stress, response to osmotic stress, acetyl-CoA metabolic process, and ethylene-mediated signaling pathway, as well as several developmental pathways, such as cell morphogenesis and development, embryo development ending in seed dormancy, Glc and starch metabolic process, and supramolecular fiber organization. These gene functions showed clear correspondence to the observed phenotypic responses of these two maize inbred lines.
Moreover, the differences in drought tolerance between B73 and Han21 can also be explored at the level of individual genes covering DMPs. VACUOLAR INVERTASE2 (VI2) is a positive regulator of root elongation in Arabidopsis (Sergeeva et al., 2006). The Han21 line showed more than a 2-fold reduction in methylation levels of m6A peaks in VI2 during drought stress (Supplemental Fig. S24). m6A peaks in Actin-7 (ACT7), a member of the actin gene family involved in root growth, cell division, and root architecture in Arabidopsis (Kandasamy et al., 2009), are also less methylated in DS samples of the maize B73 line (Supplemental Fig. S24). Furthermore, increased accumulation of epicuticular waxes is known to limit water loss and increase water deficit tolerance (Aharoni et al., 2004; Zhang et al., 2005; Kosma et al., 2009). The striking disparity in relative water content and water loss rate is accompanied by marked differences in the methylation levels of m6A peaks in genes associated with wax deposition between B73 and Han21. For example, ECERIFERUM4 and ECERIFERUM10, two genes encoding the alcohol-forming fatty acyl-CoA reductase, are involved in cuticular wax biosynthesis in Arabidopsis (Rowland et al., 2006). Methylation levels of m6A peaks in putative maize orthologs of these two genes are significantly inhibited by drought stress in Han21 and B73, respectively (Supplemental Fig. S24). Furthermore, methylation level of m6A peaks in wax ester synthase/acyl-coenzyme A: diacylglycerol acyltransferase (WSD1), a gene encoding diacylglycerol acyltransferase that is required for stem wax ester biosynthesis in Arabidopsis (Li et al., 2008), is also significantly repressed by drought stress in Han21 (Supplemental Fig. S24). Then, we performed m6A-IP-qPCR assay to further confirmed the m6A methylation levels of these DMPs. As shown in Supplemental Figure S25, the results of the m6A-IP-qPCR assay are consistent with those of m6A-seq. Together, these observations demonstrate that the concerted hypomethylation of candidate transcripts encoding proteins may associate with wax accumulation during response to drought stress.
DISCUSSION
Over the past several years, several studies have demonstrated the important roles of epigenetic modifications in the evolutionary history of eukaryotic genomes (Zemach et al., 2010; Chang and Liao, 2012; Wang et al., 2014a; Patten, 2016). However, few transcriptome-wide studies have attempted to investigate the evolutionary patterns of RNA m6A modification in the context of GD events. In this study, we used maize as a model system to study the evolution of m6A methylome following GD events. Our analysis suggested that m6A modification alteration following GD events appears to be a significant source of evolutionary novelty within plants.
The Transcriptome-wide Map of m6A in Maize
Our study presents the transcriptome-wide map of RNA m6A modifications in the leaf tissue of maize seedlings. This resource provides us an opportunity to globally characterize m6A in large, complex plant genome. In our maize m6A-seq data, the URUAY motif has a lower enrichment E value than the canonical RRACH motif. Given that the URUAY motif is identified as a plant-specific consensus motif recognized by m6A reader protein ECT2 and the UGUA motif can be methylated by endogenous Arabidopsis m6A writer proteins (Wei et al., 2018), these phenomena indicated that those m6A writer proteins with methylation activity for the URUAY motif may be conserved between Arabidopsis and maize. However, our motif enrichment analysis using previously published Arabidopsis m6A-seq data showed that the enrichment E values of the URUAY motif are generally higher than those of the RRACH motif (Supplemental Fig. S5). These differences between maize and Arabidopsis likely represent the different m6A site biases and unique biological meanings of m6A methylation between two species, or may result from the distinct technical biases in m6A-seq library preparation among these studies. Further in-depth structural and functional analysis of m6A writer and reader proteins may help to clarify these biases.
Additionally, we found that the frequency of m6A genes is positively correlated with gene length, exon number, and intron length. We infer that the longer genes may have a higher probability of containing m6A modification. Previous study has shown that gene length is increasing with evolutionary time (Grishkevich and Yanai, 2014), and longer genes are more likely to be retained as duplicates (McGrath et al., 2014; Guo, 2017). Further considering the higher retention rate of m6A duplicates compared with non-m6A genes (Fig. 4B), we suspect that gene length may be an important genic property that linked the evolutionary relationship between m6A modification and gene duplication in maize.
To benefit the readers in the future analysis, we developed a web browser named MEDB to host the transcriptome-wide m6A map and to support navigation of the map and its interactive visualization, integration, comparison, and analysis. Taking advantage of the JBrowse system (Buels et al., 2016), MEDB also allows users to transfer their private m6A methylome data to the browser as custom tracks for easy cross-study comparison. To ensure the data security, MEDB does not require the users to upload their own files to the server. Instead, it can access local files or a uniform resource locator specifying the location of a remote file. The cross-study comparison can be performed through a degree of in-browser data analysis by combining data in tracks using arithmetic and set operations, for example, finding the union, intersection, or exclusive of two tracks. Notably, combination tracks can be further used as input to other combination tracks, allowing users to build up arbitrarily complex analysis tracks.
Correlation between m6A Methylation and Biased Subgenome Fractionation
In maize, a proportion of duplicate genes were lost because of different levels of purifying selection on two subgenomes, a process known as biased fractionation. Genes in the overfractionated subgenome (maize1) are distinct with respect to overall fitness, in contrast to genes in the underfractionated subgenome (maize2; Schnable et al., 2011). Our analysis revealed that the singleton-duplicate ratio of m6A in maize1 was significantly higher than that in maize2 (Fig. 3A), suggesting that biased subgenome fractionation also occurred in m6A genes. Further investigation revealed that maize1 singletons exhibited significantly lower m6A frequency than maize2 singletons (Fig. 3B), and ω values were significantly lower in m6A singletons of maize1 than those of maize2 (Table 1), indicating significantly higher levels of purifying selection on the fractionated m6A genes in maize1 than those in maize2. These results are complementary evidences for the hypothesis that maize1 underwent stronger purifying selection that resulted in a higher frequency of fractionation (Schnable et al., 2012; Pophaly and Tellier, 2015).
