Abstract
CRISPR/Cas9-mediated mutation of NRC2, NRC3, and NRC4 genes did not affect bacterial flagellin-triggered immunity.
Plants utilize cell surface pattern recognition receptors (PRRs) and intracellular nucleotide-binding domain Leu-rich repeat containing receptors (NLRs) to fend off invading pathogens (Dodds and Rathjen, 2010; Win et al., 2012). Both types of immune receptors detect pathogen molecules directly or indirectly to activate complex immune responses and disease resistance (Kourelis and van der Hoorn, 2018). Although PRR- and NLR-triggered immunity are generally thought to activate distinct pathways, they can induce similar outputs, such as production of reactive oxygen species (ROS) and hypersensitive cell death (Peng et al., 2018). Both PRR- and NLR-activated pathways involve calcium-dependent protein kinases, mitogen-activated protein kinases (MAPKs), phytohormone signaling, and transcriptional reprogramming (Peng et al., 2018). However, whether these two pathways converge at some point to potentiate and strengthen the immune response remains unclear. A recent study suggested that the tomato (Solanum lycopersicum) NLR helper NRC4 positively regulates the ROS burst induced by the bacterial flagellin peptide flg22 (Leibman-Markus et al., 2018a, 2018b). We took advantage of the CRISPR/Cas9 system to knock out multiple NRC genes in tomato and Nicotiana benthamiana. Although these mutants failed to respond to the NRC-dependent NLRs, they remained unaltered in flg22-induced responses. We conclude that the tested NRC genes are not essential for flg22-induced responses in tomato and N. benthamiana.
Throughout evolution, a subset of NLR proteins have functionally diversified into sensors that detect pathogen molecules and helpers (also known as executors) that operate genetically downstream of sensor NLRs in mediating the hypersensitive response and disease resistance (Cesari, 2018; Adachi et al., 2019). The emerging view is that although some singleton NLRs carry both sensor and helper activities, some sensor and helper NLRs form receptor complexes that range from pairs to networks (Wu et al., 2018; Adachi et al., 2019). One example of an NLR network is formed by the NRCs (NLR required for cell death) in asterid plants (Gabriëls et al., 2007; Wu et al., 2017). Over the last ∼100 million years, the NRC network has dramatically expanded from a pair of sensor and helper genes to form a complex network of phylogenetically related sensor and helper NLRs. In N. benthamiana, the NLR helpers NRC2, NRC3, and NRC4 are partially redundant but display varying degrees of specificity toward sensor NLRs that confer resistance to oomycete, bacterial, and viral pathogens (Wu et al., 2017). Interestingly, a recent study linked the tomato NRC SlNRC4a to PRR-triggered immunity (Leibman-Markus et al., 2018a, 2018b). Leibman-Markus et al. (2018b) reported that overexpression of SlNRC4a in N. benthamiana enhances ROS production elicited by the bacterial flagellin peptide flg22 and the fungal protein ethylene-inducing xylanase (EIX). Furthermore, SlNRC4a associates with the PRRs AtFLS2 and LeEIX in coimmunoprecipitation experiments. These results led Leibman-Markus et al. (2018a, 2018b) to conclude that SlNRC4a is a positive regulator of the immune response mediated by PRRs, notably the extensively studied FLS2 receptor.
Whereas Agrobacterium-mediated transient expression of SlNRC4a (Solyc04g007070, hereafter NRC4a) in N. benthamiana can enhance flg22-induced ROS burst (Leibman-Markus et al., 2018b), it remains unclear whether knocking out NRC4a affects flg22-induced responses in tomato. NRC4a occurs in the tomato genome as a gene cluster together with two closely related paralogous genes (SlNRC4b, Solyc04g007060, NRC4b; SlNRC4c, Solyc04g007030, NRC4c; Supplemental Fig. S1A). This gene cluster also contains another gene, Solyc04g007050, that we named SlNRC5 (NRC5), which is phylogenetically related to the NRCs (Wu et al., 2017). In this study, we decided to take advantage of the CRISPR/Cas9 system to generate loss-of-function mutants in the clustered NRC genes. We reasoned that the contribution of NRC4 paralogs in FLS2-mediated responses can be addressed by deleting the entire NRC4/5 gene cluster.