A bias of gene expression dominance toward the less fractionated subgenome has been previously observed in maize (Schnable et al., 2011). Indeed, we showed that m6A genes had higher expression levels than non-m6A genes, and m6A modification divergence was correlated with gene ED between duplicate partners (Fig. 4; Supplemental Fig. S17). Considering that gene expression levels impose a strong constraint on gene duplications and subgenome fractionation, these observations indicate that m6A modification divergence of duplicate genes influences gene expression abundance and, ultimately, the divergence of subgenome dominance in maize.
Complex Interplays among m6A Modification, TE Accumulation, and Tandem Duplication
The disruptive effects of TEs have been extensively documented, as they can integrate into the regulatory or coding region of host genes or induce ectopic/nonallelic recombination, which is often associated with lower levels of gene stability (Jangam et al., 2017). In our study, m6A genes were found to be less distant from TEs than non-m6A genes, and the frequency of TEs in m6A genes was higher than that in non-m6A genes (Fig. 5, A and B). m6A genes involved in TD events also showed less distance from TEs than those not involved, and the frequency of TE-related genes was much higher in TD genes than in non-TD genes (Fig. 5, G and H). These findings suggest that genes flanked by TEs were more likely to be methylated by m6A, and then this coordination of m6A and TEs experienced a more relaxed selection (Fig. 5D). Together with former observations that m6A modification can enhance stability of target genes (Fig. 4), we propose preferential accumulation of TEs near m6A genes, as a coevolutionary outcome of m6A modification and TEs, involves a compromise between optimal levels of gene stability and prevention of the damage done by active TEs.
In addition, we found that gene members in TD arrays showed higher rates of divergence in m6A modification than those in syntenic duplication pairs (Fig. 5F), and m6A genes involved in TD events evolved more quickly and underwent stronger purifying selection than those not involved (Supplemental Table S5). This is hypothesized to be a consequence of the effects of gene balance, the theory being that genes encoding proteins that interact with large numbers of other proteins are more sensitive to changes in stoichiometry than those that do not (Birchler and Veitia, 2010). The stoichiometry of members of multisubunit complexes can affect the amount of functional complete product, which in turn affects patterns of gene expression and, ultimately, the phenotype and evolutionary fitness (Birchler and Veitia, 2012). The observation that TD genes have higher loss rates of m6A modification relative to non-TD genes (Fig. 5E) is a complementary evidence for the hypothesis that these genes may be undergoing subfunctionalization, representing an evolutionary outcome of m6A modification mediated by TD events.
Potential Roles of m6A Modification in Maize Response to Drought Stress
Differential gene expression has been proven to be responsible for drought responses in plants (Zhu, 2016; Miao et al., 2017). Differential levels of m6A modification under drought stress have also been observed in both B73 and Han21 (Supplemental Fig. S20), suggesting that m6A modification may be another important contributor for drought responses. Here, we discuss the evolutionary consequences of five duplicate gene pairs encoding m6A functional factors that may contribute to responses to drought stress (Fig. 6, B and C). The two members of each of two duplicate gene pairs (ZmHAKAIa/ZmHAKAIb and ZmECTd/ZmECTe) exhibited similar intensities of purifying selection, suggesting that they have been exposed to similar selective constraints. This explained why these members all showed mild expression. In contrast, the evolutionary rates of two members of ZmFIP37 pairs varied, but their expression values were smooth. It is possible that these two members have differential effects on other phenotypes or during other developmental stages, regardless of whether their specific roles in drought responses have diverged. Notably, variations in the evolutionary rates and levels of expression between members of the ZmALKBH10 pair were observed, in line with recent reports in Arabidopsis (Duan et al., 2017), and which could be the outcomes of neofunctionalization or subfunctionalization as the adaptive consequences of gene duplication. The upregulated expression of genes encoding demethylases (ZmALKBH10) appears to be the primary force driving m6A hypomethylation under drought stress, which indicates that m6A hypomethylation may play a positive role in drought response.
Future Perspectives: Exploring the Dynamics of RNA m6A Methylation in Different Plant Species with Spatial, Temporal, and Environmental Dimensions
In this study, we highlighted the importance of generating a transcriptome-wide m6A map in plant species and uncovered the evolutionary patterns of m6A modifications associated with genomic duplication using m6A-seq data from the leaf tissue of maize seedlings. As the most abundant internal mRNA modification, m6A has gained increasing interest in the last few years to understand its dynamic roles of posttranscriptional regulation mechanism underlying key plant developmental processes, including embryo development (Růžička et al., 2017), shoot stem cell fate (Shen et al., 2016), floral transition (Duan et al., 2017), and trichome morphogenesis (Wei et al., 2018) in Arabidopsis m6A studies. We expect that, in the future, the m6A modification dynamics will be explored using transcriptome-wide m6A maps profiled from different developmental stages and tissues of maize (as well as other important plant species) under diverse environmental conditions. Comparison of m6A modifications within and across species will be performed to further elucidate the evolutionary mode of posttranscriptional regulation in plants.
MATERIALS AND METHODS
Plant Growth and Sample Preparation
Seeds from the B73 and Han21 inbred lines of maize (Zea mays) were germinated, and seedlings were transferred to a growth chamber with controlled environmental conditions (28°C day/26°C night, 16-h light/8-h dark). The relative water content of soil was maintained at 80% of the soil moisture capacity for WW seedlings and at 40% of soil moisture capacity for DS seedlings. When seedlings developed three fully expanded leaves, leaf samples (three biological replicates for each experimental condition) were harvested, immediately frozen in liquid nitrogen, and stored at −80°C for RNA isolation and sequencing.
RNA Isolation and PolyA+ mRNA Selection
For each leaf sample, total RNA was extracted using the RNAiso Plus (code no. 9109; TaKaRa) according to the manufacturer’s instructions. Polyadenylated (PolyA+) mRNA selection was performed using oligo(dT)25 magnetic beads (code no. S1419S; New England Biolabs) following the manufacturer’s protocol.
High-Throughput m6A-seq and RNA-seq
mRNA was randomly fragmented into ∼200-nucleotide-long fragments by RNA Fragmentation Reagents (Ambion). Fragmented RNA was incubated for 2 h at 4°C with 0.5 mg/mL anti-m6A polyclonal antibody (catalog no. 202003; Synaptic Systems) in IP buffer (150 mm NaCl, 0.1% [v/v] octylphenoxypolyethoxyethanol [Igepal CA-630], and 10 mm Tris-HCl [pH 7.4]) supplemented with RNasin Plus RNase inhibitor (Promega). The mixture was then immunoprecipitated by incubation with protein-A beads at 4°C for an additional 2 h. After extensive washing, bound RNA was eluted from the beads with 0.5 mg/mL N6-methyladenosine in IP buffer and precipitated by ethanol. TruSeq standard mRNA Sample Prep Kit (Illumina) was used to construct the libraries from immunoprecipitated RNA and input RNA according to a published protocol (Dominissini et al., 2013). Sequencing was performed on an Illumina HiSeq platform (Illumina), and 50-bp single-end reads were generated. A library for RNA-seq was generated using TruSeq Stranded mRNA Sample Prep Kit (Illumina). The resulting libraries were sequenced on a HiSeq platform (Illumina) to produce 2× 122-bp paired-end reads.
Analysis of Sequencing Data
Raw reads from RNA-seq and m6A-seq were trimmed to remove adaptor sequences and low-quality bases using the Trimmomatic v0.36 tool (Bolger et al., 2014). The quality of trimmed RNA-seq and m6A-seq reads was examined using the FastQC program (https://www.bioinformatics.babraham.ac.uk/projects/fastqc).
SNPs between B73 and Han21 were identified following the GATK Best Practices workflow (Van der Auwera et al., 2013). In brief, Han21 RNA-seq reads were first mapped to the B73 reference genome using STAR with parameters set as: outFilterMultimapNmax = 1; outSAMstrandField = intronMotif; and twopassMode = Basic (Dobin et al., 2013). Then, AddOrReplaceReadGroups and MarkDuplicates functions in the Picard suite (v2.20.0; https://broadinstitute.github.io/picard) were used to add read groups and remove duplicates, respectively. Subsequently, the SplitNCigarReads function in the GATK suite (McKenna et al., 2010) was used to split reads into exon segments and hard-clip sequences overhanging into the intronic regions. SNPs were detected using the HaplotypeCaller tool in the GATK suite with parameters set as: allowPotentiallyMisencodedQuals; dontUseSoftClippedBases; stand_call_conf = 20; ERC = GVCF; and nct = 20. The CombineGVCFs and GenotypeGVCFs functions in the GATK suite were used to merge gvcf files and to generate vcf files. The results were further filtered using the VariantFiltration tool in the GATK suite with recommended parameters: window = 35; cluster = 3; filterName = FS; FS > 60; filterName = QD; and QD < 2.0. Finally, Samtools v1.9 (Li et al., 2009) was used to select highly confidence SNPs with the following requirements: (1) nonreference alleles need to be consistent, (2) SNPs supported with ≥10 reads, and (3) SNPs supported with ≥2 samples.
The Han21 pseudogenome was built based on SNPs identified from our RNA-seq data. The trimmed B73 and Han21 RNA-seq reads were aligned to the maize B73 reference genome (B73_RefGen_v4) and Han21 pseudogenome, respectively, using Tophat v2.1.1 (Kim et al., 2013) with maximum intron length set to 10 kb, with default settings for other parameters. Unique mapping reads were provided as input to Cufflinks v2.2.1 (Trapnell et al., 2013) for normalization and estimation of gene expression level in terms of FPKM mapped reads (FPKM = counts of mapped fragments × 109/[length of transcript × total count of the mapped fragments]). Differential analysis was conducted using the Cuffdiff program in Cufflinks. In this study, maize B73 reference genome sequences and annotation were downloaded from Ensembl Plants (release 41; https://plants.ensembl.org; Kersey et al., 2018).
The trimmed B73 and Han21 m6A-seq reads were aligned to the maize B73 reference genome and Han21 pseudogenome, respectively, using the STAR v2.5.3a (Dobin et al., 2013) with parameters: alignIntronMin = 20; alignIntronMax = 10000; outFilterMultimapNmax = 1; and outFilterMismatchNmax = 1. Peak calling was performed using a “sliding window” method slightly modified from previous analysis (Luo et al., 2014) and implemented with the R package PEA (Zhai et al., 2018). To call m6A peaks, the reference genome was scanned using a 25-bp sliding window. A Fisher exact test was used to identify windows enriched for m6A by comparing normalized read counts of each window for IP and input samples. Benjamini-Hochberg was implemented to adjust the P value to FDR for multiple testing using the R function “p.adjust”. Significant windows were identified if fold change of normalized read count was more than two and FDR value was less than 0.05. Adjacent significant windows were merged together to form peak regions. Peaks with length less than 100 nt in length were excluded from the analysis. The called peaks within lowly expressed genes (FPKM < 1) were discarded. For each experimental condition, peaks that overlapped in at least two of three replicates were merged as confidence m6A peaks using slice function (lower = 2, rangesOnly = TRUE) in the IRanges package (Lawrence et al., 2013). Confident m6A peaks from four experimental conditions (B73_WW, B73_DS, Han21_WW, and Han21_DS) were further merged into a unique set of m6A peaks using slice function (lower = 1, rangesOnly = TRUE) in the IRanges package. The m6A peaks with significantly differential m6A modification levels between DS and WW conditions were determined using the QNB software (Liu et al., 2017), with the criteria set as enrichment fold change ≥2 or ≤0.5, and FDR ≤ 0.05. GO enrichment analysis was performed using topGO (Alexa et al., 2006).
Identification of Enriched Motifs within m6A Peaks
Two well-known motif analysis suites, MEME (Bailey et al., 2009) and HOMER (Heinz et al., 2010), were used to perform the motif enrichment analysis. The DREME (Discriminative Regular Expression Motif Elicitation) tool in the MEME suite (http://meme-suite.org/tools/dreme) was used to discover relatively short (up to 8 bp), ungapped motifs that are enriched within a set of target sequences (m6A peak sequences) relative to a set of control sequences (shuffled m6A peak sequences). The set of target sequences (target set) is composed of m6A peak sequences extracted from the reference genome (maize: B73_RefGen_v4; Arabidopsis [Arabidopsis thaliana]: The Arabidopsis Information Resource 10) using the fastaFromBed function in BEDTools software v2.28 (Quinlan and Hall, 2010). The set of control sequences (control set) is generated by randomly shuffling each of the m6A peak sequences while preserving the nucleotide frequencies. This shuffling process is performed by using the “fasta-shuffle-letters” utility (k = 1) provided by the MEME suite. These two sets of sequences were input to DREME for discovering motifs with the following parameters: minimum length of the motif, 5; maximum length of the motif, 7; E value threshold, 1E-5.