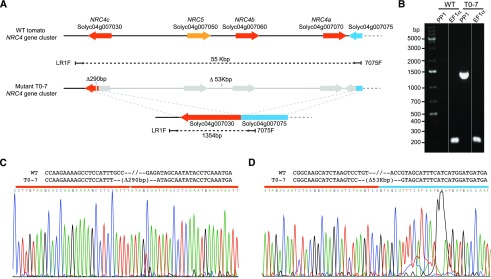
To knock out the NRC4 gene cluster in tomato, we designed four guide RNAs based on the conserved sequences in the NRC4 paralogs (Supplemental Fig. S1B). We transformed these guide RNAs together with Cas9 and a kanamycin selection marker into tomato GCR758 (Balint-Kurti et al., 1995). We recovered 13 independent transformants that are kanamycin resistant. To determine whether these transformants are mutated in the NRC4 gene cluster, we used gene-specific primers to amplify fragments of NRC4a, NRC4b, NRC4c, and NRC5 (Supplemental Fig. S1C; Supplemental Table S1). These primers amplified fragments with expected sizes when genomic DNA from wild-type plants was used as a template in the PCR reaction, but failed to amplify some of the NRCs (such as NRC4c and NRC4a) with genomic DNA from the line T0-1 (Supplemental Fig. S1C). Interestingly, we could not amplify any of the NRC4 and NRC5 fragments from the genomic DNA of the line T0-7, suggesting that this line contained multiple deletions or a large deletion in the locus of the NRC4 gene cluster (Supplemental Fig. S1C). To further confirm the genotype of the T0-7 plant, we designed four additional primers based on the sequences adjacent to NRC4c and NRC4a. Due to the distance between the primers (>50 kb based on the reference sequence), these primer pairs LR1F × 7075F and 7020R × 7075F could not amplify any fragments when the genomic DNA from the wild-type plant was used as a template (Supplemental Fig. S2). However, we successfully amplified fragments of 1.3 kb and 3.8 kb with the primer pairs LR1F × 7075F and 7020R × 7075F, respectively, using DNA from T0-7 (Fig. 1; Supplemental Fig. S2). Thus, we sequenced the 1.3-kb fragment amplified using the primer pair LR1F × 7075F by Sanger sequencing and confirmed that this plant contains a 53-kb deletion in the NRC4 locus, connecting the open reading frame (ORF) of NRC4c to the ORF of Solyc04g007075 (Fig. 1; Supplemental Fig. S3). In addition to the 53-kb deletion, we also found a 290-bp deletion in NRC4c (Fig. 1C). The remaining sequence resulted in a fusion of ORFs from Solyc04g007075 and NRC4c with multiple frameshift mutations leading to premature stop codons in NRC4c (Supplemental Fig. S3). We further obtained a homozygous T2 line (nrc4_7.4) and used this line for further experiments (Supplemental Fig. S4).
Figure 1.
The T0-7 transformant carries a large (>53 kb) deletion spanning across the NRC4 gene cluster. A, Schematic view of the tomato NRC4 gene cluster in wild-type (WT) and mutant T0-7. Orange, NRC4 paralogs; yellow, NRC5; blue, Solyc04g007075, which contains incomplete sequence information due to a sequencing gap in the reference genome. The deleted regions in the mutant T0-7 are marked in gray. B, PCR genotyping for the large deletion. PP1, amplification with primer pair 1:LR1F × 7075F indicated in A; EF1α amplification control with EF1α primers. The uncropped image is provided in Supplemental Figure S2B. C, Sequence alignment and chromatograms of Sanger DNA sequencing results. In this region, the mutant T0-7 contains a 290-bp deletion based on the reference genome and the results of sequencing. D, Sequence alignment and chromatograms of Sanger DNA sequencing results. In this region, the mutant T0-7 contains a 53-kb deletion based on the reference genome and the results of sequencing.