For a specified motif (e.g. RRACH or URUAY), the AME tool in the MEME suite (http://meme-suite.org/tools/ame) and findMotifs.pl script in the HOMER tool (http://homer.ucsd.edu/homer) were used to calculate the significance level of relative enrichment of this motif within target sequences relative to control sequences.
Synonymous (Ks) and Nonsynonymous (Ka) Substitutions in Homologous Maize Genes
The maize subgenome data (genes in maize1 and maize2) in our study were obtained from Brohammer et al. (2018) and were identified via performing syntenic analysis between the maize genome and the sorghum (Sorghum bicolor) genome. In brief, the SynMap pipeline (Lyons et al., 2008) was run to align the maize B73 reference genome (B73_RefGen_v4) against the sorghum reference genome (v3.1; http://phytozome.jgi.doe.gov) to identify maize genes in syntenic blocks relative to the ancestral state. Then, the subgenome identity of each maize chromosome was determined using a previously described method (Schnable et al., 2011). The maize tandem duplicate genes in our study were obtained from Kono et al. (2018). The coding sequences of homologous gene pairs were aligned using the MAFFT v7.271 software (Katoh and Standley, 2013). On the basis of sequence alignments, the synonymous (Ks) and nonsynonymous (Ka) substitutions and the resulting Ka/Ks values were calculated using the model-averaging method in the KaKs_calculator v2.0 (Wang et al., 2010).
Identification of Gene Transposition
The protein sequences of singleton genes in maize containing syntenic orthologs in sorghum were searched against protein sequences of the singletons without syntenic orthologs in sorghum using Protein BLAST. The BLAST hits with more than 80% similarity, which were over 80% in length, were considered potential candidate genes for transposition. When two singletons from maize were more similar to each other than they were to the sorghum syntenic orthologs, and one of the two maize genes was syntenic to the sorghum, the nonsyntenic copy of the gene was defined as a potentially transposed duplicate gene.
Phylogenetic Analysis and Identification of Genes Encoding m6A Functional Factors
The Arabidopsis writer, eraser, and reader of m6A modification were used, as previously described (Duan et al., 2017; Růžička et al., 2017; Arribas-Hernández et al., 2018; Scutenaire et al., 2018; Wei et al., 2018). The annotated protein sequences of three species (Arabidopsis Araport11, Oryza sativa v7, and S. bicolor v3.1.1) were downloaded from Phytozome V12 (https://phytozome.jgi.doe.gov/pz/portal.html). The Arabidopsis sequences were used as query sequences to obtain homologous proteins in three other species using local Protein BLAST with a cutoff as E value ≤10−5. Multiple sequence alignments of candidate full-length amino acid sequences were performed using MUSCLE with default options in the MEGA 7 software (Kumar et al., 2016). Phylogenetic trees were generated using the neighbor-joining method with 1,000 bootstraps.
Dot-Blot Assay
The dot-blot assay was performed following a previously published protocol (Nagarajan et al., 2019). In brief, we randomly chose two m6A peaks from our m6A-seq data that contained the UGUAU and UGUAC motifs, respectively. The two sequences were used as templates to synthesize four RNA oligos that contained either m6A or A at a single internal position within the URUAY motif. Oligos were denatured at 72°C in a heat block for 3 min. Then, the samples were loaded to the Amersham Hybond-N+ membrane (RPN119B; GE Healthcare). The membrane was then ultraviolet cross-linked in an HL-2000 HybriLinker Hybridization Oven (Ultraviolet Products). After cross-linking, the membrane was washed in 10 mL of wash buffer for 5 min and then blocked in 10 mL of blocking buffer for 1 h at room temperature with gentle shaking. Subsequently, the membrane was incubated with anti-m6A antibody (diluted 1:500; catalog no. 202003; Synaptic Systems) overnight at 4°C. The membrane was then washed in 10 mL of the wash buffer for 5 min three times, followed by incubation with horseradish peroxidase–conjugated mouse anti-rabbit IgG (diluted 1:10,000; catalog no. sc-2357; Santa Cruz Biotechnology) for 1 h at room temperature. The membrane was again washed in 10 mL of the wash buffer for 10 min four times. Then, the membrane was incubated with 5 mL of enhanced chemiluminescence western-blotting substrate for 5 min in darkness at room temperature and exposed with Hyperfilm enhanced chemiluminescence for a proper exposure period.
Measurement of Gene Expression Levels Using Real-Time qPCR
Total RNA was extracted following above described RNA isolation methods, and the total RNA was treated with DNaseI (code no. 2270A; TaKaRa). First-strand cDNA was synthesized using PrimeScript II First Strand cDNA Synthesis Kit (code no. 6210A; TaKaRa) according to the manufacturer’s instructions. qPCR was performed in three biological replicates × three technical replicates using CFX96 Real-Time PCR Detection System (Bio-Rad) with TB Green Premix Ex Taq II (Tli RNaseH Plus, code no. RR820A; TaKaRa). The Cyclophilin (GenBank: M55021) was used as an internal control (Lin et al., 2014). The 2-ΔΔCT method was used to calculate the gene expression levels. The primers used for real-time qPCR are listed in Supplemental Table S7.
Global m6A Quantification
Total RNA isolation and two rounds of PolyA+ mRNA selection were performed following the above described "RNA Isolation and PolyA+ mRNA Selection" section methods. The change of global m6A levels in mRNA was measured by EpiQuik m6A RNA Methylation Quantification Kit (Colorimetric; catalog no. P-9005; EpiGentek) following the manufacturer’s protocol.
Measurement of m6A Methylation Levels of Specific m6A Peaks Using m6A-IP-qPCR
For validation of m6A-seq results, m6A IP was performed using WW and DS B73 and Han21 samples, respectively. RNA samples were fragmented into ∼300-nucleotide-long fragments. Fragmented RNA was incubated for 2 h at 4°C with 0.5 mg/mL anti-m6A polyclonal antibody (catalog no. 202003; Synaptic Systems). After ethanol precipitation, the input RNA and immunoprecipitated RNA were subjected to reverse transcription and qPCR assays. The Cyclophilin (GenBank: M55021) was used as an internal control, since (1) Cyclophilin mRNA did not show any obvious m6A peak from m6A-seq data, (2) Cyclophilin showed relatively invariant expression levels between WW and DS samples, and (3) Cyclophilin is considered to be a housekeeping gene. Samples were performed in three biological replicates × three technical replicates. The m6A level of specific mRNA fragments was calculated by the ratio of RNA abundances, IP/input, as previously described by Shen et al. (2016). In brief, relative enrichment of each fragment was calculated by first normalizing the amount of a target cDNA fragment against that of internal control, and then normalizing the value for the immunoprecipitated sample against that for the input. The primers used for m6A-IP-qPCR are listed in Supplemental Table S7.
Physiological Phenotypes of Maize B73 and Han21
Seeds of the maize inbred lines, Han21 and B73, were germinated, and seedlings were transplanted into pots filled with sand and transferred to a growth chamber with controlled environmental conditions (16-h light/8-h dark cycle, 28°C day/26°C night temperature). The relative water content of soil was maintained at 80% of the soil moisture capacity for WW seedlings and at 40% of soil moisture capacity for DS seedlings.
To measure the relative water content of leaves, fresh leaves were harvested and weighed to determine the fresh weight (FW). Leaves were then saturated in water for 24 h at 4°C and weighed for the turgid weight. Last, leaves were dried in an oven at 80°C for 24 h, and the dry weight (DW) was measured. The relative water content (%) was calculated as (FW – DW)/(turgid weight – DW) × 100.
To measure the rate of water loss, fresh leaves were harvested, weighed for the FW, and then placed in a culture dish for 24 h (22°C and 70% relative humidity) to measure the dehydrated weight. Then, samples were dried in an oven at 80°C for 24 h, and the DW was measured. The rate of water loss (%) was calculated as (FW – dehydrated weight)/DW × 100.
Statistical Analysis
The Student’s t test was performed using the t.test function in R package. The χ2 test was performed using chisq.test function in R package.
Accession Numbers
All sequencing data have been deposited into the National Center for Biotechnology Information’s Sequence Read Archive database under the accession numbers SRP153627 and SRP125635.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Distribution pattern of m6A-IP reads along transcripts.
Supplemental Figure S2. Intersection among m6A peaks identified in three biological replicates of four experimental conditions.
Supplemental Figure S3. Correlation of m6A peak abundance among three biological replicates in four experimental conditions.
Supplemental Figure S4. Distribution of m6A peaks in four experimental conditions.
Supplemental Figure S5. Both RRACH and URUAY motifs are enriched within m6A peaks in maize and Arabidopsis.
Supplemental Figure S6. The enriched RRACH and URUAY motifs identified from m6A peaks in each replicated sample.
Supplemental Figure S7. Dot-blot analysis demonstrates m6A antibody specificity for URUAY motif.
Supplemental Figure S8. Statistical significance of correlation coefficients between the frequency of m6A genes with different gene features.
Supplemental Figure S9. Statistical significance of correlation coefficients between the frequency of m6A genes with different gene features in B73 and Han21.
Supplemental Figure S10. Comparison of gene features (exon number, GC content, and intron length) among different types of m6A genes in B73 and Han21, respectively.
Supplemental Figure S11. Comparison of m6A genes in B73 and Han21.
Supplemental Figure S12. Comparison of sequence variation patterns between m6A genes and non-m6A genes.
Supplemental Figure S13. Comparison of singleton-duplication ratio of m6A genes and total subgenome genes excluding tandem duplicates in maize1 and maize2.
Supplemental Figure S14. Comparison of Ka (nonsynonymous substitution), Ks (synonymous substitution), and ω (evolutionary rate) values of m6A genes and non-m6A genes.
Supplemental Figure S15. Comparison of evolutionary time (Ks) of three categories of duplicates (IM pattern, DM pattern, and non-m6A [NM] pattern).
Supplemental Figure S16. Pairwise comparison of frequencies of m6A divergence among maize1 transposition, maize2 transposition, and duplicates without transposition.
Supplemental Figure S17. Comparison of expression abundance between duplicates and singletons of genes with m6A modification.
Supplemental Figure S18. Phylogenetic relationship of m6A functional factors among maize, sorghum, and rice.
Supplemental Figure S19. Relative mRNA levels of m6A functional factors in WW and DS seedling samples of B73 and Han21.
Supplemental Figure S20. Hypomethylation of m6A induced by drought stress in B73 and Han21.
Supplemental Figure S21. Functional characteristics of DMPs in the context of genic location in B73 and Han21.
Supplemental Figure S22. Phenotypic responses of B73 and Han21 under WW and DS conditions.
Supplemental Figure S23. Genes containing drought-induced hypomethylated peaks are involved in various biological processes of plant development and abiotic stress.
Supplemental Figure S24. Dynamic m6A peaks of five drought-responsive genes (ZmVI2, ZmACT7, ZmCRE4, ZmCRE10, and ZmWSD1) in B73 and Han21.
Supplemental Figure S25. Validation of m6A peaks in five drought-responsive genes (ZmVI2, ZmACT7, ZmCRE4, ZmCRE10, and ZmWSD1).
Supplemental Table S1. Sequenced and mapped reads in m6A-seq, input RNA-seq, and mRNA-seq samples.
Supplemental Table S2. Characterization of maize genes regarding m6A modification and duplicate status.
Supplemental Table S3. Frequency of m6A peaks containing RRACH motif and URUAY motif in maize and Arabidopsis.
Supplemental Table S4. Correlation of m6A gene frequency with sequence features.
Supplemental Table S5. Comparison of evolutionary rates of m6A genes involved and not involved in tandem duplication (TD).
Supplemental Table S6. Differentially methylated peaks in B73 and Han21 in response to drought stress.
Supplemental Table S7. Primers used in this study.
Acknowledgments
We thank all our laboratory members at Northwest A&F University for their discussion on the project.
Footnotes
This work was supported by the National Natural Science Foundation of China (grant no. 31570371), the Natural Science Foundation Research Project of Shaanxi Province of China (grant no. 2019JQ–096), the Youth 1000-Talent Program of China, the Hundred Talents Program of Shaanxi Province of China, and the Fund of Northwest A&F University (grant nos. Z111021603 and Z111021403).
Articles can be viewed without a subscription.