We previously reported that the sensor NLR Rpi-blb2, which confers resistance to the potato (Solanum tuberosum) late blight pathogen Phytophthora infestans, depends on NRC4 when expressed in N. benthamiana. To test whether Rpi-blb2 signals through NRC4 in tomato, we expressed Rpi-blb2/AVRblb2, Rpi-vnt1/AVRvnt1 (NRC independent), or GFP in the wild type and the NRC4 knockout tomato line using agroinfiltration (see Supplemental Methods). Rpi-blb2-mediated cell death was compromised in the NRC4 knockout plants, whereas Rpi-vnt1 triggered strong cell death in both wild-type and NRC4 knockout plants, consistent with the earlier finding from the N. benthamiana experimental system (Supplemental Fig. S4).
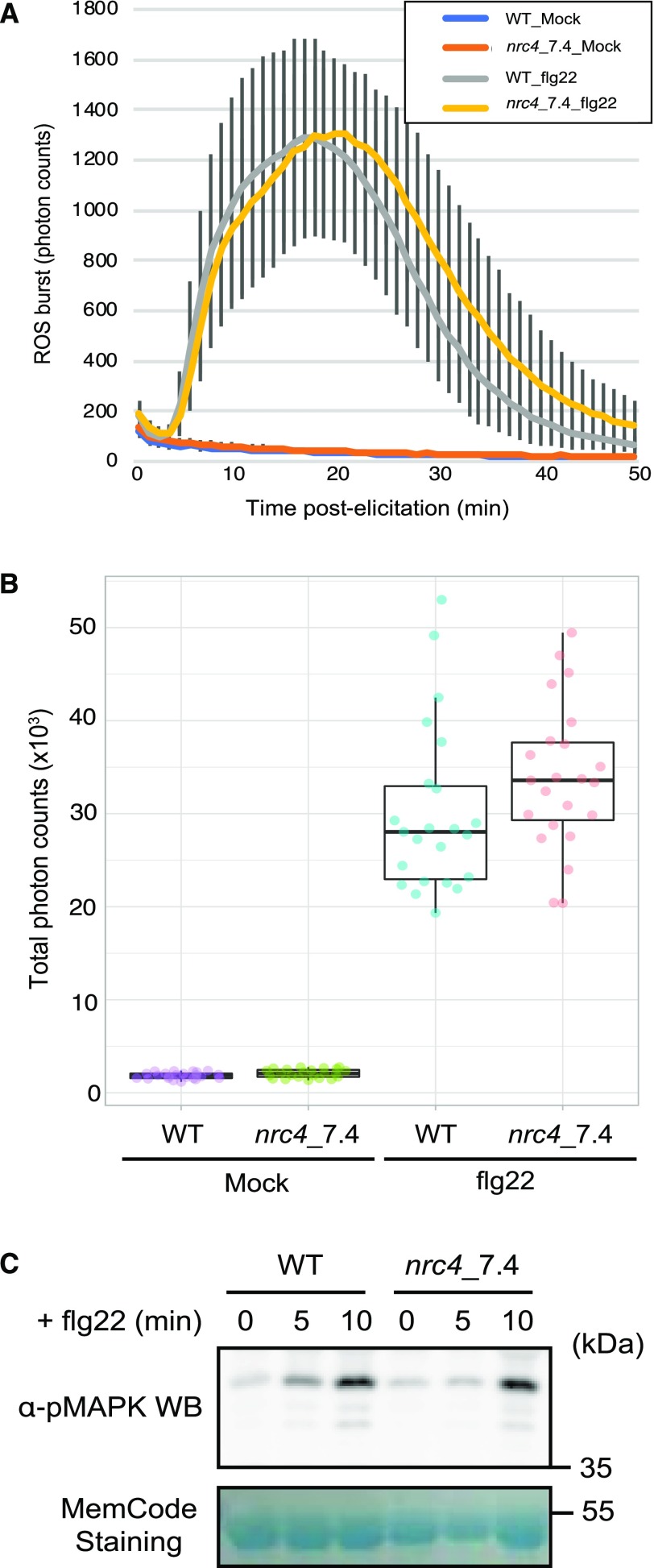
Leibman-Markus et al. (2018b) proposed that NRC4a participates in immunity mediated by FLS2 because overexpression of NRC4a in N. benthamiana enhances ROS production after flg22 treatment. Leibman-Markus et al. (2018b) obtained a CRISPR/Cas9 mutagenized tomato line that expresses a truncated variant of SlNRC4a. However, the effect of this mutation on flg22-induced responses was not reported. As NRC4a exists in a gene cluster with the highly homologous NRC4b and NRC4c, which are potentially functionally redundant, we reasoned that our NRC4/5 gene cluster deletion would be ideal for testing whether the NRC4 genes are required for FLS2-mediated responses. To test the hypothesis, we monitored apoplastic ROS production in response to flg22 peptides. We observed a transient flg22-induced ROS burst with the leaf discs from wild-type tomato plant GCR758. However, we did not observe a notable difference in terms of flg22-induced ROS burst between the wild type and the NRC4 deletion line nrc4_7.4 (Fig. 2, A and B). As MAPK activation represents another typical output in FLS2-mediated responses, we tested whether MAPK phosphorylation was impaired in the NRC4 knockout plants. We detected increased phosphorylation of MAPKs in the wild-type plants by immunoblot analysis with p-42/44 antibody after flg22 treatment. We also detected increased phosphorylation of MAPKs in the NRC4 knockout mutant, and here, too, we did not observe a significant difference between the wild type and the NRC4 deletion mutant (Fig. 2C). Our results indicate that the NRC4 genes are not essential for flg22-induced responses in tomato.
Figure 2.
NRC4 knockout tomato plants are not impaired in flg22-induced defense responses. A. Flg22-triggered ROS bursts were measured for 50 min using leaf discs of the wild type (WT) and T2 line nrc4_7.4. Data are presented as the means ± sd. B, Scatter plot and box plot of total photon counts of each treatment in A. C, Flg22-triggered MAPK activation was analyzed by immunoblots with α-pMAPK. Proteins were extracted from tomato leaf tissues of the wild type and T2 line nrc4_7.4 at 0, 5, or 10 min after treatment with flg22.
Previous studies have suggested that the NRC proteins are involved in immune responses mediated by both intracellular NLR and cell surface PRR immune receptors (Gabriëls et al., 2007; Wu et al., 2016, 2017; Brendolise et al., 2017). Silencing of NRC2 and NRC3 by virus-induced gene silencing and RNA interference reduces Cf4- and Prf-mediated cell death in N. benthamiana, indicating that NRC2 and NRC3 are involved in cell death responses activated in both pathways (Wu et al., 2016; Brendolise et al., 2017). Furthermore, silencing of NRC2, NRC3, and NRC4 together, but not individually, compromises cell death mediated by Rx, Bs2, and some other NLRs in N. benthamiana, and this phenotype can be complemented by individual NRCs (Wu et al., 2017). Given that NRC2, NRC3, and NRC4 display degrees of genetic redundancy in NLR- and PRR-mediated cell death in N. benthamiana, we sought to test whether knocking out NRC2/3/4 affects flg22-induced responses. We transformed N. benthamiana with guide RNAs targeting NRC2, NRC3, or NRC4 together with Cas9 and a phosphinothricin selection marker and obtained loss-of-function mutants (Supplemental Fig. S5A and S6A). We selected two independent T2 NRC4 knockout lines and two independent T2 NRC2/3/4 knockout lines for further characterization. Due to the complexity of the N. benthamiana genome and duplications of each NRC gene, these selected lines may express variants of NRC2, NRC3, or NRC4 proteins, ranging from 33 to 123 amino acid truncations to full-length NRCs with a 3-amino acid indel in the coiled-coil domain (Supplemental Figs. S5A and S6A). Consistent with our previous reports with virus-induced gene silencing assays, the two NRC4 knockout lines (nrc4_9.1.3 and nrc4_1.2.1) were found to be defective in Rpi-blb2-mediated cell death, and the two NRC2/3/4 knockout lines (nrc234_4.3.1 and nrc234_5.5.1) were defective in Rpi-blb2-, Prf(Pto)-, and Rx-mediated cell death (Supplemental Figs. S5 and S6). These results confirmed that the selected NRC4 and NRC2/3/4 knockout lines do not contain any NRC2, NRC3, or NRC4 variants that are still functional for the tested sensor NLR genes. Next, we tested the degree to which flg22-induced ROS burst and phosphorylation of MAPKs were affected in these NRC knockout N. benthamiana lines. Both the wild-type plants and the NRC knockout plants yielded similar results for flg22-induced ROS burst and MAPK phosphorylation assays (Supplemental Fig. S7). In conclusion, our results indicate that NRC2, NRC3, and NRC4 are not essential for flg22-induced responses in N. benthamiana.