References
- Adams JM, Cory S (1975) Modified nucleosides and bizarre 5′-termini in mouse myeloma mRNA. Nature 255: 28–33 [DOI] [PubMed] [Google Scholar]
- Aharoni A, Dixit S, Jetter R, Thoenes E, van Arkel G, Pereira A (2004) The SHINE clade of AP2 domain transcription factors activates wax biosynthesis, alters cuticle properties, and confers drought tolerance when overexpressed in Arabidopsis. Plant Cell 16: 2463–2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexa A, Rahnenführer J, Lengauer T (2006) Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinformatics 22: 1600–1607 [DOI] [PubMed] [Google Scholar]
- Amoutzias GD, He Y, Gordon J, Mossialos D, Oliver SG, Van de Peer Y (2010) Posttranslational regulation impacts the fate of duplicated genes. Proc Natl Acad Sci USA 107: 2967–2971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SJ, Kramer MC, Gosai SJ, Yu X, Vandivier LE, Nelson ADL, Anderson ZD, Beilstein MA, Fray RG, Lyons E, et al. (2018) N6-methyladenosine inhibits local ribonucleolytic cleavage to stabilize mRNAs in Arabidopsis. Cell Reports 25: 1146–1157.e3 [DOI] [PubMed] [Google Scholar]
- Arribas-Hernández L, Bressendorff S, Hansen MH, Poulsen C, Erdmann S, Brodersen P (2018) An m(6)A-YTH module controls developmental timing and morphogenesis in Arabidopsis. Plant Cell 30: 952–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS (2009) MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res 37: W202-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchler JA, Veitia RA (2010) The gene balance hypothesis: Implications for gene regulation, quantitative traits and evolution. New Phytol 186: 54–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchler JA, Veitia RA (2012) Gene balance hypothesis: Connecting issues of dosage sensitivity across biological disciplines. Proc Natl Acad Sci USA 109: 14746–14753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokar JA, Shambaugh ME, Polayes D, Matera AG, Rottman FM (1997) Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA 3: 1233–1247 [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30: 2114–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohammer AB, Kono TJY, Springer NM, McGaugh SE, Hirsch CN (2018) The limited role of differential fractionation in genome content variation and function in maize (Zea mays L.) inbred lines. Plant J 93: 131–141 [DOI] [PubMed] [Google Scholar]
- Buels R, Yao E, Diesh CM, Hayes RD, Munoz-Torres M, Helt G, Goodstein DM, Elsik CG, Lewis SE, Stein L, et al. (2016) JBrowse: A dynamic web platform for genome visualization and analysis. Genome Biol 17: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AY, Liao BY (2012) DNA methylation rebalances gene dosage after mammalian gene duplications. Mol Biol Evol 29: 133–144 [DOI] [PubMed] [Google Scholar]
- Cheng F, Wu J, Cai X, Liang J, Freeling M, Wang X (2018) Gene retention, fractionation and subgenome differences in polyploid plants. Nat Plants 4: 258–268 [DOI] [PubMed] [Google Scholar]
- Clancy MJ, Shambaugh ME, Timpte CS, Bokar JA (2002) Induction of sporulation in Saccharomyces cerevisiae leads to the formation of N6-methyladenosine in mRNA: A potential mechanism for the activity of the IME4 gene. Nucleic Acids Res 30: 4509–4518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR (2013) STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M, et al. (2012) Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485: 201–206 [DOI] [PubMed] [Google Scholar]
- Dominissini D, Moshitch-Moshkovitz S, Salmon-Divon M, Amariglio N, Rechavi G (2013) Transcriptome-wide mapping of N(6)-methyladenosine by m(6)A-seq based on immunocapturing and massively parallel sequencing. Nat Protoc 8: 176–189 [DOI] [PubMed] [Google Scholar]
- Duan HC, Wei LH, Zhang C, Wang Y, Chen L, Lu Z, Chen PR, He C, Jia G (2017) ALKBH10B is an RNA N6-methyladenosine demethylase affecting Arabidopsis floral transition. Plant Cell 29: 2995–3011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeling M. (2009) Bias in plant gene content following different sorts of duplication: Tandem, whole-genome, segmental, or by transposition. Annu Rev Plant Biol 60: 433–453 [DOI] [PubMed] [Google Scholar]
- Gout JF, Kahn D, Duret L; Paramecium Post-Genomics Consortium (2010) The relationship among gene expression, the evolution of gene dosage, and the rate of protein evolution. PLoS Genet 6: e1000944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grishkevich V, Yanai I (2014) Gene length and expression level shape genomic novelties. Genome Res 24: 1497–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B. (2017) Complex genes are preferentially retained after whole-genome duplication in teleost fish. J Mol Evol 84: 253–258 [DOI] [PubMed] [Google Scholar]
- Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK (2010) Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell 38: 576–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jangam D, Feschotte C, Betrán E (2017) Transposable element domestication as an adaptation to evolutionary conflicts. Trends Genet 33: 817–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, Yi C, Lindahl T, Pan T, Yang Y-G, et al. (2011) N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol 7: 885–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Peluso P, Shi J, Liang T, Stitzer MC, Wang B, Campbell MS, Stein JC, Wei X, Chin CS, et al. (2017) Improved maize reference genome with single-molecule technologies. Nature 546: 524–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy MK, McKinney EC, Meagher RB (2009) A single vegetative actin isovariant overexpressed under the control of multiple regulatory sequences is sufficient for normal Arabidopsis development. Plant Cell 21: 701–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol Biol Evol 30: 772–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller TE, Yi SV (2014) DNA methylation and evolution of duplicate genes. Proc Natl Acad Sci USA 111: 5932–5937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersey PJ, Allen JE, Allot A, Barba M, Boddu S, Bolt BJ, Carvalho-Silva D, Christensen M, Davis P, Grabmueller C, et al. (2018) Ensembl Genomes 2018: An integrated omics infrastructure for non-vertebrate species. Nucleic Acids Res 46(D1): D802–D808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL (2013) TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14: R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono TJY, Brohammer AB, McGaugh SE, Hirsch C (2018) Tandem duplicate genes in maize are abundant and date to two distinct periods of time. G3 8: 3049–3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosma DK, Bourdenx B, Bernard A, Parsons EP, Lü S, Joubès J, Jenks MA (2009) The impact of water deficiency on leaf cuticle lipids of Arabidopsis. Plant Physiol 151: 1918–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33: 1870–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence M, Huber W, Pagès H, Aboyoun P, Carlson M, Gentleman R, Morgan MT, Carey VJ (2013) Software for computing and annotating genomic ranges. PLOS Comput Biol 9: e1003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis R, Penman S (1978) 5′-terminal structures of poly(A)+ cytoplasmic messenger RNA and of poly(A)+ and poly(A)- heterogeneous nuclear RNA of cells of the dipteran Drosophila melanogaster. J Mol Biol 120: 487–515 [DOI] [PubMed] [Google Scholar]