The NRC network is phylogenetically restricted to asterids and caryophyllales, but is missing in Arabidopsis and other rosid species (Wu et al., 2017). Therefore, our results may not be that surprising given that FLS2 belongs to an ancient receptor-like kinase subfamily XII that broadly occurs in angiosperms (Dufayard et al., 2017; Liu et al., 2017). In contrast, NRCs may be involved in Cf-4- and LeEIX-mediated immunoresponses considering that these cell surface receptor-like proteins are phylogenetically restricted to some asterid clades (Kang and Yeom, 2018) and, unlike flg22, trigger hypersensitive cell death in plant tissues (Gabriëls et al., 2007; Wu et al., 2016; Brendolise et al., 2017). Future work will need to address how cell surface receptors mechanistically engage NLR proteins to induce cell death and other immune responses.
Supplemental Data
The following supplemental materials are available.
Supplemental Methods. Generation and phenotyping of NRC knockout tomato and N. benthamianaplants.
Supplemental Figure S1. Targeting the NRC4 gene cluster with CRISPR/Cas9 in tomato.
Supplemental Figure S2. Primer design and characterization of the large deletion in mutant T0-7.
Supplemental Figure S3. Sanger sequencing result of the NRC4 deletion allele from T0-7.
Supplemental Figure S4. The NRC4 knockout homozygous T2 line (nrc4_7.4) is impaired in Rpi-blb2-mediated cell death.
Supplemental Figure S5. Genotypes and phenotypes of NRC4 knockout N. benthamiana.
Supplemental Figure S6. Genotypes and phenotypes of NRC2/3/4 knockout N. benthamiana.
Supplemental Figure S7. Knocking out of NRCs in N. benthamiana did not affect flg22-induced defense responses.
Supplemental Table S1. Primers used in this study.
Acknowledgments
We thank the Tissue Culture and Transformation Team at The Sainsbury Laboratory for performing tomato transformation, Marta Bjornson for helping with the ROS assays, and Bruno Ngou and Hailong Guo for helping with the MAPK phosphorylation assays.
Footnotes
This research was supported by the Gatsby Charitable Foundation, the Biotechnology and Biological Sciences Research Council, the European Research Council, the Japan Society for the Promotion of Science (H.A.), and the John Innes Foundation (J.C.D.C. and R.C.-G.).
Articles can be viewed without a subscription.
References
- Adachi H, Derevnina L, Kamoun S (2019) NLR singletons, pairs, and networks: Evolution, assembly, and regulation of the intracellular immunoreceptor circuitry of plants. Curr Opin Plant Biol 50: 121–131 [DOI] [PubMed] [Google Scholar]
- Balint-Kurti PJ, Jones DA, Jones JD (1995) Integration of the classical and RFLP linkage maps of the short arm of tomato chromosome 1. Theor Appl Genet 90: 17–26 [DOI] [PubMed] [Google Scholar]
- Brendolise C, Montefiori M, Dinis R, Peeters N, Storey RD, Rikkerink EH (2017) A novel hairpin library-based approach to identify NBS-LRR genes required for effector-triggered hypersensitive response in Nicotiana benthamiana. Plant Methods 13: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari S. (2018) Multiple strategies for pathogen perception by plant immune receptors. New Phytol 219: 17–24 [DOI] [PubMed] [Google Scholar]
- Dodds PN, Rathjen JP (2010) Plant immunity: Towards an integrated view of plant-pathogen interactions. Nat Rev Genet 11: 539–548 [DOI] [PubMed] [Google Scholar]
- Dufayard JF, Bettembourg M, Fischer I, Droc G, Guiderdoni E, Périn C, Chantret N, Diévart A (2017) New insights on leucine-rich repeats receptor-like kinase orthologous relationships in angiosperms. Front Plant Sci 8: 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriëls SH, Vossen JH, Ekengren SK, van Ooijen G, Abd-El-Haliem AM, van den Berg GC, Rainey DY, Martin GB, Takken FL, de Wit PJ, et al. (2007) An NB-LRR protein required for HR signalling mediated by both extra- and intracellular resistance proteins. Plant J 50: 14–28 [DOI] [PubMed] [Google Scholar]
- Kang WH, Yeom SI (2018) Genome-wide Identification, classification, and expression analysis of the receptor-like protein family in tomato. Plant Pathol J 34: 435–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourelis J, van der Hoorn RAL (2018) Defended to the nines: 25 years of resistance gene coning identifies nine mechanisms for R protein function. Plant Cell 30: 285–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibman-Markus M, Pizarro L, Bar M, Coaker G, Avni A (2018a) NRC proteins— a critical node for pattern and effector mediated signaling. Plant Signal Behav 13: e1507404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibman-Markus M, Pizarro L, Schuster S, Lin ZJD, Gershony O, Bar M, Coaker G, Avni A (2018b) The intracellular nucleotide-binding leucine-rich repeat receptor (SlNRC4a) enhances immune signalling elicited by extracellular perception. Plant Cell Environ 41: 2313–2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PL, Du L, Huang Y, Gao SM, Yu M (2017) Origin and diversification of leucine-rich repeat receptor-like protein kinase (LRR-RLK) genes in plants. BMC Evol Biol 17: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, van Wersch R, Zhang Y (2018) Convergent and divergent signaling in PAMP-triggered immunity and effector-triggered immunity. Mol Plant Microbe Interact 31: 403–409 [DOI] [PubMed] [Google Scholar]
- Win J, Chaparro-Garcia A, Belhaj K, Saunders DG, Yoshida K, Dong S, Schornack S, Zipfel C, Robatzek S, Hogenhout SA, et al. (2012) Effector biology of plant-associated organisms: concepts and perspectives. Cold Spring Harb Symp Quant Biol 77: 235–247 [DOI] [PubMed] [Google Scholar]
- Wu CH, Abd-El-Haliem A, Bozkurt TO, Belhaj K, Terauchi R, Vossen JH, Kamoun S (2017) NLR network mediates immunity to diverse plant pathogens. Proc Natl Acad Sci USA 114: 8113–8118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CH, Belhaj K, Bozkurt TO, Birk MS, Kamoun S (2016) Helper NLR proteins NRC2a/b and NRC3 but not NRC1 are required for Pto-mediated cell death and resistance in Nicotiana benthamiana. New Phytol 209: 1344–1352 [DOI] [PubMed] [Google Scholar]
- Wu CH, Derevnina L, Kamoun S (2018) Receptor networks underpin plant immunity. Science 360: 1300–1301 [DOI] [PubMed] [Google Scholar]