- Li F, Wu X, Lam P, Bird D, Zheng H, Samuels L, Jetter R, Kunst L (2008) Identification of the wax ester synthase/acyl-coenzyme A: Diacylglycerol acyltransferase WSD1 required for stem wax ester biosynthesis in Arabidopsis. Plant Physiol 148: 97–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R; 1000 Genome Project Data Processing Subgroup (2009) The sequence alignment/map format and SAMtools. Bioinformatics 25: 2078–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Zhang C, Lan H, Gao S, Liu H, Liu J, Cao M, Pan G, Rong T, Zhang S (2014) Validation of potential reference genes for qPCR in maize across abiotic stresses, hormone treatments, and tissue types. PLoS One 9: e95445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X, et al. (2014) A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol 10: 93–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhang SW, Huang Y, Meng J (2017) QNB: Differential RNA methylation analysis for count-based small-sample sequencing data with a quad-negative binomial model. BMC Bioinformatics 18: 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo GZ, MacQueen A, Zheng G, Duan H, Dore LC, Lu Z, Liu J, Chen K, Jia G, Bergelson J, et al. (2014) Unique features of the m6A methylome in Arabidopsis thaliana. Nat Commun 5: 5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons E, Pedersen B, Kane J, Freeling M (2008) The value of nonmodel genomes and an example using SynMap Within CoGe to dissect the hexaploidy that predates the rosids. Trop Plant Biol 1: 181–190 [Google Scholar]
- Ma L, Zhao B, Chen K, Thomas A, Tuteja JH, He X, He C, White KP (2017) Evolution of transcript modification by N6-methyladenosine in primates. Genome Res 27: 385–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick RF, Truong SK, Sreedasyam A, Jenkins J, Shu S, Sims D, Kennedy M, Amirebrahimi M, Weers BD, McKinley B, et al. (2018) The Sorghum bicolor reference genome: Improved assembly, gene annotations, a transcriptome atlas, and signatures of genome organization. Plant J 93: 338–354 [DOI] [PubMed] [Google Scholar]
- McGrath CL, Gout JF, Johri P, Doak TG, Lynch M (2014) Differential retention and divergent resolution of duplicate genes following whole-genome duplication. Genome Res 24: 1665–1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, et al. (2010) The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20: 1297–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Z, Han Z, Zhang T, Chen S, Ma C (2017) A systems approach to a spatio-temporal understanding of the drought stress response in maize. Sci Rep 7: 6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan A, Janostiak R, Wajapeyee N (2019) Dot blot analysis for measuring global N6-methyladenosine modification of RNA. Methods Mol Biol 1870: 263–271 [DOI] [PubMed] [Google Scholar]
- Nichols JL. (1979) N6-methyladenosine in maize poly(A)-containing RNA. Plant Sci Lett 15: 357–361 [Google Scholar]
- Panchy N, Lehti-Shiu M, Shiu SH (2016) Evolution of gene duplication in plants. Plant Physiol 171: 2294–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson AH, Bowers JE, Bruggmann R, Dubchak I, Grimwood J, Gundlach H, Haberer G, Hellsten U, Mitros T, Poliakov A, et al. (2009) The Sorghum bicolor genome and the diversification of grasses. Nature 457: 551–556 [DOI] [PubMed] [Google Scholar]
- Patten MM. (2016) Epigenetics: Imprinting evolution in Arabidopsis. Nat Plants 2: 16152. [DOI] [PubMed] [Google Scholar]
- Ping XL, Sun BF, Wang L, Xiao W, Yang X, Wang WJ, Adhikari S, Shi Y, Lv Y, Chen YS, et al. (2014) Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res 24: 177–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pophaly SD, Tellier A (2015) Population level purifying selection and gene expression shape subgenome evolution in maize. Mol Biol Evol 32: 3226–3235 [DOI] [PubMed] [Google Scholar]
- Quinlan AR, Hall IM (2010) BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 26: 841–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roignant JY, Soller M (2017) m(6)A in mRNA: An ancient mechanism for fine-tuning gene expression. Trends Genet 33: 380–390 [DOI] [PubMed] [Google Scholar]
- Roundtree IA, Evans ME, Pan T, He C (2017a) Dynamic RNA modifications in gene expression regulation. Cell 169: 1187–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roundtree IA, Luo GZ, Zhang Z, Wang X, Zhou T, Cui Y, Sha J, Huang X, Guerrero L, Xie P, et al. (2017b) YTHDC1 mediates nuclear export of N6-methyladenosine methylated mRNAs. eLife 6: e31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland O, Zheng H, Hepworth SR, Lam P, Jetter R, Kunst L (2006) CER4 encodes an alcohol-forming fatty acyl-coenzyme A reductase involved in cuticular wax production in Arabidopsis. Plant Physiol 142: 866–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Růžička K, Zhang M, Campilho A, Bodi Z, Kashif M, Saleh M, Eeckhout D, El-Showk S, Li H, Zhong S, et al. (2017) Identification of factors required for m6 A mRNA methylation in Arabidopsis reveals a role for the conserved E3 ubiquitin ligase HAKAI. New Phytol 215: 157–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahebi M, Hanafi MM, van Wijnen AJ, Rice D, Rafii MY, Azizi P, Osman M, Taheri S, Bakar MFA, Isa MNM, et al. (2018) Contribution of transposable elements in the plant’s genome. Gene 665: 155–166 [DOI] [PubMed] [Google Scholar]
- Schnable JC, Springer NM, Freeling M (2011) Differentiation of the maize subgenomes by genome dominance and both ancient and ongoing gene loss. Proc Natl Acad Sci USA 108: 4069–4074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnable JC, Wang X, Pires JC, Freeling M (2012) Escape from preferential retention following repeated whole genome duplications in plants. Front Plant Sci 3: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S, Agarwala SD, Mumbach MR, Jovanovic M, Mertins P, Shishkin A, Tabach Y, Mikkelsen TS, Satija R, Ruvkun G, et al. (2013) High-resolution mapping reveals a conserved, widespread, dynamic mRNA methylation program in yeast meiosis. Cell 155: 1409–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scutenaire J, Deragon JM, Jean V, Benhamed M, Raynaud C, Favory JJ, Merret R, Bousquet-Antonelli C (2018) The YTH domain protein ECT2 is an m6A reader required for normal trichome branching in Arabidopsis. Plant Cell 30: 986–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeeva LI, Keurentjes JJ, Bentsink L, Vonk J, van der Plas LH, Koornneef M, Vreugdenhil D (2006) Vacuolar invertase regulates elongation of Arabidopsis thaliana roots as revealed by QTL and mutant analysis. Proc Natl Acad Sci USA 103: 2994–2999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Liang Z, Gu X, Chen Y, Teo ZW, Hou X, Cai WM, Dedon PC, Liu L, Yu H (2016) N(6)-methyladenosine RNA modification regulates shoot stem cell fate in Arabidopsis. Dev Cell 38: 186–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Liang Z, Wong CE, Yu H (2019) Messenger RNA modifications in plants. Trends Plant Sci 24: 328–341 [DOI] [PubMed] [Google Scholar]
- Shi H, Wang X, Lu Z, Zhao BS, Ma H, Hsu PJ, Liu C, He C (2017) YTHDF3 facilitates translation and decay of N6-methyladenosine-modified RNA. Cell Res 27: 315–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swigonova Z, Lai J, Ma J, Ramakrishna W, Llaca V, Bennetzen JL, Messing J (2004) On the tetraploid origin of the maize genome. Comp Funct Genomics 5: 281–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Hendrickson DG, Sauvageau M, Goff L, Rinn JL, Pachter L (2013) Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat Biotechnol 31: 46–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Auwera GA, Carneiro MO, Hartl C, Poplin R, Del Angel G, Levy-Moonshine A, Jordan T, Shakir K, Roazen D, Thibault J, et al. (2013) From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr Protoc Bioinformatics 43: 1–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vespa L, Vachon G, Berger F, Perazza D, Faure JD, Herzog M (2004) The immunophilin-interacting protein AtFIP37 from Arabidopsis is essential for plant development and is involved in trichome endoreduplication. Plant Physiol 134: 1283–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, He C (2014) Dynamic RNA modifications in posttranscriptional regulation. Mol Cell 56: 5–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Zhang Y, Zhang Z, Zhu J, Yu J (2010) KaKs_Calculator 2.0: A toolkit incorporating gamma-series methods and sliding window strategies. Genomics Proteomics Bioinformatics 8: 77–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Beyene G, Zhai J, Feng S, Fahlgren N, Taylor NJ, Bart R, Carrington JC, Jacobsen SE, Ausin I (2015) CG gene body DNA methylation changes and evolution of duplicated genes in cassava. Proc Natl Acad Sci USA 112: 13729–13734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Marowsky NC, Fan C (2014a) Divergence of gene body DNA methylation and evolution of plant duplicate genes. PLoS One 9: e110357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, Fu Y, Parisien M, Dai Q, Jia G, et al. (2014b) N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 505: 117–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Coe E, Nelson W, Bharti AK, Engler F, Butler E, Kim H, Goicoechea JL, Chen M, Lee S, et al. (2007) Physical and genetic structure of the maize genome reflects its complex evolutionary history. PLoS Genet 3: e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei LH, Song P, Wang Y, Lu Z, Tang Q, Yu Q, Xiao Y, Zhang X, Duan HC, Jia G (2018) The m6A reader ECT2 controls trichome morphology by affecting mRNA stability in Arabidopsis. Plant Cell 30: 968–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Wang X, Liu K, Roundtree IA, Tempel W, Li Y, Lu Z, He C, Min J (2014) Structural basis for selective binding of m6A RNA by the YTHDC1 YTH domain. Nat Chem Biol 10: 927–929 [DOI] [PubMed] [Google Scholar]
- Xu C, Liu K, Ahmed H, Loppnau P, Schapira M, Min J (2015) Structural basis for the discriminative recognition of N6-methyladenosine RNA by the human YT521-B homology domain family of proteins. J Biol Chem 290: 24902–24913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Hsu PJ, Chen YS, Yang YG (2018) Dynamic transcriptomic m6A decoration: Writers, erasers, readers and functions in RNA metabolism. Cell Res 28: 616–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KJ, Ringeling FR, Vissers C, Jacob F, Pokrass M, Jimenez-Cyrus D, Su Y, Kim NS, Zhu Y, Zheng L, et al. (2017) Temporal control of mammalian cortical neurogenesis by m6A methylation. Cell 171: 877–889.e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y, Liu J, He C (2015) RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes Dev 29: 1343–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemach A, McDaniel IE, Silva P, Zilberman D (2010) Genome-wide evolutionary analysis of eukaryotic DNA methylation. Science 328: 916–919 [DOI] [PubMed] [Google Scholar]
- Zhai J, Song J, Cheng Q, Tang Y, Ma C (2018) PEA: An integrated R toolkit for plant epitranscriptome analysis. Bioinformatics 34: 3747–3749 [DOI] [PubMed] [Google Scholar]
- Zhang JY, Broeckling CD, Blancaflor EB, Sledge MK, Sumner LW, Wang ZY (2005) Overexpression of WXP1, a putative Medicago truncatula AP2 domain-containing transcription factor gene, increases cuticular wax accumulation and enhances drought tolerance in transgenic alfalfa (Medicago sativa). Plant J 42: 689–707 [DOI] [PubMed] [Google Scholar]
- Zhao X, Yang Y, Sun BF, Shi Y, Yang X, Xiao W, Hao YJ, Ping XL, Chen YS, Wang WJ, et al. (2014) FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res 24: 1403–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ, Vågbø CB, Shi Y, Wang WL, Song SH, et al. (2013) ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell 49: 18–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Li H, Bodi Z, Button J, Vespa L, Herzog M, Fray RG (2008) MTA is an Arabidopsis messenger RNA adenosine methylase and interacts with a homolog of a sex-specific splicing factor. Plant Cell 20: 1278–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK. (2016) Abiotic stress signaling and responses in plants. Cell 167: 313–324 [DOI] [PMC free article] [PubMed] [Google Scholar]